Abstract
Tumor-associated carbohydrate antigens (TACA) result from the aberrant glycosylation that is seen with transformation to a tumor cell. The carbohydrate antigens that have been found to be tumor-associated include the mucin related Tn, Sialyl Tn, and Thomsen-Friedenreich antigens, the blood group Lewis related LewisY, Sialyl LewisX and Sialyl LewisA, and LewisX, (also known as stage-specific embryonic antigen-1, SSEA-1), the glycosphingolipids Globo H and stage-specific embryonic antigen-3 (SSEA-3), the sialic acid containing glycosphingolipids, the gangliosides GD2, GD3, GM2, fucosyl GM1, and Neu5GcGM3, and polysialic acid. Recent developments have furthered our understanding of the T-independent type II response that is seen in response to carbohydrate antigens. The selection of a vaccine target antigen is based on not only the presence of the antigen in a variety of tumor tissues but also on the role this antigen plays in tumor growth and metastasis. These roles for TACAs are being elucidated. Newly acquired knowledge in understanding the T-independent immune response and in understanding the key roles that carbohydrates play in metastasis are being applied in attempts to develop an effective vaccine response to TACAs. The role of each of the above mentioned carbohydrate antigens in cancer growth and metastasis and vaccine attempts using these antigens will be described.
Keywords: Tumor-associated carbohydrate antigens, T-independent antigens, Vaccines, Mucin tumor-associated antigens, ganglioside tumor-associated antigens, Lewis Ag related tumor-associated antigens, glycosphingolipid tumor-associated antigens
1. Introduction
1.1 Background
Recent successes in cancer vaccines and in monoclonal antibody cancer immunotherapy give promise and impetus to the development of vaccines targeting cancer-associated carbohydrates. Investigations into therapy or prevention of tumors using the immune system have gone on for decades yielding an acquired foundation of knowledge, but little immediate clinical benefit. The strong foundation that has been obtained will allow researchers to capitalize on the current successes and bring these successes to the more difficult carbohydrate tumor antigen target. Many tumor antigens are carbohydrates, therefore all accumulated knowledge concerning these targets must be understood so that the weaker T cell-independent responses to these antigens are improved for clinical success.
Cancer immunotherapy began in 1892, when Coley noticed that patients with cancer who developed a skin infection with Group A streptococcus called erysipelas sometimes experienced regression of their tumor [1–3]. Coley developed an extract of Streptococcus and Serratia called Coley’s toxins, which he used to treat patients. Some of these patients improved, and these experiments generated interest in the immune system as a way to fight cancer. These toxins were produced and utilized until 1953 by Park-Davis. However, others encountered problems replicating Coley’s results and since the mechanism of action of this response was not well understood, it was difficult to determine what variables were important. The mechanism of Coley’s treatment may have been through stimulation of the innate immune system through the LPS and unmethylated bacterial DNA interactions with Toll-like receptors. Other bacteria that have been used as immune enhancing agents in cancer patients include Bacille Calmette-Guerin (BCG) and Corynebacterium parvum. Of these immune enhancing agents, BCG remains as an accepted method of treatment for bladder cancer. It is important to remember the innate immune system as a possible tool to increase an anti-tumor-associated cancer antigen (TACA) response.
The initial study of tumor immunology in animal models provided data for the field of transplantation. Careful controls using normal organs allowed these initial studies in 1912 to delineate laws of organ transplant acceptance and rejection [4]. The role of T cells in transplantation rejection is clear. In 1957, Prehn and Main showed that T cells reacted to tumor antigens and could help mediate tumor regression in a methylcholanthrene-induced sarcoma [5]. The role of the T cell as a major player in the intimately related field of transplantation combined with the results of these early studies may have led to the near solo role of the T cell as the cell that tumor immunologists were banking on for the creation of an anti-tumor response. The humoral response to tumor antigens has been discounted for a number of years, with antibody to tumor antigens thought of by many as a misfortunate event dubbed “blocking antibody” for its proposed role in blocking an effective T cell response to the tumor. However, the success of the licensed Hepatitis B virus vaccine and the Human Papilloma Virus vaccine, which are preventative and reduce tumor development while relying on antibody-mediated immunity have lent support to the idea that immunological protection or therapy to tumors can occur with a humoral response due to B cell activity.
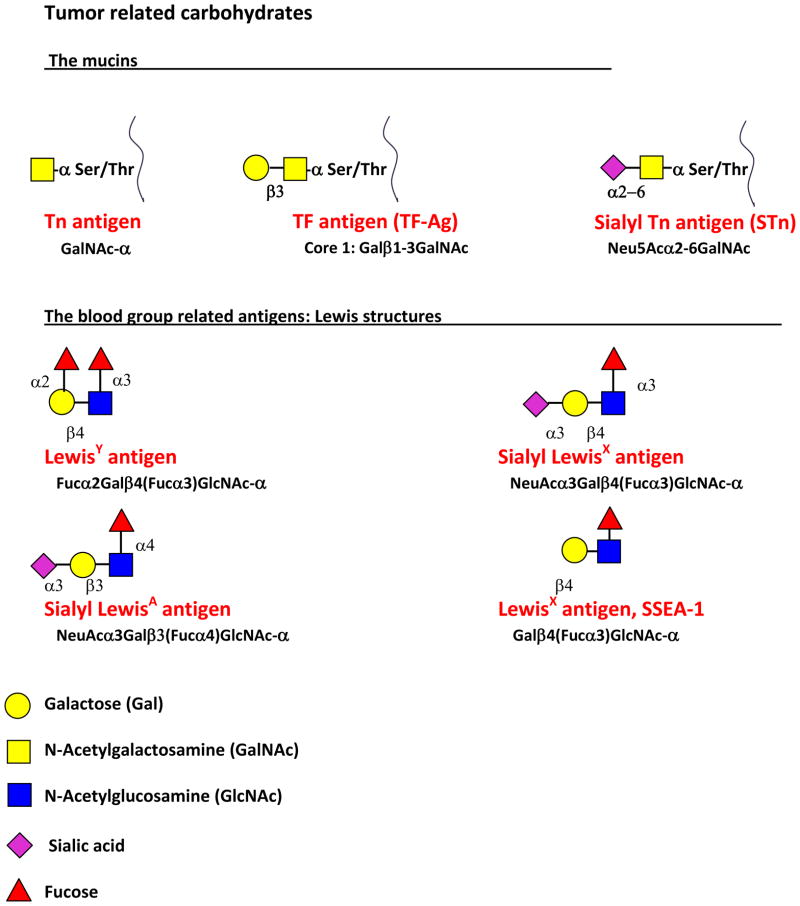
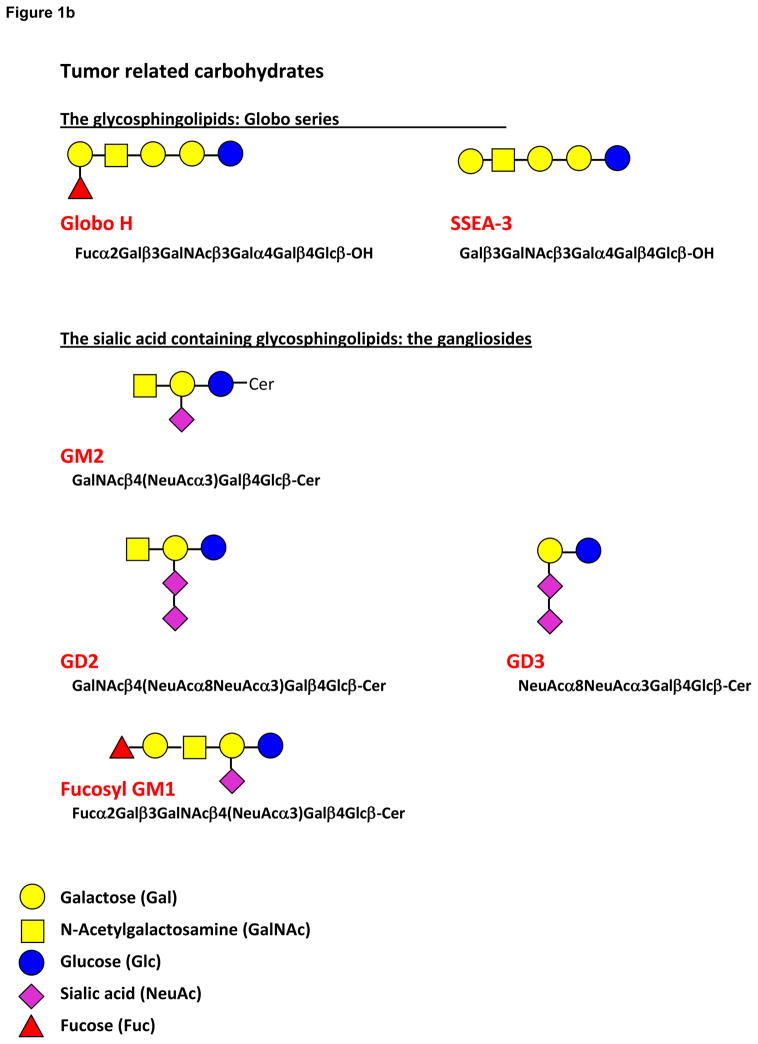
The efficacy of antibody in the treatment of disseminated cancer cells is shown in the success of Rituxan alone and with Herceptin as adjuvant therapy to help remove isolated disseminated cancer cells. These passive immunotherapy successes indicate that vaccine-induced antibodies would also be clinically relevant. Provenge, a third cancer vaccine which is used as immunotherapy rather than as immunoprevention, uses prostatic acid phosphatase linked to granulocyte-macrophage colony stimulating factor in an in vitro incubation with the patients antigen presenting cells to prime them to activate T cells. Whether a cytotoxic T cell or an antibody response against a tumor will be effective will depend on the target antigen and the extent of disease. Cytotoxic T cells would likely be required with extensive disease but antibody can play a role in blocking the spread and development of disseminated cells. Either the cytotoxic T cell response or the antibody response will require the addition of T cell help. The development of an effective B cell response facilitated in its formation by helper T cells is the most likely prospect for a vaccine outcome for carbohydrate tumor-associated antigens. Aberrant glycosylation is a hallmark of cancer cell transformation. A number of TACA have been described including the mucin related (O-linked) Tn, Sialyl Tn, and Thomsen-Friedenreich antigens, the blood group Lewis related LewisY, Sialyl LewisX, Sialyl LewisA, and LewisX (stage-specific embryonic antigen-1, SSEA-1), the glycosphingolipids Globo H, stage-specific embryonic antigen-3 (SSEA-3), and the sialic acid containing glycosphingolipids, the gangliosides GM2, GD2, GD3 and fucosyl GM1 [6,7,8,9,10] (Figure 1a and b). Many of these TACA also are expressed in fetal tissue, and are called oncofetal antigens. The oncofetal expression of these carbohydrate antigens may be related to the de-differentiation seen with malignant transformation.
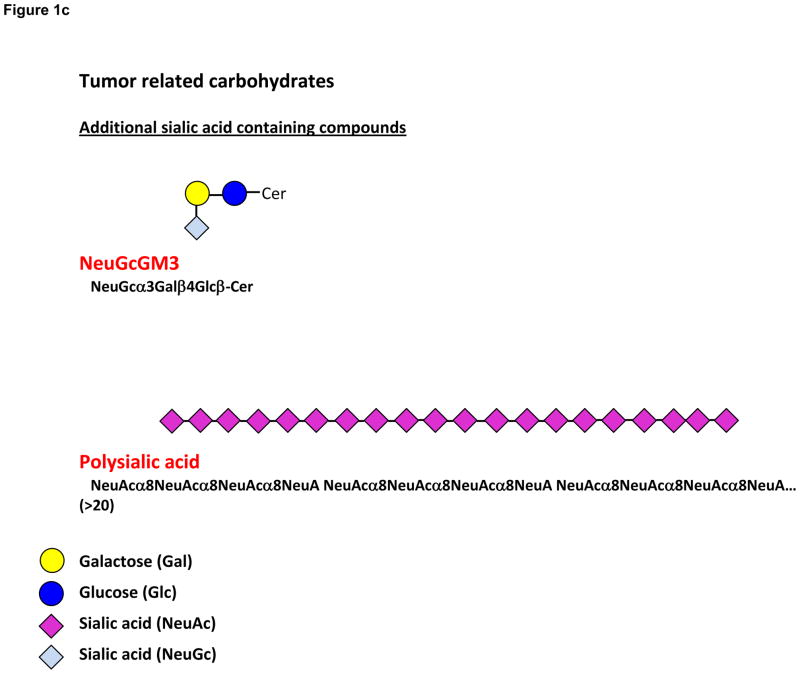
Figure 1.
Figure 1a. Structures of the mucin carbohydrate antigens Tn, Sialyl Tn, and TF, and the Lewis blood group related antigens LewisY, Sialyl LewisX and Sialyl LewisA, and LewisX.
Figure 1b. Structures of the glycosphingolipids, the globo series Globo H and SSEA-3 and the gangliosides GM2, GD2, GD3 and Fucosyl GM1.
Figure 1c. Structures of the additional sialic acid containing compounds. NeuGcGM3 and polysialic acid
1.2 The T-independent response
Carbohydrate antigens are responded to in a T cell-independent Type II manner. This response, while rapid and long-lasting often does not generate an IgM to IgG switch and the enhanced recall “memory” response is not seen. Children under 2 and the elderly have weak responses to these antigens.
T-independent Type II responses rely on antigen presentation to the B cell by dendritic cells. For this response the B cell requires a co-stimulatory signal by Transmembrane Activator and CAML Interactor (TACI), which is a Tumor Necrosis Factor (TNF) receptor homolog that binds to B Lymphocyte Stimulator (BLyS) and a proliferation inducing ligand (APRIL) [11,12]. A recent report using BLyS covalently attached to a protein antigen showed a strong adjuvant effect with production of IgG1, IgG2a, IgG2b, IgG3, and no IgA [13]. Splenic B cells, which respond with T-independent antigens are in the marginal zone and are presented antigen by dendritic cells with TACI and BLyS co-stimulation. Marginal zone B cells and B1 cells (CD5+) are required for a T-independent type II response. CD19, CD21, and CD81 act as co-receptors on the B cell. CD21 is also known as CR2 and is the C3d receptor; interaction of this receptor with C3d activates B-cells [14–17]. CR2 is expressed on B cells, follicular dendritic cells, epithelial cells, and a sub-group of T cells [16]. Interaction of CD21 with C3d activates B-cells creates immunologic memory, and causes immunoglobulin class switching but conversely is involved in B cell tolerance [17]. Enhanced immunogenicity with isotype switching is seen with chemical conjugation of C3d to the polysaccharide antigen, presumably through dual interaction of this conjugate with the CD21 molecule and the B cell receptor [18]. Interestingly, the innate immune system involvement in the stimulation of a T-independent response was exploited in experiments which showed that the Toll-like receptor agonist, CpG increased survival of antigen stimulated B cells involved in a T-independent response and increased the number of plasmablasts produced [19].
Memory B cells are generated in a T-independent response, but these are phenotypically different from the memory B cells generated in a T-dependent response [20]. IgM+ CD27+ B cells are thought to be T-independent antigen memory cells, although some controversy exists in that an alternate pathway for development of these cells would be that they are T-dependent memory cells that became memory cells prior to class switching [20]. Recent evidence concerning the high amounts of tyrosines and lysines in the CDR regions of these cells support the T-independent nature of these cells since these residues are often utilized in an anti-polysaccharide response [20]. In addition to memory cells, the long lasting production of IgM to T-independent antigens may be due to long-lived plasma cells [21]. Although IgG reaction is preferred due to the obvious association of memory development with this isotype switch, IgM reaction is important because it can result in complement-dependent cytotoxicity. In addition, since there is the possibility that long-lived IgM producing plasma cells and/or IgM+ memory cells may have developed, a T-independent response and IgM should not be completely discounted as an unsuccessful response.
Chemical conjugation of the saccharides to protein carriers allows for the switch to a T-dependent response [22]. Although this strategy came into its own in 1990 with the Haemophilus influenzae conjugate [23–25], the original paper concerning the immune enhancing influence of chemical conjugation of the saccharide to a carrier protein was published in 1929 [26]! This indicates that older literature may contain clues that the carbohydrate immunologist should not overlook. The conjugate strategy has been successful for many infectious disease vaccine targets including Haemophilus influenzae [23–25] and Streptococcus pneumoniae [26–30], but has not been very successful with TACA [31–33].
The epitope involved in binding of the carbohydrate to the antibody molecule can be 1–6 monosaccharides in size. Important in the reaction of the antibody to the carbohydrate epitope is the presence of each sugar in the saccharide (ie; a trisaccharide instead of a disaccharide), the presence of the appropriate sugar (ie: a Galactose rather than a Glucose), and the lack of substitution on the sugar at a certain hydroxyl. An antibody molecule can bind the carbohydrate structure in a groove-type or a cavity-type configuration [34]. The interaction of the hydroxyls in a groove-type interaction is more on one side of the saccharide chain and would allow flexibility in terms of substitution on the other side. The interaction in a cavity-type configuration could still allow some flexibility in substitutions but this type of binding would be more restrictive.
1.3 Key requirements for vaccine targets
Although recent developments in the cancer preventative vaccines for Hepatitis B (hepatoma) and Human Papilloma Virus (cervical cancer) and a cancer immunotherapy vaccine, Provenge (prostate cancer) along with successful monoclonal antibody therapies (Rituxan, Herceptin) inspire us, generation of vaccine-mediated anti-cancer immune responses remains a difficult challenge. For TACA targets, this challenge will only be overcome through the utilization of newly acquired knowledge in the understanding of T-independent type II responses. Attempts to add a T cell or an innate immune response to carbohydrate antigens has also been studied. Information from these studies must be combined with the knowledge of the role that the carbohydrate antigen plays in the tumor growth and spread. Tumors are slippery targets, with the ability to mutate to lose expression of the targeted antigen unless that antigen plays a key role in the tumor growth and spread, so it is essential to pick this type of target.
In addition, while a short term response may have some clinical benefit, it befits the tumor immunologist to attempt to create long term memory to the target antigen, because the patient can be challenged with a recurrence many years from the “successful” development of no evidence of disease. This challenge must drive the investigator to new and creative combinations, or perhaps drive us back to understand long ago reported successes, which were developed empirically but not understood.
Not only must we be diligent in the development of these new strategies but we must be realistic and fair in our reporting of the results. We must evaluate what the ELISA titer means in terms of possible protection. The common method of reporting titers that are 3-fold above background must be evaluated to determine if this is really significant if the background is barely detectable. If an animal metastatic model is available, it should be utilized to determine if a protective effect occurs. In vivo killing by Antibody Directed Cellular Cytotoxicity (ADCC) and Complement Dependent Cytotoxicity (CDC) should be performed with antibody at levels achieved through vaccination. Human IgG1 and IgG3 mediate ADCC with Natural Killer (NK) cells, macrophages, and neutrophils able to act as effector cells in this protective pathway. Addition of Toll-Like receptor agonists to the vaccine may improve the ability of these effector cells to function [35]. If vaccine studies are performed in the mouse model, it is important to note that effector function by mouse NK cells is mediated by mouse IgG2a≥ IgG2b>IgG1≫IgG3 [36]. In order to determine if a target would be amenable to killing by human NK cells when using antibody developed in mice, mouse IgG3 is the most effective with human NK cells [37]. CDC is mediated best by human IgG3, then in order of efficacy human IgG1, IgG2, and then IgG4, the latter of which does not mediate CDC. Mouse IgG3 is ≫ IgG2b>IgG2a>IgG1 when utilized in CDC with human complement [38]. Non-radioactive methods for measurement of ADCC and CDC include assay kits for lactate dehydrogenase release.
In terms of safety, the TACA or a cross-reactive antigen may be present on another tissue and the best animal and human safety studies prior to human use of the vaccine would be passive immunotherapy with a monoclonal antibody against the same target. If dangerous cross-reactions are seen in passive immunotherapy, the antibody can be discontinued, a way out that may be impossible with active immunotherapy. If cross-reactions occur in some individuals, and if the reason for occurrence in this subpopulation is understood, the subpopulation can be identified and not vaccinated, and the vaccine can still be utilized. Hopefully, this is the rationale for the many studies that are attempting to create an active immune response to Her-2 Neu even though the passive immunotherapy causes cardiac changes in about 9.8% of the patients treated with passive immunotherapy [39].
Although the challenge of creating a safe and protective anti-carbohydrate cancer vaccine sometimes can seem too great, the successes seen with other cancer antigen targets and the fact that anti-carbohydrate tumor-associated antigen antibodies are found naturally in the blood serum of normal individuals is driving the carbohydrate tumor immunologist forward. The presence of naturally occurring antibody indicates that tolerance will not need to be broken for these antigens. In addition, the amount of this antibody to some of the carbohydrate antigens has been found to be related to prognosis. After surgery or chemotherapy, the levels of anti-tumor-associated carbohydrate antigen (anti-TACA) antibody have been found (in some cases) to increase in patients with a good prognosis and not to increase in patients with a poor prognosis. This indicates that an effective anti-TACA vaccine would improve prognosis.
The individual carbohydrate antigens will be described below in terms of tumor association, role in the disease process, and vaccine attempts.
2. The mucin related tumor-associated antigens
Mucins are heavily glycosylated proteins that contain N-acetylgalactosamine that is O-linked to serine or threonine residues. In cancer, the truncated glycans Tn, Sialyl Tn, and TF-Ag are seen on mucins. These antigens are attached by an O-linkage to serine and/or threonine, and the presentation can be dense due to heavy glycosylation. Many of the glycosyltransferases contain peptide specific substrate preferences, so the immune response targeted with a vaccine could contain the saccharide with the associated peptide in single residues, or in clusters of the antigen linked to serine or threonine [6].
2.1 Tn and Sialyl Tn (STn) Antigens
The Tn antigen is classically defined as the monosaccharide N-acetylgalactosamine linked to serine or threonine, GalNAcα1-O-Ser/Thr. It is the simplest O-glycan, and can be modified to generate the sialyl Tn antigen, Neu5Acα2–6GalNAcα1-O-Ser/Thr (STn). These two defined antigens will be the topic of the following section.
The Tn antigen was discovered in 1957 and named ‘T antigen nouvelle’ or Tn by Moreau et al to designate its difference from T antigen (TF-Ag) which had been discovered years earlier [40,41]. The Tn antigen, also termed CD175, is added to a polypeptide chain by one of the polypeptide-α-N-acetylgalactosaminyltransferase (ppGalNAcT) enzymes. These enzymes transfer GalNAc from the sugar nucleotide donor UDP-GalNAc to the recipient serine or threonine residue [42,43]. Tn then serves as the basic precursor structure for many complex, extended O-glycans found in most tissues and as the base structure on heavily glycosylated mucins [42]. Three enzymes are responsible for most modifications to Tn. Most commonly, the T-synthase enzyme (core 1 β1–3 galactosyltransferase) adds galactose using the donor UDP-Gal to generate the T antigen, also known as core 1 (Galβ1–3GalNAcα1-O-Ser/Thr) or TF-Ag [42]. Core 1 also serves as a precursor for more complex O-glycans and will be discussed elsewhere in this review. The T-synthase enzyme requires a specific chaperone protein for activity, termed Cosmc, and T-synthase and Cosmc are found in all normal human cells, generating core 1 O-glycans in all cells available for further modifications [43, 44]. The enzyme core 3 β1–3 N-acetylglucosaminyltransferase adds GlcNAc to Tn antigen forming core 3 (GlcNAcβ1–3GalNAcα1-O-Ser/Thr), with expression restricted mostly to gastrointestinal tissues [45]. In addition, ST6GalNAc-I can add sialic acid from CMP-sialic acid to Tn to form sialyl Tn (STn), which is a terminal structure that cannot be elongated, and is not commonly found in normal tissues [46]. For a thorough review of the Tn antigen, see “The Tn Antigen- Structural Simplicity and Biological Complexity” [41].
The Tn antigen, by way of the enzymes listed above and potentially others, becomes modified in normal tissues. Therefore, it is not usually detected as the simple monosaccharide alone. However, the presence of the Tn antigen in almost any tissue signifies the presence of disease or pathology. This holds true for the STn antigen as well. Genetic alterations in the T-synthase enzyme or the Cosmc chaperone are leading causes of expression of the Tn antigen, and this has been linked to several diseases, including cancers [43, 44, 47–49]. While the T-synthase is the major enzyme that acts on Tn, the core 3 and ST6GalNAc-I enzymes mentioned above also act on Tn. Altered levels of these two enzymes, or the sugar nucleotides utilized by these enzymes, cause Tn antigen expression [6]. Decreased core 3 enzyme levels have been correlated with colon cancer [50–52], while overexpression of ST6GalNAc-I competes with T-synthase to cause expression of STn, which correlates with colon and breast tumors [53,54]. Additionally, enzymes may be able to act on core 1 and more complex O-glycans to degrade them to the Tn antigen. In many cancers, it is not clear which mechanism or mechanisms are at work to cause the altered expression of the Tn antigen.
