Abstract
Rationale: Time series studies have reported associations between ozone and daily deaths. Only one cohort study has reported the effect of long-term exposures on deaths, and little is known about effects of chronic ozone exposure on survival in susceptible populations.
Objectives: We investigated whether ozone was associated with survival in four cohorts of persons with specific diseases in 105 United States cities, treating ozone as a time varying exposure.
Methods: We used Medicare data (1985–2006), and constructed cohorts of persons hospitalized with chronic conditions that might predispose to ozone effects: chronic obstructive pulmonary disease, diabetes, congestive heart failure, and myocardial infarction. Yearly warm-season average ozone was merged to the individual follow-up in each city. We applied Cox proportional hazard model for each cohort within each city, adjusting for individual risk factors, temperature, and city-specific long-term trends.
Measurements and Main Results: We found significant associations with a hazard ratio for mortality of 1.06 (95% confidence interval [CI], 1.03–1.08) per 5-ppb increase in summer average ozone for persons with congestive heart failure; of 1.09 (95% CI, 1.06–1.12) with myocardial infarction; of 1.07 (95% CI, 1.04–1.09) with chronic obstructive pulmonary disease; and of 1.07 (95% CI, 1.05–1.10) for diabetics. We also found that the effect varied by region, but that this was mostly explained by mean temperature, which is likely a surrogate of air conditioning use, and hence exposure.
Conclusions: This is the first study that follows persons with specific chronic conditions, and shows that long-term ozone exposure is associated with increased risk of death in these groups.
Keywords: survival analysis, ozone, long-term exposure, cardiovascular disease, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
To date there is only one paper associating long-term exposure to ozone to reduced survival in a cohort, and little is known about the response of potentially susceptible populations.
What This Study Adds to the Field
This is the first cohort study that follows susceptible cohorts and shows that long-term exposure to ozone elevates the risk of mortality.
Ground level ozone is among the most widespread and dangerous air pollutants. The American Lung Association (State of the Air 2011: http://www.stateoftheair.org/2011/key-findings/2007-2009/ozone-pollution.html) reported that about 48% of the United States population lives in the 338 counties with unsafe levels of ozone.
A recent study by Anenberg and coauthors (1) estimated the global burden of mortality caused by ozone and particles with an aerodynamic diameter of 2.5 μm or less (PM2.5) from anthropogenic emissions, and found that ozone was associated with about 0.7 ± 0.3 million respiratory deaths and 6.3 ± 3 million “years of life lost” annually.
Epidemiologic studies have reported associations between short-term changes in ozone and short-term changes in deaths, especially during the warm weather months (2–5). Three recent meta-analyses reviewed the state of the ozone-mortality time-series literature (6–8). Other studies have shown higher mortality risk in more vulnerable populations, such as the elderly, persons with atrial fibrillation, and blacks (9, 10), and demonstrated the acute effects of ozone are not caused by short-term mortality displacement (11). Another study examined the question of adaptation in the acute mortality risk associated with ambient ozone (12). In contrast, only one study has examined the effects of chronic exposure to ozone in a cohort (13).
The previously mentioned epidemiologic literature is supported by studies of the biologic mechanism of ozone toxicity. There is evidence that short-term ozone exposure is associated with decrements in lung functions, increased respiratory symptoms, and lung inflammation (14–25). The effect of short- and long-term ozone exposure was associated with biomarkers of oxidative stress (26), and with reduced heart rate variability (27). Finally, a review of toxicologic studies found decreased heart rate, metabolism, blood pressure, and cardiac output when rats are exposed to typical concentrations of ozone (28).
All observational epidemiology studies are subject to potential confounding, but different study designs are usually subject to different types of confounding, or confounding by different sets of covariates. Studies of air pollution and mortality fall into two broad categories. Time-series analyses examine the association between day-to-day changes in daily death counts and day-to-day changes in air pollution concentrations. These studies are usually done separately in each geographic area studied, eliminating confounding by factors that vary across geography.
