Abstract
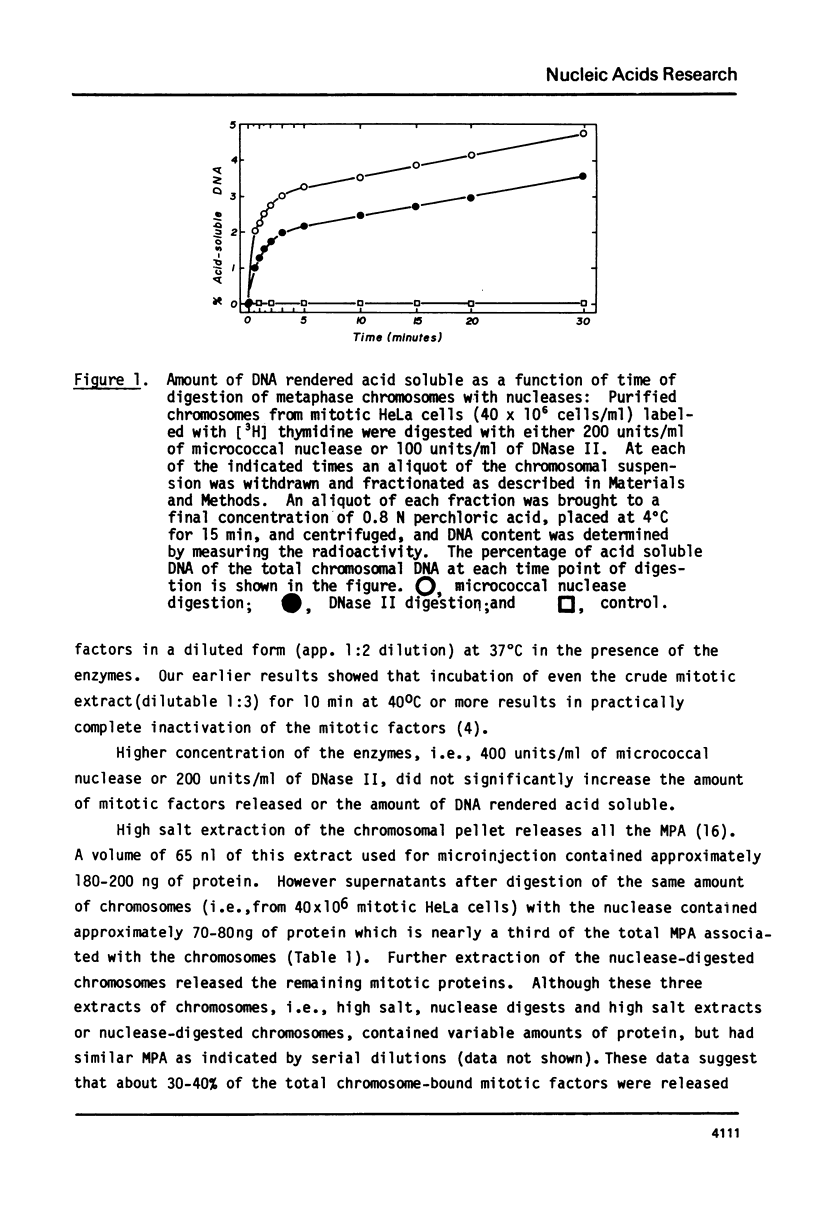
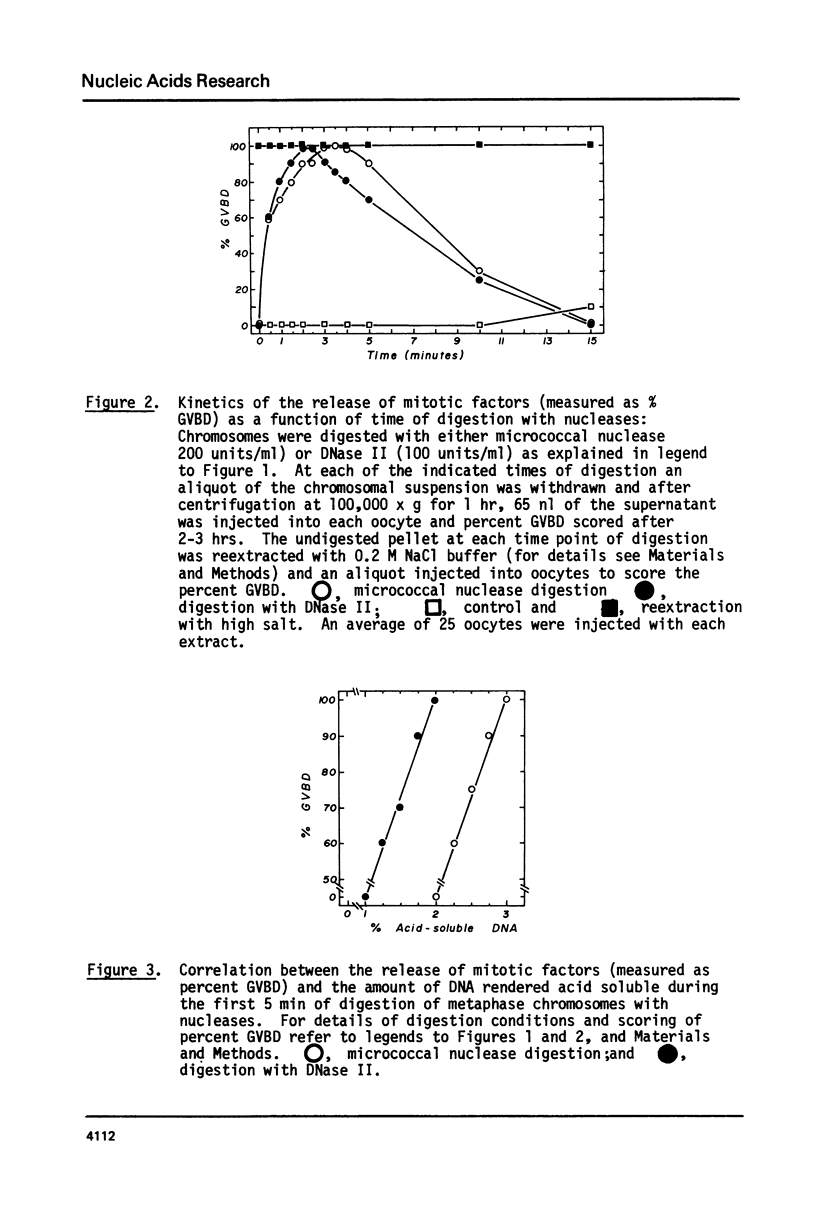
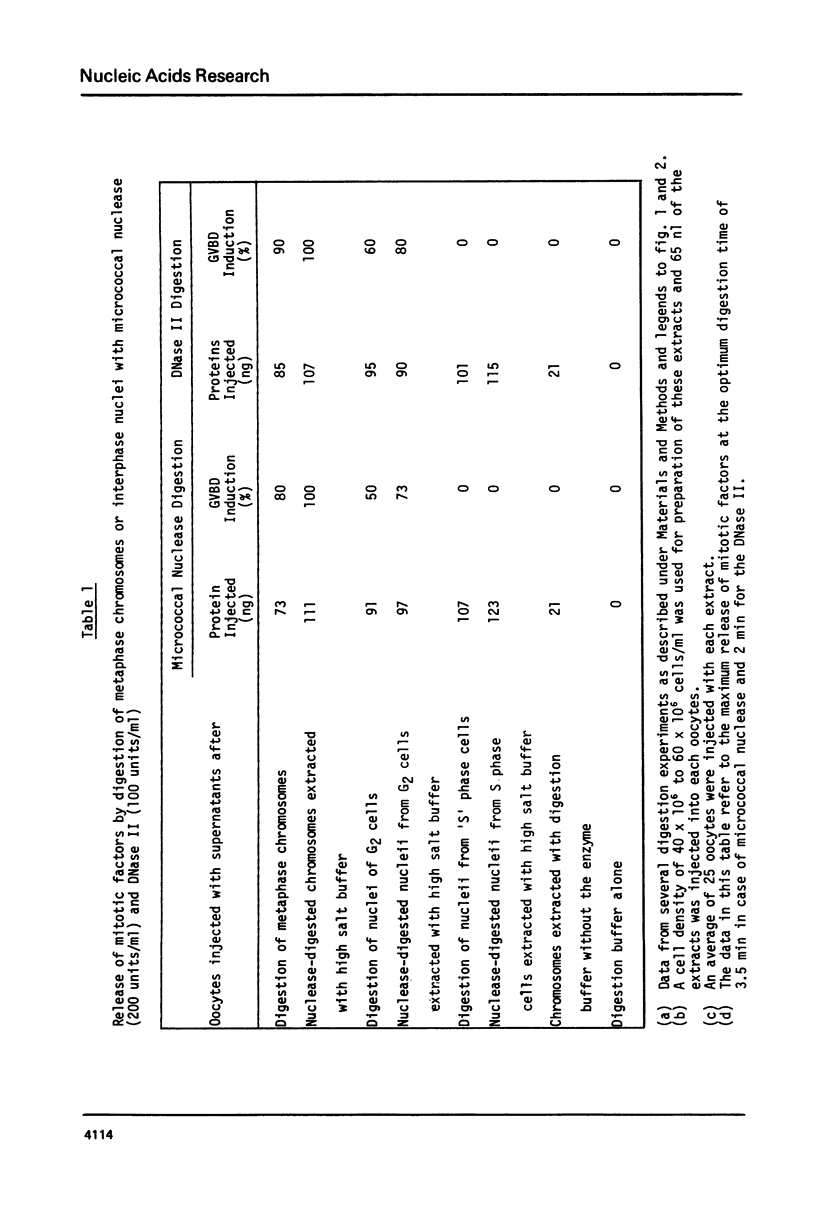
Additional evidence is presented to support our recently reported conclusion that the mitotic factors of mammalian cells, which induce germinal vesicle breakdown and chromosome condensation when injected into fully grown Xenopus laevis oocytes, are localized on metaphase chromosomes. Chromosomes isolated from mitotic HeLa cells were further purified on sucrose gradients and digested for varying periods with either the micrococcal nuclease or DNase II. At each time point of digestion the amount of mitotic factors released was determined by injecting a supernatant of these fractions, obtained by high-speed centrifugation, into oocytes. The amount of DNA rendered acid soluble under the conditions of digestion used was 3% ot 5% of the total chromosomal DNA. The extent of release of mitotic factors with both nucleases was estimated to be about 30% to 40% as evidenced by the reextraction of the undigested chromosomal pellet with 0.2 M NaC1. Similar results were obtained when nuclei from G2 cells were digested under identical conditions. The release of these chromosome-bound mitotic factors by mild digestion with these nucleases though only partial, clearly demonstrates that a significant proportion of these factors are localized on metaphase chromosomes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlakha R. C., Sahasrabuddhe C. G., Wright D. A., Lindsey W. F., Rao P. N. Localization of mitotic factors on metaphase chromosomes. J Cell Sci. 1982 Apr;54:193–206. doi: 10.1242/jcs.54.1.193. