Abstract
Abstract
Mitochondria are complex organelles constantly undergoing processes of fusion and fission, processes that not only modulate their morphology, but also their function. Yet the assessment of mitochondrial function in skeletal muscle often involves mechanical isolation of the mitochondria, a process which disrupts their normally heterogeneous branching structure and yields relatively homogeneous spherical organelles. Alternatively, methods have been used where the sarcolemma is permeabilized and mitochondrial morphology is preserved, but both methods face the downside that they remove potential influences of the intracellular milieu on mitochondrial function. Importantly, recent evidence shows that the fragmented mitochondrial morphology resulting from routine mitochondrial isolation procedures used with skeletal muscle alters key indices of function in a manner qualitatively similar to mitochondria undergoing fission in vivo. Although these results warrant caution when interpreting data obtained with mitochondria isolated from skeletal muscle, they also suggest that isolated mitochondrial preparations might present a useful way of interrogating the stress resistance of mitochondria. More importantly, these new findings underscore the empirical value of studying mitochondrial function in minimally disruptive experimental preparations. In this review, we briefly discuss several considerations and hypotheses emerging from this work.
Tanja Taivassalo (far left) is in the Department of Kinesiology and Montreal Neurological Institute at McGill University, with a background in Neuroscience and Exercise Physiology. Russ Hepple (second from left) is in the Department of Kinesiology and Department of Medicine at McGill University, with a background in Physiology. Martin Picard (second from right) and Gilles Gouspillou (far right) are a PhD candidate and Postdoctoral Fellow, respectively, in the Hepple and Taivassalo laboratories. Together, we are working on animal model and clinical projects aiming to better understand the role of mitochondria in skeletal muscle structure and function in health, ageing, and disease. Our recent work reveals that routine mitochondrial isolation procedures from skeletal muscle have a striking impact on mitochondrial function. This and other work is driving the continued evolution of how we study and interpret mitochondrial function.
 |
Mitochondria regulate critical cellular processes, from energy production to apoptosis, and measuring their function is an imperative for scientists whose research focuses on different aspects of cellular metabolism. The in-depth study of mitochondrial function in muscle tissue is most often achieved using one of two types of preparations: isolated mitochondria, where mitochondria are extracted and purified by mechanical homogenization and differential centrifugation (Frezza et al. 2007b); or permeabilized myofibres, where the plasma membrane of myofibres is selectively permeabilized leaving the mitochondria intact within their native cytoarchitectural environment and in direct contact with the incubation medium (Saks et al. 1991; Kuznetsov et al. 2008). Both methods permit the direct manipulation of mitochondrial function through the addition of specific substrates and inhibitors to the incubation medium, permitting insights into a wide range of mitochondrial functions, as detailed below.
Historically, isolated mitochondrial preparations were introduced more than half a century ago by the pioneering work of Chance & Williams (1956) and led to seminal discoveries about mitochondrial biology, including the chemiosmotic theory of oxidative phosphorylation (Mitchell, 1961) and elucidation of the Krebs cycle (Williams, 1965). To overcome important yield and selective isolation bias issues inherent to traditional isolated mitochondrial preparations, the permeabilized myofibres method was developed by Valdur Saks and colleagues several decades later (Veksler et al. 1987; Saks et al. 1998). Experimentally, mitochondria in both preparations are ultimately fed substrates and inhibitors in a carefully ordered manner allowing the experimenter to measure, among other parameters, mitochondrial respiration, reactive oxygen species (ROS) production and scavenging, and susceptibility to apoptotic events such as opening of the mitochondrial permeability transition pore (mPTP) (Zoll et al. 2003; Anderson & Neufer, 2006; Picard et al. 2008; Ljubicic et al. 2010).