Even if the exact mechanisms of Tn and STn exposure are unknown, their presence can be detected by different reagents, including antibodies and lectins, which bind to terminal GalNAc or STn [55–59]. These reagents can be used to demonstrate the presence of the antigens, which are clear signs of pathology. (On a cautionary note, many detecting reagents are not specific for Tn- they can cross-react with blood group A, and some do not discriminate well between Tn, STn, TF, and STF antigens, therefore data using these reagents should be interpreted cautiously). The Tn and STn antigen also serve as potential targets for vaccines against pathologies that result in Tn antigen expression, including cancers. It has been estimated that Tn and STn antigens occur on up to 90% of epithelial cancers, including breast, colon, lung, bladder, cervical, ovarian, stomach, and prostate [60–63]. Expression often correlates with poor prognosis of disease and an increased potential for metastasis [64,65]. The altered glycocalyx of the cell contributes to the new properties of the cell in terms of adhesiveness, survival, and others. For these reasons, as well as the fact that they are not found on most normal tissues, the Tn and STn antigens provide extremely attractive targets against cancers, which was one of Springer’s pioneering ideas in the field of carbohydrate immunology [66]. Additionally, circulating antibodies to Tn are found in most normal human sera [125].
While antibodies and lectins were being discovered and developed that could detect Tn and STn in the 1970’s, 1980’s, and beyond, they were originally regarded and used as diagnostic, prognostic, and detecting reagents, rather than having therapeutic use [55–59,65]. In 1991, Hakomori’s group used a construct of asialo-ovine submaxillary mucin (A-OSM), which contains a high concentration of Tn antigen, in mouse immunizations to actively elicit an antibody response against the Tn antigen. These immunizations generated protection against a highly invasive Tn antigen-positive tumor, TA3-HA, when used to challenge the mice [67]. This began an era of work trying to target Tn and STn by various immunological methods as therapeutics against Tn and STn-expressing cancers.
In 1992, O’Boyle et al [68] immunized colorectal patients with a partially desialylated ovine submaxillary mucin preparation, which contained Tn and STn, with 2 different adjuvants. This study was useful as a proof of concept that this type of conjugate was immunogenic to patients in that specific antibodies reactive to Tn and STn were detected and this helped to lay the foundation for further studies. One follow-up study by the same group using STn conjugated to keyhole limpet hemocyanin (KLH) plus adjuvant revealed excellent responses in patient sera against the synthetic antigen, but weak responses against sources of the natural antigen, indicating that the synthetic conjugates are not equivalent to the naturally-presented epitopes [69]. This group continued immunizations in mice to generate specific monoclonal antibodies against Tn, STn, and other glycan antigens as useful detecting and potentially therapeutic reagents [70,71].
In 1993, Longenecker’s group used STn linked to KLH with Detox adjuvant to immunize a group of metastatic breast cancer patients. All patients developed IgG and IgM antibodies against STn as measured in ELISA. While this was a promising result, the patient outcomes were difficult to assess based on the low number of subjects and the variation in disease in each [72].
Springer’s group, instrumental in defining the Tn and STn antigens and their presence in cancers, performed small studies in 1994, 1995, and subsequent years using a Tn and TF-antigen vaccine that showed a decreased recurrence of breast carcinoma and supported continued work in vaccinations against Tn and STn [66,73,74].
Studies in 2000 and 2001 generated glycopeptides carrying single or multiple Tn epitopes to imitate the clustered effect of Tn on mucins and were used alongside adjuvants to immunize mice. As demonstrated by ELISA and flow cytometry, the glycopeptides were antigenic, inducing anti-Tn antibodies without the use of a carrier protein, and these antibodies could react with Tn on cancer cell surfaces, which was a promising result and suggested that the clustering of Tn could play an important role [75,76]. A follow up study by the same group extended the results to both inbred and outbred mouse strains that could generate strong responses specific for Tn and reacted to additional cancer cells [77]. A similar study used MUC6-Tn glycoconjugate immunizations in mice. Specificity towards Tn antigen was demonstrated by inhibition studies, and anti-Tn antibodies did react with Tn positive tumor cells [78].
The Livingston group also embraced the concept of clustered Tn epitopes for vaccination and used prostate cancer patients as the subjects. When clustered Tn was given with a carrier protein (KLH) and adjuvant (QS21), IgG and IgM antibodies to Tn were seen along with a decrease in PSA [79]. In a merging of the clustered epitope theory, both research groups above collaborated to generate a dendrimeric, multiepitope Tn antigen construct for immunizations of mice and primates. Mice generated anti-Tn IgG antibodies with varying degrees of reactivity to the clustered Tn, and results were better than when Tn was linked to KLH as an immunogen. Monkeys also generated specific antibodies that could bind and generate cytotoxicity towards Tn-expressing cancer cell lines via an ADCC mechanism, a promising outcome [80].
A more chemical approach to generating Tn-containing epitopes was undertaken in 2001 and 2002 to link the Tn antigen, a classically regarded ‘B cell epitope’ since it is a carbohydrate, to an ovalbumin peptide, a classically regarded ‘T cell epitope’ known to be presented by T cell receptors. While this approach was only tested in vitro, it demonstrated the ability of such a construct to be presented efficiently by dendritic cells to T cells with the glycan present on the peptide [81,82].
Another glycopeptide approach used the Tn antigen linked to a 20-mer peptide to provide a T cell epitope and a lipopeptide, Pam3Cys, to facilitate the incorporation into liposomes. Liposomes were used to immunize Balb/c mice and IgM and IgG antibodies against Tn were generated. Liposomes in combination with QS21 adjuvant as a vaccine generated higher levels of antibody than liposomes alone, showing that the combination of glycan, peptide, lipid, and adjuvant can act as an effective vaccine [83]. The Boons group has used other unique Tn glycopeptide component conjugates to generate high titer IgG antibodies against Tn positive tumor cells with some level of success [84].
A company named Biomira conducted studies on an STn-KLH immunization called Theratope. While some of the measures of success appeared positive including an antibody response to STn, and the phase II trials indicated clinical improvement, the Phase III outcome did not show a clinical benefit to the patients, likely for a variety of reasons. They suggested that a combination of therapies may be a better approach [32,33,85]. However, although Phase III trials with this antigen did not show improved survival, inexplicably, the patients were not pre-screened to determine if their tumors were STn positive or not. Subsequently, Burchell’s group in 2009 showed that mice immunized with synthetic STn conjugated to KLH (Theratope) developed a protective immune response when they were challenged with an STn containing tumor [97]. This target warrants further investigation.
Another glycopeptide study used MUC1 peptides with and without Tn glycosylation. The Tn glycosylated MUC1-derived peptides were presented by MHC class I molecules resulting in cytotoxic immune responses. However, the glycopeptides did not provide a significant benefit against mice challenged with tumor cells possibly due to cross-reactivity to the glycosylated and non-glycosylated forms of the same peptide or a low abundance of the glycopeptides on the target tumor cells [86]. A similar line of attack was taken by Finn’s group in 2009 using glycosylated and non-glycosylated MUC1 sequences. They found that immunization with Tn-MUC1 epitopes induced glycopeptide-specific T cell and antibody responses, which involved presentation by dendritic cells. This is again a promising outcome, but the clinical benefit to patients has not been assessed [87].
A study that challenged the dogma that carbohydrates cannot act as efficient immunogens alone was conducted in 2009 using an entirely carbohydrate vaccine makeup. This idea was based on the reports that zwitterionic polysaccharides can invoke an MHC class II-mediated T cell response in the absence of a protein carrier [88–90]. High titer antibodies were generated against Tn demonstrating another potential mechanism for targeting Tn antigen on tumor cells [91].
Another strategy for targeting Tn and STn antigens on tumor cell surfaces is passive transfer of antibodies specific for the antigens, as opposed to active immunization to generate antibodies. An antibody that recognizes repeating Tn units was humanized and showed significant ADCC against Tn-expressing Jurkat cells in vitro as well as in vivo when the cells were injected into a mouse model [92]. Also capitalizing on the passive transfer approach was a 2011 study using another anti-Tn antibody, GOD3-2C4, which showed specificity for tumor cells (Jurkat, A549) and some types of tumor sections in vitro. The antibody localized to A549 cells that were seeded into mice to establish tumors, and a decreased tumor volume and size were measured in the antibody treated mice [93]. These studies lend credence to the practice of passive transfer of defined antibodies against TACAs.
A unique approach for localizing tumors and potentially delivering toxic compounds was recently tested using Morniga G lectin, which binds TF and Tn antigens. The lectin was conjugated to a photosensitizing agent, which when activated by light can cause cell death. The lectin bound to Tn positive cells and was taken up by the cells, causing a high percentage of cellular cytotoxicity. This technique may be an alternative strategy to use TACA-specific lectins to target tumor cells for death [94].
While the Tn and STn antigens have been proven as tumor-associated carbohydrate antigens that are rarely found on normal cells, the variety of vaccination strategies to target these antigens have had variable outcomes and little clinical benefit to the cancer patients tested. Still, these studies lay the groundwork for future work in developing successful, effective vaccines against Tn and STn-bearing tumors.
2.2 Thomsen-Friedenreich antigen (TF-Ag)
Thomsen-Friedenreich (TF-Ag) is a TACA that was initially reported as a red blood cell antigen which was exposed by the action of bacterial neuraminidases [95,96]. The ubiquitous nature of the corresponding anti-TF-Ag antibody was found simultaneously since red blood cells with this antigen exposed would agglutinate with the addition of any human serum. This antigen is the disaccharide galactose β1–3N-acetylgalactosamine-α–O-Ser/Thr. It is not exposed in normal tissues as it is either mono- or disialylated or further glycosylated by an N-acetylglucosamine addition to the galactose or by fucosylation. In addition TF-Ag can be modulated by sulfation [97,98]. The closely related Galβ1–3GalNAcβ is present in glycolipids of the spleen white and red pulp [99], kidney tubules [100,101], regenerating respiratory epithelial cells [100], and NK cells [102,103]. Therefore, the vaccine response must be specific in order for this antigen to be targeted. Such specificity is not impossible and for example, is seen with the monoclonal antibody JAA-F11, which has been used in immunotherapy and tumor imaging experiments in a mouse model [102,104,105].
TF-Ag is a very important tumor target because of its pancarcinoma expression, with expression on carcinomas of the breast, colon, bladder, prostate, liver, and stomach [95,96,107–155]. The potential number of people that could possibly be helped with a vaccine to a target on all of these cancers is staggering. TF-Ag is also a promising target because adhesion mediated by TF-Ag plays a role in metastasis [135,141,142,153,156,157]. This role in metastasis is important because a humoral immune response generated by a vaccine to TF-Ag could kill the tumor cells by ADCC and CDC and in addition could block the ability of the tumor cells to spread. This functional role also indicates that if a vaccine to TF-Ag causes a selective pressure in which there was survival of TF-Ag negative tumor cells, these tumor cells would have decreased ability to metastasize.
Increased TF-Ag expression in tumor cells compared to normal cells could be due to a number of factors. There could be increased synthesis from the Tn precursor. Increased synthesis could be due to increased T-synthase activity, or TF-Ag levels could be elevated be due to increased production of the UDP-Gal and the Tn Ag required for its synthesis [106,158,98]. Of these possibilities, there does not appear to be increases in the amount of T-synthase in tumor cells, but there does appear to be increased levels of Tn. Increased levels of TF-Ag could also be due to decreased subsequent glycosylation steps so that the TF-Ag remains unmodified. Decreased golgi pH seen in some tumors is thought to be linked to increased TF-Ag expression [97,98,160,161].
TF-Ag was discovered due to the ubiquitous presence of antibody to this antigen in most human serum [95,96,162]. TF-Ag was exposed in bacterially contaminated red blood cells, and when the scientist attempted to determine the ABO blood group of the individual, all the sera caused hemagglutination. This is pivotal in many ways for a vaccine candidate target. Human antibody is already present in normal people- and this indicates two things: one, that antibody to TF-Ag would not cause pathology and two, that humans are not tolerant to this antigen. The levels of TF-Ag are decreased in people with carcinoma, but they increase after curative surgery [144]. Patients with higher levels of anti-TF-Ag have a significantly better prognosis than patients with low levels [163–166]. These human studies indicate that TF-Ag would be an obtainable target, and that the antibodies would be of clinical benefit.
TF-Ag has been shown by a variety of methods to be related to metastasis. The amount of TF-Ag on the tumor cell is related to prognosis, with higher TF-Ag expression related to poorer prognosis in breast, colon, lung, ovarian, gastric, and urinary carcinomas [106,149, 166–177]. TF-Ag positive tumors were shown to be more likely to metastasize to the liver [149]. In vitro, several studies have shown a relationship of TF-Ag to the adhesion required for metastasis. TF-Ag has been shown to be involved in the binding of prostate cancer cells to endothelial cells through interaction with galectins-3 [166]. Neuraminidase-treated tumor cells with increased TF-Ag expression had 3-fold increased liver metastasis rate. JAA-F11, our monoclonal antibody to TF-Ag binds Galβ1–3GalNAcα-Ser/Thr without binding to the glycolipid Galβ1–3GalNAcβ-. JAA-F11 has been used in a number of studies [102,104,105,157,166] and has been shown to block the adhesion of human and mouse tumor cells in metastatic models in in vitro, ex vivo, and in vivo studies. The tumor cell types include human breast, colon, and prostate and mouse breast. In passive immunotherapy experiments in the 4T1 mouse breast cancer metastatic model, passive transfer with JAA-F11 blocked metastasis to the lung and improved survival [105]. This passive transfer experiment coupled with experiments which showed in vivo localization of mouse 4T1 breast cancer cells, [102] and in unpublished studies with a human triple negative breast tumor immunolocalization in SCID mice with 124I labeled antibody, indicate that JAA-F11 may be useful clinically for immunolocalization of metastasis, and may be useful once humanized for adjuvant therapy. In addition, these experiments indicate again that TF-Ag is an important vaccine target.
The first attempts at a carcinoma vaccine using TF-Ag as the target were by Georg Springer’s group in published studies in 1995 [136,139]. In one, 32 patients were vaccinated with a vaccine composed of TF-Ag derived from neuraminidase treated red blood cells (probably asialoglycophorin A), with the adjuvant Ca3(PO4)2 and the Salmonella typhi vaccine (which expresses TF-Ag). The patients were vaccinated repeated over the course of years, and all of them survived greater than 5 years [139]. In the other paper [136], 19 breast cancer patients composed of six in stage IV, six in Stage III, seven in stage II were vaccinated intradermally. Three of the stage IV, three of the stage III, and five of the stage II patients (11 total) survived greater than 10 years. All patients survived greater than 5 years post-operatively. This small number of patients does not provide conclusive evidence, but it indicates that an anti-TF-Ag vaccine and perhaps even Springer’s anti-TF-Ag vaccine would be worth further investigation.
A TF-Ag containing protein conjugate was used as a vaccine in mice with mammary adenocarcinoma [178,179] using KLH as the conjugated protein, with cyclophosphamide used to decrease the T regulatory effect [178,179,159], and with Ribi adjuvant containing trehalose dimycolate and monophosphoryl lipid A added to increase the immune response. The vaccine was protective as both a preventative therapy prior to tumor implantation and as an immunotherapeutic after tumor challenge. Maclean [179] in related studies vaccinated 10 ovarian cancer patients with TF-Ag conjugated to KLH injected with an adjuvant. These patients developed DTH responses at their vaccination sites. Nine of the patients made IgM and IgG and 8 of these 9 also made IgA against TF-Ag.
Colorectal cancer patients vaccinated with TF-Ag linked to KLH via a small crotyl linkage showed reactivity against the synthetic TF-Ag not the naturally occurring structure [180]. This may have had to do with the short linker. Slovin also immunized with TF-Ag- KLH conjugates, but used a clustered format with the adjuvant QS21. Twenty prostate cancer patients who were biochemically relapsed were immunized and an IgM and IgG response were made by these patients [181].
A multimeric vaccine was utilized which contained TF-Ag as well as Tn and GM2, Globo H, LewisY, and glycosylated MUC-1–32mer in a clustered formation, conjugated to KLH and mixed with QS21. This was administered in a Phase II setting to 30 high-risk prostate cancer patients [181]. The patients all responded to at least two of the antigens. Unfortunately, unlike the sturdy responses seen when combining vaccines to infectious diseases, the multimeric presentation was observed to lower the response to each separate antigen.
Mice immunized with TF-Ag linked to a TcR contact residue having high affinity for Class I molecules developed a cytotoxic T cell response to TF-Ag bearing tumor cells [182]. This interesting study, which showed generation of a cytotoxic T cell response to TF-Ag is continuing, and Alessandra Franco’s lab is trying to improve the peptide backbone for TF-Ag immunization.
In our laboratory, early studies had shown us that in the idiotype- anti-idiotype system a carbohydrate antigen could be mimicked by the amino acids of the anti-idiotypic antibody [104]. In this early study, the antibody that resulted from vaccination with the anti-idiotypic antibody had the same fine specificity as the original antibody raised to the carbohydrate vaccination. This lack broadening in specificity was the impetus for beginning peptide mimic research for the development of a vaccine for TF-Ag. To select the best mimic, we chose to use biopanning of a phage display library with the highly specific JAA-F11 antibody (twice), followed by biopanning with a rabbit anti-TF-Ag. Elution of the phage captured by the biopanning was performed using the TF-Ag disaccharide. These peptide mimics bound to peanut agglutinin (PNA), indicating that they were able to mimic TF-Ag. Vaccination of mice with multi-antigenic peptide constructs of these peptide mimics with Alum adjuvant and killed Bordetella pertussis resulted in antibody to TF-Ag showing successful mimicry [183].
Our work continues with structural studies with x-ray crystallography of JAA-F11 and computational docking to compare the fit of TF-Ag in the antibody with the fit of the peptide mimic in the antibody, so that the mimics can be improved. This involves collaborative studies with A. Gulick, R. Woods, S. Jadey, M. Tessier, and J. Heimburg-Molinaro. We are also resurrecting Springer’s vaccine to determine if we develop protective anti-TF-Ag in our mouse model. Attempts to further stimulate the immune response with the anti-TF-Ag vaccines will be performed with a variety of additional constructs, targeting binding to CD5+ memory cells, stimulation of the innate immune response, and/or stimulation of a T cell response. Some of these studies involve the use of nanoparticle delivery systems in collaboration with J. Barchi.
3. The blood group Lewis related tumor-associated antigens
Lewis blood group antigens contain a fucose, which is either α1–3 linked or α1–4 linked to an N-acetylglucosamine. These structures are found both on glycolipids and on glycoproteins. In individuals that are secretors, these antigens are found in body fluids. The Lewis antigens on red cells occur there due to absorption of the Lewis glycolipids from the serum [6].
3.1 LewisY
The Lewis Y (LeY) antigen structure consists of a Type II oligosaccharide Galβ1,4GlcNAc with both α1,2- and α1,3-fucose linkages [184] to form the structure Fucα(1,2)Galβ(1,4)Fucα(1,3)]GlcNAc [185]. It is also known as CD174 [186]. It is related in structure to the ABH family of blood group antigens found on human blood cells [187]. The LeY structure is formed by addition of α1,3-linked fucose to the blood group H antigen structure: Fucα(1,2)Galβ(1,4)GlcNAc [1, 4]. Fucosyltransferase I or FUT1 is responsible for attaching α1,2 fucose to Galβ(1,4)GlcNAc to form the H antigen structure [187]. α1,3-fucosyltransferases are responsible for attaching α1,3 fucose to the GlcNAc [5] of Fucα(1,2)Galβ(1,4)GlcNAc to form the LeY antigen structure. The α1,3-fucosyltransferases FUT3, 4, 5, 6, and 9 are all capable of synthesizing LeY from the type II Fucα(1,2)Galβ(1,4)GlcNAc structure [188–189].
Cancers that are known to express LeY include ovarian [185,190], breast [191], prostate [192], colon [193], and lung [194] cancers of epithelial cell origin [194]. Because the expression of LeY in normal tissues is low and it is highly expressed in many cancers it is a good potential therapeutic target [194–195]. Motzer et al. found that embryonal carcinomas, yolk sac tumors, and seminomas expressed LeY [196].
Antibodies to LeY have been generated by immunization of mice with a metastatic human breast carcinoma cell line H3396 [197]. It was confirmed by ELISA that these antibodies, known as BR64 (IgG1) and BR96 (IgG3), recognize the LeY determinant. Many other monoclonal antibodies to the antigen have been made and used commercially because it is a well-characterized blood group antigen.
Zhang et al. looked at the normal tissue expression of LeY using BR96 antibody to better understand its regulation compared with cancers [198]. The following normal tissues expressed LeY: spleen and epithelial cells from the lung, breast, prostate, colon, stomach, pancreas, uterus, and ovary, the esophagus, testes, tonsils, salivary glands, and the Paneth cells of the small intestinal epithelium [197]. Cancers that Zhang et al. found to express LeY antigen included: small cell lung, breast, prostate, lung, colon, pancreatic, gastric, ovarian, and endometrial cancers [198].
In mouse development, LeY is expressed at the morula and blastocyst stages, but not on 2–4 cell stage embryos [199], and it may be required for adhesive interactions that allow for implantation to occur because monoclonal antibodies to the antigen inhibit blastocyst implantation.
In the Jurkat human T cell line, Azuma et al. found that LeY may be involved in apoptosis along with LeX [189]. Jurkat cells were stimulated using granzyme B to induce apoptosis. This resulted in upregulation of FUT4 and FUT9 mRNAs, which code for α1,3-fucosyltransferases that synthesize both the LeX and LeY antigenic structures. Caspases, annexin V, LeX and LeY increased in the apoptotic cells, and Lewis antigens increased as apoptosis proceeded. The LeX and LeY antigens may potentially play a role in either apoptotic signaling or clearance of apoptotic cells [189].
LeY is expressed on granulocytes, which are peripheral blood cells that express the antigen normally [200], its reactivity and binding to CD66 antigens increases after stimulation, suggesting it may be involved in adhesion. Moehler et al. found that LeY/CD174 plays a role in angiogenesis [186]. In human bone marrow endothelial cells (HBMECs), stimulation with the growth factor TNFα resulted in increased surface expression of LeY and its precursor structure, H antigen, and FUT1 gene expression was also upregulated. Endothelial cells migrated to form capillary-like networks in matrigel, but this could be inhibited by anti-CD174 antibody or by FUT1 siRNA. The capillaries in a colon carcinoma tissue section were also positive for CD174. These findings suggest that LeY is involved in cell migration required for the early steps in tumor angiogenesis.
Yan et al. [185] found that overexpression of LeY antigen via overexpression of α1,2 fucosyltransferase in the ovarian cancer cell line RMG-1 resulted in upregulation of matrix metalloproteases MMP-2 and MMP-9 and increased the invasiveness of the cells in matrigel. In ovarian cancers, increased expression of the antigen correlates with poor prognosis [185]. In the colon, increased expression of the antigen correlated with increases in dysplasia and malignant potential [193].
Larrain et al. looked at the serum of breast cancer patients for circulating immune complexes of antibody to LeY and the LeY antigen expressed on Mucin1 (MUC1) [191]. The IgM LeY circulating immune complex levels correlated with both IgM/IgG MUC1 and IgG LeY circulating immune complex levels. This implies that MUC1 may carry LeY antigenic epitopes. No increase in serum immune complexes of LeY antigen was observed with different stages of breast cancer.
Different groups have tested cancer vaccines against the LeY epitope [190,201–202] with limited success due to the weak immunogenicity of the antigen.
Sabbatini et al. [190] performed a phase I pilot study on eleven cancer patients with a heptavalent vaccine that consisted of GM2, Globo-H, LeY, Tn, STn, TF, and Tn-MUC1 conjugated to keyhole limpet hemocyanin (KLH) and mixed with the adjuvant QS21. The patients had epithelial ovarian, fallopian tube, and peritoneal cancers in remission. LeY was one of the epitopes that generated very little immune response from any of the patients; only a weak response to it was formed in one patient.
Sabbatini et al. attempted a phase I trial involving a monovalent LeY-KLH conjugate vaccine with the adjuvant QS21 in 25 ovarian cancer patients who had undergone remission [202]. Although the vaccine was well tolerated with minimal side effects, the majority of the responses to the vaccine were IgM, and only four patients made a weak IgG response to the vaccine. No autoimmunity was observed although several normal tissues expressed the antigen. One single patient made IgM and IgG antibodies of a high enough titer to react with the LeY expressing tumor cell lines OVCAR3, SK-OV-3, and ovarian tumor specimens, but this patient expressed LeY antibody before immunization. However, no clinical response was indicated.
Buskas et al. [201] used an artificial amino-propyl spacer to fuse LeY to KLH that was modified by 4-(maleimidomethyl)cyclohexane-1-carboxylate (MI). Mice were immunized with KLH-MI-LeY and they formed IgG antibodies to the linker region instead of to LeY. When the amino-propyl spacer was replaced with a smaller 3-(bromoacetamido) propionate spacer, the mice formed a specific IgG antibody response to LeY instead of to the linker molecule.
Another approach by Westwood et al. [194] involved genetic redirection of T cells, which do not normally recognize the LeY antigen. LeY as a single chain humanized antibody was conjugated to the T cell signaling molecules TCRzeta and CD28 so that the T cells would recognize the antigen. The T cells generated became genetically redirected to recognize LeY and they could cause cytokine release against highly LeY+ OVCAR3 cells. This may be a more promising approach because it circumvents the difficulty of the immune system in recognizing the LeY antigen and mounting a measurable antibody response to it. Genetic redirection may be therapeutically useful in directing a focused immune response against highly invasive cancers with poor outcomes, e.g. ovarian, that ectopically express high levels of the antigen.
3.2 Sialyl LewisA (SLeA) and Sialyl LewisX (SLeX)
General Structure
Sialyl LewisA (Neu5Acα2-3Galβ1-3(Fucα1-4)GlcNAcβ-R) and Sialyl LewisX (Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ-R), belonging to the neolactoseries, are structurally similar antigens occurring predominantly on O-linked mucins. They are counted among TACAs that are upregulated in cancer cells and play a role in the invasion and metastasis of these cells. Detection of these antigens in the serum of a patient is indicative of the presence of malignancy and correlates with poor prognosis [203].
