Abstract
The most common primary brain tumor in adults is glioblastoma. These tumors are highly invasive and aggressive with a mean survival time of nine to twelve months from diagnosis to death. Current treatment modalities are unable to significantly prolong survival in patients diagnosed with glioblastoma. As such, glioma is an attractive target for developing novel therapeutic approaches utilizing gene therapy. This review will examine the available preclinical models for glioma including xenographs, syngeneic and genetic models. Several promising therapeutic targets are currently being pursued in pre-clinical investigations. These targets will be reviewed by mechanism of action, i.e., conditional cytotoxic, targeted toxins, oncolytic viruses, tumor suppressors/oncogenes, and immune stimulatory approaches. Preclinical gene therapy paradigms aim to determine which strategies will provide rapid tumor regression and long-term protection from recurrence. While a wide range of potential targets are being investigated preclinically, only the most efficacious are further transitioned into clinical trial paradigms. Clinical trials reported to date are summarized including results from conditionally cytotoxic, targeted toxins, oncolytic viruses and oncogene targeting approaches. Clinical trial results have not been as robust as preclinical models predicted; this could be due to the limitations of the GBM models employed. Once this is addressed, and we develop effective gene therapies in models that better replicate the clinical scenario, gene therapy will provide a powerful approach to treat and manage brain tumors.
Keywords: Glioma, gene therapy, dendritic cells, CD4T cells, CD8T cells, immunotherapy, cytokines, Flt3L, HSV1-TK
A. INTRODUCTION
Malignant brain tumors constitute one of the most devastating forms of human cancer. Approximately 40% of all primary brain tumors arise from transformed glial cells and are therefore classified as gliomas. Astrocytomas are a hetereogeneous group of tumors, which range from low grade to high grade anaplastic lesions, including the most aggressive variant, gliomblastoma multiforme (GBM). GBM is a progressive tumor, acquiring genetic mutations as it becomes increasingly aggressive. While primary GBM arises and progresses rapidly to death, secondary GBM develops over time evolving by mutation from lower grade tumor types into GBM. After surgical resection, the incidence of GBM recurrence is high and the mutations found in recurrent GBM differ from those in the primary lesion.. The current standard of care for GBM includes surgical debulking of the tumor mass which is accessible to the neurosurgeon, followed by temozolomide chemotherapy and radiotherapy [1–4]. In spite of advances in all these treatment modalities, mean survival after diagnosis is currently ~12–18 months post-diagnosis while the 5 year survival rate remains at <10% [1, 5]. Interestingly, recent evidence suggests that subpopulations of glioma patients may exist based on their survival time post-treatment. Characterization of these patients using gene expression and epigenetic profiling revealed long-term survival differences after conventional treatments that far surpass all expectations, even after using the most modern and aggressive forms of treatment available to date [6–10]. The better surviving gliomas displayed a more differentiated phenotype defined by overexpression of genes involved in neurogenesis [11]. Another example is the methylation status of the MGMT promoter. MGMT, O(6)-methylguanine-DNA methyltransferase, is a DNA repair enzyme that antagonizes the genotoxic effects of alkylating agents, such as temozolomide. MGMT promoter methylation leads to silencing of MGMT gene expression and is associated with a more favorable outcome in patients with glioblastoma treated with temozolomide [2, 12–15].
Due to the highly invasive nature of GBM, it is impossible for the most skilled neurosurgeon to remove all the tumor mass, usually leaving behind tumor remnants which cause the recurrences leading to the death of the patient (for a review of treatments targeting invasion see [16]). Furthermore, in some instances, the tumor is located in areas of the brain which makes total resection impossible, due to side effects such as neurological deficits and immediate morbidity. Also, increasing the field or dose of radiation therapy will yield unacceptable tissue damage, necrosis, edema and long term neurological deficits.
Due to the limitations of current treatment modalities, efforts are being directed at improving chemotherapeutic agents and more efficient delivery techniques which will improve the diffusion of the drugs through the blood brain barrier and the tumor mass. In addition, novel treatment modalities based on the delivery and expression of therapeutic genes which can induce tumor cell death, inhibit tumor angiogenesis, and induce an effective immune response against the GBM are being very actively pursued. In this review we will cover gene therapy approaches which harness the effects of cytotoxic tumor cell death, caused by either conditional cytotoxic genes, or direct cytotoxic approaches using toxins, in combination with immune stimulatory approaches to induce the generation of an effective systemic immune response against the tumor. These techniques in combination with current treatment modalities will greatly improve the prognosis and extend the lifespan of patients affected with this devastating form of brain cancer.
B. MODELS OF GLIOMA
The study of tumorigenesis and the evaluation of new therapies requires accurate and reproducible brain tumor animal models, which reduce the exposure of patients to non efficacious or unsafe drugs. Ideally, models of glioma should exhibit key features of the human disease state including glial differentiation of tumor cells, diffuse infiltration, neovascular proliferation, regional necrosis, and resemble progression kinetics and antitumor immune responses [17, 18].
In vivo tumor models developed after intracranial or subcutaneous implantation of glioma cell lines in rodents are widely used in cancer therapy research. The advantages of these glioma models are their highly efficient gliomagenesis, reproducible growth rates and an accurate knowledge of the site of the tumor. Some of the most widely used rat brain tumor models include 9L gliosarcoma, C6 glioma, CNS-1 glioma, F98 glioma, RG2 glioma and RT-2 induced glioma [19, 20]. CNS-1, F98 and RG2 glioma cells are excellent sources for brain tumor models, due to their glial phenotype, reproducible in vivo growth rates and histological features that closely resemble human glioma, being nonimmunogenic in syngeneic rats [20–23]. These models display some of the histopathological features of human GBM, including infiltration of neo-plastic cells throughout the surrounding brain parenchyma, areas of necrosis, pseudo-pallisade structures, micro-vascular hyperplasia, and hemorrhages. These models they are technically straightforward and highly reproducible [21, 24–28], constituting good systems to test therapeutic efficacy in vivo. The fact that these animal models have an intact immune system, makes them a valuable tool to test immunotherapeutic approaches [24, 25, 29–32].
Mouse glioma models are also available for brain cancer research. Human glioma xenografts, including SF-295, U-251, D54 and U87, are implanted in immunocompromised mice are extensively used [33–44]. However, the impairment of immune-mediated events that occur during tumorigenesis and anti-cancer therapies limits their usefulness for evaluating novel immunotherapeutics. Syngeneic mouse models, including GL26 [45] and GL261 [46] cell lines, which are non-immunogenic when injected into C57BL6 mice, and SMA-560 cells in in VMDK mice [47] have shown to be useful for studying the response of brain tumors to immunotherapy [20, 30, 46]. A recent syngeneic glioma cell line derived from spontaneous tumor in a transgenic animal called 4C8, shows histological features of human gliomas and constitutes a promising animal model for anti-cancer therapy experimentation [48].
To more accurately represent the spontaneous development of glioma, genetically engineered mouse models have also been generated by modifying genes known to be altered in human gliomas [49–51], including downregulation of tumor suppressor genes such as p53 and PTEN as well as elevated expression of growth factors, and their cognate tyrosine kinase receptors, such as PDGF and EGFR are found in a high percentage of human GBM tumors [52–55]. Genetic glioma models have advantages over cell implantation models, in that they mimic molecular and histological features of human brain tumors, as well as the tumorigenic process itself [56]. Although cell implantation allows probing site-specific effects and offers an easy and reliable model to test therapies, genetic glioma models simulate the interactions between the tumor and the surrounding brain tissue as well as the time course of gliomagenesis and progression [56].
Different approaches have been used to develop genetic models of glioma. Trangenic mice have been developed with germline deletions of the tumor suppressor genes p53 or NF1 were found to increase the susceptibility to astrocytoma and glioblastoma in mice [57]. Another approach is to deliver tumorgenic genes into the brain of pre-natal or adult rodents to induce the formation of endogenous brain tumors. These tumors harbor the genetic abnormalities found in human GBM, as well as the histopathological hallmarks of human GBM, including an aggressive invasive phenotype. The degree of penetrance, tumor latency, and histopathological characteristics are dependant on the species and age of animals, the identity of specific genetic alterations and the vector system used to deliver them, and the anatomical location of genetic alterations. Examples of viral vector mediated brain tumor models include retroviral-mediated delivery of PDGF [58, 59], retroviral-mediated delivery a constitutively active form of epidermal growth factor receptor gene (EGFR) in combination with basic fibroblast growth factor (bFGF) or ckd4 into the brain of neo-natal transgenic mice [60], and lentiviral vector delivery of H-Ras or AKT into the brains of neo-natal transgenic mice [61].
Another recent approach to induce endogenous GBM in mice is the use of the Sleeping Beauty (SB) transposable element to achieve integration of human oncogenes into the genome of brain cells of neo-natal immune competent mice [62, 63]. Plasmids harboring up to three genetic alterations (AKT, N-RAS, EGRFvIII, and/or shRNA specific for p53) in combination with a plasmid encoding for the SB transposase enzyme were delivered into the brain of three different neonatal mice strains [62]. The histological characteristics of the tumors were dependant of the combination of genetic lesions introduced to the mice, although most resembled human astrocytoma or GBM. In some mice, multifocal tumors, another hallmark of human GBM, were observed. These tumors were highly invasive and immunoreactive for nestin and GFAP indicating heterogeneity in the tumor mass.
C. TARGETS FOR GENE THERAPY OF GLIOMA
Preclinical progress using animal models has led to the characterization of potential gene therapeutic approaches for glioma. Conditional cytotoxic approaches introduce non-cytotoxic enzymes into the glioma which upon prodrug administration convert the prodrugs into toxic compounds capable of killing tumors. Anti-angiogenic paradigms are designed to prevent the vascularization of tumors which is required for growth and metastasis. Immune stimulatory approaches seek to use the patient’s own immune system to target and destroy tumors; this approach ideally also would involve induction of immunological memory to protect against disease recurrence. Targeted toxin strategies utilize receptors specifically overexpressed on glioma cells to target the toxins directly into tumor cells, specifically destroying these cells. Also, tumor suppressor and oncogenes are targets for gene therapy and utilize the genetic abnormalities of the tumor as a therapeutic target. Substantial progress characterizing potential treatments preclinically has occurred in all five target areas and will be summarized in subsequent sections.
1. Replication-Deficient Vectors Encoding Conditional Cytotoxic Genes to Treat Brain Tumors
In targeting brain tumors with conditionally cytotoxic therapies the goal is to achieve highly specific destruction of tumor cells without toxicity to normal tissue or induction of a systemic immune response against healthy tissues/organs. Conditionally cytotoxic gene therapy delivers an enzyme into tumor cells which is non-cytotoxic until the administration of a likewise, non-cytotoxic prodrug. Upon prodrug administration, the therapeutic enzyme converts the non-cytotoxic prodrug into a toxic metabolite able to induce cell death.
Initial investigations sought to exploit prodrug activation using endogenous enzymes expressed at higher levels in tumor cells [64, 65], however; clinical application was limited since such enzymes were expressed in normal cells and only a small number of human cancers had high enough levels of activating enzymes to elicit efficacy in cancer therapy. To overcome these problems, identification of non-mammalian enzyme/prodrug combinations was undertaken. Use of viruses to specifically deliver enzymes to tumors has produced promising results in vitro and in vivo.
For therapy to be successful the enzyme must be expressed exclusively within the tumor cells and its catalytic activity be high enough for clinical benefit without toxicity to normal tissue. Since expression will not occur in all tumor cells, a significant bystander effect is essential. Bystander effects occur when the cytotoxic metabolite is transmitted to cells not originally transduced with the enzyme. This may occur via transport through gap junctions or by diffusion through the extracellular space. In addition to delivery of the enzyme, delivery of the prodrug must be delayed sufficiently to allow expression of the enzyme in target cells.
A large number of enzyme/prodrug combinations have been discovered and characterized in brain tumor treatment. The most well characterized conditionally cytotoxic combinations are herpes simplex virus type-1 thymidine kinase (HSV1-TK)/ganciclovir (GCV) and cytosine deaminase (CD)/ 5-fluorocytosine (5-FC). In addition to these well characterized pairings, cytochrome P450/CPA, E. coli purine nucleoside phosphorylase/6-methyl-purine-2’-deoxynucleo-side, carboxypeptidase/methotrexate-α-phenylalanine have all been under investigation for use in brain tumor treatment (for review see [66, 67]).
a. Herpes Simplex Virus Type-1 Thymidine Kinase/ Ganciclovir (GCV)
HSV1-TK was first developed as a prodrug-activating enzyme by Moolten and has been studied intensively in preclinical and clinical studies to treat a wide range of solid tumors [68, 69]. In addition to wild-type TK, several TK mutants have shown increased TK mediated effects in glioma models [70, 71]. The prodrugs gancyclovir (GCV) or valacyclovir (Vatrex®, VCV), are acyclic analogs of DNA nucleoside 2-deoxyguanosine which HSV1-TK phosphorylates to convert into a toxic DNA analog which triggers tumor cell death.
HSV1-TK/GCV pairing was the first in which bystander effects were described [72]. In murine glioma studies, total tumor regression was observed when at least 10% of tumor cells were transduced with HSV1-TK [72–75]. GCV-triphosphate moves between cells via gap junctions [76–79] and triggers cell death through cell:cell contact.
Delivery of HSV1-TK into intracranial tumors has been successfully accomplished using replication-deficient retroviral vectors [80], retroviral packaging cells [80–82], HSV vectors [64, 83, 84] replication deficient adenoviral vectors (Fig 1) [31, 85–88], and adeno-associated vectors [89, 90]. Treatment triggered infiltration of CD4+ and CD8+ T cells and macrophages as well as increased expression of a host of cytokines [86, 91]. Induction of the immune system resulted in tumor regression locally at the site of HSV1-TK/GCV action and at distant sites in both normal and immuno-compromised animals [92–94] (Fig. 1). CTL mediated regression of tumors produced long-term immunity to subcutaneous tumors. Likewise, treatment of subcutaneous tumors triggered regression of intracranial tumors even if the intracranial tumor was established before CTL response to the subcutaneous tumor was fully activated [90]. While HSV1-TK efficiently destroys tumor cells in the brain, long-term expression of HSV1-TK can result in chronic inflammatory [26, 95] responses making the use of regulatable vectors a promising approach [96, 97]. Transduction of cells with HSV1-TK and treatment with GCV renders cells more sensitive to both chemotherapy and radiation suggesting that using multiple treatment modalities will produce more effective tumor regression [98–101]. In addition to combining standard therapies, combining HSV1- TK with immune stimulatory strategies is under investigation and shows promise for more efficient tumor destruction. HSV1-TK has been combined with TNFα [102–104], IL-4 [105, 106], Flt3L [23–25, 29, 30, 32, 107], decorin [108] and connexin 43 [109] to attempt increased efficacy in preclinical GBM models.
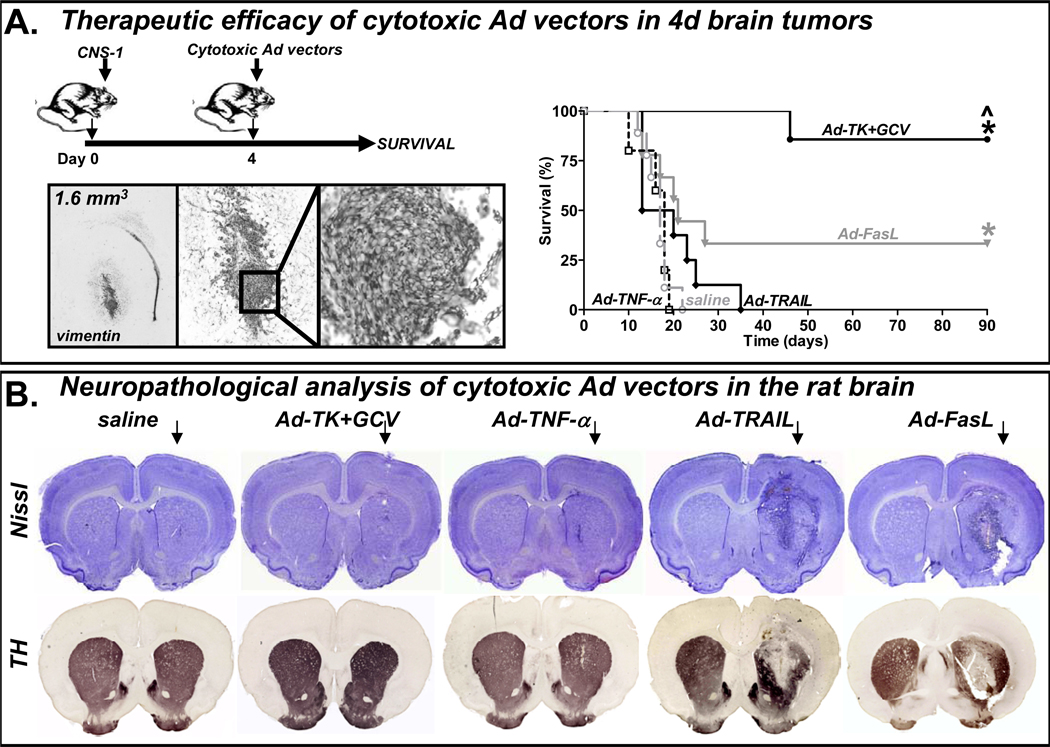
Figure 1. Comparative assessment of in vivo efficacy and toxicity of adenovrial vectors encoding cytotoxic transgenes after injection into normal brain.
