Abstract
The glutamate system has been strongly implicated in the pathophysiology of psychotic illnesses, including schizophrenia and schizoaffective disorder. We recently found that knockout (KO) mice lacking the AMPA GluA1 subunit displayed behavioral abnormalities relevant to some of the positive symptoms of these disorders. Here we phenotyped GluA1 KO mice for behavioral phenotypes pertinent to negative and cognitive/executive symptoms. GluA1 KO mice were tested for conspecific social interactions, the acquisition and extinction of an operant response for food-reward, operant-based pairwise visual discrimination and reversal learning, and impulsive choice in a delay-based cost/benefit decision-making T-maze task. Results showed that GluA1 KO mice engaged in less social interaction than wildtype (WT) controls when tested in a non-habituated, novel environment, but, conversely, displayed more social interaction in a well habituated, familiar environment. GluA1 KO mice were faster to acquire an operant stimulus-response for food reward than WT and were subsequently slower to extinguish the response. Genotypes showed similar pairwise discrimination learning and reversal, although GluA1 KO mice made fewer errors during early reversal. GluA1 KO mice also displayed increased impulsive choice, being less inclined to choose a delayed, larger reward when given a choice between this and a smaller, immediate reward, compared to WT mice. Finally, sucrose preference did not differ between genotypes. Collectively, these data add to the growing evidence that GluA1 KO mice display at least some phenotypic abnormalities mimicking those found in schizophrenia/schizoaffective disorder. Although these mice, like any other single mutant line, are unlikely to model the entire disease, they may nevertheless provide a useful tool for studying the role of GluA1 in certain aspects of the pathophysiology of major psychotic illness.
Keywords: Glutamate, Schizophrenia, Schizoaffective, Cognition, Executive function, Knockout
1. Introduction
L-glutamate is the major excitatory neurotransmitter in the brain, and glutamatergic dysfunction is strongly implicated in neuropsychiatric diseases including anxiety, depression, bipolar disorder, and schizophrenia (Coyle, 2006). Glutamatergic neurotransmission is mediated by a complex array of receptors belonging to the ionotropic (α-amino-3-hydroxy-5-methyl-4isoxazole propionic acid [AMPA], N-methyl-D-aspartate [NMDA], kainate) and metabotropic receptor (mGluR) subfamilies. AMPA receptors are postsynaptic heteromeric proteins composed of one or more glutamate receptor GluA1-GluA4 subunits (Shi et al., 2001) and serve as key regulators of synaptic function and plasticity (Malenka and Bear, 2004). The functional properties of AMPA receptors are determined by subunit composition. For example, when GluA1-containing AMPA receptors lack the GluA2 subunit, they become calcium permeable and exert major contributions to synaptic plasticity (Liu and Zukin, 2007) and reward-related behaviors (Conrad et al., 2008; Engblom et al., 2008).
In the absence of subunit-specific pharmacological tools, insight into the contribution of specific AMPA receptor subunits to various behavioral processes has come from studies of mutant mice with targeted subunit deletions. Previous studies have shown that GluA1 ‘knockout’ (KO) mice have impaired synaptic plasticity (Zamanillo et al., 1999), and display a complex pattern of cognitive abnormalities. GluA1 KO mice exhibit normal development, life expectancy and fine structure of neuronal dendrites and synapses. They do, however, exhibit a marked reduction in the number of functional AMPA receptors and reduced AMPA receptor-mediated excitatory synaptic currents (Andrasfalvy et al., 2003; Jensen et al., 2003; Romberg et al., 2009). Furthermore, deletion of GluA1 also affects hippocampal synaptic plasticity directly. Initial electrophysiological characterization of GluA1 KO mice showed that hippocampal long-term potentiation (LTP), induced by high frequency tetanic stimulation, was abolished at Schaffer collateral – CA1 pyramidal cell synapses during in vitro recordings made in slice preparations from adult animals (Zamanillo et al., 1999). However, more recent studies have revealed different results. Frey et al. (2009) recently showed that spike-timing dependent plasticity in CA1 pyramidal cells is GluA1-independent, and studies using theta-burst stimulation paradigms have revealed a gradually developing form of LTP in the GluA1 KO mice that was indistinguishable from that in wildtype (WT) mice 20–45 min after induction (Hoffman et al., 2002; Romberg et al., 2009). Thus, following theta-burst stimulation, at least, GluA1 appears to contribute more to the early, rapidly decaying component of LTP, which might actually be considered more akin to a form of short-term potentiation (STP). Consistent with these findings, Erickson et al. (2009) have recently shown that weaker stimuli, which are insufficient to induce LTP, result in STP in WT mice, and that this is greatly reduced in the GluA1 KO mice.
The behavior of GluA1 KO mice has also been studied extensively. They appear normal on simple measures of physical health, and neurological and sensory function (e.g. Wiedholz et al., 2008), but we have consistently found a pronounced locomotor hyperactivity in these animals (Bannerman et al., 2004; Fitzgerald et al., 2010; Wiedholz et al., 2008). GluA1 KO mice also exhibit a pattern of cognitive abnormalities which includes impaired spatial working/short-term memory (Bannerman et al., 2003; Reisel et al., 2002; Schmitt et al., 2003, 2005), but intact associative, long-term spatial reference memory (Schmitt et al., 2003; Zamanillo et al., 1999). For example, GluA1 KO mice are impaired at remembering which arm of a Y- maze they have just recently visited during a test of spatial novelty preference (Sanderson et al., 2007), but are perfectly capable of learning the spatial location of a hidden escape platform in the standard version of the Morris watermaze task (Reisel et al., 2002; Zamanillo et al., 1999). This dissociation between impaired short-term but spared long-term spatial memory has been demonstrated simultaneously, within a single task, using the radial arm maze (Schmitt et al., 2003). Importantly, this dissociation argues for a very selective cognitive deficit and against a general disturbance in sensorimotor or motivational aspects of performance. Indeed, under certain conditions, long-term spatial memory is actually enhanced in GluA1 KO mice (Sanderson et al., 2009). We have suggested that GluA1 KO mice have a deficit in short-term habituation (Sanderson et al., 2010), an impairment that is observed with both spatial (Sanderson et al., 2010) and non-spatial stimuli (Sanderson et al., 2011a,b), and in both male and female mice (e.g. Bannerman et al., 2004; Fitzgerald et al., 2010).
In addition, GluA1 KO mice exhibit deficits in Pavlovian fear conditioning (Feyder et al., 2007; Humeau et al., 2007), although the performance of GluA1 KO mice on unconditioned tests of anxiety is more complex and may be difficult to interpret given the differences in short-term habituation and locomotor activity seen in these animals (e.g. Bannerman et al., 2004; Wiedholz et al., 2008). GluA1 KO mice (and mutants lacking the GluA1 Ser 831 phosphorylation site) have also been shown to perform abnormally on reward- and addiction-related tasks. These abnormalities include impaired extinction of cocaine-seeking (Mead et al., 2007) and conditioned reinforcement, and enhanced cued operant responding for food reward (Crombag et al., 2008b; Mead and Stephens, 2003; Mead et al., 2007). GluA1 KO mice also display an impaired outcome-specific devaluation of instrumental responding but, by contrast, normal cocaine self-administration (Mead et al., 2007), cued reinstatement of cocaine-seeking (Mead et al., 2007), (food) conditioned place preference (Mead et al., 2005) and Pavlovian instrumental transfer (Crombag et al., 2008a; Mead and Stephens, 2003). These data are consistent with an inability in GluA1 KO mice to encode the relationship between sensory-specific aspects of reward and their incentive value, leading to reduced goal-directed behavior and resulting in habitual responding (Johnson et al., 2005).
