Abstract
Using the R6/1 mouse model of Huntington disease (HD), we have recently shown that voluntary physical activity was able to correct the depressive-like behaviours exhibited by the HD animals at a pre-motor symptomatic stage of the disease. Using the high performance liquid chromatography system, we have now evaluated the effect of exercise on monoamine metabolism in HD mice. We found that serotonin and its metabolite as well as dopamine and noradrenaline were reduced across several brain regions in female R6/1 animals. Our data also suggest that some of these neurochemical deficits were modulated by physical activity, in a genotype-region dependent manner. These newly identified changes could account for some of the behavioural effects of exercise previously reported in HD mice.
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disorder caused by expansion of CAG repeats in exon 1 of the huntingtin gene [1]. Clinical onset of HD is determined on the basis of motor symptoms; however the pre-motor stages of the disease are commonly associated with cognitive deficits as well as psychiatric manifestations such as depression [2] [3] [4] [5]. Similar impaired emotionality-related behaviors have been found in transgenic mouse models of HD including R6/1 [6] [7] [8] and YAC128 [9] HD mice.
Serotonin (5-HT) and catecholamines (including noradrenaline (NA) and dopamine (DA)) are known to play a key role in the development of mood disorders [10] [11]. The serotonin-noradrenaline hypothesis of depression is primarily supported by the mechanism of action of the tricyclic antidepressants, which by binding to the serotonin transporter (SERT) and the noradrenaline transporter (NET) result in an increased extracellular concentration of these neurotransmitters. There is also clinical evidence implicating dopaminergic dysfunctions in the pathophysiology of both depression [11] [12] and HD [13]. Interestingly, alterations in 5-HT and/or DA systems have been found in pre-motor symptomatic R6 HD animals [6] [7] [8] [14] [15], and we have recently shown that the associated depressive-like behaviours exhibited by R6/1 female mice were corrected by chronic treatment with the serotonin reuptake inhibitor (SSRI) sertraline as well as voluntary physical activity [7].
Many brain disorders (including cognitive and affective abnormalities as well as HD) could potentially benefit from enhanced physical activity. However the effects of exercise on monoamine metabolism have been poorly characterized, especially in motor asymptomatic HD mice. Therefore using the high performance liquid chromatography (HPLC) system, we measured tissue levels of 5-HT, DA and NA in several brain regions (hippocampus, cortex and striatum) of animals (WT and R6/1 HD mice) exposed to running-wheels versus standard housing from 8 to 12 weeks of age.
Materials and Methods
Mice
R6/1 transgenic hemizygote males [16] were originally obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and bred with CBB6 (CBA×C57/B6) F1 females to establish the R6/1 (HD) colony. Animals housed in standard (SH) cages (15×30×12 cm) were compared with mice housed in large cages (25×37×16 cm) with elevated lids and provided with two running wheels (RW, 12 cm diameter) from 8 to 12 weeks of age. All animals used in this study were group-housed (2 mice from each genotype per cage) and maintained on a 12 h light/dark cycle with access to food and water ad libitum. Therefore, we were not able to individually measure the total distance run during the 4 weeks with free access to running wheels. However, we have evidence that female mice used the wheels regardless of genotype (unpublished data using animals grouped by genotype). Furthermore, the cages were monitored regularly and this confirmed that the wheels were frequently used by mice, with either one or two mice observed to run on a single wheel. All experiments were performed on female 12-week mice in accordance with the guidelines of the HFI Animal Ethics Committee and the National Health and Medical Research Council (NHMRC).
Whole tissue measurements of serotonin, dopamine, noradrenaline and their metabolites
Tissue levels of endogenous serotonin (5-HT) and its metabolite 5-hydroxyindolacetic acid (5-HIAA) as well as the catecholamines (dopamine and noradrenaline), were determined as previously published [17]. Dissected brain structures were homogenized in 5-10 volumes (v/w) of ice-cold 0.1 M HClO4 containing 1.34 mM disodium EDTA and 0.05% Na2S2O5. Homogenates were centrifuged at 30,000 g for 20 min at 4°C. After neutralization with 2 M KH2PO4/K2HPO4, pH 7.4, containing 0.01 mg per mL ascorbate oxidase (Boehringer Mannheim, Meylan, France), supernatants were further centrifuged at 30,000 g for 20 min. Aliquots (10 µL) of clear supernatants were injected into a high performance liquid chromatography (HPLC) column (Ultrasphere IP, Beckman, Gagny, France ; 25x0.46 cm, C18 reversed phase, particle size 5 µm) protected with a Brownlee pre-column (3 cm, 5 µm). The mobile phase for the elution (at a flow rate of 1 mL per min) consisted of (in mM) : KH2PO4, 70 ; triethylamine, 3.1 ; disodium EDTA, 0.1 ; octane sulphonate, 1.05 ; methanol, 16%, adjusted to pH 3.02 with solide citric acid. The electrochemical detection system (ESA 5011, Bedford, MA , USA) comprises an analytical cell with dual coulometric monitoring electrodes (+50 and +350 mV). The generated signals were integrated by a computing integrator (Millenium 32, Waters, Saint Quentin Fallavier, France).