(A) Lewis rats were implanted with CNS1 cells in the brain and then treated 4 days later with an intratumoral injection of either saline, or adenoviral vectors encoding TNF-α (Ad-TNF-α), TRAIL (Ad-TRAIL), FasL (Ad-FasL) or thymidine kinase (Ad-TK). Rats treated with Ad-TK received GCV. Kaplan Meier survival curves are shown *p<0.05 vs saline, ^p<0.05 vs Ad-FasL (Mantel log-rank test). Representative microphotographs show the appearance of the tumor at the time of treatment (day 4), as assessed by vimentin staining. (B) To assess neuropathology, each vector was injected into the naïve Lewis rat brain. 7 days later neuropathological analysis of the brain was assessed by Nissl staining and immunocytochemistry using antibodies against tyrosine hydroxylase (TH).
b. Cytosine Deaminase/5-Fluorocytosine
As with HSV-ITK, cytosine deaminase (CD) produces a toxic nucleotide analog that triggers cell death. CD is not found in mammalian cells but occurs in bacteria and fungi catalyzing the conversion of cytosine to uracil. When combined with the prodrug 5-fluorocytosine (5-FC), deamination generates 5-fluorouracil (5-FU) which ultimately triggers cell death through inhibition of thymidylate synthase. CD/5-FC results in a strong bystander effect that is not cell contact specific [110]. Transduction of only 2–4% of cells resulted in significant regression of tumor as toxic metabolites diffuse freely[111, 112].
Delivery of CD either by replication-deficient adenovirus, oncolytic adenovirus or retrovirus caused tumor regression of both C6 and 9L rat models of glioma [42, 113–115]. Areas of necrosis surrounded by apoptotic cells were observed [114] as was demylenation and gliosis within areas of normal brain tissue. Both HSV1-TK and CD therapeutics result in apoptosis of cells that is independent of p53 or death receptors [116]. Mitochondrial caspase activation is required in both modalities to induce apoptosis[117]. To increase efficacy combination of CD/5FC with HSV1-TK/GCV results in faster and more complete tumor regression than either single therapy alone [118, 119]. Likewise CD cytotoxicity is enhanced by radiation therapy although damage to normal brain can also occur requiring strict definition of both therapeutic modalities [120]. Recent studies have demonstrated that human neural stem cells transduced with retrociral vectors encoding cytosine deaminase displayed remarkable 'bystander killer effect' on the glioma cells [121].
c. Cytochrome P450/Cyclophosphamide
Cytochrome P450 converts cyclophosphamide (CPA) into a mustard like toxin which triggers DNA crosslinking and protein alkylation [122]. CPA can be activated by endogenous Cytochrome P450 in human liver requiring monitoring of liver function in studies involving this enzyme prodrug combination [123]. Cytochrome 450/CPA bystander effects do not require cell contact as metabolites released from the cell can trigger cytotoxicity in cells not directly transduced with cytochrome P450 [124]. Intracranial delivery of cytochrome P450 by adenovirus or retrovirus into either 9L or C6 glioma models resulted in at least a partial regression of tumor and prolonged survival [125, 126]. In addition to CPA, cytochrome P450 produces cytotoxic effects in glioma cells when other prodrugs are used alone or in combination with CPA [66, 127–130]. Also, chemotherapy combined with cytochrome p450 gene therapy showed greater efficacy than either treatment alone[131]. A recent study demonstarted that primary neural stem/progenitor cells (NSPC) expressing cytochrome p450 2B6 (CYP2B6) can migrate to the tumor bearing hemisphere when implanted at distant sites in the brain parenchyma to hinder tumor growth through local activation of CPA [132] .
d. E. coli Purine Nucleoside Phosphorylase
E. coli purine nucleoside phosphorylase (PNP) converts nontoxic purine nucleoside analogs into toxic adenine analogs to block both mRNA and protein synthesis. PNP can be combined with multiple prodrugs including 6-methylpurine and F-araAMP[133, 134]. High bystander activity which is cell contact independent may allow widespread tumor death from a relatively small dose of PNP. Delivery of PNP by adenovirus into subcutaneous glioma cells tumors resulted in tumor elimination when only 2–5% of cells were directly transduced [135]. Subcutaneous tumors generated from glioma cells retrovirally transduced to express PNP showed regression upon prodrug administration [133, 136].
e. Carboxypeptidase G2
Carboxypeptidase G2 (CPG2) is found in bacteria but not humans and removes glutamic acid moieties from folic acid, inhibiting cell growth. When combined with the prodrug [137] 4-benzoyl-L-glutamic acid (CMDA), a DNA-crosslinking mustard drug is released [138]. Unlike HSV1-TK and CD, catalysis of the prodrug with CPG2 does not require further enzymatic processing to become the final toxic compound. Mustard-alkylating agents are not cell-cycle dependent enabling the killing of proliferating and non-proliferating cells [139]. As with other enzyme/prodrugs, CPG2/CMDA produces a robust bystander effect. Only 10–12% transduction resulted in 50–100% killing in vitro or in vivo [137, 140]. Replication-deficient adenoviral vector delivery of CPG2 into glioma cells which were resistant to chemotherapeutic drugs and not killed by HSV-TK/GCV showed 70% cell killing [141].
2. Suppression of Angiogenesis
Large tumors consist of poorly vascularized but densely packed cells through which nutrients and oxygen do not permeate readily. Angiogenesis involves the rapid proliferation of endothelial vascular cells, culminating in the formation of new blood vessels, and is tightly regulated in adults. This regulation is coordinated by the expression of both activators and inhibitors of angiogenesis. As tumors increase in size, a need arises for vascularization within the tumor mass. Therefore, a selective pressure is placed on the tumor cells to alter the expression of promoters and inhibitors of angiogenesis and in doing so to stimulate the development of new vasculature.
Glioblastoma is among the most highly vascularized of all tumors; consequently, angiogenesis has received much attention as a potential therapeutic target. These therapies are expected to have few serious side effects because angiogenesis in healthy adult humans usually only occurs in response to pathological insults from wounds or hypoxia. Several of these angiogenic inhibitors have been shown to reduce tumor growth in vitro and in vivo [142]. However, a number of disadvantages limit the potential of angiogenic inhibitors in clinical setting. First, production of sufficient quantities of angiogenic inhibitors is expensive limiting their availability for large clinical trials. Synthetic small molecule inhibitors of angiogenesis are being developed to overcome this problem [143] but the side effects of these drugs are unknown.. Second, angiogenic inhibitors are believed to be cytostatic, not cytotoxic requiring long-term treatment to control and ultimately reduce tumor size [142]. Third, toxic side effects have been observed with systemic delivery of some angiogenic inhibitors [144]. Gene therapy offers distinct advantages to deliver clinically effective doses of angiogenic inhibitors to the tumor and has been successfully employed in the treatment of a variety of tumors in preclinical studies [145].
a. Targets That Promote Angiogenesis
The first growth factor identified as a positive regulator of angiogenesis was basic fibroblast growth factor (bFGF) [146] and increased expression of bFGF correlates with progression of a wide variety of solid tumors [147]. Adenoviral gene transfer of bFGF was found to promote angiogenesis in rat brains [148]. However, a clear correlation between increased bFGF expression and glioma progression has not been demonstrated in glioma suggesting that bFGF is not the main mediator of angiogenesis [149]. Another promoter of angiogenesis is vascular endothelial growth factor (VEGF) which was found to be overexpressed in high grade gliomas [150]. Expression of the receptors for VEGF, Flt-1 (VEGFR-1) and Flk-1 (VEGFR-2), are also elevated in glioblastoma in comparison with surrounding normal tissue and Flk-1 in particular is believed to promote angiogenesis in response to VEGF [151]. Transfection of anti-sense VEGF cDNA into rat glioma C6 cells in vitro impaired C6 tumor cells growth in comparison to controls when subsequently implanted into nude mice [152]. Recombinant viruses have also been used to transfer anti-sense VEGF cDNA sequence and rats with intracranial neoplasms showed a statistically significant improvement in survival when treated with this retrovirus [153]. Recenly, a lentiviral vector delivery of shRNA sequences specific for VEGF and IL-6 showed promise in an in vivo model of GBM [154]. A VEGF receptor that displays dominant negative function when overexpressed in cells has also been developed and was expressed by a retrovirus. Survival was successfully prolonged in rats with intracranial tumors and these tumors displayed many classical signs of impaired angiogenesis including reduced vascular density and elevated necrosis [155, 156]. Urokinase Plasminogen activated receptor (uPAR) and Cathepsin B are also overexpressed during glioma progression and have been implicated in promoting angiogenesis. Adenovirus expressing anti-sense uPAR and Cathepsin B and injection of plasmid DNA encoding siRNA sequences targeting uPAR and Cathepsin B inhibit glioma growth, invasion and angiogenesis [157, 158]; downregulation of uPAR using plasmids encoding uPAR and Cathepsin B specific shRNA sequences induces caspase-8-mediated apoptosis in the human glioma cell line SNB19 [159].
b. Targeting Inhibitors of Angiogenesis
The relatively low percentage of cells transduced by recombinant viral vectors is a limiting factor in inhibiting targets which promote angiogenesis. Inhibitors of angiogenesis overcome this problem and have been the subject of numerous pre-clinical studies. Many naturally occurring inhibitors of angiogenesis are derived from proteolytic degradation of the extracellular matrix. Endostatin and angiostatin are generated following the proteolytic cleavage of plasminogen and collagen respectively and are potent inhibitors of angiogenesis [160, 161]. These peptides are difficult to generate in sufficient quantities in vitro, and thus are ideal candidates as transgenes for gene therapy. Recombinant viral vectors that express endostatin [162, 163] or angiostatin [164, 165] have been developed and tested in preclinical models of glioma. Improved survival of animals with intracranial neoplasms was observed in all cases and tumor growth rates were reduced by as much as 90%. A recent study has demonstrated that a combined gene therapy approach using adenoviral vectors to deliver soluble endostatin and soluble VEGFR2 leads to inhibition of tumor progression in mice bearing human xenografts [166]. Other anti-angiogenic protein fragments have also been studied for effectiveness in animal models of glioma and these include soluble human platelet factor 4 and the N-terminal fragment of rat prolactin. It appears that these transgenes are not as effective as endostatin and angiostatin in significantly improving survival [167, 168]. A number of proteins associated with immune system function also have anti-angiogenic properties. IL-4 and interferon gamma have been studied in rat models of glioma [169, 170] with improved survival and reduced angiogenesis and tumor growth rates. However, the principal function of these transgenes is in recruiting and modulating various cellular and humoral aspects of the immune response and will be dealt with in the following section.
c. Inhibiting Angiogenesis: the Clinical Experience
Most clinical trials to date designed to study the inhibition of angiogenesis involve the use of monoclonal antibodies that target VEGF or its receptors [171]. The most widely studied antiangiogenic drug is bevacizumab (Avastin; Genentech), which is a humanized antivascular endothelial growth factor (anti-VEGF) monoclonal antibody. Based on improved outcomes in humans with other types of tumors such as colorectal, breast, and small cell lung cancer [172–176], and from improved objective response rates in two phase 2 clinical trials for GBM [177, 178], bevacizumab was granted accelerated approval by the US Food and Drug Administration for use in patients with previously treated GBM. To date, the available clinical data assessing the efficacy of bevacizumab for the treatment of GBM are derived from phase 2 clinical trials and retrospective studies which indicate that the responce rates with bevacizumab-based combination therapy ranged between 38% and 62% with a median response duration of 4.3 months [171]. Gene therapy approaches to deliver these anti-angiogenic compounds could also be implemented clinically if the clinical data would warrant this therapeutic intervention.
3. Immune Stimulation
Histological analysis of tumors reveals that an immune response is often elicited against the tumor. Inflammation, and even tumor-specific lymphocytes are often evident, and in some rare cases, tumor regression spontaneously occurs in response to autoimmune paraneoplastic syndromes [179, 180]. This is believed to be caused by tumor specific antigen expression and underscores a role for the immune system in cancer immunosurveillance and control of disease progression. Unfortunately, most tumors develop counter measures that hamper an effective immune response developing against the growing tumor. As a result, there is significant interest in developing immunotherapies to improve the response of the immune system to the tumor. Gene therapy offers numerous different mechanisms to stimulate an immune response against tumors. We shall briefly outline progress in the four most promising areas.
a. Tumor Antigens Delivered through Adenoviral Expression
Most if not all tumors express proteins that are recognized by the immune system and are called tumor antigens. Adenoviral vectors can be engineered to express these antigens as transgenes and subsequently used to prime an immune response against that target antigen if injected systemically. Promising results from preclinical trials have been reported for renal cell carcinoma among others, where adenovirus expresses the tumor antigen carbonic anhydrase IX protein [181]. However, it is unclear whether this approach would be effective for mounting an effective immune response against gliomas. A recent study identified a glioma neo-antigen GARC-1 in the GL261 cell line with a point mutation that changed the amino acid coding sequence. Moreover, T cell epitope analysis revealed that the point mutation was recognized by CTLs [182]. Recent integrated genomic analysis of over 200 human GBM tumors revealed numerous point mutations and frame-shift mutations in genes such as TP53, RB1, EGFR, PTEN, NF1, IDH1, PIK3Ca, PIK3R1 and ERBB2 [183, 184]. Furthermore, there is evidence that the expression levels of numerous genes are altered in recurrent GBM tumors, i.e., the tumor suppressor gene TP53, the cellular oncogene MDM2, EGFR, and the mismatch repair gene MSH2 [185]. Finally, a recent analysis of GBM tissue from patient samples post-chemotherapy revealed the presence of mutations in the mismatch repair gene MSH6, which are selected during temozolomide therapy and are causally associated with temozolomide resistance [186, 187]. Thus, both viral and non-viral gene delivery systems could potentially be used to deliever GBM neo-antigens to enhance antitumor immune responses.
b. Enhancement of the Immune Response Using Interferons
Interferons are secreted ligands involved in immunity and inflammation. They are potentially valuable targets in gene therapy due to the highly specific immune-stimulatory function of many of these molecules. Type I interferons, including IFN-α, IFN-β and IFN-ω are produced primarily by a specialized population of dendritic cells in response to viral infection and other immune modulators. IFN-α has been shown to elicit numerous antitumor effects including inhibition of cell cycle progression, induction of apoptosis and stimulation of the immune system to destroy tumor cells [188, 189]. In addition, treatment of human glioblastoma cell lines with IFN-α increased cell surface expression of MHC-1 [190]. Intramuscular delivery of plasmid DNA encoding IFN-α significantly reduced the tumor volume in a mouse model of glioma when compared with control animals [191]. IFN-α also promoted regression of intracranial gliomas when co-delivered with dendritic cells directly into the tumor mass [192]. Another type I interferon called IFN-α provides systemic antitumor immunity against GL261 cells when delivered intracranially. This reduces tumor growth and improves survival in C57 BL6 mice through a combination of anti-proliferative effects and also the activation of CD8+ but not CD4+ cells [193]. In another report, combination of IFN-α and dendritic cells was found to suppress tumor growth. This was mediated by a highly effective CTL response against the tumor and was far more efficient that either therapy alone [194]. An adeno-associated virus designed to deliver this transgene has also been developed and completely inhibits growth of exogenous human tumor xenografts in nude mice, further supporting the potential of IFN-α as a novel therapy for treating human glioma [195]. A recent study by the Naldani group exploited the tumor-homing ability of proangiogenic Tie2-expressing monocytes (TEMs) to deliver IFN-alpha to mice bearing intracranial human xenograft tumors tumors [196].
Interferon-gamma (IFNγ) is a Type II interferon that has been shown to increase tumor immunogenicity, disrupt mechanisms of tumor cell proliferatation, and inhibit tumor angiogenesis. Tumor cells and local T cells that comprise the brain tumor microenvironment produce sparse amounts IFNγ [197]. Consequently, gene therapy meadietd delivery of IFNγ into the brain tumor microenvironment could be used to enhance antitumor immune reposnes [198]. IFNγ has been shown to upregulate the expression of MHCI, MHCII, and NK cell activating ligands in both human and murine GBM cell lines [199, 200]. Pre-clinical experiments have shown that intratumoral delivery of IFNγ using either adenoviral vectors [200]or transposon elements [201] enhances the recruitment of lymphocytes to the brain tumor site in orthotopic mouse models of GBM, but does not lead to long-term survival. A recent study using a canine spontaneous model of brain cancer, has demonstrated long-term survival following delivery of an adenoviral vector encoding IFNγ when used in combination with multiple vaccinations with autologous tumor cells mixed with CpG oligodeoxynucleotides. While transient left-sided blindness and hemiparesis occurred following the latter vaccinations, neurological abnormalaties resolved and dog remains tumor-free over 450 days following surgery [202].
c. Enhancing T Cell Activation
A number of cytokines are believed to activate various subclasses of T lymphocytes. For example, IL-12 plays a central role in the induction of T helper 1 (TH1) cells which play a critical role in effective antitumor immunity [203]. Adenovirus expressing IL-12 has been reported to enhance the immune response to brain tumors and improve survival in mice inoculated with GL26 glioma cells intracranially. Increased CD4+ and increased CD8+ T cells were identified at the tumor site [204]. Allogenic cells genetically engineered to secrete IL-2, were found to significantly improve survival in a mouse glioma model [27, 205]. The immune response was found to be predominantly mediated by CD8+ and natural killer cells (NK) and was highly specific for the glioma cells above non-neoplastic cells [205]. Other experimental approaches under investigation to deliver IL-12 for the treatment of brain tumors including using HSV1 vectors [206, 207], oncolytic adenoviral vectors [208, 209], AAV viral vectors [210], Semliki Forest virus virus-like particle (SFV VLP) vectors [211], and PPC polymers [212].
d. Mobilizing Dendritic Cells
Dendritic cells (DCs) are the most powerful antigen presenting cells and are required for the development of an antigen-dependent immune response. DCs differentiate from precursor cells in response to Flt3L expression through a STAT3 dependent mechanism [213]. Expression of Flt3L has been demonstrated to induce tumor regression and significantly improve survival [214]. Furthermore, DCs are highly effective inducers of tumor specific killer and helper T lymphocytes in animal models of cancer [215]. Therefore, interest has been generated surrounding the use of DCs and Flt3L in immunotherapy.