Extending the phenotypic profile of the GluA1 KO mouse, we recently reported that GluA1 KO caused behavioral abnormalities considered relevant to schizophrenia (e.g., novelty-induced locomotor hyperactivity, impaired prepulse inhibition, Wiedholz et al., 2008), bipolar disorder (e.g., lithium-reversible approach behavior, Fitzgerald et al., 2010) and depression (e.g., behavioral ‘despair’ after repeated stress, Chourbaji et al., 2008). These observations led to the suggestion that gene deletion of GluA1 may produce a phenotype modeling some of the positive symptoms and at least one negative symptom (depressed mood) of schizoaffective disorder – an illness with a complex clinical presentation comprising elements of schizophrenia and bipolar mania (Fitzgerald et al., 2010; Sanderson et al., 2010). The aforementioned cognitive and reward-related alterations in these mutant mice would be broadly congruent with this hypothesis.
Schizophrenia and schizoaffective disorder both present clinically with executive and cognitive dysfunction (Carter et al., 2008). Moreover, schizophrenia is frequently co-morbid with drug addictions (DSM-IV, 1994). The manic component of schizoaffective disorder in particular is often typified by ‘excessive involvement in pleasurable activities with a high potential for painful consequences,’ including substance abuse (DSM-IV, 1994), suggesting a deficit in executive control over reward-seeking.
The aim of the current study was to extend the characterization of GluA1 KO mice to include behaviors associated with the negative and cognitive/executive symptoms of schizophrenia and schizoaffective disorder. To this end we tested the mutants for the acquisition and extinction of an operant response for food-reward, for operant-based pairwise visual discrimination and reversal learning, and for impulsive choice in a delay-based cost/benefit decision-making T-maze task. We extended our analysis to encompass other measures relevant to negative symptoms of schizophrenia and schizoaffective disorder. We tested for the propensity to consume a freely available pleasurable reward in the form of sucrose preference. Because schizophrenic and schizoaffective patients exhibit a ‘restricted range of social contact’ (DSM-IV, 1994) we also tested for conspecific social interactions.
2. Materials and methods
2.1. Subjects
GluA1 KO mice were generated as previously described (Zamanillo et al., 1999) on a 129S1/Sv-p+Tyr+KitlSl−J/+ × 129X1/SvJ background. For the experiments conducted in Oxford (sucrose preference, social interaction, impulsive choice), mice were maintained on a C57BL/6J × CBA/J background. WT and KO mice were littermates bred in-house from heterozygous (HET) × HET parents. Males and females were used. Mice were housed in same-sex groupings in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0700 h) and tested in the light phase. Experimental procedures were approved by the Home Office.
For the experiments conducted at the NIH (acquisition and extinction of operant responding, acquisition and reversal of visual discrimination learning), mice were backcrossed to produce a >75% C57BL/6J background, as verified by genome scan (Wiedholz et al., 2008). WT and KO were littermates bred from HET × HET parents, either bred at The Jackson Laboratory (Bar Harbor, ME) and transported to NIH at ~8 weeks of age, or bred in-house. Males and females were used. Mice at the NIH were housed in same-sex groupings in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0600 h) and tested in the light phase. Experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the local Animal Care and Use Committee.
2.2. Sucrose preference
Preference for a naturally rewarding substance such as sucrose has been used to investigate pleasure-seeking and a reduction in this behavior is taken to be indicative of anhedonia, a possible negative symptom of schizophrenia and related disorders (El Yacoubi et al., 2003; Strekalova et al., 2004). Mice in this experiment (24 male WT, 21 female WT, 21 male KO, 26 female KO, usually housed 3–5 per cage) had previously been tested on the elevated plus maze (as reported in Fitzgerald et al., 2010). Mice were individually housed in large cages (41.5 × 25.5 ×11.5 cm) with enrichment for 8 days with ad libitum access to food and 2 bottles. During the first 4 days, both bottles contained water. During the subsequent 4 days, 1 bottle contained water and the other contained an 8% (weight/volume) sucrose solution. The relative positions of the bottles were counter-balanced across mice and switched after every 2 days to prevent development of a place preference. Bottles were weighed at 0800 and 1800 h each day. Average sucrose consumption across the 4-day period was calculated by dividing bottle weights by 1.08 (weight in grams of 1 ml 8% sucrose w/v solution). Sucrose preference was also calculated by dividing sucrose consumption by total fluid consumption (sucrose/(sucrose + water)).
2.3. Social behavior
To attain a measure relating to the restricted social interactions associated with schizophrenia and schizoaffective sufferers, we assessed the social activity of GluA1 KO mice. Mice in this experiment had previously been tested for sucrose preference (see above), as well as on the elevated plus maze, and monitored for home cage locomotor activity (as reported in Fitzgerald et al., 2010). Mice were first tested for free dyadic social interaction in a novel cage environment. Two mice of the same genotype and sex, that had previously been housed in separate cages, were placed in a clean cage (41.5 × 25.5 ×11.5 cm) with bedding sufficient to cover the bottom of the cage. Behavior was video recorded and manually scored off-line for the duration of social (defined as sniffing, grooming and following within 1 cm of the stimulus mouse) and aggressive (defined as biting, scratching and chasing the stimulus mouse) interactions over a 10 min session. The procedure was repeated the following day in a clean cage with a new social pairing. Behavior on these 2 days was averaged and categorized as the ‘pre-habituation’ condition.
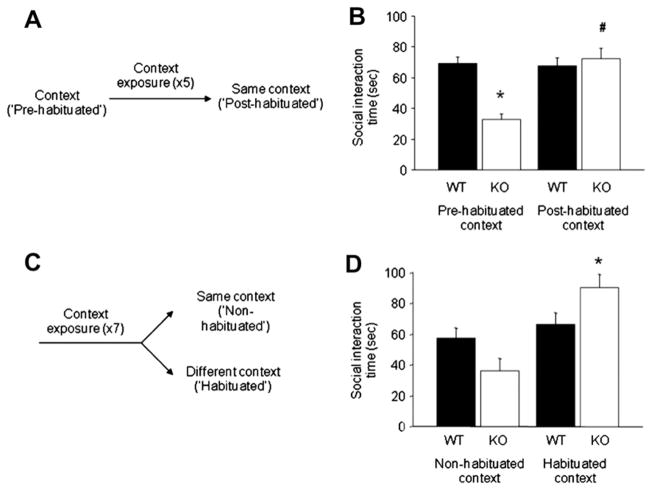
The same mice were then tested in a familiar cage environment. We have previously shown that GluA1 KO mice are slower to habituate to a novel context (Sanderson et al., 2009, 2007), and therefore they tend to explore the environment more than their littermate wild-type controls. Any differences in social investigation/behavior could therefore reflect differences in exploration of the testing environment which might be expected to compete with social exploration (Sanderson et al., 2011a). Therefore, the mice were extensively habituated to the same test cage environment by repeated, individual exposures (10 min per day for 5 consecutive days, bedding changed for each trial). On the following 2 days, social interaction was tested as above, with a novel pairing used on each trial. These 2 days were averaged as the ‘post-habituation’ condition (for schematic, see Fig. 1A). The effects of genotype, sex and context condition (pre- versus post-habituation) were analyzed using 3-factor ANOVA followed by Fisher’s LSD post hoc tests.
Fig. 1.
GluA1 KO mice show reduced social behavior in non-habituated, but not in habituated, tests contexts. (A) Schematic of experimental design. (B) GluA1 KO mice engaged in less social interaction with a novel conspecific mouse than WT controls during the pre-habituation but not the post-habitation test. Social interaction increased from the pre- to post-habituation conditions in GluA1 KO mice but not WT controls (n = 44–46 per genotype). (C) Schematic of experimental design. (D) GluA1 KO mice display a trend to engage in less social interaction than WT controls in a non-habituated context, but engage is significantly more social exploration in a habituated test context. Levels of social interaction increased from the pre- to post-habituation conditions in GluA1 KO mice but not WT controls (n = 9–12 per genotype). Data are Means ± SEM. *p < .05 KO vs. WT, #p < 0.05 KO pre-habituation vs. post-habituation.