Despite the development of the permeabilized myofibre method, isolated mitochondria continue to be the more widely used method of investigating mitochondrial function in skeletal muscle. It seems likely that the assumption that isolated organelles preserve the function of mitochondria in vivo stems from the old ‘textbook’ view of mitochondrial structure, where these organelles were considered as bean-shaped spheroids that became liberated from their intracellular tethers upon mechanical isolation. However, we have known for more than a quarter of a century that skeletal muscle mitochondria exhibit a markedly diverse architecture (Bakeeva et al. 1978; Kirkwood et al. 1986; Kayar et al. 1988; Ogata & Yamasaki, 1997), with some mitochondria exhibiting elongated tubular branched structures (Ogata & Yamasaki, 1997) and extensive functional connections (Fang et al. 2011) between what would appear to be individual mitochondria in tissue cross-sections. With this view in mind, it is clear that mitochondrial structure after isolation procedures would be radically altered, owing to the ripping apart of the more elaborate tubular structures to yield relatively homogeneously sized spheroid organelles during mechanical homogenization. Based on the growing appreciation for the fact that mitochondrial structure is intricately linked to their function, alteration of mitochondrial function should be an expected outcome following routine isolation procedures. An overview of our current understanding of the events occurring during routine mitochondrial isolation procedures and their impact on mitochondrial morphology is depicted in Fig. 1.
Figure 1. Mitochondrial isolation fragments mitochondria and alters mitochondrial function.
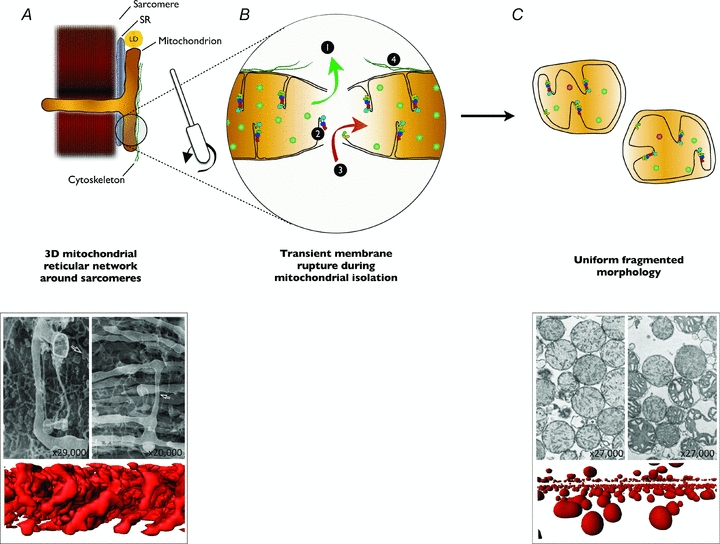
A, in vivo and in situ, mitochondria exhibit a complex three-dimensional network arrangement around sarcomeres (Kirkwood et al. 1986; Ogata & Yamasaki, 1997). In addition, mitochondria maintain functional interactions with the sarcoplasmic reticulum (SR), lipid droplets (LD) (Goodman, 2008) and cytoskeleton (Saks et al. 2010). B, during mitochondrial isolation, muscle is homogenized using scissors and a Teflon pestle, which separates mitochondria and ruptures mitochondrial membranes. This transient rupture of mitochondrial membranes has four potential direct effects: allowing soluble mitochondrial molecules to escape the mitochondria, thereby diluting matrix components necessary for oxidative phosphorylation or reactive oxygen species detoxification (1); causing disruption of the inner mitochondrial membrane and the structural integrity of its residing supramolecular complexes (2); allowing soluble molecules from the homogenization medium to enter the mitochondria to alter aspects of mitochondrial function, or allowing the medium itself to enter the matrix causing swelling and dilution of matrix constituents (3); and disrupting functional interactions between mitochondria, SR, LDs and cytoskeleton (4). C, the end result of mitochondrial isolation is a relatively homogeneous population of spherical organelles with swollen morphology, disorganized cristae and diluted matrix content. Together, these effects result in altered functional characteristics of isolated mitochondria when compared to intact mitochondria from permeabilized myofibres. EM images reproduced from Ogata & Yamasaki (1997) and Hackenbrock (1968), with permission from Wiley and Rockefeller University Press, respectively. Three dimensional reconstructions are from MitoTracker-labelled confocal imaging of mitochondria in permeabilized myofibres or isolated mitochondria, as described in Picard et al. (2011).
