Abstract
Background
Prognostic risk stratification according to acquired or inherited genetic alterations has received increasing attention in acute myeloid leukemia in recent years. A germline Janus kinase 2 haplotype designated as the 46/1 haplotype has been reported to be associated with an inherited predisposition to myeloproliferative neoplasms, and also to acute myeloid leukemia with normal karyotype. The aim of this study was to assess the prognostic impact of the 46/1 haplotype on disease characteristics and treatment outcome in acute myeloid leukemia.
Design and Methods
Janus kinase 2 rs12343867 single nucleotide polymorphism tagging the 46/1 haplotype was genotyped by LightCycler technology applying melting curve analysis with the hybridization probe detection format in 176 patients with acute myeloid leukemia under 60 years diagnosed consecutively and treated with curative intent.
Results
The morphological subtype of acute myeloid leukemia with maturation was less frequent among 46/1 carriers than among non-carriers (5.6% versus 17.2%, P=0.018, cytogenetically normal subgroup: 4.3% versus 20.6%, P=0.031), while the morphological distribution shifted towards the myelomonocytoid form in 46/1 haplotype carriers (28.1% versus 14.9%, P=0.044, cytogenetically normal subgroup: 34.0% versus 11.8%, P=0.035). In cytogenetically normal cases of acute myeloid leukemia, the 46/1 carriers had a considerably lower remission rate (78.7% versus 94.1%, P=0.064) and more deaths in remission or in aplasia caused by infections (46.8% versus 23.5%, P=0.038), resulting in the 46/1 carriers having shorter disease-free survival and overall survival compared to the 46/1 non-carriers. In multivariate analysis, the 46/1 haplotype was an independent adverse prognostic factor for disease-free survival (P=0.024) and overall survival (P=0.024) in patients with a normal karyotype. Janus kinase 2 46/1 haplotype had no impact on prognosis in the subgroup with abnormal karyotype.
Conclusions
Janus kinase 2 46/1 haplotype influences morphological distribution, increasing the predisposition towards an acute myelomonocytoid form. It may be a novel, independent unfavorable risk factor in acute myeloid leukemia with a normal karyotype.
Keywords: acute myeloid leukemia, prognosis, Janus kinase 2, 46/1 haplotype
Introduction
Acute myeloid leukemia (AML) is a clonal hematopoietic stem cell disorder with diverse clinical symptoms, a heterogeneous genetic background and variable response to treatment. Several acquired genetic abnormalities influence the outcome of the disease; prognostic risk stratification according to genetic alterations present at diagnosis in leukemic cells has, therefore, received increasing attention in AML in recent years.1 Current prognostic factors are not sufficient to predict prognosis accurately, especially in AML with a normal karyotype (NK-AML). Inherited functional genomic polymorphisms are among the candidates explaining the heterogeneity of the prognoses.
Somatic gain-of-function mutation of the Janus kinase 2 (JAK2) gene located on chromosome 9p (c.1849G>T resulting in V617F) has been identified in BCR-ABL-negative myeloproliferative neoplasms.2–4 Previous studies demonstrated that the presence of JAK2 V617F is associated with an inherited JAK2 haplotype designated as the 46/1 haplotype.5–7 The 46/1 haplotype stretches through a 280-kb-long linkage disequilibrium block involving the JAK2, INSL4 and INSL6 genes. Carriers of the 46/1 haplotype have a higher risk of developing V617F-positive and -negative myeloproliferative neoplasms.5–9 On rare occasions, the same mutation occurs in myelodysplastic syndromes and de novo AML.10–14 In our recent study,15 an inherited predisposition to JAK2 V617F-negative NK-AML was demonstrated in 46/1 haplotype carriers. To extend these observations, the current study was aimed at: (i) comparing disease characteristics (age, morphology and acquired cytogenetic or molecular genetic alterations at diagnosis of AML) between JAK2 46/1 haplotype carriers and non-carriers; and (ii) testing the prognostic impact of the 46/1 haplotype on AML outcome (remission and relapse rate, overall and disease-free survival, cause of death).
