Abstract
BTBR T+tf/J (BTBR) mice have emerged as strong candidates to serve as models of a range of autism-relevant behaviors, showing deficiencies in social behaviors; reduced or unusual ultrasonic vocalizations in conspecific situations; and enhanced, repetitive self grooming. Recent studies have described their behaviors in a seminatural Visible Burrow System (VBS); a social proximity test in which avoidance of a conspecific is impossible; and in an object approach and investigation test evaluating attention to specific objects and potential stereotypies in the order of approaching/investigating objects. VBS results confirmed strong BTBR avoidance of conspecifics and in the social proximity test, BTBR showed dramatic differences in several close-in behaviors, including specific avoidance of a nose-to-nose contact that may potentially be related to gaze-avoidance. Diazepam normalized social avoidance by BTBRs in a three-chamber test, and some additional behaviors –but not nose to nose avoidance- in the social proximity test. BTBR also showed higher levels of preference for particular objects, and higher levels of sequences investigating 3- or 4- objects in the same order. Heparan sulfate (HS) associated with fractal structures in the subventricular zone of the lateral ventricles was severely reduced in BTBR. HS may modulate the functions of a range of growth and guidance factors during development, and HS abnormalities are associated with relevant brain (callosal agenesis) and behavioral (reductions in sociality) changes; suggesting the value of examination of the dynamics of the HS system in the context of autism.
Keywords: Autism, Heparan Sulfate, BTBR T+tf/J, Social Behavior, VBS, Social Proximity, Object preference, stereotypy, diazepam, gaze aversion
1.0 Introduction
Diagnoses of autism spectrum disorders (ASD) have increased dramatically in the past decade (Fombonne, 2003; Maughan et. al., 2005, Rutter, 2005), and now may involve nearly 1% of young children (Maenner & Durkin, 2010). ASD are defined by three major symptom clusters: social interaction deficits, communication deficits, and ritualistic-repetitive behaviors, that are typically detectable in early childhood and continue throughout life (APA, 1994; Baird et. al., 2003; Folstein & Rosen-Sheidley, 2001). ASD is primarily a heritable disorder (DiCicco-Bloom et. al., 2006; Gupta & State, 2007), but its transmission appears to be polygenic, with 15 or more contributing loci (Gupta & State, 2007). This genetic complement may additionally interact with environmental or experiential factors, putting an extraordinary emphasis on use of animal research to clarify its etiology and provide a basis for development and preclinical testing of therapeutic approaches. As ASD are defined solely in terms of behavior, suitable animal models require a clear relationship to the types of social and communicatory deficits and behavioral stereotypies that are considered to be its core symptoms.
L. C. Dunn at Columbia University originally developed the BTBR strain by crossing stock carrying the T (brachyury) gene (Dobrovolskaia-Zavadskaia, 1928) with mice carrying the tufted (T) mutation (Lyon, 1956) and then maintained the resulting strain by continual inbreeding. BTBR is now officially an inbred strain, readily available from The Jackson Laboratory and is one of the strains included in the Mouse Phenome Project (see http://jax.org/phenome for more information). Behaviorally, BTBR mice display a number of noteworthy features, relative to most other inbred strains, including superior performance on the accelerating rotorod (Rustay et al., 2003) although this may relate to characteristics of the protocol and equipment used (see Moy et al 2007), increased sensitivity to the antidepressant effects of citalopram in the tail suspension assay (Crowley et al., 2005) and a deficit in reversal learning in the Morris water maze (Wahlsten et al., 2005; Moy et al., 2007).
More recently, the BTBR T+tf/J (BTBR) mouse has received a great deal of attention as a potential model for social deficiencies in general, and more specifically for the social and stereotypical changes that are characteristic of ASD (Bolivar et. al., 2007; McFarlane et. al., 2008; Moy et. al., 2007). BTBR mice display low levels of social behavior (Bolivar et. al., 2007; Chadman, 2011; Gould et. al., 2011; McFarlane et. al., 2008; Moy et. al., 2007). They also show poor social learning in the transmission-of-food-preference assay (McFarlane et. al., 2008); a learning deficit that may be related to low levels of sniffing behavior. Adult BTBR males show reduced ultrasonic vocalization to female urine (Wöhr et. al., 2011). Also, compared to C57BL/6J (B6), FVB/NJ, and 129X1/SvJ mouse pups, BTBR pups produced an unusual repertoire of ultrasonic vocalizations in response to separation from the mother, suggesting a link to communication deficits (Scattoni et. al., 2008). BTBR pups, like adults, were also less social than B6 mice and showed more grooming: Neither the sociability of BTBR pups nor their high level of grooming was rescued by cross-fostering with B6 mothers (Yang et. al., 2007b), although longer-term post-weaning maintenance of BTBR with B6 did produce a diminution in social deficits for the former (Yang et. al., 2010). Adult BTBR also showed lower levels of vocalization compared to B6 mice, and, unlike B6, failed to show significant correlations between vocalization and social investigation in both male-male and female-female pairs (Scattoni et. al., 2010). Although ritualistic, repetitive or stereotyped behaviors have been less investigated than social behaviors in BTBR, high levels of repetitive self-grooming have been consistently reported for these mice (McFarlane et. al., 2008; Yang et. al., 2009; Yang et. al., 2007a,b).
These social, vocalization and repetitive behavior changes in BTBR mice do not appear to be the result of alterations in activity, or, emotionality. BTBR social behavior changes do not correlate with low locomotor activity (Bolivar 2009), a factor that may play a role in low social activity in strains such as A/J (Cook et. al., 2001; Moy et. al., 2007). Studies of anxiety-like behavior in BTBR mice had have mixed results, with indications of no difference, enhanced, or reduced, anxiety-like behavior in comparison to several other mouse strains, in particular the B6 line, often used as controls in these studies (Benno et. al., 2009; Moy et. al., 2007; McFarlane et. al., 2008; Silverman et. al. 2010b; Yang et. al., 2009). One explanation for the variability of BTBR anxiety-like behavior compared to that of B6 may be that these two strains are actually very similar on this measure (Moy et. al., 2007) such that minor variations in stimuli, situations, or measures, can alter their relative anxiety-like behavior in either direction. Thus, although differences of the two strains are not uncommon, findings that they may be in either direction make it unlikely that a change in general levels of anxiety can account for the consistent social deficiencies of BTBR mice (Silverman et. al., 2010b; Pobbe et. al., 2011).
These findings make a strong case for the suitability of BTBR mice as a model for ASD-relevant behaviors. They also suggest that BTBRs may be extremely useful in terms of validating additional tests of social, communicatory, and repetitive or stereotypical behavior patterns for use in further preclinical work on the biology of behaviors potentially related to ASD. This report reviews the behaviors of BTBR mice, compared to B6 mice, in a number of recent tests designed specifically to provide additional information on behaviors that may show functional parallels to the core symptoms of autism. When possible, the same studies also included replications of previously utilized tests of sociality, anxiety-like behavior, etc. in conjunction with the new tests, or tests newly applied to analysis of BTBR behavior, compared to that of B6 mice. These comparisons facilitated comparisons of similarities/differences between the BTBR bred in this laboratory, and those used in other labs.
2.0 General Procedures
2.1 Subjects
In all of these tests, the BTBR and B6 mice were bred in the animal facilities of the University of Hawaii Laboratory Animal Service from breeding animals purchased from Jackson Laboratories (Bar Harbor, ME). Except as noted, all subjects were male. Outbred CD-1 mice, used as stimuli in some studies, were purchased from Charles River Labs (San Diego, CA). After weaning at 25 days of age, subjects and stimulus mice were reared in standard polypropylene cages, in groups of three to five male littermates.
2.2 Group Size
Except as noted, each study involved BTBR or B6 groups of 11 or more mice per group. All mice were adults, with a minimum age of 70 days.