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Billett M. A. Protein distribution amongst chromatin particles following DNAse II digestion. Cell Biol Int Rep. 1981 Jan;5(1):55–58. doi: 10.1016/0309-1651(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Dieden J. D., Kapp L. N., Sedat J. W. Rapid isolation of metaphase chromosomes containing high molecular weight DNA. J Cell Biol. 1979 Apr;81(1):255–259. doi: 10.1083/jcb.81.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet J., Baltus E., De Schutter-Pays A., Hanocq-Quertier J., Hubert E., Steinert G. Induction of maturation (meiosis) in Xenopus laevis oocytes by three organomercurials. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1574–1578. doi: 10.1073/pnas.72.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- El-Etr M., Schorderet-Slatkine S., Baulieu E. E. Meiotic maturation in Xenopus laevis oocytes initiated by insulin. Science. 1979 Sep 28;205(4413):1397–1399. doi: 10.1126/science.472755. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Partington G. A. Distribution of messenger RNA-coding sequences in fractionated chromatin. Cell. 1977 Dec;12(4):953–962. doi: 10.1016/0092-8674(77)90160-x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. Structure of chromosome fibers and chromosomes. Annu Rev Biochem. 1973;42:355–378. doi: 10.1146/annurev.bi.42.070173.002035. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Rao P. N. Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei. Nature. 1970 May 23;226(5247):717–722. doi: 10.1038/226717a0. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Koontz J. W. A study of the induction of cell division in amphibian oocytes by insulin. Dev Biol. 1981 Jul 30;85(2):309–316. doi: 10.1016/0012-1606(81)90262-1. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Masui Y. Relative roles of the pituitary, follicle cells, and progesterone in the induction of oocyte maturation in Rana pipiens. J Exp Zool. 1967 Dec;166(3):365–375. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- Nelkin B., Nichols C., Vogelstein B. Protein factor(s) from mitotic CHO cells induce meiotic maturation in Xenopus laevis oocytes. FEBS Lett. 1980 Jan 14;109(2):233–238. doi: 10.1016/0014-5793(80)81094-5. [DOI] [PubMed] [Google Scholar]