Cohort analyses of long-term exposure to air pollution and survival are very different. In these, long-term average air pollution concentrations in a particular geographic place are assigned to subjects in that place, and their mortality experience across place is compared with the air pollution variation across place. Here season and other short-term fluctuations are not a source of confounding, because the exposure contrast is long-term exposure across place. Potential confounders, therefore, are those that likewise vary across place. These include such factors as diet and socioeconomic position. These are precisely the factors that are not confounders in the time-series studies.
Both types of studies have reported associations of particulate air pollution with mortality risk. However, only one cohort study has examined the association between mortality and warm season average ozone concentrations (13), and the dearth of confirmatory reports prompted the US Environmental Protection Agency's Science Advisory Board to recommend not using that study to estimate the health benefits of reducing ozone concentrations.
Recently, we introduced a variant of the cohort study that seeks to capture some of the advantages of both time series and cross-sectional cohort studies, and that is subject to a different and, we argued, smaller set of potential confounders. The approach focuses on year-to-year differences in exposure from their long-term trend within city. In this approach a time varying Cox proportionate hazard model is used with annual follow-ups, and year-to-year variations in exposure are used as risk factors for the year-to-year survival of the cohort, controlling for long-term trend within city. This analysis can be repeated in multiple cities and the results combined across cities in a second stage. Using that approach, we have demonstrated that particle concentrations were risk factors for reduced survival in persons discharged alive after hospitalization for either myocardial infarctions (MIs) (29) or chronic obstructive pulmonary disease (COPD) (30). We here apply that approach to the question of whether longer-term exposure to ozone is associated with reduced survival in cohorts of putatively susceptible populations, including persons with heart disease, chronic lung disease, or diabetes, using data from 105 cities.
Methods
Study Population
The US Medicare program covers hospitalization for all residents aged 65 and older. Using data for the years 1985–2006, we constructed four cohorts of persons with potentially predisposing conditions. These were defined as persons discharged alive after emergency admission for the specific conditions we hypothesized might render subjects at greater risk, defining cases as a primary discharge diagnosis of COPD (International Classification of Disease 9th revision [ICD-9] 490–496, except 493); diabetes (ICD-9 250); congestive heart failure (CHF; ICD-9 428); and MI (ICD-9 410).
We obtained date of death for each subject, or whether they were still alive as of the end of 2006, and information on age, sex, race, severity of the index admission expressed by the number of coronary and medical intensive care days, and on medical conditions that might affect the risk of survival. We defined these as previous admissions with diagnoses of atrial fibrillation (ICD-9 427.3) or MI, and secondary (on the index admission) or previous diagnoses for COPD, diabetes, CHF, and essential hypertension (ICD-9 401).
Subjects alive the first of May of the year following the index admission entered into the cohort, and follow-up periods were 1-year periods (May to December) until the year in which they die or until December 2006 (censoring). We excluded subjects whose death occurred within the first 3 months of their index admission, and those who were admitted in 2006. This method has been previously described (29, 30).
Environmental Data
We obtained ozone (8-h mean) data from the US Environmental Protection Agency's Air Quality System Technology Transfer Network in 105 cities. For each subject and follow-up period we created yearly averages of the 8-hour mean daily ozone concentrations for the summer (May to September) and transitional season (Spring and Autumn) for that year. We choose 105 cities with at least 100,000 inhabitants, monitoring data for ozone, and representing a geographic distribution across the United States (see Table E1 in the online supplement).
We created the yearly average of summertime (June to August) and wintertime (December to February) temperature in each year in each city.
We examined if the risk differed by prevailing climate by dividing the United States into regions based on the Köppen climate classification (31), which is one of the most widely used climate classification systems (see online supplement).
Statistical Methods
To avoid cross-sectional confounding we fit separate survival analyses in each city and each cohort. The exposure was warm season (or transitional season) ozone, which was treated as a time-varying covariate. We controlled for long-term time trends with a linear term for year of follow-up, therefore examining whether year-to-year variations in survival around its long-term trend were associated with year-to-year variations in ozone, around its long-term trend. We also adjusted for season, weather, and individual risk factors. More details are in the online supplement.