In the experimental design above, the different outcomes in the pre- and post-habituation conditions could be due to either (i) the relative novelty/familiarity of the test environment, or (ii) the prior experience of social interaction testing. To distinguish between these possibilities, we conducted another experiment in a separate cohort of naïve mice (16 male WT mice, 16 male KO mice). A test cage was placed in each of 2 separate rooms with different distal spatial cues, and mice were individually exposed to one of the cages for 10 min per day for 7 days. Beginning the following day, mice were tested in a social dyadic encounter (as above) for 5 min. There were 3 social interaction tests on 3 consecutive days (each day in a clean cage with a novel pairing), and behavior was averaged across days. Shorter test encounters were used in this paradigm to reduce the amount of habituation to the novel testing environment. For these sessions, half of the mice (counterbalanced for genotype) were tested in the habituated context and the other half in the non-habituated context (for schematic, see Fig. 1C). The effects of genotype and context were analyzed using 2-factor ANOVA followed by Fisher’s LSD post hoc tests. Note: data from 3 pairs of mice (KO/different context) were lost in this experiment due to a technical fault.
2.4. Acquisition and extinction of reward-seeking
As a further test of reward-seeking behavior, test-naïve mice were assessed for acquisition and extinction of a stimulus-response reward-seeking behavior using a touchscreen-based operant system (22 male WT, 12 female WT, 22 male KO, 12 female KO, housed 3–5 per cage). The apparatus was an operant chamber measuring 21.6 ×17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT), housed within a sound and light attenuating box (model #ENV-022MD, Med Associates). The grid floor of the chamber was covered with solid Plexiglas to facilitate ambulation. A pellet dispenser delivering 14 mg dustless pellets (#F05684, BioServ, Frenchtown, NJ) into a magazine was located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Light Industrial Metal Cased TFT LCD Monitor, Craft Data Limited, Chesham, U.K.), a house-light, and a tone generator. The touchscreen was covered by a black Plexiglas panel that had 5 × 5 cm2 windows separated by 0.5 cm and located at a height of 6.5 cm from the floor of the chamber. Stimuli presented on the screen were controlled by custom software (‘MouseCat’, L.M. Saksida) and visible through the windows (1 stimulus/window). Nosepokes at the stimuli were detected by the touchscreen and recorded by the software.
Body weight was reduced and maintained at 85% free feeding weight throughout testing to motivate instrumental responding for food reward. Mice were progressively shaped to respond to visual stimuli presented on a touch-sensitive screen. First, variously shaped stimuli were presented on the touchscreen and their disappearance resulted in delivery of a single food reward in a magazine located at the opposite end of the operant chamber to the touchscreen. From this stage onwards, food reward coincided with the presentation of 2 Pavlovian cues: a 2 s 65 dB pure tone and a light in the magazine. Mice were required to eat 30 pellets within a 30 min session to attain criterion. Next, a visual stimulus appeared on screen until a response was made. Criterion was, again, 30 responses in less than 30 min. To shape mice to self-initiate trials, mice were next required to produce stimuli on the screen by making a head entry into the magazine. Mice were required to make 30 responses in less than 30 min. In the final phase of acquisition, mice were required to respond to 30 presentations of the onscreen stimuli (1 ×2.8 cm2 white rectangles a square is presented in each window at every trial) (15 s inter-trial interval) in less than 12.5 min over 5 consecutive sessions. Trials to reach acquisition criterion was analyzed by ANOVA with sex and genotype as between-subject factors.
Next, extinction of the operant response was measured as described previously in various inbred strains (Hefner et al., 2008; Lederle et al., 2011) and other glutamate mutant mice (NR2A KO: Brigman et al., 2008; GLAST KO: Karlsson et al., 2009). Responses to stimuli, each presented for 9 s, produced no food reward (nor Pavlovian cues). Extinction training continued until mice omitted ≥77% responses per session on 2 consecutive sessions. The effect of genotype, sex and session were analyzed by ANOVA, with repeated measures for session, followed by Fisher’s PLSD post hoc tests. In order to examine within-session extinction, data from the first and last 3-trial blocks were calculated and we analyzed the effect of genotype and trial-block using 2-factor ANOVA, with repeated measures for trial-block, followed by Fisher’s PLSD post hoc tests.
2.5. Discrimination and reversal learning
To assess further possible correlates of the cognitive symptoms of schizophrenia and related disorders, test-naïve mice were tested for pairwise visual discrimination and reversal learning using the touchscreen-based operant system, as previously described (Brigman et al., 2010; Izquierdo et al., 2006). Mice (5 male WT, 5 female WT, 3 male KO, 5 female KO) first underwent pre-training as for the acquisition procedure described above. They then received an additional phase in which responses to a blank window during stimulus presentation produced a 5 s timeout (signaled by extinction of the house light) to discourage indiscriminate screen responding. Each of the responses at the blank window was followed by correction trials in which the same stimulus and spatial configuration was presented until a correct response was made. Performance criterion on this phase was ≥75% correct responses (excluding correction trials) over a 30-trial session.
For discrimination learning, 2 novel equiluminescent stimuli were presented in a spatially pseudorandomized manner over 30-trial sessions (15 s ITI). Responses at 1 stimulus (correct) resulted in reward; responses at the other stimulus (incorrect) resulted in a 5 s timeout (signaled by extinction of the house light) and were followed by a correction trial. Stimuli remained on screen until a response was made. Designation of the correct and incorrect stimulus was counterbalanced across genotype. Performance criterion was an average of 85% correct responses (excluding correction errors) over 2 consecutive sessions, with a minimum of 25 correct responses on any given session. These measures were analyzed using ANOVAs with sex and genotype as between-subject factors.
After attaining the discrimination criterion, the designation of stimuli as correct versus incorrect was reversed for each mouse and performance tested over 30-trial daily sessions to a criterion of 85% correct (excluding correction trials) over 2 consecutive sessions. The number of trials, errors and correction errors committed to attain discrimination and reversal criteria, as well as trials omitted, average stimulus reaction time, average reward retrieval latency, and average session completion time, were analyzed. We also examined the early stage of reversal (when behavior is relatively more perseverative) and late stage of reversal by separately analyzing trials, errors and correction errors for sessions where performance was below 50% and performance from 50% to criterion, as previously described (Brigman et al., 2008). These measures were analyzed using ANOVAs with sex and genotype as between-subject factors.
We and others have previously found complex alterations in anxiety-related behavior in GluA1 KO mice (Bannerman et al., 2004; Fitzgerald et al., 2010; Vekovischeva et al., 2004), including locomotor hyperactivity in response to stress (Fitzgerald et al., 2010). Therefore, we tested a naïve cohort of mice for the effects of an acute stress exposure on reversal performance. Mice were subjected to a single 10 min forced swim stress as previously described (Boyce-Rustay et al., 2007) by individually placing them in a 20 cm-diameter cylinder filled to ~13 cm with 24 ±1.0 °C water. 30 min later, mice were tested on a single reversal session (as above). Non-stressed controls remained in their home cage.
2.6. Impulsive choice
Impulsive choice behavior was assessed using a non-spatial T-maze task, as previously described (Mariano et al., 2009), but modified for mice. The apparatus was an enclosed T-maze consisting of a start arm (30 ×10 cm) and 2 removable goal arms (30 ×10 cm), surrounded by 30 cm high walls (see Fig. 4A). A raised metal food well (12 mm diameter) was situated at the far end of each goal arm. The start arm, which was unpainted (natural wood color), led to the two goal arms which were painted in different colors: one with alternate black and white stripes (~2 cm wide) and the other uniform grey. The black/white or grey color covered the entire walls and floor of the goal arm from the entrance to the back wall behind the food well. Within each goal arm were two 30 cm high guillotine doors (also painted grey or with black and white stripes as appropriate), one at the entrance to the goal arm (Gate A), and one 5 cm from the end wall of the goal arm, just in front of the food well (Gate B). These doors could be independently moved so as to restrict or allow access to various portions of the goal arm as required. The doors were used to contain the mouse in the goal arm in order to be able to impose a delay between making a choice and receiving the reward.
