Abstract
Sufficient pulmonary surfactant production is required for the fetal–neonatal transition, especially in preterm infants. Neuregulin (NRG) and its transmembrane receptor ErbB4 positively regulate the onset of fetal surfactant synthesis. Details of this signaling process remain to be elucidated. ErbB4 is known to regulate gene expression in the mammary gland, where the receptor associates with the signal transducer and activator of transcription Stat5a to transactivate the β-casein gene promoter. We hypothesized that in the fetal lung, ErbB4 functions as a transcriptional regulator for surfactant protein B (Sftpb), the most critical surfactant protein gene. Re-expressing full-length ErbB4 in primary fetal ErbB4-depleted Type II epithelial cells led to an increased expression of Sftpb mRNA. This stimulatory effect required the nuclear translocation of ErbB4 and association with Stat5a, with the resultant binding to and activation of the Sftpb promoter. We conclude that ErbB4 directly regulates important aspects of fetal lung maturation that help prepare for the fetal–neonatal transition.
Keywords: Surfactant, Type II cell, Stat5a, lung development
Clinical Relevance
Because surfactant protein B is the most important protein for the surface tension–lowering function of surfactant, and is also the most important component of the surfactant preparation given to infants with surfactant deficiency stimulating Sftpb expression by an up-regulation of the ErbB4 signaling pathway through the application of neuregulin may be considered by those interested in designing new interventions to prevent surfactant deficiency in the newborn.
The differentiation of the neuronal (1, 2) and mammary (3) systems requires proper signaling between the growth factor neuregulin (NRG) and its cell-surface receptor, ErbB4. In the fetal lung, NRG is required for the initiation of fetal surfactant synthesis (4), and ErbB receptors (named because of their homology to the erythroblastoma viral gene product, v-erbB), also known as human epidermal growth factor receptors (HERs), are involved in the timely progression of fetal lung cell differentiation (4–6).
Surface-active agents (surfactants) prevent pulmonary alveoli from collapsing at end-expiration. Therefore, the synthesis of surfactants by fetal pulmonary Type II cells is a crucial part of prenatal lung development (7), in preparation for the transition at birth from the intrauterine (aquatic) to the extrauterine (aerobic) environment. ErbB4, the signaling receptor for NRG, is the most prominent receptor dimerization partner in fetal Type II epithelial cells (8) and the down-regulation of ErbB4 inhibits fetal surfactant synthesis (9).
Fetal ErbB4 transgenic mice that are rescued from their lethal heart defect by expressing human ErbB4 cDNA under the control of the cardiac-specific myosin heavy chain (α-HMC) promoter (HER4heart) (3) exhibit changes in lung function and structure, resulting in a hyperreactive airway system and alveolar simplification in adult HER4heart (−/−) animals (10). In the fetal lung, the deletion of pulmonary ErbB4 leads to an overall delayed progression of structural and functional lung development, including the delayed expression of surfactant protein B (Sftpb). This delay is most prominent at the transition from the canalicular to the saccular stage on Embryonic Day 17. Delayed saccular development, with an increased mesenchymal area around the airspaces and a delayed onset of surfactant synthesis and Sftpb expression in HER4heart (−/−) lungs, confirms the important role of the ErbB4 receptor in the regulation of the timely progression of fetal lung development (5). We here expand on the exact ErbB4 signals that regulate the expression of Sftpb in the isolated fetal Type II epithelial cell.
ErbB4, known for its involvement in processes of differentiation (1, 2, 11, 12), is unique among transmembrane tyrosine kinase receptors, because its intracellular domain (4ICD) is translocated to the nucleus after proteolytic cleavage by the TNFα-converting enzyme (13). The 80-kD 4ICD is released into the cytosol (14), and is further processed by presenilin-dependent γ-secretase activity (15, 16). In the developing breast, 4ICD functions as a nuclear chaperone for the signal transducer and activator of transcription family member, Stat5a (17). The relevance of the nuclear translocation of 4ICD and its interaction with Stat5a in the developing lung remain to be determined. Here we show how ErbB4 interacts and coregulates Stat5a in its effects on the developing fetal pulmonary surfactant system, using a unique model of primary fetal ErbB4-naive Type II cells.
Materials and Methods
The Sftpb promoter luciferase reporter plasmid was kindly provided by Philip L. Ballard (University of California, San Francisco, CA) (18). pEGFP N3 (control), pHER4 GFP (full-length ErbB4 receptor), pHER4 GFP-muNLS (mutant with a defective nuclear localization signal), and pRed Stat5a (full-length Stat5a) were used for transfection experiments (17). NRG-1β producer cells were kindly provided by Dr. Kermit Carraway III (University of California, Davis, CA), and NRG was purified by Ann Kane at the Phoenix Laboratory (Tufts Medical Center, Boston, MA).
Materials and detailed methods of Type II cell isolation, electron microscopy, coimmunoprecipitation, Western blotting, MTT assay, confocal microscopy, RNA isolation, quantitative real-time PCR, and nuclear subfractionation are described in the online supplement.
