Abstract
A combination of several enzymes, RNase-T1, nuclease S1, T4-polynucleotide kinase and T4-RNA ligase were used to prepare and modify different fragments of yeast tRNAAsp (normal anticodon G U C). This allowed us to reconstitute, in vitro, a chimeric tRNA that has any of the four bases G, A, U or C, as the first anticodon nucleotide, labelled with (32p) in its 3' position. Such reconstituted (32p) labelled yeast tRNAAsp were microinjected into the cytoplasm or the nucleus of the frog oocyte and checked for their stability as well as for their potential to work as a substrate for the maturation (modifying) enzymes under in vivo conditions. Our results indicate that the chimeric yeast tRNAsAsp were quite stable inside the frog oocyte. Also, the G34 was effectively transformed inside the cytoplasm of frog oocyte into Q34 and mannosyl-Q34; U34 into mcm5s2U and mcm5U. In contrast, C34 and A34 were not transformed at all neither in the cytoplasm nor in the nucleus of the frog oocyte. The above procedure constitutes a new approach in order to detect the presence of a given modifying enzyme inside the frog oocyte; also it provides informations about its cellular location and possibility about its specificity of interaction with foreign tRNA.
Full text
PDF



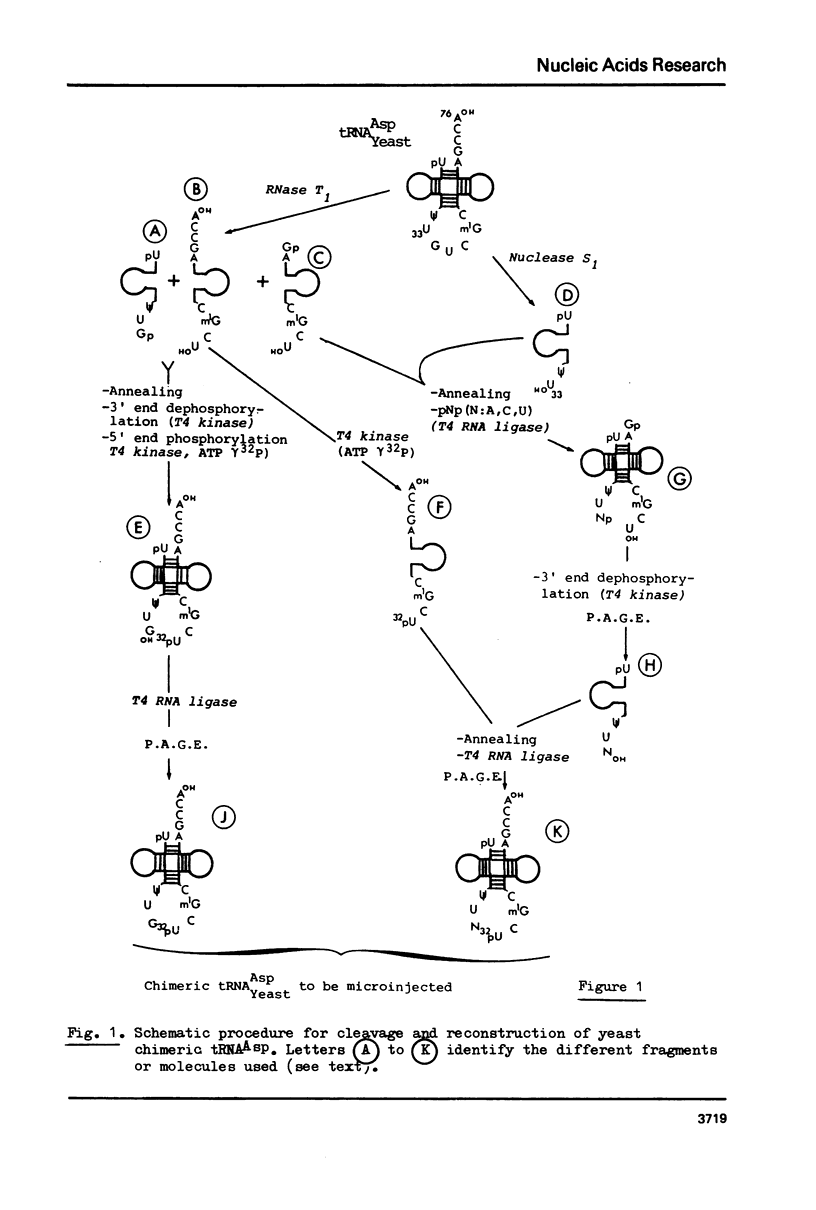






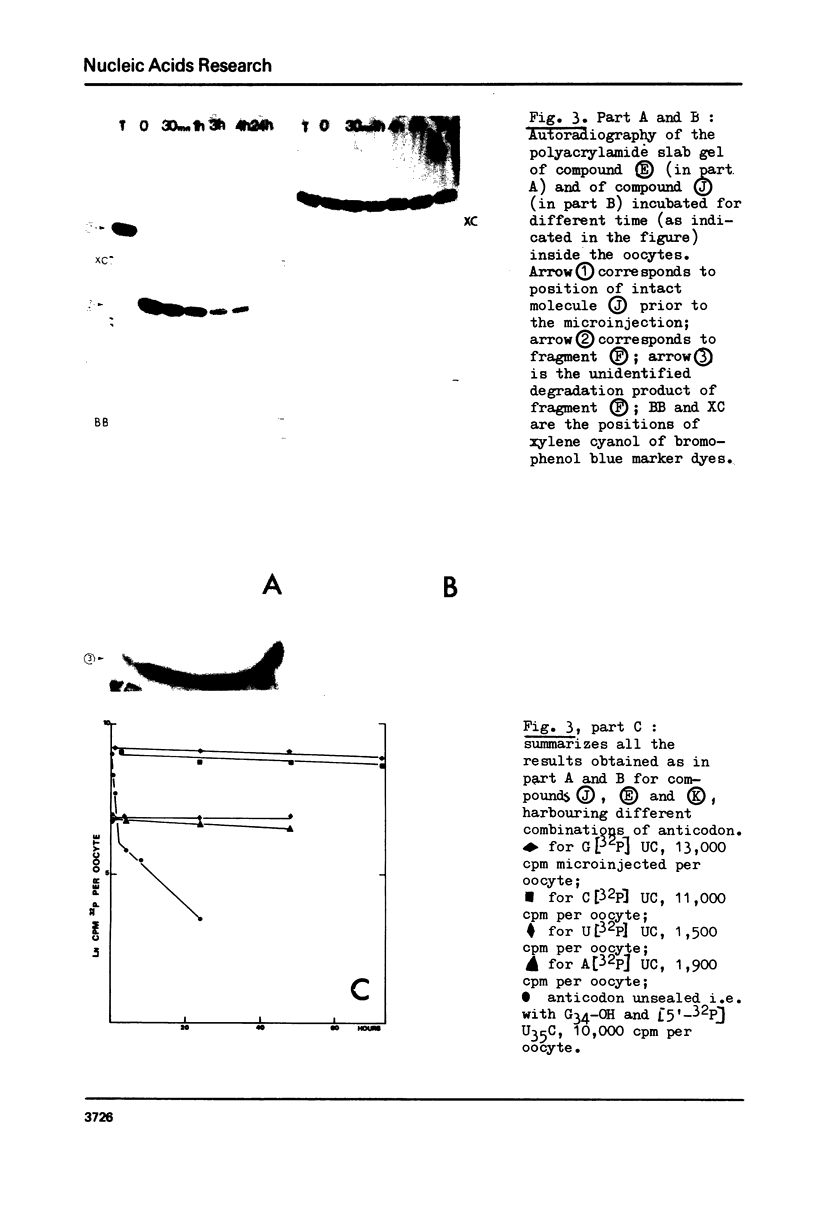

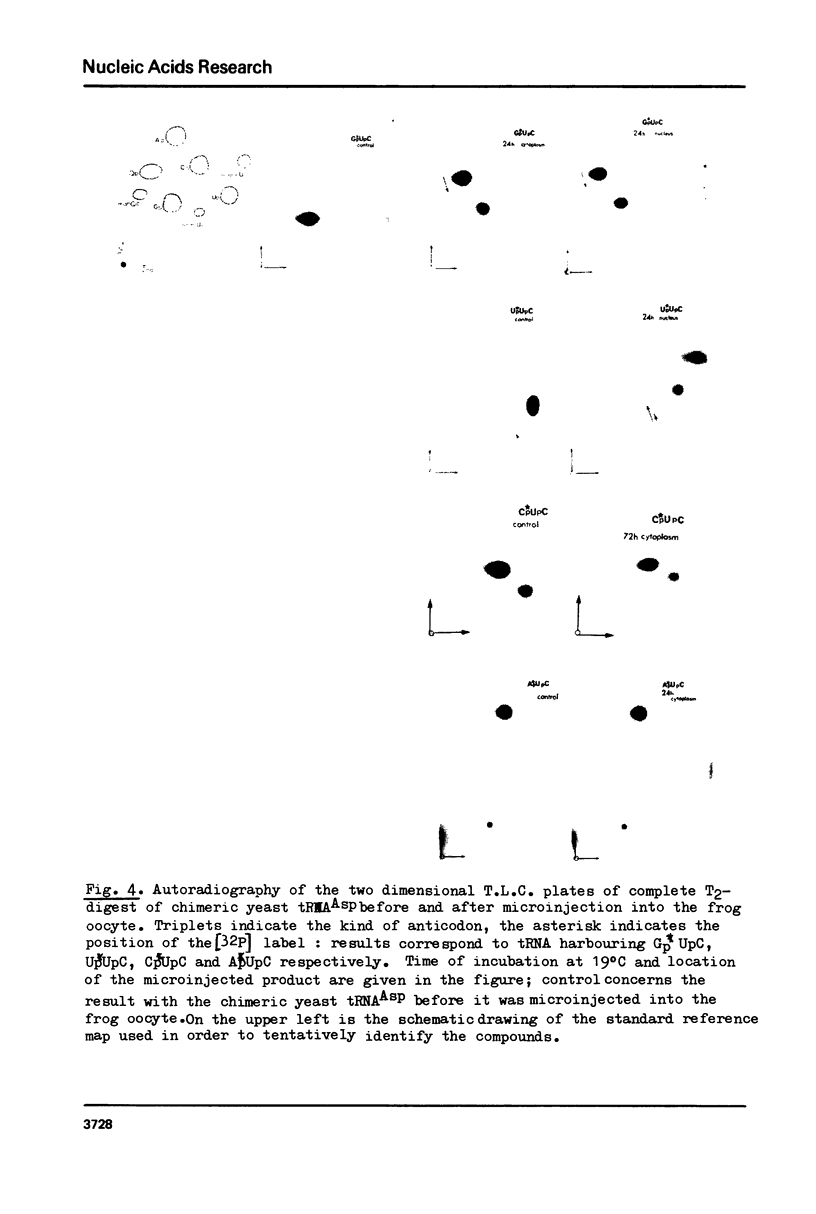




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Soltis D., Uhlenbeck O. C. Polynucleotide kinase from a T4 mutant which lacks the 3' phosphatase activity. Nucleic Acids Res. 1978 Mar;5(3):825–833. doi: 10.1093/nar/5.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Piper P. W. Compilation of mutant suppressor tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r83–r91. doi: 10.1093/nar/10.2.762-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi M. S., Melton D. A., Cortese R. Site-directed mutagenesis of a tRNA gene: base alterations in the coding region affect transcription. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1388–1392. doi: 10.1073/pnas.79.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugré M., Cedergren R. J. Origine de l'inosine dans les tRNA de levure. Can J Biochem. 1974 May;52(5):417–422. [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Keith G., Ebel J. P., Dirheimer G. The primary structure of aspartate transfer ribonucleic acid from brewer's yeast. II. Partial digestions with pancreatic ribonuclease and T 1 ribonuclease and derivation of complete sequence. Biochim Biophys Acta. 1972 Jan 31;259(2):210–222. [PubMed] [Google Scholar]
- Ghosh R. K., Deutscher M. P. Identification of an Escherichia coli nuclease acting on structurally altered transfer RNA molecules. J Biol Chem. 1978 Feb 25;253(4):997–1000. [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hassur S. M., Whitlock H. W., Jr UV shadowing--a new and convenient method for the location of ultraviolet-absorbing species in polyacrylamide gels. Anal Biochem. 1974 May;59(1):162–164. doi: 10.1016/0003-2697(74)90020-7. [DOI] [PubMed] [Google Scholar]
- Jukes T. H. How many anticodons? Science. 1977 Oct 21;198(4314):319–320. doi: 10.1126/science.910132. [DOI] [PubMed] [Google Scholar]
- Kammen H. O., Spengler S. J. The biosynthesis of inosinic acid in transfer RNA. Biochim Biophys Acta. 1970 Aug 8;213(2):352–364. doi: 10.1016/0005-2787(70)90043-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Littauer U. Z. Covalent joining of phenylalanine transfer ribonucleic acid half-molecules by T4 RNA ligase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3741–3745. doi: 10.1073/pnas.71.