Morphology–function relationship
As evidence of the impact of mitochondrial structure on function (Heath-Engel & Shore, 2006), some have shown that enhanced network branching induced by upregulating mitochondrial fusion (Sugioka et al. 2004; Ong et al. 2010) or downregulating fission (Ong et al. 2010) can reduce or prevent apoptotic signalling. Blocking mitochondrial fission also prevents fission-induced ROS release in hyperglycaemic conditions (Yu et al. 2006). The opposite also appears to occur: enhanced network fragmentation by upregulating mitochondrial fission (Frank et al. 2001; Ong et al. 2010) or downregulating mitochondrial fusion (Lee et al. 2004; Sugioka et al. 2004) can promote pro-apoptotic signalling in live cells, although this causal link has not always been observed (Youle & Karbowski, 2005). Further to this, promoting mitochondrial fission and network fragmentation has been associated with reduced respiratory capacity and increased ROS production (Koopman et al. 2005; Benard et al. 2007; Frezza et al. 2007a; Yu et al. 2008). In addition, the major protein involved in mitochondrial fusion – mitofusin 2 – influences expression of oxidative phosphorylation genes (Pich et al. 2005), indicating overlap at the genetic level between regulatory pathways for mitochondrial morphology and metabolism (Zorzano et al. 2010). Mitochondrial membrane potential is also closely associated with reversible changes in mitochondrial morphology (Guillery et al. 2008), and additional findings demonstrate an intricate relationship between mitochondrial dynamics, structure and function (Chen & Chan, 2010; McBride & Soubannier, 2010). Clear demonstration that mitochondrial fusion plays a major physiological role in skeletal muscle function comes from skeletal muscle-specific Mfn2−/− mice, which accumulate mutations in mitochondrial DNA, exhibit severe deficits in growth (body weight 30–50% of Mfn2+/+) and muscle oxidative capacity, and have impaired thermogenesis (Chen et al. 2010). This profound interdependence among mitochondrial morphology and function, emerging decades following the establishment of isolation methods as the gold standard to study mitochondrial function, obligates re-consideration of the functional characteristics of isolated organelles.
If we compare the morphology of mitochondria in intact skeletal muscle fibres – branched elongated structures, of heterogeneous size and shape (Bakeeva et al. 1978; Kirkwood et al. 1986; Kayar et al. 1988; Ogata & Yamasaki, 1997) – to that of isolated mitochondria – quasi-spherical units, of relatively homogeneous size and shape (Figueiredo et al. 2008; Picard et al. 2010) – we must logically conclude that membranes of the mitochondrial network are ruptured during muscle homogenization and must rapidly re-seal to yield apparently ‘intact’ organelles. Consistent with this, our results (Picard et al. 2011) and those of others (Schwerzmann et al. 1989) suggest that soluble matrix enzymes are lost from the mitochondrial matrix during isolation, probably during the transient rupturing–resealing of mitochondrial membranes that occurs during isolation procedures. Until recently, the functional consequences of this loss of matrix constituents and altered organelle morphology resulting from mitochondrial isolation have been largely unknown.
Mitochondrial isolation: functional consequences
Saks and colleagues first reported that the Km for ADP during mitochondrial respiration is considerably lower in isolated organelles versus those left in situ within permeabilized myofibre bundles (Saks et al. 1991). Lack of interactions between the mitochondrial voltage-dependent anion channel (VDAC) and cytoskeletal components such as tubulin, as well as an absence of ADP diffusion limitations appear to cause this effect (Saks et al. 2010). However, until recently the full magnitude of differences in respiratory capacity under different states, ROS production, and susceptibility to mPTP opening between these two preparations was unknown.
In an attempt to better understand the impact of isolation on mitochondrial function, we recently conducted a series of experiments directly comparing isolated mitochondria and permeabilized myofibres from the rat gastrocnemius muscle. Strikingly, isolated mitochondria exhibited a 47-fold sensitization of the mPTP to Ca2+ challenge (shorter time to mPTP opening), altered respiratory responses to stepwise substrate addition (lower state 2 respiration, higher N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD)-driven complex IV respiration in isolated mitochondria), and increased ROS production by 9- to 23-fold under different energized states (Picard et al. 2011). One could argue that these considerable differences in key indices of mitochondrial function may arise from a selection of a specific mitochondrial population yielded by the isolation procedure. Indeed, muscle cells contain two different mitochondrial populations – subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria – that not only differ in their intracellular localization but also in their functional properties (Adhihetty et al. 2005) and in their relative ease of extraction. However, the magnitude of difference between SS and IMF mitochondria in mPTP sensitivity to Ca2+ is only around 30–40% (IMF > SS) (Adhihetty et al. 2005); and the magnitude of difference for ROS production is only around 2- to -3-fold (IMF < SS) (Adhihetty et al. 2005), suggesting that selective isolation of subpopulations of mitochondria is unlikely to account for the observed differences between mitochondria from isolated and permeabilized preparations. Finally, in the experiments that we employed with isolated mitochondria we used the protease nagarse to facilitate harvest of both SS and IMF mitochondrial populations (Picard et al. 2011). Thus, the differences in function between isolated mitochondria and permeabilized myofibre bundles are most likely a consequence of the isolation procedures on mitochondrial physiology.