Statistical analysis
Statistical analyses were performed using SPSS statistics 17.0 and GraphPad Prism 5.0. Two-way analysis of variance (ANOVAs) were used to examine possible effect of genotype and/or exercise. To determine specific group differences in case of significant main effects (or interaction), the two-way ANOVAs were followed by Fisher’s LSD or Bonferroni post-hoc tests. In all cases, the significance level was set at p<0.05.
Results
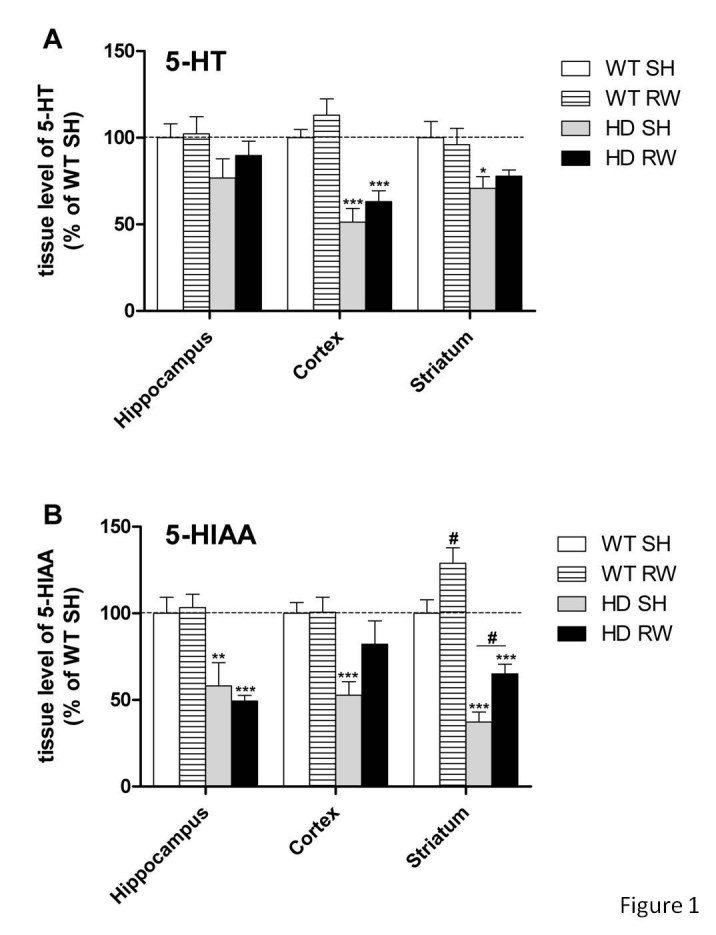
Effects of HD mutation and exercise on levels of serotonin and its metabolite
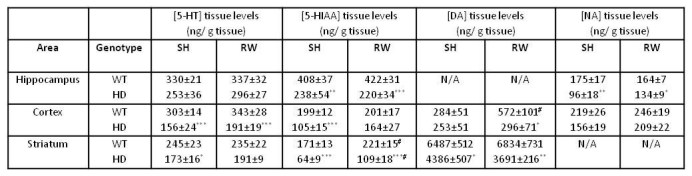
As shown in figure 1, under standard-housing (SH) conditions the HD mutation produced a decrease of tissue levels of serotonin (5-HT) in the hippocampus (-25%), the cortex (-50%) and the striatum (-30%). However compared to WT controls, two-way ANOVA of hippocampal tissue from HD animals did not reach significance (F(1,23)=3.98, p=0.057). Significant reductions were observed in both cortex (F(1,23)=45.8, p<0.001) and striatum (F(1,23)=8.82, p<0.01) of HD mice. Similar significant deficits of the metabolite 5-HIAA were also displayed by HD animals in all brain regions studied (Figure 1 and Table 1). Finally exercise on running wheel (RW) increased 5-HIAA striatal levels regardless of genotype (F(1,23)=15.1, p<0.001).