Design and Methods
Patients
The patients studied were 176 consecutively diagnosed individuals with AML [discovery cohort: 77 males/99 females; median age of disease onset: 48 years (range, 18–60)] treated between January 2001 and December 2007 at the Department of Hematology and Stem Cell Transplantation, St. Istvan and St. Laszlo Hospital (Hungary). The minimum follow–up was 24 months (maximum, 107 months). A second independent cohort of 74 patients with AML [validation cohort: 40 males/34 females; median age at disease onset: 45 years (range, 20–60)] diagnosed between January 2008 and December 2009 at the 1st Department of Internal Medicine, St. Istvan and St. Laszlo Hospital] was also studied. In this latter group, the minimum follow-up was 16 months (maximum, 32 months). Clinical data were collected retrospectively. Complete remission, early death (<28 days after the start of therapy), resistant disease, disease-free survival and overall survival were defined according to recommended criteria.16 Infection as a cause of death was recorded in cases without any signs of leukemia (in remission or in aplasia) when ante-mortem clinical findings or autopsy results were conclusive for an infectious origin. During cytogenetic analysis, at least 20 metaphases were karyotyped and abnormalities were described according to the International System for Human Cytogenetic Nomenclature (ISCN 2005). Fms-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) and nucleophosmin 1 (NPM1) insertion mutations were tested by polymerase chain reaction followed by Genescan analysis.17,18 Participants signed informed consent, and the study was approved by the Regional Ethics Committee.
The 46/1 haplotype
The JAK2 rs12343867 single nucleotide polymorphism (NT_008413.17: g.5064189T>C in intron 14) tagging the 46/1 haplotype was genotyped from genomic DNA by LightCycler technology (LightCycler 480II, Roche Diagnostics) applying melting curve analysis with hybridization probe detection format. Amplification primers and hybridization probes were designed using LightCycler Probe Design software (Roche Diagnostics).
Statistics
JAK2 rs12343867 allele frequencies are presented as percentages ± 95% confidence intervals (95% CI) as JAK2 46/1 haplotype frequency. Testing for Hardy-Weinberg equilibrium and linkage disequilibrium studies (D’ statistics, haplotype frequency estimations) were performed by the SNPstats web tool (http://bioinfo.iconcologia.net/Snpstats).19 Comparisons of dichotomous variables were performed using the χ2 or Fisher’s exact test. Odds ratios (OR) and 95% CI were estimated. A log-rank test was performed to compare disease-free survival and overall survival between groups. Following univariate analysis, variables with P values less than or equal to 0.05 in the entire AML or NK-AML groups were included in a Cox proportional hazards model for disease-free survival and overall survival. Hazard ratios (HR) and 95% CI values were computed. The analyses were carried out with the SPSS (version 13.0) software package.
Results
Patients’ characteristics in relation to the 46/1 haplotype
The frequency of the 46/1 haplotype was 29.3±4.8% in the discovery cohort (n=176). Genotype distribution did not deviate from the Hardy-Weinberg equilibrium (P=0.7): the TT genotype was detected in 87 cases (49.4%), the CT genotype in 75 cases (42.6%) and the CC genotype in 14 cases (8.0%). For further statistical analyses, a dominant model was applied: individuals with at least one minor allele (CT and CC genotypes) were grouped together as 46/1 haplotype carriers and compared to individuals homozygous for the major allele (TT genotype as 46/1 non-carriers).