Normal Function
SLeA is mostly expressed on normal fibroblasts, on the lumenal side of ductal epithelial cells, and some parenchymatous cells [203,206]. SLeX, on the other hand is a commonly found antigen on the surface of neutrophils and other critical immune cells and facilitates the extravasation to sites of inflammation [207,208]. Allahverdian et. al have demonstrated that SLeX plays a critical role in the repair of epithelial wounds in the airway by modifying and hence regulating the function of EGFRs [209]. SLeA antigen is a ligand for E-selectin present on the endothelium [210], while SLeX found on P-selectin glycoprotein 1 (PSGL-1) is a major ligand for P- and L-selectin [208]. The sialic acid residue of SLeX antigen has been shown to play a major role in its interaction with E-selectin present on endothelial cells while CD15 (LeX) hampers the interaction of the antigen with E-selectin [211]. The absence of the sialic acid at the terminal end of the glycan decreases the affinity with which P-selectin binds to SLeX on PSGL-1, its major biologically relevant receptor [212]. The presence of sulfated SLeX favors binding to L-Selectin [208]. This interaction between the ligand and the endothelial cells is augmented by the activation of endothelial cells by cytokines such as IL-1 [213].
SLeX is synthesized from the core 2 structure and is predominantly present as a terminal epitope on mucin molecules [214]. Core 2 β1–6 N-acetylglucosaminyltransferase, which catalyses the synthesis of core 2 from core 1, along with β1–4 Galactosyltransferase, α2–3 Sialyltransferase, and α1–3 Fucosyltransferase enzymes contribute to the synthesis of SLeX [214,215].
Overexpression in cancer
SLeA and SLeX are aberrantly expressed in several tumor cells [207,210]. Overexpression of SLeX is found in breast, ovarian, melanoma, colon, liver, lung, and prostate cancer, mimicking their presence on the migrating blood cells [207,211,213,214]. SLeA has been found to be upregulated in the malignancies of the breast, colon, and pancreas and recognized as a tumor differentiation antigen in melanomas [207,210]. The overexpression of these ligands for E-selectin seems to favor the hematogenous metastasis of the overexpressing tumor cells leading to poor prognosis [207,213]. Supporting this hypothesis are reports of the presence of higher levels of SLeX in metastatic cells than in primary tumor cells [213]. The increased expression of SLeX and SLeA antigens on tumor cells could be due to the upregulation of the genes encoding the enzymes responsible for the production of the antigen, or due to deficiency in the enzymes responsible for sulfation, which normally lead to the generation of Sialyl 6-Sulfo-LewisX antigen and 2–6 sialylation resulting in the production of disialyl Lewis A, present on the normal epithelium [216]. These alterations may be due to epigenetic effects [214–217]. Other causes such as an increase in the UDP-galactose transporter, GLUT 1 and changes in galactose metabolism have also been implicated in the overexpression of the Sialyl Lewis antigens [217].
Treatment with antibodies against these antigens has proven to be efficient in inhibiting metastasis in pancreatic tumor mouse models [204]. Moreover, NK cells have been shown to have the ability to detect the overexpression of SLeX antigen while tolerating the moderate or normal distribution of the antigen present on normal cells [206].
Strategies for vaccine development and outcomes
Development of vaccines to stimulate the immune system to react to the overexpressed or unique antigen is an ideal way to target a disease including cancer. Though aberrant glycosylation is a typical hallmark of several malignancies, vaccine production based on these carbohydrate antigens have been plagued with problems of low immunogenicity and the presence of these antigens on normal cells. Several strategies have been developed to circumvent these hurdles.
The overexpression of SLeX and SLeA antigens can be exploited to differentiate between normal and tumor cells and hence can be utilized as biomarkers for diagnosis, treatment, and development of therapeutic vaccines against tumors overexpressing these antigens [215]. Antibodies against the Sialyl Lewis antigens have been detected in patients afflicted by melanomas that overexpress these antigens, showing that these antigens are immunogenic. However, this immune response is not sufficient to defeat the tumor, but augmenting this immune response by treatment with therapeutic vaccines may help battle against tumor cells [207].
One of the mechanisms of inducing an immunogenic reaction in melanoma patients is the injection of SLeX overexpressing polyvalent Melanoma Cell Vaccine (MCV), which consists of 3 melanoma cell lines: M10-v, M24, and M101 which were cryopreserved and irradiated [207,218]. These tumor cells express high levels of several TACAs including SLeA, and gangliosides GM2, GD2, and GD3 with M10-v overexpressing SLeX as well [207]. The rationale behind this vaccine is that SLeX is present at a higher density on the melanoma cells as compared to normal melanocytes. This facilitates the binding and aggregation of the tumor cells by the antibodies generated against them, inducing an immune response to eliminate the malignant cells. It is anticipated that the lower density of the antigen on the surface of the normal melanocytes will prevent aggregation, preventing a pathologic reaction with normal cells [207]. There was a good response in the form of anti-SLeX IgM antibodies which played an important role in inducing complement and antibody-dependent cytotoxicity and in the opsonization of the tumor cells, thus augmenting the immune mediated clearance of the tumor [207]. These antibodies also act as ligand traps to bind to free antigens, which may otherwise have immunosuppressive properties [207]. When administered with adjuvants such as BCG derived from Mycobacterium bovis or MPL derived from Salmonella minnesota R595, MCV caused a total loss of SLeX antigen on the surface of the tumor cells and led to a reduction in the growth of the tumor [217]. Of interest was their finding that the induction of high titers of IgM and low IgG was efficient in clearing this tumor with these patients having better prognosis, while patients with high IgG:IgM had worse prognosis [217]. This is in agreement with their previous studies that suggested that unlike the case of proteins, the generation of IgM against carbohydrate antigens leads to persistent and effective immune reaction to the tumor cells and supports the observation that IgGs against these carbohydrate antigens correlate inversely with survival [207]. The response elicited by MCV favored delayed tumor progression and reduction in the tumor growth [217]. This study indicates that cancer vaccinologists that obtain an IgM response should continue their studies to determine if a clinical effect is seen. There is not enough data to know if the high IgM and low IgG would be better with other tumor types, but it is at least important not to discount a study if the IgM to IgG switch is not made.
Another technique employed in the development of vaccines against these carbohydrate antigens is the chemical synthesis of the antigen conjugated with a carrier protein and an adjuvant that will boost the immune response. SLeA is normally present on the inner surface of the ductal epithelium which make it inaccessible to the antibodies and cells of the immune system. These otherwise restricted molecules are expressed on the surface of the cancer cells thus making it possible to target only the malignant cells [203]. Ragupathi et al demonstrated the effectiveness of a synthetic pentaglycoside of the hexasaccharide, SLeA with KLH produced by one-pot synthetic glycosylation as an anti-tumor antigen. When this vaccine was administered with GPI-0100 as an adjuvant, high titers of both IgG and IgM antibodies were obtained, especially IgG that were capable of inducing ADCC without the induction of toxicity or cross reactivity to other similar antigens in the animals. The induction of CDC is evident from the observation of lysis of the human ovarian cancer cell line SW626 in the presence of the sera from immunized animals and human complement. Moreover, this approach of vaccination was found to be devoid of toxicity and off target effects but has yet to be tested clinically [203].
To switch to a T-dependent response, mimicry of these carbohydrate antigenic epitopes with peptides has been performed [220]. Using epitope mapping techniques, the 3–5 amino acid peptide motif to which antibodies to carbohydrate antigens bind were determined and the Lewis antigens sharing similarity in the epitope structure were found to be efficiently mimicked by the peptide motif of W/YXY [220]. In order to mimic the TACAs, tandem repeats of the peptide motif were synthesized on polylysine groups using Fmoc synthesis [204,220]. The tandem repeats served to increase the robustness of the immune response and also resulted in clustering to the epitopes similar to the clustering of the antigens on the surface of the cells [220]. Immunizing mice with the peptide mimic along with the adjuvant QS21 proved to be effective in stimulating an anti-SLeX specific immune response and complement dependent cytotoxicity (CDC) when challenged with Meth-A fibrosarcoma cells that are known to aberrantly express SLeX [220]. Introduction of the SLeX antigen promoted T-lymphocyte activation and the secretion of IFN-γ [204]. However, the protection offered by this mechanism has been so far dose dependent i.e. it is incapable of protecting mice when a large amount of tumor cells are present. Hence its application as a therapeutic vaccine is not efficient especially in the advanced stages of the disease [204,220].
Future directions
Achievements in the development of carbohydrate vaccines against Sialyl Lewis antigens have been slow. One of the major disadvantages is the stimulation of a T-cell independent immune response. Moreover, several of these studies have been performed with pre-immunization with the vaccine followed by challenge with the tumors cells, which does not correlate with the real life scenario. There is also variability in the response of the patients to the vaccine and therefore the response elicited cannot be generalized to all patients. Importantly, these antigens are found on normal cells and have critical regulatory functions, so general responses to these antigens could have detrimental effects to patients.
3.3 SSEA-1 (Lewisx)
Stage-specific embryonic antigen-1 (SSEA-1) is a type two [221] neolacto-series glycan [222]. It is known by many names because other groups were researching its roles outside of embryonic development before it was realized that these structures all had the same carbohydrate epitope in common. Lewis X, LeX, CD15, and the SSEA-1 trisaccharide share the structure Galβ(1–4)Fucα(1–3)GlcNAc- [223–225]. The epitope that SSEA-1 antibody recognizes corresponds to the LeX antigen [222]. SSEA-1 can be found on glycolipids and glycoproteins. In this review, SSEA-1 will refer to the non-sialylated form of the carbohydrate because sialyl-SSEA-1 (Sialyl LeX) has different adhesion properties, is found on different tissues and tumors, and is discussed in a different section of this review.
SSEA-1 was first identified and defined by the reactivity of a monoclonal antibody generated in a mouse immunized with F9 teratocarcinoma cells [226]. The antibody bound to late 8-cell stage embryos, morula stage embryos, and blastocysts with increasing intensity in staining as development progressed; it also stained both mouse and human teratocarcinoma cell lines. No staining was present on unfertilized eggs, zygotes, or before the 8-cell stage, and all differentiated cell lines were also negative for staining [226]. The study showed that the antigen was involved in embryonic development at specific stages. This structure was identified as Galβ(1–4)Fucα(1–3)GlcNAc- [227], which was determined to be the structure of LeX [225]. Childs et al. [228] discovered that glycoproteins carry the SSEA-1 antigen in mouse teratocarcinoma cells.
The role of SSEA-1 in development has yet to be elucidated. Fenderson et al. [229] proposed that SSEA-1 plays a role in compaction of preimplantation mouse embryos through use of a multivalent LeX-lysyl lysine conjugate that caused decompaction in preimplantation embryos. The LeX structure was also found to potentially regulate murine sperm to zona pellucida binding during fertilization [230]. FUT9 knockout mice do not express SSEA-1 but still develop normally, therefore it is not essential in mouse development [231].
D’Costa et al. [232] researched SSEA-1 expression in turkey embryos and found that SSEA-1+ cells become expressed in the blastoderm stage and increase in number during development, then the SSEA-1+ cells circulate through the vasculature. The antigen becomes expressed in multipotent, proliferating neural epithelial cells of the developing central nervous system, and is later expressed in the dorsal neural tube and eventually only in migrating neural crest cells [232].
SSEA-1 plays a role in development of the central nervous system in vertebrates [233–235]. The FORSE-1 monoclonal antibody also recognizes the SSEA-1 structure in rat forebrain [233]. FORSE-1 staining was found in the undifferentiated dividing cells of the cortical ventricular zone, but later in gestation, staining was weaker. Because of this decline in staining after differentiation, Allendoerfer et al. [233] proposed that the antigen may function in adhesion and axon guidance in the developing central nervous system. SSEA-1 was found to be involved in adhesion and neurite outgrowth in the developing central nervous systems of Xenopus [234] and rat [235].
SSEA-1 also functions as an adhesion molecule in polymorphonuclear neutrophils (PMNs) of the immune system [223]. Spooncer et al. [236] found SSEA-1 to be an epitope of a large, polyfucosylated glycan expressed on human PMNs, demonstrated by My1 monoclonal antibody recognition [236]. Using monoclonal antibodies to the epitope Galβ(1–4)Fucα(1–3)GlcNAcβ1–3Galβ1–4Glc on neutrophils, Skubitz et al. [237] found that SSEA-1 functions in adhesion to endothelium, phagocytosis, stimulating degranulation, and respiratory bursts as shown by antibody inhibition of these functions. The CD15 antigen was first identified as SSEA-1 before it was identified in neutrophils [236, 238]. Stocks et al. [239] found that the antigen associates with the neutrophil adhesion molecules CR3 and NCA-160. Stimulation of neutrophils with anti-CD15 antibody causes them to become activated, adhere to each other, and form aggregates [223]. Elola et al. [223] found that SSEA-1 (LeX) mediated adhesion of PMNs, as well as MCF-7 breast cancer cells, to activated endothelium. CD15/SSEA-1 antibodies are specific for neutrophils in normal blood and tissues, can label leukocytes in Hodgkin’s disease, and these antibodies can inhibit platelet-PMN adhesion [224]. CD15/SSEA-1 is expressed on normal human neutrophils, monocytes, and promyelocytes [240]. The α1,3-fucosyltransferases that synthesize the antigenic structure have been identified as fucosyltransferase IX (FUT9) in mature granulocytes and fucosyltransferase IV (FUT4) in promyelocytes and monocytes [240].
Pathogenic organisms such as Helicobacter pylori and Schistosoma mansoni express the SSEA-1/LeX epitope [241]. S. mansoni expresses LeX on its surface to prevent the immune system from recognizing the parasitic trematode larvae once it has made its way into a human or animal host [242]. The overexpression of the same carbohydrate structure on H. pylori also serves to weaken the host immune response [243].
Fox et al. [244] characterized the normal expression of SSEA-1 in human tissues in an attempt to better understand how it is regulated in cancers and found it expressed mostly on epithelial cells of the stomach, colon, ducts of the salivary glands, kidneys, bladder, skin glands, epididymis, uterus, cervix, alveoli during pregnancy, and medulla. It weakly stained the epithelial cells of the small intestine, pelvis, ureter, fallopian tube, and vagina. In the hematopoietic and lymphoid organs, SSEA-1 was found on PMN leukocytes, bone marrow, tissue macrophages, and in spleen cells. In the nervous system, only the cerebrum, cerebellum, pons, and spinal cord reacted with antibody to SSEA-1, but no peripheral nervous system components reacted. No SSEA-1 expression was found on mesenchymal cell-derived tumors. SSEA-1 expression was also found in colon adenomatous polyps and adenocarcinomas, stomach adenocarcinomas, kidney carcinomas, transitional cell carcinoma of the kidney, uterine adenocarcinoma, and in myelogenous leukemia--all tissues that normally expressed SSEA-1 before neoplasias developed. SSEA-1 was also found in several cancers in tissues that are normally SSEA-1 negative, and these included breast carcinomas and an ovarian adenocarcinoma [244].
Shi et al. [245] found SSEA-1 to be expressed at low levels in normal colon and at increased levels in developing fetal colon. The group looked at SSEA-1 expression in 20 early phase human colon adenocarcinomas and found it to be expressed in all of the specimens, with strong staining in 11 of the specimens; the poorly differentiated tumors had heterogeneous patterns of SSEA-1 staining as well [245]. Aberrant expression of SSEA-1 is one of the oncofetal antigenic changes that occur in early colon cancer development that has been identified by this group. Itzkowitz et al. [246] also found SSEA-1 to be expressed in human colon cancers and found it was present in more differentiated tumors.
Bladder cancer is one of the most widely studied cancers that express SSEA-1 [247–250]. Sheinfeld et al. [249] looked at the tumors from 89 bladder tumor patients and found that 76 of the 89 (92.3%) tumors were positive for SSEA-1 staining using the P-12 monoclonal antibody. These tumors consisted of low grade tumors such as papillomas, carcinoma in situ, and transitional cell carcinomas of the bladder. A few umbrella cells were positive; these are multinucleated normal urothelial cells with convex plasma membranes, and this gives them a characteristic umbrella-like morphology [249]. Of the control specimens, 34 of 40 were negative for SSEA-1, but the six false positives were from patients who had cancers at other sites.
Shirahama et al. [250] also investigated the expression of SSEA-1 in bladder cancer, in 78 patients with transitional cell carcinoma of the bladder (TCCB). In this study, the SSEA-1 antibody did not react with the surrounding normal urothelium except for a few umbrella cells. The grade II and III tumors were significantly more reactive with the anti-SSEA-1 antibody than the grade I tumors. The initial step of lymph node permeation occurred in tumors that expressed high or low SSEA-1, but metastasis occurred significantly more frequently in tumors that expressed high SSEA-1, indicating that SSEA-1 may play a role in adhesion to or colonization of the lymph nodes in cancer. The overall findings of the study showed that increased SSEA-1 expression correlated with poor prognosis and increased lymph node metastasis in TCCB.
Konety et al. [248] also looked at SSEA-1 staining in TCCB and found positive staining in 76% of the tumors, but only found a weak correlation of SSEA-1 expression with tumor recurrence in grade II tumors, while Kajiwara et al. [247] found that loss of SSEA-1 correlates with an increased invasiveness and increased incidence of recurrence of TCCB.
SSEA-1 is also expressed in renal tumors [251], Reed-Sternberg cells in Hodgkin’s lymphoma [252], acute promyelocytic leukemia [253], and primary testicular germ cell tumors [254]. Ohana-Malka et al. [252] found that SSEA-1 expressed on Reed-Sternberg cells of malignant Hodgkin’s lymphoma is associated with a better prognosis when the epitope was non-sialylated because the non-sialylated form could not adhere to E-selectin on endothelium as well as when it was sialylated (SLeX). In addition, brain and neuronal tumors also express SSEA-1 [255–257].
Read et al. [256] used the Patched mutant mouse model Ptc+/− to generate a model of medulloblastoma and found that SSEA-1 was a marker for tumor propagating cells or tumor stem cells. The SSEA-1 cells gave rise to tumors in SCID mice and generated all other cell types that made up the tumors. They also found that SSEA-1 was expressed in a subset of human medulloblastomas and those with the highest expression of the antigen had the shortest survival time [256]. SSEA-1 is also found in malignant human glio-neuronal tumors [257].
Son et al. [257] found that SSEA-1+ cells enrich human glioma tumor-initiating cell populations and stem cell populations, are highly tumorigenic in SCID mice, and they could generate multilineage cells that were both SSEA-1+ or SSEA-1−. Its role as a cancer stem cell enrichment marker in other types of human cancer than glioma is unknown.
Various studies have been performed involving targeting the SSEA-1 antigen in different ways to investigate its therapeutic potential. Katagihallimath et al. generated a LeX glycomimetic peptide to see if it could induce regeneration after spinal cord injury in mice but had no success [258]. They did not investigate its potential use in generating an anti-cancer vaccine to the SSEA-1 epitope or other uses.
LeX is expressed in cisplatin-resistant ovarian carcinoma cell lines that do not express the antigen before drug treatment [260]; this shows that a potential strategy in combating drug resistance could involve inhibiting glycosyltransferase genes.
One trial involving SSEA-1 in therapy used an antibody called FC-2.15, which was later determined to recognize the LeX epitope [260]. Mordoh et al. generated this IgM monoclonal antibody by immunizing Balb/c mice with tumor cells from an undifferentiated human primary breast carcinoma [261]. FC-2.15 recognized tumor proliferating cells and stem cells in most breast carcinomas and recognized a carbohydrate antigen that is absent in most tissues except the kidney and colon [261]. In a phase I clinical trial, the antibody was given to 11 patients with advanced stage IV cancers consisting of: five with breast cancer, two with colon cancer, one with melanoma, one with lung cancer, one with medullary thyroid cancer, and one with skin squamous cell carcinoma [262]. The trial was carried out before it was known that the antibody recognized the SSEA-1 epitope, so it would not be expected that the vaccine would have been effective in patients whose tumors did not express the antigen. Because the antibody was mouse in origin, the patients made human anti-murine antibodies to FC-2.15, which made successive infusions less effective, but only mild toxicities were observed. The antibody induced neutropenia due to killing of peripheral granulocytes, which returned to the circulation after antibody infusions stopped. One of the breast cancer patients had remission of over 50% of liver metastases during the trial, so the vaccine showed some promise [262]. An IgG3 antibody MCS-1, did not induce PMN aggregation or the neutropenia that occurred with FC-2.15; it also reduced MCF-7 cell adhesion just as efficiently, so it is potentially a better therapeutic agent [223].
Ballou et al. [263] injected mice with MH-15 teratocarcinoma cells that carry SSEA-1 and SSEA-1 negative myeloma cells. Radiolabeled SSEA-1 antibody was injected into the mice and only the MH-15 cells were labeled by 3–5 days. Radiolabeled SSEA-1 antibody was also injected into nude mice with xenografts of human myeloma or BeWO choriocarcinoma cells. The weakly bound radiolabeled antibody was not retained in normal SSEA-1 expressing tissues after a period of 3–5 days, but was retained in the tumors. This showed that targeting SSEA-1 in tumors may potentially work without having adverse effects on normal tissues that express the antigen.
4. The glycosphingolipids: globo series
The carbohydrate antigens Globo H, stage-specific embryonic antigen-3 (SSEA-3), and stage-specific embryonic antigen-1 (SSEA-1) are closely related to one another in either structure or in function. Globo H and SSEA-3 are both globoseries glycosphingolipids [264–266], with SSEA-3 being the non-fucosylated pentasaccharide precursor structure of Globo H [264–265]. SSEA-1 described above in the Lewis blood group related antigens is a neolactoseries glycosphingolipid [221–222] whose expression is closely related to the expression of SSEA-3 in mammalian development [222], with each being a tightly regulated stage-specific embryonic antigen. Glycosphingolipids are glycans attached to the hydroxyl group of the lipid ceramide. Ceramide is sphingosine, a long-chain amino alcohol, linked by an amide linkage to a fatty acid. Most glycolipids in humans are glycosphingolipids [267]. Glycosphingolipids appear clustered together in lipid rafts on the cell membrane [267].
4.1 Globo H
Globo H is a glycosphingolipid hexasaccharide with the structure Fucα(1–2)Galβ(1–3)GalNAcβ(1–3)Galα(1–4)Galβ(1–4)Glcβ(1) [264,266], and SSEA-3 is its pentasaccharide precursor without the fucose [264–265]. Both structures are considered globoseries glycosphingolipids.
Globo H was first isolated from human MCF-7 breast cancer cells [264,267]. Antibodies were generated against Globo H using fusion of myeloma cells with spleen cells from mice immunized with MCF-7 cells. Of the resulting clones, one produced an IgM monoclonal antibody, MBr1, which reacted with a carbohydrate structure found on normal and neoplastic mammary gland epithelium and in postpartum milk [268]. Mariani-Costantini et al. [269] showed that the MBr1 antibody stained metastatic breast carcinomas, was poorly reactive with non-epithelial breast tumors, and had the potential to be used as a diagnostic tool in breast cancer. Canevari et al. [270] ruled out that the MBr1 antibody binds to Forssman-like antigen or TF-Ag and showed that the antibody recognized a different tumor-associated antigen. Bremer et al. [222] identified the structure that MBr1 antibody recognizes as: Fucα(1–2)Galβ(1–3)GalNAcβ(1–3)Galα(1–4)Galβ(1–4)Glcβ(1)-Cer [271], which is Globo H.
Globo H is known to be expressed in breast and small cell lung carcinomas [272]. Zhang et al. [273] performed a survey of tumor antigens, such as Globo H, in various tumors and normal tissues. Globo H was found to be expressed on the epithelial lumen of normal lung, breast, prostate, stomach, pancreas, uterus, and ovary. MBr1 staining for Globo H was positive in small cell lung, breast, prostate, lung, pancreas, gastric, ovarian, and endometrial tumor specimens [273]. Zhang et al. [266] also found Globo H expression in both primary and metastatic prostate cancer glandular epithelium.
Chang et al. [264] looked at the Globo H and SSEA-3 expression in breast cancer specimens and found both Globo H and its precursor SSEA-3 to be expressed in breast cancer tumors but they were each expressed at lower levels on breast cancer stem cells (BCSCs). SSEA-3 was expressed more highly on both tumors and in the BCSCs than Globo H [264]. Because FUT1 and FUT2 were identified as α(1,2) fucosyltransferase genes [274], Chang et al. also looked to see if either of these two genes were either upregulated or downregulated. In MCF-7 and MB-157 breast cancer cells, FUT2 mRNA expression was almost undetectable, which suggested that FUT1 was the fucosyltransferase used by these cell lines to synthesize the Globo H structure. In MB-157 cells, this was validated by FUT1 siRNA knockdown, which resulted in decreased Globo H expression. In T47D breast cancer cells, FUT2 mRNA was highly expressed and FUT2 siRNA knockdown decreased Globo H expression, showing that FUT2 was the fucosyltransferase involved. From these results, the fucosyltransferase involved in generation of the Globo H structure appeared to be dependent on the cell line [264]. In addition, they also found that human BCSCs expressed lower levels of FUT2 than the non-BCSCs and this was likely why the BCSCs expressed lower levels of Globo H and higher levels of the incomplete SSEA-3 precursor of Globo H.