In the second stage of the analysis, the results of these city-specific analyses (for each predisposing condition, for the average ozone during summer and transitional period) were combined using a random effect meta-regression (32).
Results
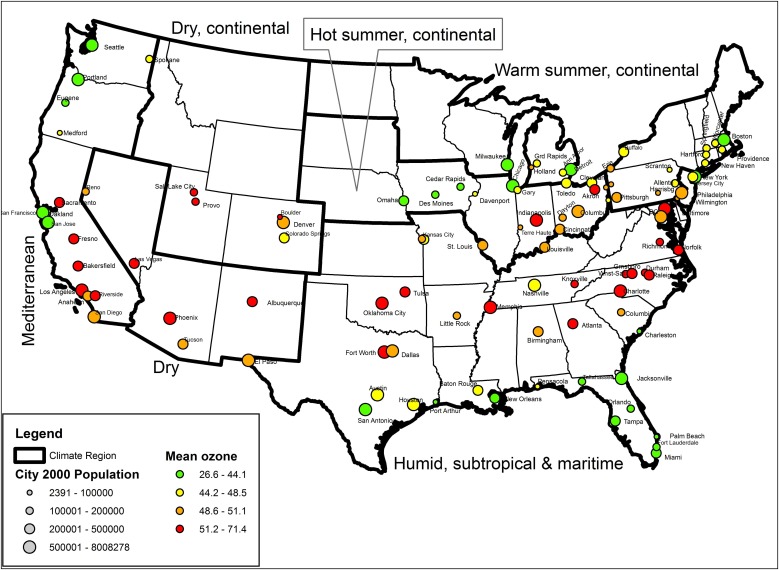
The cities used in the study, together with the city-specific distribution of the average ozone during summer (May to September) and transitional season (Spring and Autumn) are listed in Table E1. Figure 1 shows the map of the United States with the location of the 105 cities included in the study; the symbol size represents the population in each city, whereas the color represents the average ozone in the city. There were 43 cities in region 1, 21 cities in region 2, 16 cities in region 3, 5 cities in region 4, 5 cities in region 5, and 15 cities in region 6.
Figure 1.
Map of the United States with the location of the 105 cities included in the study; the symbol size represents the population in each city, whereas the color represents the average ozone in the city.
Table 1 shows characteristics of the study population for all the cities together for each of the four cohorts. Overall, our cohorts consisted of 3,210,511 persons with COPD, 1,561,819 with CHF, 2,935,647 with diabetes, and 1,186,496 with MI. Of these, 45% of the COPD population, 55.4% of the CHF population, 38.6% of the diabetes population, and 38.1% of the MI population died before the end of follow-up.
TABLE 1.