Dendritic cells are scarce within the brain parenchyma except under conditions of inflammation and it is believed that this a major reason for immune privilege of the brain [216–221]. This places limitations on the ability of dendritic cells to migrate to intracranial tumors. One strategy for circumventing this problem is to deliver dendritic cells directly into the intracranial tumor mass [192]. Another approach is to pulse dendritic cells with glioma antigens or autologous tumor cell lysates in vitro, before re-administering these cells in the periphery [7, 222–231]. Our group has developed an alternative strategy to to recruit DCs into the brain tumor mass where they will undergo in situ priming against brain tumor antigens. To do so, we have engineered an adenoviral vector (Ad) expressing human soluble fms-like tyrosine kinase 3 ligand (Flt3L) [24, 232], which recruits bone marrow-derived dendritic cells (DCs) to the brain tumor microenvironment in orthotopic syngeniec mouse [30, 233] and rat models of GBM [24, 25, 232]. In our therapeutic approach, we also administer a second Ad encoding thymidine kinase (TK) [33, 37] to kill the brain tumor cells thereby releasing exposing endogenous brain tumor antigens and innate inflammatory signals, such as HMGB1 [29, 30]. In this combined gene therapy approach, both adenoviral vectors are delivered directly into the tumor mass and cause a potent, antitumor immune response resulting in the rejection of the tumor in 60–80% of animals where all other therapies tested fail (Fig 2). Depletion of either CD4+ and/or CD8+T cells or antigen presenting cells caused the therapy to fail completely, suggesting that by presenting antigen to TH cells, DCs’ primed a potent antitumor immune response. This data highlight the promise of immuno-therapies in enhancing the efficacy of current therapies and the potential of curing the disease.
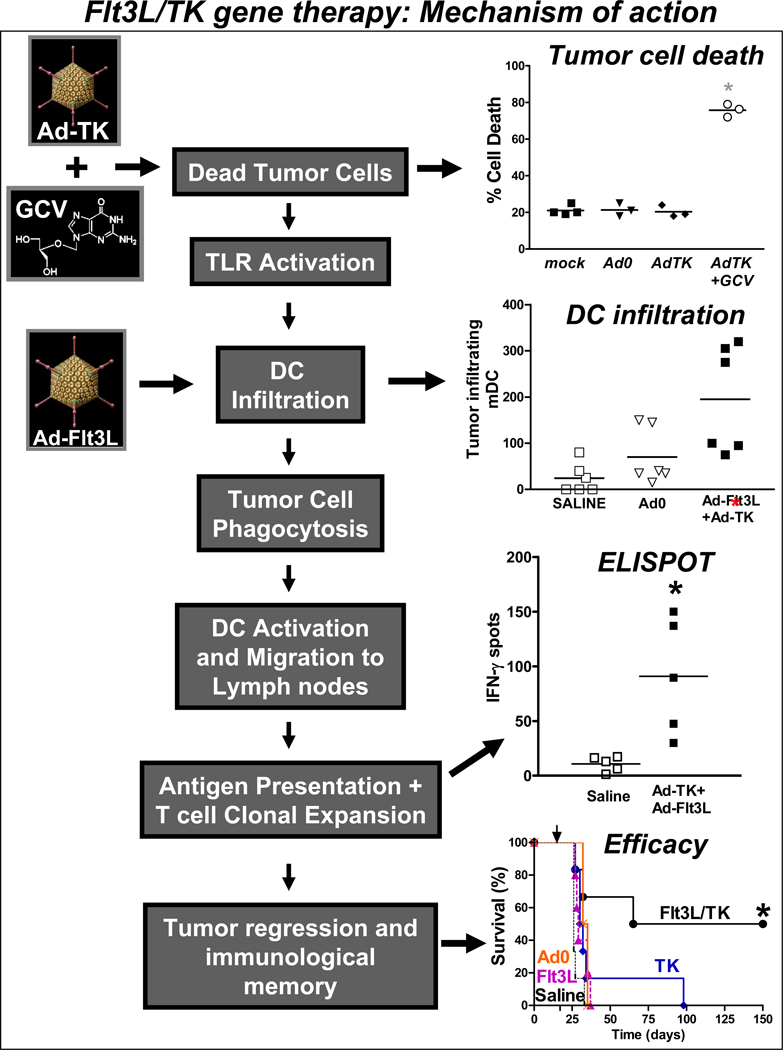
Figure 2. Immunological mechanism underlying the efficacy of combined gene therapy using Ad-Flt3L and Ad-TK (+GCV).
Transduction of tumor cells with Ad-TK (+GCV treatment) leads to tumor cell death and the release of endogenous brain tumor antigens and TLR ligands. Concomittant delivery of Ad-Flt3L into the brain tumor causes the infiltration of dendritic cells (DCs) into the tumor mass. The release of TLR ligands, including the TLR2 agonist HMGB1, results in TLR activation on on tumor infiltrating DCs. DC’s phagocytose tumor cells remnants which lead to their activation and maturation. Mature, loaded DCs migrate to the draining cervical lymph nodes where they present brain tumor antigens to naïve T cells, thereby causing the proliferation of anti-GBM specific T cells. The expansion of anti-GBM specific T cells kills residual brain tumor cells leading to tumor regression and immunological memory.
4. Targeted Toxins
Several cellular receptors are exclusively overexpressed on brain tumor cells have been used to target anti-cancer therapy. Human gliomas in situ overexpress several membrane molecules, including variants of the IL-13 receptor, IL13Rα2 [234–239], the urokinase-type plasminogen activator (uPA) receptor [237, 240] the epidermal growth factor (EGF) receptor [241, 242], and transferrin receptor [243, 244]. These receptors are virtually absent in the normal brain; thus, they have been targeted in preclinical and clinical trials for the treatment of brain tumors, with minimal side effects to normal brain tissue. Natural ligands of IL13Rα2, uPA receptor, EGF receptor, and transferrin receptor, i.e., IL-13, uPA, EGF/transforming growth factor α (TGF-α), and transferrin, respectively, have been fused to the catalytic and translocation domains of highly cytotoxic bacterial products, such as Pseudomonas [242, 245, 246] and Diphteria exotoxins [204, 237, 240, 241, 247–250]. These fusion toxins have shown to be selectively internalized by glioma cells. Once internalized the toxins inhibit protein synthesis, which induces cell death of the targeted cell without affecting normal brain cells. In vitro and in vivo experiments in murine glioma models have shown the efficacy of these approaches (Fig 3) [204, 237, 240–242, 245, 246, 249].
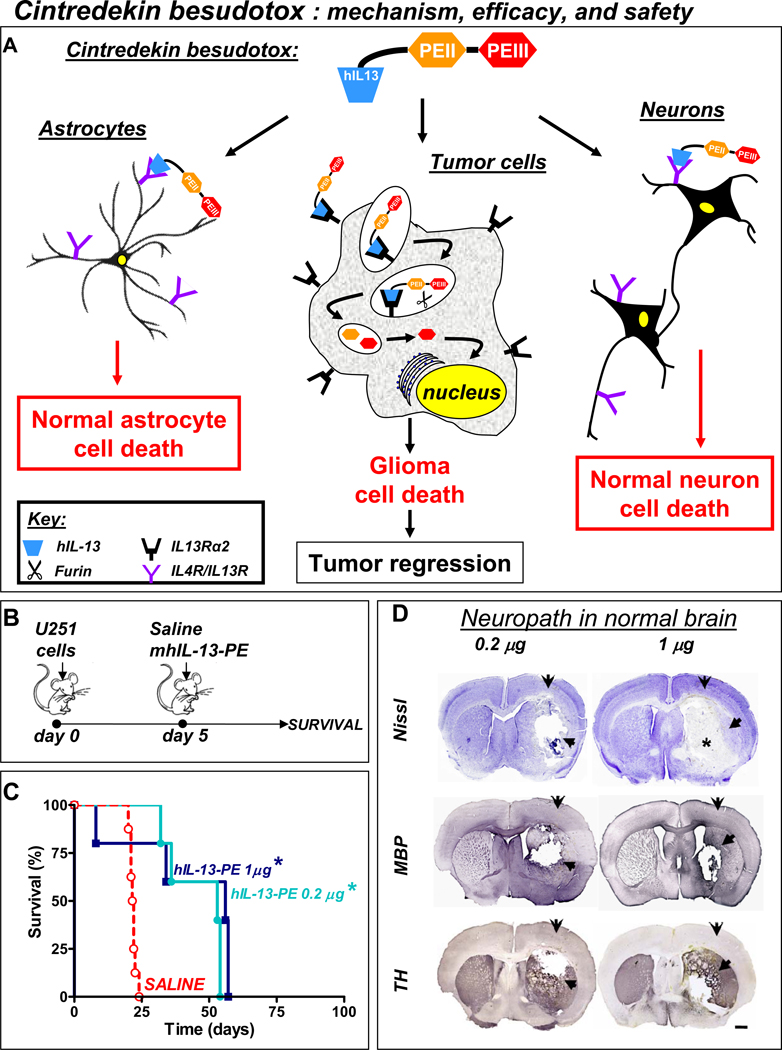
Figure 3. Intratumoral delivery of Cintredekin besudotox improves survival in nude mice bearing orthotopic U251 brain tumors but leads to severe neurotoxicity.
A. Illustration depicting the mechanism of action of the protein formulation Cintredekin besudotox mediated cytotoxicity in glioma cells. B. Nude mice were implanted with human U251 glioma cells in the striatum and 5 days later, animals received an intratumoral injections of either Cintredekin besudotox at a high dose (1 µg) or a low dose (0.2 µg) or saline as control. B. Kaplan Meier survival curves of nude mice bearing intracranial U251 and treated as indicated in A. C. Three days post-delivery of Cintredekin besudotox, mice were euthanized due to severe neurological deficits and neuropathological analysis was assessed by Nissl staining and immunocytochemistry using antibodies against tyrosine hydroxylase (TH) and myelin basic protein (MBP). Note the severe local neurotoxicity of both doses of Cintredekin besudotox when injected into the normal brain parenchyma (arrows).
IL-13 is a cytokine that binds in normal cells to a heterodimeric receptor complex composed of IL-13 receptor and IL-4 receptor. Although this receptor is widely expressed in normal peripheral tissues, it is virtually absent in normal brain tissue [251, 252]. However, IL-13 binds with high affinity to glioma cells [245, 253] due to the overexpression of IL-13Rα2, a restricted monomeric receptor with affinity for IL-13, but not for IL-4 [234, 235, 252, 254–256]. This feature of IL-13Rα2 can be used as a therapeutic target for GBM.
Pseudomonas exotoxin is a cytotoxic bacterial protein which encompasses three functional domains. Domain I binds the α2-macroglobulin receptor, which is ubiquitously expressed in normal tissues, and the exotoxin-α2-macroglobulin receptor complex undergoes receptor-mediated endocytosis [257]. Domain II is a site of proteolytic cleavage that activates the resulting exotoxin and is necessary to catalyze the translocation of the toxin into the cytosol. Domain III directs the processed fragment of the toxin to the endoplasmic reticulum and possesses an ADP ribosylation activity that inactivates elongation factor 2, inhibiting protein synthesis and leading to cell death [257]. The mutant exotoxin, PE38QQR (PE), does not bind to the ubiquitous α2-macroglobulin receptor due to the deletion of domain I [258], and can be linked to various ligands in order to promote its internalization into target tumor cells. In order to target the PE toxin to human glioma cells, a fusion protein was developed by linking the mutated form of Pseudomonas exotoxin to hIL-13 throughout its N-terminal domain, to generate hIL-13-PE [259]. This recombinant protein, also termed IL-13 toxin, is cytotoxic to human glioblastoma cells expressing the IL-13α2 receptor in culture [245, 255, 259] and in human xenograft glioma cells implanted in the flank of nude mice [260]. The targeting of IL-13α2 receptor has been improved by the engeneering of the human IL-13 gene, leading to a mutated IL-13 toxin with higher cytotoxicity and affinity for the IL-13α2 receptor when compared to the wild type IL-13 toxin [261, 262]. The fusion of this muIL-13 to PE resulted in an even more active cytotoxin on glioma tumors both in vitro and in vivo with negligible affinity to IL-13 receptor of normal cells [261]. Intratumoral administration of IL13-PE toxin into intracranial human glioma xenografts in mice showed highly cytotoxic effects without undesirable side effects [39]. Recently our group developed a novel third-generation IL-13-based cytotoxin. To do so, a single high-capacity adenoviral vector (HC-Ad) was engineered to encode mIL13-PE under a bi-cistronic regulatable promoter. To further increase the safety of this therapeutic vector, we also encoded a mutated IL-4 (mIL4, IL-4.Y124D). Since mIL-4 has been found to bind and block the IL13R/IL4R present in normal cells without interacting with IL-13α2R [261, 262], we hypothesized that it would block any potential binding of the mIL-13-PE to normal cells, without affect the binding of the chimeric toxin to neo-plastic cells in the brain. The expression of these transgenes is under the control of the regulatable bidirectional TRE promoter, which leads to tight control of transgene expression, allowing the inhibition of transgene expression by withdrawal of the inducer Dox if adverse side effects were to occur [263, 264]. This approach has several advantages over traditional protein formulations of IL-13 cytotoxins: i) mIL13-PE released from trabsduced cells will exert a powerful by-stander effect, inducing apoptosis of GBM cells expressing the IL-13α2R located within the diffusion range of the toxin thus amplifying the therapeutic efficiency of our approach; ii) this approach is highly specific and exhibits negligible toxicity towards normal brain tissue [265], since mIL-13-PE specifically binds to GBM cells expressing IL-13α2R, sparing normal brain cells and the co-expression of mIL4 blocks any putative negligible binding of the toxin to normal cells. We demonstrated that a single intratumoral injection of the therapeutic vector in intracranial human GBM xenografts and syngeneic GL26 tumors implanted in immune-competent mice leads to tumor regression and long-term survival in 50–70% of the animals (Fig 4) [246].
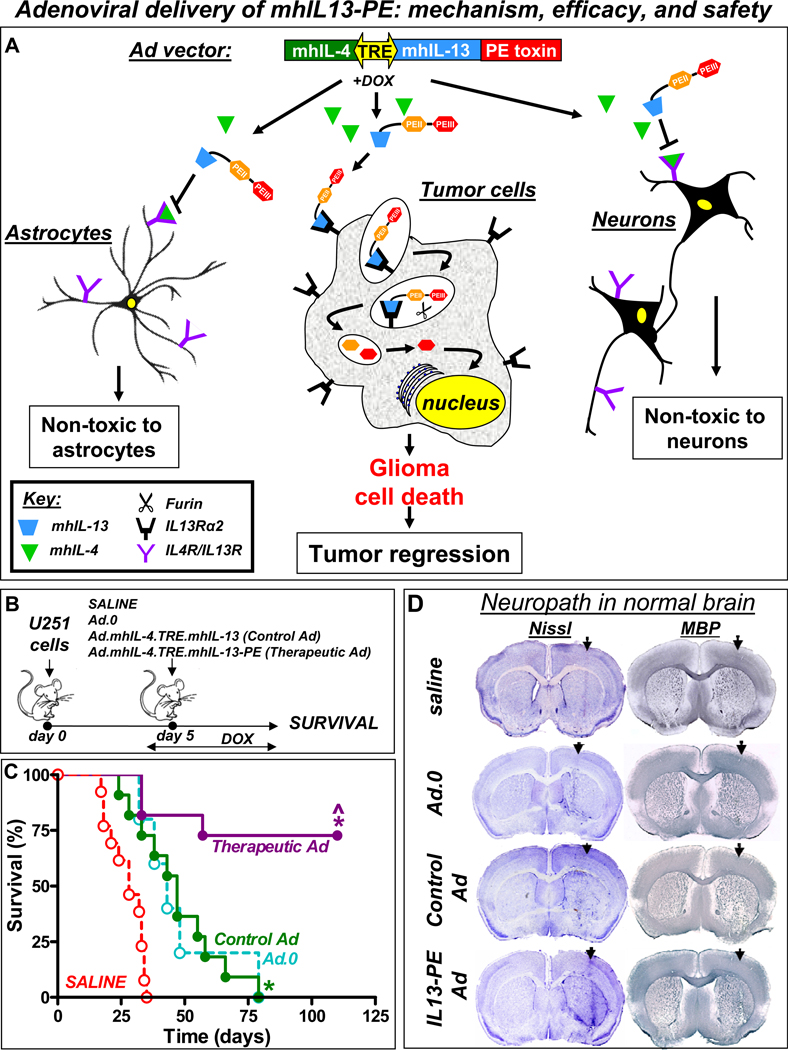
Figure 4. Intratumoral delivery of an adenoviral vector encoding mhIL-13-PE and mIL-4 leads to long-term survival with an absence of neurotoxicity.
A. Illustration depicting the mechanism of action of the adenovirus encoded IL13-PE in brain tumor cells. B. Nude mice were implanted with human U251 glioma cells in the striatum and 5 days later, animals received an intratumoral injection of either a control vector Ad.mhIL-4.TRE.mhIL-13 (without PE) or the therapeutic vector Ad.mhIL-4.TRE.mhIL-13-PE. To “turn on” transgene expression, animals also received a simultaneous injection of an adenoviral vector encoding the TetOn expression cassette (Ad.TetON). Control animals received saline or an empty Ad vector (Ad.0). Animals were fed Dox-chow to switch on transgene expression. B. Kaplan Meier survival curves of nude mice bearing intracranial U251 and treated as indicated in A. *p<0.05 vs saline, ^p<0.05 vs control Ad.mhIL-4.TRE.mhIL-13. Mantel log rank test. C. Naïve wild type Balb/c mice were intracranially injected with saline, control vector Ad.mhIL-4.TRE.mhIL-13 or therapeutic vector Ad.mhIL-4.TRE.mhIL-13-PE and Ad.TetON. Animals were fed Dox-chow to activate transgene expression. Seven days post-vector delivery, neuropathological analysis of the brain was assessed by Nissl staining and immunocytochemistry using antibodies against MBP.