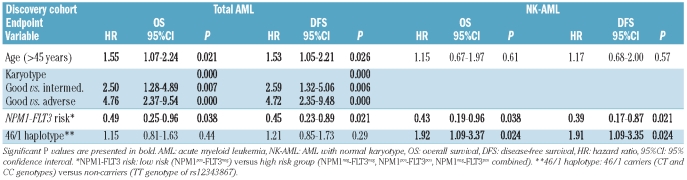
The clinical characteristics of the AML patients in the discovery cohort are presented in Table 1. The distributions of age, sex and AML etiology were similar between 46/1 carriers and non-carriers. There were considerably fewer cases with AML with maturation (FAB M2) morphology within the group of 46/1 carriers than in the group of non-carriers [5.6% (5/89 CT and CC) versus 17.2% (15/87 TT); P=0.018], and conversely acute myelomonocytic leukemia (FAB M4) was more frequent among 46/1 carriers [28.1% (25/89 CT and CC) versus 14.9% (13/87 TT); P=0.044]. A similar distribution was observed in NK-AML (FAB M2: P=0.031; FAB M4: P=0.035: Table 1). The distributions of FLT3 ITD and NPM1 mutation among 46/1 carriers and non-carriers were the same.
Table 1.
Pre-treatment, clinical and molecular characteristics in the discovery cohort according to 46/1 haplotype. Comparisons are presented between 46/1 carriers (CT and CC genotypes of the single nucleotide polymorphism rs12343867) and 46/1 non-carriers (TT genotype of single nucleotide polymorphism rs12343867) in all AML patients independently of karyotype (total AML) and in the subgroup of AML patients with normal karyotype (NK-AML).
In order to confirm our observations, we included an independent validation cohort of AML patients (n=74) in the study. The pretreatment clinical characteristics of the validation cohort are presented in Online Supplementary Table S1.) In agreement with our observations in the discovery cohort, 46/1 haplotype frequency was 31.8±7.7% in the validation cohort (Hardy-Weinberg equilibrium: P=0.8). Similarly, FAB M2 morphological subtype was less frequent among 46/1 carriers than among non-carriers [5.1% (2/39 CT and CC) versus 22.9% (8/35 TT), P=0.039], while the morphological distribution shifted towards FAB M4 among 46/1 haplotype carriers [43.6% (17/39 CT and CC) versus 20% (7/35 TT), P=0.046]. Myelomonocytic morphology was also more common among 46/1 carriers in NK-AML (FAB M4: 62.5% in carriers versus 30.8% in non-carriers) but the difference did not reach the level of statistical significance probably because of the low number of cases (n=29, P=0.14).
Prognostic impact of the 46/1 haplotype
Disease outcome data according to 46/1 haplotype are presented in Table 2 for the discovery cohort. In the entire discovery cohort, 46/1 haplotype carriers and non-carriers had similar complete remission rates, while there was a tendency towards a lower complete remission rate among 46/1 carriers than among non-carriers in NK-AML [78.7% (37/47 CT and CC) versus 94.1% (32/34 TT); OR (95% CI): 4.32 (0.88–21.22); P=0.064]. Relapse rate was similar among 46/1 carriers and non-carriers both in all AML patients and in the NK-AML subgroup. Among NK-AML cases, the rate of deaths in remission or in aplasia caused by infections established by autopsy or ante-mortem clinical signs of infections was significantly higher in 46/1 carriers than in non-carriers [46.8% (22/47 CT and CC) versus 23.5% (8/34 TT); OR (±95%CI): 2.86 (1.08–7.61); P=0.038].
Table 2.
Outcome data for all AML patients independently of karyotype (total AML) and of cytogenetically normal AML (NK-AML) patients in the discovery cohort according to 46/1 haplotype. Comparisons are presented between 46/1 carriers (CT and CC genotypes of the single nucleotide polymorphism rs12343867) and 46/1 non-carriers (TT genotype of the single nucleotide polymorphism rs12343867).