Chang et al. [264] found Globo H expression on colon, ovarian, gastric, pancreatic, lung, prostate, and breast cancers; in normal tissues it was present in breast, colon, esophagus, small intestine, prostate, rectum, testis, and uterine cervix, but only on apical surfaces [264]. Wang et al. found that both normal donors and breast cancer patients expressed high levels of antibodies to SSEA-3, but breast cancer patients expressed much higher levels of antibodies to Globo H than normal donors by glycan array [265]. Because the sites where either SSEA-3 or Globo H could be found in normal tissues are in areas that are considered generally inaccessible to immune cells [265,266,275], both SSEA-3 and Globo H make attractive targets for a cancer vaccine. Several groups have performed or are in the process of carrying out various stages of clinical trials with monovalent and polyvalent vaccines of Globo H because of its attractiveness as a therapeutic target [275–280].
Slovin et al. [275] performed a phase I trial using a synthetic KLH-conjugated Globo H vaccine in patients with relapsed prostate cancer and found the vaccine to be well tolerated as well as having evidence of a slight anti-tumor effect.
Gilewski et al. [276] examined a similar synthetic Globo H-KLH conjugate in a phase I trial consisting of 27 patients with a history of metastatic breast cancer and found it could induce mostly an IgM response and was well tolerated with only mild side effects; 14 of 15 patients who had no evidence of disease at the beginning of the trial remained that way after two years of follow up. Of the 14 patients who had disease at the beginning of the trial, 10 had no evidence of disease and 4 progressed. The group planned to include the antigen as part of a polyvalent vaccine to target heterogeneic tumors due to the success of the trial.
Ragupathi et al. [277] also generated a synthetic KLH-Globo H vaccine. Five patients with recurrent progressing prostate cancer were immunized with this vaccine; all made IgM antibody, two made IgG, and these antibodies recognized Globo H conjugated to ceramide, as well as prostate cancer and breast cancer cell extracts. The sera from the patients also caused CDC in MCF-7 cells, which are highly positive for Globo H, but not in melanoma cells, which lack Globo H.
Sabbatini et al. [278] performed a pilot study to look at the safety of a heptavalent vaccine consisting of Globo H, GM2, LeY, Tn, STn, TF, and Tn-MUC1 conjugated to KLH. The idea behind using so many epitopes is to target heterogeneic tumor cells that express one but possibly not all of these antigens. The vaccine was given to 11 patients with documented stage II to IV ovarian, fallopian tube, or peritoneal cancers in remission. All of the antigens except for LeY and GM2 induced an immune response even though all 7 are expressed in ovarian cancers, and CDC was not observed.
Slovin et al. [279] administered a bivalent vaccine consisting of Globo H and MUC2 conjugated to KLH to 43 patients with relapsed prostate cancer in a phase I trial. The vaccine was found to be safe, and while IgM, but no IgG, antibodies to Globo H were induced, this does not preclude clinical efficacy and a larger phase II trial has begun.
Zhu et al. [280] made a pentavalent synthetic vaccine consisting of Globo H, GM2, STn, TF, and Tn antigens conjugated to the carrier protein KLH in an effort to target antigens commonly overexpressed in breast and prostate cancers. This vaccine was given to mice and antibodies were made to all of the carbohydrate antigens in the vaccine, and these antibodies reacted with MCF-7 cells. This vaccine is now scheduled for phase I clinical trials.
4.2 SSEA-3
Stage-specific embryonic antigen-3 (SSEA-3) was first identified and defined by the reactivity of an IgM monoclonal antibody generated in a rat immunized with 4- to 8-cell stage mouse embryos. This monoclonal antibody reacted with all mouse preimplantation embryos from oocytes up to the early blastocyst stage where its expression became more restricted, in the primitive endoderm after implantation, and only in the kidney of adult mice. The SSEA-3 antigenic determinant was determined to be a carbohydrate present on glycolipids and glycoproteins; it was also found on human teratocarcinoma cells and human erythrocytes. The study showed that SSEA-3 was expressed differently in the developing germ layer or organ and at the stage of embryonic development than other stage specific embryonic antigens that had been characterized at the time [281]. On the structure NeuAcα(2–3)Galβ(1–3)GalNAcβ(1–3)Galα(1–4)Galβ(1–4)Glcβ(1–1)Cer, the previously generated antibody to SSEA-3, referred to as MC631 by Kannagi et al. [282], showed the highest binding affinity to the portion of this structure that lacked sialic acid [282]. In a panel of structures isolated from the 2102Ep human teratocarcinoma cell line, the SSEA-3 antibody had the highest affinity for Galβ(1–3)GalNAcβ(1–3)Galα(1–4)Galβ(1–4)Glcβ(1)Cer [267]. This structure is also known as Gb5 [283], galactosyl-globoside, or globopentaosylceramide [264]. In differentiating 2102Ep human teratocarcinoma cells, SSEA-3 expression decreased as SSEA-1 expression began to increase during differentiation [282].
Synthesis of SSEA-3 occurs when β1,3-galactosyltransferase V (β3GalT-V) transfers galactose to the GalNAc of globoside to form Gb5 or galactosyl-globoside [284]. In more recent studies, attempts were made to determine if SSEA-3 could be used as a marker to identify stem cells in umbilical cord blood [283]. It was determined that SSEA-3 was not expressed in hematopoietic or mesenchymal stem cells and therefore was not a good marker of multipotent cells [283].
Schrump et al. [285] immortalized lymph node lymphocytes from primary lung cancer patients, generated hybridomas, and selected for antibody secreting clones. Monoclonal antibodies were then generated from two of these clones--J309 and D579, which recognized the SSEA-3 antigenic determinant. The antibodies recognized SSEA-3 on several tumor cell lines including lung and breast cancer cell lines, and a teratocarcinoma cell line; in an immune adherence assay, rodent monoclonal SSEA-3 antibody, also referred to as MC631, reacted against the same cell lines as the J309 and D579 antibodies [285]. SSEA-3 has also been found on testicular germ cell tumors [254], as well as in breast cancer and in BCSCs [264].
Chang et al. [264] looked at SSEA-3 expression on normal tissues using a tissue microarray because its location outside of cancer and development was largely unknown. The group found SSEA-3 to be expressed on normal epithelium of colon, esophagus, small intestine, kidney, prostate, rectum, skin, testis, thymus, and uterine cervix. Expression was located only on the apical surfaces of epithelial cells or in the cytoplasm, which are considered immune system restricted or inaccessible sites [264]. In an experiment using a KLH-conjugated Globo H monovalent vaccine in mice, an antibody response was made to only the Globo H antigen. When α-GalCer was added as an adjuvant, the amount of overall antibody production increased and the mice made polyclonal antibodies to both the Globo H and the SSEA-3 antigen structures, which vaccination was unable to generate in the absence of the adjuvant [264]. This result showed that SSEA-3 and Globo H could make promising targets for cancer vaccines and could be targeted simultaneously.
SSEA-3 is developmentally regulated and its expression is closely related to that of another stage-specific embryonic antigen, SSEA-1 [286]. In mice, SSEA-3 is expressed highly in the fertilized egg and at the late 8-cell embryo stage, it is found only on the inner cell mass at the blastocyst stage, and becomes absent once the embryo has reached the early egg cylinder stage [286]. SSEA-1 begins to be expressed at the late 8-cell embryo stage, increases on the inner cell mass of the blastocyst to form embryonic stem (ES) cells, and becomes expressed on the primitive ectoderm to form the primordial germ cells at the early egg cylinder stage [286]. In humans, ES cells and embryonal carcinoma cells express SSEA-3 instead of SSEA-1 [286].
5. The sialic acid containing glycosphingolipids, the gangliosides
5.1 Introduction
Gangliosides are complex glycosphingolipids that contain a sialic acid [287]. In the nomenclature, the G stands for ganglioside and the M, D, or T stands for mono, di, or tri number of sialic acids. The number 1, 2, or 3 has to do with the order of the distance of migration in thin layer chromatography, in which the 3 travels the furthest, then the 2, then 1. Studies indicate that gangliosides play a role in cell-to-cell and cell-to-extracellular matrix interactions. In addition, antibodies to gangliosides have been shown to decrease cell growth, inhibit metastasis, and induce cell death. Thus the mechanistic role of gangliosides in the pathogenesis of tumors indicates that these molecules would be excellent vaccine targets. These molecules can be targeted by the immune system since they are expressed on the tumor cell surface. The first report concerning ganglioside expression as a tumor associated marker was in 1966 on differential expression in brain tumor tissue when compared to normal brain [288]. Care must be taken with animal tumor models studying ganglioside antigens because the rat and mouse have N-glycolylneuraminic acid rather than N-acetylneuraminic acid and this could affect antigen presentation [287]. Vaccination to target ganglioside antigens has involved anti-idiotypic antibodies, the adjuvants BCG and QS21 and conjugation to a T-dependent carrier.
The Lewis blood group related antigen, SLea is also considered a ganglioside but it was described above with the Lewis related antigens.
5.2. GD2, GD3, GM2, fucosyl GM1
GD2, GD3, and GM2 are overexpressed in tumors, but have some expression in normal tissues. Fucosylated GM1 (Fuc-GM1) has a more tumor restricted expression, with Fuc-GM1 expressed on small cell lung tumors and some expression in sensory nerves.
In neuroblastoma treatment with anti-GD2, the GD2 antigen was not lost from the tumor cells, so antigenic modulation of this antigen did not occur [287,289]. GD2 is highly expressed on neuro-ectodermal tumors but also expressed in low levels in normal peripheral nerve tissue. In monoclonal antibody passive immunotherapy in patients with melanoma using antibody against GD2 considerable toxicity occurred due to expression of this antigen on normal tissue. The toxicity included sensorimotor polyneuropathy and additionally some patients had the syndrome of inappropriate antidiuretic hormone [291]. Biopsy showed nerve demyelination in patients affected with sensory neuropathy [292]. This information should preclude any monoclonal antibody therapy to GD2 to treat these diseases and should preclude attempts to use this ganglioside as a cancer vaccine in melanoma or neuroblastoma. Even with these results in the literature, a group in 2002 attempted active immunotherapy to GD2 using a GD2-KLH/MPL-A conjugate [292]. Fortunately for the patients, no anti-GD2 antibody was seen post-immunization and no side effects were seen. Another trial to target GD2, utilized a vaccine composed of an anti-idiotype mimicking GD2 injected with the adjuvant QS21, in a preparation called TriGem and antibody development was seen, and the safety studies seemed successful, but efficacy in terms of patient outcome was not reported, and no further information has been published [293].
GD3 plays a role in the regulation of cell growth; antibodies to this ganglioside inhibit cell growth, and in addition these antibodies decrease angiogenesis [287,294,295]. In in vitro models of metastasis, GD3 was associated with tumor cell attachment of melanoma to the matrices. Monoclonal antibodies to GD3 were infused into 37 melanoma patients, and one showed a complete response for 2 years in one patient and in one other showed a response for 2 months. However, a pulmonary capillary leak occurred at high antibody dose, probably due to the normal expression of this antigen [287,294,295,296]. Active immunotherapy trials have been performed with patients immunized [297] with GD3-KLH with QS21 or followed by an anti-idiotypic antibody BEC2, a mimic of GD3 plus BCG, or the mimic followed by the GD3-KLH. 10 patients responded with the production of anti-GD3 antibody. A phase III trial was performed with BEC2/BCG in small-cell lung cancer patients with limited disease. 515 patients were randomly assigned to vaccination or no vaccination, and the vaccination had no impact on outcome of these patients [297,298,299].
GM2 is found in the areas of tumor cell-to-tumor cell contact indicating a role in cellular interactions and adhesion [287]. About 5% of melanoma patients have anti-GM2 antibodies, and the presence of these antibodies is related to longer disease free survival [300,301]. In small-cell lung cancer an antibody to GM2 (3F8) was utilized in radioimmunolocalization studies and successfully found most tumor sites [287]. GM2 was injected along with BCG into 58 melanoma patients and 64 patients received BCG only as a control. 50 of the 58 developed anti-GM2 compared to 7 of the 64 vaccinated with BCG alone. If compared as randomized, no statistically significant difference was seen in either disease free survival or overall survival. If the patients that entered the study with anti-GM2 antibodies (1 in the test group and 5 in the control group) were removed from the study there was a significant difference in disease free survival but not in overall survival [300]. The next study compared GM2-KLH immunization using the adjuvant QS21 in one treatment arm with high dose interferon alpha-2b in the other treatment arm. 880 patients were randomized to receive either vaccine or high dose interferon and there was statistically significant lower disease free survival and overall survival in the GM2-KLH group [302], however this did not study whether vaccine in addition to interferon would have been helpful, nor did it compared vaccine treated to no treatment. Fifty percent of the patients developed at least a 1:80 titer to GM2. Another study utilizing 1314 patients in a GM1 vaccine trail has been published as an abstract only, and in this study GM2-KLH conjugate immunization was compared to observation alone. In this study, vaccination was worse than observation alone. Since this was an abstract it is not clear whether the immunizations led to an antibody mediated response and whether antibody levels were also associated with a decrease in overall survival. Concomitant with any vaccination is the possibility of boosting the T regulatory cell population and decreasing the antibody and cytotoxic T cell response, in which case the outcome would be worse. This may have been the case but the data is not available. Certainly, it would have been interesting and important to evaluate in order to determine the mechanism of the poorer outcome, but with these results it is unlikely that further analysis should or could be done with this antigen.
Fucosyl GM1 is a unique structure that is found in small cell lung cancers (SCLC) with a very limited expression in normal tissues [302–304]. Immunization of SCLC patients with either bovine derived or synthetic fucosyl GM1 resulted in antibody production. No description of efficacy or safety were given. These authors have decided to continue the immunotherapy of SCLC patients using a quadrivalent vaccine containing GM2, fucosyl GM1, globo H and polysialic acid as vaccine targets [302–304]. Unfortunately, the very limited expression of this antigen includes expression in sensory nerves, and sera from patients with sensory nerve pathology were found to react with Fucosyl GM1 [305–307].
5.3 NeuGcGM3 variant GM3
NeuGcα2–3Galβ1–4Glc-Cer ganglioside, NeuGcGM3 is a glycosphingolipid that contains an N-glycolylated version of neuraminic acid, differing from N-acetylneuraminic acid by the addition of an oxygen atom on the acetyl group usually by a specific hydroxylase that is ineffective in humans due to an exon deletion. An important distinguishing factor is that this sialic acid variant is selectively expressed in tumor cells, whilst being absent or in extremely low amounts in normal tissues [308–318]. NeuGcGM3 expression has been detected up-regulated in various tumors including colon, breast, melanoma, retinoblastoma and germ cell tumors. However, conflicting reports concerning expression or lack of expression in various tumor cell types exist but may be due to the cross-reactivities of antibodies used for the detection of the NeuGcGM3. Improved antibody specificity analysis due to glycan array testing is settling these discrepancies as are GC-MS studies [308]. The consensus is that Neu5Gc is overexpressed in human tumors [308]. Currently, most literature attributes the tumor expression of NeuGcGM3 to be a result of metabolic assimilation of NGc taken in diet, although endogenous synthesis from glycolyl-CoA is a possibility [308]. However why either synthesis or uptake occurs more in human tumor tissue than in normal tissue has not been elucidated. Antibodies to Neu5Gc are produced by humans and are called Hanganutziu-Deicher antibodies. These antibodies were discovered and named when they were found in patients that had been injected with serum from another species, and are also called serum sickness antibodies [307]. The presence of antibodies to Neu5Gc indicates that this is a promising immunotherapy target, since the cancer patient will not be tolerant to it and the selective tumor cell association indicates that autoimmunity would not be caused by reaction to this antigen.
Alfonso et al. prepared P3 (Ab1), an IgMκ mouse monoclonal antibody (mAb) specific to gangliosides including NeuGc in breast tumor cells. They immunized Balb/c mice with P3 (Ab1) attached with KLH, and with Freund’s adjuvant, to produce a γ-type anti-idiotype monoclonal antibody (Ab2). This antibody was called 1E10, or racotumomab [308–318]. A clinical trial was performed in 2002 with this Ab2 in advanced stage malignant melanoma patients. Selective NeuGcGM3 reaction occurred in all patients, not showing any cross-reactivity. The study proposed that 1E10 triggers generation of Ab3 antibody that has the same idiotype as P3 mAb, thereby making Ab3 antigen selective. ELISA, TLC immunostaining, and inhibition assays validated the NeuGcGM3 and NeuGcGM2 selective response of Ab3 antibody in most patients. Minimal side effects were observed, thus 1E10 is a promising therapeutic vaccine that targets NeuGcGM3 for various tumors, including breast tumor and SCLC [308–318].
Neninger et al. used the 1E10 vaccine for a phase I trial in 2007 for a group of 9 SCLC patients, usually having a four month life expectancy post-diagnosis without therapy. The trial was based on favorable anti-tumor response in mice using the same vaccine on lung metastases of breast tumors and melanomas during previous experiments. The schedule for the trial included ten doses, and eight patients were considered relevant for data analysis. Seven out of the eight patients developed NeuGcGM3 exclusive response, with some patients generating NeuGcGM3 specific IgM and IgG response as well. Anti-1E10 and anti-NeuGcGM3 reaction was also detected during the response, which continued for more than three months once the dosage schedule was complete. With no major side effects from the vaccine, re-immunizations followed after three months. 1E10 presents itself as a safe candidate for treating small metastases in SCLC patients, in conjunction with the initial therapy for the major tumor [311]. In 2011, Hernandez et al. performed a clinical trial with 1E10 vaccine in a group of 20 patients with non-small cell lung cancer, where the anti-NeuGcGM3 antibodies identified the NeuGcGM3 expressing tumor cells and selectively killed them by a mechanism that is independent of complement activation. The authors suggest that anti-NeuGcGM3 can initiate oncotic necrosis by having a cytotoxic effect on antigen expressing tumor cells. The cytotoxicity is directly related to the antigen expression level, which reasserts NeuGcGM3 as an important tumor target [312].
Carr et al. formed a very small size proteoliposome (VSSP) by assimilating a ganglioside in combination with the external membrane protein complex of Neisseria meningitidis. They showed that VSSP enhanced GM3 and NeuGcGM3 immunogenicity in mice. In 2003, they did a clinical trial in advanced stage (III or IV) breast cancer patients using a heterophilic NeuGcGM3/VSSP/Montanide ISA 51 vaccine. Montanide ISA 51 served as the adjuvant in the vaccine. To be considered usable from data and final analysis perspective, patients had to have at least 5 doses. With minimalistic side effects post-immunization, all stage III group patients and half of the stage IV group patients generated high anti-NeuGcGM3 IgM response, and detectable serum IgA antibodies. Two patients with pulmonary metastases prior to entering the trial, did not have tumor progression for 18 and 40 months, respectively. The knowledge about the role of IgA in the first line of mucosal defense, coupled with the elevated immunogenic response in stage III patients, gives a promising outlook. The therapy can be improved with the possibility of vaccinations being done in an adjuvant setting, as well as re-immunizations [313]. Osorio et al. used VSSP for a phase Ib/IIa trial in 2009 in 22 patients with advanced cutaneous and ocular malignant melanomas. Two dose groups were formed, and after the first levels of immunization, some discoloration of the skin was observed in all patients. Specific IgG, IgM, and IgA antibodies were generated in the patients by VSSP, and their titers were similar to the ones in stage IV breast cancer patients in the study by Carr et al. in 2003. An unanticipated, yet favorable outcome of the trial was the survival of seven patients for more than two years post-immunization [314]. Labrada et al. in 2010 performed immunotherapy using VSSP vaccine in mouse 3LL-D122 lung carcinoma. VSSP generated results similar to the human breast tumor model, thereby establishing NeuGcGM3 as a therapeutic target [35].
In 2006, de Leon et al. added pure NeuGcGM3 to mouse or human T lymphocytes, and showed that this caused a concentration dependent down-regulation of CD4 expression. The most affected T lymphocytes were native cells. Though CD4 expression resumed normalcy within two days after NeuGcGM3 was eliminated, the T cells remained responsive to NeuGcGM3-initiated CD4 down-regulation for a prolonged period of time post NeuGcGM3 removal. This suggests that a NeuGcGM3 expressing tumor can lower CD4 expression for extended periods, affecting the normal functionality of T cells, thus aiding growth of the tumor [316].
5.4 Polysialic Acid (PSA)
Polysialic acid (PSA) is a linear homopolymer carbohydrate made up of more than 20 units of α 2,8-N-acetyl neuraminic acid residues which is found attached to the fifth immunoglobulin domain of the membrane bound neural cell adhesion molecule (NCAM). The association of PSA with NCAM was discovered in 1982 [319–323]. Embryonic brain has high amounts of Polysialylated NCAM (PSA-NCAM) and there is upregulated PSA expression during embryonic development [321-,326]. The interactions between cells during synaptic formation and cell migration processes involve PSA-NCAM [322,–324]. PSA also has ubiquitous presence in areas of adult mammalian brain and it plays roles in neural generation and plasticity [322–325]. PSA is an oncofetal tumor-associated antigen, being present in tumor tissue, embryonic tissue, with some expression in normal brain. Its expression in tumor cells is explained by Hildebrandt et al. in studies of human neuroblastoma cells, which identified two distinct polysialyltransferases, ST8SiaII and ST8SiaIV. Using RT-PCR, these tumor cells were found positive for expressing both sialyltransferases, suggesting their possible role in PSA production. Further study and classification of these polysialyltransferases could aid in developing better therapy and diagnosis [326].
PSA attached to NCAM hinders cell adhesion and thus blocks cell-surface interactions [323]. The surfaces of various growing and metastatic human tumors like small cell lung carcinoma (SCLC), rhabdomyosarcoma, Wilms’ tumor, and neuroblastoma have been found to have PSA overexpression [327]. Prior work has detected that tumor metastasizing ability and growth is positively affected by NCAM polysialylation, implying that PSA-NCAM reduces the probability of patient survival [327]. In 2003, Seidenfaden et al. used endoneuraminidase N, which is a PSA cleaving enzyme, to remove only PSA from the cell surface without impacting NCAM expression. They successfully demonstrated that cell growth could be stopped in rhabdomyosarcoma and neuroblastoma cells. Their explanation for this outcome is that PSA decreases NCAM signaling at contact sites between cells [327]. Petridis et al. in 2009 showed a direct correlation between levels of PSA expression and metastatic ability of astrocytomas. A similar correlation is validated for SCLC, with mortality rates for patients expressing PSA-NCAM elevated when compared to patients not expressing PSA-NCAM [328]. The results obtained from this study combined with previous work by Seidenfaden et al. puts forth endoneuraminidase N as a possible therapeutic strategy [327–328]. In 2001, Miyahara et al. demonstrated that the chances of survival of patients with SCLC expressing both NCAM and PSA are considerably less than the ones not expressing PSA [328]. In 2004, Petridis et al. treated neuroblastoma cells with endoneuraminidase N to remove PSA from the cell surface. It was known from previous research that PSA helps with the cell differentiation process. Thus, upon enzymatic treatment, development of synaptic growth from neuroblastoma cells was observed [322, 334]. Korja et al. in 2009 suggested a direct relation between PSA-NCAM expression and metastasis. They used neuroblastoma cell samples from patients and performed tumor tissue microarray studies to classify PSA-NCAM expression, and showed that patients overexpressing PSA-NCAM had more metastases. This study also matched this data against different clinical parameters including age, stage, etc. and established the diagnostic and prognostic value of PSA and NCAM. Counter-intuitively, in the case of patients with no PSA-NCAM expression although they had less metastasis, the prognosis for complete survival was adverse, especially during late stages. Despite limitations, PSA-NCAM expression may have a role as a molecular marker as well as a therapeutic target for neuroblastoma [329].
In 2010, Amoureux et al. analyzed 56 glioblastoma multiforme samples to quantify PSA-NCAM using ELISA and immunohistochemistry. The results showed PSA-NCAM overexpression in over two-thirds of the samples. Their immunohistochemistry analysis was able to differentiate PSA-NCAM expression amounts for a cluster of cells versus the amount expressed per cell. Heterogeneity was demonstrated with cells spread over tissues showing low expression versus highly expressing cellular islets. The study also found that the expression of Olig2, a glioma cell transcription factor required for gliomagenesis, was directly related to PSA-NCAM expression. The results of this study make PSA a strong candidate as a biomarker for glioblastoma multiforme [323].
Despite the fact that PSA expression is upregulated on the surface of tumor cells like SCLC and absent in normal cells, it is very difficult to develop PSA vaccine targeting therapies. The reason for such difficulty is the human immune tolerance against PSA that develops due to PSA expression in embryonic development and its continuous presence in some segments of the brain. The key to breaking tolerance is the presentation of an altered self-like molecule. In 2000, Krug et al. used a precursor, N-propionylated mannosamine, and metabolically engineered PSA synthesis cause production of N-propionylated-PSA (NP-PSA) glycoconjugates on leukemic cell surfaces in mice models to determine if modified PSA would make a good therapeutic target. Immunotherapy was performed using monoclonal antibody prepared against NP-PSA. Therapy was either by passive administration or a vaccine like NP-PSA conjugated with a protein carrier such as KLH. The study generated exciting results because although cell growth was attenuated only partially, the containment of metastasis was obtained. Since PSA is only present on tumor cell surfaces, embryonic tissue, and the areas in the brain immune responses would have tumor selectivity [330]. The areas in the brain may seem troublesome if an immune response is created, but the blood brain barrier should prevent antibody entrance into the brain in most cases. Studies on polysialic acid as a vaccine should include assays to determine if any pathology is caused due to any passage of antibody through the blood-brain barrier. Passage of antibody through this barrier can occur in inflammatory responses so this may be a consideration [331–332].