5. Tumor Suppressors, Oncogenes, and other replication competent vectors
All cancerous cells were originally derived from normal precursors. However, cancerous cells harbor harmful mutations in key genes, either tumor suppressors or oncogenes, which regulate proliferation and/or apoptosis. It is widely accepted that tumorigenesis is a multi-step process that requires mutations in many different genes in the DNA of an individual cell, such as genes that promote cell cycle progression, growth factor independence, angiogenesis, increased motility, anchorage independence, decreased levels of apoptosis and reduced sensitivity to chemotherapeutic agents. The genetics of gliomagenesis is well characterized in comparison with other cancers and this information can be used to develop gene therapy that repairs these genetic aberrations. Mutations in four pathways in particular are commonly associated with glioma formation in humans; the P53/ARF/human MDM2 pathway, the P16/Rb/cyclinD/ CDK4 pathway, the receptor tyrosine kinase (RTK)/Ras pathway and the PI3K/PTEN/Akt pathway [51, 266–268]. Viral vectors have been designed that express transgenes commonly mutated in glioma in an attempt to correct the genetic mutations.
a. Tumor Suppressors: p53
P53 is often referred to as “the guardian of the genome” and is mutated or absent in over 50% of all human tumors. Other proteins known to regulate P53 expression such as c-Jun and MDM2, and downstream effectors of p53 including P21 and E2F1 are also frequently mutated in cancer. In fact, mutations in components of the p53 pathway are believed to occur in >90% of all human tumors, including human gliomas. The principal role of p53 as a tumor suppressor is to detect gross genetic abnormalities during DNA synthesis. Expression of p53 is absent in quiescent cells but is expressed in cells during cell cycle progression or in response to genotoxic insults. Once a genetic abnormality has been detected, p53 arrests cell cycle progression and monitors the tumor repair process. If the DNA damage is too great, p53 may induce apoptosis. This altruistic behavior is vital to the collective well being of the organism and greatly reduces the frequency of tumor formation.
Allelic loss of chromosome 17p or mutations in p53 gene are observed with equal frequency in low grade gliomas and high grade glioblastomas [269] suggesting that inactivation of p53 occurs early during gliomagenesis and may be an important target for gene therapy. Re-introduction of wild-type p53 into glioma with p53 mutations has been the subject of intense scientific research. Early results suggested that the re-introduction of p53 reduced the proliferation of glioma cells in vitro and suppressed tumor formation when implanted into nude mice [270]. Adenovirus expressing p53 was later demonstrated to reduce tumor volume by 40% over 14 days in rats, [271, 272]. P53 as a therapeutic transgene is not limited to glioma that have lost P53 function. Overexpression of p53 using viral vectors improved survival against challenge with wild type p53 expressing glioma cell lines, indicating a versatile function for this transgene in treating all forms of glioma [273]. P53 overexpression increases the sensitivity of drug and radiation resistant glioma cell lines to cisplatin and radiotherapy in vitro [274] and adenovirus expressing p53 restored the sensitivity of 9L glioblastoma cells to cisplatin [275] and radiotherapy [276] in pre-clinical models of glioma. P53 increases the expression of numerous apoptotic proteins in cells, including BAX activators Bim and DP5, and the death receptor ligand FasL. In a recent study, adenoviral vectors expressing p53 under the control of the CMV promoter were demonstrated to induce significant levels of apoptosis as measured by DNA ladder when injected intracranially into the tumor. Furthermore, a 100% survival rate was observed in these animals 100 days following viral injection [277]. A number of downstream effectors of p53 such as P21, E2F1 and P16 have also shown promising results in pre-clinical glioma models [278, 279]. In fact, vectors expressing P16 and P21 were more effective than P53 at improving survival [280], although this has yet to be validated in human clinical trials.
An alternative strategy was originally conceived by Bischoff JR and others and takes advantage of the anti-viral properties of p53. The human AdE1B gene is expressed during adenovirus infection and codes for the 55 kDa protein that binds with and inactivates p53. E1B is essential for a successful viral replication cycle within the host cell and adenoviruses lacking the E1B gene are unable to replicate inside cells expressing normal p53. One widely studied oncolytic viral vector is ONYX-015 which is cytopathic against p53-deficient human tumor cell lines implanted in nude mice [281–286].
b. Rb Pathway
The P16/Rb/cyclinD/CDK4 pathway is the most frequently mutated pathway in glioma, and mutations generally characterize a transition from low-grade tumors with relatively slow rate of proliferation to intermediate-grade gliomas with dramatically increased cell proliferation [17]. In normal quiescent cells, Rb is present in a hypophosphorylated form and is bound by the transcription factor E2F1. This prevents transcription of genes important for mitosis and prevents progression of the cell through the G1/S phase restriction point [287]. In gliomagenesis, allelic losses on chromosome 9q or 13q, or amplification of 12q usually accompany transition of glioma from low grade to intermediate grade [21, 288]. This was later found to correspond with loss of Rb (13q14), loss of INK4A and ARF (9p21), or amplification of CDK4 (12q13–14). Adenovirus mediated Rb gene therapy has been successfully used in pre-clinical models of glioma, where it was found to decrease the proliferation of spontaneous pituitary tumors in Rb+/− mice and prolonged survival of animals [289]. In a similar strategy to ONYX-15, a recombinant adenovirus lacking AdE1A (Δ24) can only replicate in cells expressing phospho-RB and is preferentially cytotoxic to glioma cells. A single injection of Delta24 reduced growth of flank tumors by 66%, and multiple injections reduced tumor growth by 84% [290].
Several groups are actively pursuing second generation oncolytic adenoviruses. One such example is Ad5-Δ24RGD, which has a genetically modified capsid that incorporates an Arg-Gly-Asp (RGD) motif into the HI loop of the viral fiber knob. The RGD motif enhances the virus’s affinity for αv integrins, which are abundant in glioma cells [291, 292]. Ad5-Δ24RGD has shown promise in pre-clinical studies using human GBM bearing xenograft nude mice in combination with low dose radiation [291]. Recent studies demonstrate the utility of these third generation oncolytic vectors which incorporate tissue specific promoters into targeted oncolytic adenoviral vectors (CRAd-Survivin-pk7) resulting in improved long-term survival with evidence of enhanced adenovirus infectivity, decreased mitotic activity, and enhanced tumor apoptosis [293–296].
Substantial research has also investigated the potential of P16INK4A to reduce tumor proliferation and improve survival in rodent models of glioma. P16INK4A inhibits Rb phosphorylation and is mutated in more than 50% of glioblastomas [297]. P16INK4A expressing vectors were demonstrated to improve survival in animal models of glioma, even when compared with P53 expressing vectors [280]. In spite of these promising results, caution is warranted with all therapies designed to repair common genetic lesions in glioma. In a recent report, P16INK4A was expressed in glioma cell lines under the control of the Tet repressor system. Elevated P16INK4A reduced tumor proliferation in vivo initially, supporting work published by others. However, long term transgene expression induced a decrease in the expression of Rb suggesting that gene therapy approaches involving P16INK4A may ultimately lead to the selection of Rb deficient tumors [298]. In fact, this is a potential problem of all approaches designed to correct genetic lesions in cancer. Tumor cells are genetically unstable and undergo accelerating genetic mutation. Unfortunately, this accelerates natural selection and will select for tumor cells that overcome this transgene insertion. The possibility of tumor cells compensating for transgene insertion through one or more subsequent mutations must be explored in all promising therapies that repair the primary genetic lesion in cancer.
b. Other replication competent viral vectors
In addition to oncolytic adenoviral and HSV derived viral vectors, other replication competent viral vectors have been used to kill GBM cells including replication-competent retrovirus (RCR), oncolytic reovirus and oncolytic measles virus vectors. RCR vectors are based on murine leukemia virus (MLV) and are only able to infect quiescent cells, thus RCR exhibit high selectivity for tumor cells. RCR and has been shown to achieve highly selective and stable gene transfer throughout entire solid tumors in vivo [299, 300]. In contrast to oncolytic HSV and adenovirus, RCR are not selectively lytic in tumor cells and instead, are engineered to encode conditionally cytotoxic transgenes such as cytosine deaminase (CD) [301] or Escherichia coli purine nucleoside phosphorylase (PNP) [302].
Reovirus can also be used an an oncolytic vector to selectively replicate in GBM, where stimulation of RAS pathway by PDGFR or EGFR inhibits RNA-activated protein kinase activation, thereby enabling synthesis of viral proteins leading to tumor regression in preclinical studies using nude mice bearing orthotopic human glioma xenografts [303]. Safety and efficacy of reoviral vectors in humans was recently demonstrated in a Phase I clinical trial. Delivery of live, replication competent, and genetically unmodified reovirus directly into the tumors of patients with malignant gliomas demonstrated that oncolytic reoviruses are safe and well tolerated with no evidence of clinical encephalitis [304].
Finally, strains of the attenuated measles virus have been shown to preferentially infect and kill tumor cells and not adjacent non-tumor cells. [305]. The measlesvector backbone has been engineered to express soluble marker peptides, such as the human carcinoembryonic antigen (CEA; MV-CEA) gene and the human thyroidal sodium iodide symporter (NIS; MV-NIS virus) gene to masses onitor the in vivo dissemination and elimination of the the viral vector over time [306, 307]. In pre-clinical experiments, MV showed cytotoxicity in the U87, U118, and U251 human glioma cell lines [308]. A Phase I clinical trial of intratumoral and administration into the resection cavity of MV-CEA in patients with recurrent glioblastoma multiforme is currently recruiting patients (http://www.clinicaltrials.gov/ct2/show/NCT00390299).
D. CLINICAL TRIALS OF GENE THERAPY FOR GLIOMA
The current standard of care for GBM involves surgical resection combined with temozolomide chemotherapy and radiotherapy. Even with aggressive conventional therapeutic approaches, mean patient survival is currently steady at 18–21 months [309]. Numerous gene therapy approaches have moved from preclinical studies to clinical trials with the goal of delivering gene-based therapeutics into the tumor mass to trigger tumor elimination and long standing protection against recurrence.
Multiple approaches to specifically target brain tumor cells have been developed and will be discussed. Conditional cytotoxicity delivers non-cytotoxic therapies into tumor cells where upon administration of a prodrug, cytotoxic metabolites are produced which trigger tumor cell death. Targeted toxins specifically deliver toxins like pseudomonas endotoxin or diphtheria toxin into tumor cells by targeting receptors upregulated only on tumor cells. Oncolytic viruses cause tumor cell lysis and viral spread after infection by specifically infecting tumor cells with genetic/metabolic alterations relative to normal tissue
Delivery of conventional and experimental therapeutics into the brain poses a significant challenge in the development of novel treatments for GBM; the bone structure of the cranium, the blood brain barrier, an immune suppressive tumor microenvironment, the brain’s immune privledge all constitute formaidable challenges. The use of convection enhanced delivery (CED) may presently represent the best option to achieve safe widespread distribution of the therapeutic vectors/compounds. By this method several catheters are placed within the target brain area and infusion of the therapeutic is conducted at a continuous and slow rate. CED has been utilized in clinical trials utilizing targeted toxins [310, 311].
1. Conditional Cytotoxicity
The majority of brain tumor related clinical trials utilize viral vectors delivering a Herpes Simplex virus type 1 thymidine kinase (HSV1-TK) gene (mechanism of action described above). To date clinical trials using HSV1-TK to transduce brain tumors have been completed using liposomes, replication-deficient retrovirus producing cells or replication-deficient adenoviruses.
Retroviruses selectively target actively dividing cells making them an attractive vector in the brain where tumor cells are the only rapidly dividing cells. However low titers and unstable virus particles have required the use of virus producing cells (VPCs) instead of direct viral injection into brain [69, 312, 313]. VPCs continuously produce replication-deficient retrovirus vectors with a very low risk of wild-type virus production from recombination events. VPCs are short lived vector producers incapable of migration, limiting their usefullness [312].
Phase one/two clinical trials to determine maximum tolerable dose (MTD) and toxicity of VPCs producing retroviruses expressing HSV1-TK in treatment of brain cancer have been extensively performed. Most studies involve implanting VPCs into the cavity of resected tumors. After VPCs implantation, virus diffused into surrounding tissue and ganciclovir was administered; patients were evaluated for survival and toxicity [69, 314–319]. VPCs in small tumors produced antitumor effects [69, 314] and individual case studies showed increased immune response following treatment [314, 320, 321]. In general however, survival increases were marginal and limited to a small number of the total patients treated in a trial. Bystander and tumor transduction rates were considerably lower than that observed in preclinical studies [69, 322, 323]. The MTD was not determined as all doses used were well tolerated. Concerns for safety resulted in evaluation of anti-virus antibody titers as a systemic immune response to the virus could cause a life threatening situation. While some studies show no change, others showed a small number of patients with increased antibody titers [69, 318], however, no systemic effects caused by the treatment were observed. Evaluation of peripheral blood lymphocytes for wild-type or replication-deficient therapeutic virus showed low or transient presence of therapeutic virus and no wild-type virus outside of the brain [324, 325]. To evaluate survival, a larger randomized controlled trial was conducted once safety and toxicity had been established. A randomized controlled, multicenter trial involving 248 patients found that while VPC-expressing therapeutic vectors were safe, no significant difference in survival was evident [326] requiring further refinement of treatment strategies to reproduce the preclinical effects observed in a clinical setting.
To increase clinical efficacy, combinations of HSV1-TK with immune stimulatory factors have also reached clinical trial stages. VPCs expressing both Interleukin 2 and HSV1-TK and Interleukin 4 and HSV1-TK have been injected into patients [106, 327, 328]. Results combining IL-2 and HSV1-TK indicate that the treatment is safe and causes increased infiltration of immune cells and tumor necrosis [328].
Adenoviral vectors are non integrating, nonenveloped viruses which express transgenes at high levels, are producible at high titers, and infect both dividing and non-dividing cells. Studies comparing either retrovirus producing cells and replication deficient adenoviral vectors’ efficiency in transducing human glioma tumors found higher gene transfer efficiency and greater survival times with replication deficient adenoviral vectors [75, 329].
Phase one trials using replication deficient adenovirus to deliver HSV1-TK into resected tumor beds or intratumorally followed by ganciclovir administration have established that no systemic toxicicty occurs when viral vector administration remain below 1012 viral particles [330, 331]. When 2×1012 vp were injected intratumorally, toxicity with confusion hypoatremia and seizures resulted. Post mortem tumors examined following treatment show areas of necrosis and infiltration of macrophages and lymphocytes consistent with an immune response to the tumor [331].
The primary concern in the use of adenovirus is a systemic immune response to the virus, since the majority of adults have been exposed to and have mounted an immune response to wild-type adenovirus. No systemic or local symptoms consistent with overt inflammatory processes were observed [330–332]. Likewise, while increased anti-adenoviral vector antibodies were reported in some patients, no symptoms associated with this increase were reported [75, 332, 333].
With promising results from toxicity studies, a randomized controlled study was conducted involving 36 patients injecting replication-deficient adenovirus into the tumor bed following tumor resection. Mean survival time of adenoviral treated patients was 70.6 weeks compared to 39 weeks with controls [333]. While not curative, these results were statistically significant and encouraging enough to move to a Phase III clinical trial sponsored by Ark Therapeutics, a U.K. based biotech company.
In April 2009, Ark Therapeutics released an update on promising results from their multi-center Phase III clinical trial using Cerepro®, an adenoviral vector encoding TK [334, 335]. Unfortunately, the European Medicines Agency (EMEA) recently rejected Ark Therapeutics's marketing application for Cerepro® after deciding that the study was statistically underpowered and failed to show sufficient efficacy in terms of postponing death or re-intervention. The decision by the EMEA is currently under appeal by Ark Therapeutics [336].
With the concerns of immunogenecity and inflammation associated with the use of viral vectors for gene therapy, development of non viral vectors to deliver therapeutic genes has also lead to clinical trials. In a phase I/II study of the safety and MTD using liposome mediated delivery of HSV1-TK in patients with recurrent glioma, no systemic side effects or immune response associated with the treatment were observed. HSV1-TK cDNA was detectable in cells up to 70 days after infusion. While treatment was not curative, tumor regression was observed in a majority of patients [337]. In addition to HSV1-TK delivery, clinical trials are underway to deliver interferon β to brain tumors utilizing liposome technology [338].
2. Targeted Toxins
The ability to deliver targeted therapeutics to treat brain tumors is highly desirable to limit the toxic side effects of novel therapies. Specificity in gene therapy can be achieved with the use of targeted toxins. Utilizing biological features unique to tumor cells, delivery of cytotoxic substances can be refined. By, selectively targeting receptors expressed at high levels on tumor cells, vectors can then carry a toxin into the cell to trigger tumor specific cell death.
a. IL-13 Receptor
The interleukins, a class of cytokines, are produced by T cells and mediate immune system activation acting on nearly all immune cell types. To target glioma cells while sparing normal brain tissue, chimeric IL-13 with mutated Pseudomonas endotoxin (PE) [257] has been utilized in clinical trials. Upon binding to the IL-13α2R, receptor-mediated endocytosis occurs and the toxin translocates to the cytosol to ultimately trigger cell death [257].
Phase I/II studies to determine MTD and toxic effects utilizing the protein formulation of IL-13 targeted cytotoxin have been reported in patients diagnosed with malignant glioma [319, 339–344]. Multiple injections or continued delivery was necessary to achieve therapeutic effects [39, 343]. Intratumoral infusions by convection-enhanced delivery caused steroid responsive edema in 1 out of 3 patients. Dose escalation studies have not yet identified a MTD [341]. The average intraparenchymal distribution of the protein formulation of IL-13 targeted cytotoxin ranged from 10 to 15 mm radially from the tip of catheter. Therefore, poor drug distribution could have contributed to the lack of significant clinical responses [345].
To overcome the short half life of the hIL-13-PE protein formulation, we developed regulatable first generation adenoviral vectors to deliver IL-13.E13K, a mutated variant of the hIL13 [261] with a high binding affinity to the GBM-associated IL13Rα2 [246]. In pre-clinical experiments using human GBM xenografts, we demonstrated that adenoviral vector mediated delivery of mhIL-13-PE led to tumor regression and long term survival in ~70% of the animals without causing apparent neurotoxicity [246]
b. IL-4 Receptor
Interleukin 4 (IL-4) is produced by activated T cells, mast cells and basophils and acts synergistically in the early stages of hematopoesis and B cell activation [346, 347]. As with IL-13, linkage to the cytotoxin PE by replacing the binding domain of PE with IL-4 enables targeted killing of IL-4R expressing cells [257, 348].