Preparation of Fibroblast-Conditioned Medium and Primary Fetal Murine Type II Epithelial Cell Cultures
Transgenic ErbB4 mice, rescued from their lethal cardiac defects by expressing a human ErbB4 (HER4heart) cDNA under the cardiac-specific α-HMC promoter (3) (kindly provided by Carmen Birchmeier, in agreement with Dr. Martin Gassmann), were used. The animal research protocol was approved by the institutional IACUC (Institutional Animal Care and Use Committee) at Hannover Medical School (Hannover, Germany). Time-dated pregnant HER4heart mice were killed on Embryonic Day 17.5 for cell isolation. Wild-type mice were killed on Embryonic Day 18 for the preparation of fibroblast-conditioned medium (FCM), by CO2 inhalation. Fetal lungs were removed, washed in sterile Hanks' buffered salt solution, and minced with a razorblade. The minced lungs were incubated with collagenase Type II for 2 hours at 37°C, and than transferred onto ice for 30 minutes. The tissue was centrifuged, and the pellet was resuspended in DMEM and incubated for 30 minutes on ice. After a second centrifugation, the pellet was resuspended in DNase and trypsin, and incubated for 12 minutes at 37°C. The reaction was stopped using DMEM containing 10% FCS. The cells were filtered, centrifuged, resuspended in DMEM containing 10% FCS, and plated in culture dishes for 60 minutes at 37°C (21% O2/5% CO2) to allow for differential adherence of lung fibroblasts. Fibroblasts were grown to confluence and serum-starved for 24 hours, to prepare the conditioned media.
For the isolation of Type II cells, supernatants from the first differential adherence were centrifuged again. The cell pellet was resuspended in DMEM containing 10% FCS, and plated in culture flasks for 60 minutes at 37°C for a second differential adherence. Supernatants were again removed and centrifuged. Cells were plated in DMEM containing 20% FCS and grown until they were used. To verify the Type II cell identity of isolated cells, their morphology was studied by electron microscopy (Figure 1) and the expression of Sftpc was analyzed by quantitative real time PCR (Figure E1).
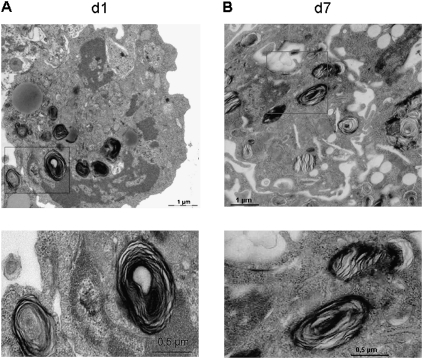
Figure 1.
Fetal Type II cells keep their cell characteristics in vitro. Fetal Type II cells were isolated on Gestational Day 17.5 from HER4heart animals and analyzed by electron microscopy after 1 day (A) and 7 days (B) in culture. Higher magnifications of lamellar bodies (LBs, as indicated in red frames) are shown below. LBs remain present in cells for up to 7 days in culture, confirming their Type II cell character in vitro. d, day.
Cell Transfection
Type II cells were cultured in six-well plates for real-time PCR and the luciferase assay, or on coverslips in 24-well plates for confocal microscopy, at a cell density of 1 × 106cells/ml. Transfections were performed after 24 hours, as described by the manufacturer (PAA-Laboratories, Pasching, Austria). Briefly, 1 μg DNA of pEGFP N3, pHER4 GFP, pHER4 GFP-muNLS, and pRed Stat5a was diluted with the Nanofectin solution (transfection reagent) and incubated for 30 minutes before added to the serum-containing medium on the cells. Cells were harvested after 48 hours of transfection.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) analyses were performed using a ChIP assay kit, as described by the manufacturer (USB, Staufen, Germany). Type II cells of HER4heart (+/+) animals were cultured in culture dishes, serum-starved for 4 hours, and treated with DMEM (control) or DMEM/FCM (1:1) for an additional 24 hours. Cells were cross-linked with 1% formaldehyde and chromatin was sonicated to an average size of 200–1,000 base pairs (bp). Chromatin was incubated with 5 μg antibody (anti-ErbB4 or anti-Stat5a) overnight. Input DNA was used as a positive control (input). A negative control without antibody (mock) was also included. To capture the immune complexes, 50 μl of preblocked protein Agarose beads were added. The DNA was isolated after the immune complexes were washed and eluted, and protein–DNA cross-links were reverse-transcribed. Primers for PCR amplification for the Sftpb promoter are listed in Table 1. The PCR products were sequenced using BigDye chemistry (ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit, Applied Biosystems, Darmstadt, Germany) and the Avant 3100 Genetic Analyzer (Applied Biosystems, Darmstadt, Germany), to show product specificity.
TABLE 1.
PRIMER FOR SURFACTANT PROTEIN B PROMOTER
| Primer Sequences | ||
| Sftpb 1 | Forward primer | 5′-TCTGCTTCTGGGTAAAGCTAC-3′ |
| Reverse primer | 5′-TTATGCAACGCCCAAGAGAG-3′ | |
| Sftpb 2 | Forward primer | 5′-CACTTACCCTGCGTCAAGAG-3′ |
| Reverse primer | 5′-GCCTGACTTTGTTCACGTC-3′ | |
| Sftpb 3 | Forward primer | 5′-AAGGACTAGGAACCGACATC-3′ |
| Reverse primer | 5′-ACTGCAGTAGGTGCGACCTTG-3′ |
Definition of abbreviation: Sftb, surfactant protein B.
Luciferase Reporter Gene Assay
Murine lung epithelial (MLE)–12 cells were transfected as already described, with the Sftpb promoter luciferase reporter plasmid alone or with the enhanced green fluorescent protein (EGFP), pHER4 or pRed Stat5a plasmid. Forty-eight hours after transfection, cells were starved for 4 hours and treated with DMEM (control) or NRG (33 nM) for 24 hours. Cell lysates were prepared in 100 μl of lysis buffer, and a luciferase assay was performed in duplicate, using a Berthold LB 9507 luminometer (Berthold, Bundoora Vic, Australia).
Data Analysis
All treatment values are presented as the mean ± SEM of experiment-specific control samples, unless otherwise stated. To evaluate the results in terms of statistical significance, we used a two-tailed t test with post hoc Bonferroni correction for multiple comparisons.