CHARACTERISTICS OF THE STUDY POPULATION FOR EACH COHORT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE, DIABETES, MYOCARDIAL INFARCTION, AND CONGESTIVE HEART FAILURE, AMONG RESIDENTS OF 105 UNITED STATES CITIES
| CHF |
MI |
Diabetes |
COPD |
|||||
| N* | % | N* | % | N* | % | N* | % | |
| Events | 1,562 | 100 | 1,186 | 100 | 2,936 | 100 | 3,211 | 100 |
| Deaths | 865 | 55.4 | 453 | 38.1 | 1,134 | 38.6 | 1,445 | 45 |
| Sex | ||||||||
| Male | 632 | 40.5 | 604 | 50.9 | 1,211 | 41.3 | 1,470 | 45.8 |
| Female | 930 | 59.5 | 583 | 49.1 | 1,724 | 58.7 | 1,741 | 54.2 |
| Race | ||||||||
| White | 1,249 | 80 | 1,019 | 85.9 | 2,145 | 73.1 | 2,728 | 85 |
| Black | 226 | 14.5 | 104 | 8.7 | 538 | 18.3 | 312 | 9.7 |
| Other | 87 | 5.6 | 64 | 5.4 | 252 | 8.6 | 170 | 5.3 |
| Age† | 79.1 (66.9, 92.3) | 76.5 (66.7, 89.6) | 75.7 (66, 88.6) | 76.8 (66.4, 89.9) | ||||
| N days in coronary care† | 0.67 (0–4) | 1.4 (0–6.5) | 0.3 (0–2.3) | 0.4 (0–2.6) | ||||
| N days in intensive care† | 0.85 (0–4.7) | 1.5 (0–6.7) | 0.5 (0–3.3) | 0.7 (0–4.4) | ||||
| Secondary or previous diagnoses | ||||||||
| COPD | 421 | 27 | 182 | 15.3 | 341 | 11.6 | ||
| CHF | 387 | 32.7 | 434 | 14.8 | 644 | 20 | ||
| Diabetes | 425 | 27.2 | 256 | 21.5 | 432 | 13.4 | ||
| Hypertension | 639 | 40.9 | 454 | 38.3 | 1,278 | 43.5 | 973 | 30.3 |
| Previous admissions | ||||||||
| Atrial fibrillation | 250 | 16 | 69 | 5.8 | 129 | 4.4 | 200 | 6.2 |
| MI | 161 | 10.3 | 70 | 2.4 | 103 | 3.2 | ||
Definition of abbreviations: CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; MI = myocardial infarction.
N divided by 1,000.
Expressed as mean (5–95%).
The average duration of the follow-up was 5.1 years for CHF, 5.6 years for COPD, 5.6 years for diabetes, and 6.1 years for MI. The range of survival times in all the cohorts varied from 1–21 years.
The four cohorts differed little in terms of population characteristics with a higher percentage of female, and more whites. The CHF cohort was the older with a mean age of 79 years, whereas the diabetes cohort was younger with a mean age of 76 years.
Table 2 presents the results of the analyses across all cities for ozone averages during summer and transitional seasons. The average interquartile range of ozone over all the cities was 5.5 for the summer season and 4.3 for the transitional season. Therefore, the results are expressed as hazard ratio for 5 ppb of seasonal average ozone. For summertime ozone we found significant associations in the four survival analyses with a hazard ratio of 1.06 (95% confidence interval [CI], 1.03–1.08) for the heart failure cohort; of 1.09 (95% CI, 1.06–1.12) for the MI cohort; of 1.07 (95% CI, 1.05–1.10) for the diabetes cohort; and 1.07 (95% CI, 1.04–1.09) for the COPD cohort. The associations using ozone during the transitional season are lower but still significant.
TABLE 2.
HAZARD RATIO AND 95% CONFIDENCE INTERVALS FOR 5-ppb INCREASE IN 8-HOUR OZONE FOR THE YEAR OF FAILURE, ACROSS THE 105 CITIES
| Ozone Average May to September |
Ozone Average Spring and Autumn |
|||
| HR | 95% CI | HR | 95% CI | |
| CHF | 1.06 | 1.03–1.08 | 1.02 | 0.99–1.05 |
| MI | 1.09 | 1.06–1.12 | 1.04 | 1.00–1.08 |
| Diabetes | 1.07 | 1.05–1.10 | 1.03 | 1.00–1.07 |
| COPD | 1.07 | 1.04–1.09 | 1.03 | 1.00–1.06 |
Definition of abbreviations: CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio; MI = myocardial infarction.
We then performed a sensitivity analysis by expanding the length of the ozone season by 2 months. That is, we used the average ozone for the months April until October. Person time at risk was therefore April to December. These results (not shown) are similar to the May to September estimates.
In a previous paper, Franklin and coauthors (33) found that the association between PM2.5 mass and mortality was modified by the seasonal average temperature, showing an inverted U-shaped relationship between the PM2.5-mortality effect estimates and temperature. In that study temperature was used as a surrogate to explain ventilation of ambient air to the indoor environment (34).