Krug et al. in 2004 administered a PSA-KLH vaccine in conjunction with the adjuvant QS21 to generate immune response in SCLC patients. The trial was divided into two groups, one receiving vaccine made from native PSA, while the other received vaccine made from chemically tailored NP-PSA. Previous work has confirmed that NP-PSA causes an improvement in the reaction of mouse IgG to meningococcal group B polysaccharide. In the group vaccinated with NP-PSA, IgM antibodies that cross-reacted with native PSA were produced in all patients except one. In the control group that was treated with native PSA, only one patient produced an IgM response. This approach is of particular interest for SCLC patients, who respond well to the therapy in early stages but ultimately succumb to metastases. Antigen targeting antibodies can be used as vaccines, and they have been shown to have minimal side effects. This approach could help with treating micro- metastases, and increasing the life expectancy of SCLC patients [330].
PSA has a selective tumor cell surface presence and has been found in small cell lung carcinoma, rhabdomyosarcoma, Wilms’ tumor, astrocytoma, neuroblastoma, and glioblastoma multiforme. PSA appears to be an excellent potential candidate for tumor targeting and therapy as long as attention is paid to possibilities of autoimmunity due to reaction with brain antigens [319].
6. Miscellaneous antigens
Precursor type 1 of the Lewis blood group system which is also called Lacto-N-tetraose (LNT) with the structure Galβ1–4GlcNAcβ1–3Galβ1–4Glc has been found to be upregulated in tumors of the pancreas, regardless of secretor status of the patient. However, LNT is present in the acini and ducts of non-secretors, but not in the pancreas of secretors. [333]. Similarly, LNT was found on the gastric mucosa of non-secretors but not on the gastric mucosa of secretor, and was found on the gastric adenocarcinomas of both [334]. LNT could be used as a TACA target in secretors but not in non-secretors because it is in their normal tissue [333–334]. Tk is an antigen similar to TF and Tn in that this antigen was discovered by the polyagglutinability of red blood cells following infection. This antigen is the trisaccharide GlcNAcβ1–3Galβ1–4Glc. It has been found on colorectal cancer cells, but not on normal tissue. In normal tissue this structure is elongated with either galactose or fucose. An immunization study performed in tumor bearing rats showed significant protection from a Tk positive but not a TK negative colorectal cancer following immunization with endo-β-galactosidase treated (TK expressing) red blood cells [335]. This interesting study shows that TK has promise as a vaccine for colorectal cancer and further studies are warranted.
Gb3 is a trihexosylceramide in the Globo series with the structure αGal-α1-4Galβ1-4Glcβ1-1Cer and is found in testicular, colorectal, and breast carcinoma as well as Burkitt’s lymphoma [336,337]. This is the antigen that Shiga toxin binds to, so a recent study proposed targeting tumor cells using Shiga toxin binding delivery system [337]. Levels of Gb3 are not correlated with amount of metastasis. A vaccine containing Gb3 and the MUC5AC peptide epitope was prepared by Danishefsky’s group [338], but we must await the results of this vaccine [see Table 5 for a summary of NeuGc GM3 and polysialic acid] .
Table 5.
| Antigen Group | Antigen | Function in tumor growth and spread | Tumor types | Naturally occurring antibody in patients | Efficacy and safety of passive immunotherapy, animal experiments | Vaccination of patients with |
|---|---|---|---|---|---|---|
| Additional Sialic acid containing | NeuGcGM3 | Decrease CD4 T cell response | Colon, breast, melanoma, small cell lung cancer, neuroblastoma, germ cell tumors | Hanganutziu-Deicher antibodies These are related to serum sickness | Minimal side effects |
|
| Polysialic acid linked to NCAM | Blocks cell-cell interactions Related to cell growth and metastasis | small cell lung carcinoma rhabdo-myosarcoma, Wilms’ tumor, neuroblastoma glioblastoma multiforme | Autoimmunity to brain possible but not seen |
|
7. Conclusion
Aberrant expression of carbohydrate structures is a natural occurrence with the transformation from a normal cell to a tumor cell, so these carbohydrate antigens have been the targets of interest for possible immunotherapy of cancer. Problems in utilizing these antigens to target tumors include both low immunogenicity of TACA and, for most of them, expression on normal tissues. Expression on normal tissues could cause tolerance in any vaccine situation or could result in autoimmune pathology in the vaccinated. The potential of immunotherapy fosters the pursuit of the ideal anti-TACA vaccine. Prior to utilization of a carbohydrate vaccine, passive immunotherapy with antibody to the target, first in an animal model, and then in humans should show safety and efficacy. Naturally occurring antibody in the patient is a positive indicator for safety of a vaccine, and if this antibody is related to prognosis, it is a better indicator. Although cytotoxic T cells would be required with bulky disease, antibody can be important in cancer immunotherapy, as is shown by the success of the monoclonal antibodies Rituxan and Herceptin. This is important because the anti-carbohydrate vaccine is most likely to yield an antibody response. A role for the carbohydrate antigen in the pathogenesis of the tumor is also a positive indicator for this marker to be used as an immunotherapy target. If antigenic modulation occurred after use of the molecule as a target, the role that the TACA played in pathogenesis would be eliminated and a better prognosis would result.
The TACA discussed include the mucin related Tn, Sialyl Tn, and Thomsen-Friedenreich antigens, the blood group Lewis related LewisY, Sialyl LewisX and Sialyl LewisA, and LewisX, (SSEA-1), the glycosphingolipids Globo H, stage-specific embryonic antigen-3 (SSEA-3), and the sialic acid containing glycosphingolipids, gangliosides GD2, GD3, GM2, and fucosyl GM1, and additional sialic acid containing compounds NeuGcGM3 and polysialic acid (Table 1–5). Most of these molecules have roles in tumor cell metastasis through their involvement in adhesive interactions. Tumor expression of these molecules ranges from the pancarcinoma expression of the mucin related molecules (Table 1), to the more restrictive melanoma and small cell lung cancer of the gangliosides (Table 4). The first goal in the selection of a vaccine target is to do no harm, thus GD2 should be eliminated as a target since monoclonal antibody caused sensorimotor polyneuropathy and nerve demyelination. In addition GD3 should be approached carefully since a pulmonary capillary leak occurred at high antibody dose in passive transfer experiments, and fucosyl GM1 should also be approached carefully since patients with sensory nerve pathology have anti-fucosyl GM1. Patients vaccinated with GM2-KLH conjugate actually fared worse than the control patients, so unless a mechanism for this is understood and it can be avoided, this target should be avoided. SSEA-1 LewisX negative tumors have increased pathogenicity, and since targeting this antigen may cause antigenic modulation this possibility must be thoroughly explored prior to use of this target. This leaves as most promising, the mucin associated antigens Tn, Sialyl Tn and TF, Globo H, SSEA-3, LewisY, Sialyl LewisX, and Sialyl LewisA as the first choices to target with an anti-TACA response (Tables 1–3). Human antibodies to these remaining structures have been produced, so these represent possible vaccine targets. All of these structures play a mechanistic role in cancer pathogenesis, and thus immunomodulation is less likely to occur. To further differentiate, passive transfer of antibody to Tn, TF, STn, SLeX, and SLeA was protective in mice, while Globo H and SSEA-3 have not been studied in this way. Lastly, naturally occurring antibody in cancer patients seems to have a protective role in TF. These carbohydrate antigens all show promise, and as we better understand the immune response to T-independent antigens, it is more likely that an effective response to these antigens will result. We look forward to the future of this field that is ripe for clinical progress.
Table 1.
| Antigen Group | Antigen | Function in tumor growth and spread | Tumor types | Naturally occurring antibody in patients | Efficacy and safety of passive immunotherapy, animal experiments | Vaccination of patients with |
|---|---|---|---|---|---|---|
| Mucin | Tn | Adhesive-ness Survival | ~ 90% of epithelial cancers, including breast, colon, lung, bladder, cervical, ovarian, stomach, and prostate | Yes | Vaccination in mice with asialo-bovine submaxillary mucin protective passive transfer caused decrease tumor size |
|
| SialylTn | Adhesive- ness Survival | ~ 90% of epithelial cancers, including breast, colon, lung, bladder, cervical, ovarian, stomach, and prostate | No | Vaccination of mice with STn-KLH was protective |
|
|
| TF | Metastatic spread | Pancarcinoma Cancers of breast, colon, bladder, prostate, liver, and stomach | Yes, and it is related to improved prognosis |
|
|
Table 4.
| Antigen Group | Antigen | Function in tumor growth and spread | Tumor types | Naturally occurring antibody in patients | Efficacy and safety of passive immunotherapy, animal experiments | Vaccination of patients with |
|---|---|---|---|---|---|---|
| Ganglio-sides | GD2 | tumor cell attachment to matrices | Neuroblastoma | Monoclonal antibody caused sensorimotor polyneuropathy nerve demyelination |
|
|
| GD3 | a role in the regulation of cell growth angiogenesis tumor cell attachment to matrices | melanoma small-cell lung cancer patients | a pulmonary capillary leak occurred at high antibody dose, |
|
||
| GM2 | a role in cellular interactions and adhesion | melanoma small-cell lung cancer | 5% of melanoma patients have anti- GM2 antibodies, and the presence of these antibodies is related to longer disease free survival |
|
||
| Fucosyl GM1 | small-cell lung cancer | patients with sensory nerve pathology have anti- Fucosyl GM1 | Monoclonal antibody protective in animal experiments |
|
Table 3.
| Antigen Group | Antigen | Function in tumor growth and spread | Tumor types | Naturally occurring antibody in patients | Efficacy and safety of passive immunotherapy, animal experiments | Vaccination of patients with |
|---|---|---|---|---|---|---|
| Glyco-sphingo-lipids | Globo H | Related to metastatic spread | small cell lung, breast, prostate, lung, colonpancreas, gastric, prostate, ovarian, and endometrial tumors | Yes | Anti-Globo H made after tumor cell vaccination |
|
| SSEA-3 | Related to metastatic spread | Testicular, lung, breast, teratocarcinoma | Yes Human hybridomas made from lung cancer patient nodes |
|
Table 2.
| Antigen Group | Antigen | Function in tumor growth and spread | Tumor types | Naturally occurring Ab in patients | Efficacy and safety of passive immunotherapy, animal experiments | Vaccination of patients with |
|---|---|---|---|---|---|---|
| Lewis related antigens | LewisY CD174 |
|
Ovarian, breast, prostate, colon and lung cancers of epithelial cell origin, embryonal carcinomas, yolk sac tumors, and seminomas | Yes, unknown if it is protective |
|
|
| Sialyl LewisX | role in the invasion and metastasis | in breast, ovarian, melanoma, colon, liver, lung, and prostate cancer | Yes |
|
|
|
| Sialyl LewisA CA 19.9 | role in the invasion and metastasis | breast, colon, and pancreas and in melanomas | Yes |
|
|
|
| SSEA-1 LewisX | Potential role in adhesive interactions involved in tumor metastasis | Colon, stomach, uterine, breast, ovarian adenocarcinomas, kidney carcinomas, kidney, bladder transitional cell carcinomas, AML | Only in H. pylori associated gastric cancers, protective response to bacteria not the tumors |
|
|
Highlights.
Tumor-associated carbohydrate antigens (TACA) result from the aberrant glycosylation that is seen with transformation to a tumor cell.
-
The carbohydrate antigens that have been found to be tumor-associated include
the mucin related Tn, Sialyl Tn, and Thomsen Friedenreich antigens
the blood group Lewis related Lewisy, Sialyl LewisX and Sialyl LewisA, and LewisX, (SSEA-1),
the glycosphingolipids Globo H, stage-specific embryonic antigen-3 (SSEA-3)
the sialic acid containing compounds including glycosphingolipids, the gangliosides GD2, GD3, GM2, and fucosyl GM1, and NeuGcGM3 and polysialic acid.
Recent developments have furthered our understanding of the T-independent type II response that is seen in response to carbohydrate antigens.
The selection of a vaccine target antigen is on the presence of the antigen in a tumor tissues and on the role this antigen plays in tumor growth and metastasis.
Acknowledgments
We would like to thank the following funding sources: CDMRP W81XWH-04-1-0342 (J.H.M., K.R.O.), NIAID R15 AI 49210-01(K.R.O.), UB STOR Product Development Fund (K.R.O.), Oishei Foundation (K.R.O.) and The King Saud University Chairs Research Fund (K.R.O., A.A.) for their support of the TF-Ag projects summarized herein. We would also like to thank Kshipra Gharpure, Kimiko Ferguson and Susan Morey for proof-reading the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. Journal of Leukocyte Biology. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 2.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 3.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 4.The University of Texas MD Anderson Cancer Center Coley Toxins Detailed Scientific Review. [Accessed 5/8/11]; http://www.mdanderson.org/education-and-research/resources-for-professionals/clinical-tools-and-resources/cimer/therapies/nonplant-biologic-organic-pharmacologic-therapies/coley-toxins-scientific.html.
- 5.Silverstein AM. Chapter 11 Transplantation and Immunogenetics. In: Silverstein AM, editor. A History of Immunology. New York: Elsevier Academic Press; 2009. [Google Scholar]
- 6.Cummings Richard D, Varki Adjit, Esko Jeffery D, Stanley Pamela, Bertozzi Carolyn, Hart Gerald, Etzler Marilynn E. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. [PubMed] [Google Scholar]
- 7.Cazet A, Julien S, Bobowski M, et al. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Research. 2010;12:204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobata A, Amano J. Altered glycosylation of proteins produced by malignant cells, and application for the diagnosis and immunotherapy of tumours. Immunology & Cell Biology. 2005;83:429–439. doi: 10.1111/j.1440-1711.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Warren JD, Danishefsky SJ. Synthetic carbohydrate-based anticancer vaccines: the Memorial Sloan-Kettering experience. Expert Review of Vaccines. 2009;8:1399–1413. doi: 10.1586/erv.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Z, Wang Q. Recent development in carbohydrate-based cancer vaccines. Current Opinion in Chemical Biology. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida T, Mei H, Dörner T, Hiepe F, Radbruch A, Fillatreau S, Hoyer BF. Memory B and memory plasma cells. Immunological Reviews. 2010;23:1. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 12.Bulow GV, Deursen JM, van Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 13.Gor DO, Ding X, Li Q, Sultana D, Mambula SS, Bram RJ, Greenspan NS. Enhanced immunogenicity of pneumococcal surface adhesin A (PsaA) in mice via fusion to recombinant human B lymphocyte stimulator (BLyS) Biology Direct. 2011;6:9. doi: 10.1186/1745-6150-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen BJ, et al. Structure of C3b reveals conformational changes that underlie complement activity. Nature; 2006;444(7116):213–6. doi: 10.1038/nature05172. [DOI] [PubMed] [Google Scholar]
- 15.Szakonyi G, et al. Structure of complement receptor 2 in complex with its C3d ligand. Science. 2001;292(5522):1725–8. doi: 10.1126/science.1059118. [DOI] [PubMed] [Google Scholar]
- 16.Bower JF, Ross TM. A minimum CR2 binding domain of C3d enhances immunity following vaccination. Adv Exp Med Biol. 2006;586:249–64. doi: 10.1007/0-387-34134-X_17. [DOI] [PubMed] [Google Scholar]
- 17.Llopis M-JP, Harms G, Hardonk MJ, Timens W. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. Journal of Allergy and Clinical Immunology. 1996;97(4):1015–1024. doi: 10.1016/s0091-6749(96)80078-9. [DOI] [PubMed] [Google Scholar]
- 18.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased Immunogenicity and Induction of Class Switching by Conjugation of Complement C3d to Pneumococcal Serotype 14 Capsular Polysaccharide. Infect Immun. 2001;69(5):3031–3040. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan T, Ghina H, Paul M, Marie-Jeanne A, Thomas PP, Nicolas B, Thierry D, Laurent G. Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. The Journal of infectious diseases. 2010;202(3):470–9. doi: 10.1086/653739. [DOI] [PubMed] [Google Scholar]
- 20.Wu YC, Kipling D, Leong HS, Martin V, Ademokun AA, Dunn-Walters DK. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 2010;116(7):1070–1078. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taillardet M, Haffar G, Mondiere P, Asensio M-J, Gheit H, Burdin N, Defrance T, Genestier L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114(20):4432–4440. doi: 10.1182/blood-2009-01-200014. [DOI] [PubMed] [Google Scholar]
- 22.Finn A. Bacterial polysaccharide–protein conjugate vaccines. British Medical Bulletin. 2004;70:1–14. doi: 10.1093/bmb/ldh021. [DOI] [PubMed] [Google Scholar]
- 23.Anderson P, Pichichero ME, Insel RA. Immunogens consisting of oligosaccharides from Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Invest. 1985;76:52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madore DV, Phipps DC, Eby R, et al. Immune response of young children vaccinated with Haemophilus influenzae type b conjugate vaccines. In: Cruse JM, Lewis RE, editors. Contributions to Microbiology and Immunology: Conjugate Vaccines. Vol. 10. New York, NY: Karger Medical and Scientific Publishers; 1989. pp. 125–150. [PubMed] [Google Scholar]
- 25.Madore DV, Phipps DC, Eby R, et al. Safety and immunologic response to Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in 1- to 6-month-old infants. Pediatrics. 1990;85:331–337. [PubMed] [Google Scholar]
- 26.Avery OT, Geobel WF. Chemo-immunological studies on conjugated carbohydrate- proteins. II Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–50. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayhtyand H, Eskola J. New vaccines for the prevention of pneumococcal infections. Emerging Infectious Diseases. 1996;2:289–298. doi: 10.3201/eid0204.960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978–1994: implications for development of a conjugate vaccine. Journal of Infectious Diseases. 1995;171:885–889. doi: 10.1093/infdis/171.4.885. [DOI] [PubMed] [Google Scholar]
- 29.Siber GR. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 30.Rennels Margaret B, Edwards Kathryn M, Keyserling Harry L, Reisinger Keith S, Hogerman Deborah A, Madore Dace V, Chang Ih, Paradiso Peter R, Malinoski Frank J, Kimura Alan. Safety and Immunogenicity of Heptavalent Pneumococcal Vaccine Conjugated to CRM197 in United States Infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 31.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: A phase I trial. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3270–5. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3(Suppl 4):S134–S138. doi: 10.3816/cbc.2003.s.002. [DOI] [PubMed] [Google Scholar]
- 33.Moase EH, Qi W, Ishida T, Gabos Z, Longenecker BM, Zimmermann GL, Ding L, Krantz M, Allen TM. Clinical outcome of breast and ovarian cancer patients treated with high- dose chemotherapy, autologous stem cell rescue and THERATOPE: STn-KLH cancer vaccine. Bone Marrow Transplant. 2000;25(12):1233–1241. doi: 10.1038/sj.bmt.1702430. [DOI] [PubMed] [Google Scholar]
- 34.Padlan EA, Kabat EA. Model-building study of the combining sites of two antibodies to alpha (1----6)dextran. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6885–9. doi: 10.1073/pnas.85.18.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Mensdorff-Pouilly S. Vaccine-induced antibody responses in patients with carcinoma. Expert Review of Vaccines. 2010;9:579–594. doi: 10.1586/erv.10.51. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Ravetch JV. Divergent Immunoglobulin G Subclass Activity Through Selective Fc Receptor Binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 37.Shenoy AM, Brahmi Z. Human IgG1 and mouse IgG3 but not monomeric IgM or IgE facilitate antibody-dependent cell-mediated cytotoxicity by human natural killer cells. Nat Immun Cell Growth Regul. 1989;8(6):338–48. [PubMed] [Google Scholar]
- 38.Michaelsen TE, Kolberg J, Aase A, et al. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scandinavian Journal of Immunology. 2004;59:34–39. doi: 10.1111/j.0300-9475.2004.01362.x. [DOI] [PubMed] [Google Scholar]
- 39.Morris PG, Hudis CA. Trastuzumab-Related Cardiotoxicity Following Anthracycline- Based Adjuvant Chemotherapy: How Worried Should We Be? Journal of Clinical Oncology. 2010;28:3407–3410. doi: 10.1200/JCO.2009.26.0125. [DOI] [PubMed] [Google Scholar]
- 40.Dausset J, Moullec J, Bernard J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn) Blood. 1959 Oct;14:1079–93. [PubMed] [Google Scholar]
- 41.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011 Feb 18;50(8):1770–91. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans. 2009 [PubMed] [Google Scholar]
- 43.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16613–8. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9228–33. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N- acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002 Apr 12;277(15):12802–9. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 46.Kurosawa N, Takashima S, Kono M, Ikehara Y, Inoue M, Tachida Y, et al. Molecular cloning and genomic analysis of mouse GalNAc alpha2, 6-sialyltransferase (ST6GalNAc I) J Biochem. 2000 May;127(5):845–54. doi: 10.1093/oxfordjournals.jbchem.a022678. [DOI] [PubMed] [Google Scholar]
- 47.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005 Oct 27;437(7063):1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 48.Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008 Mar 15;68(6):1636–46. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 49.Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006 Oct 13;314(5797):304–8. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 50.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007 Jun 11;204(6):1417–29. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vavasseur F, Dole K, Yang J, Matta KL, Myerscough N, Corfield A, et al. O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. Eur J Biochem. 1994 Jun 1;222(2):415–24. doi: 10.1111/j.1432-1033.1994.tb18880.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang JM, Byrd JC, Siddiki BB, Chung YS, Okuno M, Sowa M, et al. Alterations of O- glycan biosynthesis in human colon cancer tissues. Glycobiology. 1994 Dec;4(6):873–84. doi: 10.1093/glycob/4.6.873. [DOI] [PubMed] [Google Scholar]
- 53.Burchell JM, Mungul A, Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001 Jul;6(3):355–64. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- 54.Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, et al. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem. 2001 Apr 6;276(14):11007–15. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 55.Hammarstrom S, Murphy LA, Goldstein IJ, Etzler ME. Carbohydrate binding specificity of four N-acetyl-D-galactosamine- “specific” lectins: Helix pomatia A hemagglutinin, soy bean agglutinin, lima bean lectin, and Dolichos biflorus lectin. Biochemistry. 1977 Jun 14;16(12):2750–5. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- 56.Kjeldsen T, Clausen H, Hirohashi S, Ogawa T, Iijima H, Hakomori S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2----6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988 Apr 15;48(8):2214–20. [PubMed] [Google Scholar]
- 57.Takahashi HK, Metoki R, Hakomori S. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Res. 1988 Aug 1;48(15):4361–7. [PubMed] [Google Scholar]
- 58.Tollefsen SE, Kornfeld R. Isolation and characterization of lectins from Vicia villosa. Two distinct carbohydrate binding activities are present in seed extracts. J Biol Chem. 1983 Apr 25;258(8):5165–71. [PubMed] [Google Scholar]