2.3 Behavior ratings
All procedures were videotaped and later rated by experimenters who had been trained to a minimum 90% agreement on each test to be rated. While raters were not informed of the strain of animals, this was relatively obvious due to the patches where many BTBR mice have lost hair due to mutation of the tufted (tf) allele. However, the ratings were made by a range of students, most of whom were taking research classes for undergraduate credit. These raters were not informed of any hypotheses relevant to the behavior of BTBR vs B6 mice.
3.0 Studies of Social Behaviors in BTBR and B6 males: The Visible Burrow System (Pobbe et. al., 2010)
3.1 Apparatus and Procedures
Visible burrow systems (VBS), constructed as previously described (Arakawa et al, 2008), are bins containing about one-half m square of floor space, with an open area to which 3 chambers are connected by tunnels. Mice maintained in these habitats tend to sleep in the chambers during an inactive period (lights-on) and to utilize both the chambers and the open area, showing a range of active social, consummatory, and self-grooming behaviors during the active period (lights-off). Groups of 3 age-matched but previously unfamiliar BTBR or B6 mice, none run in previous tests, were placed in each VBS. Subjects in each group were unfamiliar to each other prior to group formation. A total of 24 hours of videorecordings, 4 hours each on days 1, 2 and 3 in the dark period and days 2, 3 and 4 in the light period, were made and behaviors were analyzed by time sampling. Individual mice had been marked with hair dye prior to colony formation, and behaviors were entered for each animal separately. Behaviors analyzed included: huddle, being alone, allogrooming, self-grooming, approaches to the front or to the back of another animal, flight, chase and follow.
3.2 Results
3.2.1. Front and Back Approach, Flight, and Chase/Follow
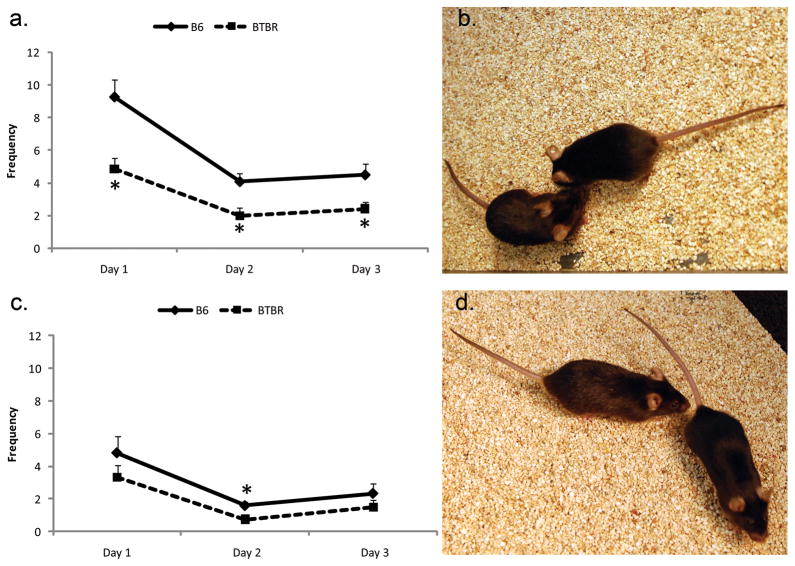
Fig. 1 presents frontal and back approach measures for B6 and BTBR mice as a function of time in the VBS, during the dark periods. The strain effect was significant for both, with BTBR mice making fewer of each type of approach. Flight, and Chase/Follow (not shown) were both significantly reduced in BTBR mice. The BTBR reductions in frontal approach, Flight, and Chase/Follow were greater in the dark period.
Figure 1.
BTBR mice tested in the Visible Burrow System showed decreased approach front (a,b) and approach back (c,d) behaviors in the dark periods when compared to B6 (pictured in b and d). *p<0.05.
3.2.2. Self- and allogrooming
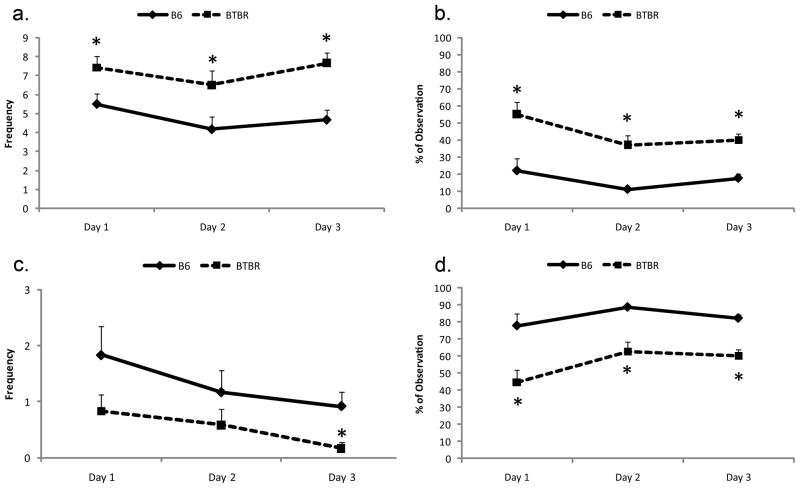
Self-grooming was significantly increased, and allogrooming significantly reduced, in the BTBR strain (Fig 2).
Figure 2.
BTBR mice tested in the Visible Burrow System showed increased self grooming (a) and duration of time alone (b); and decreased allogrooming (c) and duration of time huddling (d) in the light periods, *p<0.05.
3.2.3. Huddle and alone
All subjects huddled more during the inactive (lights on) period. Huddling was significantly reduced in the BTBR strain, and Alone significantly increased, during both dark and light periods (Fig 2; light periods only are shown).
3.3 Discussion
The VBS is a seminatural habitat in which rodent groups show considerable consistency in terms of time and activity budgets and patterns, even though there are few external constraints on their behavior aside from a standard light/dark cycle (Arakawa et. al., 2008; Blanchard & Blanchard, 1989). The finding that BTBR mice show consistent and highly significant reductions in a range of social activities in the VBS is in agreement with previous reports of reduced sociality in this strain, and also serves to indicate a potential usefulness for VBS measures as additional, and ethologically valid, indices of social activity.
4.0 The Social Approach or Three-Chamber Test (Pobbe et. al., 2010, 2011)
4.1 Apparatus and procedures
The Social Approach or Social Preference (3 chamber) test has been extensively utilized in studies of sociality in a variety of mouse strains (Moy et. al., 2004, 2007, 2008; Nadler et. al., 2004). Naïve BTBR or B6 mice were run individually in a three-chambered arena, constructed as previously described (Moy et. al., 2004). The two outside chambers contained an inverted empty black wire cup which housed a CD-1 stimulus mouse. Following an initial habituation period, a naïve subject mouse (BTBR or B6) was placed in the arena, and the duration of time in each of the two outside stimulus compartments was scored with stopwatches, during a 10-min session. While the performance of BTBR and B6 mice in the Social Approach Test was analyzed in both Pobbe et. al., 2010 and 2011, Pobbe et. al., 2011 additionally utilized a diazepam condition (reported under 7.0) and only the results for animals receiving a vehicle injection in that test are reported here.
4.2 Results and Discussion
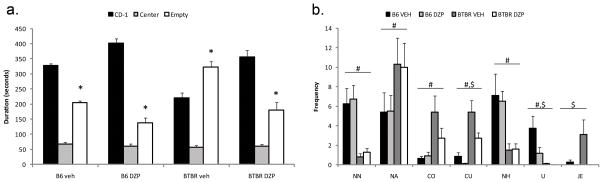
In Pobbe et. al., 2011, B6 mice showed a significant preference for spending time in the side of the test box containing the unfamiliar CD-1 mouse stimulus vs. the opposite side (p<0.05). BTBR mice typically do not show a significant preference for one side over the other, and failed to show such a preference in Pobbe et al, 2010. However, in Pobbe et. al., 2011, in which mice received an injection prior to being run, BTBR mice under the control condition showed a significant preference for the empty side – the side opposite the one in which an unfamiliar conspecific was located (see vehicle control groups in Fig. 4a). This difference, discussed under 7.1 below, raises the possibility that injection just prior to testing might have produced greater stress for BTBR mice, resulting in enhanced avoidance of a conspecific (but see Silverman et. al, 2010b). Notably, Benno et. al. (2009) reported that tail suspension just prior to testing produced an enhanced anxiety-like response on the elevated plus maze for BTBR compared to B6 mice.