We therefore used a meta-regression between the city-specific effect estimates for ozone and city average temperature (and temperature squared) and we also found an inverted U-shaped relationship. Temperature is a proxy measure for ventilation and therefore also of air conditioning use and may play a role in explaining differences among regions. We then added dummy variables for region to the meta-regression to determine whether there was any remaining regional variation in ozone effect not explained by temperature.
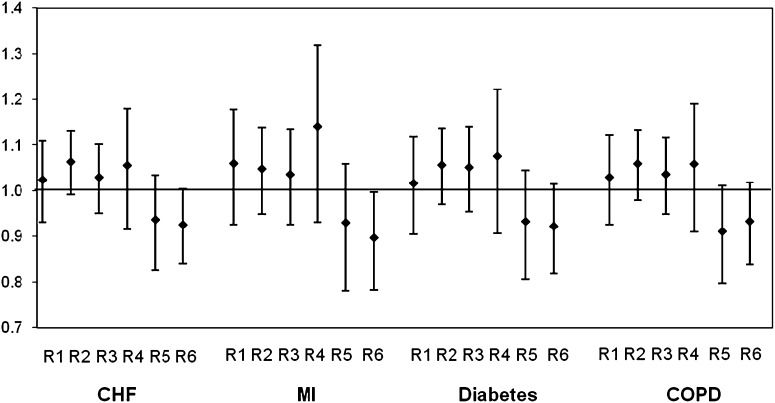
Figure 2 shows the estimated deviation from the overall effect of ozone in each region, as predicted by temperature. The regional effects, then, are the difference of that region from expected, given temperature. In general, there is no significant variation remaining, except for a lower effect of ozone in Region 6 (West Coast), which is significant for the MI cohort and marginal for the others.
Figure 2.
Estimated effect of region in the meta-regression after controlling for temperature and temperature squared in each city. The effects are the regional difference from expected given the city mean temperature. In general the differences are not significant, indicating that the regional differences in city-specific estimates are explained by city-specific mean temperature. The regions are as follows: region 1 (R1), humid subtropical climates and maritime temperate climates, which includes FL, LA TX, GA, AL, MS, AR, OK, KS, MO, TN, SC, NC, VA, WV, and KY; region 2 (R2), warm summer continental climates, including ND, MN, WI, MI, PA, NY, CT, RI, MA, VT, NH, and ME; region 3 (R3), hot summer continental climates with SD, NE, IA, IL, IN, and OH; region 4 (R4), dry climates, including NM, AZ, and NV; region 5 (R5), dry climates together with continental climate with MT, ID, WY, UT, and CO; and region 6 (R6), Mediterranean climates, which includes CA, OR, and WA. CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; MI = myocardial infarction.
Discussion
Our study is in part consistent with the results of Jerrett and coworkers (13) that longer-term exposure to ozone is associated with reduced survival. In addition, we have extended that finding in several ways. We find the ozone exposure in the transitional season exerts an independent effect on survival, controlling for summertime ozone. Importantly, we find this association with year-to-year variations in the exposure around its long-term trend within each city, demonstrating that year-to-year fluctuations, and not just very long-term exposures, can influence survival. We also found regional differences that were explained by mean temperature, likely reflecting the use of air conditioning, which reduces exposure.
A key feature of this study is the nature of the exposure contrast. City-specific regressions eliminated potential confounding by factors that vary across city, which is a common concern in most air pollution cohort studies. Rather, we examined whether within-city year-to-year fluctuations in ozone concentrations about their long-term trend were associated with within-city fluctuations of survival rates about their long-term trend. This study design avoids both confounding by cross-sectional factors that vary by city and the short-term factors that confound daily times series, but are not present in annual analyses. It also costs considerable amounts of power, because much of the exposure variation (short-term within city, and long-term across city) is thrown away. However, the large cohorts available using Medicare data allow such an approach. A key advantage is that fluctuations from summer to summer in ozone concentrations around the long-term level and trend in a specific city is unlikely to be correlated with most other predictors of mortality risk, except for temperature, which is controlled in the regression. For example, smoking rates may have fallen differently in different cities, but we cannot see a mechanism that would induce a correlation whereby year-to-year fluctuations around that trend in smoking would be correlated with year-to-year fluctuations in ozone around its trend. Some factors, such as unemployment rates, may fluctuate with emissions of air pollution. However, our cohorts are retired populations unlikely to be effected by unemployment, and ozone is a secondary pollutant whose concentrations vary with emissions in upwind communities, not the local one. However, a key limitation of our study was the inability to control for PM2.5, because it was not available in these cities until 1999. This may have resulted in confounding.