To highlight differences between the isolated mitochondria and permeabilized myofibre preparations, here we analyse all of these experiments collectively by using a hierarchical clustering analysis (Fig. 2). This unsupervised analysis – describing similarities between samples and functional parameters – clearly shows that isolated mitochondria and permeabilized myofibres segregate into two distinct clusters. These two clusters reveal that isolated mitochondria and permeabilized myofibres possess strikingly distinct functional signatures across a broad range of mitochondrial functions. It is, however, important to note that state 3 respiratory capacity (Vmax), the most commonly reported index of mitochondrial function from isolated organelles, was not different between preparations. This is likely to explain, at least partially, why the magnitude of altered function in isolated mitochondria has not been appreciated heretofore.
Figure 2. Isolated mitochondria and permeabilized myofibres have different functional signatures.
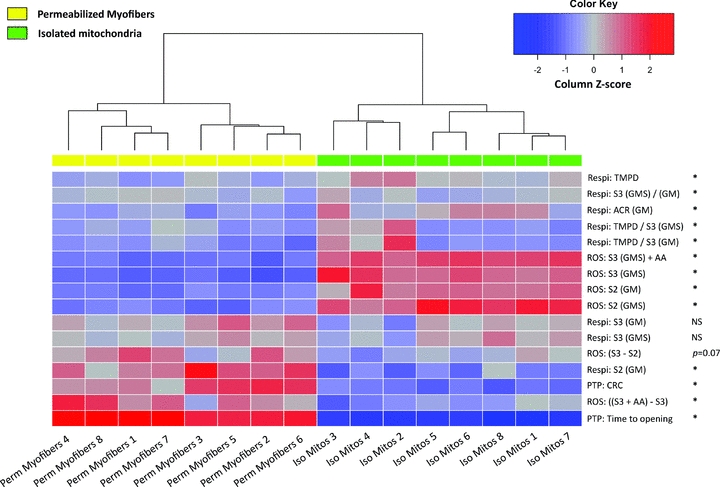
Heat map depicting results from an unsupervised hierarchical clustering analysis, whereby samples with the most similar functional signatures are grouped together. Functional signatures are defined here as the integration of functional measures of respiratory capacity (Respi) and H2O2 production (ROS) under various stimulatory conditions, as well as mitochondrial permeability transition pore (mPTP) responses to a Ca2+ challenge. The dendrogram above the heat map shows two primary clusters of similar compactness and exhibiting marked distinctiveness, corresponding to permeabilized myofibre and isolated mitochondrial preparations. The heat map analysis was performed with data obtained from mixed gastrocnemius muscles of Fisher 344/Brown Norway F1-hybrid rats (data reported in: Picard et al. 2011). To allow direct comparison of both preparations, all data are presented and normalized per international unit of citrate synthase activity, except for time to PTP opening expressed in seconds. The z-score for each value was computed from the mean for each measure. Measures which significantly differ (P < 0.05) between preparations are marked with an asterisk. Statistical analysis was performed using ‘R’ (R Foundation for Statistical Computing, version 2.12.1). List of abbreviations: TMPD, TMPD-driven complex IV respiration; S2, State 2 – Substrates-driven respiration; S3, State 3 – ADP-driven respiration with glutamate + malate (GM) or GM + succinate (GMS); ACR, acceptor control ratio; AA, antimycin A; CRC, calcium retention capacity; NS, not significant. Details of experimental procedures are reported in Picard et al. (2011).