Figure 4.
Diazepam was administered to independent groups in the social proximity tests. Social approach testing (a) showed that in subjects administered the vehicle (VEH), B6 mice spend more time in the chamber containing a CD-1 stimulus mouse and BTBR mice spent more time in the chamber containing an empty cup, *p<0.05. Administration of diazepam (DZP) reversed the social approach behavior of the BTBR mice. In social proximity testing (b), subjects administered DZP displayed decreased crawl under (CU), upright (U) and jump escape (JE) behaviors, $p<0.05. Main effects for strain showed decreased nose tip-to-nose tip (NN), nose-to-head (NH) and U behaviors; and increased nose-to-anus (NA), crawl over (CO) and CU behaviors in BTBR mice compared to B6 mice, #p<0.05.
These studies, using the extensively investigated three-chamber apparatus to measure social approach or social preference, were included to verify that the BTBRs used in the experiments from this laboratory show a deficit in sociality similar to that obtained in previous studies. The finding of social deficits for these mice provided such evidence and support a view that the behaviors of BTBR used in these and other tests are typical of the pattern of deficits and hyperexpressed actions associated with this strain; facilitating an evaluation of the validity of the new ethological measures reported here.
5.0 Anxiety Tests in BTBR and B6 mice. (Pobbe et. al., 2011)
5.1 Elevated plus maze (EPM)
The EPM test apparatus (Handley & Mithani, 1984) has two open arms and two closed arms extending from a common central platform. Naïve B6 and BTBR mice were placed individually on the central platform of the EPM, and entries and durations in the open and closed arms were measured from videorecordings. The results were expressed as mean ratio of entries into open arms to total entries into both open and closed arms, mean ratio of time spent in open arms to total time spent in both open and closed arms and mean total number of closed arm entries. Ethological measures included frequencies of stretched attend postures, head-dipping, head-out, and stretched head-out.
There was no significant strain effect on the percentage of open arm entries, but BTBR mice showed significantly reduced open arm time. BTBR mice made significantly more stretch attend postures and stretched head-outs when compared to B6.
5.2 Mouse defense test battery (MDTB)
The MDTB (Griebel et. al., 1995) was conducted in an oval runway, consisting of two straight segments joined by curved segments and separated by a median wall The floor of the apparatus was marked to facilitate measurement of locomotion distances. Two ceiling-mounted video cameras were used to record the test.
BTBR and B6 Mice were run in the MDTB one week after being run in the EPM. In several subtests of the MDTB a hand-held and hand-moved anesthetized Sprague-Dawley rat (predator) slowly approached the subject (Predator Avoidance Test); chased it in the (endless) oval runway (Chase/Flight Test); remained at a distance from it in a straight alley formed by the closing of doors in the runway (Straight Alley Test); and was brought up to contact the subject in the same straight alley (Forced Contact Test). A range of measures reflecting typical defensive behaviors seen in each such situation were taken.
BTBR mice showed a significant decrease in the frequency of line crossings and wall rears assessed in the pre-test; reduced escape distances in the predator avoidance test; increased vocalizations and a decrease in defensive uprights in the forced contact test; and increased line-crossings but decreased wall rears in the runway after the rat stimulus was removed. This complex set of findings does not provide a clear interpretation in terms of anxiety or defensiveness. For example, although increased vocalizations and defensive uprights are typically interpreted as representing enhanced defensiveness; the former increased while the latter decreased. Similarly, enhanced line crossings but reduced uprights (escape attempts?) after the threat stimulus is encountered and then removed, are difficult to interpret.
5.3 Elevated Zero Maze
The Elevated Zero Maze (EZM) Test was run in an elevated circular maze, consisting of open and closed segments of similar length. The closed segments were surrounded by a 20 cm high wall. The apparatus was originally designed to include features of the elevated plus maze, while the lack of a central area between the open and closed arms simplifies comparisons of open and closed area time (Shepherd et. al., 1994). In contrast to other tests in these series, the BTBR and B6 mice run in the Elevated Zero Maze were not test-naïve, having been run three weeks earlier in the MDTB. Results of these 5 min EZM tests were calculated as open arm entries and time as percentages of total arm entries or time, respectively.
BTBR mice had a higher proportion of entries into the open segments of the EZM and their open segment times were also increased in comparison to than B6 mice. These differences were obtained against a background of higher numbers of entries for BTBR into both open and closed segments of the maze.
5.4 Discussion: anxiety tests
Results from these three tests of anxiety provide little consistent support for a view of either enhanced or reduced anxiety-like behavior for BTBR mice in nonsocial situations. This finding is in agreement with a recent review of the increasingly substantial literature comparing BTBR and B6 mice in a range of anxiety tests (Silverman et al, 2010b). What the Pobbe et al, 2011 study added was a test, the MDTB, that had not previously been run with BTBR mice. Although several differences between BTBR and B6 on this test were significant, as with comparisons of the EPM and the EZM test, the differences tended to be in opposite directions. Thus the MDTB adds to a view that in nonsocial, and nonpainful, situations BTBR mice show levels of anxiety-like behaviors that are roughly equivalent to those of B6 mice. This is of interest because it suggests that the social differences of BTBR and B6 males are not due to a general enhancement of anxiety-like behavior in the former. However, fear or anxiety-like behavior in response to social stimuli may be very different, and these data cannot rule out that such anxiety-like behavior may modulate behavioral differences of BTBR and B6 in social situations.
6.0 Social Proximity Tests in BTBR and B6 mice (Defensor et. al., 2011)
Although the VBS and the three chamber tests produced consistent results indicating that BTBR mice are less social than B6, both of these tests utilized relatively large enclosures configured to permit effective avoidance of social stimuli. Because avoidance was readily available, neither test provided satisfactory measures of potential behavioral differences for BTBR mice when in close contact with other mice, such as might permit an analysis of the microstructure of contact for BTBRs. The Social Proximity Test was devised specifically to afford such close contact, enabling an assessment of how BTBR social behaviors might differ in this situation. The chamber used enabled both mice in a pair to stand normally on 4 feet without touching. However, any substantial locomotor movement would put the moving mouse in contact with the other pair member.
6.1 Social Proximity in same-strain pairs
Pairs of naïve, BTBR or B6 mice, previously unfamiliar to each other, were evaluated during a single 10 min test in an upended rectangular tube forming a 7 × 7 cm. chamber. These dimensions allowed both mice of a pair to stand normally on the substrate but ensured a substantial degree of contact as they moved. Mice were marked for individual identification, and measures taken reflected specific types of contact by each mouse to the other. Nose tip-to-nose tip (NN): Nose-to-head (NH): Nose-to-Anogenital (NA): Crawl Over (CO): Crawl Under (CU. Upright (U) and Jump Escape (JE) responses were analyzed for each subject.
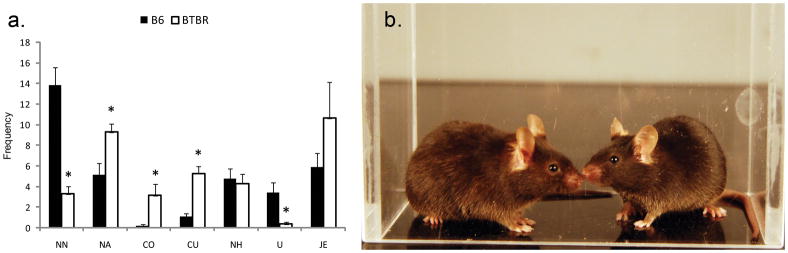
BTBR and B6 mice showed markedly different behaviors in the social proximity test. Two separate studies in Defensor et al, 2011 compared male same sex, same strain pairs in the social proximity test, and data from these tests are combined, for convenience, in Fig. 3. In each of these tests, for which statistics were analyzed separately, BTBR mice showed lower nose tip-to-nose tip contact than B6 mice, while displaying reliably higher nose-to-anogenital contact. Upright behaviors were significantly decreased in BTBR, which also displayed significantly more crawl over and crawl under behaviors than B6 mice. B6 mice appeared to avoid crawl under and crawl over behaviors, instead orienting to the other mouse and moving around it. The BTBR nose-to-head contacts, in contrast to the nose tip-to-nose tip contacts, were not statistically significant in either of the separate studies.