In phase I testing in patients with recurrent malignant glioma, were treated with convection enhanced delivery of cpIL4-PE using doses based on preclinically efficient dosages to determine toxicity in humans [349]. Following infusion increased intracranial pressure and edema were observed in most of those treated however it caused no permanent neurological deficits and responded to treatment. No other systemic toxicity was noted and biopsy of the treated area showed no toxicity to normal brain. Increased survival was observed.
No systemic effects were observed in phase II studies to determine the MTD and therapeutic volume [350, 351]. IL-4 cytotoxin was not detectible outside of the CNS however increased IgG antibodies to PE were measured. In ongoing trials, a MTD and increased survival have yet to be observed.
c. TGFα
While expressed at low levels on normal brain, epidermal growth factor receptor (EGFR) is often overexpressed in malignant gliomas. Transforming growth factor alpha (TGFα) binds to EGFR. As with IL-13 and IL-3, TGFα replacement of the PE binding domain may allow destruction of brain tumors by selectively targeting the overexpressed receptor present only on tumor cells[352].
In phase I trials TP-38 (TGFα-PE) was evaluated for toxicity and MTD [225, 352–354]. TP-38 was infused into brain tumors and corticosteroids were administered up to 72 hours after infusion to prevent edema. While no systemic toxicity was observed, seizure, headache, neuropathy, fatigue, and visual and speech problems were observed, all of which resolved. The MTD of TP-38 was never reached, however, two dose-limiting toxicities (DLT) were observed and were of neurologic nature [353]. While overall survival was not different between all patients in the study, 3 of 15 patients showed decreased tumor burden.
d. Transferrin Receptor
Transferrin receptors mediate iron transport into cells and are increased on rapidly dividing cells with increased iron requirements [355]. Tumor cells including glioma have increased levels of transferrin receptor on their cells surface .
Diphtheria toxin (DT) is a multiple subunit protein composed of binding and catalytic domains [247, 248]. The catalytic subunit catalyzes the addition of adenosine diphosphateribose onto elongation factor 2 to inhibit protein synthesis and kill cells. Replacement of the binding subunit with transferrin alters the binding specificity and carries DT into glioma cells. An additional mutation (CRM107) decreased nonspecific binding.
Phase I clinical trial of Tf-CRM107 was completed to determine toxicity and MTD [356]. Patients with recurrent malignant brain glioma were infused with the TF cytotoxin, antibiotics and dexamethasone. The MTD was determined to be 0.67ug/ml in a 40ml total infusion volume. Transient increased intracerebral pressure was observed and treated. At the MTD, no local toxicity was observed. No systemic toxicity was observed although transient elevation in serum transaminases and mild hypoalbumnemia were observed. Survival increased from 36 weeks in non responders to 74 weeks in responding patients.
Phase II clinical trial of Tf-CRM107 was undertaken to further study the safety and efficacy of treatment [250, 356]. Forty four patients enrolled in the study. Infusion related edema and seizures were reported both of which responded to therapy. While all patients entered the study in a progressive disease state, 48% of patients saw disease arrest at the time of evaluation and 30% survived beyond one year.
3. Tumor Suppressors and Oncogenes
P53 – In addition to delivery of HSV1-TK, adenoviral vectors have been used to deliver p53 into brain tumors. The p53 gene is critical to normal cell cycle and apoptosis. In human glioma, mutation of p53 or its inactivating proteins are the most frequently found genetic mutations. Inactivation of p53 allows tumor cells to circumvent normal cellular growth controls. Replication-deficient adenoviral viral vectors deliver p53 into glioma cells to inhibit tumor growth and trigger apoptosis [357].
In phase one trials, patients were implanted with catheters to deliver Ad-p53 into their tumor mass. Several days after virus injection, tumors were resected to evaluate the extent of Ad-p53 biological effects. After resection further Ad-p53 was injected into the tumor cavity to determine toxicity [358].
Resected tumors showed p53 transduction however transduced cells were found no farther than 5–8mm from the injection site. Evidence of apoptosis was restricted to a small number of cells. While no active virus outside of the CNS was detected, there were increased anti-adenoviral antibody titers. Some patients developed neurological side effects which responded to corticosteroid treatment. Survival to recurrence was a median of seven months [358].
4. Oncolytic Viruses
While concerns about toxicity to normal tissue with the use of replicating viral vectors have limited their study in humans, the decrease in therapeutic efficiency seen with replication-deficient vectors as studies transition from preclinical to clinical has caused a resurgence in the study of the use of oncolytic or replicating viral vectors. Since widespread distribution of gene therapy products is essential for therapeutic efficacy, development of vectors which promote targeted but high level transduction efficiency are desired. Oncolytic virus infection results in viral replication and cell lysis such that no therapeutic transgene is need as the virus infection itself destroys the tumor mass. Herpes simplex virus, adenovirus, influenza virus, vaccinia virus, vesicular stomatitis virus, Newcastle disease virus, poliovirus, and reovirus are all being investigated for clinical oncolytic virus therapeutics, meanwhile adenovirus and herpes simplex virus have already entered clinical trials[359].
a. Adenovirus
While injection of wild-type virus may be detrimental to normal tissue, selective targeting by mutation of the virus has allowed for more selective killing of tumor cells. Mutated viruses are used to act directly as cytotoxic agents to destroy tumor cells and further spread newly replicated viral particles [357].
ONYX-15
Among the adenoviruses, ONYX-15 has been used in clinical trials of glioma. The E1b region of wild-type adenovirus inactivates host cell p53 preventing apoptosis induction and allowing for viral replication. Originally, mutations in the E1b region of ONYX-15 were thought to render it unable to replicate in cells with normal p53 function [359]. While thought to only to kill cells exhibiting p53 mutations which result in deficits in p53 function, the mechanism by which ONYX-15 induces cell death is under intense investigation [360–363]. In phase one clinical trials to examine toxicity and MTD in resected glioma patents, a maximum dose was not identified with up to 1×1010 pfu being well tolerated [286]. No systemic toxicity was observed even with elevated levels of anti-adenoviral antibodies in several patients. While median survival was 6.2 months from recurrence several patients survived over a year. Development of other oncolytic adenoviruses in addition to ONYX-15 are underway [364].
b. Herpes Simplex Virus
HSV vectors have also been used as replication competent vectors to treat brain tumors. HSV is a non integrating, neurotropic virus with oncolytic properties that may be exploited in targeting brain tumors.
G207
HSV-G207 vectors contain two mutations separating them from wild-type HSV. The HSV γ134.5 gene blocks activation of anti-viral defenses within a cell allowing viral protein synthesis to occur. γ134.5 vectors only infect and replicate in cells without normal protein synthesis controls [365]. The second gene mutated in G207 is UL39 which is required to synthesize nucleotides in nondividing cells [366, 367]. Disabling UL39 with a lacZ insertion disables nucleotide synthesis such that viral replication can only be carried out in actively dividing cells. While the two mutations within the virus confer specificity to G207, intact thymidine kinase gene provides a mechanism to control any herpetic infection that may arise from use of these replicating vectors.
In phase one clinical trials with G207, MTD and toxicity were evaluated [149]. Patents with progressive or recurrent glioma were injected with a single dose of virus. MTD was not established as the highest level 3×109pfu was well tolerated. No herpetic, encephalic or inflammatory effects were observed. While one patient seroconverted showing positive anti-HSV1 antibodies after treatment, no systemic toxicity attributable to G207 treatment was observed. Excluding four surviving patients, mean survival from diagnosis to death was 15.9 months. Phase 1b/2 trials are underway.
A Phase Ib clinical trial of G207 was recently published in which six patients with resectable, recurrent GBM were treated with two administrations of G207, the first administration was directly injected into the tumor mass followed by surgical resection several days later. After tumor debulking, a second administration of G207 was given by multiple injections into the tumor cavity post surgical resection [368]. Results from this trial demonstrated the high safety profile of multiple administrations of G207 with no evidence of encephalitis. Although the Phase Ib study was not designed to demonstrate therapeutic efficacy, viral replication was observed and limited evidence of antitumor activity was reported.
HSV1716
HSV1716 contains a single mutation of the y134.5 gene rendering it unable to replicate in neurons while targeting glioma cells. Phase one clinical trials in brain tumor patients were unable to determine a MTD as up to 1×105 pfu were tolerated well [369]. No encephalitis or herpetic complications were observed and all patients remained seronegative for HSV-1. In an additional trial, recurrent tumor patient tumors were examined after injection of HSV1716 and virus was detectible by semiquantitative PCR [370]. Even in inoculated tumors for which virus was not detectible, reinfection in vitro triggered low level HSV1716 viral shedding indicating persistent long-term effects may be possible [371]. Following trials in which HSV1716 was injected intratumorally, HSV1716 was injected after resection into the rim of the tumor cavity to determine if virus administration could act to eliminate residual tumor cells responsible for rapid tumor regrowth after resection [372]. No treatment related toxcicities were observed. Of twelve patients, 3 survived, one died of non related events, and 8 died after tumor progression. Further clinical trials are ongoing.
c. Newcastle Disease Virus
NDV replication-competent viruses have also been used in clinical trials to attempt treatment of GBM. Preclinically these viruses showed promising tumor regression without recurrence[373, 374]. In a nonrandomized study of glioma patients, tumor cells taken from patients were infected with NDV, irradiated and used to vaccinate the patient. Vaccinated patients survived significantly longer than non-vaccinated controls and the therapy was well tolerated [375, 376].
With promising results from replication competent adeno- and Newcastle disease virus interest in replication competent viruses continues to grow. Preclinical studies indicate the replication-competent retroviral vectors infect and selectively destroy glioma cells while sparing normal brain tissue[115]. These vectors could also be translated into phase I clinical trials.
E. CONCLUSION AND FUTURE PROSPECTS
The treatment of tumors of the central nervous system represents a formidable challenge, further magnified by the fact that the brain is isolated by the blood brain barrier which makes the delivery of high doses of chemotherapeutic agents or gene delivery vectors to the tumor very difficult. This posses the challenge of unacceptable toxic systemic levels of the drugs or vectors [377, 378]. The approaches which propose combinations of local tumor delivery such as immune-stimulation and cytotoxic gene therapies within the tumor mass and/or surrounding tumor cavity are very promising for the effective treatment of GBM. Also, with the introduction of new technologies such as the microchip and convection enhanced drug delivery, it will be possible to achieve local delivery of high doses of the vectors carrying the therapeutic genes, both within the tumor mass and the surrounding area, where the infiltrating tumor cells are localized. These technologies should enable a more effective and constant delivery of the therapies, such as drugs or gene therapy vectors.
Taking gene therapies for GBM and other types of brain cancers to the clinic constitutes a very exciting prospect. One would envisage that when a patient undergoes surgical resection or debulking of the tumor, the neurosurgeon could deliver the gene therapy vectors encoding for a combination of therapeutic genes. By delivering genes which will enhance tumor cells’ death and cytokines/chemokines which will elicit a systemic antitumor immune response, it will be possible to further eliminate tumor cells which have escaped the neurosurgical resection and also mount an effective antitumor immune response. The power of this approach is that the local antigen presenting cells (APCs) will be exposed to tumor antigens in situ as opposed to ex vivo. This will have the added advantage that the APCs will be loaded with the antigens released from the tumor in situ and will have more chance to mount an effective immune response when compared to loading of APCs with tumor extracts ex vivo. This is due to the fact that what kills the patients are the tumor recurrences and the tumor that recurs is very different from the primary tumor which was resected and used to load APCs ex vivo. Using gene delivery vectors with the capability of expressing therapeutic genes long term [95, 379] and under the control of regulatable promoter systems [380], one would eliminate the need of re-delivery of the gene therapies and also, by following the progression of the tumor by MRI or PET techniques, the neuro-oncologist will be able to make an informed decision as to whether the gene therapies will need to be turned on or reactivated for each individual patient. While these exciting prospects for GBM treatment will become a reality in the near future, more work is needed to perfect the gene delivery techniques and also in the development of powerful therapeutic targets. Results from the approaches described in this review will become available after phase I and II gene therapy trials are completed.
Table 1.
Overview of Clinical Trials for Gene Therapy and Targeted Toxins
| Treatment type | Molecule | Vector/Delivery method | Authors of study |
|---|---|---|---|
| Conditional cytotoxcity | TK | Retrovirus | Izquierdo et al., 1997 |
| Ram et al., 1997 | |||
| Klatzmann et al., 1998 | |||
| Palu et al., 1999 | |||
| Shand et al., 1999 | |||
| Harsh et al., 2000 | |||
| Packer et al., 2000 | |||
| Rainov et al., 2000 | |||
| Prados et al., 2003 | |||
| Adenovirus | Sandmair et al., 2000 | ||
| Trask et al., 2000 | |||
| Germano et al., 2003 | |||
| Smitt et al., 2003 | |||
| Immonen et al., 2004 | |||
| Osborne R, 2008 | |||
| Mitchell P, 2010 | |||
| Nonviral | Jacobs et al., 2001 | ||
| Voges et al., 2003 | |||
| Targeted toxins | IL-13R–PE | CED | Weingart et al., 2002 |
| Kuwar et al., 2003 | |||
| Prados et al., 2003 | |||
| Kuwar et al., 2005 | |||
| Kuwar et al., 2006 | |||
| Kuwar et al., 2007 | |||
| Kuwar et al., 2010 | |||
| IL-4R–PE | CED | Rand et al., 2000 | |
| Weber et al., 2003 | |||
| TGFα−PE | CED | Sampson et al., 2003 | |
| Sampson et al., 2005 | |||
| Sampson et al., 2008 | |||
| Sampson et al., 2008 | |||
| Transferrin receptor - DT | CED | Laske et al., 1997 | |
| Tumor suppressor | p53 | Adenovirus | Weaver et al., 2003 |
| Lang et al., 2003 | |||
| Oncolytic viruses | ONYX-015 | Adenovirus | Shah et al., 2003 |
| Chiocca et al., 2004 | |||
| G207 | Herpes Simples Virus | Markert et al., 2000 | |
| Markert et al., 2009 | |||
| HSV1716 | Herpes Simplex Virus | Rampling et al., 2000 | |
| Papanastassiou et al., 2002 | |||
| Harrow et al., 2004 | |||
| Newcastle disease virus | Schneider et al., 2001 | ||
| Steiner et al., 2004 |
ACKNOWLEDGEMENTS
This work is supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grant 1UO1 NS052465.01; NIH/NINDS Grants 1RO1-NS057711; 1RO1-NS074387; 1R21-NS054143 to M.G.C.; NIH/NINDS Grants 1RO1-NS 054193; 1RO1-NS061107 to P.R.L. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to PRL and MGC, respectively, The Drown Foundation; The Linda Tallen & David Paul Kane Foundation Annual Fellowship and the Board of Governors at CSMC. M.C was supported by an NIH/NINDS 1F32 NS058156 fellowship. G.D.K was supported by an NIH/NINDS postdoctoral NRSA fellowship F32NS053034.