The study also differs from most other air pollution cohort studies in that the entire population over 65 was part of the initial sampling frame, rather than a convenience sample. This avoids selection bias, and confounding by possible selection of more or less healthy samples of the population in towns with more or less air pollution.
The finding of an inverted U-shaped relationship between the ozone effect in a city and its mean temperature, control for which renders regional differences insignificant, suggests that those regional differences are mostly related to temperature. The inverted U shape suggests that temperature is mostly capturing exposure differences. Effects are highest in mild temperatures, when windows are most likely to be open. Other possibilities for regional differences also exist, such as differences in medication, coexposures, and so forth. Future studies should address this issue.
This is the first study of longer-term exposure to ozone that focused on cohorts of susceptible subjects–persons with chronic conditions (specifically COPD, heart failure, diabetes, and MI) and as expected the hazard ratios were higher in these high-risk cohorts than in the American Cancer Society cohort. We should recognize, however, that there are differences between the exposure measures in the different studies. Time-series studies examine the more immediate risk on a high-pollution day. In contrast, long-term studies, such as Jerrett and coworkers (13), should capture the integrated effects of these acute responses, the effects of semi-chronic effects (response to a high ozone summer), and the additional responses resulting from long-term exposure to ozone over years. Our study focuses on the semi-chronic effect. Hence, comparisons of effect sizes across such studies should recognize that they are examining different things (and in the case of our study, susceptible populations).
In this context, the risks of semi-chronic exposures in this cohort analysis were higher than in studies analyzing the acute effects of ozone on the risk of mortality. For example, in a previous study (11) we reported a 0.32% increase in total mortality for 10 ppb of 8-hour average ozone, and the meta-analyses of Levy and coworkers (7) and Ito and coworkers (8) reported effect size estimates of 0.41% and 0.39% for a 10-ppb increase in maximum hourly ozone. This study report around a 7% (depending on the cohort) increase in mortality per 5 ppb (which is approximately the interquartile range) increase in summer average of 8-hour mean ozone.
Only one cohort study has investigated the effect of warm season average ozone on the risk of death in a large cohort (13). The authors used data from the American Cancer Society Cancer Prevention Study II cohort and found a 1.1% increase in cardiovascular causes, a 1.5% increase in ischemic heart disease, and a 2.9% increase in respiratory causes, which increase when adjusting for PM2.5. In that study, the authors investigated a younger population that was more educated and socially advantaged than the general population, which may account for some of the difference in effect size compared with our analysis of older, sicker subjects. They also found the effect primarily in respiratory deaths. Susceptibility alone cannot explain the difference. Given that respiratory deaths are a small fraction of all deaths, our results also suggest an effect of ozone on nonrespiratory deaths.
The biologic mechanism by which ozone can affect mortality is still under examination. A review of toxicologic studies found decreased heart rate, metabolism, blood pressure, and cardiac output when rats are exposed to typical concentrations of ozone (28).
A study (22) found a possible ongoing inflammatory response in the lungs of recreational joggers exposed to ozone and associated copollutants during the summer months. A previous paper (23) showed that airway inflammation persists after repeated ozone exposure, despite attenuation of some inflammatory markers in bronchoalveolar lavage fluid and adaptation of lung function.
