Abstract
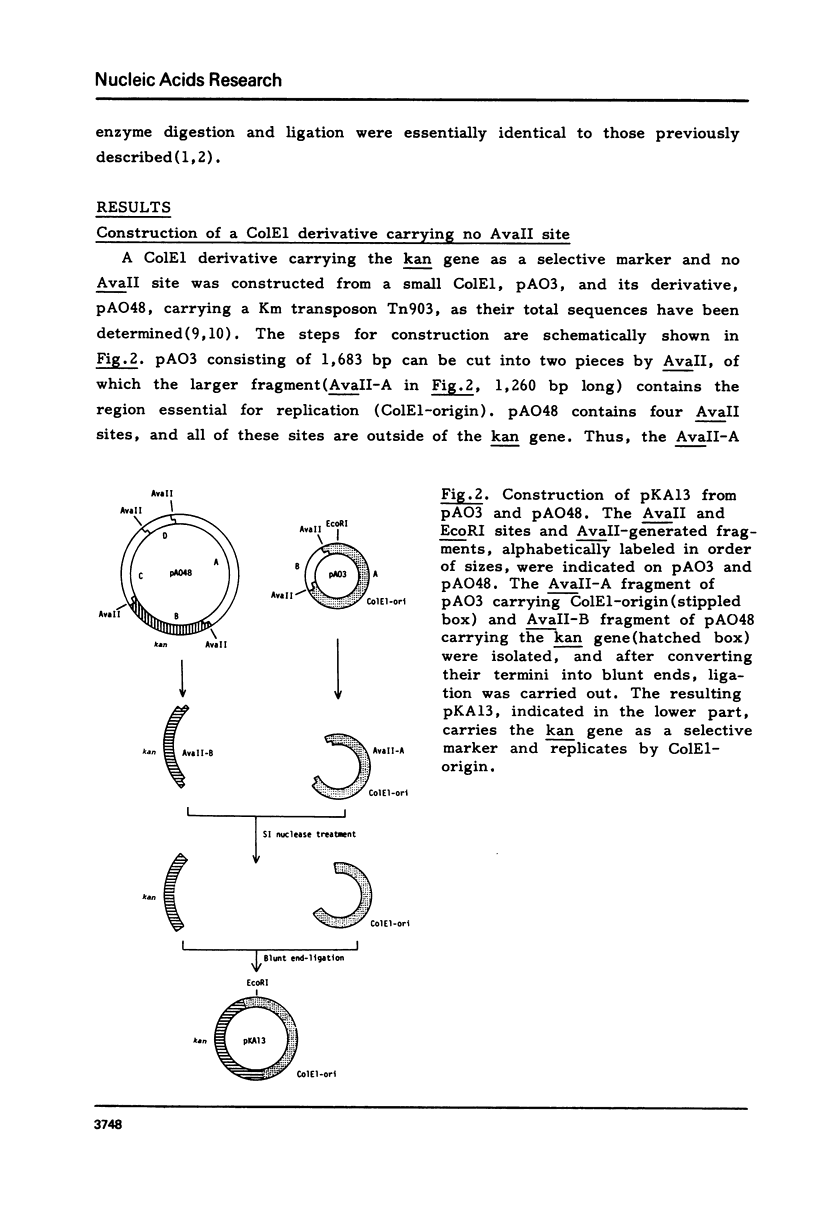
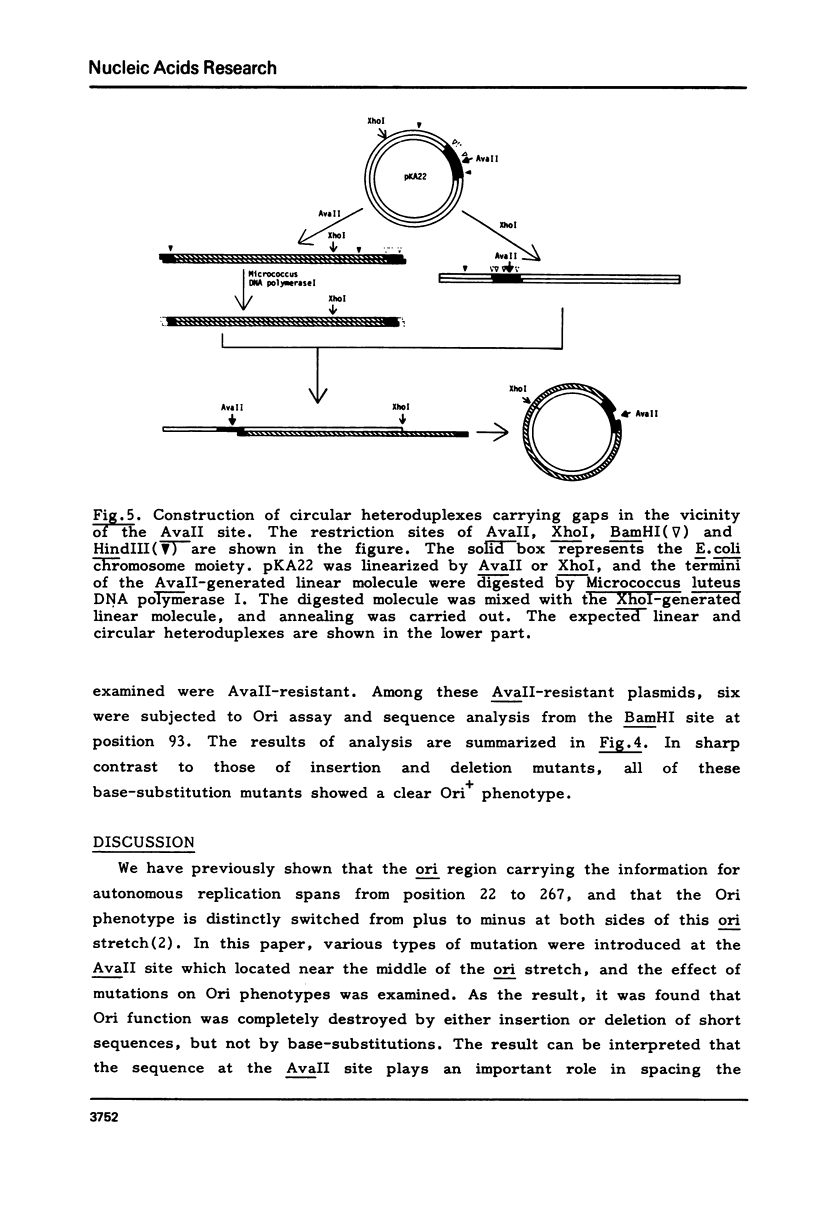
The replication origin region of the Escherichia coli K-12 chromosome has been cloned, and a region of 245 base-pairs has been shown to contain all the information for autonomous replication (defined ori). In order to obtain further information on the sequence organization in the defined ori stretch, various types of mutation were introduced by in vitro techniques at a restriction site (AvaII site) which locates near the middle of ori. When the correlation between these mutations and replicating function was examined, different effects were obtained with the types of mutation: the replicating function was completely destroyed by either insertion or deletion of short sequences, but not by base-substitutions. Based on these observations and on the fact that multi-gene products are involved in the initiation of replication, we assume that two categories of sequences are present within the ori stretch, one specifying interaction with initiation factors (recognition sequences) and the other spacing the recognition sequences in appropriate distances (spacer sequences), and that the AvaII site is located in the spacer region.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Chow L. T., Dugaiczyk A., Hedgpeth J., Goodman H. M. DNA substrate site for the EcoRII restriction endonuclease and modification methylase. Nat New Biol. 1973 Jul 11;244(132):40–43. doi: 10.1038/newbio244040a0. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Oka A., Nomura N., Sugimoto, Sugisaki H., Takanami M. Nucleotide sequence at the insertion sites of a kanamycin transposon. Nature. 1978 Dec 21;276(5690):845–847. doi: 10.1038/276845a0. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Braverman B., Louis J. B., Servis R. E. Nucleic acid reactivity and conformation. II. Reaction of cytosine and uracil with sodium bisulfite. J Biol Chem. 1973 Jun 10;248(11):4060–4064. [PubMed] [Google Scholar]
- Shapiro R., Cohen B. I., Servis R. E. Specific deamination of RNA by sodium bisulphite. Nature. 1970 Sep 5;227(5262):1047–1048. doi: 10.1038/2271047a0. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]