Figure 3.
Same strain social proximity testing of male subjects (a) showed increased nose-to-anus (NA), crawl under (CU) and crawl over (CO) behavior; and decreased nose tip-to-nose tip (NN) and upright (U) behaviors in BTBR mice compared to B6 mice, *p<0.05. There were no significant strain differences in nose-to-head (NH) and jump escape (JE) behaviors. (b) B6 mice performing nose tip-to-nose tip behavior in the social proximity chamber.
6.2 Social Proximity in different-strain pairs
The apparatus and procedures of this test were identical to those of 6.1, except that each pair tested consisted of one BTBR and one B6. Again, these animals were naïve to testing and previously unfamiliar to each other. In these mixed BTBR – B6 pairs, there was no difference in nose tip-to-nose tip contact between pair members. However, this reflects that nose tip-to-nose tip contacts are necessarily the same for each of the two animals in a pair, regardless of which pair member initiated the contact. Moreover, the level of nose tip-to-nose tip in these mixed-strain pairs was similar to that in BTBR pairs, and much lower than for B6 pairs. Observation of the sequence of events leading to a nose tip-to-nose tip contact suggested that these were typically initiated by the B6 pair member, and that the BTBR often made a rapid head withdrawal in response to such investigation by the B6 mouse; reducing the number of successful nose tipto-nose tip encounters and suggesting that mutually oriented nose tip-to-nose tip contact is aversive to these mice. This interpretation is in agreement with the very low levels of nose tip-to-nose tip contact among BTBR pairs.
Nose-to-head contact, which does reflect the initiating pair-member, was decreased in BTBR pair members compared to their B6 partners, and nose-to-anogenital contact was increased in BTBR mice over B6 mice. BTBRs continued to display an elevated crawl under behavior, but not an enhanced crawl over, when confined with a B6 partner. This may reflect that when BTBR mice crawl under B6 mice and remain there, locomotion by the B6 mouse, now on top of its BTBR partner, constitutes crawl over behavior. This sharply increases crawl overs for the B6 partner, in comparison to that seen when B6 pairs were used. The results from mixed strain pairs were consistent with results from within strain pairs in showing that BTBR and B6 mice are different in terms of the BTBR aversion to some aspects of facial contact, and that BTBR display elevated anogenital investigation and crawl under behaviors.
6.3 Social Approach in same-strain female-female vs. male-male pairs
Naïve pairs of BTBR and B6 mice were run in a social approach test identical to that described in 3.3 above, and with similar procedures, except that same-strain female-female pairs were used, in addition to same-strain male-male pairs.
Results of the Social Approach in male B6 pairs and male BTBR pairs replicated our previous findings (Pobbe et al, 2010, 2011) for these strains: B6 males showed enhanced social approach, while BTBR males did not. However, B6 females failed to show a social preference, in contrast to previous reports (Riedel et. al., 2009) while BTBR females did show such a preference.
6.4 Social proximity tests in same-strain male-male and female-female pairs
The apparatus and procedures of this test were identical to those of 6.1, except that the mice used had previously served as the subjects of the social approach tests described in 6.3. Each pair run in the social proximity test consisted of sex and strain matched animals, previously unfamiliar to each other, but not naïve with regard to previous testing.
As noted above, data from the male-male BTBR and B6 pairs provided a full replication of the results from naïve male same strain pairs (6.1), with significant reductions for BTBR mice in nose tip-to-nose tip contacts and upright postures; and elevations in nose-to-anogenital, crawl over and crawl under behaviors. However, correlations of each behavior measured, with time in the chamber in the social proximity test, for the same animals, failed to reveal any significant relationships.
The effect of sex was significant for nose tip-to-nose tip contacts (higher in females) and nose-to-anogenital contacts, which were lower in females. The only significant interaction was for nose-to-anogenital contacts, with BTBR males higher than any other group.
6.5 Discussion of BTBR vs B6 results on the social proximity test
The social proximity test was specifically devised to provide a description of social behaviors for BTBR vs B6 mice in situations of forced contact, i.e. when avoidance was not an option. In this extremely small test enclosure, where two mice standing normally on all four limbs would find it difficult to avoid physical contact while moving, the test was aimed at determining whether mice of the two strains would orient differently to each other, or show other differences in behavior. This series of tests provided three independent analyses of same-strain male-male pairs, and one of mixed strain male pairs. The three studies of same sex pairs, with test-naïve (6.1), non-naïve (6.4), and vehicle-injected (see section 7.2) animals as subjects, showed remarkable similarities in results. In each study, BTBR males showed reduced nose tipto-nose tip contact and upright postures, and enhanced nose-to-anogenital contact, crawl under, and crawl over. Jump escapes appeared to be higher in BTBR in all tests, but were significantly so only in vehicle controls in the diazepam study (see 7.2 below), while nose-to-head contacts were reduced for BTBRs only in controls in the diazepam studies. The consistent differences in nose tip-to-nose tip contact and in uprights appear to be related, in that upright postures are strongly associated with frontal contact with the mystacial vibrissae (Blanchard et al, 1977). Mice do have a zone of binocular vision for objects directly in front of the nose (Antonini et. al., 1999) and these data suggest that BTBR mice may be specifically avoiding such visual contact with other mice.
The between-strain pairs (6.2) were used in order to provide some indication of the interactive dynamics of social behaviors in BTBR and B6 males. A striking finding was that when pairs consisted of one BTBR and one B6 male, the frequency of nose tip-to-nose tip contact was much lower (about 85% less) than the level seen in B6 pairs. While this frequency was necessarily identical for the two members of a pair, observations of videorecordings of these interactions suggested that the B6 pair member often attempted to initiate such contacts, but the BTBR pair member moved its nose away from such frontal approaches, usually before contact occurred; resulting in a lower frequency of success in achieving a mutual orientation. The other notable difference, compared to within-strain tests, was in the crawl under/crawl over behaviors. In the mixed pairs, BTBRs continued to crawl under at higher rates, but crawl over rates were not significantly higher than those of B6; the latter were substantially higher than in same-strain B6 pairs. These differences appeared to have been initiated by the BTBR pair member, in that its crawl under behaviors resulted in the BTBR being partly underneath the B6 animal, with the later, in attempting to move off of the BTBR, meeting the criterion for crawl over. This, in turn, suggests that crawl under may also have been a major initiating factor in high levels for crawl overs in the BTBR same-strain pairs, with high levels of crawl over being, at least in part, a response to being positioned over the other pair member.
7.0 Diazepam Effects in the Social Approach (Three Chamber) and Social Proximity Tests
A great deal of attention has been given to the possibility that BTBR mice show aberrant social behavior because they are more anxious, a factor that might explain the lower proportionate approach levels to a social stimulus seen in the three chamber test, and also the VBS. A thorough recent review of anxiety-like behavior in BTBR compared to B6 mice in a range of standard anxiety tests (Silverman et al, 2010b) indicated few differences between the two, and the findings described in 5.0 above were broadly in agreement with this view, in that the differences obtained in the EPM and the EZM were opposite in nature, with the MDTB showing differences that were internally inconsistent.
A different approach to evaluating the effects of anxiety on social changes in BTBR mice utilized an anxiolytic drug, diazepam, to determine if anxiety reduction alters social behavior in these tests.
7.1 Diazepam in the Social Approach Test
Naïve BTBR and B6 mice were run in a social approach test identical to that described in 4.1 above, with the addition of a diazepam or vehicle injection component (Pobbe et al, 2011). Diazepam (RBI, USA) was injected in a dose of 2 mg/kg) intraperitoneally (i.p.) 30 minutes before the start of the social approach test.