Results
Fetal Type II Cells Keep Their Cell Characteristics In Vitro
The presence of Type II cell–specific organelles was analyzed in primary fetal murine Type II cells after 1 day (Figure 1A) and after 7 days (Figure 1B) in culture. These cells keep their Type II cell phenotype (i.e., lamellar bodies) for up to 7 days in culture. The expression of Sftpc mRNA (19, 20) remains stable in the in vitro setting for the first 5 days (5.8 ± 1.7, n = 3, on Day 1; 9.6 ± 0.5, n = 3, on Day 2; 10.8 ± 0.6, n = 3, on Day 3; and 10.6 ± 0.1, n = 3, on Day 4), and slowly decreases afterward (14.1 ± 1.8, n = 3, on Day 6; 14.2 ± 1.8, n = 3, on Day 7; and 13.6 ± 0.6, n = 3, on Day 8). The mRNA expression is lowest after 9 days of cell culture (18.1 ± 2.1, n = 3) (Figure E1 in the online supplement). No Sftpc mRNA expression is evident in fetal fibroblasts (data not shown). Results are presented as numbers of cycles (Ct values), normalized by the number of cycles for actin. Ct levels are inversely proportional to the amount of target nucleic acid in the sample.
Homogeneity of Isolated Type II Cells
A lower-power electron micrograph was obtained to show the purity of isolated fetal Type II cells (Figure E2A). Epithelial cell specificity was detected by thyroid transcription factor (TTF)–1 staining (Figure E2B). TTF-1 is a homeodomain-containing transcription factor, and is essential for the expression of surfactant proteins. It is expressed predominantly in alveolar Type II cells and epithelial cells in the lung (21). Type II cell specificity was evaluated by Sftpc immunofluorescence staining (Figure E2B).
ErbB4 Significantly Stimulates the mRNA Expression of All Surfactant Proteins
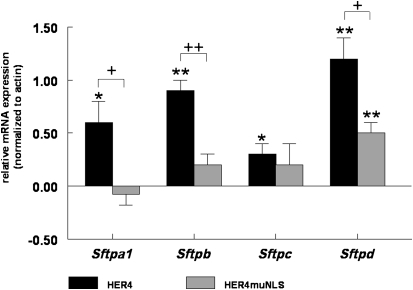
The expression of full-length human ErbB4 (HER4) leads to a significant increase in concentrations of Sftpa1 (0.6 ± 0.2, P = 0.01, n = 4), Sftpb (0.9 ± 0.1, P < 0.0001, n = 3), Sftpc (0.3 ± 0.1, P = 0.01, n = 4), and Sftpd (1.2 ± 0.2, P < 0.0001, n = 4) mRNA in primary Fetal Day 17.5 ErbB4-naive HER4heart (−/−) Type II cells, compared with cells transfected with an EGFP control plasmid (Figure 2).
Figure 2.
ErbB4 significantly stimulates the mRNA expression of all surfactant proteins. ErbB4-naive Gestational Day 17.5 Type II cells were isolated from HER4heart (−/−) animals transfected with full-length ErbB4 (HER4; black bars) or an ErbB4 mutant lacking the nuclear localization sequence (HER4 muNLS; gray bars). The effects of ErbB4 on the expression of surfactant protein A1 (Sftpa1), Sftpb, Sftpc, and Sftpd were measured by real-time PCR. Full-length ErbB4 induced a significant increase in Sftp mRNA concentrations for all four surfactant proteins (*P < 0.001, **P < 0.0001). Preventing the nuclear localization of ErbB4 significantly inhibited the stimulatory effect of ErbB4 on Sftp mRNA concentrations (+P < 0.001, ++P < 0.0001; n = 3).
ErbB4 Nuclear Localization Is Required for Its Effects on All Surfactant Proteins
The transfection of ErbB4-naive fetal Type II cells with an ErbB4 mutant lacking the nuclear localization signal (HER4 muNLS) exerts no significant effect on the mRNA expression of Sftpa1 (−0.08 ± 0.1, P = 0.58, n = 4), Sftpb (0.2 ± 0.1, P = 0.14, n = 3), and Sftpc (0.2 ± 0.2, P = 0.28, n = 3) expression, although it significantly increases Sftpd (0.5 ± 0.1, P < 0.0001, n = 3). Compared with transfection with full-length ErbB4, transfection with HER4 muNLS is associated with a significant decrease in the mRNA expression of Sftpa1 (−0.7 ± 0.2, P = 0.0114, n = 4), Sftpb (−0.4 ± 0.1, P = 0.0005, n = 3), and Sftpd (−0.6 ± 0.3, P = 0.0111, n = 3), suggesting that ErbB4 nuclear localization seems to be important for the mRNA expression of Sftpa1, Sftpb, and Sftpd in the fetal Type II cell. No significant change is evident in the effect on the mRNA expression of Sftpc when using the HER4 muNLS construct (0.2 ± 0.2, P = 0.5851, n = 3), compared to the full-length HER4 construct (Figure 2), implying that nuclear localization is not critical for its effect on this Sftp.
Transfection with HER4 (19 ± 0.1, P = 0.3, n = 3) or HER4 muNLS (19 ± 0.2, P = 0.4, n = 3) does not alter the mRNA expression of actin (Actb), when compared to cells transfected with EGFP (18.7 ± 0.2, n = 3) (data not shown).
ErbB4 Does Not Change Cell Proliferation
The expression of full-length ErbB4 (HER4) leads to an insignificant increase in cell viability, to 114% ± 9% (n = 6, P = 0.4), in HER4heart (+/+) Type II cells. This result is similar for the expression of the ErbB4 mutant (HER4 muNLS), increasing cell viability to 106% ± 5% (n = 6, P = 0.7) compared to EGFP transfected cells (100% ± 11%, n = 6) (Figure E3).