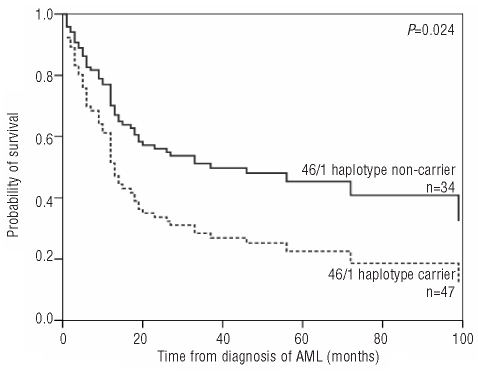
In the discovery cohort, univariate analysis (Online Supplementary Table S2) revealed significantly longer disease-free survival and overall survival in younger patients (<45 years) and in patients with intermediate and favorable karyotypes, while sex, FLT3 ITD and NPM1 mutations alone were not associated with altered disease-free survival or overall survival in the entire discovery cohort (n=176). As NPM1 and FLT3 ITD mutations frequently associate and influence prognosis in an opposite way,20 we analyzed the combined effect of NPM1, FLT3 ITD on survival. Mutant NPM1 without FLT3 ITD (NPM1pos-FLT3neg: low risk group) proved to be associated with longer disease-free survival compared to other combinations (NPM1neg-FLT3neg, NPM1pos-FLT3pos, NPM1neg-FLT3pos combined: high risk group). The 46/1 haplotype did not influence survival in the entire discovery cohort. Performing univariate analysis in the NK-AML subgroup of the discovery cohort (Online Supplementary Table S2), significant differences in disease-free survival and overall survival were observed after stratification by 46/1 haplotype and NPM1-FLT3 risk combined, but not by age, sex, FLT3 ITD or NPM1 mutations alone. 46/1 carriers within the normal karyotype group had significantly shorter disease-free survival and overall survival than their 46/1 non-carrier counterparts [disease-free survival: HR (95% CI): 1.88 (1.07–3.29), P=0.028; overall survival: HR (95%CI): 1.88 (1.07–3.29), P=0.027], while there was no significant difference within the abnormal karyotype group [disease-free survival: HR (95% CI): 0.86 (0.53–1.38), P=0.53; overall survival: HR (95% CI): 0.78 (0.48–1.25), P=0.30]. In multivariate analyses (Table 3), 46/1 haplotype was associated with shorter disease-free survival and overall survival independently of age and NPM1-FLT3 status in NK-AML [disease-free survival: HR (95% CI): 1.91 (1.09–3.35), P=0.024; overall survival: HR (95% CI): 1.92 (1.09–3.37), P=0.024] (Figure 1). Within the NPM1- and FLT3-ITD-negative NK-AML subgroup of patient in the discovery cohort, 46/1 carriers had shorter disease-free and overall survival than non-carriers [disease-free survival: HR (95% CI): 2.55 (1.14–5.68), P=0.022; overall survival: HR (95% CI): 2.79 (1.82–6.39), P=0.015], while there was no such difference in the NPM1- or FLT3-ITD-positive subgroups. Treatment modalities were not different between 46/1 carriers and non-carriers in the entire discovery cohort or in the NK-AML subgroup (Online Supplementary Table S3), thus therapeutic variability is unlikely to be responsible for the observed differences in outcome.
Table 3.
Multivariate analysis for overall survival and disease-free survival in all AML patients independently of karyotype (total AML) and in the NK-AML subgroup of the discovery cohort.
Figure 1.
Overall survival in cytogenetically normal AML according to 46/1 haplotype in the discovery cohort.
Disease outcome data according to 46/1 haplotype for the validation cohort are presented in Online Supplementary Table S4. We were not able to confirm our observations on overall survival, disease-free survival or rate of death caused by infection in the NK-AML subgroup within the validation cohort, probably because of the low number of cases, the shorter maximum follow-up period and the more pronounced heterogeneity of treatment protocols (less intensive).