Figure 1: Effect of HD mutation and voluntary physical exercise on tissue content of 5-HT and 5-HIAA
Figure 1 illustrates the levels of (A) serotonin (5-HT) and (B) its metabolite (5-HIAA) in various brain regions of WT vs. HD animals housed in standard conditions (SH) or exposed to running wheel (RW). Tissue levels are expressed in percentage (%) of standard-housed WT. Values represent means (± SEM) of independent determinations in 6-8 mice of each genotype. WT vs. HD: (*) p<0.05, (**) p<0.01, (***) p<0.001. SH vs. RW: (#) p<0.05.
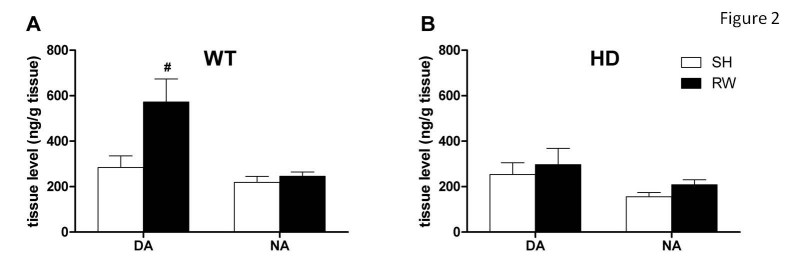
Effects of HD mutation and exercise on levels of catecholamines
Tissue content of dopamine (DA) in cortex (F(1,23)=4.67, p<0.05) and striatum (F(1,23)=24.9, p<0.001) as well as levels of noradrenaline (NA) in hippocampus (F(1,23)=14.2, p<0.001) and cortex (F(1,23)=4.94, p<0.05) were all decreased in HD mice when compared to WT animals (Table 1). Assessing the effect of running wheel (RW) exercise on brain levels of catecholamines (Figure 2), we found a significant effect of exercise on DA (but not NA) cortical levels in WT mice (F(1,23)=6.94, p<0.05). Interestingly, physical activity had no effect on HD animals (F(1,23)=1.29, p=0.27).
Figure 2: Effect of HD mutation and voluntary physical exercise on cortical levels of catecholamines Figure 2 illustrates the levels of dopamine (DA) and noradrenaline (NA) in the cortex of (A) WT vs. (B) HD animals housed in standard conditions (SH) or exposed to running wheel (RW). Tissue levels are expressed in ng/g tissue. Values represent means (± SEM) of independent determinations in 6-8 mice of each genotype. SH vs. RW: (#) p<0.05.
Discussion
As previously shown in R6/2 mice [15], we found that serotonin (5-HT) and its metabolite (5-HIAA) as well as dopamine (DA) and noradrenaline (NA) were reduced across several brain regions in female R6/1 animals. This is the first report of such dopaminergic alterations at the pre-motor symptomatic stage of disease progression. Indeed using a similar number of animals per groups (n=6-8), Reynolds et al. (1999) found reduced DA levels in striatum of R6/2 mice only from 12 weeks of age, paralleling motor impairment and decreased D1/D2 receptor expression [18] observed in these transgenic mice at this stage. Previous studies on R6/1 animals failed to show any effect of the HD mutation on striatal DA tissue content [19] [20], however these contradictory observations were more likely due to statistical limitations (they used n=3 animals per group only) since they nevertheless revealed a strong trend toward a reduced DA levels in HD mice.
Interestingly we found that DA was decreased in the striatum but not in the cortex of R6/1 animals, as recently suggested by another study on R6/2 mice using higher numbers of animals per group (n=15-18) [14]. Altogether our observations are consistent with reduced monoamine levels in the cerebrospinal fluid (CSF) collected from depressed patients [21] (but also see [22]) and could potentially be involved in the development of the depressive-like behaviors we recently reported in pre-symptomatic female R6/1 mice [6] [7] [8].