- 59.Tollefsen SE, Kornfeld R. The B4 lectin from Vicia villosa seeds interacts with N- acetylgalactosamine residues alpha-linked to serine or threonine residues in cell surface glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5172–6. [PubMed] [Google Scholar]
- 60.Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer. 1997 Jul 3;72(1):119–27. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 61.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 62.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997 Aug;75(8):594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 63.Wang BL, Springer GF, Carlstedt SC. Quantitative computerized image analysis of Tn and T (Thomsen-Friedenreich) epitopes in prognostication of human breast carcinoma. J Histochem Cytochem. 1997 Oct;45(10):1393–400. doi: 10.1177/002215549704501007. [DOI] [PubMed] [Google Scholar]
- 64.Springer GF. Tn epitope (N-acetyl-D-galactosamine alpha-O-serine/threonine) density in primary breast carcinoma: a functional predictor of aggressiveness. Mol Immunol. 1989 Jan;26(1):1–5. doi: 10.1016/0161-5890(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 65.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989 Jan 1;49(1):197–204. [PubMed] [Google Scholar]
- 66.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev. 2000 Oct;14(4):312–25. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 67.Singhal A, Fohn M, Hakomori S. Induction of alpha-N-acetylgalactosamine-O- serine/threonine (Tn) antigen-mediated cellular immune response for active immunotherapy in mice. Cancer Res. 1991 Mar 1;51(5):1406–11. [PubMed] [Google Scholar]
- 68.O’Boyle KP, Zamore R, Adluri S, Cohen A, Kemeny N, Welt S, et al. Immunization of colorectal cancer patients with modified ovine submaxillary gland mucin and adjuvants induces IgM and IgG antibodies to sialylated Tn. Cancer Res. 1992 Oct 15;52(20):5663–7. [PubMed] [Google Scholar]
- 69.Adluri S, Helling F, Ogata S, Zhang S, Itzkowitz SH, Lloyd KO, et al. Immunogenicity of synthetic TF-KLH (keyhole limpet hemocyanin) and sTn-KLH conjugates in colorectal carcinoma patients. Cancer Immunol Immunother. 1995 Sep;41(3):185–92. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, Lloyd KO, et al. Specificity analysis of murine monoclonal antibodies reactive with Tn, sialylated Tn, T, and monosialylated (2-->6) T antigens. Hybridoma. 1996 Dec;15(6):401–8. doi: 10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- 71.Reddish MA, Jackson L, Koganty RR, Qiu D, Hong W, Longenecker BM. Specificities of anti-sialyl-Tn and anti-Tn monoclonal antibodies generated using novel clustered synthetic glycopeptide epitopes. Glycoconj J. 1997 Aug;14(5):549–60. doi: 10.1023/a:1018576224062. [DOI] [PubMed] [Google Scholar]
- 72.MacLean GD, Reddish M, Koganty RR, Wong T, Gandhi S, Smolenski M, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36(4):215–22. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer GF, Desai PR, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced human breast carcinoma. Cancer Biother. 1994 Spring;9(1):7–15. doi: 10.1089/cbr.1994.9.7. [DOI] [PubMed] [Google Scholar]
- 74.Springer GF, Desai PR, Spencer BD, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced breast carcinoma. Cancer Detect Prev. 1995;19(4):374–80. [PubMed] [Google Scholar]
- 75.Vichier-Guerre S, Lo-Man R, Bay S, Deriaud E, Nakada H, Leclerc C, et al. Short antigen. J Pept Res. 2000 Feb;55(2):173–80. doi: 10.1034/j.1399-3011.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- 76.Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol. 2001 Feb 15;166(4):2849–54. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 77.Vichier-Guerre S, Lo-Man R, BenMohamed L, Deriaud E, Kovats S, Leclerc C, et al. Induction of carbohydrate-specific antibodies in HLA-DR transgenic mice by a synthetic glycopeptide: a potential anti cancer vaccine for human use. J Pept Res. 2003 Sep;62(3):117–24. doi: 10.1034/j.1399-3011.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 78.Freire T, Lo-Man R, Piller F, Piller V, Leclerc C, Bay S. Enzymatic large-scale synthesis of MUC6-Tn glycoconjugates for antitumor vaccination. Glycobiology. 2006 May;16(5):390–401. doi: 10.1093/glycob/cwj082. [DOI] [PubMed] [Google Scholar]
- 79.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003 Dec 1;21(23):4292–8. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 80.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004 Jul 15;64(14):4987–94. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 81.Peri F, Cipolla L, Rescigno M, La Ferla B, Nicotra F. Synthesis and biological evaluation of an anticancer vaccine containing the C-glycoside analogue of the Tn epitope. Bioconjug Chem. 2001 May-Jun;12(3):325–8. doi: 10.1021/bc000143a. [DOI] [PubMed] [Google Scholar]
- 82.Cipolla L, Rescigno M, Leone A, Peri F, La Ferla B, Nicotra F. Novel Tn antigen- containing neoglycopeptides: synthesis and evaluation as anti tumor vaccines. Bioorg Med Chem. 2002 May;10(5):1639–46. doi: 10.1016/s0968-0896(01)00433-3. [DOI] [PubMed] [Google Scholar]
- 83.Buskas T, Ingale S, Boons GJ. Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated tn antigen. Angew Chem Int Ed Engl. 2005 Sep 19;44(37):5985–8. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 84.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007 Oct;3(10):663–7. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holmberg LA, Sandmaier BM. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004 Dec;3(6):655–63. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 86.Stepensky D, Tzehoval E, Vadai E, Eisenbach L. O-glycosylated versus non-glycosylated MUC1-derived peptides as potential targets for cytotoxic immunotherapy of carcinoma. Clin Exp Immunol. 2006 Jan;143(1):139–49. doi: 10.1111/j.1365-2249.2005.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryan SO, Gantt KR, Finn OJ. Tumor antigen-based immunotherapy and immunoprevention of cancer. Int Arch Allergy Immunol. 2007;142(3):179–89. doi: 10.1159/000097020. [DOI] [PubMed] [Google Scholar]
- 88.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004 May 28;117(5):677–87. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006 Nov;6(11):849–58. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 90.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993 Oct 15;262(5132):416–9. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 91.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc. 2009 Jul 22;131(28):9622–3. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 92.Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, et al. Mouse- human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull. 2008 Sep;31(9):1739–44. doi: 10.1248/bpb.31.1739. [DOI] [PubMed] [Google Scholar]
- 93.Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B. A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti tumour activity. Glycobiology. 2011 Apr 5; doi: 10.1093/glycob/cwr048. [DOI] [PubMed] [Google Scholar]
- 94.Poiroux G, Pitie M, Culerrier R, Segui B, Van Damme EJ, Peumans WJ, et al. Morniga G: a plant lectin as an endocytic ligand for photosensitizer molecule targeting toward tumor-associated T/Tn antigens. Photochem Photobiol. 2011 Mar-Apr;87(2):370–7. doi: 10.1111/j.1751-1097.2010.00858.x. [DOI] [PubMed] [Google Scholar]
- 95.Thomsen OZ. Ein vermehrungsfahiges Agens als verandererdes isoagglutinatorischen verhaltens der roten Blutkorperchen,eine bisher unbekannta Quelle der Fehlbestimmungen. Zeitschrift fuÈr ImmunitaÈts Forschung. 1927;52:85–90. [Google Scholar]
- 96.Friedenreich V. The Thomsen hemagglutination phenomenon. Levin and Munksgaard; Copenhagen, Denmark: 1930. [Google Scholar]
- 97.Julien S, Picco G, Sewell R, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santos-Silva F, Fonseca A, Caffrey T, et al. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology. 2005;15:511–517. doi: 10.1093/glycob/cwi027. [DOI] [PubMed] [Google Scholar]
- 99.Kovacic N, Muthing J, Marusic A. Immunohistological and flow cytometric analysis of glycosphingolipid expression in mouse lymphoid tissues. Journal of Histochemistry & Cytochemistry. 2000;48:1677–1690. doi: 10.1177/002215540004801211. [DOI] [PubMed] [Google Scholar]
- 100.de Bentzmann S, Roger P, Dupuit F, et al. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infection & Immunity. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shysh A, Eu SM, Noujaim AA, et al. In vivo localization of radioiodinated peanut lectin in a murine TA3/Ha mammary carcinoma model. European Journal of Nuclear Medicine. 1985;10:68–74. doi: 10.1007/BF00261767. [DOI] [PubMed] [Google Scholar]
- 102.Chaturvedi R, Heimburg J, Yan J, et al. Tumor immunolocalization using 124 I-iodine- labeled JAA-F11 antibody to Thomsen-Friedenreich alpha-linked antigen. Applied Radiation & Isotopes. 2008;66:278–287. doi: 10.1016/j.apradiso.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller GM, Andres ML, Gridley DS. NK cell depletion results in accelerated tumor growth and attenuates the antitumor effect of total body irradiation. International Journal of Oncology. 2003;23:1585–1592. [PubMed] [Google Scholar]
- 104.Rittenhouse-Diakun K, Xia Z, Pickhardt D, et al. Development and characterization of monoclonal antibody to T-antigen: (gal beta1–3GalNAc-alpha-O) Hybridoma. 1998;17:165–173. doi: 10.1089/hyb.1998.17.165. [DOI] [PubMed] [Google Scholar]
- 105.Heimburg J, Yan J, Morey S, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia (New York, NY) 2006;8:939–948. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu L-G. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconjugate Journal. 2007;24:411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 107.Springer GF, Desai PR, Scanlon EF. Blood group MN precursors as human breast carcinoma-associated antigens and “naturally” occurring human cytotoxins against them. Cancer. 1976;37:169–176. doi: 10.1002/1097-0142(197601)37:1<169::aid-cncr2820370124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 108.Springer GF, Desai PR, Murthy MS, Scanlon EF. Delayed-type skin hypersensitivity reaction (DTH) to Thomsen-Friedenreich (T) antigen as diagnostic test for human breast adenocarcinoma. Klinische Wochenschrift. 1979;57:961–963. doi: 10.1007/BF01478554. [DOI] [PubMed] [Google Scholar]
- 109.Springer GF, Desai PR, Murthy MS, Scanlon EF. Human carcinoma-associated precursor antigens of the NM blood group system. Journal of Surgical Oncology. 1979;11:95–106. doi: 10.1002/jso.2930110204. [DOI] [PubMed] [Google Scholar]
- 110.Springer GF, Desai PR, Murthy MS, et al. Human carcinoma-associated precursor antigens of the blood group MN system and the host’s immune responses to them. Progress in Allergy. 1979;26:42–96. [PubMed] [Google Scholar]
- 111.Springer GF, Desai PR, Murthy MS, et al. Precursors of the blood group MN antigens as human carcinoma-associated antigens. Transfusion. 1979;19:233–249. doi: 10.1046/j.1537-2995.1979.19379204204.x. [DOI] [PubMed] [Google Scholar]
- 112.Desai PR, Springer GF. Sialylation of Thomsen-Friedenreich(T) and Blood Type NM antigens by transferases in human sera measured by [14C]NANA uptake. International Journal of Immunogenetics. 1980;7:149–155. doi: 10.1111/j.1744-313x.1980.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 113.Springer GF, Murthy MS, Desai PR, Scanlon EF. Breast cancer patient’s cell-mediated immune response to Thomsen-Friedenreich (T) antigen. Cancer. 1980;45:2949–2954. doi: 10.1002/1097-0142(19800615)45:12<2949::aid-cncr2820451210>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 114.Springer GF, Tegtmeyer H. On the origin of anti-Thomsen-Friedenreich (T) antibodies. Naturwissenschaften. 1980;67:317–318. doi: 10.1007/BF01153511. [DOI] [PubMed] [Google Scholar]
- 115.Uhlenbruck G. The Thomsen-Friedenreich (TF) receptor: an old history with new mystery. Immunological Communications. 1981;10:251–264. doi: 10.3109/08820138109093459. [DOI] [PubMed] [Google Scholar]
- 116.Springer GF, Tegtmeyer H. Origin of Anti-Thomsen-Friedenreich (T) and Tn Agglutinins in Man and in White Leghorn Chicks. British Journal of Haematology. 1981;47:453–460. doi: 10.1111/j.1365-2141.1981.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 117.Springer GF, Desai PR. Extent of desialation of blood group MM, NN, and MN antigens required for reactivity with human anti-T antibody and Arachis hypogaea lectin. Journal of Biological Chemistry. 1982;257:2744–2746. [PubMed] [Google Scholar]
- 118.Springer GF, Desai PR. Detection of lung- and breast carcinoma by quantitating serum anti-T IgM levels with a sensitive, solid-phase immunoassay. Naturwissenschaften. 1982;69:346–348. doi: 10.1007/BF00480463. [DOI] [PubMed] [Google Scholar]
- 119.Springer GF, Desai PR. Similarity between Ca antigen and Thomsen-Friedenreich antigen. Lancet. 1982;2:660. doi: 10.1016/s0140-6736(82)92761-1. [DOI] [PubMed] [Google Scholar]
- 120.Springer GF, Murthy SM, Desai PR, et al. Patients’ immune response to breast and lung carcinoma-associated thomsen-friedenreich (T) specificity. Journal of Molecular Medicine. 1982;60:121–131. doi: 10.1007/BF01711276. [DOI] [PubMed] [Google Scholar]
- 121.Fry WA, Springer GF, Desai PR. Lung cancer patients’ autoimmune responses to Thomsen-Friedenreich (T) antigen: diagnostic utility. Klinische Wochenschrift. 1983;61:817–818. doi: 10.1007/BF01496727. [DOI] [PubMed] [Google Scholar]
- 122.Goodale RL, Springer GF, Shearen JG, et al. Delayed-type cutaneous hypersensitivity to Thomsen-Friedenreich (T) antigen in patients with pancreatic cancer. Journal of Surgical Research. 1983;35:293–297. doi: 10.1016/0022-4804(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 123.Ignatoff JM, Springer GF, Blum MD, et al. Patients mount an autoimmune response to their bladder cancer-associated t antigen. Naturwissenschaften. 1983;70:42. doi: 10.1007/BF00365959. [DOI] [PubMed] [Google Scholar]
- 124.Schirrmacher V, Dzarlieva R, Altevogt P, et al. Phenotypic and genotypic differences between high- and low-metastatic related tumor lines and the problem of tumor progression and variant generation. Symposium on Fundamental Cancer Research. 1983;36:81–99. [PubMed] [Google Scholar]
- 125.Springer GF. Blood group T and Tn antigens are universal, clonal, epithelial cell-adhesive, autoimmunogenic carcinoma markers. Progress in Clinical & Biological Research. 1983;133:157–166. [PubMed] [Google Scholar]
- 126.Springer GF. Tn and T blood group precursor antigens are universal, clonal, epithelial cell-adhesive, autoimmunogenic carcinoma (CA) markers. Naturwissenschaften. 1983;70:369–370. doi: 10.1007/BF00444219. [DOI] [PubMed] [Google Scholar]
- 127.Springer GF, Desai PR, Fry WA, et al. T antigen, a tumor marker against which breast, lung and pancreas carcinoma patients mount immune responses. Cancer Detection & Prevention. 1983;6:111–118. [PubMed] [Google Scholar]
- 128.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 129.Springer GF, Fry WA, Desai PR, et al. Further studies on the detection of early lung and breast carcinoma by T antigen. Cancer Detection & Prevention. 1985;8:95–100. [PubMed] [Google Scholar]
- 130.Springer GF, Desai PR, Robinson MK, et al. The fundamental and diagnostic role of T and Tn antigens in breast carcinoma at the earliest histologic stage and throughout. Progress in Clinical & Biological Research. 1986;204:47–70. [PubMed] [Google Scholar]
- 131.Clausen H, Stroud M, Parker J, et al. Monoclonal antibodies directed to the blood group a associated structure, galactosyl-A: Specificity and relation to the thomsen-friedenreich antigen. Molecular Immunology. 1988;25:199–204. doi: 10.1016/0161-5890(88)90068-5. [DOI] [PubMed] [Google Scholar]
- 132.Thomson DM, Springer GF, Desai PR, et al. Comparison by leukocyte adherence inhibition of human immune response to cancer-associated immunogens, Thomsen- Friedenreich (T) and Tn, myelin basic protein, and organ-specific cancer neoantigens. Clinical Immunology & Immunopathology. 1988;49:231–241. doi: 10.1016/0090-1229(88)90113-4. [DOI] [PubMed] [Google Scholar]
- 133.Desai PR, Ujjainwala LH, Carlstedt SC, Springer GF. Anti-Thomsen-Friedenreich (T) antibody-based ELISA and its application to human breast carcinoma detection. Journal of Immunological Methods. 1995;188:175–185. doi: 10.1016/0022-1759(95)00246-4. [DOI] [PubMed] [Google Scholar]
- 134.Springer GF, Desai PR, Ghazizadeh M, Tegtmeyer H. T/Tn pancarcinoma autoantigens: fundamental, diagnostic, and prognostic aspects. Cancer detection and prevention. 1995;19:173–182. [PubMed] [Google Scholar]
- 135.Springer GF. T and Tn pancarcinoma markers: autoantigenic adhesion molecules in pathogenesis, prebiopsy carcinoma-detection, and long-term breast carcinoma immunotherapy. Critical Reviews in Oncogenesis. 1995;6:57–85. doi: 10.1615/critrevoncog.v6.i1.50. [DOI] [PubMed] [Google Scholar]
- 136.Springer GF, Desai PR, Spencer BD, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced breast carcinoma. Cancer detection and prevention. 1995;19:374–380. [PubMed] [Google Scholar]
- 137.Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochemistry & Cell Biology. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- 138.Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model [Erratum appears in Int J Cancer 1997 Sep 4;72(5):918] International Journal of Cancer. 1997;72:119–127. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 139.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. Journal of Molecular Medicine. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 140.Wang B-L, Springer GF, Carlstedt SC. Quantitative Computerized Image Analysis of Tn and T (Thomsen–Friedenreich) Epitopes in Prognostication of Human Breast Carcinoma. Journal of Histochemistry & Cytochemistry. 1997;45:1393–1400. doi: 10.1177/002215549704501007. [DOI] [PubMed] [Google Scholar]
- 141.Wang BL, Springer GF, Harwick LC. T (Thomsen-Friedenreich) and Tn epitope location and their spatial relations to adhesion plaques on human breast carcinoma cells: immunogold-silver staining studies at scanning electron microscopic level. Journal of submicroscopic cytology and pathology. 1998;30:503–509. [PubMed] [Google Scholar]
- 142.Kishikawa T, Ghazizadeh M, Sasaki Y, Springer GF. Specific role of T and Tn tumor- associated antigens in adhesion between a human breast carcinoma cell line and a normal human breast epithelial cell line. Japanese Journal of Cancer Research. 1999;90:326–332. doi: 10.1111/j.1349-7006.1999.tb00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garratty G. A tribute to Georg Springer, MA, MD, DSc (Hon)--Who laid a foundation for using the relationship of blood groups to disease as a basis for the diagnosis and treatment of patients. Transfusion Medicine Reviews. 2000;14:289–290. doi: 10.1053/tmrv.2000.16226. [DOI] [PubMed] [Google Scholar]
- 144.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prog]nosis, and immunotherapy. Transfusion Medicine Reviews. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 145.Abdi EA, Kamitomo VJ, McPherson TA, et al. Radioiodinated peanut lectin: Clinical use as a tumour-imaging agent and potential use in assessing renal-tubular function. European Journal of Nuclear Medicine and Molecular Imaging. 1986;11:350–354. doi: 10.1007/BF00253300. [DOI] [PubMed] [Google Scholar]
- 146.Baldus SE, Hanisch FG, Kotlarek GM, et al. Coexpression of MUC1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer. 1998;82:1019–1027. doi: 10.1002/(sici)1097-0142(19980315)82:6<1019::aid-cncr3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 147.Demichelis SO, Alberdi CG, Servi WJ, et al. Comparative immunohistochemical study of MUC1 and carbohydrate antigens in breast benign disease and normal mammary gland. Applied Immunohistochemistry & Molecular Morphology. 2010;18:41–50. doi: 10.1097/PAI.0b013e3181ac1c20. [DOI] [PubMed] [Google Scholar]
- 148.Kannan S, Lakku RA, Niranjali D, et al. Expression of peanut agglutinin-binding mucin- type glycoprotein in human esophageal squamous cell carcinoma as a marker. Molecular Cancer. 2003;2:38–48. doi: 10.1186/1476-4598-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cao Y, Karsten UR, Liebrich W, et al. Expression of thomsen-friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76:1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 150.Cao Y, Merling A, Karsten U, et al. Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. International Journal of Cancer. 2008;123:89–99. doi: 10.1002/ijc.23493. [DOI] [PubMed] [Google Scholar]
- 151.Ryder SD, Smith JA, Rhodes JM. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. Journal of the National Cancer Institute. 1992;84:1410–1416. doi: 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- 152.Gabius HJ, Schroter C, Gabius S, et al. Binding of T-antigen-bearing neoglycoprotein and peanut agglutinin to cultured tumor cells and breast carcinomas. Journal of Histochemistry & Cytochemistry. 1990;38:1625–1631. doi: 10.1177/38.11.2212620. [DOI] [PubMed] [Google Scholar]
- 153.Rhodes JM, Campbell BJ, Yu L-G. Lectin-epithelial interactions in the human colon. Biochemical Society Transactions. 2008;36:1482–1486. doi: 10.1042/BST0361482. [DOI] [PubMed] [Google Scholar]
- 154.Yu LG, Milton JD, Fernig DG, Rhodes JM. Opposite effects on human colon cancer cell proliferation of two dietary Thomsen-Friedenreich antigen-binding lectins. Journal of Cellular Physiology. 2001;186:282–287. doi: 10.1002/1097-4652(200102)186:2<282::AID-JCP1028>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 155.Campbell BJ, Finnie IA, Hounsell EF, Rhodes JM. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. The Journal of Clinical Investigation. 1995;95:571–576. doi: 10.1172/JCI117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu L-G, Andrews N, Zhao Q, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. Journal of Biological Chemistry. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 157.Gassmann P, Kang M-L, Mees ST, Haier J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell--endothelial cell interaction. BMC Cancer. 2010;10:177. doi: 10.1186/1471-2407-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park Y-S, Bae J-H, Son C-H, et al. Cyclophosphamide Potentiates the Antitumor Effect of Immunization with Injection of Immature Dendritic Cells into Irradiated Tumor. Immunological Investigations. 2011;40:383–399. doi: 10.3109/08820139.2011.552141. [DOI] [PubMed] [Google Scholar]
- 160.Cummings Richard D, Varki Adjit, Esko Jeffery D, Stanley Pamela, Bertozzi Carolyn, Hart Gerald, Etzler Marilynn E. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. [PubMed] [Google Scholar]
- 161.Singh R, Campbell BJ, Yu LG, et al. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology. 2001;11:587–592. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- 162.Klaamas K, Kurtenkov O, Rittenhouse-Olson K, et al. Expression of Tumor-Associated of Tumor-Associated Thomsen-Friedenreich Antigen (T Ag) in Helicobacter Ag in Helicobacter Pylori and Modulation of T Ag Specific Helicobacter Pylori and Modulation of T Ag Specific Immune Response of Infected Individuals. Immunological Investigations. 2002;31:191–204. doi: 10.1081/imm-120016240. [DOI] [PubMed] [Google Scholar]
- 163.Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, et al. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: Relation to survival. Acta Oncologica. 2007;46:316–323. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 164.Smorodin EP, Kurtenkov OA, Sergeyev BL, Chuzmarov VI, Afanasyev VP. The relation of serum anti-(GalNAc beta) and -para-Forssman disaccharide IgG levels to the progression and histological grading of gastrointestinal cancer. Experimental Oncology. 2007;29:61–66. [PubMed] [Google Scholar]
- 165.Kurtenkov O, Klaamas K, Rittenhouse-Olson K, Vahter L, Sergejev B, Miljukhina L, Shljapnikova L. IgG immune response to tumor-associated carbohydrate antigens (TF, Tn, alphaGal) in patients with breast cancer: impact of neoadjuvant chemotherapy and relation to the survival. Experimental Oncology. 2005;27:136–140. [PubMed] [Google Scholar]
- 166.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Research. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 167.Baldus SE, Zirbes TK, Glossmann J, et al. Immunoreactivity of monoclonal antibody BW835 represents a marker of progression and prognosis in early gastric cancer. Oncology. 2001;61:147–155. doi: 10.1159/000055366. [DOI] [PubMed] [Google Scholar]