Discussion
The inherited genetic background of an individual may not only increase disease susceptibility, but genomic polymorphisms may also play an important role in determining treatment outcome. Until recently, genetic variants involved in drug metabolism or DNA repair were considered the main possible modifiers of treatment outcome.21–23 Single nucleotide polymorphisms of genes involved in hematopoietic signal-transduction were investigated less frequently.24,25
In this study, we report an association of the JAK2 46/1 haplotype with disease characteristics and treatment outcome in AML patients. The 46/1 haplotype was found to be a factor predisposing to the development of acute myelomonocytic leukemia. In NK-AML, a considerably lower remission rate and more deaths caused by infections in remission or aplasia were observed in 46/1 haplotype carriers than in non-carriers, resulting in shorter disease-free survival and overall survival in the carriers. An association of another JAK2 single nucleotide polymorphism (rs2230724, NT_008413.18: g.5071780G>A, p.830I>I) with disease outcome was previously reported in Chinese AML patients.26,27 Retrieving haplotype information from the HapMap Project on rs12343867 and rs2230724 in a Chinese population revealed that the above polymorphisms are not in complete linkage (estimated haplotype frequencies of rs12343867/rs2230724: T/G: 57%, C/A: 23%, T/A: 18%, C/G: 2%, D’: 0.892; P<0.000).28 In Chinese AML patients, the AA genotype of rs2230724 was significantly associated with higher complete remission rate, and the GG genotype with early death. The different findings in our study may be explained by the incomplete linkage of the polymorphisms tested, by different ethnic backgrounds of the cohorts tested or by different therapeutic regimens.
The exact pathomechanism of how the 46/1 haplotype predisposes to the acquisition of acute myelomonocytic leukemia, and how it influences the outcome of NK-AML, is unknown. Two possible hypotheses have been proposed in the case of myeloproliferative neoplasms and both theories may be applicable to AML.5,29 The “hypermutability hypothesis”, suggesting genomic instability at the JAK2 locus, is supported by the preferential acquisition of JAK2 V617F on the 46/1 allele in myeloproliferative neoplasms. On the other hand, the JAK2 gene was thoroughly resequenced in AML, and no acquired JAK2 mutations were observed in AML.30 Enhanced susceptibility to DNA damage was estimated by comet assays in peripheral blood leukocytes of healthy 46/1 carriers indicating genomic instability affecting not exclusively the JAK2 locus.31 The “fertile ground hypothesis” suggests that 46/1 haplotype carriers may have subtle differences in JAK2 function compared to 46/1 non-carriers, which increase the predisposition to clonal myeloproliferation. Indeed, healthy 46/1 haplotype carriers were reported to have reduced granulocyte-macrophage colony formation.5 The marked difference in death rate caused by infections (46.8% in carriers versus 23.5% in non-carriers, P=0.038) observed in our study may be explained by a functional alteration of the JAK2 associated with the 46/1 haplotype. Since JAK2 plays a central role in the signaling of diverse myeloid and lymphoid cytokine receptors (including granulocyte-macrophage colony-stimulating factor, GM-CSF), altered JAK2 function might delay bone marrow reconstruction and cause impaired immune defense after chemotherapy, resulting in more severe infectious complications.
Interestingly, genome-wide association studies found an association between JAK2 46/1 haplotype and inflammatory bowel disease. The impairment of JAK2 function was suggested to affect interleukin-23 signaling and the differentiation of pro-inflammatory T cells (Th17) in inflammatory bowel disease.31–34 On the other hand, several lines of evidence suggest that there is defective GM-CSF signaling in inflammatory bowel disease: decreased GM-CSF secretion,35 neutralizing autoantibodies against GM-CSF,36 and defective GM-CSF receptor expression and function37 were described as characteristic features of inflammatory bowel disease. Compromised GM-CSF signaling resulting in functional abnormality of neutrophils and macrophages facilitates the invasion of the epithelial barrier by intestinal bacteria. The association of JAK2 46/1 haplotype with inflammatory bowel disease further supports the hypothesis that the 46/1 haplotype may be associated with a functional variant of JAK2.