Diazepam significantly increased time in the stimulus mouse side of the apparatus for both B6 and BTBR mice, compared to their respective vehicle groups (Fig. 4). BTBR mice treated with vehicle spent significantly more time in the empty cup side of the apparatus, and less time in the stimulus mouse side, than did any of the other three groups, which did not differ. What is particularly interesting is that for the diazepam groups, in contrast to the vehicle groups, there was no significant difference between time in the compartment with the mouse stimulus for B6 and BTBR mice, indicating that diazepam treatment normalized social preference for BTBR mice.
7.2 Diazepam effects on social proximity measures
The apparatus and procedures of this test were identical to those of 6.1 except that subjects received an intraperitoneal injection of either 2 mg/kg diazepam or vehicle 30 minutes before the start of the social proximity test.
The vehicle control animals of this study provide another replication of the findings of 6.1 (Fig. 4). BTBR males showed fewer nose tip-to-nose tip contacts and upright postures, with more nose-to-anogenital, crawl over and crawl under behaviors. The main effect of diazepam was significant for crawl under, upright, and jump escape, all of which declined with drug administration. The only interaction was for upright, which was higher in the B6 control group than either the B6 diazepam group, or the BTBR control group (Defensor et al, 2011).
7.3 Discussion: Diazepam Effects
These findings are in agreement with two very recent studies (Chadman, 2011; Gould et. al., 2011) indicating that fluoxetine can normalize the social approach of BTBR mice in the three-chamber test. These, and the present diazepam findings that anxiolytics normalize social approach in BTBR suggests that, in contrast to anxiety tests utilizing nonsocial stimuli, anxiety-like behavior may play a role in BTBR differences from controls in situations involving other mice. In the Social Proximity Test, diazepam reductions in crawl under, upright, and jump escape suggest that anxiety-like behavior to social stimuli may modulate some of the “close in” behaviors measured in this test. These findings agree with earlier work (Blanchard et al, 2003; Griebel et al, 1995, 1998, 2005) indicating that upright and jump escape responses are associated with defensiveness, fear, or anxiety, and they additionally suggest that crawl under may represent a defensive response to social proximity, higher in the BTBR strain. However, two aspects of these results are particularly interesting. First, the diazepam effects in this situation, except for jump escape, did not involve interactions with strain. Thus evidence for specifically heightened anxiety-like behavior to social stimuli for BTBR mice in this test is relatively modest. Second, one of the most striking responses of BTBR mice in this test, a reduction in nose tip to nose tip behaviors, did not show any response to diazepam. This behavior, which involved the BTBR mouse moving the head away from such oriented approach by a B6, in the mixed strain pairs (6.2), suggests that BTBR mice avoid a specific type of sensory contact with conspecifics, and that this specific avoidance does not depend on anxiety. Thus while the diazepam studies indicate that social anxiety may modulate some general measures of social avoidance in BTBR, this finding indicates that the social differences of BTBR and B6 mice go beyond social anxiety.
8.0 Tests of Motor and Cognitive Stereotypies
Criteria for diagnosis of ASD include stereotyped interests or behaviors, in addition to impaired social behavior (APA, 2000). BTBR mice show enhancement of repetitive self-grooming behavior in their home cage, clean novel cages, and within semi-natural environments (McFarlane et. al., 2008; Pobbe et. al., 2010; Yang et. al., 2007a,b, 2009). While “lower-order” motor stereotypies such as movements and self-injurious behavior are not uncommon in ASD, “higher-order”, or cognitive stereotypies are also relevant (Bodfish et al, 2000; Moy et. al., 2008). In a hole board task, BTBR mice display inflexibility in exploratory behavior and fail to shift exploration away from a familiar bedding stimulus to a palatable food odor (Moy et. al., 2008), suggesting that the BTBR mouse displays both aberrant motor and cognitive stereotypies.
8.1 Grooming analyses in BTBR and B6 mice: (Pearson et. al., 2011)
Subjects were individually assessed for grooming microstructure in a clear Plexiglas chamber measuring 14 × 7 × 30 (H) cm, the same chamber that was used in social proximity testing (6.1). Videotapes of spontaneous grooming episodes were scored for the frequency and duration of paw licking, head washing, body grooming, leg licking, and tail/genital grooming. Mice typically show a clear sequential pattern of grooming, following the order of body contact listed above (e.g. Paw Licking through Tail/Genital contact: Fentress & Stilwell, 1973), and transitions from one body part to another that did not follow this sequence (“incorrect transitions”) were measured, as were “interrupted bouts” grooming bouts that were interrupted, but for less than 6 seconds: Interruptions of more than 6 seconds resulted in the designation of a new bout. Following a previously validated syntactical grooming analysis (Kalueff et. al., 2007), we calculated the number of bouts, the number of interrupted bouts, and the proportion of interrupted bouts, as well as the number of transitions, the number of incorrect transitions, and the proportion of incorrect transitions. At the end of the 30 minute session, the number of hairs left on the bottom surface was manually counted for each individual.
BTBR mice displayed elevated frequencies of each stage of grooming: paw lick, head wash, body groom, leg lick, and tail/genital groom, relative to B6 mice, and higher durations of all but paw lick. They also showed a significant increase in the proportions of interrupted bouts, but proportionately fewer incorrect transitions between grooming stages. These results suggest that while the BTBR mice show more overall grooming, and a disproportionate increase in the percentage of interrupted bouts, the transitions between body-site stages for BTBR mice are proportionately less likely than those of the B6 mice to deviate from a rigorous pattern of targeting of specific body sites.
BTBR mice also showed a significant increase in the number of hairs lost during the 30 minute self grooming session, but none of the grooming variables significantly correlated with the number of hairs lost. This finding does not support one specific interpretation of enhanced grooming in BTBR mice, that it reflects itching from abnormal hair follicles. Because mice with the tufted mutation also display hair loss, it is notable that the hairs they shed appear normal under microscopic examination (Lyon, 1956). To our knowledge there have been no reports of mice with the tufted mutation displaying enhanced grooming.
8.2 Bar-biting in BTBR and B6 mice: Apparatus and Procedures (Pearson et. al., 2011)
Four-hour videorecordings of the same mice, in their individual home cages, were examined for biting of the wire mesh cage tops. Videotapes were time-sampled for the presence or absence of bar-biting during a 60 second scan every 10 minutes for the entire duration. BTBR mice showed higher incidences of bar-biting behavior than B6 mice, providing evidence of an additional form of motor stereotypy in these mice.
8.3 Novel Object Contact Task in BTBR and B6 mice. Apparatus and Procedure (Pearson et. al., 2011)
Naïve B6 and BTBR mice were assessed for the frequency of repetitive contacts with novel objects, in a previously habituated polypropylene cage, 26.5 × 17 × 11.5 (H) cm, with the floor covered by a layer of sawdust bedding (1 cm). The cage contained four small novel objects (children’s toys, 1.5 to 4 cm in length) made of high density plastic. These were located 4 cm from each of the four corners of the cage, in a fixed pattern. Recorded DVDs were scored for the occurrence of investigation of the four toys, defined as clear facial or vibrissae contact with or burying of each object. Occurrences of identical patterns of sequential contact with three, or of four toys were counted, as was total frequency of object investigation. Also, to determine if there was a strain effect on the tendency to display preferences for particular toys, the frequencies of contact with each object were ranked in decreasing order from maximum to minimum preference (contact) values for each subject, and the frequencies were averaged by strain, and compared.
8.4 Novel Object Contact Task in BTBR and B6 mice. Results and Discussion
The frequency of object investigation did not significantly differ between the strains. However, when the percentage preference for each object for each mouse was ranked, and the strength of those ranks compared between strains, BTBR mice showed significantly stronger preferences for the first two ranks and a significantly lower preference for the last ranked object compared to B6 mice. These results suggest that BTBRs show stronger spontaneous object preference than do B6 mice. Also, the number of specific sequential approaches to three or four particular toys was significantly higher for BTBR mice, indicating more repetitive patterns of object investigation. Notably, this pattern was not mediated by higher numbers of visits overall, as these were not significantly different for the two groups.