Stat5a Coprecipitates ErbB4
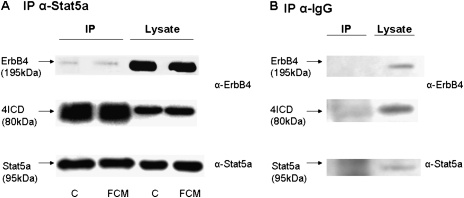
The immunoprecipitation (IP) of Stat5a was performed in wild-type HER4heart (+/+) Type II cells, using coimmunoprecipitation conditions. Stat5a coimmunoprecipitates the ErbB4 intracellular domain (4ICD) in primary fetal murine lung Type II cells under control conditions and after stimulation with FCM, confirming the interactions of these two proteins (Figure 3A). The IgG control IP confirms antibody specificity (Figure 3B).
Figure 3.
The signal transducer and activator of transcription Stat5a coprecipitates ErbB4. Interactions of the ErbB4 intracellular domain (4ICD) with Stat5a were studied in wild-type Type II cells isolated from HER4heart (+/+) animals, using coimmunoprecipitation conditions. Representative blots were probed with ErbB4 (upper and middle blots) and Stat5a (lower blots) antibodies. (A) Stat5a coimmunoprecipitates the ErbB4 intracellular domain (4ICD) in fetal Type II cells under control conditions (C) and after stimulation with fibroblast-conditioned medium (FCM) . (B) The IgG control immunoprecipitation (IP) confirms antibody specificity.
NRG Induces ErbB4 and Stat5a Intranuclear Colocalization
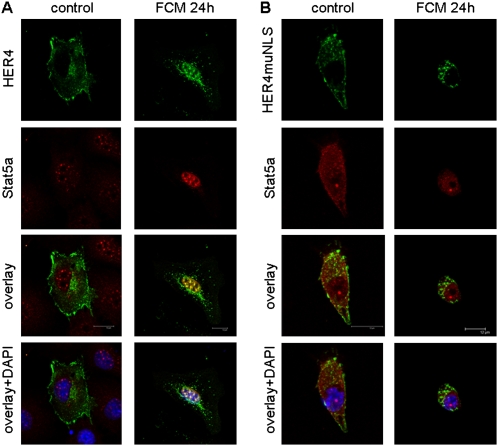
We then studied the intracellular localization and colocalization of ErbB4 and Stat5a in fetal ErbB4-naive Type II cells, using confocal microscopy. DAPI(4′,6-diamidino-2-phenylindole) staining was used to confirm nuclear localization. Full-length ErbB4 (HER4) is localized predominantly to the cell membrane and the cytoplasm, whereas endogenous Stat5a localizes to the nucleus, even in nonstimulated cells. A 24-hour stimulation with mature Day 18 fetal FCM, which is known to contain NRG (4) and is the most efficient stimulator of surfactant synthesis in fetal Type II cells (22), leads to an enrichment of transfected ErbB4 in the nucleus and a colocalization of ErbB4 with the endogenous Stat5a (Figure 4A).
Figure 4.
Neuregulin (NRG) induces the intranuclear colocalization of ErbB4 and Stat5a. Full-length ErbB4 (HER4) (green) localizes to the membrane when expressed in ErbB4-naive Gestational Day 17.5 Type II cells isolated from HER4heart (−/−) lungs. Stat5a (red) localizes diffusely in the cytoplasm and nucleus. (A) Twenty-four–hour exposure to mature, NRG-containing, fibroblast-conditioned medium (FCM, right) induces the localization of ErbB4 to the nucleus and colocalization with Stat5a. (B) The prevention of nuclear localization, using the mutant ErbB4 (HER4 muNLS), leads to a more diffuse localization of Stat5a in the cytoplasm, and prevents FCM-induced colocalization with Stat5a. Nuclear localization is confirmed by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue).
In cells transfected with HER4 muNLS (the mutant lacking the nuclear localization signal), endogenous Stat5a is more diffusely located in the cytoplasm in nonstimulated cells, suggesting that this mutant may prevent the localization of Stat5a to the nucleus under control conditions. Despite the absence of ErbB4 nuclear localization, stimulation with FCM induces the nuclear localization of Stat5a. No colocalization of HER4 muNLS with endogenous Stat5a occurs, even in the perinuclear region of the cytoplasm, where activated ErbB4 is found (Figure 4B). This finding confirms the need for ErbB4 nuclear entry for FCM-induced ErbB4 nuclear interactions with Stat5a. It also suggests the involvement of other factors in Stat5a nuclear transport.
ErbB4 Is a Nucleic Acid–Binding Protein
The observed effects of ErbB4 on the expression of Sftpb and the interaction of the receptor with the transcription factor Stat5a prompted us to investigate whether the transmembrane receptor ErbB4 is a nucleic acid–binding protein. Using nuclear subfractionation, we observed that in fetal Type II cells, 4ICD associates with nucleic acid in nonstimulated and FCM-stimulated cells (Figure E4, nucleic acid binding fraction). A 24-hour stimulation with FCM leads to an enrichment of 4ICD in the cytosolic fraction (Figure E4), implying an increased cleavage after FCM-induced receptor activation.