Fig. 4.
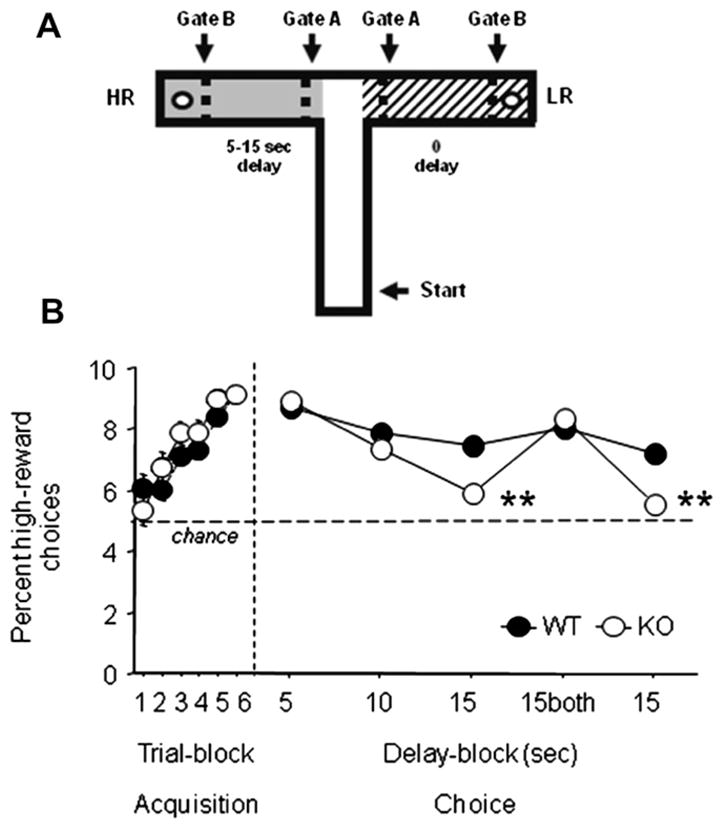
GluA1 KO mice show increased impulsive choice in a delay-based cost/benefit decision-making task. (A) T-maze apparatus. Mice were placed in the start arm of the T-maze and allowed to choose (Gates A open) between the 2 visually-distinct goal arms, associated with either a high- (HR) or low- (LR) milk reward. On entry into an arm, Gate A was immediately closed and a forced waiting period (5–15 s for HR arm, 0 s for LR arm) imposed before Gate B was opened to allow access to the reward. (B) GluA1 KO mice and WT controls exhibited preference for the HR arm over 6-training sessions (0 delay for HR arm). In both genotypes, HR arm preference was maintained at a 5 s delay and slightly reduced at a 10 s delay. At a 15 s delay, GluA1 KO mice showed less HR-arm preference than WT controls. When both arms were HR at the 15 s delay, genotypes did not differ, but GluA1 KO mice again showed less HR arm preference when the 15 s delay was reinstated. n = 15–20 per genotype. Data are Means ± SEM. **p < .01 WT vs. KO.
Mice (8 male WT,12 female WT, 7 male KO, 8 female KO) were maintained under food restriction in order to keep them at 85–90% of their free-feeding weight. They were first habituated to drinking sweetened condensed milk (diluted 50:50 with water) in their home cages before being fully habituated to the maze. During this stage of pre-training, goal arms of natural wood color were used. Mice were initially habituated to the maze in groups of two or three, with milk rewards freely available in both goal arms. They were then made to run individually for milk in each goal arm by preventing access to the alternate goal arm using Gate A. Once all the animals were running freely and readily consuming the rewards, training on the reward task began.
Mice were first trained to choose between a low reward (LR) arm which contained a 0.05 ml milk reward and a high reward (HR) arm which contained 0.25 ml. For half of the mice, the HR was associated with the black/white striped goal arm and the LR with the grey goal arm. For the remaining mice, the associations were reversed. There were no delays present during this stage of training and mice had immediate access to either reward. On each day of testing, mice first received two forced trials at the start of each training session (by closing the appropriate Gate A), one to the HR arm and one to the LR arm (the order of the forced trials was according to a pseudorandom sequence). The mice then received 5 free choice trials and their choices were recorded. The left/right orientation of the HR/LR arms was varied according to a pseudorandom sequence with no more than two consecutive trials with the arms in the same configuration. The number of trials in which the HR was on the left or the right was balanced across 2 consecutive test sessions (i.e., across each block of 10 choice trials).
Training in this zero delay condition continued for 12 days for a total of 60 trials and averaged into 10-trial blocks (Trial-blocks 1–6 in Fig. 4A). Next, a 5 s delay was introduced into the HR arm. The doors (Gate B) at the end of both goal arms were now initially closed. If the mouse chose the HR arm, Gate B was kept shut and the animal was detained in the goal arm by closing Gate A. After a delay of 5 s, Gate B was raised allowing access to the HR. If the mouse chose the LR, Gate B was raised immediately and thus there was no additional delay to reinforcement. Each test session involved 2 forced trials and 5 choice trials.
After 6 training sessions with the 5 s delay, the delay in the HR arm was further increased to 10 s. Mice then received 6 test sessions (2 forced/5 choice trials per session) with a 10 s delay to reinforcement in the HR arm, and with immediate access to the food in the LR arm (3 blocks of 10 trials). Subsequently, mice then received a further 6 sessions with a 15 s delay in the HR arm and immediate access to the milk in the LR arm (3 blocks of 10 trials). In Phase E, an identical 15 s delay was also introduced into the LR arm. Therefore, the mouse was now detained for 15 s prior to reinforcement, irrespective of which arm was chosen. In Phase F, the contingencies from Phase D were re-introduced, with again no delay in the LR arm. By this stage the majority of mice were choosing the HR arm on the majority of trials, and rarely chose the LR arm. Because this resulted in little exposure to the change in contingency (i.e., that there was now no longer a delay present in the LR arm), all animals were first given 2 days of forced trials. They received 10 forced trials per day, 5 to the HR arm including a 15 s delay, and 5 to the LR arm with no delay. Thereafter, the mice received 6 days of testing (2 forced and 5 choice trials per day) with a 15 s delay in the HR arm and no delay to reinforcement in the LR arm (Phase F; Blocks 19–21). The effect of genotype, sex and block were analyzed using 3-factor ANOVA, with repeated measures for block, followed by Fisher’s LSD post hoc tests.
3. Results
3.1. GluA1 KO mice show normal sucrose preference
GluA1 KO mice did not differ from WT controls in the amount of sucrose solution consumed or the preference for the sucrose solution over water (percent sucrose preference: WT 71.8% ± 2.1, KO 73.3% ± 2.0). Total fluid consumption also did not differ between genotypes (WT 38.2 ml ± 1.5, KO 36.8 ml ± 1.5). No effects of genotype, sex or sex × genotype interactions were seen.