On the one hand, because these findings demonstrate that isolation profoundly impacts several aspects of mitochondrial function, this raises concerns regarding the interpretation of results obtained in studies using isolated mitochondria. For example, age-related changes in mitochondrial function are exaggerated when assessed in isolated mitochondria compared to permeabilized myofibres (Picard et al. 2010). On the other hand, because isolated mitochondria exhibit qualitatively similar characteristics of mitochondria undergoing fission during apoptosis in vivo (Youle & Karbowski, 2005; Frezza et al. 2007a), including marked fragmented morphology, elevated ROS production and sensitization of the mPTP (Picard et al. 2011), isolated mitochondria may better represent stressed organelles than mitochondria functioning under normal circumstances in vivo, suggesting a novel application of mitochondrial isolation to interrogate the stress resistance of the organelle.
Interestingly, mitochondrial respiration was selectively impaired in isolated mitochondria when complex I (NADH:ubiquinone oxidoreductase) substrates were used, compared to conditions where substrates acting more directly on a given complex were used (succinate to activate complex II; the artificial electron donor, TMPD, used to directly activate complex IV). To this end, we note that complex I-driven respiration with malate and glutamate as substrates depends on the presence and function of soluble components of the Krebs cycle to produce the reducing equivalent NADH, and consequently also depends on the presence of soluble precursor molecules such as the coenzyme NAD+. In addition, complex I activity is potentiated by its assembly with complex III and IV within the inner mitochondrial membrane into functional supercomplexes (Schafer et al. 2006; Vonck & Schafer, 2009). Based on this and on the model illustrated in Fig. 1, we speculate that this preferential loss of Complex I activity could be explained by a combination of the following factors: (i) partial loss of matrix constituents like NAD+ and Krebs cycle components during isolation (as originally suggested by Schwerzmann et al. (1989); (ii) proteolytic damage to ETC proteins by nagarse, which may enter the mitochondria during isolation, resulting in protein degradation (Wilson, 1987; Patel et al. 2009); and/or (iii) cristae remodelling and disruption of supramolecular structures induced by isolation, secondary to either swelling or mitochondrial inner membrane (IMM) rupture. The first two possibilities are discussed in detail elsewhere (Picard et al. 2011). The third is supported by electron microscopy (EM) imaging of isolated mitochondria showing disrupted architecture of the IMM cristae in isolated organelles compared to in vivo (Fig. 3).
Figure 3. Muscle mitochondrial ultrastructure is disrupted in isolated mitochondria (in vitro) compared to normal mitochondria in situ.
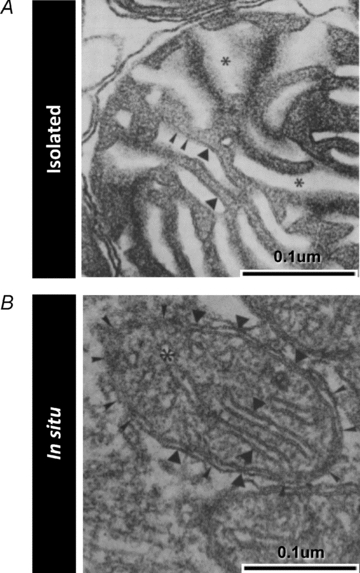
Electron micrographs of mitochondria from skeletal muscle depicting the cristae morphology in isolated mitochondria (A) compared to mitochondria fixed in situ (B). Several differences are noted in isolated mitochondria, including: lower electron density of the matrix space, dysmorphic and irregular inner mitochondrial membrane cristae structure, absence of consistent contact sites near cristae junctions, and swelling of inter-membrane spaces. Images are from Schwerzmann et al. (1989) and are reproduced here with permission from the National Academy of Sciences of the USA.