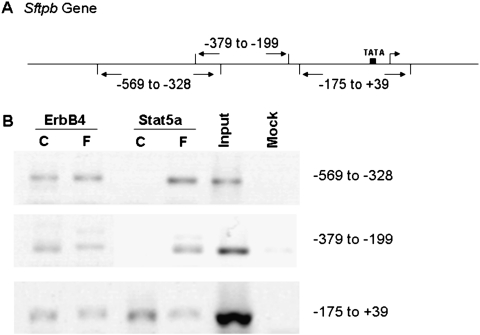
ErbB4 and Stat5a Bind to the Sftpb Promoter
To study whether ErbB4 and Stat5a bind to the Sftpb promoter, fetal wild-type Type II cells (HER4heart [+/+]) were stimulated with FCM, and chromatin immunoprecipitation experiments were performed. Primers were designed for the Sftpb promoter region, encompassing approximately 200-bp intervals (Figure 5A). The ErbB4 antibody precipitates three Sftpb promoter regions (nt −569 to −328, nt −379 to −199, and nt −175 to +39) under control conditions, whereas the Stat5a antibody precipitates only the region at nt −175 to +39. Stimulation with FCM sustains the presence of ErbB4 at all three promoter regions, and promotes the binding of Stat5a to all three promoter regions (nt −569 to −328, nt −379 to −199, and nt −175 to +39) (Figure 5B).
Figure 5.
ErbB4 and Stat5a bind to the Sftpb promoter. (A) Primers were designed for the Sftpb promoter, encompassing approximately 200–base pair intervals. (B) Cells were mock-stimulated (C) or FCM-stimulated (F). A chromatin immunoprecipitation (ChIP) assay was performed, and DNA bound to ErbB4 or Stat5a was analyzed by semiquantitative PCR amplification. The chromatin was immunoprecipitated, using antibodies directed against ErbB4 and Stat5a. The input DNA was used as positive control (Input), and a negative control with no antibody (Mock) was included. ErbB4 binds to all three sites in the Sftpb promoter region. Stat5a requires stimulation by FCM to bind to all three sites.
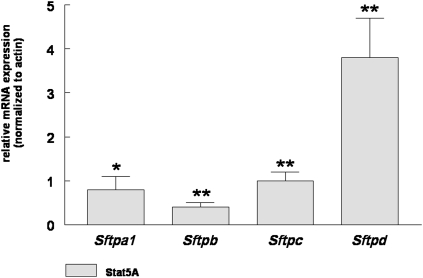
Overexpression of Stat5a Stimulates Sftp mRNA Expression
The effect of Stat5a on the expression of fetal Sftp was analyzed by overexpressing the transcription factor in ErbB4-naive Type II cells isolated from HER4heart (−/−) fetal lungs. Real-time PCR reveals a significant increase in mRNA concentrations of Sftpa1 (0.8 ± 0.3, mean ± SE, P = 0.015, n = 3), Sftpb (0.4 ± 0.1, mean ± SE, P = 0.002, n = 3), Sftpc (1.0 ± 0.2, mean ± SE, P = 0.001, n = 3), and Sftpd (3.8 ± 0.9, mean ± SE, P = 0.002, n = 3), compared to cells transfected with a control EGFP plasmid (Figure 6). Interestingly, some differences are evident in comparison to cells expressing full-length HER4 (Figure 2). The expression of Sftpb is stimulated to a much lower extent, compared to cells expressing ErbB4. This decrease in effect on Sftpb expression is similar to cells where the nuclear transport of ErbB4 is impaired, implying that both the nuclear localization of ErbB4 and nuclear interactions between ErbB4 and Stat5a are needed for the expression of Sftpb in the fetal Type II cell.
Figure 6.
Stat5a induces Sftp mRNA expression. The overexpression of Stat5a leads to a significant increase in Sftp mRNA concentrations in ErbB4-naive, Gestational Day 17.5 fetal Type II cells isolated from HER4heart (−/−) lungs (*P < 0.001, **P < 0.0001; n = 3).
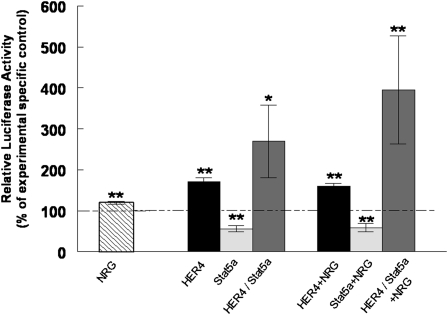
NRG Stimulates Sftpb Promoter Activity, Which Is Further Enhanced by ErbB4–Stat5a Interactions
To determine whether ErbB4 contributes to the activation of the Sftpb promoter, we transfected MLE-12 cells with a Sftpb promoter luciferase reporter plasmid. NRG significantly stimulated luciferase activity up to 120% ± 4% (P = 0.003, n = 10), compared to nonstimulated control cells (100% ± 2%, n = 11). Cotransfection of the Sftpb promoter luciferase reporter with full-length HER4 leads to an even more pronounced increase in luciferase activity, up to 170% ± 10% (P < 0.0001, n = 5), compared to cells transfected with the control plasmid (100% ± 4%, n = 13). Cotransfection with Stat5a alone decreases luciferase activity to 55% ± 8% (P < 0.0001, n = 6), whereas cotransfection with both HER4 and Stat5a increases luciferase activity up to 269% ± 89% (P = 0.04, n = 10). NRG further stimulates luciferase activity up to 159% ± 7% (P < 0.0001, n = 5) in HER4-overexpressing MLE-12 cells, compared to NRG-stimulated cells transfected with a control plasmid (100% ± 1%, n = 13). NRG does not overcome the inhibitory effect on luciferase activity in cells transfected with Stat5a (58% ± 11%, P < 0.0001, n = 4), and exerts only a slightly additive stimulatory effect on cells already cotransfected with both HER4 and Stat5a, increasing luciferase activity up to 395% ± 132% (P = 0.0016, n = 5) (Figure 7).
Figure 7.