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski R. A., Clarkson S. G., Kurjan J., Hall B. D., Smith M. Mutations of the yeast SUP4 tRNATyr locus: transcription of the mutant genes in vitro. Cell. 1980 Nov;22(2 Pt 2):415–425. doi: 10.1016/0092-8674(80)90352-9. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Pirrotta V., Birnstiel M. L. Transcription of cloned tRNA gene fragments and subfragments injected into the oocyte nucleus of Xenopus laevis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1176–1180. doi: 10.1073/pnas.75.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I., Leineweber M., RajBhandary U. L. Site-specific mutagenesis on cloned DNAs: generation of a mutant of Escherichia coli tyrosine suppressor tRNA in which the sequence G-T-T-C corresponding to the universal G-T-pseudouracil-C sequence of tRNAs is changed to G-A-T-C. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4753–4757. doi: 10.1073/pnas.78.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., De Robertis E. M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol. 1981 Jan 15;145(2):405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Kurjan J., Hall B. D., De Robertis E. M. Genetic analysis of the processing of a spliced tRNA. EMBO J. 1982;1(2):263–268. doi: 10.1002/j.1460-2075.1982.tb01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Tanaka T., Miyake T., Markham A. F., Nakagawa E., Wakabayashi T., Taniyama Y., Nishikawa S., Fukumoto R. Total synthesis of a RNA molecule with sequence identical to that of Escherichia coli formylmethionine tRNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5493–5497. doi: 10.1073/pnas.78.9.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Nishimura S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J Biol Chem. 1979 Apr 25;254(8):3061–3066. [PubMed] [Google Scholar]