Whether the processes involved in the mitochondrial membrane resealing that must occur during mitochondrial isolation are passive, involving spontaneous resealing of phospholipid bilayer, or active, involving the mitochondrial fusion proteins mitofusin 1 and 2 (Mfn2 and Mfn2) and optic atrophy 1 (OPA1), is currently unknown. In our recent experiments, we also observed that the functional alterations induced by the isolation of mitochondria from skeletal muscles were greater in aged muscles (Picard et al. 2010). If the successful resealing of disrupted mitochondria during isolation depends upon fusion proteins, these results could in part be explained by dysfunction in the fusion aspect of mitochondrial dynamics that may occur with ageing (Seo et al. 2010), handicapping the ability of mitochondria to reconstitute during isolation and rendering them more susceptible to isolation damage. This would also exacerbate loss of matrix constituents during isolation procedures in aged mitochondria, a point consistent with the elevation in ratio of the biochemical activities of the mitochondrial inner membrane enzyme, complex IV, and the mitochondrial matrix enzyme, citrate synthase, that we observed in isolated mitochondria from aged muscle (Picard et al. 2010). Further work will be necessary to more completely address these issues.
Live cell and in vivo methods to assess mitochondrial function
While we favour the use of the permeabilized myofibre over the isolated mitochondrial technique based on its preservation of mitochondrial morphology and its flexibility of use in assessing a wide range of mitochondrial functional outputs, there are methods which permit measurement of certain parameters of mitochondrial function from live cells where the sarcolemma is left intact. For instance, modern microscopy-based imaging methods such as confocal (for cultured cells or in vivo imaging) (Duchen, 2004; Frezza et al. 2007a; Fang et al. 2011) and two-photon fluorescence microscopy (for in vivo imaging in tissues) (Rudolf et al. 2004; Pozzan & Rudolf, 2009; Romanello et al. 2010) have been used to monitor mitochondrial function in different types of muscle cells (Duchen, 2004; Saks et al. 2010; Fang et al. 2011). Microscopic imaging is used in conjunction with specific fluorescent probes sensitive to mitochondrial membrane potential, [Ca2+], or ROS (e.g. O2.−); by exploiting the autofluorescent properties of intracellular molecules such as NAD(P)H and flavoproteins (i.e. FADH) at specific excitation wavelengths (Duchen, 2004), the assessment of intracellular fluxes and relative levels of biochemical parameters linked to mitochondrial function is made possible.
A definite advantage of imaging techniques over in vitro preparations (i.e. isolated mitochondria and permeabilized myofibres) is that mitochondrial morphology and certain aspects of function can be assessed simultaneously (Frezza et al. 2007a; Fang et al. 2011). Additionally, in the in vivo situation, mitochondria are not only morphologically intact but also in their native intracellular and systemic environment, which allows one to evaluate certain mitochondrial function parameters in the context of putative soluble cytoplasmic factors that may impact how mitochondria function within the intact muscle. However, imaging techniques do not provide information on one of the most important mitochondrial functional parameters: the oxygen consumption rate. Oxygen consumption can nevertheless be measured in intact cultured cells using high sensitivity systems (such as the Seahorse (Gerencser et al. 2009), or Oroboros systems (Gnaiger, 2009)), with the compromise being that these systems do not permit simultaneous microscopic measurements. Alternatively, oxygen consumption has also been monitored using a standard  electrode in single intact myocytes (Elzinga & van der Laarse, 1988), and this method can probably be adapted to permit monitoring of other aspects of cellular function simultaneously using confocal imaging methods (Stary & Hogan, 2000, 2005).
electrode in single intact myocytes (Elzinga & van der Laarse, 1988), and this method can probably be adapted to permit monitoring of other aspects of cellular function simultaneously using confocal imaging methods (Stary & Hogan, 2000, 2005).
A significant drawback of using imaging methods on cells with an intact plasma membrane lies in the fact that the micro-environment surrounding mitochondria cannot be readily and precisely controlled. Consequently, these methods preclude the accurate determination of the effect of certain factors of interest on mitochondrial function. For example, live cell experiments using microscopy prevents one testing the direct effect of specific substrates (e.g. glutamate, malate, succinate, long-chain fatty acids) or non-freely diffusible molecules (ADP) on mitochondrial function. Determining the effect of these different molecules on mitochondrial function is an important step in the characterization of mitochondrial alterations/adaptations induced by physiological or pathophysiological events, but such level of experimental control is currently only possible with isolated mitochondria and permeabilized myofibre methods. Confocal microscopy techniques can nevertheless be applied to permeabilized myofibres with the added benefit of spatial resolution, allowing investigation of subcellular and organelle-level dynamics in [Ca2+] and redox state (Isaeva et al. 2005). Given the breadth of techniques preserving mitochondrial morphology that are available, researchers must determine how much experimental control is necessary to answer physiological questions of interest. Furthermore, one may wish to use intact cells in tandem with more invasive approaches, with the latter being used to provide greater detail of the nature of specific mitochondrial defects identified in intact cells.