- RAO P. N., ENGELBERG J. HELA CELLS: EFFECTS OF TEMPERATURE ON THE LIFE CYCLE. Science. 1965 May 21;148(3673):1092–1094. doi: 10.1126/science.148.3673.1092. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Pathak S., Hsu T. C. Responses of mammalian metaphase chromosomes to endonuclease digestion. Chromosoma. 1978 Dec 6;69(3):331–338. doi: 10.1007/BF00332136. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Saunders G. F. Salt-induced structural changes in nucleosomes. Nucleic Acids Res. 1977 Apr;4(4):853–866. doi: 10.1093/nar/4.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. Initiation of meiotic maturation in Xenopus laevis oocytes by lanthanum. Nature. 1976 Jul 22;262(5566):289–290. doi: 10.1038/262289a0. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Corden J. L., Sahasrabuddhe C. G., Van Holde K. E. Chromatographic separation of chromatin subunits. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1193–1198. doi: 10.1016/s0006-291x(74)80410-9. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2799–2802. doi: 10.1073/pnas.76.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells: a preliminary characterization. J Supramol Struct. 1979;11(2):189–195. doi: 10.1002/jss.400110208. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Smith L. D. Calmodulin triggers the resumption of meiosis in amphibian oocytes. J Cell Biol. 1981 Jun;89(3):389–394. doi: 10.1083/jcb.89.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Axel R. Nucleosomes in metaphase chromosomes. Nucleic Acids Res. 1976 Jun;3(6):1463–1471. doi: 10.1093/nar/3.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]