3.2. GluA1 KO mice show reduced social behavior in non-habituated but not habituated environments
In the first experiment comparing social behavior pre- and post-habituation to the test context, there was a significant interaction between genotype and habituation state (novel vs. familiar) for social interaction (F(1,172) = 22.17, p < .01). Post hoc tests showed that GluA1 KO mice displayed less social interaction than WT in the non-habituated, novel context but not in the habituated, familiar test environment (Fig. 1B). Aggression (seen only in male mice) was low in the pre-habituation environment and modestly, but significantly increased in the post-habituation environment (main effect of context: F(1,84) = 4.61, p < .05). Overall, aggression was significantly lower in GluA1 KO mice than WT controls (main effect of genotype: F(1,84) = 4.75, p < .05, genotype × environment interaction: ns) (data not shown). Male mice also spent more time in social encounters than female mice (main effect of sex F(1,172) = 50.70, p < 0.01), but this did not interact with genotype or environment.
In the second experiment comparing social behavior in habituated and non-habituated contexts (but now equating for the presence/absence of prior social testing), there was once again a genotype × environment interaction for duration of social interaction (F(1,41) = 7.87, p < .01). Analysis of simple main effects revealed that there was a strong trend for reduced social interaction in the KO mice in the non-habituated, novel test context (F(1,41) = 3.34; p = 0.08), broadly consistent with the data from the first experiment. In contrast, simple main effects analysis also showed that there was a significant effect of genotype when testing took place in the well-habituated, familiar context (F(1,41) = 4.65; p < 0.05), which now reflected the fact that there was actually more social interaction in the GluA1 KO mice than in WT under these conditions (Fig. 1D). Simple main effects also showed that whereas social interaction was significantly greater in the habituated context relative to the non-habituated context in GluA1 KO mice, this was not the case for the WT controls. Virtually no aggressive behavior was observed during this test, regardless of genotype or context (data not shown).
3.3. GluA1 KO mice show faster acquisition but impaired extinction of an instrumental response for reward
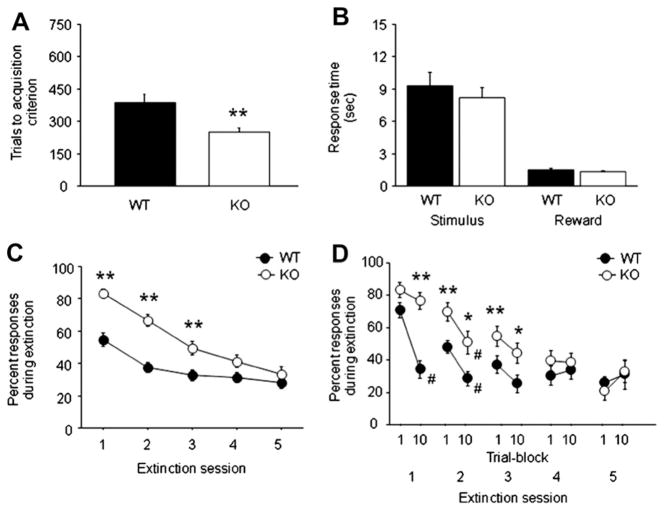
GluA1 KO mice required significantly fewer trials than WT controls to reach criterion for acquisition of the operant response (F(1,65) = 8.97, p < .01) (Fig. 2A). Genotypes did not differ in either stimulus reaction time or reward-retrieval latency during acquisition (Fig. 2B). No effect of sex or interactions with sex were seen on any measure.
Fig. 2.
GluA1 KO mice show faster acquisition and impaired extinction of reward-seeking behavior. (A) GluA1 KO mice required fewer trials than WT controls to acquire a stimulus-response behavior. (B) Stimulus response and reward-retrieval latency was not different between genotypes during acquisition. (C) GluA1 KO mice made significantly more non-rewarded responses than WT during the first 3 session of extinction training. (D) GluA1 KO mice failed to show significant within-session extinction during the first extinction session, and made significantly more non-rewarded responses than WT controls during the first and last trial-blocks during the second and third extinction sessions. n = 34–35 per genotype. Data are Means ± SEM. **p < .01, *p < .05 vs. WT, #p < .05 vs. Trial-block 1.
There was a significant genotype × session interaction for percent responding during extinction (F(4,192) = 3.17, p < .05). Post hoc tests showed that GluA1 KO mice made significantly more responses than WT controls during the first 3 extinction sessions (Fig. 2C). Within-session analysis of extinction (Fig. 2D) revealed a significant reduction in responding from first to last trial-block of session 1 in WT controls (t = 7.05, df = 33, p < .01) but not GluA1 KO mice, such that GluA1 KO mice were responding more than WT controls by the last block. On the second session, both WT (t = 3.52, df = 33, p < .01) and GluA1 KO (t = 2.26, df = 34, p < .05) mice showed a significant within-session reduction in responding, but responding remained higher in the KO than the WT mice on both blocks. On the third session, responding in GluA1 KO mice remained higher than WT controls on both the first and last blocks, and neither genotype showed a significant reduction across blocks. Genotype differences in responding were absent by the fourth session.
3.4. GluA1 KO mice show altered performance during reversal learning
Discrimination learning did not differ between GluA1 KO mice and WT controls, as measured by the number of trials, errors and correction errors to reach criterion (Table 1). Genotypes also did not differ on the number of trials omitted or reward-retrieval response times, while GluA1 KO mice showed a non-significant trend for faster stimulus-response times than WT controls (Table 1). No main effect of sex or interactions with sex were seen.
Table 1.
GluA1 KO mice show normal pairwise visual discrimination learning. Genotypes did not significantly differ on performance measures during discrimination learning. n = 8–10 per genotype. Data are Means ± SEM.
| WT | KO | |
|---|---|---|
| Trials to criterion | 283 ± 48 | 232 ± 37 |
| Errors to criterion | 66.6 ± 13.3 | 65.8 ± 11.9 |
| Correction errors to criterion | 107 ± 26 | 141 ± 25 |
| Trials omitted to criterion | 16.9 ± 10.7 | 4.5 ± 4.5 |
| Stimulus reaction time (s) | 15.6 ± 4.9 | 8.4 ± 1.6 |
| Reward retrieval latency (s) | 3.8 ± 1.3 | 2.7 ± 1.5 |
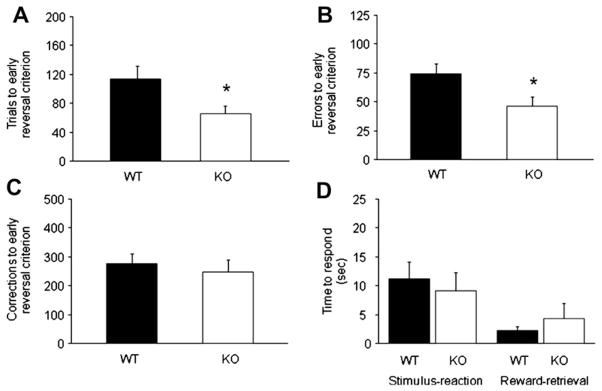
Genotypes were also statistically similar on performance measures to the final reversal criterion (trials: WT = 414 ± 81, KO = 278 ± 64; errors: WT = 143 ±17, KO = 96 ±15; correction errors: WT = 422 ± 45, KO = 331 ±42; stimulus-reaction time: WT = 12.4 ± 3.4 s, KO = 8.5 ± 2.7; reward-retrieval latency: WT = 2.1 ±0.3 s, KO = 1.7 ± 0.3; t-tests all ns). However, GluA1 KO mice required significantly fewer trials (t = 2.27, df = 16, p < .05; Fig. 3A) and made significantly fewer errors (t = 2.35, df = 16, p < .05; Fig. 3B), but not correction errors (Fig. 3C), than WT controls during the early reversal stage when correct responding was sub-chance. Stimulus-reaction times and reward-retrieval latencies were no different between genotypes (Fig. 3D). During the late reversal stage, when performance was above chance levels, genotypes did not significantly differ, although trends for superior performance were again evident in the GluA1 KO mice (trials: WT = 300 ± 69, KO = 212 ± 65; errors: WT = 69.2 ±13.7, KO = 49.9 ±14.7; correction errors: WT = 145.3 ± 34.0, KO = 83.9 ± 25.0; stimulus-reaction time: WT = 13.8 ± 4.4 s, KO = 8.5 ± 2.8; reward-retrieval latency: WT = 2.2 ± 0.4 s, KO = 1.7 ± 0.3; t-tests all ns).