These findings suggest that BTBR mice may show an enhancement of interest in specific objects, and a stereotyped pattern of approaches to several different objects that goes beyond motor repetition. They are consonant with previous reports that BTBR mice show a reluctance to investigate new stimuli over familiar ones in a hole board task (Moy et. al., 2008).
9.0 Ventricular and Extracellular Matrix Abnormalities (Blanchard et al, submitted)
BTBR mice consistently show agenesis of the corpus callosum and, more variably, impaired development of the hippocampal commissure (Kusek et. al., 2007; Wahlsten et. al., 2003). Callosal agenesis itself may not be a crucial factor for the social deficiencies displayed by BTBR mice, as social preference remains intact in B6 mice in which the corpus callosum has been cut at P7 (Yang et al, 2009). In accord with this interpretation, disturbances in social/cognitive skills do not appear to depend on callosal disruption in autistic individuals (Booth et al, 2011). This suggests that disruption of mechanisms involved in normal growth or guidance of developing neurons may be responsible for both callosal agenesis, and for some or all of the behavioral changes associated with autism. Accordingly, several components of the extracellular matrix of the subventricular zone (SVZ), known to influence the expression of growth and guidance factors, were examined in BTBR vs. B6 mice. These included fractones, fractal-shaped structures visualized by laminin immunoreactivity (LAM-ir) (Kerever et al, 2008) and, heparan sulfate (HS), a linear polysaccharide with multiple functions that is expressed on the surface and extracellular matrix of most cells (Turnbull, 2010): The number and sizes of fractones were analyzed, as was LAM-ir and HS-ir in association with these fractones, which have been hypothesized to aggregate HS and facilitate its binding to and modulation of growth and guidance factors in the extracellular matrix (ECM) (Douet & Mercier, in press).
9.1 Procedure
Immunohistochemistry was performed directly on 25μm-thick coronal sections from the brains of 3 B6 and 3 BTBR adult male mice. Anti-laminin (L9393; 1/1,000; Sigma-Aldrich) and anti-N-sulfate glycosamines (10E4; 1/300; Seikagaku Co., East Falmouth, MA) antibodies were used as primary antibodies to localize laminin and heparin sulfate according to previously published parameters (Kerever et al, 2007; Lundin et al, 2000; Mercier and Hatton, 2001). Anti-rabbit 647 (A21244; 1/400; Invitrogen) and anti-rat IgM 488 (A11006; 1/400; Invitrogen) secondary antibodies recognized the laminin and heparin sulfate primaries, respectively. A Zeiss Pascal confocal laser scanning microscope was used to collect double-labeled (LAM, HS, Fig. 5, C and G) images for fractone size and HS and LAM intensity analysis. For quantification of ventricle size and the length of subventricular zone (SVZ) an inverted Leica DMIL epifluorescent microscope was utilized. All quantification was done using ImageJ software (NIMH).
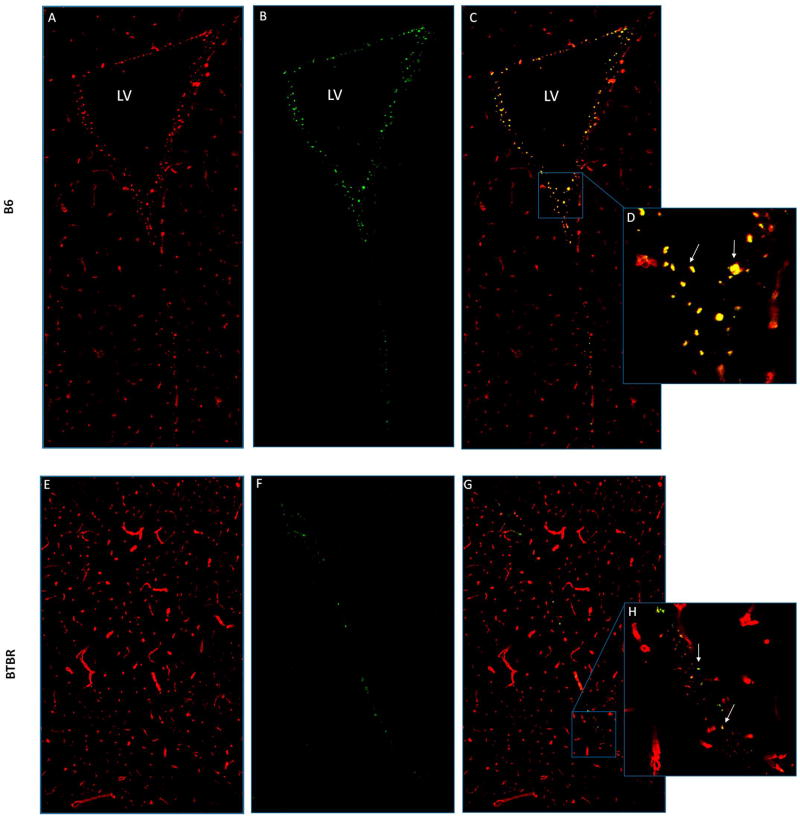
Figure 5.
Laminin and n-sulfated heparan sulfate in the SVZ of B6 and BTBR mice. Laminin (red) labels meninges and vasculature in the SVZ and surrounding tissue of the intact and collapsed lateral ventricles, respectively of B6 (a) and BTBR (e) mice. Images (b) and (f) represent n-sulfated heparan sulfate, and (c) and (g) present merged laminin and heparan channels with magnified images of (d) and (h) illustrating the reductions in fractone number (arrows), size and heparan immunoreactivity of BTBR mice relative to B6 controls.
9.2 Results
BTBR mice showed a strikingly reduced volume of the lateral ventricle cavity, particularly in its anterior portion (Fig. 5, compare B and F) where the ventricle was compressed to a slit rather than an open space. The SVZ, measured in its midline, was also about 40% shorter in BTBR mice, but the difference was less pronounced than for ventricle area.
Fractones were visualized by laminin immunoreactivity (LAM-ir) and their punctate appearance at this scale. BTBR mice had significantly fewer, and smaller, fractones than B6 mice. The intensity of fractone-associated LAM-ir staining in the SVZ was lower in BTBR than in B6 mice. Also, in contrast to B6 in which LAM-ir intensity was greatest in slices around AP +0.5 (from bregma), the AP distribution of LAM-ir in BTBR mice was not different over a range from +1.0 to −1.0. Heparan sulfate immunoreactivity (HS-ir) was also reduced in BTBR mice (Fig. 5F) and again, no AP differences were found in BTBR mice, while in B6 the highest content of HS-ir occurred in brain slices from Bregma 0.0.
9.3 Discussion of ECM differences
Findings of reductions in BTBR lateral ventricles and their adjacent subventricular zones open a wide range of possibilities for differences between BTBR and B6 mice. The SVZ of the lateral ventricles is one of two brain areas that show neurogenesis in the adult brain (Mudo et. al., 2009). Its diminution in BTBRs, to about 60% of the B6 length, along with reductions in each of the constituents of the SVZ that were measured in these mice – fractones and fractone-associated laminin, and heparan sulfates, suggests the possibility of ongoing deficiencies related to neurogenesis, neuronal differentiation, migration, and guidance in BTBR mice.
