Abstract
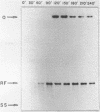
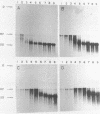
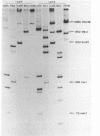
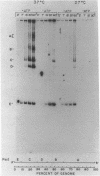
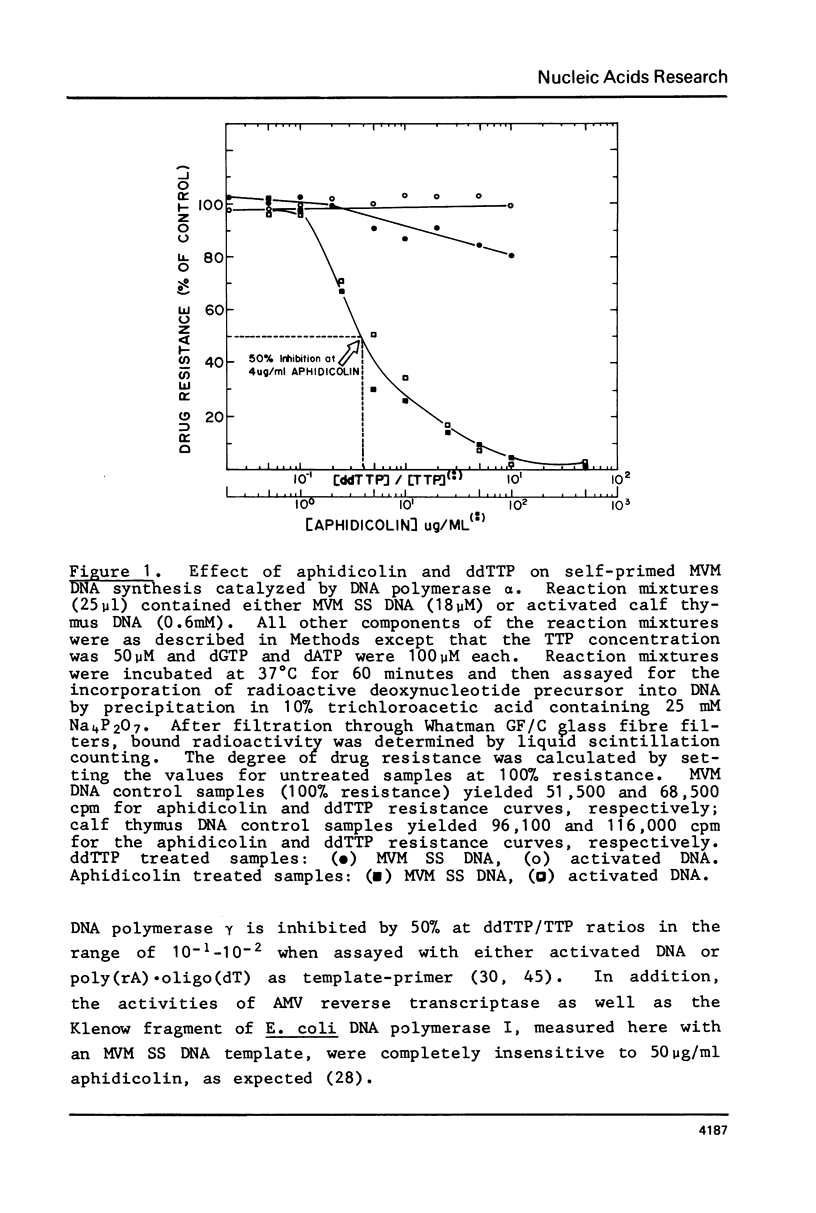
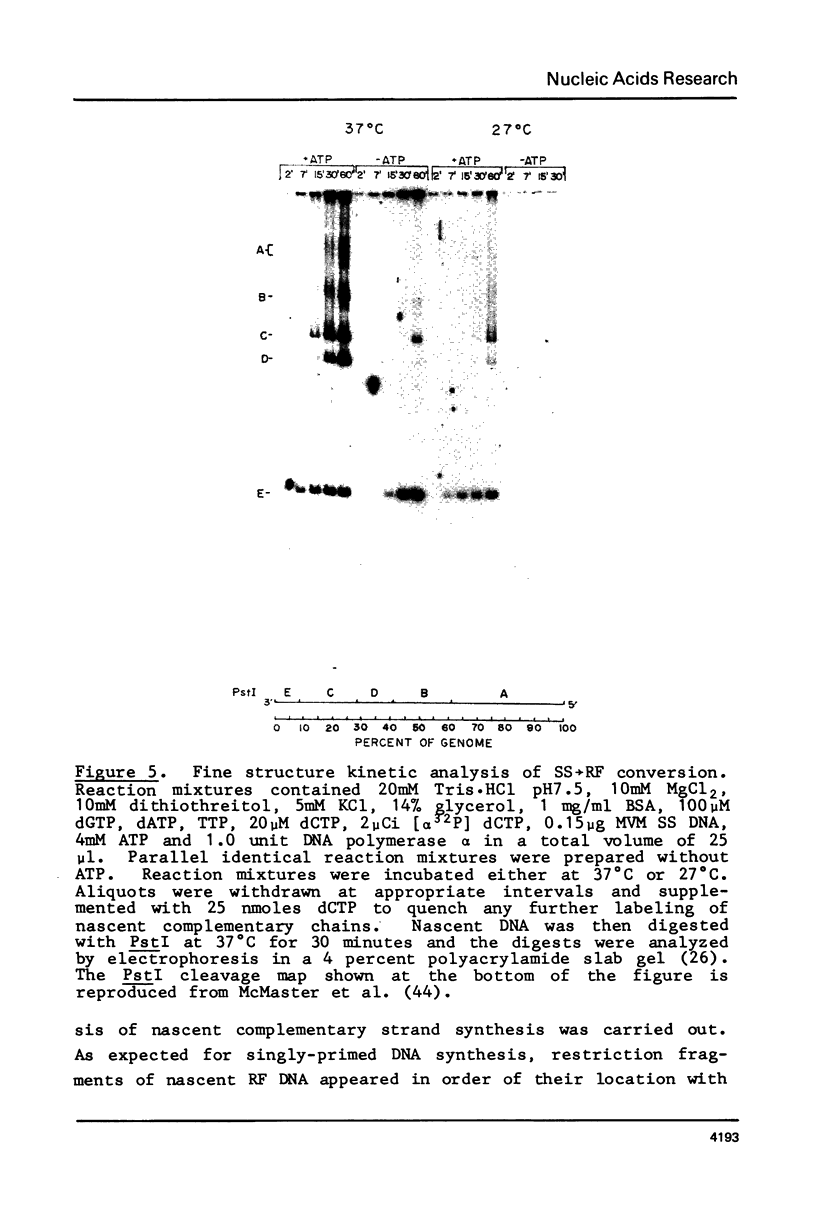
A partially purified preparation of DNA polymerase alpha, obtained from the cytosol of Ehrlich ascites tumour cells, has been found to catalyze the conversion of MVM parvovirus, SS DNA (5 kilobases) to RF in vitro. The reaction initiates at a natural 55 base pair hairpin which exists at the 3' terminus of MVM SS DNA. The SS leads to RF conversion is sensitive to aphidicolin, resistant to ddTTP and is promoted by purine ribonucleoside 5' triphosphates, a phenomenon which could not be explained simply by stabilization effects on the in vitro deoxynucleotide precursor pool. In the absence of rNTPs, nascent complementary strands frequently terminate prematurely at a preferred location, between 1300 and 1700 nucleotides from the initiating 3' hairpin terminus. This in vitro system, involving self-primed parvovirus DNA synthesis, provides a convenient assay for those components of the mammalian replicative DNA polymerase complex which are required for the elongation of nascent DNA chains.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Smith M., Chow M. B., Ward D. C. Sequence of the 3' terminus of the genome from Kilham rat virus, a nondefective parvovirus. Virology. 1979 Jul 30;96(2):669–674. doi: 10.1016/0042-6822(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Smith M., Chow M. B., Ward D. C. Structure of the 3' hairpin termini of four rodent parvovirus genomes: nucleotide sequence homology at origins of DNA replication. Cell. 1979 Jul;17(3):691–703. doi: 10.1016/0092-8674(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Plummer J., Huberman A. J., Evans M. J. Restriction fragment primed phi X174 single-stranded DNA as template for DNA polymerase alpha and beta. Detection and partial purification of a polymerase alpha stimulating factor. Biochim Biophys Acta. 1980 Sep 19;609(2):205–223. doi: 10.1016/0005-2787(80)90232-4. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Chen J. T., Korn D. Enzymological characterization of KB cell DNA polymerase-alpha. Regulation of template binding by nucleic acid base composition. J Biol Chem. 1981 Jan 10;256(1):133–141. [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Fisher P. A., Korn D. Enzymological characterization of KB cell DNA polymerase-alpha. III. The polymerization reaction with single-stranded DNA. J Biol Chem. 1979 Nov 10;254(21):11040–11046. [PubMed] [Google Scholar]
- Fisher P. A., Wang T. S., Korn D. Enzymological characterization of DNA polymerase alpha. Basic catalytic properties processivity, and gap utilization of the homogeneous enzyme from human KB cells. J Biol Chem. 1979 Jul 10;254(13):6128–6137. [PubMed] [Google Scholar]
- Handa H., Carter B. J. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J Biol Chem. 1979 Jul 25;254(14):6603–6610. [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E., Alberts B. M. Two types of replication proteins increase the rate at which T4 DNA polymerase traverses the helical regions in a single-stranded DNA template. J Biol Chem. 1981 Apr 25;256(8):4087–4094. [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Fine mapping of secondary structures of fd phage DNA in the region of the replication origin. Nucleic Acids Res. 1981 Nov 11;9(21):5587–5599. doi: 10.1093/nar/9.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Pauses at positions of secondary structure during in vitro replication of single-stranded fd bacteriophage DNA by T4 DNA polymerase. Anal Biochem. 1980 Mar 15;103(1):127–139. doi: 10.1016/0003-2697(80)90246-8. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Longiaru M., Horwitz M. S., Hurwitz J. Elongation of primed DNA templates by eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5827–5831. doi: 10.1073/pnas.77.10.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Goulian M. Synthesis of parvovirus H-1 replicative form from viral DNA by DNA polymerase gamma. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6206–6210. doi: 10.1073/pnas.78.10.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Tseng B. Y., Goulian M. DNA polymerase requirements for parvovirus H-1 DNA replication in vitro. J Virol. 