Fig. 3.
GluA1 KO mice show slightly faster reversal learning. (A) KO required significantly fewer trials than WT to complete the early stage of reversal (=correct performance < 50%) when perseverative responding is relatively high. (B) KO made significantly fewer errors than WT during the early reversal stage. (C) Correction errors were not significantly different between genotypes during the early reversal stage. (D) Stimulus-reaction time and reward-retrieval latency did not differ between genotypes. n = 8–10 per genotype. Data are means ± SEM. *p < .05 vs. WT.
Stress did not affect (first session) reversal performance, regardless of genotype (Table 2). GluA1 KO mice made more correction errors than WT controls on this session (main effect of genotype F(1,26) = 4.59, p < .05).
Table 2.
Stress had minimal effects on reversal performance in WT and GluA1 KO mice. Acute swim stress did not affect (first session) reversal performance in either genotype. GluA1 KO mice made more correction errors than WT controls during this session, irrespective of stress. n = 6–10 per genotype per stress group. Data are Means ± SEM.
| WT
|
KO
|
|||
|---|---|---|---|---|
| Control | Stressed | Control | Stressed | |
| Correct responses (%) | 11.4 ± 4.6 | 8.0 ± 3.6 | 17.2 ± 8.0 | 8.6 ± 3.5 |
| Errors | 10.0 ± 2.6 | 6.2 ± 1.1 | 10.0 ± 1.4 | 8.1 ± 2.7 |
| Correction errors* | 90.0 ± 15.3 | 93.1 ± 4.4 | 121.7 ± 8.5 | 107.6 ± 13.9 |
| Trials omitted | 16.6 ± 3.4 | 21.4 ± 1.7 | 14.8 ± 3.8 | 19.3 ± 3.5 |
| Stimulus reaction time (s) | 4.6 ± 1.1 | 9.9 ± 1.5 | 4.3 ± 0.5 | 5.3 ± 0.7 |
| Reward retrieval latency (s) | 1.2 ± 0.2 | 1.5 ± 0.7 | 1.0 ± 0.3 | 1.3 ± 0.2 |
p < .05 KO vs. WT.
3.5. GluA1 KO mice show increased impulsive choice
GluA1 KO mice and WT controls both showed a gradually increasing preference for the HR arm during training when no delay was present in either goal arm of the maze (main effect of trial block: F(5,155) = 40.75, p < 0.001; main effect of genotype: ns; trial block × genotype interaction: ns). All mice learned to discriminate between the two patterned goal arms and were choosing the HR option on the majority of trials by the end of training (Phase A). During subsequent test phases, in which the delay to reinforcement was manipulated, there was a significant genotype × delay-block interaction for HR arm preference (F(4,124) = 29.0.022, p < 0.001). Post hoc tests showed that genotypes did not differ in terms of HR arm preference with a 5 or 10 s delay in this arm, but GluA1 KO mice showed significantly less preference for the HR arm than WT controls under the 15 s delay condition (Fig. 4B). Genotype differences were not, however, evident if access to HR and LR in both arms were similarly delayed by 15 s, confirming that GluA1 KO mice were still able to discriminate between arms as well as WT during this phase of testing. On resumption of the condition with a 15 s delay in the HR-arm only, GluA1 KO mice once again showed significantly less preference than WT controls for the delayed, HR arm. No effects of sex or interactions with sex were seen during any stage of task.
4. Discussion
The current study found that GluA1 KO mice exhibited a context-dependent reduction in social behavior, showed faster acquisition and slower extinction of an instrumental stimulus-response, and displayed increased impulsive choice. By contrast, pairwise visual discrimination was intact, and, although there was some suggestion that GluA1 KO mice were initially faster to reverse this discrimination when performance levels were sub-chance, overall the two genotypes did not differ across the entire reversal phase of the experiment. Sucrose preference was normal in these mice.
Thus, the GluA1 null mutation produced a highly specific set of behavioral abnormalities, some of which are relevant to the negative and cognitive symptoms of schizophrenia and schizoaffective disorder. Although no mouse model can recapitulate the full symptom spectrum of a human neuropsychiatric disorder, GluA1 KO mouse display an interesting accumulation of schizoaffective-relevant phenotypic abnormalities.
4.1. GluA1 KO mice show reduced social behavior in novel but not familiar environments
Social interaction with an unfamiliar conspecific mouse was markedly reduced in GluA1 KO mice when tested in a novel, unfamiliar context to which the experimental subjects had not been extensively habituated. This is consistent with a prior report of GluA1 KO mice showing reduced directed (anogenital) social investigation in a novel context, albeit over a longer (sixty minute) session than used here (Wiedholz et al., 2008). The novel finding here was that GluA1 KO mice actually engaged in significantly more social interaction than WT controls when tested in a familiar, well habituated context.
Profound deficits in short-term habituation have been seen in other test settings with GluA1 KO mice. For example, GluA1 KO mice exhibit deficits in habituation to novel spatial environments (Sanderson et al., 2009, 2007; Wiedholz et al., 2008), and also to non-spatial stimuli, including object (Sanderson et al., 2011a,b). The pattern of results in the social interaction studies may also be explained in terms of differences in short-term habituation. The reduced social behavior seen in an unfamiliar (non-habituated) environment in GluA1 KO mice could be another manifestation of a failure to habituate to the context, and a resultant increase in environmental exploration, that competes with, and thus reduces, levels of social investigation. We have observed a similar pattern with object investigation levels as animals become familiar with the test context (Sanderson et al., 2011a). Initially, object exploration levels were lower in GluA1 KO mice, presumably reflecting competition from exploration of the context, but then as the animals habituated to the context across repeated test sessions, object exploration levels were higher in the knockout animals. Consistent with this account, social interaction levels were rescued when mice were subsequently habituated to the environment prior to further testing. In fact, in the second experiment, levels of social investigation were actually higher in the GluA1 knockouts in the familiar environment. Of course, this increase in social investigation may also reflect a deficit in short-term habituation to social stimuli, although it could also reflect enhanced long-term habituation to the text context (Sanderson et al., 2009). Thus, in these experimental settings the levels of social investigation observed reflect the complex interactions between the various exploratory responses to both the testing context and the target stimuli, and the relative rates at which these competing responses habituate.
Thus, the reduction in social behavior exhibited by the GluA1 KO mice in the novel test context is unlikely to reflect a fundamental deficit in social behavior, but rather may be secondary to deficits in short-term habituation to the testing context and the altered allocation of attentional resources. Nevertheless, the possibility that deficits in social behavior in unmedicated schizophrenic patients may, in part, reflect a core attentional or cognitive impairment should not be completely ruled out (Goldberg et al., 2010). Further studies are required to investigate this issue.
4.2. GluA1 KO mice show impaired extinction of an instrumental response for reward
In contrast to deficits in short-term habituation, certain forms of associative learning are preserved, or even enhanced in GluA1 KO mice (see also Sanderson et al., 2009). The current study found that GluA1 KO mice acquired a simple instrumental stimulus-response more rapidly than WT controls. Subsequently, the mutants appeared to be slower to extinguish this behavior. Unlike WT mice, the KO mice initially showed no within-session extinction during the first session and maintained elevated levels of responding over subsequent sessions. However, it is impossible to separate an account of these data based on impaired extinction from one based on stronger acquisition of the operant response preceding the extinction phase. Therefore, while the GluA1 KO mice clearly show impaired extinction, this may be a function of stronger acquisition. It is important to note that even though all mice were individually trained to a fixed performance criterion during acquisition, the possibility that the impaired extinction in GluA1 KO mice was a consequence of stronger initial learning cannot be ruled out. It does not, however, appear that faster acquisition and slower extinction were simply due to hyperactivity or increased motivation to obtain the reward because neither stimulus response times nor reward-retrieval latencies during acquisition differed between genotypes. Also of note in this regard is that GluA1 KO mice showed no increased preference for (freely available) sucrose, further arguing against a generalized hyperhedonia, although it is also important to recognize the limitations of using sucrose preference as a simple measure of pleasure seeking. In summary, the apparent impairment in extinction could reflect a loss of inhibitory control over this behavior, and as such would extend the earlier finding that these mice also exhibit deficient extinction of cocaine-seeking (Mead et al., 2007), or alternatively it could reflect stronger initial learning.