In summary, the JAK2 46/1 haplotype influences morphological distribution increasing a predisposition to the acute myelomonocytoid form. Our data indicate that the JAK2 germline 46/1 haplotype may be an independent adverse prognostic factor affecting the severity of infections occurring during treatment in NK-AML. Prospective studies with larger numbers of AML patients are warranted to confirm our result. This finding may be useful in selecting risk-adapted treatment strategies in intermediate-risk AML, or in identifying a possible treatment modifying factor in therapies targeting the JAK2 pathway in AML.
Acknowledgments
This work was supported by grants from OTKA (K69102), OTKA (PF63953), KMOP 1.1.2-07/1-2008-0003, and COST Action BM0801. HA is a recipient of a Janos Bolyai Research Scholarship from the Hungarian Academy of Sciences.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Stone RM. Prognostic factors in AML in relation to (ab)normal karyotype. Best Pract Res Clin Haematol. 2009;22(4):523–8. doi: 10.1016/j.beha.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41(4):446–9. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with pre-disposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41(4):455–9. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–4. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 8.Pardanani A, Lasho TL, Finke CM, Gangat N, Wolanskyj AP, Hanson CA, et al. The JAK2 46/1 haplotype confers susceptibility to essential thrombocythemia regardless of JAK2V617F mutational status-clinical correlates in a study of 226 consecutive patients. Leukemia. 2010;24(1):110–4. doi: 10.1038/leu.2009.226. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Lasho TL, Patnaik MM, Finke CM, Hussein K, Hogan WJ, et al. JAK2 germline genetic variation affects disease susceptibility in primary myelofibrosis regardless of V617F mutational status: nullizygosity for the JAK2 46/1 haplotype is associated with inferior survival. Leukemia. 2010;24(1):105–9. doi: 10.1038/leu.2009.225. [DOI] [PubMed] [Google Scholar]
- 10.Frohling S, Lipka DB, Kayser S, Scholl C, Schlenk RF, Dohner H, et al. Rare occurrence of the JAK2 V617F mutation in AML subtypes M5, M6, and M7. Blood. 2006;107(3):1242–3. doi: 10.1182/blood-2005-09-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram W, Lea NC, Cervera J, Germing U, Fenaux P, Cassinat B, et al. The JAK2 V617F mutation identifies a subgroup of MDS patients with isolated deletion 5q and a proliferative bone marrow. Leukemia. 2006;20(7):1319–21. doi: 10.1038/sj.leu.2404215. [DOI] [PubMed] [Google Scholar]
- 12.Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106(10):3370–3. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 14.Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106(10):3377–9. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrikovics H, Nahajevszky S, Koszarska M, Meggyesi N, Bors A, Halm G, et al. JAK2 46/1 haplotype analysis in myeloproliferative neoplasms and acute myeloid leukemia. Leukemia. 2010;24(10):1809–13. doi: 10.1038/leu.2010.172. [DOI] [PubMed] [Google Scholar]
- 16.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 17.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 18.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107(10):4011–20. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 19.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–9. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 20.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 21.Kuptsova N, Kopecky KJ, Godwin J, Anderson J, Hoque A, Willman CL, et al. Polymorphisms in DNA repair genes and therapeutic outcomes of AML patients from SWOG clinical trials. Blood. 2007;109(9):3936–44. doi: 10.1182/blood-2006-05-022111. [DOI] [PubMed] [Google Scholar]
- 22.Monzo M, Brunet S, Urbano-Ispizua A, Navarro A, Perea G, Esteve J, et al. Genomic polymorphisms provide prognostic information in intermediate-risk acute myeloblastic leukemia. Blood. 2006;107(12):4871–9. doi: 10.1182/blood-2005-08-3272. [DOI] [PubMed] [Google Scholar]