- Schlegel R. A., Iversen P., Rechsteiner M. The turnover of tRNAs microinjected into animal cells. Nucleic Acids Res. 1978 Oct;5(10):3715–3729. doi: 10.1093/nar/5.10.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Dingermann T., Farrell P., Söll D. Internal control regions for transcription of eukaryotic tRNA genes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6657–6661. doi: 10.1073/pnas.78.11.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A., Gatica M., Allende J. E. In vivo repair of the 3'terminus of transfer RNA injected into amphibian oocytes. Nucleic Acids Res. 1977 Jun;4(6):1873–1880. doi: 10.1093/nar/4.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of sequences of tRNA genes. Nucleic Acids Res. 1982 Jan 22;10(2):r57–r81. [PMC free article] [PubMed] [Google Scholar]
- Waldron C., Cox B. S., Wills N., Gesteland R. F., Piper P. W., Colby D., Guthrie C. Yeast ochre suppressor SUQ5-ol is an altered tRNA Ser UCA. Nucleic Acids Res. 1981 Jul 10;9(13):3077–3088. doi: 10.1093/nar/9.13.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. H., Zhu L. Q., Yuan J. G., Liu F., Zhang L. F. Joining of yeast alanine transfer ribonucleic acid half molecules to form a whole molecule by T4 RNA ligase. Biochim Biophys Acta. 1981 Jan 29;652(1):82–89. doi: 10.1016/0005-2787(81)90211-2. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Breeden L. Mutants of Su+7 tRNA include a functional tRNA with an altered T pseudo uracil CG sequence. Cell. 1981 Sep;25(3):815–823. doi: 10.1016/0092-8674(81)90189-6. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Hamer D. H. TRNA precursor transcribed from a mutant human gene inserted into a SV40 vector is processed incorrectly. Nature. 1982 Feb 11;295(5849):533–535. doi: 10.1038/295533a0. [DOI] [PubMed] [Google Scholar]