REFERENCES
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms-an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hottinger AF, van den Bent MJ, Dietrich PY, Brandes AA. Frequently asked questions in the medical management of high-grade glioma: a short guide with practical answers. Ann Oncol. 2008;19 Suppl 7:vii209–vii216. doi: 10.1093/annonc/mdn474. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Roila F. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19 Suppl 2:ii83–ii85. doi: 10.1093/annonc/mdn099. [DOI] [PubMed] [Google Scholar]
- 5.Grossman SA, Ye X, Piantadosi S, et al. Current survival statistics for patients with newly diagnosed glioblastoma treated with radiation and temozolomide on research studies in the United States. ASCO Annual Meeting. 2009 doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mischel PS, Cloughesy TF, Nelson SF. DNA-microarray analysis of brain cancer: molecular classification for therapy. Nat Rev Neurosci. 2004;5:782–792. doi: 10.1038/nrn1518. [DOI] [PubMed] [Google Scholar]
- 7.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T cell infiltration and survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res Dec 6. 2010 doi: 10.1158/1078-0432.CCR-10-2563. (Epub ahead of full print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rich JN, Hans C, Jones B, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65:4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan RP, Costello JF. Molecular epigenetics and genetics in neuro-oncology. Neurotherapeutics. 2009;6:436–446. doi: 10.1016/j.nurt.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudreau CR, Yang I, Liau LM. Gliomas: advances in molecular analysis and characterization. Surg Neurol. 2005;64:286–294. doi: 10.1016/j.surneu.2005.03.033. discussion 94. [DOI] [PubMed] [Google Scholar]
- 11.Freije WA, Castro-Vargas FE, Fang Z, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 12.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 13.Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 14.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 15.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 16.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 17.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 18.Kleihues P, Zulch KJ, Matsumoto S, Radke U. Morphology of malignant gliomas induced in rabbits by systemic application of N-methyl-N-nitrosourea. Z Neurol. 1970;198:65–78. doi: 10.1007/BF00316136. [DOI] [PubMed] [Google Scholar]
- 19.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 20.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WF. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 22.Tzeng JJ, Barth RF, Orosz CG, James SM. Phenotype and functional activity of tumor-infiltrating lymphocytes isolated from immunogenic and nonimmunogenic rat brain tumors. Cancer Res. 1991;51:2373–2378. [PubMed] [Google Scholar]
- 23.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Hong MJ, Park SD, et al. Enhancement of antitumor immunity specific to murine glioma by vaccination with tumor cell lysate-pulsed dendritic cells engineered to produce interleukin-12. Cancer Immunol Immunother. 2006:1–11. doi: 10.1007/s00262-006-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens GC, Orr EA, DeMasters BK, Muschel RJ, Berens ME, Kruse CA. Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res. 1998;58:2020–2028. [PubMed] [Google Scholar]
- 29.Candolfi M, Yagiz K, Foulad D, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtin J, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King GD, Muhammad AG, Xiong W, et al. High-Capacity adenoviral vector-mediated anti-glioma gene therapy in the presence of systemic anti-adenovirus immunity. J Virol. 2008;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King GD, Muhammad AKM, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch M, Strasser J, Allende R, Bello L, Zhang J, Black PM. Angiostatin suppresses malignant glioma growth in vivo. Cancer Res. 1998;58:4654–4659. [PubMed] [Google Scholar]
- 36.Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res. 2000;6:971–978. [PubMed] [Google Scholar]
- 37.Schmidt NO, Ziu M, Carrabba G, et al. Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and survival in an orthotopic human glioblastoma model. Clin Cancer Res. 2004;10:1255–1262. doi: 10.1158/1078-0432.ccr-03-0052. [DOI] [PubMed] [Google Scholar]
- 38.Tentori L, Leonetti C, Scarsella M, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370–5379. [PubMed] [Google Scholar]
- 39.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H, Gomez-Manzano C, Alemany R, et al. Comparative effect of oncolytic adenoviruses with E1A-55 kDa or E1B-55 kDa deletions in malignant gliomas. Neoplasia. 2005;7:48–56. doi: 10.1593/neo.04391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 42.Conrad C, Miller CR, Ji Y, et al. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12:284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 43.Lamfers ML, Gianni D, Tung CH, et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005;65:9398–9405. doi: 10.1158/0008-5472.CAN-04-4264. [DOI] [PubMed] [Google Scholar]
- 44.Samoto K, Ehtesham M, Perng GC, et al. A herpes simplex virus type 1 mutant with gamma 34.5 and LAT deletions effectively oncolyses human U87 glioblastomas in nude mice. Neurosurgery. 2002;50:599–605. doi: 10.1097/00006123-200203000-00031. discussion −6. [DOI] [PubMed] [Google Scholar]
- 45.Albright L, Madigan JC, Gaston MR, Houchens DP. Therapy in an intracerebral murine glioma model, using Bacillus Calmette-Guerin, neuraminidase-treated tumor cells, and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1975;35:658–665. [PubMed] [Google Scholar]
- 46.Akbasak A, Oldfield EH, Saris SC. Expression and modulation of major histocompatibility antigens on murine primary brain tumor in vitro. J Neurosurg. 1991;75:922–929. doi: 10.3171/jns.1991.75.6.0922. [DOI] [PubMed] [Google Scholar]
- 47.Sampson JH, Ashley DM, Archer GE, et al. Characterization of a spontaneous murine astrocytoma and abrogation of its tumorigenicity by cytokine secretion. Neurosurgery. 1997;41:1365–1372. doi: 10.1097/00006123-199712000-00024. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 48.Weiner NE, Pyles RB, Chalk CL, et al. A syngeneic mouse glioma model for study of glioblastoma therapy. J Neuropathol Exp Neurol. 1999;58:54–60. doi: 10.1097/00005072-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Huse JT, Holland EC. Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol. 2009;19:132–143. doi: 10.1111/j.1750-3639.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonabend AM, Ulasov IV, Lesniak MS. Emerging role of new transgenic mouse models in glioma research. Expert Rev Anticancer Ther. 2007;7:S7–S13. doi: 10.1586/14737140.7.12s.S7. [DOI] [PubMed] [Google Scholar]
- 51.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 53.Sanson M, Thillet J, Hoang-Xuan K. Molecular changes in gliomas. Curr Opin Oncol. 2004;16:607–613. doi: 10.1097/01.cco.0000142485.81849.cc. [DOI] [PubMed] [Google Scholar]
- 54.Nagane M, Lin H, Cavenee WK, Huang HJ. Aberrant receptor signaling in human malignant gliomas: mechanisms and therapeutic implications. Cancer Lett. 2001;162 Suppl:S17–S21. doi: 10.1016/s0304-3835(00)00648-0. [DOI] [PubMed] [Google Scholar]
- 55.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. quiz 1 p following 16. [DOI] [PubMed] [Google Scholar]
- 56.Lampson LA. New animal models to probe brain tumor biology, therapy, and immunotherapy: advantages and remaining concerns. J Neurooncol. 2001;53:275–287. doi: 10.1023/a:1012230113527. [DOI] [PubMed] [Google Scholar]
- 57.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 58.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–5279. [PubMed] [Google Scholar]
- 60.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marumoto T, Tashiro A, Friedmann-Morvinski D, et al. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiesner SM, Decker SA, Larson JD, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69:431–439. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohlfest JR, Freese AB, Largaespada DA. Nonviral vectors for cancer gene therapy: prospects for integrating vectors and combination therapies. Curr Gene Ther. 2005;5:629–641. doi: 10.2174/156652305774964749. [DOI] [PubMed] [Google Scholar]
- 64.Cobb LM, Connors TA, Elson LA, et al. 2,4-dinitro-5-ethyleneiminobenzamide (CB 1954): a potent and selective inhibitor of the growth of the Walker carcinoma 256. Biochem Pharmacol. 1969;18:1519–1527. doi: 10.1016/0006-2952(69)90267-6. [DOI] [PubMed] [Google Scholar]
- 65.Connors TA, Whisson ME. Cure of mice bearing advanced plasma cell tumours with aniline mustard: the relationship between glucuronidase activity and tumour sensitivity. Nature. 1966;210:866–867. doi: 10.1038/210866b0. [DOI] [PubMed] [Google Scholar]
- 66.Aghi M, Hochberg F, Breakefield XO. Prodrug activation enzymes in cancer gene therapy. J Gene Med. 2000;2:148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 67.Greco O, Dachs GU. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol. 2001;187:22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 68.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 69.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 70.Cowsill C, Southgate TD, Morrissey G, et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Ther. 2000;7:679–685. doi: 10.1038/sj.gt.3301147. [DOI] [PubMed] [Google Scholar]
- 71.Wiewrodt R, Amin K, Kiefer M, et al. Adenovirus-mediated gene transfer of enhanced Herpes simplex virus thymidine kinase mutants improves prodrug-mediated tumor cell killing. Cancer Gene Ther. 2003;10:353–364. doi: 10.1038/sj.cgt.7700589. [DOI] [PubMed] [Google Scholar]
- 72.Freeman SM, Abboud CN, Whartenby KA, et al. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 73.Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci U S A. 1993;90:7024–7028. doi: 10.1073/pnas.90.15.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen CY, Chang YN, Ryan P, Linscott M, McGarrity GJ, Chiang YL. Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the "bystander effect". Hum Gene Ther. 1995;6:1467–1476. doi: 10.1089/hum.1995.6.11-1467. [DOI] [PubMed] [Google Scholar]
- 75.Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 76.Dilber MS, Abedi MR, Christensson B, et al. Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res. 1997;57:1523–1528. [PubMed] [Google Scholar]
- 77.Elshami AA, Saavedra A, Zhang H, et al. Gap junctions play a role in the 'bystander effect' of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3:85–92. [PubMed] [Google Scholar]
- 78.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci U S A. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Touraine RL, Vahanian N, Ramsey WJ, Blaese RM. Enhancement of the herpes simplex virus thymidine kinase/ganciclovir bystander effect and its antitumor efficacy in vivo by pharmacologic manipulation of gap junctions. Hum Gene Ther. 1998;9:2385–2391. doi: 10.1089/hum.1998.9.16-2385. [DOI] [PubMed] [Google Scholar]
- 80.Vincent AJ, Vogels R, Someren GV, et al. Herpes simplex virus thymidine kinase gene therapy for rat malignant brain tumors. Hum Gene Ther. 1996;7:197–205. doi: 10.1089/hum.1996.7.2-197. [DOI] [PubMed] [Google Scholar]
- 81.Izquierdo M, Cortes M, de Felipe P, et al. Long-term rat survival after malignant brain tumor regression by retroviral gene therapy. Gene Ther. 1995;2:66–69. [PubMed] [Google Scholar]
- 82.Takamiya Y, Short MP, Moolten FL, et al. An experimental model of retrovirus gene therapy for malignant brain tumors. J Neurosurg. 1993;79:104–110. doi: 10.3171/jns.1993.79.1.0104. [DOI] [PubMed] [Google Scholar]
- 83.Boviatsis EJ, Park JS, Sena-Esteves M, et al. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751. [PubMed] [Google Scholar]
- 84.Boviatsis EJ, Scharf JM, Chase M, et al. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 85.Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Cruet MJ, Trask TW, Chen SH, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39:506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- 87.Nanda D, Vogels R, Havenga M, Avezaat CJ, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61:8743–8750. [PubMed] [Google Scholar]
- 88.Candolfi M, Kroeger KM, Muhammad AK, et al. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr Gene Ther. 2009;9:409–421. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizuno M, Yoshida J, Colosi P, Kurtzman G. Adeno-associated virus vector containing the herpes simplex virus thymidine kinase gene causes complete regression of intracerebrally implanted human gliomas in mice, in conjunction with ganciclovir administration. Jpn J Cancer Res. 1998;89:76–80. doi: 10.1111/j.1349-7006.1998.tb00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okada H, Pollack IF, Lieberman F, et al. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther. 2001;12:575–595. doi: 10.1089/104303401300042528. [DOI] [PubMed] [Google Scholar]
- 91.Vile RG, Castleden S, Marshall J, Camplejohn R, Upton C, Chong H. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer. 1997;71:267–274. doi: 10.1002/(sici)1097-0215(19970410)71:2<267::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 92.Bi W, Kim YG, Feliciano ES, et al. An HSVtk-mediated local and distant antitumor bystander effect in tumors of head and neck origin in athymic mice. Cancer Gene Ther. 1997;4:246–252. [PubMed] [Google Scholar]
- 93.Dilber MS, Abedi MR, Bjorkstrand B, et al. Suicide gene therapy for plasma cell tumors. Blood. 1996;88:2192–2200. [PubMed] [Google Scholar]
- 94.Wilson KM, Stambrook PJ, Bi WL, Pavelic ZP, Pavelic L, Gluckman JL. HSV-tk gene therapy in head and neck squamous cell carcinoma. Enhancement by the local and distant bystander effect. Arch Otolaryngol Head Neck Surg. 1996;122:746–749. doi: 10.1001/archotol.1996.01890190042011. [DOI] [PubMed] [Google Scholar]
- 95.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 96.Puntel M, Muhammad AK, Candolfi M, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–6017. doi: 10.1128/JVI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghulam Muhammad AKM, Puntel M, Candolfi M, et al. Study of the efficacy, biodistribution and safety profile of therapeutic gutless adenovirus vectors as a prelude to a Phase I Clinical Trial for Glioblastoma. Clin Pharmacol Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SH, Kim JH, Kolozsvary A, Brown SL, Freytag SO. Preferential radiosensitization of 9L glioma cells transduced with HSV-tk gene by acyclovir. J Neurooncol. 1997;33:189–194. doi: 10.1023/a:1005764324900. [DOI] [PubMed] [Google Scholar]
- 99.Nestler U, Wakimoto H, Siller-Lopez F, et al. The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neurooncol. 2004;67:177–188. doi: 10.1023/b:neon.0000021897.53969.ca. [DOI] [PubMed] [Google Scholar]
- 100.Rainov NG, Fels C, Droege JW, Schafer C, Kramm CM, Chou TC. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8:662–668. doi: 10.1038/sj.cgt.7700355. [DOI] [PubMed] [Google Scholar]
- 101.Valerie K, Brust D, Farnsworth J, et al. Improved radiosensitization of rat glioma cells with adenovirus-expressed mutant herpes simplex virus-thymidine kinase in combination with acyclovir. Cancer Gene Ther. 2000;7:879–884. doi: 10.1038/sj.cgt.7700185. [DOI] [PubMed] [Google Scholar]
- 102.Moriuchi S, Oligino T, Krisky D, et al. Enhanced tumor cell killing in the presence of ganciclovir by herpes simplex virus type 1 vector-directed coexpression of human tumor necrosis factor-alpha and herpes simplex virus thymidine kinase. Cancer Res. 1998;58:5731–5737. [PubMed] [Google Scholar]
- 103.Niranjan A, Moriuchi S, Lunsford LD, et al. Effective treatment of experimental glioblastoma by HSV vector-mediated TNF alpha and HSV-tk gene transfer in combination with radiosurgery and ganciclovir administration. Mol Ther. 2000;2:114–120. doi: 10.1006/mthe.2000.0101. [DOI] [PubMed] [Google Scholar]
- 104.Niranjan A, Wolfe D, Tamura M, et al. Treatment of rat gliosarcoma brain tumors by HSV-based multigene therapy combined with radiosurgery. Mol Ther. 2003;8:530–542. doi: 10.1016/s1525-0016(03)00232-6. [DOI] [PubMed] [Google Scholar]
- 105.Benedetti S, Dimeco F, Pollo B, et al. Limited efficacy of the HSV-TK/GCV system for gene therapy of malignant gliomas and perspectives for the combined transduction of the interleukin-4 gene. Hum Gene Ther. 1997;8:1345–1353. doi: 10.1089/hum.1997.8.11-1345. [DOI] [PubMed] [Google Scholar]
- 106.Okada H, Pollack IF, Lotze MT, et al. Gene therapy of malignant gliomas: a phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum Gene Ther. 2000;11:637–653. doi: 10.1089/10430340050015824. [DOI] [PubMed] [Google Scholar]
- 107.King GD, Kroeger KM, Bresee CJ, et al. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther. 2008;16:682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biglari A, Bataille D, Naumann U, et al. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marconi P, Tamura M, Moriuchi S, et al. Connexin 43-enhanced suicide gene therapy using herpesviral vectors. Mol Ther. 2000;1:71–81. doi: 10.1006/mthe.1999.0008. [DOI] [PubMed] [Google Scholar]
- 110.Domin BA, Mahony WB, Zimmerman TP. Transport of 5-fluorouracil and uracil into human erythrocytes. Biochem Pharmacol. 1993;46:503–510. doi: 10.1016/0006-2952(93)90527-4. [DOI] [PubMed] [Google Scholar]
- 111.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808–4812. [PubMed] [Google Scholar]
- 113.Ge K, Xu L, Zheng Z, Xu D, Sun L, Liu X. Transduction of cytosine deaminase gene makes rat glioma cells highly sensitive to 5-fluorocytosine. Int J Cancer. 1997;71:675–679. doi: 10.1002/(sici)1097-0215(19970516)71:4<675::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 114.Ichikawa T, Tamiya T, Adachi Y, et al. In vivo efficacy and toxicity of 5-fluorocytosine/cytosine deaminase gene therapy for malignant gliomas mediated by adenovirus. Cancer Gene Ther. 2000;7:74–82. doi: 10.1038/sj.cgt.7700086. [DOI] [PubMed] [Google Scholar]
- 115.Wang WJ, Tai CK, Kasahara N, Chen TC. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum Gene Ther. 2003;14:117–127. doi: 10.1089/104303403321070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurozumi K, Tamiya T, Ono Y, et al. Apoptosis induction with 5-fluorocytosine/cytosine deaminase gene therapy for human malignant glioma cells mediated by adenovirus. J Neurooncol. 2004;66:117–127. doi: 10.1023/b:neon.0000013494.98345.80. [DOI] [PubMed] [Google Scholar]
- 117.Fischer U, Steffens S, Frank S, Rainov NG, Schulze-Osthoff K, Kramm CM. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/ 5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005;24:1231–1243. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- 118.Aghi M, Kramm CM, Chou TC, Breakefield XO, Chiocca EA. Synergistic anticancer effects of ganciclovir/thymidine kinase and 5-fluorocytosine/cytosine deaminase gene therapies. J Natl Cancer Inst. 1998;90:370–380. doi: 10.1093/jnci/90.5.370. [DOI] [PubMed] [Google Scholar]
- 119.Chang JW, Lee H, Kim E, Lee Y, Chung SS, Kim JH. Combined antitumor effects of an adenoviral cytosine deaminase/thymidine kinase fusion gene in rat C6 glioma. Neurosurgery. 2000;47:931–938. doi: 10.1097/00006123-200010000-00026. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 120.Kambara H, Tamiya T, Ono Y, et al. Combined radiation and gene therapy for brain tumors with adenovirus-mediated transfer of cytosine deaminase and uracil phosphoribosyltransferase genes. Cancer Gene Ther. 2002;9:840–845. doi: 10.1038/sj.cgt.7700506. [DOI] [PubMed] [Google Scholar]