- 23.Seedhouse CH, Grundy M, White P, Li Y, Fisher J, Yakunina D, et al. Sequential influences of leukemia-specific and genetic factors on p-glycoprotein expression in blasts from 817 patients entered into the National Cancer Research Network acute myeloid leukemia 14 and 15 trials. Clin Cancer Res. 2007;13(23):7059–66. doi: 10.1158/1078-0432.CCR-07-1484. [DOI] [PubMed] [Google Scholar]
- 24.Damm F, Heuser M, Morgan M, Yun H, Grosshennig A, Gohring G, et al. Single nucleotide polymorphism in the mutational hotspot of WT1 predicts a favorable outcome in patients with cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2010;28(4):578–85. doi: 10.1200/JCO.2009.23.0342. [DOI] [PubMed] [Google Scholar]
- 25.Wagner K, Damm F, Gohring G, Gorlich K, Heuser M, Schafer I, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010;28(14):2356–64. doi: 10.1200/JCO.2009.27.6899. [DOI] [PubMed] [Google Scholar]
- 26.Chen BA, Zhong YJ, Feng JF, Li YF, Ding JH, Gao C, et al. Association of Janus kinase 2(JAK2) A830G polymorphism with acute leukemia susceptibility. Blood. 2009;114(22):629. [Google Scholar]
- 27.Zhong Y, Chen B, Feng J, Cheng L, Li Y, Qian J, et al. The associations of Janus kinase-2 (JAK2) A830G polymorphism and the treatment outcomes in patients with acute myeloid leukemia. Leuk Lymphoma. 2010;51(6):1115–20. doi: 10.3109/10428191003774960. [DOI] [PubMed] [Google Scholar]
- 28.The International HapMap Consortium. The International HapMap Project. Nature. 2003;426(6968):789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 29.Campbell PJ. Somatic and germline genetics at the JAK2 locus. Nat Genet. 2009;41(4):385–6. doi: 10.1038/ng0409-385. [DOI] [PubMed] [Google Scholar]
- 30.Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20(6):971–8. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, et al. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat Res. 2010;690(1–2):108–15. doi: 10.1016/j.mrfmmm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43(3):246–52. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58(8):1152–67. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 34.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011 Feb 7; doi: 10.1136/gut.2009.199679. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Brosbol-Ravnborg A, Hvas CL, Agnholt J, Dahlerup JF, Vind I, Till A, et al. Toll-like receptor-induced granulocyte-macrophage colony-stimulating factor secretion is impaired in Crohn’s disease by nucleotide oligomerization domain 2-dependent and -independent pathways. Clin Exp Immunol. 2009;155(3):487–95. doi: 10.1111/j.1365-2249.2008.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology. 2009;136(4):1261–71. e1–3. doi: 10.1053/j.gastro.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein JI, Kominsky DJ, Jacobson N, Bowers B, Regalia K, Austin GL, et al. Defective leukocyte GM-CSF receptor (CD116) expression and function in inflammatory bowel disease. Gastroenterology. 2011;141(1):208–16. doi: 10.1053/j.gastro.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thalhammer F, Geissler K, Jager U, Kyrle PA, Pabinger I, Mitterbauer M, et al. Duration of second complete remission in patients with acute myeloid leukemia treated with chemotherapy: a retrospective single-center study. Ann Hematol. 1996;72(4):216–22. doi: 10.1007/s002770050163. [DOI] [PubMed] [Google Scholar]
- 39.Montillo M, Mirto S, Petti MC, Latagliata R, Magrin S, Pinto A, et al. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am J Hematol. 1998;58(2):105–9. doi: 10.1002/(sici)1096-8652(199806)58:2<105::aid-ajh3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.Steinmetz HT, Schulz A, Staib P, Scheid C, Glasmacher A, Neufang A, et al. Phase-II trial of idarubicin, fludarabine, cytosine arabinoside, and filgrastim (Ida-FLAG) for treatment of refractory, relapsed, and secondary AML. Ann Hematol. 1999;78(9):418–25. doi: 10.1007/s002770050541. [DOI] [PubMed] [Google Scholar]
- 41.Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) pilot study. Blood. 1996;88(4):1390–8. [PubMed] [Google Scholar]
- 42.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]