NRG stimulates Sftpb promoter activity, which is further enhanced by ErbB4–Stat5a interactions. Murine lung epithelial–12 cells were transfected with the Sftpb promoter luciferase reporter. Luciferase activity was measured after stimulation with NRG (striped bar) and compared to nonstimulated control cells. Cells were cotransfected with the Sftpb promoter luciferase reporter and full-length ErbB4 (HER4) (black bar), Stat5a (light gray bar), or a combination of ErbB4 and Stat5a (dark gray bar). Effects were compared with effects observed in nonstimulated cells transfected with a control plasmid (epidermal growth factor receptor; EGFP). Cells were stimulated with NRG, and luciferase activity in ErbB4 (black bar), Stat5a (light gray bar), or ErbB4 and Stat5a (dark gray bar) transfected cells was compared to NRG-stimulated, EGFP-transfected control cells. NRG, ErbB4, and the combination of ErbB4 and Stat5a expression stimulates Sftpb promoter activity. This stimulatory effect is further enhanced by treatment with NRG (*P < 0.001, **P < 0.0001; n = 5–13).
Discussion
NRG-induced ErbB signaling plays an important role in the development and homeostasis of the nervous system (2, 23, 24), and may even be a candidate for neuroprotection (25, 26). Its role in the developing lung is largely unknown. Here we present evidence that NRG-induced ErbB4 interactions with Stat5a regulate the expression of fetal Sftpb. We previously showed that the deletion of pulmonary ErbB4 leads to lung disease in adult animals (10), and these defects originate in the developing lung (5). Here we provide detailed insights into the cell biology of ErbB4 function in primary isolated fetal Type II cells by using an in vitro approach in a unique transgenic murine model.
Surfactant deficiency and the structural immaturity of the lung contribute to long-term pulmonary morbidity in preterm infants. The prevalence of bronchopulmonary dysplasia (BPD) has not changed significantly, despite the widespread use of postnatal surfactant and prenatal surfactant-stimulating glucocorticoids (27–29). At least in part, this ongoing prevalence of BPD may be attributable to the negative side effects of glucocorticoid treatment, and also to intrauterine exposure to inflammation and the postnatal need for ventilatory support and exposure to oxygen (30, 31), all of which affect the normal progression of structural lung development.
One way to advance lung maturity with fewer negative side effects may involve stimulating an endogenous pathway that promotes the maturation of the fetal surfactant system and lung structure. NRG-1 is secreted by lung fibroblasts (4), and the NRG receptor ErbB4 is expressed by fetal Type II cells (8, 9). Both proteins play an important role in fetal Type II cell maturation (4, 32), promoting the synthesis of fetal surfactant.
In the present study, we explored the role of ErbB4 nuclear translocation in murine fetal Type II cells, using a unique model of ErbB4-naive primary fetal Type II cells, isolated on Gestational Day 17.5 from HER4heart mice, which are rescued from their lethal heart defect by re-expressing a human ErbB4 cDNA in the heart by a cardiac-specific α-HMC promoter (3). Day 17.5 of gestation was chosen for the isolation of fetal Type II cells, because at that time, the fetal lungs advance from the canalicular to the saccular stage, and surfactant synthesis is initiated (33).
Adult Type II cells are difficult to grow on tissue culture plastic because they lose their Type II cell phenotype, and transdifferentiate by gaining Type I cell markers (34, 35). The expression of Sftpc is restricted to Type II cells, and can be used as a cellular marker (19, 20). Using electron microscopy, the mRNA expression of Sftpc, and immunofluorescence staining, we demonstrated that isolated fetal Type II cells do not transdifferentiate, but keep their Type II cell–specific characteristics (i.e., lamellar bodies, which are the major surfactant phospholipid storage organelles) and maintain the expression of Sftpc for up to 7 days in culture.
Sftpb and Sftpc are secreted by lamellar bodies, whereas Sftpa1 and Sftpd are secreted independently (36). Surfactant synthesis is a crucial component of fetal lung development in preparation for the aquatic–aerobic transition at birth (7). A genetic deficiency of Sftpb results in neonatal respiratory failure and death (37). Because Sftpb is the surfactant protein most critical for surfactant dynamics and for the reduction of surface tension (38), and because the ErbB4 receptor plays an important role in structural and functional lung development (5, 10), we focused on the role of ErbB4 in its function on Sftpb gene regulation. The expression of full-length ErbB4 induced Sftpb mRNA expression, but the nuclear localization of ErbB4 was a critical requirement for this up-regulation, underlining the critical role of ErbB4 nuclear function in the fetal Type II cell.
In adult pulmonary epithelial cells, the ErbB4 ligand NRG activates the JAK–STAT (Janus Kinase-Signal Transducer and Activator of Transcription) signal transduction pathway through ErbB2/ErbB3 receptor heterodimers, leading to increased cell proliferation (39). The role of NRG and ErbB4 signaling in primary adult Type II cells has not been well studied. In fetal Type II cells, ErbB4 is a preferred dimerization partner for ErbB1, also known as epidermal growth factor receptor (EGFR) (8). ErbB4 actively regulates the most important functional maturation of the fetal Type II cell, namely, fetal surfactant synthesis (9). The deletion of ErbB4 leads to delayed fetal lung development (5) and structural and functional disturbances in the adult lung (10). Our results suggest that the nuclear translocation of ErbB4 plays a critical role in its effects on the expression of Sftpb.
ErbB4 is known to be cleaved at the cell membrane by presenilin-dependent γ-secretase activity, liberating the cytoplasmic domain of ErbB4 (4ICD) from the plasma membrane into the cytosol, which translocates to the nucleus (15, 16). In the developing breast, 4ICD appears to function as a nuclear chaperone for Stat5a, to regulate milk gene expression (17). Stat5a is involved in diverse cellular functions such as proliferation, apoptosis, and differentiation (40). Until now, the relevance of 4ICD and its interactions with transcription factors in the developing fetal Type II cell are, to the best of our knowledge, unknown. Here we show that Stat5a is expressed in fetal Type II cells, and mature FCM induces the colocalization of Stat5a and ErbB4 within the nucleus.