Long-term exposure to ozone was associated with reduced lung function in a study of freshmen at the University of California, Berkeley (24). The associations were independent of any effects related to PM and NO2. In the same cohort (26) the effect of short- and long-term ozone exposures on biomarkers of oxidative stress were examined, and the authors found that estimated 2-week before, 1-month before, and lifetime O3 exposures of young healthy individuals were significantly and independently associated with increased 8-iso-prostaglandin F levels, while controlling for sex and ethnicity.
Another study (25) examined the association between current respiratory health status and long-term ozone exposure histories in 520 Yale University students. The authors found that lung function was significantly lower in the group with high ozone exposures, and chronic phlegm wheeze and respiratory symptom index were increased in the ozone-exposed group. They conclude that living for 4 or more years in regions of the country with high levels of ozone is associated with diminished lung function and more frequent reports of respiratory symptoms. Ozone exposure has also been associated with reduced heart rate variability (27).
Our study presents some major limitations. One is that Medicare does not provide the underlying cause of death. In addition, our definitions of persons with COPD are restrictive. Doubtless there are other persons in the 105 cities, who were covered by Medicare, who had the conditions we were interested in, and who were not included in the cohort either because they were not hospitalized for the condition or because it was misdiagnosed. Although this limits generalizability and moves our focus to a frailer subset of the persons with the conditions, it does not limit internal validity. The main limitation, however, is the absence of information on subject characteristics, such as smoking, body mass index, or medicine use. However, because of our study design, differences in these variables across cities, or long-term trends in smoking within city, cannot confound our estimates. In our model we controlled for all the available personal characteristics, such as age, race, sex, severity of the index admission, and detailed data on previous and secondary diagnosis. Moreover, we conducted a city-specific analysis to remove location-specific differences from the analyses, and removed long-term time trends. Hence, differences across cities in smoking rates cannot confound the association, and neither can long-term time trends in smoking rates within city.
Our findings suggest that long-term exposure to ozone elevates the risk of mortality in different subgroups of susceptible populations.
Supplementary Material
Footnotes
Supported by NIH ES-000002 and the US Environmental Protection Agency through STAR grants RD 832416 and RD 83479801 to Harvard University. It has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Author's contributions: A.Z. participated in the conception and design of the study, prepared the data, and did the statistical analysis with interpretation of data. She also drafted the manuscript. J.S. supervised and obtained funding for the study, participated in the conception and design of the study and in the interpretation of data, and he did a critical revision of the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201102-0227OC on June 23, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Anenberg SC, Horowitz LW, Tong DQ, West JJ. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect 2010;118:1189–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinney PL, Ito K, Thurston GD. A sensitivity analysis of mortality/PM-10 associations in Los Angeles. Inhal Toxicol 1995;7:59–69 [Google Scholar]
- 3.Kinney PL, Ozkaynak H. Associations of daily mortality and air pollution in Los Angeles County. Environ Res 1991;54:99–120 [DOI] [PubMed] [Google Scholar]
- 4.Touloumi G, Katsouyanni K, Zmirou D, Schwartz J, Spix C, de Leon AP, Tobias A, Quennel P, Rabczenko D, Bacharova L, et al. Short-term effects of ambient oxidant exposure on mortality: a combined analysis within the APHEA project. Air pollution and health: A European approach. Am J Epidemiol 1997;146:177–185 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz J. How sensitive is the association between ozone and daily deaths to control for temperature? Am J Respir Crit Care Med 2005;171:627–631 [DOI] [PubMed] [Google Scholar]
- 6.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology 2005;16:436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric Bayes metaregression analysis. Epidemiology 2005;16:458–468 [DOI] [PubMed] [Google Scholar]