- 121.Ito S, Natsume A, Shimato S, et al. Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010;17:299–306. doi: 10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- 122.Dachs GU, Tupper J, Tozer GM. From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anticancer Drugs. 2005;16:349–359. doi: 10.1097/00001813-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- 124.Wei MX, Tamiya T, Rhee RJ, Breakefield XO, Chiocca EA. Diffusible cytotoxic metabolites contribute to the in vitro bystander effect associated with the cyclophosphamide/cytochrome P450 2B1 cancer gene therapy paradigm. Clin Cancer Res. 1995;1:1171–1177. [PubMed] [Google Scholar]
- 125.Manome Y, Wen PY, Chen L, et al. Gene therapy for malignant gliomas using replication incompetent retroviral and adenoviral vectors encoding the cytochrome P450 2B1 gene together with cyclophosphamide. Gene Ther. 1996;3:513–520. [PubMed] [Google Scholar]
- 126.Wei MX, Tamiya T, Chase M, et al. Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther. 1994;5:969–978. doi: 10.1089/hum.1994.5.8-969. [DOI] [PubMed] [Google Scholar]
- 127.Frank S, Steffens S, Fischer U, Tlolko A, Rainov NG, Kramm CM. Differential cytotoxicity and bystander effect of the rabbit cytochrome P450 4B1 enzyme gene by two different prodrugs: implications for pharmacogene therapy. Cancer Gene Ther. 2002;9:178–188. doi: 10.1038/sj.cgt.7700422. [DOI] [PubMed] [Google Scholar]
- 128.Huang Z, Raychowdhury MK, Waxman DJ. Impact of liver P450 reductase suppression on cyclophosphamide activation, pharmacokinetics and antitumoral activity in a cytochrome P450-based cancer gene therapy model. Cancer Gene Ther. 2000;7:1034–1042. doi: 10.1038/sj.cgt.7700200. [DOI] [PubMed] [Google Scholar]
- 129.Jounaidi Y, Waxman DJ. Combination of the bioreductive drug tirapazamine with the chemotherapeutic prodrug cyclophosphamide for P450/P450-reductase-based cancer gene therapy. Cancer Res. 2000;60:3761–3769. [PubMed] [Google Scholar]
- 130.Rainov NG, Dobberstein KU, Sena-Esteves M, et al. New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol. Hum Gene Ther. 1998;9:1261–1273. doi: 10.1089/hum.1998.9.9-1261. [DOI] [PubMed] [Google Scholar]
- 131.Chen L, Waxman DJ. Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res. 1995;55:581–589. [PubMed] [Google Scholar]
- 132.Mercapide J, Rappa G, Anzanello F, King J, Fodstad O, Lorico A. Primary gene-engineered neural stem/progenitor cells demonstrate tumor-selective migration and antitumor effects in glioma. Int J Cancer. 2010;126:1206–1215. doi: 10.1002/ijc.24809. [DOI] [PubMed] [Google Scholar]
- 133.Gadi VK, Alexander SD, Waud WR, Allan PW, Parker WB, Sorscher EJ. A long-acting suicide gene toxin, 6-methylpurine, inhibits slow growing tumors after a single administration. J Pharmacol Exp Ther. 2003;304:1280–1284. doi: 10.1124/jpet.102.044743. [DOI] [PubMed] [Google Scholar]
- 134.Parker WB, Allan PW, Hassan AE, Secrist JA, 3rd, Sorscher EJ, Waud WR. Antitumor activity of 2-fluoro-2'-deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase. Cancer Gene Ther. 2003;10:23–29. doi: 10.1038/sj.cgt.7700520. [DOI] [PubMed] [Google Scholar]
- 135.Hong JS, Waud WR, Levasseur DN, et al. Excellent in vivo bystander activity of fludarabine phosphate against human glioma xenografts that express the escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 2004;64:6610–6615. doi: 10.1158/0008-5472.CAN-04-0012. [DOI] [PubMed] [Google Scholar]
- 136.Parker WB, King SA, Allan PW, et al. In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Hum Gene Ther. 1997;8:1637–1644. doi: 10.1089/hum.1997.8.14-1637. [DOI] [PubMed] [Google Scholar]
- 137.Marais R, Spooner RA, Light Y, Martin J, Springer CJ. Gene-directed enzyme prodrug therapy with a mustard prodrug/carboxypeptidase G2 combination. Cancer Res. 1996;56:4735–4742. [PubMed] [Google Scholar]
- 138.Springer CJ, Antoniw P, Bagshawe KD, Searle F, Bisset GM, Jarman M. Novel prodrugs which are activated to cytotoxic alkylating agents by carboxypeptidase G2. J Med Chem. 1990;33:677–681. doi: 10.1021/jm00164a034. [DOI] [PubMed] [Google Scholar]
- 139.Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest. 2000;105:1161–1167. doi: 10.1172/JCI10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stribbling SM, Friedlos F, Martin J, et al. Regressions of established breast carcinoma xenografts by carboxypeptidase G2 suicide gene therapy and the prodrug CMDA are due to a bystander effect. Hum Gene Ther. 2000;11:285–292. doi: 10.1089/10430340050016021. [DOI] [PubMed] [Google Scholar]
- 141.Cowen RL, Williams JC, Emery S, et al. Adenovirus vector-mediated delivery of the prodrug-converting enzyme carboxypeptidase G2 in a secreted or GPI-anchored form: High-level expression of this active conditional cytotoxic enzyme at the plasma membrane. Cancer Gene Ther. 2002;9:897–907. doi: 10.1038/sj.cgt.7700514. [DOI] [PubMed] [Google Scholar]
- 142.Kirsch M, Schackert G, Black PM. Anti-angiogenic treatment strategies for malignant brain tumors. J Neurooncol. 2000;50:149–163. doi: 10.1023/a:1006487412567. [DOI] [PubMed] [Google Scholar]
- 143.Sebti SM, Hamilton AD. Design of growth factor antagonists with antiangiogenic and antitumor properties. Oncogene. 2000;19:6566–6573. doi: 10.1038/sj.onc.1204121. [DOI] [PubMed] [Google Scholar]
- 144.Puduvalli VK, Sawaya R. Antiangiogenesis -- therapeutic strategies and clinical implications for brain tumors. J Neurooncol. 2000;50:189–200. doi: 10.1023/a:1006469830739. [DOI] [PubMed] [Google Scholar]
- 145.Chen QR, Zhang L, Gasper W, Mixson AJ. Targeting tumor angiogenesis with gene therapy. Mol Genet Metab. 2001;74:120–127. doi: 10.1006/mgme.2001.3223. [DOI] [PubMed] [Google Scholar]
- 146.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Szabo S, Sandor Z. The diagnostic and prognostic value of tumor angiogenesis. Eur J Surg Suppl. 1998:99–103. doi: 10.1080/11024159850191526. [DOI] [PubMed] [Google Scholar]
- 148.Yukawa H, Takahashi JC, Miyatake SI, et al. Adenoviral gene transfer of basic fibroblast growth factor promotes angiogenesis in rat brain. Gene Ther. 2000;7:942–949. doi: 10.1038/sj.gt.3301182. [DOI] [PubMed] [Google Scholar]
- 149.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 150.Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 151.Stratmann A, Machein MR, Plate KH. Anti-angiogenic gene therapy of malignant glioma. Acta Neurochir Suppl (Wien) 1997;68:105–110. doi: 10.1007/978-3-7091-6513-3_20. [DOI] [PubMed] [Google Scholar]
- 152.Saleh M, Stacker SA, Wilks AF. Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence. Cancer Res. 1996;56:393–401. [PubMed] [Google Scholar]
- 153.Sasaki M, Wizigmann-Voos S, Risau W, Plate KH. Retrovirus producer cells encoding antisense VEGF prolong survival of rats with intracranial GS9L gliomas. Int J Dev Neurosci. 1999;17:579–591. doi: 10.1016/s0736-5748(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 154.Saidi A, Hagedorn M, Allain N, et al. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int J Cancer. 2009;125:1054–1064. doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 155.Machein MR, Risau W, Plate KH. Antiangiogenic gene therapy in a rat glioma model using a dominant-negative vascular endothelial growth factor receptor 2. Hum Gene Ther. 1999;10:1117–1128. doi: 10.1089/10430349950018111. [DOI] [PubMed] [Google Scholar]
- 156.Heidenreich R, Machein M, Nicolaus A, et al. Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Int J Cancer. 2004;111:348–357. doi: 10.1002/ijc.20260. [DOI] [PubMed] [Google Scholar]
- 157.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 158.Gondi CS, Lakka SS, Yanamandra N, et al. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res. 2004;64:4069–4077. doi: 10.1158/0008-5472.CAN-04-1243. [DOI] [PubMed] [Google Scholar]
- 159.Gondi CS, Kandhukuri N, Kondraganti S, et al. RNA interference-mediated simultaneous down-regulation of urokinase-type plasminogen activator receptor and cathepsin B induces caspase-8-mediated apoptosis in SNB19 human glioma cells. Mol Cancer Ther. 2006;5:3197–3208. doi: 10.1158/1535-7163.MCT-05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 161.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 162.Peroulis I, Jonas N, Saleh M. Antiangiogenic activity of endostatin inhibits C6 glioma growth. Int J Cancer. 2002;97:839–845. doi: 10.1002/ijc.10115. [DOI] [PubMed] [Google Scholar]
- 163.Yamanaka R, Zullo SA, Ramsey J, et al. Induction of therapeutic antitumor antiangiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001;8:796–802. doi: 10.1038/sj.cgt.7700367. [DOI] [PubMed] [Google Scholar]
- 164.Ma HI, Lin SZ, Chiang YH, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9:2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka T, Cao Y, Folkman J, Fine HA. Viral vector-targeted antiangiogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res. 1998;58:3362–3369. [PubMed] [Google Scholar]
- 166.Szentirmai O, Baker CH, Bullain SS, et al. Successful inhibition of intracranial human glioblastoma multiforme xenograft growth via systemic adenoviral delivery of soluble endostatin and soluble vascular endothelial growth factor receptor-2: laboratory investigation. J Neurosurg. 2008;108:979–988. doi: 10.3171/JNS/2008/108/5/0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 168.Witte JS, Palmer LJ, O'Connor RD, Hopkins PJ, Hall JM. Relation between tumour necrosis factor polymorphism TNFalpha-308 and risk of asthma. Eur J Hum Genet. 2002;10:82–85. doi: 10.1038/sj.ejhg.5200746. [DOI] [PubMed] [Google Scholar]
- 169.Saleh M, Jonas NK, Wiegmans A, Stylli SS. The treatment of established intracranial tumors by in situ retroviral IFN-gamma transfer. Gene Ther. 2000;7:1715–1724. doi: 10.1038/sj.gt.3301273. [DOI] [PubMed] [Google Scholar]
- 170.Saleh M, Wiegmans A, Malone Q, Stylli SS, Kaye AH. Effect of in situ retroviral interleukin-4 transfer on established intracranial tumors. J Natl Cancer Inst. 1999;91:438–445. doi: 10.1093/jnci/91.5.438. [DOI] [PubMed] [Google Scholar]
- 171.Chamberlain MC. Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer. 2010;116:3988–3999. doi: 10.1002/cncr.25256. [DOI] [PubMed] [Google Scholar]
- 172.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 173.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 174.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 175.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 176.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 178.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 179.Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet. 1993;341:21–22. doi: 10.1016/0140-6736(93)92485-c. [DOI] [PubMed] [Google Scholar]
- 180.Nagel GA, Piessens WF, Stilmant MM, Lejeune F. Evidence for tumor-specific immunity in human malignant melanoma. Eur J Cancer. 1971;7:41–47. doi: 10.1016/0014-2964(71)90093-4. [DOI] [PubMed] [Google Scholar]
- 181.Jongmans W, van den Oudenalder K, Tiemessen DM, et al. Targeting of adenovirus to human renal cell carcinoma cells. Urology. 2003;62:559–565. doi: 10.1016/s0090-4295(03)00378-9. [DOI] [PubMed] [Google Scholar]
- 182.Iizuka Y, Kojima H, Kobata T, Kawase T, Kawakami Y, Toda M. Identification of a glioma antigen, GARC-1, using cytotoxic T lymphocytes induced by HSV cancer vaccine. Int J Cancer. 2006;118:942–949. doi: 10.1002/ijc.21432. [DOI] [PubMed] [Google Scholar]
- 183.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stark AM, Witzel P, Strege RJ, Hugo HH, Mehdorn HM. p53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatry. 2003;74:779–783. doi: 10.1136/jnnp.74.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66:3987–3991. doi: 10.1158/0008-5472.CAN-06-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15:4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kemp TJ, Elzey BD, Griffith TS. Plasmacytoid dendritic cell-derived IFN-alpha induces TNF-related apoptosis-inducing ligand/Apo-2L–mediated antitumor activity by human monocytes following CpG oligodeoxynucleotide stimulation. J Immunol. 2003;171:212–218. doi: 10.4049/jimmunol.171.1.212. [DOI] [PubMed] [Google Scholar]
- 189.Tosi D, Valenti R, Cova A, et al. Role of cross-talk between IFN-alpha-induced monocyte-derived dendritic cells and NK cells in priming CD8+ T cell responses against human tumor antigens. J Immunol. 2004;172:5363–5370. doi: 10.4049/jimmunol.172.9.5363. [DOI] [PubMed] [Google Scholar]
- 190.Yang I, Kremen TJ, Giovannone AJ, et al. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. 2004;100:310–319. doi: 10.3171/jns.2004.100.2.0310. [DOI] [PubMed] [Google Scholar]
- 191.Horton HM, Anderson D, Hernandez P, Barnhart KM, Norman JA, Parker SE. A gene therapy for cancer using intramuscular injection of plasmid DNA encoding interferon alpha. Proc Natl Acad Sci U S A. 1999;96:1553–1558. doi: 10.1073/pnas.96.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tsugawa T, Kuwashima N, Sato H, et al. Sequential delivery of interferon-alpha gene and DCs to intracranial gliomas promotes an effective antitumor response. Gene Ther. 2004;11:1551–1558. doi: 10.1038/sj.gt.3302300. [DOI] [PubMed] [Google Scholar]
- 193.Natsume A, Tsujimura K, Mizuno M, Takahashi T, Yoshida J. IFN-beta gene therapy induces systemic antitumor immunity against malignant glioma. J Neurooncol. 2000;47:117–124. doi: 10.1023/a:1006441030976. [DOI] [PubMed] [Google Scholar]
- 194.Nakahara N, Pollack IF, Storkus WJ, Wakabayashi T, Yoshida J, Okada H. Effective induction of antiglioma cytotoxic T cells by coadministration of interferon-beta gene vector and dendritic cells. Cancer Gene Ther. 2003;10:549–558. doi: 10.1038/sj.cgt.7700598. [DOI] [PubMed] [Google Scholar]
- 195.Yoshida J, Mizuno M, Nakahara N, Colosi P. Antitumor effect of an adeno-associated virus vector containing the human interferon-beta gene on experimental intracranial human glioma. Jpn J Cancer Res. 2002;93:223–228. doi: 10.1111/j.1349-7006.2002.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.De Palma M, Mazzieri R, Politi LS, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 197.Kane A, Yang I. Interferon-gamma in brain tumor immunotherapy. Neurosurg Clin N Am. 2010;21:77–86. doi: 10.1016/j.nec.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 198.Selznick LA, Shamji MF, Fecci P, Gromeier M, Friedman AH, Sampson J. Molecular strategies for the treatment of malignant glioma--genes, viruses, and vaccines. Neurosurg Rev. 2008;31:141–155. doi: 10.1007/s10143-008-0121-0. discussion 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu A, Wiesner S, Xiao J, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 200.Ehtesham M, Samoto K, Kabos P, et al. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 201.Wu A, Oh S, Ericson K, et al. Transposon-based interferon gamma gene transfer overcomes limitations of episomal plasmid for immunogene therapy of glioblastoma. Cancer Gene Ther. 2007;14:550–560. doi: 10.1038/sj.cgt.7701045. [DOI] [PubMed] [Google Scholar]
- 202.Pluhar GE, Grogan PT, Seiler C, et al. antitumor immune response correlates with neurological symptoms in a dog with spontaneous astrocytoma treated by gene and vaccine therapy. Vaccine. 2010;28:3371–3378. doi: 10.1016/j.vaccine.2010.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 204.Liu Y, Ehtesham M, Samoto K, et al. In situ adenoviral interleukin 12 gene transfer confers potent and long-lasting cytotoxic immunity in glioma. Cancer Gene Ther. 2002;9:9–15. doi: 10.1038/sj.cgt.7700399. [DOI] [PubMed] [Google Scholar]
- 205.Lichtor T, Glick RP. Cytokine immunogene therapy for treatment of brain tumors. J Neurooncol. 2003;65:247–259. doi: 10.1023/b:neon.0000003654.83272.4a. [DOI] [PubMed] [Google Scholar]
- 206.Parker JN, Meleth S, Hughes KB, Gillespie GY, Whitley RJ, Markert JM. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther. 2005;12:359–368. doi: 10.1038/sj.cgt.7700784. [DOI] [PubMed] [Google Scholar]
- 207.Hellums EK, Markert JM, Parker JN, et al. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro-oncol. 2005;7:213–224. doi: 10.1215/S1152851705000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee YS, Kim JH, Choi KJ, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res. 2006;12:5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 209.Huang JH, Zhang SN, Choi KJ, et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther. 2010;18:264–274. doi: 10.1038/mt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chiu TL, Lin SZ, Hsieh WH, Peng CW. AAV2-mediated interleukin-12 in the treatment of malignant brain tumors through activation of NK cells. Int J Oncol. 2009;35:1361–1367. doi: 10.3892/ijo_00000454. [DOI] [PubMed] [Google Scholar]
- 211.Roche FP, Sheahan BJ, O'Mara SM, Atkins GJ. Semliki Forest virus-mediated gene therapy of the RG2 rat glioma. Neuropathol Appl Neurobiol. 2010;36:648–660. doi: 10.1111/j.1365-2990.2010.01110.x. [DOI] [PubMed] [Google Scholar]
- 212.Sonabend AM, Velicu S, Ulasov IV, et al. A safety and efficacy study of local delivery of interleukin-12 transgene by PPC polymer in a model of experimental glioma. Anticancer Drugs. 2008;19:133–142. doi: 10.1097/CAD.0b013e3282f24017. [DOI] [PubMed] [Google Scholar]
- 213.Laouar Y, Crispe IN. Functional flexibility in T cells: independent regulation of CD4+ T cell proliferation and effector function in vivo. Immunity. 2000;13:291–301. doi: 10.1016/s1074-7613(00)00029-7. [DOI] [PubMed] [Google Scholar]
- 214.Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 215.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 216.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 217.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 218.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 219.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 220.Santambrogio L, Belyanskaya SL, Fischer FR, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schmittling RJ, Archer GE, Mitchell DA, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Methods. 2008;339:74–81. doi: 10.1016/j.jim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 224.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 227.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 228.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 229.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 230.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 232.Curtin JF, King GD, Barcia C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg Depletion Inhibits Efficacy of Cancer Immunotherapy: Implications for Clinical Trials. PLoS ONE. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 235.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 236.Li C, Hall WA, Jin N, Todhunter DA, Panoskaltsis-Mortari A, Vallera DA. Targeting glioblastoma multiforme with an IL-13/diphtheria toxin fusion protein in vitro and in vivo in nude mice. Protein Eng. 2002;15:419–427. doi: 10.1093/protein/15.5.419. [DOI] [PubMed] [Google Scholar]
- 237.Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17:157–164. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 238.Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88:245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 240.Mori T, Abe T, Wakabayashi Y, et al. Up-regulation of urokinase-type plasminogen activator and its receptor correlates with enhanced invasion activity of human glioma cells mediated by transforming growth factor-alpha or basic fibroblast growth factor. J Neurooncol. 2000;46:115–123. doi: 10.1023/a:1006339717748. [DOI] [PubMed] [Google Scholar]
- 241.Liu TF, Hall PD, Cohen KA, et al. Interstitial diphtheria toxin-epidermal growth factor fusion protein therapy produces regressions of subcutaneous human glioblastoma multiforme tumors in athymic nude mice. Clin Cancer Res. 2005;11:329–334. [PubMed] [Google Scholar]
- 242.Phillips PC, Levow C, Catterall M, Colvin OM, Pastan I, Brem H. Transforming growth factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38) treatment of subcutaneous and intracranial human glioma and medulloblastoma xenografts in athymic mice. Cancer Res. 1994;54:1008–1015. [PubMed] [Google Scholar]