Fibroblast–Type II cell interactions play an important role in the developing fetal surfactant system. Mature fetal fibroblasts secrete soluble factors into the FCM, to initiate fetal surfactant synthesis in the fetal Type II cell (22). We recently reported that NRG constitutes one component of these factors (4). Our present study further elucidates the role of NRG as an intracellular signal involved in the maturation of the fetal surfactant system. In particular, we found that the NRG-induced nuclear localization of ErbB4 and the intracellular colocalization of Stat5a with ErbB4 are required for the effect on Sftpb promoter activity.
The Sftpb gene is encoded on murine chromosome 6 (41), and is composed of 11 exons and 10 introns, flanked by promoter-enhancer consensus sequences (42). Highly conserved regions in the Sftpb gene are located 100 and 300 bp downstream from the transcription start site, and these highly conserved regions contain DNase hypersensitivity and footprinting sites, suggesting the presence of transcription factor binding sites. The expression of Sftpb is known to be influenced by hormonal, developmental, and microenvironmental factors (43). Here we show for the first time, to the best of our knowledge, that ErbB4 binds to the Sftpb promoter, and although FCM stimulates Stat5a binding to this promoter region, Stat5a requires the presence of ErbB4 for its stimulatory effect on Sftpb promoter activity. Other members of the ErbB receptor family are known to be involved in transcriptional regulation. EGFR, for example, binds to the cyclin D1 promoter (44), and is able to associate with Stat5a on the ATRS motif (AT-rich sequence sites), to transactivate the Aurora-A promoter (45). ErbB2 forms a complex at the COX-2 (Cyclooxygenase-2) promoter, and stimulates its transcription (46). Because ErbB receptors lack any known structural signatures required for associations with DNA (47), they are not likely to interact directly with genomic DNA. For EGFR, an association with a histone 3 isoform was reported (48).
The effects of Stat5a overexpression that we observed on surfactant proteins were independent of ErbB4, and may be attributable to interactions with other ErbB receptors, e.g., ErbB1, mainly because ErbB1 homodimers are known to activate Stats sufficiently (49). On the other hand, ErbB4 is known for its transcriptional function through interactions with other DNA binding nuclear factors (17, 50, 51). Further studies are needed to elucidate these pathways in greater detail in fetal Type II cells.
In conclusion, we demonstrate that ErbB4 functions as a transcription factor for the Sftpb gene. ErbB4 signaling in Sftpb gene expression involves the coregulation of Stat5a. Because Sftpb is the most important protein for the surface tension–lowering function of surfactant, and is the most important component of the surfactant preparation given to infants with surfactant deficiency, an up-regulation of the ErbB4 signaling pathway through an application of NRG may be considered by those interested in designing new interventions to prevent surfactant deficiency in the newborn. Systemic treatment with NRG was successfully used to enhance brain remyelination in mice (52) and to improve cardiac function in humans (53). Therefore, systemic treatment with NRG may conceivably enhance fetal lung development.
Supplementary Material
Acknowledgments
The authors thank Dr. Martin Gassmann and Carmen Birchmeier for providing the HER4heart murine line, and Dr. Philip L. Ballard for supplying the Sftpb promoter luciferase reporter plasmid. The authors also thank S. Rozek for assistance in breeding the experimental animals, and D. von Mayersbach for processing and cutting samples for electron microscopy.
Footnotes
This study was supported by the German Research Foundation grants Da 378/3-1 and Da 378/3-2 and by the National Heart, Lung, and Blood Institute grant HL-04436 (C.E.L.D.) from the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0179OC on February 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res 2009;315:611–618 [DOI] [PubMed] [Google Scholar]
- 2.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science 2004;304:700–703 [DOI] [PubMed] [Google Scholar]
- 3.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA 2003;100:8281–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dammann CE, Nielsen HC, Carraway KL. Role of neuregulin1{beta} in the developing lung. Am J Respir Crit Care Med 2003;167:1711–1716 [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Purevdorj E, Zscheppang K, von Mayersbach D, Behrens J, Brinkhaus MJ, Nielsen HC, Schmiedl A, Dammann CE. ErbB4 regulates the timely progression of late fetal lung development. Biochim Biophys Acta 2010;1803:832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen HC. Epidermal growth factor influences the development clock regulating maturation of the fetal lung fibroblast. Biochim Biophys Acta 1989;1012:201–206 [DOI] [PubMed] [Google Scholar]
- 7.Langston C, Kida K, Reed M. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis 1984;129:607–613 [PubMed] [Google Scholar]
- 8.Liu W, Zscheppang K, Murray S, Nielsen HC, Dammann CE. The ErbB4 receptor in fetal rat lung fibroblasts and epithelial Type II cells. Biochim Biophys Acta 2007;1772:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zscheppang K, Liu W, Volpe MV, Nielsen HC, Dammann CE. ErbB4 regulates fetal surfactant phospholipid synthesis in primary fetal rat Type II cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L429–L435 [DOI] [PubMed] [Google Scholar]
- 10.Purevdorj E, Zscheppang K, Hoymann HG, Braun A, von Mayersbach D, Brinkhaus MJ, Schmiedl A, Dammann CE. ErbB4 deletion leads to changes in lung function and structure similar to bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2008;294:L516–L522 [DOI] [PubMed] [Google Scholar]
- 11.Prevot V, Lomniczi A, Corfas G, Ojeda SR. ErbB-1 and ErbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology 2005;146:1465–1472 [DOI] [PubMed] [Google Scholar]