In particular, heparan sulfate appears to be strongly involved in the development of the CNS (Yamaguchi et al, 2010), and Douet & Mercier (in press) suggest that HS aggregation by fractones is a core mechanism in this range of effects. Conway et al (2011) note that postsynthetic sugar modifications of HS proteoglycans increase their molecular complexity and variability, potentially permitting differential modulation of cell responsivity to an array of specific signaling molecules. This view suggests that HS may have a core organizational role in the neural connections formed during development. In the present context, there is substantial evidence that reductions in the magnitude of HS (Inatani et al, 2003), or of specific heparan sulfotransferases (Conway et al, 2011) are associated with abnormalities in the corpus callosum. There are also potential links between disruptions in HS and autism, or autism-related behavior changes: Li et al (2002) reported a possible link between autism associated with mental retardation and novel deletion mutations in EXT1, a gene expressed in protein that is involved in the biosynthesis of heparan sulfate. This interpretation, that alterations of HS magnitude or composition can influence both callosal development, and autism-relevant behaviors, with some positive relationship but no causal link between the two latter phenotypes, is compatible with the report of Booth et al (2011) that some individuals with callosal agenesis show autism-like cognitive changes, while others without a corpus callosum develop good social/cognitive skills.
One set of growth/guidance factors known to be influenced by HS is the FGF (fibroblast growth factor) family. Several of these, including FGF8 and FGF17 are expressed by a rostral patterning center in the developing forebrain, and have a major role in the development of the cortex, (Borello et. al., 2008). FGF17-deficient mice have been reported to show reduced social behaviors, as well as reduced c-Fos expression in frontal cortex following social interactions in a novel environment (Scearce-Levie et. al., 2008). FGF8 hypomorphic mice show alterations of the epithalamus, including the habenula, potentially involved in motor learning, and the pineal gland (Martinez-Ferre and Martinez, 2009) which is strongly involved in the regulation of sleep-waking cycles. While sleep disturbances are not among the criteria for ASD, between 40 and 80% of autistic individuals do show disturbed sleep patterns (Doyen et. al., 2011; Souders et. al., 2009). It is also of interest that FGF8 hypomorphic mice show reductions in oxytocin in some hypothalamic nuclei (Brooks et. al., 2010). Oxytocin may be involved in social recognition and social reward functions related to autism (Insel, 2010).
10.0 General Summary and Discussion
The behavioral studies in this series compared BTBR and B6 mice in a variety of situations designed to provide parallels to two of the three major symptom groupings of ASD. The third such symptom grouping, deficiencies in communication, was not treated here. However, a series of studies by Maria Luisa Scattoni (Scattoni et al, 2008, 2010) outline some substantial differences in vocalizations for BTBR compared to B6 infant and adult mice.
Disruption of reciprocal social interactions, the first major symptom of ASD, was evaluated in a seminatural social situation affording many of the features of the natural environment for mice; a substantial area permitting spacing and avoidance; tunnels and burrows; a consistent social group; an extended period in which the animals could become familiar to each other. In this context, BTBR mice showed consistent reductions in social behaviors that persisted over several days in the situation, providing little suggestion that these behaviors would converge with the levels shown by B6 mice over a more extended period.
In contrast to the seminatural VBS, and the three-chamber social approach test that is often used to measure sociality in mice, the social proximity test was designed to eliminate the possibility of avoidance of a social stimulus in order to permit evaluation of other, perhaps more specific, changes in social behavior. Results from three separate experiments using same-strain male pairs of BTBR and B6 mice provided an exceptionally consistent picture of high-magnitude differences in close-in social behaviors for BTBR mice, with particular reductions in nose tip to nose tip behaviors for BTBR. When BTBR were paired with B6 males, B6 frequently attempted to initiate the nose tip to nose tip orientation, while the BTBR partner immediately withdrew. In addition, the pattern of changes in crawl under/crawl over suggested that BTBR, in this close situation, might be using crawl under to escape from frontal contact with the partner mouse. Diazepam reductions in crawl under for BTBR supported the suggestion that this was an escape/avoidance behavior. Diazepam also generally reduced jump escapes, and uprights (largely in B6, as BTBR showed few uprights), consonant with a view that these behaviors also reflect an aversive or avoidance response (Griebel et al, 1998, 2005). Notably, BTBR reductions in social approach in the three-chamber test were also normalized by diazepam, suggesting a role for social anxiety of BTBR mice in that test, even though the BTBR-B6 differences in tests of nonsocial anxiety did not suggest a consistent difference between the two strains. However, diazepam did not normalize nose tip to nose tip behaviors for BTBR, suggesting that avoidance of this behavior or orientation is independent of social anxiety, representing a more fundamental change in the social responsivity of BTBR mice. Notably, nose-to-head (typically involving orientation to the side of the head) was generally not decreased in BTBR pairs, suggesting that nose tip to nose tip may be avoided because of its eye to eye orientation; possible parallel to gaze aversion, an early symptom that is often associated with ASD (Kleinke, 1986).
In addition to indicating that BTBR mice, compared to B6 mice, may show social deficiencies –some normalizable by diazepam while others are not—that suggest parallels to major symptoms of ASD, these studies broaden the range of stereotypical behaviors shown by BTBR mice, replicating previously reported (McFarlane et al, 2008; Silverman et al, 2010a,b; Yang et al, 2007a, 2008a, 2010) increases in allogrooming; also reporting increased bar-biting; and adding a more detailed analysis of stereotypies in several aspects of object interest and approach. While the first two of these are consonant with an interpretation of motor stereotypy, the last of these suggests that the stereotypies of BTBR mice are not confined to motor behaviors, but encompass object preferences and more invariant patterns of attention to objects that may provide striking parallels to the stereotyped patterns of behavior, interests, and activities seen in autistic children (APA, 2000). These include an “encompassing preoccupation with one or more stereotyped and restricted patterns of interest…inflexible adherence to specific, nonfunctional routines or rituals… (and) a persistent preoccupation with parts of objects (APA, 2000).”
Finally, it is notable that several of the behaviors from the social proximity test differentiated not only groups, but all or almost all individuals of the two groups (Defensor et al, 2011). Nose-to-anogenital behavior was higher for the BTBR partner of every BTBR-B6 pair tested, while crawl under and nose-to-head showed overlaps between strains in only 1, or 2, respectively, of 14 such pairs. While nose tip-to-nose tip was, by definition, the same for both members of a pair, in mixed strain pairs the B6 member always appeared to be the initiator, while the BTBR mouse appeared to avoid such contact. The consistency of these differences suggest the possibility that they might be combinable into a profile or pattern of social aberration for individual animals, for possible comparison to variables of potential interest in terms of the etiology of autism-relevant behaviors.
The work with ECM differences of BTBR and B6 mice suggest one such potential variable. While the overall dynamics of heparan sulfate action as a modulator of growth and guidance factors in the ECM are just beginning to be outlined, recent literature (e.g. Inatani et. al., 2003; Conway et al, 2011) makes it clear that variations in HS magnitude or composition can produce abnormalities in brain structure (e.g. callosal agenesis) similar to that seen in BTBR mice, while some of the factors modulated by HS, such as members of the FGF family, may influence relevant behaviors (Brooks et al.,2010; Scearce-Levy et al, 2007). Scattered case reports (Ishikawa-Brush et. al., 1997; Li et. al., 2002; Verhoeven et. al., 2010) have suggested that HS may be associated with ASD, or with aberrations in social behaviors, and the finding that BTBR mice show reductions in HS associated with fractones suggests that these mice may provide a particularly important model for analysis of the dynamics of the HS system and its potential relationship to important biomarkers or endophenotypes for autism.
Highlights.
BTBR mice show reduced social behaviors, more “alone”, and enhanced autogrooming in a semi-natural VBS, and
reductions in nose tip-to-nose tip behaviors (gaze avoidance?) and enhanced crawl under/over in a social proximity test
Diazepam normalizes social preference and crawl under/over but not nose tip-to-nose tip behaviors of BTBR
BTBR object preferences and patterned investigations of several objects in order are enhanced
BTBR show sharply reduced fractone-associated heparan sulfate, suggesting disturbances of growth and guidance factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D. Caroline Blanchard, Pacific Biosciences Research Center, University of Hawaii, 1993 East-West Road, Honolulu, HI 96822, USA.