Summary
Over the last several decades, preparations of ‘intact’ isolated mitochondria from skeletal muscle have been widely employed to study the active function of these organelles. However, we now have convincing evidence that mitochondrial isolation severely disturbs mitochondrial morphology, and not unexpectedly, that this is associated with marked impairments of mitochondrial function. Although these differences urge caution in the interpretation of data collected from isolated mitochondria, a more thorough understanding of the basis for these differences in function could lead to novel applications of this technique. Indeed, developing assays allowing one to experimentally enhance or impair reconstitution of mitochondria during isolation from tissues may provide new insights into the rapidly evolving understanding of structure–function relationships in mitochondria.
In this era where neurodegenerative, muscular, cardiovascular, malignant and metabolic diseases are frequently proposed to involve altered mitochondria, and where several versions of a mitochondrial theory of ageing dominates the current thinking in gerontology, there is a pressing need to test these theories with the most appropriate and relevant experimental methods. Characterizing the current methods – isolated mitochondria, permeabilized cells and intact cell microscopy-based methods – to permit a clearer understanding of how function is influenced by the preparation used, is a necessary step in this process. This will allow researchers to better exploit the current methods and hopefully pave the way for the development of new ways of investigating mitochondrial function and their impact on physiological/pathophysiological processes, with the ultimate goal of identifying molecular targets for interventions. In this latter respect, while the current review underscores the effect of isolation on mitochondrial function, we must also consider how mitochondrial function may be altered from the in vivo condition even in the permeabilized myofibre technique, where the intracellular environment is altered from the native state by the use of standard incubation buffers. This alteration could mask changes in mitochondrial function that occur in vivo secondary to disease- and/or ageing-induced alterations in the intracellular milieu in which the mitochondria reside. This critical evaluation of the methods for interrogating mitochondrial function is fundamental to advancing our understanding of how mitochondria may be involved in health, ageing and disease.
Glossary
Abbreviations
- IMM
inner mitochondrial membrane
- mPTP
mitochondrial permeability transition pore
- ROS
reactive oxygen species
References
- Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289:C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol. 2006;290:C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta. 1978;501:349–369. doi: 10.1016/0005-2728(78)90104-4. [DOI] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J Biol Chem. 1956;221:477–489. [PubMed] [Google Scholar]
- Chen H, Chan DC. Physiological functions of mitochondrial fusion. Ann N Y Acad Sci. 2010;1201:21–25. doi: 10.1111/j.1749-6632.2010.05615.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Elzinga G, van der Laarse WJ. Oxygen consumption of single muscle fibres of Rana temporaria and Xenopus laevis at 20 degrees C. J Physiol. 1988;399:405–418. doi: 10.1113/jphysiol.1988.sp017088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X, Zheng M, Wang W, Cheng H. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 2011 doi: 10.1038/cr.2011.81. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo PA, Ferreira RM, Appell HJ, Duarte JA. Age-induced morphological, biochemical, and functional alterations in isolated mitochondria from murine skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:350–359. doi: 10.1093/gerona/63.4.350. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Measuring mitochondrial shape changes and their consequences on mitochondrial involvement during apoptosis. Methods Mol Biol. 2007a;372:405–420. doi: 10.1007/978-1-59745-365-3_29. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007b;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Goodman JM. The gregarious lipid droplet. J Biol Chem. 2008;283:28005–28009. doi: 10.1074/jbc.R800042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery O, Malka F, Frachon P, Milea D, Rojo M, Lombes A. Modulation of mitochondrial morphology by bioenergetics defects in primary human fibroblasts. Neuromuscul Disord. 2008;18:319–330. doi: 10.1016/j.nmd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968;37:345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath-Engel HM, Shore GC. Mitochondrial membrane dynamics, cristae remodelling and apoptosis. Biochim Biophys Acta. 2006;1763:549–560. doi: 10.1016/j.bbamcr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayar SR, Hoppeler H, Mermod L, Weibel ER. Mitochondrial size and shape in equine skeletal muscle: a three-dimensional reconstruction study. Anat Rec. 1988;222:333–339. doi: 10.1002/ar.1092220405. [DOI] [PubMed] [Google Scholar]
- Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol. 1986;251:C395–402. doi: 10.1152/ajpcell.1986.251.3.C395. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Visch HJ, Verkaart S, van den Heuvel LW, Smeitink JA, Willems PH. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol. 2005;289:C881–890. doi: 10.1152/ajpcell.00104.2005. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibres, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubicic V, Menzies KJ, Hood DA. Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech Ageing Dev. 2010;131:79–88. doi: 10.1016/j.mad.2009.12.004. [DOI] [PubMed] [Google Scholar]
- McBride H, Soubannier V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol. 2010;20:R274–276. doi: 10.1016/j.cub.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibres. Anat Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Patel SP, Gamboa JL, McMullen CA, Rabchevsky A, Andrade FH. Lower respiratory capacity in extraocular muscle mitochondria: evidence for intrinsic differences in mitochondrial composition and function. Invest Ophthalmol Vis Sci. 2009;50:180–186. doi: 10.1167/iovs.08-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol. 2008;295:R659–668. doi: 10.1152/ajpregu.90357.2008. [DOI] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Wright KJ, Romestaing C, Melissa MT, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9:1032–1046. doi: 10.1111/j.1474-9726.2010.00628.x. [DOI] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MT, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rudolf R. Measurements of mitochondrial calcium in vivo. Biochim Biophys Acta. 2009;1787:1317–1323. doi: 10.1016/j.bbabio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V, Guzun R, Timohhina N, Tepp K, Varikmaa M, Monge C, Beraud N, Kaambre T, Kuznetsov A, Kadaja L, Eimre M, Seppet E. Structure-function relationships in feedback regulation of energy fluxes in vivo in health and disease: Mitochondrial interactosome. Biochim Biophys Acta. 2010;1797:678–697. doi: 10.1016/j.bbabio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Saks VA, Belikova YO, Kuznetsov AV. In vivo regulation of mitochondrial respiration in cardiomyocytes: specific restrictions for intracellular diffusion of ADP. Biochim Biophys Acta. 1991;1074:302–311. doi: 10.1016/0304-4165(91)90168-g. [DOI] [PubMed] [Google Scholar]
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fibre techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- Schafer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, Vonck J. Architecture of active mammalian respiratory chain supercomplexes. J Biol Chem. 2006;281:15370–15375. doi: 10.1074/jbc.M513525200. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER. Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci U S A. 1989;86:1583–1587. doi: 10.1073/pnas.86.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JCY, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2532–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Impairment of Ca2+ release in single Xenopus muscle fibres fatigued at varied extracellular PO2. J Appl Physiol. 2000;88:1743–1748. doi: 10.1152/jappl.2000.88.5.1743. [DOI] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Intracellular pH during sequential, fatiguing contractile periods in isolated single Xenopus skeletal muscle fibres. J Appl Physiol. 2005;99:308–312. doi: 10.1152/japplphysiol.01361.2004. [DOI] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibres. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- Vonck J, Schafer E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim Biophys Acta. 2009;1793:117–124. doi: 10.1016/j.bbamcr.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Williams GR. Dynamic aspects of the tricarboxylic acid cycle in isolated mitochondria. Can J Biochem Physiol. 1965;43:603–615. doi: 10.1139/o65-070. [DOI] [PubMed] [Google Scholar]
- Wilson EJ. Should nagarse be used during the isolation of brain mitochondria? Neurochem Res. 1987;12:831–834. doi: 10.1007/BF00971523. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll J, N'Guessan B, Ribera F, Lampert E, Fortin D, Veksler V, Bigard X, Geny B, Lonsdorfer J, Ventura-Clapier R, Mettauer B. Preserved response of mitochondrial function to short-term endurance training in skeletal muscle of heart transplant recipients. J Am Coll Cardiol. 2003;42:126–132. doi: 10.1016/s0735-1097(03)00499-6. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol. 2010;21:566–574. doi: 10.1016/j.semcdb.2010.01.002. [DOI] [PubMed] [Google Scholar]