- 168.Chung YS, Yamashita Y, Kato Y, et al. Prognostic significance of T antigen expression in patients with gastric carcinoma. Cancer. 1996;77:1768–1773. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1768::AID-CNCR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 169.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. Journal of Pathology. 1996;179:358–369. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 170.Fukatsu H, Nonomura H, Miyagawa Y, et al. The study on cell surface antigens in epithelial tumor of the upper urinary tract: ABH-isoantigen and Thomsen-Friedenreich antigen. Hinyokika Kiyo - Acta Urologica Japonica. 1989;35:949–954. [PubMed] [Google Scholar]
- 171.Ghazizadeh M, Oguro T, Sasaki Y, et al. Immunohistochemical and ultrastructural localization of T antigen in ovarian tumors. American Journal of Clinical Pathology. 1990;93:315–321. doi: 10.1093/ajcp/93.3.315. [DOI] [PubMed] [Google Scholar]
- 172.Juhl BR. Blood group antigens in transitional cell tumours of the urinary bladder. An immunohistochemical study. Danish Medical Bulletin. 1994;41:1–11. [PubMed] [Google Scholar]
- 173.Takanami I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncology Reports. 1999;6:341–344. doi: 10.3892/or.6.2.341. [DOI] [PubMed] [Google Scholar]
- 174.Wolf MF, Ludwig A, Fritz P, Schumacher K. Increased expression of Thomsen- Friedenreich antigens during tumor progression in breast cancer patients. Tumor Biology. 1988;9:190–194. doi: 10.1159/000217561. [DOI] [PubMed] [Google Scholar]
- 175.Schindlbeck C, Jeschke U, Schulze S, et al. Characterisation of disseminated tumor cells in the bone marrow of breast cancer patients by the Thomsen-Friedenreich tumor antigen. Histochemistry & Cell Biology. 2005;123:631–637. doi: 10.1007/s00418-005-0781-6. [DOI] [PubMed] [Google Scholar]
- 176.Moriyama H, Nakano H, Igawa M, Nihira H. T antigen expression in benign hyperplasia and adenocarcinoma of the prostate. Urologia internationalis. 1987;42:120–123. doi: 10.1159/000281868. [DOI] [PubMed] [Google Scholar]
- 177.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. Journal of the National Cancer Institute. 1998;90:118–123. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 178.Fung PY, Madej M, Koganty RR, Longenecker BM. Active specific immunotherapy of a murine mammary adenocarcinoma using a synthetic tumor-associated glycoconjugate. Cancer Research. 1990;50:4308–4314. [PubMed] [Google Scholar]
- 179.MacLean GD, Bowen-Yacyshyn MB, Samuel J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen-Friedenreich) determinant using a synthetic carbohydrate antigen. Journal of Immunotherapy. 1992;11:292–305. doi: 10.1097/00002371-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 180.Adluri S, Helling F, Ogata S, et al. Immunogenicity of synthetic TF-KLH (keyhole limpet hemocyanin) and sTn-KLH conjugates in colorectal carcinoma patients. Cancer Immunology Immunotherapy. 1995;41:185–192. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Slovin SF, Ragupathi G, Fernandez C, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunology, Immunotherapy. 2007;56:1921–1930. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu Y, Gendler SJ, Franco A. Designer Glycopeptides for Cytotoxic T Cell–based Elimination of Carcinomas. The Journal of Experimental Medicine. 2004;199:707–716. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heimburg-Molinaro J, Almogren A, Morey S, et al. Development, Characterization, and Immunotherapeutic Use of Peptide Mimics of the Thomsen-Friedenreich Carbohydrate Antigen. Neoplasia. 2009;11:780–792. doi: 10.1593/neo.09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fukuda M. Carbohydrate-dependent cell adhesion. Bioorg Med Chem. 1995;3:207–15. doi: 10.1016/0968-0896(95)00014-8. [DOI] [PubMed] [Google Scholar]
- 185.Yan L, Lin B, Gao L, Gao S, Liu C, Wang C, Wang Y, Zhang S, Iwamori M. Lewis (y) antigen overexpression increases the expression of MMP-2 and MMP-9 and invasion of human ovarian cancer cells. Int J Mol Sci. 2010;11:4441–51. doi: 10.3390/ijms11114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moehler TM, Sauer S, Witzel M, Andrulis M, Garcia-Vallejo JJ, Grobholz R, Willhauck-Fleckenstein M, Greiner A, Goldschmidt H, Schwartz-Albiez R. Involvement of α1-2-fucosyltransferase I (FUT1) and surface-expressed Lewisy (CD174) in first endothelial cell-cell contacts during angiogenesis. J Cell Physiol. 2008;215:27–36. doi: 10.1002/jcp.21285. [DOI] [PubMed] [Google Scholar]
- 187.D’Adamo PJ, Kelly GS. Metabolic and immunologic consequences of ABH secretor and Lewis subtype status. Altern Med Rev. 2001;6:390–405. [PubMed] [Google Scholar]
- 188.Nishihara S, Iwasaki H, Kaneko M, Tawada A, Ito M, Narimatsu H. α1,3-Fucosyltrasferase 9 (FUT9; Fuc-TIX) preferentially fucosylates the distal GlcNAc residue of polylactosamine chain while the other four α1,3FUT members preferentially fucosylate the inner GlcNAc residue. FEBS Letters. 1999;462:289–94. doi: 10.1016/s0014-5793(99)01549-5. [DOI] [PubMed] [Google Scholar]
- 189.Azuma Y, Kurusu Y, Sato H, Higai K, Matsumoto K. Increased expression of Lewis x and y antigens on the cell surface and FUT4 mRNA during granzyme B-induced Jurkat cell apoptosis. Biol Pharm Bull. 2007;30:655–60. doi: 10.1248/bpb.30.655. [DOI] [PubMed] [Google Scholar]
- 190.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR, Tew WP, Konner J, Clausen H, Rustum NA, Danishefsky SJ, Livingston PO. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 191.Isla Larrain M, Demichelis S, Crespo M, Lacunza E, Barbera A, Creton A, Terrier F, Segal-Eiras A, Croce MV. Breast cancer humoral immune response: involvement of Lewis y through the detection of circulating immune complexes and association with Mucin I (MUC I) J Exp Clin Cancer Res. 2009;28:121. doi: 10.1186/1756-9966-28-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Myers R, Srivastava S, Grizzle WE. Lewis Y antigen as detected by the monoclonal antibody BR96 is expressed strongly in prostatic adenocarcinoma. J Urol. 1995;153:1572–4. [PubMed] [Google Scholar]
- 193.Waldock A, Ellis IO, Armitage N, Turner DR, Hardcastle JD, Embleton J. Differential expression of the Lewis antigen defined by monoclonal antibody C14/1/46/10 in colonic polyps. Cancer. 1989;15:414–21. doi: 10.1002/1097-0142(19890715)64:2<414::aid-cncr2820640213>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 194.Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L, Ball D, Michael M, Burman A, Mayura-Guru P, Trapani JA, Peinert S, Honemann D, Prince HM, Scott AM, Smyth MJ, Darcy PK, Kershaw MK. The Lewis-y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J Immunother. 2009;32:292–301. doi: 10.1097/CJI.0b013e31819b7c8e. [DOI] [PubMed] [Google Scholar]
- 195.Yuriev E, Farrugia W, Scott AM, Ramsland PA. Three-dimensional structures of carbohydrate determinants of Lewis system antigens: implications for effective antibody targeting of cancer. Immunol Cell Biol. 2005;83:709–17. doi: 10.1111/j.1440-1711.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 196.Motzer RJ, Reuter VE, Cordon-Cardo C, Bosl GJ. Blood group-related antigens in human germ cell tumors. Cancer Res. 1988;48:5342–7. [PubMed] [Google Scholar]
- 197.Hellstrom I, Garrigues J, Garrigues U, Hellstrom KE. Highly tumor-reactive, internalizing, mouse monoclonal antibody to Ley-related cell surface antigens. Cancer Res. 1990;50:2183–90. [PubMed] [Google Scholar]
- 198.Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–6. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 199.Williams S, Stanley P. Complex N-glycans or core 1-derived O-glycans are not required for the expression of stage-specific antigens SSEA-1, SSEA-3, SSEA-4, or LeY in the preimplantation mouse embryo. Glycoconj J. 2009;26:335–47. doi: 10.1007/s10719-008-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dettke M, Palfi G, Loibner H. Activation-dependent expression of the blood group-related Lewis Y antigen on peripheral blood granulocytes. J Leukoc Biol. 2000;68:511–4. [PubMed] [Google Scholar]
- 201.Buskas T, Li Y, Boons G-J. The immunogenicity of the tumor-associated antigen Lewisy may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem Eur J. 2004;10:3517–24. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 202.Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O’Flaherty C, Curtin J, Lloyd KO. Immunization of ovarian cancer patients with a synthetic LewisY-protein conjugate vaccine:a phase I trial. Int J Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 203.Ragupathi G, Damani P, Srivastava G, Srivastava O, Sucheck S, Ichikawa Y, Livinton P. Synthesis of Sialyl Lewis a (sLea, CA 19–9) And Construction of An Immunogenic sLea Vaccine. Cancer Immunology and Immunotherapy. 2009;58(9):1397–405. doi: 10.1007/s00262-008-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Monzavi-Larbassi B, Cunto-Amesty G, Luo P, Shamloo S, Blaszcyk-Thurin M, Keiber-Emmons T. Immunization with a carbohydrate mimicking peptide augments tumor-specific cellular responses. International Immunology. 2001;13(11):1361–71. doi: 10.1093/intimm/13.11.1361. [DOI] [PubMed] [Google Scholar]
- 205.Ohyama C, Tsuboi S, Fukuda M. Dual Roles of Sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. The EMBO Journal. 1999;18(6):1516–25. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hakomori S. Tumor-Associated Carbohydrate Antigens Defining Tumor Malignancy: Basis For Development of Anti-Cancer Vaccines. In: Wu A, editor. The Molecular Immunology of Complex Carbohydrates 2. Kluwer Academic/Plenum; 1999. pp. 369–402. [DOI] [PubMed] [Google Scholar]
- 207.Ravindranath MH, Amiri AA, Bauer PM, Kelley MC, Essner R, Morton DL. Endothelial-Selectin Ligands Sialyl Lewis x and Sialyl Lewisa Are Differentiation Antigens Immunogenic in Human Melanoma. Cancer. 1997;79(9):1686–97. doi: 10.1002/(sici)1097-0142(19970501)79:9<1686::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 208.Fukuda M, Hiraoka N, Yeh J-C. C-Type Lectins and Sialyl Lewis X Oligosaccharides: Versatile Roles in Cell-Cell Interaction. The Journal of Cell Biology. 1999;147(3):467–70. doi: 10.1083/jcb.147.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Allahverdian S, Wang A, Singhera GK, Wong BW, Dorsheid DR. Sialyl Lewis X modification of the epidermal growth factor receptor regulates receptor functionduring airway epithelial wound repair. Clinical and Experimental Allergy. 2010;40:607–18. doi: 10.1111/j.1365-2222.2010.03455.x. [DOI] [PubMed] [Google Scholar]
- 210.Ugorski M, Laskowska A. Sialyl Lewis a: a tumor-associated carbohydrate antigen involved in adhesion and metastatic potential of cancer cells. Acta Biochimica Polonica. 2002;49(2):303–11. [PubMed] [Google Scholar]
- 211.Groves RW, Allen MH, Ross EL, Ahsan G, Barker JNWN, MacDonald DM. Expression of Selectin Ligands by Cutaneous Squamous Cell Carcinoma. American Journal of Pathology. 1993;143(4):1220–25. [PMC free article] [PubMed] [Google Scholar]
- 212.Larsen GR, Sako D, Ahern TJ, Shaffer M. P-selectin and E-selectin Distinct But Overlapping Leukocyte Ligand SPecificities. The Journal of Biological Chemistry. 1992;267(16):11104–10. [PubMed] [Google Scholar]
- 213.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of Carbohydrate Antigens Sialyl Lewis A and Sialyl Lewis X to Adhesion of Human Cancer Cells to Vascular Endothelium. Cancer Research. 1993;53(2):354–61. [PubMed] [Google Scholar]
- 214.Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. Journal of Clinical Pathology. 2010;63(4):322–29. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 215.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Science. 2004;95(5):377–84. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kannagi R. Carbohydrate Antigen Sialyl Lewis a – Its Pathophysiological Significance and Induction Mechanism in Cancer Progression. Chang Gung Medical Journal. 2007;30(3):189–209. [PubMed] [Google Scholar]
- 217.Kannagi R. Molecular Mechanism for cancer-associated induction of Sialyl Lewis X and Sialyl Lewis A expression - The Warburg effect revisited. Glycoconjugate Journal. 2004;20:353–64. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- 218.Morton DL, Foshag LJ, Hoon DSB, Nizze JA, Wanek LA, Chang C, Davtyan DG, Gupta RK, Elashoff R, Irie RF. Prolongation of Survival in Metastatic Melanoma After Active Specific Immunotherapy With a New Polyvalent Melanoma Vaccine. Annals of Surgery. 1992;216(4):463–82. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ravindranath MH, Kelley MC, Jones RC, Amiri AA, Bauer PM, Morton DL. Ratio of IgG: IgM Antibodies To Sialy; Lewis X And GM3 Correlates With Tumor Growth After Immunization With Melanoma-Cell Vaccine With Different Adjuvants In Mice. International Journal of Cancer. 1998;75:117–24. doi: 10.1002/(sici)1097-0215(19980105)75:1<117::aid-ijc18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 220.Kieber-Emmons T, Luo P, Qiu J, Chang TYOI, Blaszcyk-Thurin M, Steplewski Z. Vaccination with carbohydrate peptide minotpoes promotes anti-tumor responses. Nature Bioltechnology [Research] 1999 July;17:660–65. doi: 10.1038/10870. [DOI] [PubMed] [Google Scholar]
- 221.Hittelet A, Camby Isabelle, Nagy Nathalie, Legendre Hugues, Bronkart Yves, Decaestecker Christine, Kaltner Herbert, Nifant’ev Nikolay E, Bovin Nicolai V, Pector Jean-Claude, Salmon Isabelle, Gabius Hans-Joachim, Kiss Robert, Yeon Paul. Binding Sites for Lewis Antigens are Expressed by Human Colon Cancer Cells and Negatively Affect Their Migration. Laboratory Investigation. 2003;83(6):777–87. doi: 10.1097/01.lab.0000073129.62433.39. [DOI] [PubMed] [Google Scholar]
- 222.Yanagisawa M. Stem Cell Glycolipids. Neurochemical Research. 2010 doi: 10.1007/s11064-010-0358-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 223.Elola MT, Capurro Mariana Isabel, Barrio Maria Marcela, Coombs Peter J, Taylor Maureen E, Drickamer Kurt, Mordon Jose. Lewis X Antigen Mediates Adhesion of Human Breast Carcinoma Cells to Activated Endothelium: Possible Involvement of the Endothelial Scavenger Receptor C-type Lectin. Breast Cancer Research and Treatment. doi: 10.1007/s10549-006-9286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kerr MA, Craig Stocks S. The Role of CD15-(Lex)-related Carbohydrates in Neutrophil Adhesion. Histochemical Journal. 1992;24:811–26. doi: 10.1007/BF01046353. [DOI] [PubMed] [Google Scholar]
- 225.Hakomori S-i, Nudelman Edward, Levery Steven, Solter Davor, Knowles Barbara B. The Hapten Structure of a Developmentally Regulated Glycolipid Antigen (SSEA-1) Isolated From Human Erythrocytes and Adenocarcinoma: A Preliminary Note. Biochemical and Biophysical Research Communications. 1981;100(4):1578–86. doi: 10.1016/0006-291x(81)90699-9. [DOI] [PubMed] [Google Scholar]
- 226.Solter D, Knowles Barbara B. Monoclonal Antibody Defining a Stage-specific Mouse Embryonic Antigen (SSEA-1) PNAS. 1978;75(11):5565–9. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gooi HC, Feizi T, Kapadia A, Knowles BB, Solter D, Evans MJ. Stage-specific Embryonic Antigen Involves a1–3 Fucosylated Blood Group Chains. Nature. 1981;292:156–8. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- 228.Childs RA, Pennington Jane, Uemura Kei-ichi, Scudder Peter, Goodfellow Peter N, Evans Martin J, Feizi Ten. High-molecular-weight Glycoproteins are the Major Carriers of the Carbohydrate Antigens I, i, and SSEA-1 of Mouse Teratocarcinoma Cells. Biochemical Journal. 1983;215:491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fenderson BA, Zehavi Uri, Hakomori Sen-Itiroh. A Multivalent Lacto-N-Fucopentaose III-Lysyllysine Conjugate Decompacts Preimplamtation Mouse Embryos, While the Free Oligosaccharide is Ineffective. Journal of Experimental Medicine. 1984;160:1591–6. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Johnston DS, Wright William W, Shaper Joel H, Hokke Cornelius H, Van den Eijnden Dirk H, Joziasse David H. Murine Sperm-Zona Binding, A Fucosyl Residue Is Required for a High Affinity Sperm-binding Ligand. The Journal of Biological Chemistry. 1998;273(4):1888–95. doi: 10.1074/jbc.273.4.1888. [DOI] [PubMed] [Google Scholar]
- 231.Kudo T, Kaneko Mika, Iwasaki Hiroko, Togayachi Akira, Nishihara Shoko, Abe Kuniya, Narimatsu Hisashi. Normal Embryonic and Germ Cell Development in Mice Lacking a1,3-Fucosyltransferase IX (Fut9) Which Show Disappearance of Stage-Specific Embryonic Antigen 1. Molecular and Cellular Biology. 2004;24(10):4221–8. doi: 10.1128/MCB.24.10.4221-4228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.D’Costa S, Petitte James N. Characterization of Stage-specific Embryonic Antigen-1 (SSEA-1) Expression During Early Development of the Turkey Embryo. International Journal of Developmental Biology. 1999;43:349–56. [PubMed] [Google Scholar]
- 233.Allendoerfer KL, Durairaj Arun, Matthews Glennis A, Patterson Paul H. Morphological Domains of Lewis-X/FORSE-1 Immunolabeling in the Embryonic Neural Tube Are Due to Developmental Regulation of Cell Surface Carbohydrate Expression. Developmental Biology. 1999;211:208–19. doi: 10.1006/dbio.1999.9308. [DOI] [PubMed] [Google Scholar]
- 234.Yoshida-Noro C, Heasna Janet, Goldstone Kim, Vickers Lucinda, Wylie Chris. Expression of Lewis Group Carbohydrate Antigens During Xenopus Development. Glycobiology. 1999;9(12):1323–30. doi: 10.1093/glycob/9.12.1323. [DOI] [PubMed] [Google Scholar]
- 235.Brito C, Danglot Lydia, Galli Thierry, Costa Julia. Subcellular Localization of the Carbohydrate Lewisx Adhesion Structure in Hippocampus Cell Cultures. Brain Research. 2009;1287:39–46. doi: 10.1016/j.brainres.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 236.Spooncer E, Fukuda Minoru, Klock John C, Oates Jane E, Dell Anne. Isolation and Characterization of Polyfucosylated Lactosaminoglycan from Human Granulocytes. The Journal of Biological Chemistry. 1984;259(8):4792–801. [PubMed] [Google Scholar]
- 237.Skubitz KM, Snook Robert W., II Monoclonal Antibodies That Recognize Lacto-N-Fucopentaose III (CD15) React with the Adhesion-Promoting Glycoprotein Family (LFA-1/HMAC-1/GP 150,95) and CR1 on Human Neutrophils. The Journal of Immunology. 19887;139(5):1631–9. [PubMed] [Google Scholar]
- 238.Stocks SC, Albrechtsen Merete, Kerr Michael A. Expression of the CD15 Differentiation Antigen (3-fucosyl-N-acetyl-lactosamine, LeX) on Putative Neutrophil Adhesion Molecules CR3 and NCA-160. Biochemical Journal. 1990;268:275–80. doi: 10.1042/bj2680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Stocks SC, Kerr Michael A. Stimulation of Neutrophil Adhesion by Antibodies Recognizing CD15 (LeX) and CD-15-expressing Carcinoembryonic Antigen-related Glycoprotein NCA-160. Biochemical Journal. 1992;288:23–7. doi: 10.1042/bj2880023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nakayama F, Nishihara Shoko, Iwasaki Hiroko, Kudo Takashi, Okubo Reiko, Kaneko Mika, Nakamura Mitsuru, Karube Masataka, Sasaki Katsutoshi, Narimatsu Hisashi. CD15 Expression in Mature Granulocytes Is Determined by a1,3-Fucosyltransferase IX, but in Promyelocytes and Monocytes by a1,3-Fucosyltransferase IV. The Journal of Biological Chemistry. 2001;276(19):16100–6. doi: 10.1074/jbc.M007272200. [DOI] [PubMed] [Google Scholar]
- 241.Wang W, Hu Tianshun, Frantom Patrick A, Zheng Tianqing, Gerwe Brian, Soriano del Amo David, Garret Sarah, Seidel Ronald D, III, Wu Peng. Chemoenzymatic Synthesis of GDP-L-fucose and the Lewis X Glycan Derivatives. PNAS. 2009;106(38):16096–101. doi: 10.1073/pnas.0908248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ko AI, Drager Ursula C, Harn Donald A. A Schistosoma mansoni Epitope Recognized by a Protective Monoclonal Antibody is Identical to the Stage-specific Embryonic Antigen 1. PNAS. 1990;87:4159–63. doi: 10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sherburne R, Taylor Diane E. Helicobacter pylori Expresses a Complex Surface Carbohydrate, Lewis X. Infection and Immunity. 1995;63(12):4564–8. doi: 10.1128/iai.63.12.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fox N, Damjanov Ivan, Knowles Barbara B, Solter Davor. Immunohistochemical Localization of the Mouse Stage-specific Embryonic Antigen 1 in Human Tissues and Tumors. Cancer Research. 1983;43:669–78. [PubMed] [Google Scholar]
- 245.Shi ZR, McIntyre Laurence J, Knowles Barbara B, Solter Davor, Kim Young S. Expression of a Carbohydrate Differentiation Antigen, Stage-specific Embryonic Antigen 1, in Human Colonic Adenocarcinoma. Cancer Research. 1984;44:1142–7. [PubMed] [Google Scholar]
- 246.Itzkowitz SH, SHi ZR, Kim YS. Heterogeneous Expression of Two Oncodevelopmental Antigens, CEA and SSEA-1, in Colorectal Cancer. Histochemical Journal. 1986;18(4):155–63. doi: 10.1007/BF01676115. [DOI] [PubMed] [Google Scholar]
- 247.Kajiwara H, Yasuda Masanori, Kumaki Nobue, Shibayama Taro, Osamura Yoshiyuki. Expression of Carbohydrate Antigens (SSEA-1, Sialyl-Lewis X, DU-PAN-2 and CA19–9) and E-selectin in Urothelial Carcinoma of the Renal Pelvis, Ureter, and Urinary Bladder. The Tokai Journal of Experimental and Clinical Medicine. 2005;30(3):177–82. [PubMed] [Google Scholar]
- 248.Konety BR, Ballou Byron, Jaffe Ronald, Singh Jagjit, Reiland Jean, Hakala Thomas R. Expression of SSEA-1 (Lewisx) on Transitional Cell Carcinoma of the Bladder. Urologia Internationalis. 1996;58:69–74. doi: 10.1159/000282953. [DOI] [PubMed] [Google Scholar]
- 249.Sheinfeld J, Reuter Victor E, Melamed Myron R, Fair William R, Morse Michael, Sogani Pramod C, Herr Harry W, Whitmore Willet F, Cordon-Cardo Carlos. Enhanced Bladder Cancer Detection with the Lewis X Antigen as a Marker of Neoplastic Transformation. The Journal of Urology. 1990;143:285–8. doi: 10.1016/s0022-5347(17)39935-4. [DOI] [PubMed] [Google Scholar]
- 250.Shirahama T, Ikoma Michiaki, Muramatsu Takashi, Ohi Yoshitada. Expression of SSEA-1 Carbohydrate Antigen Correlates with Stage, Grade, and Metastatic Potential of Transitional Cell Carcinoma of the Bladder. The Journal of Urology. 1992;148:1319–22. doi: 10.1016/s0022-5347(17)36900-8. [DOI] [PubMed] [Google Scholar]
- 251.Liebert M, Jaffe Ronald, Taylor Rodney J, Ballou Byron T, Soltor Davor, Hakala Thomas R. Detection of SSEA-1 on Human Renal Tumors. Cancer. 1987;59:1404–8. doi: 10.1002/1097-0142(19870415)59:8<1404::aid-cncr2820590804>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 252.Ohana-Malka O, Benharroch Daniel, Isakov Noah, Prinsloo Isebrand, Shubinsky Giora, Sacks Martin, Gopas Jacob. Selectins and Anti-CD15 (Lewis x/a) Antibodies Transmit Activation Signals in Hodgkin’s Lymphoma-derived Cells. Experimental Hematology. 2003;31:1057–65. doi: 10.1016/s0301-472x(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 253.Rizzatti EG, Portieres Fernando L, Martins Sergio LR, Rego Eduardo M, Zago Marco A, Falcao Roberto P. Microgranular and t(11;17)/PLZF-RARa Variants of Acute Promyelocytic Leukemia Also Present the Flow Cytometric Pattern of CD13, CD34, and CD15 Expression Characteristic of PML-RARa Gene Rearrangement. American Journal of Hematology. 2004;76:44–51. doi: 10.1002/ajh.20055. [DOI] [PubMed] [Google Scholar]
- 254.Patru C, Romao Luciana, Varlet Pascale, Coulombel Laure, Raponi Eric, Cadusseau Josette, Renault-Mihara Francois, Thirant Cecile, Leonard Nadine, Berhneim Alain, Mihalescu-Maingot Maria, Haiech Jacques, Bieche Ivan, Moura-Neto Vivaldo, Daumas-Duport Catherine, Junier Marie-Pierre, Chneiweiss Herve. CD133, CD15/SSEA-1, CD34 or Side Populations Do Not Resume Tumor-initiating Properties of Long-term Cultured Cancer Stem Cells From Human Malignant Glio-neuronal Tumors. BMC Cancer. 2010;10:art. no. 66. doi: 10.1186/1471-2407-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Read T-A, Fogarty Marie P, Markant Shirley L, McLendon Roger E, Wei Zhengzheng, Ellison David W, Febbo Phillip G, Wechsler-Reya Robert J. Identification of CD15 as a Marker for Tumor-Propagating Cells in a Mouse Model of Medulloblastoma. Cancer Cell. 2009;15(2):135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Son MJ, Woolard Kevin, Nam Do-Hyun, Lee Jeongwu, Fine Howard A. SSEA-1 Is an Enrichment Marker for Tumor-Initiating Cells in Human Glioblastoma. Cell Stem Cell. 2009;4:440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Katagihallimath N, Mehanna Ali, Guseva Daria, Kleene Ralf, Schachner Melitta. Identification and Validation of a Lewisx Glycomimetic Peptide. European Journal of Cell Biology. 2010;89:77–86. doi: 10.1016/j.ejcb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 259.Iwamori M, Iwamori Yuriko, Kubushiro Kaneyuki, Ishiwata Isamu, Kiguchi Kazushige. Characteristic Expression of Lewis-antigenic Glycolipids in Human Ovarian Carcinoma-derived Cells with Anticancer Drug-resistance. The Journal of Biochemistry. 2007;141(3):309–17. doi: 10.1093/jb/mvm031. [DOI] [PubMed] [Google Scholar]