- 12.Jones FE. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J Mammary Gland Biol Neoplasia 2008;13:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub MS, Germann SL, Lloyd KCK. Behavioral characteristics of a nervous system–specific ErbB4 knock-out mouse. Behav Brain Res 2004 2004;153:159–170 [DOI] [PubMed] [Google Scholar]
- 14.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor–alpha–converting enzyme is required for cleavage of ErbB4/HER4. J Biol Chem 2000;275:10379–10387 [DOI] [PubMed] [Google Scholar]
- 15.Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 2001;294:2179–2181 [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, Kim TW. Presenilin-dependent gamma-secretase–like intramembrane cleavage of ErbB4. J Biol Chem 2002;277:6318–6323 [DOI] [PubMed] [Google Scholar]
- 17.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol 2004;167:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Zhu NL, Tan RC, Ballard PL, Derynck R, Minoo P. Transforming growth factor–beta inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NKX2.1 and HNF-3 transcription factors. J Biol Chem 2002;277:38399–38408 [DOI] [PubMed] [Google Scholar]
- 19.Glasser SW, Korfhagen TR, Bruno MD, Dey C, Whitsett JA. Structure and expression of the pulmonary surfactant protein SP-C gene in the mouse. J Biol Chem 1990;265:21986–21991 [PubMed] [Google Scholar]
- 20.Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar Type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol 1992;6:594–600 [DOI] [PubMed] [Google Scholar]
- 21.Boggaram V. Thyroid transcription factor–1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009;116:27–35 [DOI] [PubMed] [Google Scholar]
- 22.Smith BT. Lung maturation in the fetal rat: acceleration by injection of fibroblast pneumocyte factor. Science 1979;204:1094–1095 [DOI] [PubMed] [Google Scholar]
- 23.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002;71:877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, et al. Loss of ErbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA 2007;104:8131–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dammann O, Cesario A, Hallen M. NEOBRAIN: an EU-funded project committed to protect the newborn brain. Neonatology 2007;92:217–218 [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Xu Z, Ford GD, Croslan DR, Cairobe T, Li Z, Ford BD. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res 2007;1184:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 1995;173:322–335 [DOI] [PubMed] [Google Scholar]
- 28.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643 [DOI] [PubMed] [Google Scholar]
- 29.Bonanno C, Wapner RJ. Antenatal corticosteroid treatment: what's happened since Drs. Liggins and Howie? Am J Obstet Gynecol 2009;200:448–457 [DOI] [PubMed] [Google Scholar]
- 30.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729 [DOI] [PubMed] [Google Scholar]
- 31.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed 2008;93:F455–F461 [DOI] [PubMed] [Google Scholar]
- 32.Zscheppang K, Korenbaum E, Bueter W, Ramadurai SM, Nielsen HC, Dammann CE. ErbB receptor dimerization, localization, and co-localization in mouse lung Type II epithelial cells. Pediatr Pulmonol 2006;41:1205–1212 [DOI] [PubMed] [Google Scholar]
- 33.Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol 1984;46:617–628 [DOI] [PubMed] [Google Scholar]
- 34.Adamson IY, Bowden DH. Derivation of Type 1 epithelium from Type 2 cells in the developing rat lung. Lab Invest 1975;32:736–745 [PubMed] [Google Scholar]
- 35.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar Type 2 cells to Type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150 [DOI] [PubMed] [Google Scholar]
- 36.Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol 2001;129:233–243 [DOI] [PubMed] [Google Scholar]
- 37.Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 1993;328:406–410 [DOI] [PubMed] [Google Scholar]
- 38.Hamm H, Kroegel C, Hohlfeld J. Surfactant: a review of its functions and relevance in adult respiratory disorders. Respir Med 1996;90:251–270 [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Kern JA. Neuregulin-1 activates the JAK–STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 2002;27:306–313 [DOI] [PubMed] [Google Scholar]
- 40.Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev 1999;10:131–157 [DOI] [PubMed] [Google Scholar]
- 41.D'Amore-Bruno MA, Wikenheiser KA, Carter JE, Clark JC, Whitsett JA. Sequence, ontogeny, and cellular localization of murine surfactant protein B mRNA. Am J Physiol 1992;262:L40–L47 [DOI] [PubMed] [Google Scholar]
- 42.Margana RK, Boggaram V. Rabbit surfactant protein B gene: structure and functional characterization of the promoter. Am J Physiol 1996;270:L601–L612 [DOI] [PubMed] [Google Scholar]
- 43.Pryhuber GS. Regulation and function of pulmonary surfactant protein B. Mol Genet Metab 1998;64:217–228 [DOI] [PubMed] [Google Scholar]
- 44.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 2001;3:802–808 [DOI] [PubMed] [Google Scholar]
- 45.Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, Chuang YH, Lai CH, Chang WC. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res 2008;36:4337–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell 2004;6:251–261 [DOI] [PubMed] [Google Scholar]
- 47.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res 2009;15:6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blagoev B, Kratchmarova I, Ong SE, Nielsen M, Foster LJ, Mann M. A proteomics strategy to elucidate functional protein–protein interactions applied to EGF signaling. Nat Biotechnol 2003;21:315–318 [DOI] [PubMed] [Google Scholar]
- 49.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor–induced activation of Stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem 1999;274:17209–17218 [DOI] [PubMed] [Google Scholar]
- 50.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res 2006;66:6412–6420 [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, Vadlamudi RK, Jones FE. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res 2006;66:7991–7998 [DOI] [PubMed] [Google Scholar]
- 52.Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, Gwynne D, Raine CS. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci USA 1998;95:10100–10105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 2011;13:83–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.