The beneficial effects of physical activity in alleviating depressive mood are well known and exercise has been often suggested as a form of therapy for depressed patients [23] [24] [25]. Unfortunately similar clinical studies on HD patients (especially at early stage of the disease) have not been undertaken, yet these are crucial to test the relevance of physical activity as a potential therapeutic tool. Promisingly, together with the initiation of the first pre-manifest HD clinical studies [26] [27], recent human data suggest that avoiding a passive lifestyle may significantly delay the onset of HD [[28]. Exercise has been studied in several rodent models of depression [29] [30] [31] [32]. We also have shown than locomotor impairments as well as cognitive and affective deficits displayed by R6/1 animals were ameliorated by wheel running [7] [33] [34], and similar observations have been reported using the R6/2 mice [35] (but also see [36]). Furthermore, environmental enrichment, which also enhances physical activity, has been shown to delay onset of affective, cognitive and motor deficits in R6/1 HD mice [[6] [37] [38]] . Interestingly, our present HPLC data suggest the turnover of serotonin in the striatum as a possible process to establishing antidepressant effects at a behavioural level since wheel-running resulted in higher concentrations (~70% increase in HD mice) of 5-HIAA in the striatum. The striatum has traditionally been associated with the specific cognitive and motor symptoms of HD; it would be interesting to study the role of striatal pathology in HD symptomatology including affective-associated disorders. Surprisingly, exercise did not correct the striatal DA deficit of HD mice. We also report that cortical DA levels were unchanged in female R6/1 mice after wheel-running, although this same measure was enhanced in WT animals. Finally, NA levels did not seem to be affected by physical activity.
Altogether we show that tissue levels of 5-HT, DA and NA were all reduced in R6/1 mice, even at a pre-motor symptomatic stage of the disease. Our findings also suggest that some of these neurochemical deficits were modulated by physical activity, in a genotype-region dependent manner. However, whether these region-specific identified changes could account for the antidepressant-like effects of exercise (as well as the amelioration of the cognitive deficits and the delayed onset of locomotor symptoms observed in HD mice with access to running wheels) remain unclear. Importantly, experimental prerequisites (e.g. initiating interventions at clear pre-motor stages and designing investigations so as to optimize statistical power) need to be carefully evaluated before undertaking such studies.
Acknowledgments
Leah Leang, Michelle Zajac and Terence Pang for their valuable assistance.
Funding information
This work was funded by NHMRC Project Grants and ARC Future Fellowship (AJH), NHMRC-INSERM Exchange Fellowship (TR).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors do not have any conflicts of interests to disclose.
Correspondence
To Thibault Renoir, Behavioral Neurosciences, Florey Neuroscience Institutes, University of Melbourne, Parkville, VIC 3010, Australia. E-mail: tibo.renoir@gmail.com, thibault.renoir@florey.edu.au
References
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993 Mar 26;72(6):971-83. [DOI] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC; Predict-HD Investigators of the Huntington Study Group. Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007 Dec 15;62(12):1341-6. Epub 2007 May 3. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, Craufurd D. Psychiatric disorders in preclinical Huntington's disease. J Neurol Neurosurg Psychiatry. 2007 Sep;78(9):939-43. Epub 2006 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, White K, Weaver M, Flury Wetherill L, Hui S, Stout JC, Johnson SA, Beristain X, Gray J, Wojcieszek J, Foroud T. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007 Jan;64(1):116-21. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005 Fall;17(4):496-502. [DOI] [PubMed] [Google Scholar]
- Pang TY, Du X, Zajac MS, Howard ML, Hannan AJ. Altered serotonin receptor expression is associated with depression-related behavior in the R6/1 transgenic mouse model of Huntington's disease. Hum Mol Genet. 2009 Feb 15;18(4):753-66. Epub 2008 Nov 13. [DOI] [PubMed] [Google Scholar]
- Renoir T, Pang TY, Zajac MS, Chan G, Du X, Leang L, Chevarin C, Lanfumey L, Hannan AJ. Treatment of depressive-like behaviour in Huntington's disease mice by chronic sertraline and exercise. Br J Pharmacol. 2011 Jun 30. doi: 10.1111/j.1476-5381.2011.01567.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir T, Zajac MS, Du X, Pang TY, Leang L, Chevarin C, Lanfumey L, Hannan AJ. Sexually dimorphic serotonergic dysfunction in a mouse model of Huntington's disease and depression. PLoS One. 2011;6(7):e22133. Epub 2011 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersén A, Hayden MR. Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain. 2009 Apr;132(Pt 4):919-32. Epub 2009 Feb 18. [DOI] [PubMed] [Google Scholar]