- 260.Capurro M, Bover Laura, Portela Paula, Livingston Philip, Mordoh J. FC-2.15, a Monoclonal Antibody Active Against Human Breast Cancer, Specifically Recognizes LewisX Hapten. Cancer Immunology and Immunotherapy. 1998;45(6):334–9. doi: 10.1007/s002620050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mordoh J, Leis S, Bravo AI, Podhajcer OL, Ballare C, Capurro M, Kairiyama C, Bover L. Description of a New Monoclonal Antibody, FC-2.15, Reactive with Human Breast Cancer and Other Human Neoplasias. The International Journal of Biological Markers. 1994;9(3):125–34. doi: 10.1177/172460089400900301. [DOI] [PubMed] [Google Scholar]
- 262.Mordoh J, SIlva C, Albarellos M, Bravo AI, Kairiyama C. Phase I Clinical Trial in Cancer Patients of a New Monoclonal Antibody FC-2.15 Reacting with Tumor Proliferating Cells. Journalf of Immunology. 1995;17:151–60. doi: 10.1097/00002371-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 263.Ballou B, Reiland Jean M, Levine Geoffrey, Taylor Rodney, Shen Wei-Chiang, Ryser Hughes J-P, Solter Davor, Hakala Thomas R. Tumor Location and Drug Targeting Using a Monoclonal Antibody (anti-SSEA-1) and Antigen-Binding Fragments. Journal of Surgical Oncology. 1986;31:1–12. doi: 10.1002/jso.2930310102. [DOI] [PubMed] [Google Scholar]
- 264.Chang W-W, Lee Chien Hsin, Lee Peishan, Lin Juway, Hsu Chun-Wei, Hung Jung-Tung, Lin Jin-Jin, Yu Jyh-Cherng, Shao Li-en, Yu John, Wong Chi-Huey, Yu Alice L. Expression of Globo H and SSEA3 in Breast Cancer Stem Cells and the Involvement of Fucosyl Transferases 1 and 2 in Globo H Synthesis. PNAS. 2008;105(33):11667–72. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang C-C, Huang Yen-Lin, Ren Chien-Tai, Lin Chin-Wei, Hung Jung-Tung, Yu Jyh-Cherng, Yu Alice L, Wu Chung-Yi, Wong Chi-Huey. Glycan Microarray of Globo H and Related Structures for Quantitative Analysis of Breast Cancer. PNAS. 2008;105(33):11661–6. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang S, Zhang Helen S, Reuter Victor E, Slovin Susan F, Scher Howard I, Livingston Philip O. Expression of Potential Target Antigens for Immunotherapy on Primary and Metastatic Prostate Cancers. Clinical Cancer Research. 1998;4:295–302. [PubMed] [Google Scholar]
- 267.Kannagi R, Levery Steven B, Ishigami Fumitsugu, Hakomori Sen-itiroh, Shevinsky Lynne H, Knowles Barbara B, Solter Davor. New Globoseries Glycosphingolipids in Human Teratocarcinoma Reactive with the Monoclonal Antibody Directed to a Developmentally Regulated Antigen, Stage-specific Embryonic Antigen 3. The Journal of Biological Chemistry. 1983;258(14):8934–42. [PubMed] [Google Scholar]
- 268.Menard S, Tagliabue Elda, Canevari Silvana, Fossati Guiseppe, Colnaghi Maria I. Generation of Monoclonal Antibodies Reacting with Normal and Cancer Cells of Human Breast. Cancer Research. 1983;43:1295–300. [PubMed] [Google Scholar]
- 269.Mariani-Costantini R, Barbanti Paolo, Colnaghi Maria Ines, Menard Sylvie, Clemente Claudio, Rilke Franco. Reactivity of a Monoclonal Antibody With Tissues and Tumors from the Human Breast. American Journal of Pathology. 1984;115(1):47–56. [PMC free article] [PubMed] [Google Scholar]
- 270.Canevari S, Fossati Guiseppe, Balsari Andrea, Sonnino Sandro, Colnaghi Maria I. Immunochemical Analysis of the Determinant Recognized by a Monoclonal Antibody (MBr1) Which Specifically Binds to Human Mammary Epithelial Cells. Cancer Research. 1983;43:1301–5. [PubMed] [Google Scholar]
- 271.Bremer EG, Levery Steven B, Sonnino Sandro, Ghidoni Riccardo, Canevari Silvana, Kannagi Reiji, Hakomori Sen-itiroh. Characterization of a Glycosphingolipid Antigen Defined by the Monoclonal Antibody MBr1 Expressed in Normal and Neoplastic Epithelial Cells of Human Mammary Gland. The Journal of Biological Chemistry. 1984;259(23):14773–7. [PubMed] [Google Scholar]
- 272.Adobati E, Panza Luigi, Russo Giovanni, Colnaghi Maria I, Canevari Silvana. In vitro Mimicry of CaMBr1 Tumor-associated Antigen by Synthetic Oligosaccharides. Glycobiology. 1997;7(2):173–8. doi: 10.1093/glycob/7.2.173. [DOI] [PubMed] [Google Scholar]
- 273.Zhang S, Cordon-Cardo Carlos, Zhang Helen S, Reuter Victor E, Adluri Sucharita, Hamilton Wm Bradley, Lloyd Kenneth O, Livingston Philip O. Selection of Tumor Antigens as Targets for Immune Attack Using Immunohistochemistry: I. Focus on Gangliosides. International Journal of Cancer. 1997;73:42–9. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 274.Rouquier S, Lowe John B, Kelly Robert J, Fertitta Anne L, Lennon Gregory G, Giorgi Dominique. Molecular Cloning of a Human Genomic Region Containing the H Blood Group a(1,2)Fucosyltransferase Gene and Two H Locus-related DNA Restriction Fragments. The Journal of Biological Chemistry. 1995;270(9):4632–9. doi: 10.1074/jbc.270.9.4632. [DOI] [PubMed] [Google Scholar]
- 275.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Carbohydrate Vaccines in Cancer: Immunogenicity of a Fully Synthetic Globo H Hexasaccharide Conjugate in Man. PNAS. 1999;96:5710–5. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gilewski T, Ragupathi Govindaswami, Bhuta Sonal, WIlliams Lawrence J, Musselli Cristina, Zhang Xu-Fang, Bencsath Kalman P, Panageas Katherine S, Chin Jeanette, Hudis Clifford A, Norton Larry, Houghton Alan N, Livingston Philip O, Danishefsky Samuel J. Immunization of Metastatic Breast Cancer Patients with a Fully Synthetic Globo H Conjugate: A Phase I Trial. PNAS. 2001;98(6):3270–5. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ragupathi G, Slovin Susan F, Adluri Sucharita, Sames Dalibor, Kim Jong, Kim Hyunjin M, Spassova Maria, Bornmann William G, Lloyd Kenneth O, Scher Howard I, Livingston Philip O, Danishefsky Samuel J. A Fully Synthetic Globo H Carbohydrate Vaccine Induces a Focused Humoral Response in Prostate Cancer Patients: A Proof of Principle. Angewandte Chemie International Edition. 1999;38(4):563–6. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 278.Sabbatini PJ, Ragupathi Govind, Hood CHandra, Aghajanian Carol A, Juretzka Margrit, Iasonos Alexia, Hensley Martee L, Spassova Maria K, Ourfelli Ouathek, Spriggs David R, Tew William P, Konner Jason, Clausen Henrik, Abu Rustum Nadeem, Danishefsky Samuel J, Livingston Philip O. Pilot Study of a Heptavalent Vaccine-Keyhole Limpet Hemocyanin Conjugate plus QS21 in Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer. Clinical Cancer Research. 2007;13(14):4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 279.Slovin SF, Ragupathi Govind, Fernandez Celina, Jefferson Matthew P, Diani Meghan, Wilton Andrew S, Powell Shemeeakah, Spassova Maria, Reis Celso, Clausen Henrick, Danishefsky Samuel, Livingston Philip, Scher Howard I. A Bivalent Conjugate Vaccine in the Treatment of Biochemically Relapsed Prostate Cancer: A Study of Glycosylated MUC-2-KLH and Globo H-KLH Conjugate Vaccines Given with the New Semi-synthetic Saponin Immunological Adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–22. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 280.Zhu J, Wan Qian, Lee Dongjoo, Yang Guangbin, Spassova Maria K, Ouerfelli Ouathek, Ragupathi Govind, Damani Payal, Livingston Philip O, Danishefsky Samuel J. From Synthesis to Biologics: Preclinical Data on a Chemistry Derived Anticancer Vaccine. Journal of the American Chemical Society. 2009;131(26):9298–303. doi: 10.1021/ja901415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shevinsky LH, Knowles Barbara B, Damjanov Ivan, Solter Davor. Monoclonal Antibody to Murine Embryos Defines a Stage-Specific Embryonic Antigen Expressed on Mouse Embryos and Human Teratocarcinoma Cells. Cell. 1982;30(3):697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- 282.Kannagi R, Cochran Nancy A, Ishigami Fumitsugu, Hakomori Sen-itiroh, Andrews Peter W, Knowles Barbara B, Solter Davor. Stage-specific Embryonic Antigens (SSEA3 and -4) are Epitopes of a Unique Globo-series Ganglioside Isolated from Human Teratocarcinoma Cells. The EMBO Journal. 1983;2(12):2355–61. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Suila H, Pitkanen Virve, Hirvonen Tia, Heiskanen Annamari, Anderson Heidi, Laitinen Anita, Natunen Suvi, Miller-Podraza Halina, Satomaa Tero, Natunen Jari, Laitinen Saara, Valmu Leena. Are Globoseries Glycosphingolipids SSEA-3 and -4 Markers for Stem Cells Derived from Human Umbilical Cord Blood. Journal of Molecular and Cell Biology. 2010 doi: 10.1093/jmcb/mjq041. Article in Press. [DOI] [PubMed] [Google Scholar]
- 284.Zhou D, Henion Timothy R, Jungalwala Firoze B, Berger Eric G, Hennet Thierry. The b1,3-galactosyltransferase b3GalT-V Is a Stage-specific Embryonic Antigen-3 (SSEA-3) Synthase. The Journal of Biological Chemistry. 2000;275(30):22631–4. doi: 10.1074/jbc.C000263200. [DOI] [PubMed] [Google Scholar]
- 285.Schrump DS, Furukawa Koichi, Yamaguchi Hiroshi, Lloyd Kenneth O, Old Lloyd J. Recognition of Galactosylgloboside by Monoclonal Antibodies Derived from Patients with Primary Lung Cancer. PNAS. 1988;85:4441–5. doi: 10.1073/pnas.85.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Muramatsu T, Muramatsu Hisako. Carbohydrate Antigens Expressed on Stem Cells and Early Embryonic Cells. Glycoconjugate Journal. 2004;21:41–5. doi: 10.1023/B:GLYC.0000043746.77504.28. [DOI] [PubMed] [Google Scholar]
- 287.Fredman P, Hedberg K, Brezicka T. Gangliosides as therapeutic targets for cancer. Biodrugs: Clinical Immunotherapeutics, Biopharmaceuticals And Gene Therapy. 2003;17:155–167. doi: 10.2165/00063030-200317030-00002. [DOI] [PubMed] [Google Scholar]
- 288.Seifert H. On an additional ganglioside characteristic of brain tumors. Klin Wochenschr. 1966;44(8):469–470. doi: 10.1007/BF01727468. [DOI] [PubMed] [Google Scholar]
- 289.Kramer K, Gerald WL, Kushner BH, et al. Disialoganglioside G(D2) loss following monoclonal antibody therapy is rare in neuroblastoma. Clinical Cancer Research. 1998;4:2135–2139. [PubMed] [Google Scholar]
- 290.Grant SC, Kostakoglu L, Kris MG, Yeh SD, Larson SM, Finn RD, Oettgen HF, Cheung NV. Targeting of small-cell lung cancer using the anti-GD2 ganglioside monoclonal antibody 3F8: a pilot trial. Eur J Nucl Med. 1996 Feb;23(2):145–9. doi: 10.1007/BF01731837. [DOI] [PubMed] [Google Scholar]
- 291.Yuki N, Yamada M, Tagawa Y, et al. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. Journal of the Neurological Sciences. 1997;149:127–130. doi: 10.1016/s0022-510x(97)05390-2. [DOI] [PubMed] [Google Scholar]
- 292.Becker R, Eichler MK, Jennemann R, Bertalanffy H. Phase I clinical trial on adjuvant active immunotherapy of human gliomas with GD2-conjugate. British Journal Of Neurosurgery. 2002;16:269–275. doi: 10.1080/02688690220148860. [DOI] [PubMed] [Google Scholar]
- 293.Foon Kenneth A, Lutzky Jose, Baral Rathindra N, Yannelli John R, Hutchins Laura, Teitelbaum April, Kashala Oscar L, Das Ruma, Garrison Juanita, Reisfeld Ralph A. Bhattacharya-Chatterjee, Malaya Clinical and Immune Responses in Advanced Melanoma Patients Immunized With an Anti-Idiotype Antibody Mimicking Disialoganglioside GD2. Journal of Clinical Oncology Issue. 2000 January;18(2):376–384. doi: 10.1200/JCO.2000.18.2.376. [DOI] [PubMed] [Google Scholar]
- 294.Hedberg KM, Dellheden B, Wikstrand CJ, Fredman P. Monoclonal anti-GD3 antibodies selectively inhibit the proliferation of human malignant glioma cells in vitro. Glycoconj J. 2000 Oct;17(10):717–26. doi: 10.1023/a:1011026823362. [DOI] [PubMed] [Google Scholar]
- 295.Zeng G, Gao L, Birklé S, Yu RK. Suppression of Ganglioside GD3 Expression in a Rat F-11 Tumor Cell Line Reduces Tumor Growth, Angiogenesis, and Vascular Endothelial Growth Factor Production. Cancer Research. 2000;60:6670–6676. [PubMed] [Google Scholar]
- 296.Kirkwood JM, Mascari RA, Edington HD, Rabkin MS, Day RS, Whiteside TL, Vlock DR, Shipe-Spotloe JM. Analysis of therapeutic and immunologic effects of R(24) anti-GD3 monoclonal antibody in 37 patients with metastatic melanoma. Cancer. 2000 Jun 15;88(12):2693–702. [PubMed] [Google Scholar]
- 297.Giaccone G, Debruyne C, Felip E, et al. Phase III Study of Adjuvant Vaccination With Bec2/Bacille Calmette-Guerin in Responding Patients With Limited-Disease Small-Cell Lung Cancer (European Organization for Research and Treatment of Cancer 08971–08971B; Silva Study) Journal of Clinical Oncology. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 298.Bitton RJ, Guthmann MD, Gabri MR, et al. Cancer vaccines: an update with special focus on ganglioside antigens. Oncology Reports. 2002;9:267–276. [PubMed] [Google Scholar]
- 299.Chapman PB, Wu D, Ragupathi G, et al. Sequential Immunization of Melanoma Patients with GD3 Ganglioside Vaccine and Anti-Idiotypic Monoclonal Antibody That Mimics GD3 Ganglioside. Clinical Cancer Research. 2004;10:4717–4723. doi: 10.1158/1078-0432.CCR-04-0345. [DOI] [PubMed] [Google Scholar]
- 300.Livingston P, Wong G, Adluri S, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. Journal of Clinical Oncology. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 301.Alexandrescu DT, Ichim TE, Riordan NH, et al. Immunotherapy for Melanoma: Current Status and Perspectives. Journal of Immunotherapy. 2010;33:570–590. doi: 10.1097/CJI.0b013e3181e032e8. 510.1097/CJI.1090b1013e3181e1032e1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-Dose Interferon Alfa-2b Significantly Prolongs Relapse-Free and Overall Survival Compared With the GM2-KLH/QS-21 Vaccine in Patients With Resected Stage IIB-III Melanoma: Results of Intergroup Trial E1694/S9512/C509801. Journal of Clinical Oncology. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 303.Tokuda N, Zhang Q, Yoshida S, et al. Genetic mechanisms for the synthesis of fucosyl GM1 in small cell lung cancer cell lines. Glycobiology. 2006;16:916–925. doi: 10.1093/glycob/cwl022. [DOI] [PubMed] [Google Scholar]
- 304.Krug LM, Ragupathi G, Hood C, et al. Vaccination of Patients with Small-Cell Lung Cancer with Synthetic Fucosyl GM-1 Conjugated to Keyhole Limpet Hemocyanin. Clinical Cancer Research. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 305.Livingston PO, Hood C, Krug LM, et al. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunology, Immunotherapy. 2005;54:1018–1025. doi: 10.1007/s00262-005-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yoshino H, Ariga T, Latov N, et al. Fucosyl-GM1 in Human Sensory Nervous Tissue Is a Target Antigen in Patients with Autoimmune Neuropathies. Journal of Neurochemistry. 1993;61:658–663. doi: 10.1111/j.1471-4159.1993.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 307.Eggermont AMM. Therapeutic vaccines in solid tumours: Can they be harmful? European Journal of Cancer. 2009;45:2087–2090. doi: 10.1016/j.ejca.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 308.Malykh YN, Schauer R, Shaw L. N- Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–34. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 309.Hernandez AM, Rodr guez M, Lopez-Requena A, Beausoleil I, Perez R, Vazquez AM. Generation of Anti-Neu-Glycolyl-Ganglioside Antibodies by Immunization with an Anti-Idiotype Monoclonal Antibody: A Self versus Non-Self-Matter. Immunobiology. 2005;210:11–21. doi: 10.1016/j.imbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 310.Alfonso M, Diaz A, Hernandez AM, Perez A, Rodriguez E, Bitton R, Perez R, Vazquez AM. An Anti-Idiotype Vaccine Elicits a Specific Response to N-Glycolyl Sialic Acid Residues of Glycoconjugates in Melanoma Patients. The Journal of Immunology. 2002;168:2523–29. doi: 10.4049/jimmunol.168.5.2523. [DOI] [PubMed] [Google Scholar]
- 311.Neninger E, Diaz RM, de la Torre A, Rives R, Diaz A, Saurez G, Gabri MR, Alonso DF, Wilkinson B, Alfonso AM, Combet T, Perez R, Vazquez AM. Active Immunotherapy with 1E10 Anti-Idiotype Vaccine in Patients with Small Cell Lung Cancer: Report of a Phase I Trial. Cancer Biology & Therapy. 2007;6 (2):145–50. doi: 10.4161/cbt.6.2.3574. [DOI] [PubMed] [Google Scholar]
- 312.Hernandez AM, Rodriguez N, Gonzalez JE, Reyes E, Rondon T, Grinan T, Macias A, Alfonso S, Vazquez AM, Perez R. Anti-NeuGcGM3 Antibodies, Actively Elicited by Idiotypic Vaccination in Nonsmall Cell Lung Cancer Patients, Induce Tumor Cell Death by an Oncosis-like Mechanism. Journal of Immunology. 2011;186 (6):3735–44. doi: 10.4049/jimmunol.1000609. [DOI] [PubMed] [Google Scholar]
- 313.Carr A, Rodrıguez E, del Carmen Arango M, Camacho R, Osorio M, Gabri M, Carrillo G, Valdes Z, Bebelagua Y, Perez R, Fernandez LE. Immunotherapy of Advanced Breast Cancer With a Heterophilic Ganglioside (NeuGcGM3) Cancer Vaccine. Journal of Clinical Oncology. 2003;21 (6):1015–21. doi: 10.1200/JCO.2003.02.124. [DOI] [PubMed] [Google Scholar]
- 314.Osorio M, Gracia E, Rodríguez E, Saurez G, del Carmen Arango M, Noris E, Torriella A, Joan A, Gomez E, Anasagasti L, Gonzalez JL, Melgares MDLA, Torres I, Gonzalez J, Alonso D, Rengifo E, Carr A, Perez R, Fernandez LE. Heterophilic NeuGcGM3 Ganglioside Cancer Vaccine in Advanced Melanoma Patients: Results of a Phase Ib/IIa Study. Cancer Biology & Therapy. 2008;7 (4):488–95. doi: 10.4161/cbt.7.4.5476. [DOI] [PubMed] [Google Scholar]
- 315.Labrada M, Clavell M, Bebelagua Y, Leon J, Alonso DF, Gabri MR, Veloso RC, Vérez V, Fernandez LE. Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy. Expert Opinion on Biological Therapy. 2010;10 (2):153–62. doi: 10.1517/14712590903443084. [DOI] [PubMed] [Google Scholar]
- 316.de Leon J, Fernandez A, Mesa C, Clavel M, Fernandez LE. Role of Tumour-Associated N-Glycolylated Variant of GM3 Ganglioside in Cancer Progression: Effect over CD4 Expression on T Cells. Cancer Immunology, Immunotherapy. 2006;55:443–50. doi: 10.1007/s00262-005-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fernandez-Marrero Y, Hernandez T, Roque-Navarro L, Talavera A, Moreno F, Grinan T, Vazquez AM, de Acosta CM, Perez R, Lopez-Requena A. Switching on Cytotoxicity by a Single Mutation at the Heavy Chain Variable Region of an Anti-Ganglioside Antibody. Molecular Immunology. 2011;48:1059–67. doi: 10.1016/j.molimm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 318.Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, Fainboim L, Gomez DE, Alonso DF. NGcGM3 Ganglioside: A Privileged Target for Cancer Vaccines. Clinical and Developmental Immunology 2010. 2010 doi: 10.1155/2010/814397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Angata K, Fukuda M. Polysialyltransferases: Major Players in Polysialic Acid Synthesis on the Neural Cell Adhesion Molecule. Biochimie. 2003;85:195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 320.Finne J. Occurrence of Unique Polysialosyl Carbohydrate Units in Glycoproteins of Developing Brain. The Journal of Biological Chemistry. 1982;257(20):11966–70. [PubMed] [Google Scholar]
- 321.Rothbard JB, Brackenbury R, Cunningham BA, Edelman GM. Differences in the Carbohydrate Structures of Neural Cell-Adhesion Molecules from Adult and Embryonic Chicken Brains. The Journal of Biological Chemistry. 1982;257 (18):11064–69. [PubMed] [Google Scholar]
- 322.Petridis AK, El Maarouf A, Rutishauser U. Polysialic Acid Regulates Cell Contact Dependent Neuronal Differentiation of Progenitor Cells from the sub-ventricular zone. Developmental Dynamics. 2004;230:675–84. doi: 10.1002/dvdy.20094. [DOI] [PubMed] [Google Scholar]
- 323.Amoureux MC, Coulibaly B, Chinot O, Loundou A, Metellus P, Rougon G, Figarella-Branger D. Polysialic Acid Neural Cell Adhesion Molecule (PSA-NCAM) is an Adverse Prognosis Factor in Glioblastoma, and Regulates olig2 Expression in Glioma Cell Lines. BMC Cancer. 2010;10:91. doi: 10.1186/1471-2407-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Rutishauser U, Landmesser L. Polysialic Acid in the Vertebrate Nervous System - a Promoter of Plasticity in Cell-Cell Interactions. Trends in Neurosciences. 1996;19:422–27. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 325.Durbec P, Cremer H. Revisiting the Function of PSA-NCAM in the Nervous System. Molecular Neurobiology. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- 326.Hildebrandt H, Becker C, Gluer S, Rösner H, Gerardy-Schahn R, Rahmann H. Polysialic Acid on the Neural Cell Adhesion Molecule Correlates with Expression of Polysialyltransferases and Promotes Neuroblastoma Cell Growth. Cancer Research. 1998;58 (4):779–84. [PubMed] [Google Scholar]
- 327.Seidenfaden R, Krauter A, Schertzinger F, Gerardy-Schahn R, Hildebrandt H. Polysialic Acid Directs Tumor Cell Growth by Controlling Heterophilic Neural Cell Adhesion Molecule Interactions. Molecular and Cellular Biology. 2003;23 (16):5908–18. doi: 10.1128/MCB.23.16.5908-5918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Miyahara R, Tanaka F, Nakagawa T, Matsuoka K, Isii K, Wada H. Expression of Neural Cell Adhesion Molecules (Polysialylated form of Neural Cell Adhesion Molecule and L1-cell Adhesion Molecule) on Resected Small Cell Lung Cancer Specimens: In relation to proliferation state. Journal of Surgical Oncology. 2001;77 (1):49–54. doi: 10.1002/jso.1065. [DOI] [PubMed] [Google Scholar]
- 329.Korja M, Jokilammi A, Salmi TT, Kalimo H, Pelliniemi TT, Isola J, Rantala I, Haapasalo H, Finne J. Absence of Polysialylated NCAM is an Unfavorable Prognostic Phenotype for Advanced Stage Neuroblastoma. BMC Cancer. 2009;9:57. doi: 10.1186/1471-2407-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Krug LM, Ragupathi G, Ng KK, Hood C, Jennings HJ, Guo Z, Kris MG, Miller V, Pizzo B, Tyson L, Baez V, Livingston PO. Vaccination of Small Cell Lung Cancer Patients with Polysialic Acid or N-Propionylated Polysialic Acid Conjugated to Keyhole. Clinical Cancer Research. 2004;10 (3):916–23. doi: 10.1158/1078-0432.ccr-03-0101. [DOI] [PubMed] [Google Scholar]
- 331.Dube DH, Bertozzi CR. Glycans in Cancer and Inflammation - Potential for Therapeutics and Diagnostics. Nature Reviews Drug Discovery. 2005;4:477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 332.Simka M. Blood Brain Barrier Compromise with Endothelial Inflammation may Lead to Autoimmune Loss of Myelin during Multiple Sclerosis. Current Neurovascular Research. 2009;6:132–9. doi: 10.2174/156720209788185605. [DOI] [PubMed] [Google Scholar]
- 333.Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical Engineering of Surface a2–8 Polysialic Acid for Immunotargeting Tumor Cells. The Journal of Biological Chemistry. 2000;275 (42):32832–36. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- 334.Petridis AK, Wedderkopp H, Hugo HH, Mehdorn HM. Polysialic Acid Overexpression in Malignant Astrocytomas. Acta Neurochirurgica. 2009;151:601–4. doi: 10.1007/s00701-009-0324-3. [DOI] [PubMed] [Google Scholar]
- 335.Schuessler MH, Pintado S, Welt S, Real FX, Xu M, Melamed R, Lloyd KO, Oettge HF. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991 January 21;47(2):180–187. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- 336.Blasco E, Gutierrez-Hoyos A, Lojendio M, Cosme A, Arenas JI. Expression of type 1 blood group precursor in human gastric carcinoma. Eur J Cancer. 1991;27(4):501–3. doi: 10.1016/0277-5379(91)90396-u. [DOI] [PubMed] [Google Scholar]
- 337.Meichenin M, Rocher J, Galanina O, Bovin N, Nifant’ev N, Sherman A, Cassagnau E, Heymann MF, Bara J, Fraser RH, Le Pendu J. Tk, a New Colon Tumor-associated Antigen Resulting from Altered O-Glycosylation. Cancer Research. 2000;60:5499–507. [PubMed] [Google Scholar]; 336 Adlercreutz D, Weadge JT, Petersen BO, Duus JØ, Dovichi NJ, Palcic MM. Enzymatic synthesis of Gb3 and iGb3 ceramides. Carbohydrate Research. 2010;345:1384. doi: 10.1016/j.carres.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Falguières T, Maak M, von Weyhern C, Sarr M, Sastre X, Poupon M-F, Robine S, Johannes L, Janssen K-P. Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Molecular Cancer Therapeutics. 2008;7:2498–508. doi: 10.1158/1535-7163.MCT-08-0430. [DOI] [PubMed] [Google Scholar]
- 339.Zhu J, Wan Q, Ragupathi G, George CM, Livingston PO, Danishefsky SJ. Biologics through Chemistry: Total Synthesis of a Proposed Dual-Acting Vaccine Targeting Ovarian Cancer by Orchestration of Oligosaccharide and Polypeptide Domains. Journal of the American Chemical Society. 2009;131:4151–8. doi: 10.1021/ja810147j. [DOI] [PMC free article] [PubMed] [Google Scholar]