- 243.Hall WA, Godal A, Juell S, Fodstad O. In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J Neurosurg. 1992;76:838–844. doi: 10.3171/jns.1992.76.5.0838. [DOI] [PubMed] [Google Scholar]
- 244.Martell LA, Agrawal A, Ross DA, Muraszko KM. Efficacy of transferrin receptor-targeted immunotoxins in brain tumor cell lines and pediatric brain tumors. Cancer Res. 1993;53:1348–1353. [PubMed] [Google Scholar]
- 245.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 246.Candolfi M, Xiong W, Yagiz K, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics: efficacy in the absence of neurotoxicity. Proc Natl Acad Sci U S A. 2010;107:20021–20026. doi: 10.1073/pnas.1008261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Johnson VG, Wrobel C, Wilson D, et al. Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. J Neurosurg. 1989;70:240–248. doi: 10.3171/jns.1989.70.2.0240. [DOI] [PubMed] [Google Scholar]
- 248.Greenfield L, Johnson VG, Youle RJ. Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity. Science. 1987;238:536–539. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- 249.Johnson VG, Wilson D, Greenfield L, Youle RJ. The role of the diphtheria toxin receptor in cytosol translocation. J Biol Chem. 1988;263:1295–1300. [PubMed] [Google Scholar]
- 250.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 251.Abramovitch R, Meir G, Neeman M. Neovascularization induced growth of implanted C6 glioma multicellular spheroids: magnetic resonance microimaging. Cancer Res. 1995;55:1956–1962. [PubMed] [Google Scholar]
- 252.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271:22428–22433. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 254.Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48:103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 255.Liu TF, Willingham MC, Tatter SB, et al. Diphtheria toxin-epidermal growth factor fusion protein and Pseudomonas exotoxin-interleukin 13 fusion protein exert synergistic toxicity against human glioblastoma multiforme cells. Bioconjug Chem. 2003;14:1107–1114. doi: 10.1021/bc034111+. [DOI] [PubMed] [Google Scholar]
- 256.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neurooncol. 2003;64:117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 257.Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 258.Debinski W, Pastan I. An immunotoxin with increased activity and homogeneity produced by reducing the number of lysine residues in recombinant Pseudomonas exotoxin. Bioconjug Chem. 1994;5:40–46. doi: 10.1021/bc00025a006. [DOI] [PubMed] [Google Scholar]
- 259.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 260.Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 261.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 262.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xiong W, Candolfi M, Kroeger KM, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable Gutless Adenovirus Vectors Sustain Inducible Transgene Expression in the Brain in the Presence of an Immune Response against Adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Candolfi M, Xiong W, Yagiz K, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1008261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Merlo A. Genes and pathways driving glioblastomas in humans and murine disease models. Neurosurg Rev. 2003;26:145–158. doi: 10.1007/s10143-003-0267-8. [DOI] [PubMed] [Google Scholar]
- 267.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Louis DN. The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol. 1994;53:11–21. doi: 10.1097/00005072-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 270.Asai A, Miyagi Y, Sugiyama A, et al. Negative effects of wild-type p53 and s-Myc on cellular growth and tumorigenicity of glioma cells. Implication of the tumor suppressor genes for gene therapy. J Neurooncol. 1994;19:259–268. doi: 10.1007/BF01053280. [DOI] [PubMed] [Google Scholar]
- 271.Badie B, Drazan KE, Kramar MH, Shaked A, Black KL. Adenovirus-mediated p53 gene delivery inhibits 9L glioma growth in rats. Neurol Res. 1995;17:209–216. doi: 10.1080/01616412.1995.11740314. [DOI] [PubMed] [Google Scholar]
- 272.Kock H, Harris MP, Anderson SC, et al. Adenovirus-mediated p53 gene transfer suppresses growth of human glioblastoma cells in vitro and in vivo. Int J Cancer. 1996;67:808–815. doi: 10.1002/(SICI)1097-0215(19960917)67:6<808::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 273.Li H, Alonso-Vanegas M, Colicos MA, et al. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin Cancer Res. 1999;5:637–642. [PubMed] [Google Scholar]
- 274.Gjerset RA, Turla ST, Sobol RE, et al. Use of wild-type p53 to achieve complete treatment sensitization of tumor cells expressing endogenous mutant p53. Mol Carcinog. 1995;14:275–285. doi: 10.1002/mc.2940140408. [DOI] [PubMed] [Google Scholar]
- 275.Dorigo O, Turla ST, Lebedeva S, Gjerset RA. Sensitization of rat glioblastoma multiforme to cisplatin in vivo following restoration of wild-type p53 function. J Neurosurg. 1998;88:535–540. doi: 10.3171/jns.1998.88.3.0535. [DOI] [PubMed] [Google Scholar]
- 276.Badie B, Kramar MH, Lau R, Boothman DA, Economou JS, Black KL. Adenovirus-mediated p53 gene delivery potentiates the radiation-induced growth inhibition of experimental brain tumors. J Neurooncol. 1998;37:217–222. doi: 10.1023/a:1005924925149. [DOI] [PubMed] [Google Scholar]
- 277.Cirielli C, Inyaku K, Capogrossi MC, Yuan X, Williams JA. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of experimental intracranial human malignant glioma. J Neurooncol. 1999;43:99–108. doi: 10.1023/a:1006289505801. [DOI] [PubMed] [Google Scholar]
- 278.Chen J, Willingham T, Shuford M, et al. Effects of ectopic overexpression of p21(WAF1/CIP1) on aneuploidy and the malignant phenotype of human brain tumor cells. Oncogene. 1996;13:1395–1403. [PubMed] [Google Scholar]
- 279.Fueyo J, Gomez-Manzano C, Yung WK, et al. Overexpression of E2F-1 in glioma triggers apoptosis and suppresses tumor growth in vitro and in vivo. Nat Med. 1998;4:685–690. doi: 10.1038/nm0698-685. [DOI] [PubMed] [Google Scholar]
- 280.Wang TJ, Huang MS, Hong CY, Tse V, Silverberg GD, Hsiao M. Comparisons of tumor suppressor p53, p21, and p16 gene therapy effects on glioblastoma tumorigenicity in situ. Biochem Biophys Res Commun. 2001;287:173–180. doi: 10.1006/bbrc.2001.5565. [DOI] [PubMed] [Google Scholar]
- 281.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 282.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 283.Geoerger B, Grill J, Opolon P, et al. Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX-015) in human malignant glioma xenografts. Br J Cancer. 2003;89:577–584. doi: 10.1038/sj.bjc.6601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chiocca EA, Aghi M, Fulci G. Viral therapy for glioblastoma. Cancer J. 2003;9:167–179. doi: 10.1097/00130404-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 285.Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B–Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 287.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 288.Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- 289.Riley DJ, Nikitin AY, Lee WH. Adenovirus-mediated retinoblastoma gene therapy suppresses spontaneous pituitary melanotroph tumors in Rb+/− mice. Nat Med. 1996;2:1316–1321. doi: 10.1038/nm1296-1316. [DOI] [PubMed] [Google Scholar]
- 290.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 291.Lamfers ML, Grill J, Dirven CM, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- 292.Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 293.Ulasov IV, Zhu ZB, Tyler MA, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 294.Ulasov IV, Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009;100:1154–1164. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ulasov IV, Tyler MA, Rivera AA, Nettlebeck DM, Douglas JT, Lesniak MS. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–1603. doi: 10.1002/jmv.00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ulasov IV, Tyler MA, Zhu ZB, Han Y, He TC, Lesniak MS. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. Int J Oncol. 2009;34:729–742. doi: 10.3892/ijo_00000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lee SH, Kim MS, Kwon HC, et al. Growth inhibitory effect on glioma cells of adenovirus-mediated p16/INK4a gene transfer in vitro and in vivo. Int J Mol Med. 2000;6:559–563. [PubMed] [Google Scholar]
- 298.Simon M, Simon C, Koster G, Hans VH, Schramm J. Conditional expression of the tumor suppressor p16 in a heterotopic glioblastoma model results in loss of pRB expression. J Neurooncol. 2002;60:1–12. doi: 10.1023/a:1020226130478. [DOI] [PubMed] [Google Scholar]
- 299.Jespersen T, Duch M, Carrasco ML, Warming S, Pedersen FS. Expression of heterologous genes from an IRES translational cassette in replication competent murine leukemia virus vectors. Gene. 1999;239:227–235. doi: 10.1016/s0378-1119(99)00402-3. [DOI] [PubMed] [Google Scholar]
- 300.Logg CR, Logg A, Tai CK, Cannon PM, Kasahara N. Genomic stability of murine leukemia viruses containing insertions at the Env-3' untranslated region boundary. J Virol. 2001;75:6989–6998. doi: 10.1128/JVI.75.15.6989-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hiraoka K, Kimura T, Logg CR, et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007;67:5345–5353. doi: 10.1158/0008-5472.CAN-06-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tai CK, Wang W, Lai YH, et al. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 17:614–623. doi: 10.1038/cgt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 304.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 305.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 306.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 307.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 308.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 309.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Qureshi NH, Bankiewicz KS, Louis DN, Hochberg FH, Chiocca EA, Harsh GRt. Multicolumn infusion of gene therapy cells into human brain tumors: technical report. Neurosurgery. 2000;46:663–668. doi: 10.1097/00006123-200003000-00027. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 311.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 312.Rainov NG, Ren H. Clinical trials with retrovirus mediated gene therapy--what have we learned? J Neurooncol. 2003;65:227–236. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 313.Short MP, Choi BC, Lee JK, Malick A, Breakefield XO, Martuza RL. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990;27:427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- 314.Izquierdo M, Martin V, de Felipe P, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther. 1996;3:491–495. [PubMed] [Google Scholar]
- 315.Klatzmann D, Valery CA, Bensimon G, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase "suicide" gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9:2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 316.Packer RJ, Raffel C, Villablanca JG, et al. Treatment of progressive or recurrent pediatric malignant supratentorial brain tumors with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration. J Neurosurg. 2000;92:249–254. doi: 10.3171/jns.2000.92.2.0249. [DOI] [PubMed] [Google Scholar]
- 317.Rainov NG, Kramm CM, Banning U, et al. Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Ther. 2000;7:1853–1858. doi: 10.1038/sj.gt.3301311. [DOI] [PubMed] [Google Scholar]
- 318.Shand N, Weber F, Mariani L, et al. A phase 1–2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10:2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 319.Prados MD, McDermott M, Chang SM, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 320.Floeth FW, Shand N, Bojar H, et al. Local inflammation and devascularization--in vivo mechanisms of the "bystander effect" in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther. 2001;8:843–851. doi: 10.1038/sj.cgt.7700382. [DOI] [PubMed] [Google Scholar]
- 321.Kramm CM, Korholz D, Rainov NG, et al. Systemic activation of the immune system during ganciclovir treatment following intratumoral herpes simplex virus type 1 thymidine kinase gene transfer in an adolescent ependymoma patient. Neuropediatrics. 2002;33:6–9. doi: 10.1055/s-2002-23592. [DOI] [PubMed] [Google Scholar]
- 322.Harsh GR, Deisboeck TS, Louis DN, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. J Neurosurg. 2000;92:804–811. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- 323.Izquierdo M, Cortes ML, Martin V, et al. Gene therapy in brain tumours: implications of the size of glioblastoma on its curability. Acta Neurochir Suppl. 1997;68:111–117. doi: 10.1007/978-3-7091-6513-3_21. [DOI] [PubMed] [Google Scholar]
- 324.Long Z, Li LP, Grooms T, et al. Biosafety monitoring of patients receiving intracerebral injections of murine retroviral vector producer cells. Hum Gene Ther. 1998;9:1165–1172. doi: 10.1089/hum.1998.9.8-1165. [DOI] [PubMed] [Google Scholar]
- 325.Long Z, Lu P, Grooms T, et al. Molecular evaluation of biopsy and autopsy specimens from patients receiving in vivo retroviral gene therapy. Hum Gene Ther. 1999;10:733–740. doi: 10.1089/10430349950018490. [DOI] [PubMed] [Google Scholar]
- 326.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 327.Colombo F, Zanusso M, Casentini L, et al. Gene stereotactic neurosurgery for recurrent malignant gliomas. Stereotact Funct Neurosurg. 1997;68:245–251. doi: 10.1159/000099933. [DOI] [PubMed] [Google Scholar]
- 328.Palu G, Cavaggioni A, Calvi P, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther. 1999;6:330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- 329.Puumalainen AM, Vapalahti M, Agrawal RS, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 330.Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–858. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 331.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 332.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 333.Immonen A, Vapalahti M, Tyynela K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 334.Osborne R. Ark floats gene therapy's boat, for now. Nat Biotechnol. 2008;26:1057–1059. doi: 10.1038/nbt1008-1057. [DOI] [PubMed] [Google Scholar]
- 335.Ark-Therapeutics. Cerepro® Phase III trial update: Analyses strengthen as more patients reach endpoint. 2009 [9/28/10]; Press Release]. Available from: http://investors.arktherapeutics.com/servlet/HsPublic?context=ir.access&ir_option=RNS_NEWS&item=126418067393456&ir_client_id=4553&transform=newsitem_new.
- 336.Mitchell P. Ark’s gene therapy stumbles at the finish line. Nat Biotechnol. 2010;28:183–184. doi: 10.1038/nbt0310-183. [DOI] [PubMed] [Google Scholar]
- 337.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 338.Yoshida J, Mizuno M, Wakabayashi T. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004;95:858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Weingart J, Strauss L, Grossman S, et al. Phase I/II study: intra-tumoral infusion of IL13-PE38QQR cytotoxin for recurrent supratentorial malignant glioma. Neuro - oncology. 2002;4:379. [Google Scholar]
- 340.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 341.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 342.Kunwar S, Chang SM, Prados MD, et al. Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg Focus. 2006;20:E15. [PubMed] [Google Scholar]
- 343.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 344.Parney IF, Kunwar S, McDermott M, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 345.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 347.Puri RK. Structure and function of interleukin-4 and its receptor. Cancer Treat Res. 1995;80:143–185. doi: 10.1007/978-1-4613-1241-3_6. [DOI] [PubMed] [Google Scholar]
- 348.Puri RK, Hoon DS, Leland P, et al. Preclinical development of a recombinant toxin containing circularly permuted interleukin 4 and truncated Pseudomonas exotoxin for therapy of malignant astrocytoma. Cancer Res. 1996;56:5631–5637. [PubMed] [Google Scholar]
- 349.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–2165. [PubMed] [Google Scholar]
- 350.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64:125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 351.Weber FW, Floeth F, Asher A, et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl. 2003;88:93–103. doi: 10.1007/978-3-7091-6090-9_15. [DOI] [PubMed] [Google Scholar]
- 352.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 353.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sampson JH, Reardon DA, Friedman AH, et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol. 2005;7:90–96. doi: 10.1215/S1152851703000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg. 1990;72:941–945. doi: 10.3171/jns.1990.72.6.0941. [DOI] [PubMed] [Google Scholar]
- 356.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 357.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neurooncol. 2003;65:237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 358.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 359.Shah AC, Benos D, Gillespie GY, Markert JM. Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J Neurooncol. 2003;65:203–226. doi: 10.1023/b:neon.0000003651.97832.6c. [DOI] [PubMed] [Google Scholar]
- 360.Edwards SJ, Dix BR, Myers CJ, et al. Evidence that replication of the antitumor adenovirus ONYX-015 is not controlled by the p53 and p14(ARF) tumor suppressor genes. J Virol. 2002;76:12483–12490. doi: 10.1128/JVI.76.24.12483-12490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Hann B, Balmain A. Replication of an E1B 55-kilodalton protein-deficient adenovirus (ONYX-015) is restored by gain-of-function rather than loss-of-function p53 mutants. J Virol. 2003;77:11588–11595. doi: 10.1128/JVI.77.21.11588-11595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 363.Petit T, Davidson KK, Cerna C, et al. Efficient induction of apoptosis by ONYX-015 adenovirus in human colon cancer cell lines regardless of p53 status. Anticancer Drugs. 2002;13:47–50. [PubMed] [Google Scholar]
- 364.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 365.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 367.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 368.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 370.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 371.Harland J, Papanastassiou V, Brown SM. HSV1716 persistence in primary human glioma cells in vitro. Gene Ther. 2002;9:1194–1198. doi: 10.1038/sj.gt.3301782. [DOI] [PubMed] [Google Scholar]
- 372.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 373.Lorence RM, Katubig BB, Reichard KW, et al. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54:6017–6021. [PubMed] [Google Scholar]
- 374.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 375.Schneider T, Gerhards R, Kirches E, Firsching R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J Neurooncol. 2001;53:39–46. doi: 10.1023/a:1011856406683. [DOI] [PubMed] [Google Scholar]
- 376.Steiner HH, Bonsanto MM, Beckhove P, et al. Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22:4272–4281. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 377.Burton E, Prados M. New chemotherapy options for the treatment of malignant gliomas. Curr Opin Oncol. 1999;11:157–161. doi: 10.1097/00001622-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 378.Burton EC, Prados MD. Malignant gliomas. Curr Treat Options Oncol. 2000;1:459–468. doi: 10.1007/s11864-000-0073-2. [DOI] [PubMed] [Google Scholar]
- 379.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Goverdhana S, Puntel M, Xiong W, et al. Regulatable gene expression systems for gene therapy applications:progress and future challenges. Molecular Therapy. 2005 doi: 10.1016/j.ymthe.2005.03.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]