- Haenisch B, Bönisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2011 Mar;129(3):352-68. Epub 2010 Dec 13. Review. [DOI] [PubMed] [Google Scholar]
- Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007 Apr;12(4):331-59. Epub 2007 Jan 16. Review. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Prospect of a dopamine contribution in the next generation of antidepressant drugs: the triple reuptake inhibitors. Curr Drug Targets. 2009 Nov;10(11):1069-84. Review. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Piccini P, Harding AE, Brooks DJ. Striatal D1 and D2 dopamine receptor loss in asymptomatic mutation carriers of Huntington's disease. Ann Neurol. 1996 Jul;40(1):49-54. [DOI] [PubMed] [Google Scholar]
- Mochel F, Durant B, Durr A, Schiffmann R. Altered dopamine and serotonin metabolism in motorically asymptomatic R6/2 mice. PLoS One. 2011 Mar 31;6(3):e18336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GP, Dalton CF, Tillery CL, Mangiarini L, Davies SW, Bates GP. Brain neurotransmitter deficits in mice transgenic for the Huntington's disease mutation. J Neurochem. 1999 Apr;72(4):1773-6. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996 Nov 1;87(3):493-506. [DOI] [PubMed] [Google Scholar]
- Renoir T, Païzanis E, El Yacoubi M, Saurini F, Hanoun N, Melfort M, Lesch KP, Hamon M, Lanfumey L. Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int J Neuropsychopharmacol. 2008 Dec;11(8):1149-62. Epub 2008 Jul 9. [DOI] [PubMed] [Google Scholar]
- Cha JH, Frey AS, Alsdorf SA, Kerner JA, Kosinski CM, Mangiarini L, Penney JB Jr, Davies SW, Bates GP, Young AB. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington's disease. Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):981-9. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersén A, Puschban Z, Lotharius J, NicNiocaill B, Wiekop P, O'Connor WT, Brundin P. Evidence for dysfunction of the nigrostriatal pathway in the R6/1 line of transgenic Huntington's disease mice. Neurobiol Dis. 2002 Oct;11(1):134-46. [DOI] [PubMed] [Google Scholar]
- Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, Ernfors P, Alberch J. Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington's disease. J Neurochem. 2005 Jun;93(5):1057-68. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann N Y Acad Sci. 1997 Dec 29;836:158-81. Review. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000 Sep-Oct;62(5):633-8. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005 Jan;28(1):1-8. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002 Aug 15;156(4):328-34. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Leavitt BR. The clinical and genetic features of Huntington disease. J Geriatr Psychiatry Neurol. 2010 Dec;23(4):243-59. Epub 2010 Oct 5. Review. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Sills T, Anderson KE, Bachoud-Lévi AC, Borowsky B, Craufurd D, Duff K, Giuliano J, Groves M, Guttman M, Kupchak P, Ho AK, Paulsen JS, Pedersen KF, van Duijn E, van Kammen DP, Evans K. Assessment of Depression, Anxiety and Apathy in Prodromal and Early Huntington Disease. PLoS Curr. 2011 Jun 17;3:RRN1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath MK, Horton ZA, Tippett L, Hogg V, Collins VR, Churchyard A, Velakoulis D, Roxburgh R, Delatycki MB. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord. 2010 Jul 30;25(10):1444-50. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124(4):985-92. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Mathé AA, Brené S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2010 Jul;20(7):820-8. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003 Apr 1;23(7):2889-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999 Jan;276(1 Pt 2):R152-61. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience. 2006 Aug 25;141(2):569-84. Epub 2006 May 22. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC Neurosci. 2008 Apr 1;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NI, Glynn D, Morton AJ. "Brain training" improves cognitive performance and survival in a transgenic mouse model of Huntington's disease. Neurobiol Dis. 2011 Jun;42(3):427-37. Epub 2011 Feb 13. [DOI] [PubMed] [Google Scholar]
- Potter M, Yuan C, Ottenritter C, Mughal M, van Praag H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington's disease. PLoS Curr. 2010 Dec 7;2:RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Barkus C, Murphy M, Hannan AJ. Gene-environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington's disease transgenic mice. Neurobiol Dis. 2008 Mar;29(3):490-504. Epub 2007 Nov 24. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington's in mice. Nature. 2000 Apr 13;404(6779):721-2. [DOI] [PubMed] [Google Scholar]