Erwin B. Defensor, Department of Psychology, University of Hawaii, 2430 Campus Road, Honolulu, HI 96822, USA
Ksenia Z. Meyza, Department of Psychology, University of Hawaii, 2430 Campus Road, Honolulu, HI 96822, USA
Roger L.H. Pobbe, Department of Psychology, University of Hawaii, 2430 Campus Road, Honolulu, HI 96822, USA
Brandon L. Pearson, Department of Psychology, University of Hawaii, 2430 Campus Road, Honolulu, HI 96822, USA
Valerie J. Bolivar, Wadsworth Center, New York State Department of Health, and Department of Biomedical Sciences, School of Public Health, State University of New York at Albany, Albany, NY 12208, USA
Robert J. Blanchard, Department of Psychology, University of Hawaii, 2430 Campus Road, Honolulu, HI 96822, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 1994. DSM-IV. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. DSM-IV-TR. [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. Anatomical Correlates of Functional Plasticity in Mouse Visual Cortex. J Neurosci. 1999;19(11):4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32(7):1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G, Cass H, Slonims V. Diagnosis of autism. BMJ. 2003;327:488–93. doi: 10.1136/bmj.327.7413.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197(2):462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Meyza KZ, Pearson BL, Pobbe RLH, Blanchard RJ. Abnormal lateral ventricle and subventricular extracellular matrix formation in BTBR T+tf/J mice; Submitted for the 2011 Society for Neuroscience Annual Meeting. [Google Scholar]
- Blanchard RJ, Blanchard DC. Anti-predator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Takahashi T, Kelley MJ. Attack and defensive behaviour in the albino rat. Anim Behav. 1977;25(3):622–634. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463(1–3):97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol Learn Mem. 2009;92(2):206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Wallace GL, Happe F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011;189:303–317. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- Borello U, Cobos I, Long JE, McWhirter JR, Murre C, Rubenstein JL. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Dev. 2008;3:17. doi: 10.1186/1749-8104-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks LR, Chung WC, Tsai PS. Abnormal hypothalamic oxytocin system in fibroblast growth factor 8-deficient mice. Endocrine. 2010;38(2):174–180. doi: 10.1007/s12020-010-9366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Conway CD, Howe KM, Nettleton NK, Price DJ, Mason JO, Pratt T. Heparan sulfate sugar modifications mediate the functions of slits and other factors needed for mouse forebrain commissure development. J Neurosci. 2011;31(6):1955–1970. doi: 10.1523/JNEUROSCI.2579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci. 2001;115:468–476. [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217(2):302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia-Zavasdkaia N. L’irradiation des testicules et l’heredite chez la souris. Arch Biol (Liege) 1928;38:457–501. [Google Scholar]
- Douet V, Mercier F. Control of cell division in the adult brain by heparan sulfates in fractones and vascular basement membranes. In press. [Google Scholar]
- Doyen C, Mighiu D, Kaye K, Colineaux C, Beaumanoir C, Mouraeff Y, Rieu C, Paubel P, Contejean Y. Melatonin in children with autistic spectrum disorders: recent and practical data. Eur Child Adolesc Psychiatry. 2011;20(5):231–239. doi: 10.1007/s00787-011-0162-8. [DOI] [PubMed] [Google Scholar]
- Fentress JC, Stilwell FP. Grammar of a movement sequence in inbred mice. Nature. 1973;244:52–53. doi: 10.1038/244052a0. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nature Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Jung A, Lee JC, Masuda CK, Blanchard RJ. Further evidence that the mouse defense test battery is useful for screening anxiolytic and panicolytic drugs: effects of acute and chronic treatment with alprazolam. Neuropharmacol. 1995;34:1625–1633. doi: 10.1016/0028-3908(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents: comparison with diazepam and buspirone. Psychopharmacology. 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiat. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Gupta AR, State MW. Recent Advances in the Genetics of Autism. Biol Psychiatry. 2007;61(4):429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327(1):1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302(5647):1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Brush Y, Powell JF, Bolton P, Miller AP, Francis F, Willard HF, et al. Autism and multiple exostoses associated with an X;8 translocation occurring within the GRPR gene and 3′ to the SDC2 gene. Hum Mol Genet. 1997;6(8):1241–1250. doi: 10.1093/hmg/6.8.1241. [DOI] [PubMed] [Google Scholar]
- Jackson Laboratory. Mouse Phenome Database (MPD) 2011 Retrieved on February 11, 2011 from http://phenome.jax.org/
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2(10):2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: a research review. Psychol Bull. 1986;100:78–100. [PubMed] [Google Scholar]
- Kusek GK, Wahlsten D, Herron BJ, Bolivar VJ, Flaherty L. Localization of two new X-linked quantitative trait loci controlling corpus callosum size in the mouse. Genes Brain Behav. 2007;6(4):359–363. doi: 10.1111/j.1601-183X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Li H, Yamagata T, Mori M, Momoi MY. Association of autism in two patients with hereditary multiple exostoses caused by novel deletion mutations of EXT1. J Hum Genet. 2002;47(5):262–265. doi: 10.1007/s100380200036. [DOI] [PubMed] [Google Scholar]
- Lundin L, Larsson H, Kreuger J, Kanda S, Lindahl U, Salmivirta M, Claesson-Welsh L. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem. 2000;275(32):24653–24660. doi: 10.1074/jbc.M908930199. [DOI] [PubMed] [Google Scholar]
- Lyon MF. Hereditary hair loss in the tufted mutant of the house mouse. J Hered. 1956;47:101–103. [Google Scholar]
- Maenner MJ, Durkin MS. Trends in the prevalence of autism on the basis of special education data. Pediatrics. 2010;126(5):1018–1025. doi: 10.1542/peds.2010-1023. [DOI] [PubMed] [Google Scholar]
- Martinez-Ferre A, Martinez S. The development of the thalamic motor learning area is regulated by Fgf8 expression. J Neurosci. 2009;29(42):13389–13400. doi: 10.1523/JNEUROSCI.2625-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Iervolino AC, Collishaw S. Time trends in child and adolescent mental disorders. Curr Opin Psychiatry. 2005;18:381–5. doi: 10.1097/01.yco.0000172055.25284.f2. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and bFGF are primarily expressed in subpial and subependymal layers in adult brain parenchyma: Roles in stem cell proliferation and morphological plasticity? J Comp Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188(1):178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudò G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009;116(8):995–1005. doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10(2):228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216(1):446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214(2):443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Kang SH, Choi DY, Platt B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res. 2009;204(1):217–225. doi: 10.1016/j.bbr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci USA. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Autism research: lessons from the past and prospects for the future. J Autism Dev Disord. 2005;35:241–257. doi: 10.1007/s10803-004-2003-9. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Res. 2008;187:371–78. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00623.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7(3):344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Psycho-pharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T(+)tf/J mouse model of autism. Neuroscience. 2010b;171(4):1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, Weaver TE, Pinto-Martin J. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull JE, Miller RL, Ahmed Y, Puvirajesinghe, Guimond SE. Glycomics profiling of heparin sulfate structure and activity. Methods Enzymol. 2010;480:65–85. doi: 10.1016/S0076-6879(10)80004-7. [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Csepán R, Marcelis CL, Lefeber DJ, Egger JI, Tuinier S. Sanfilippo B in an elderly female psychiatric patient: a rare but relevant diagnosis in presenile dementia. Acta Psychiatr Scand. 2010;122(2):162–165. doi: 10.1111/j.1600-0447.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- Walhsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971(1):47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10(1):35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Inatani M, Matsumoto Y, Ogawa J, Irie F. Roles of heparan sulfate in mammalian brain development current views based on the findings from Ext1 conditional knockout studies. Prog Mol Biol Transl Sci. 2010;93:133–152. doi: 10.1016/S1877-1173(10)93007-X. [DOI] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2010 doi: 10.1002/aur.163. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, MacFarlane HG, Crawley JN. Social Approach Behaviors are Similar on Conventional Versus Reverse Lighting Cycles, and in Replications Across Cohorts, in BTBR T+ tf/J, C57BL/6J, and Vasopressin Receptor 1B Mutant Mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25(8):515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]