More generally, GluA1 KO mice might be considered to have a deficit in the ability to moderate reward-seeking behaviors across a range of behavioral assays. Taken together with the finding that these mutants also have an increased propensity for forming habitual responses for reward (Johnson et al., 2005), their phenotype could be tentatively conceptualized as being ‘compulsive-like’. This notion is broadly congruent with compelling evidence that GluA1-containing AMPAR are key mediators of reward- and addiction-related behaviors, and compulsive drug-seeking in particular (Bowers et al., 2010). This, in turn, has implications for a number of psychiatric disorders involving altered reward processing and risk-seeking behaviors, including schizophrenia-like and affective disorders, as well as drug-dependent states.
4.3. GluA1 KO mice show increased impulsive choice but normal reversal
We also assessed GluA1 KO mice in two other major domains of executive function: impulsivity and cognitive flexibility. Impulsive choice was assessed in a non-spatial T-maze task in which mice were required to choose between a delayed, high reward and an immediate, low reward, which were associated with visually distinct goal arms. The mutants were less likely than WT controls to choose a large reward over a small reward when there was a long, forced delay to access the larger reward. Genotypes did not differ in reward choice under either shorter delay or free-choice conditions, or when there was an equivalent 15 s delay in both goal arms, demonstrating that the mutant deficit was specifically driven by delay-intolerance (i.e., increased impulsive choice). These data are in line with our previous finding that GluA1 KO mice also display what might be considered an impulsive phenotype on a differential reinforcement of low rates of responding (DRL) operant task (Reisel et al., 2005), and support a robust impulsivity phenotype in these mutants across tasks.
We have previously reported a mild spatial reversal deficit in an appetitively motivated cross-maze task (Bannerman et al., 2003) but normal reversal in a water-maze task (Schmitt et al., 2004) in GluA1 KO mice. In the current study, we found that GluA1 KO mice were not impaired on reversal of a pairwise visual discrimination in a touchscreen-based procedure and that this was not affected by stress, although the group sizes for this experiment were small than in other tasks detailed in this paper. There was actually a suggestion for modestly superior reversal learning during the early stages of reversal, relative to WT controls. The GluA1 knockout animals made significantly fewer errors in the early stages of reversal when performance levels were sub-chance. However, we are cautious not to over-emphasize this particular finding as such an improvement from sub-chance levels of performance up to chance levels is difficult to interpret. Thus, effects of GluA1 deletion on reversal learning may depend upon either the test parameters (e.g., visual versus spatial, maze-based versus operant-based), and/or the underlying neural systems being recruited to subserve performance. These data also show that, unlike the increased rate of stimulus-response acquisition, mutants did not exhibit improved learning of the initial discrimination task. Nevertheless, these data also provide further evidence that GluA1 KO mice do not have a generalized cognitive impairment. It also underscores how different measures of cognitive/executive function tested in the touchscreen system are highly dissociable within the same mutant mouse or pharmacological manipulation (Bussey et al., 2011).
4.4. The GluA1 KO mouse phenotype
On the basis of the pattern of behavioral and neurochemical abnormalities found in the GluA1 KO mouse in a number of previous studies, we recently suggested that these mutant mice may model certain features relevant to schizophrenia or schizoaffective disorder (Fitzgerald et al., 2010). There are further potential parallels between these disorders and some of the phenotypes observed in the GluA1 KO mice in the current study. Although speculative, this could include the social deficits seen in GluA1 KO mice, which might, in part, bear some resemblance to the social deficits in schizophrenia and schizoaffective disorder. Notably, the dependence of this deficit on the novelty of the experimental context may better represent the narrowing of social activity in schizoaffective patients rather than an overall diminished ability to interact. This dissociation of effects in familiar and novel environments, more broadly, is in keeping with the impaired short-term habituation exhibited by the GluA1 KO mice in a number of tests and consequent alterations in the allocation of attentional resources (Sanderson et al., 2011a, 2010).
With regards to the pattern of cognitive and executive alterations we found in these mice, the apparently impaired operant extinction could be reminiscent of the deficient extinction of fear responses in schizophrenia (Holt et al., 2009), although it could also simply reflect stronger initial acquisition of the operant response. Note that it was not possible to test fear extinction in GluA1 KO mice because these mice have an impairment in fear learning per se (Feyder et al., 2007; Humeau et al., 2007). Moreover, rates of co-morbid substance abuse are higher in patients with schizophrenia or schizoaffective disorder who score high for impulsivity, disinhibition and novelty-seeking (Blanchard et al., 1999; Dervaux et al., 2001) – phenotypes which characterize the GluA1 KO mutant mouse. As noted earlier, however, even with these overt similarities, we caution against drawing too strong a connection between the behavior of this mouse and the human clinical picture until additional work to elucidate underlying their respective neural bases.
Finally, the present data also demonstrate that at least some positive, negative and cognitive symptoms could potentially derive from disruption to a single, core psychological process. For example, we have argued that GluA1 may play a very specific role in short-term habituation processes and, thus, in modulating attentional intensity and perceived stimulus salience. In particular, we have argued that in GluA1 knockout mice the attentional intensity of stimuli remains stronger for longer (Sanderson et al., 2010). This, of course, may have important implications for certain psychiatric disorders, most notably schizophrenia. Attentional deficits are a core symptom of this disease. Furthermore, it has been suggested that hallucinations and delusions may reflect the formation of inappropriate associations between stimuli, something that would not normally occur in healthy individuals (Frith, 1996; Gray et al., 1991; Hemsley, 1994; Kapur, 2003). In this respect, it is interesting that in certain situations, including the acquisition of the stimulus-response reward seeking behavior in the present study, associations can form more readily in GluA1 KO mice (see also Sanderson et al., 2009). Thus, an inability to reduce the attentional intensity of stimuli, as a result of deficits in GluA1-mediated short-term habituation, may result in associations being formed more readily (or indeed inappropriately), which, in turn, may have consequences for the patient’s perception of his or her external environment. Furthermore, the present data also show how deficits in social behavior could manifest as a result of differences in the perceived salience of target and contextual cues, and the rate at which habituation occurs to these stimuli. Deficits in short-term habituation and modulation of stimulus salience cannot explain all of the symptoms associated with schizophrenia or schizoaffective disorder, but they may contribute to a number of the core features of these conditions. Of course, the role of GluA1 in other neural circuits, such as in those required for representing sensory specific aspects of outcomes, and thus in regulating the balance between goal-directed and habitual responding, are also likely to be of great importance (Johnson et al., 2005; Mead and Stephens, 2003).
5. Conclusions
The goal of the current study was to extend the phenotypic characterization of GluA1 KO mice for a range of social and cognitive/executive behaviors thought to be relevant to the negative symptomatology of schizophrenia and schizoaffective disorder, given earlier work indicating that these mice model aspects of the positive symptoms of these disorders. We found GluA1 KO mice exhibit reduced social behavior in a novel context, faster acquisition and impaired extinction of a reward-seeking response, and increased impulsive choice. It is of course important to emphasize that this mouse model involves constitutive deletion of GluA1. Although this is not necessarily a shortcoming from a modeling perspective, given evidence that glutamatergic influences on schizophrenia-related phenotypes are developmentally regulated (Belforte et al., 2010), it does not allow us to differentiate between effects of GluA1 loss in adult brain versus those arising from developmental disturbances. Nonetheless, these mice provide a valuable tool for studying the role of GluA1 in the pathophysiology of major psychotic illness, especially as pharmacological tools do not exist to probe selectively the roles of individual AMPA receptor subunits in the way that is possible with these mice.