1982 Mar;41(3):982–989. doi: 10.1128/jvi.41.3.982-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Novak B., Baril E. F. HeLa DNA polymerase alpha activity in vitro: specific stimulation by a non-enzymic protein factor. Nucleic Acids Res. 1978 Jan;5(1):221–239. doi: 10.1093/nar/5.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Effect of aphidicolin on viral and human DNA polymerases. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1194–1202. doi: 10.1016/0006-291x(79)91106-9. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planck S. R., Wilson S. H. Studies on the structure of mouse helix-destabilizing protein-1. DNA binding and controlled proteolysis with trypsin. J Biol Chem. 1980 Dec 10;255(23):11547–11556. [PubMed] [Google Scholar]
- Pritchard C., Stout E. R., Bates R. C. Replication of parvoviral DNA. I. Characterization of a nuclear lysate system. J Virol. 1981 Jan;37(1):352–362. doi: 10.1128/jvi.37.1.352-362.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos A., Sedgwick S. G., Yarranton G. T., Hübscher U., Banks G. R. Detection of the catalytic activities of DNA polymerases and their associated exonucleases following SDS-polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Apr 24;9(8):1825–1839. doi: 10.1093/nar/9.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Villani G., Fay P. J., Bambara R. A., Lehman I. R. Elongation of RNA-primed DNA templates by DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1981 Aug 10;256(15):8202–8207. [PubMed] [Google Scholar]
- Villani G., Sauer B., Lehman I. R. DNA polymerase alpha from Drosophila melanogaster embryos. Subunit structure. J Biol Chem. 1980 Oct 10;255(19):9479–9483. [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Nature. 1978 Nov 30;276(5687):532–534. doi: 10.1038/276532a0. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bouché J. P., Méchali M., Girard M. Involvement of both DNA polymerases alpha and gamma in the replication of adenovirus deoxyribonucleic acid in vitro. Virology. 1980 Jul 15;104(1):56–72. doi: 10.1016/0042-6822(80)90365-7. [DOI] [PubMed] [Google Scholar]