- 8.Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology 2005;16:446–457 [DOI] [PubMed] [Google Scholar]
- 9.Bell ML, Dominici F. Effect modification by community characteristics on the short-term effects of ozone exposure and mortality in 98 us communities. Am J Epidemiol 2008;167:986–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina-Ramon M, Schwartz J. Who is more vulnerable to die from ozone air pollution? Epidemiology 2008;19:672–679 [DOI] [PubMed] [Google Scholar]
- 11.Zanobetti A, Schwartz J. Mortality displacement in the association of ozone with mortality: an analysis of 48 cities in the United States. Am J Respir Crit Care Med 2008;177:184–189 [DOI] [PubMed] [Google Scholar]
- 12.Zanobetti A, Schwartz J. Is there adaptation in the ozone mortality relationship? A multi-city case-crossover analysis. Environ Health 2008;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerrett M, Burnett RT, Pope CA, III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 2009;360:1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazucha MJ. Relationship between ozone exposure and pulmonary function changes. J Appl Physiol 1987;62:1671–1680 [DOI] [PubMed] [Google Scholar]
- 15.Hoppe P, Peters A, Rabe G, Praml G, Lindner J, Jakobi G, Fruhmann G, Nowak D. Environmental ozone effects in different population subgroups. Int J Hyg Environ Health 2003;206:505–516 [DOI] [PubMed] [Google Scholar]
- 16.Arjomandi M, Witten A, Abbritti E, Reintjes K, Schmidlin I, Zhai W, Solomon C, Balmes J. Repeated exposure to ozone increases alveolar macrophage recruitment into asthmatic airways. Am J Respir Crit Care Med 2005;172:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G, Ostro BD. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health 2006;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath SM, Gliner JA, Folinsbee LJ. Adaptation to ozone: duration of effect. Am Rev Respir Dis 1981;123:496–499 [DOI] [PubMed] [Google Scholar]
- 19.Hackney JD, Linn WS, Mohler JG, Collier CR. Adaptation to short-term respiratory effects of ozone in men exposed repeatedly. J Appl Physiol 1977;43:82–85 [DOI] [PubMed] [Google Scholar]
- 20.Folinsbee LJ, Bedi JF, Horvath SM. Respiratory responses in humans repeatedly exposed to low concentrations of ozone. Am Rev Respir Dis 1980;121:431–439 [DOI] [PubMed] [Google Scholar]
- 21.Bedi JF, Horvath SM, Drechsler-Parks DM. Adaptation by older individuals repeatedly exposed to 0.45 parts per million ozone for two hours. JAPCA 1989;39:194–199 [DOI] [PubMed] [Google Scholar]
- 22.Kinney PL, Nilsen DM, Lippmann M, Brescia M, Gordon T, McGovern T, El-Fawal H, Devlin RB, Rom WN. Biomarkers of lung inflammation in recreational joggers exposed to ozone. Am J Respir Crit Care Med 1996;154:1430–1435 [DOI] [PubMed] [Google Scholar]
- 23.Jorres RA, Holz O, Zachgo W, Timm P, Koschyk S, Muller B, Grimminger F, Seeger W, Kelly FJ, Dunster C, et al. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med 2000;161:1855–1861 [DOI] [PubMed] [Google Scholar]
- 24.Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Kunzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 2005;16:751–759 [DOI] [PubMed] [Google Scholar]
- 25.Galizia A, Kinney PL. Long-term residence in areas of high ozone: associations with respiratory health in a nationwide sample of nonsmoking young adults. Environ Health Perspect 1999;107:675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect 2007;115:1732–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SK, O'Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect 2005;113:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watkinson WP, Campen MJ, Nolan JP, Costa DL. Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ Health Perspect 2001;109:539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect 2007;115:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanobetti A, Bind MA, Schwartz J. Particulate air pollution and survival in a COPD cohort. Environ Health 2008;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z 2006;15:259–263 [Google Scholar]
- 32.Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Stat Med 1998;17:2537–2550 [DOI] [PubMed] [Google Scholar]
- 33.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology 2008;19:680–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutrakis P, Sax SN, Sarnat JA, Coull B, Demokritou P, Oyola P, Garcia J, Gramsch E. Analysis of pm10, pm2.5, and pm2.5–10 concentrations in Santiago, Chile, from 1989 to 2001. J Air Waste Manag Assoc 2005;55:342–351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.