Acknowledgments
We are very grateful to Guoxiang Luo for genotyping. Research supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism and The Wellcome Trust (Grant no. 087736). Chris Barkus was supported by a BBSRC (UK) Case Studentship in collaboration with Glaxo Smith Kline.
References
- Andrasfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Brady S, Bruce A, Sprengel R, Seeburg PH, Rawlins JN. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behav Neurosci. 2004;118:643–647. doi: 10.1037/0735-7044.118.3.643. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Squires D, Henry T, Horan WP, Bogenschutz M, Lauriello J, Bustillo J. Examining an affect regulation model of substance abuse in schizophrenia. The role of traits and coping. J Nerv Ment Dis. 1999;187:72–79. doi: 10.1097/00005053-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, Geyer M, Green M, Nuechterlein KH, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T, Heinssen R. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hortnagl H, Riva MA, Sprengel R, Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur J Neurosci. 2008a;27:3284–3291. doi: 10.1111/j.1460-9568.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Lee HK, Holland PC, Gallagher M, Huganir RL. A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav Brain Res. 2008b;191:178–183. doi: 10.1016/j.bbr.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervaux A, Bayle FJ, Laqueille X, Bourdel MC, Le Borgne MH, Olie JP, Krebs MO. Is substance abuse in schizophrenia related to impulsivity, sensation seeking, or anhedonia? Am J Psychiatry. 2001;158:492–494. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. APA Press; Washington, D.C: 1994. [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Maramara LA, Lisman J. A single 2-spike burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M, Wiedholz L, Sprengel R, Holmes A. Impaired associative fear learning in mice with complete loss or haploinsufficiency of AMPA GluR1 receptors. Front Behav Neurosci. 2007;1:4. doi: 10.3389/neuro.08.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM, Machado-Vieira R, Graybeal C, Sharp T, Zarate C, Harvey-White J, Du J, Sprengel R, Gass P, Bannerman D, Holmes A. Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis. 2010;40:608–621. doi: 10.1016/j.nbd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MC, Sprengel R, Nevian T. Activity pattern-dependent long-term potentiation in neocortex and hippocampus of GluA1 (GluR-A) subunit-deficient mice. J Neurosci. 2009;29:5587–5596. doi: 10.1523/JNEUROSCI.5314-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. Neuropsychology of schizophrenia, what are the implications of intellectual and experiential abnormalities for the neurobiology of schizophrenia? Br Med Bull. 1996;52:618–626. doi: 10.1093/oxfordjournals.bmb.a011571. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Keefe RS, Goldman RS, Robinson DG, Harvey PD. Circumstances under which practice does not make perfect: a review of the practice effect literature in schizophrenia and its relevance to clinical treatment studies. Neuropsychopharmacology. 2010;35:1053–1062. doi: 10.1038/npp.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlins JNP, Hemsley DR, Smith AD. The neuropsychology of schizophrenia. Behav Brain Sci. 1991;14:1–84. [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley DR. A cognitive model for schizophrenia and its possible neural basis. Acta Psychiatr Scand Suppl. 1994;384:80–86. doi: 10.1111/j.1600-0447.1994.tb05895.x. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Sprengel R, Sakmann B. Molecular dissection of hippocampal theta-burst pairing potentiation. Proc Natl Acad Sci USA. 2002;99:7740–7745. doi: 10.1073/pnas.092157999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Reisel D, Johnson AW, Borchardt T, Jensen V, Gebhardt C, Bosch V, Gass P, Bannerman DM, Good MA, Hvalby O, Sprengel R, Luthi A. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J Neurosci. 2007;27:10947–10956. doi: 10.1523/JNEUROSCI.2603-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jensen V, Kaiser KM, Borchardt T, Adelmann G, Rozov A, Burnashev N, Brix C, Frotscher M, Andersen P, Hvalby O, Sakmann B, Seeburg PH, Sprengel R. A juvenile form of postsynaptic hippocampal long-term potentiation in mice deficient for the AMPA receptor subunit GluR-A. J Physiol. 2003;553:843–856. doi: 10.1113/jphysiol.2003.053637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Bannerman DM, Rawlins NP, Sprengel R, Good MA. Impaired outcome-specific devaluation of instrumental responding in mice with a targeted deletion of the AMPA receptor glutamate receptor 1 subunit. J Neurosci. 2005;25:2359–2365. doi: 10.1523/JNEUROSCI.4146-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederle L, Weber S, Wright T, Feyder M, Brigman JL, Crombag HS, Saksida LM, Bussey TJ, Holmes A. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS One. 2011;6:e15536. doi: 10.1371/journal.pone.0015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JNP, Walton ME, Rushworth MF, Baxter MG, Campbell TG. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Brown G, Le Merrer J, Stephens DN. Effects of deletion of gria1 or gria2 genes encoding glutamatergic AMPA-receptor subunits on place preference conditioning in mice. Psychopharmacology (Berl) 2005;179:164–171. doi: 10.1007/s00213-004-2071-8. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci. 2003;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology. 2007;32:343–353. doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- Reisel D, Bannerman DM, Deacon RM, Sprengel R, Seeburg PH, Rawlins JN. GluR-A-dependent synaptic plasticity is required for the temporal encoding of nonspatial information. Behav Neurosci. 2005;119:1298–1306. doi: 10.1037/0735-7044.119.5.1298. [DOI] [PubMed] [Google Scholar]
- Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- Romberg C, Raffel J, Martin L, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM, Paulsen O. Induction and expression of GluA1 (GluR-A)-independent LTP in the hippocampus. Eur J Neurosci. 2009;29:1141–1152. doi: 10.1111/j.1460-9568.2009.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Good MA, Skelton K, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for a dual-process memory model. Learn Mem. 2009;16:379–386. doi: 10.1101/lm.1339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RM, Seeburg PH, Sprengel R, Good MA, Rawlins JN, Bannerman DM. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Hindley E, Smeaton E, Denny N, Taylor A, Barkus C, Sprengel R, Seeburg PH, Bannerman DM. Deletion of the GluA1 AMPA receptor subunit impairs recency-dependent object recognition memory. Learn Mem. 2011a;18:181–190. doi: 10.1101/lm.2083411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, McHugh SB, Good MA, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: evidence for Wagner’s dual-process memory model. Neuropsychologia. 2010;48:2303–2315. doi: 10.1016/j.neuropsychologia.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Sprengel R, Seeburg PH, Bannerman DM. Deletion of the GluA1 AMPA receptor subunit alters the expression of short-term memory. Learn Mem. 2011b;18:128–131. doi: 10.1101/lm.2014911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WB, Deacon RM, Reisel D, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Spatial reference memory in GluR-A-deficient mice using a novel hippocampal-dependent paddling pool escape task. Hippocampus. 2004;14:216–223. doi: 10.1002/hipo.10168. [DOI] [PubMed] [Google Scholar]
- Schmitt WB, Deacon RM, Seeburg PH, Rawlins JN, Bannerman DM. A within-subjects, within-task demonstration of intact spatial reference memory and impaired spatial working memory in glutamate receptor-Adeficient mice. J Neurosci. 2003;23:3953–3959. doi: 10.1523/JNEUROSCI.23-09-03953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, Rawlins JN, Bannerman DM. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-Aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3:253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R, Celikel T, Daws LC, Holmes A. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]