Abstract
The Human Papillomavirus type-16 (HPV-16) E6 and E7 oncogenes are selectively retained and expressed in cervical carcinomas, and expression of E6 and E7 is sufficient to immortalize human cervical epithelial cells. Expression of the epidermal growth factor receptor (EGFR) is often increased in cervical dysplasia and carcinoma, and HPV oncoproteins stimulate cell growth via the EGF-R pathway. We found that erlotinib, a specific inhibitor of EGFR tyrosine kinase activity, prevented immortalization of cultured human cervical epithelial cells by the complete HPV-16 genome or the E6/E7 oncogenes. Erlotinib stimulated apoptosis in cells that expressed HPV-16 E6/E7 proteins and induced senescence in a subpopulation of cells that did not undergo apoptosis. Since immortalization by HPV E6/E7 is an important early event in cervical carcinogenesis, the EGFR is a potential target for chemoprevention or therapy in women who have a high risk for cervical cancer.
Keywords: epidermal growth factor receptor, erlotinib, immortalization, apoptosis, senescence, papillomavirus, cervical cancer, chemoprevention
INTRODUCTION
Cervical cancer is a major cause of death in women worldwide. The incidence is high in underdeveloped countries and in individuals who have low socioeconomic status or are immune compromised (Schiffman and Brinton, 1995). The major risk factor for cervical cancer is persistent infection with high-risk types of human papillomavirus (HPV) (Schiffman and Brinton, 1995). The HPV E6 and E7 genes from high-risk HPV types (HPV-16 and -18) are sufficient to immortalize human epithelial cells (Hawley-Nelson et al., 1989; Munger et al., 1989), and these genes are selectively retained and expressed in most cervical cancers (von Knebel Doeberitz et al., 1992). The majority of HPV infections are eliminated by the host’s immune response, but individuals who have compromised immunity (AIDS patients or transplant recipients) develop persistent infections with multiple HPV types (Palefsky, 2009). Persistent infection with high risk HPVs and immortalization of infected cells are important early events in the development of cervical cancer.
Therapy for cervical cancer involves surgery, chemotherapy or radiation, and the five year survival for women with invasive cervical cancer is poor. In contrast, if the disease is detected early by PAP screening or HPV testing, the prognosis is good (Arbyn et al., 2009). Chemoprevention of cervical cancer has been explored in several clinical trials, but most agents have not been effective (Vlastos, Schottenfeld, and Follen, 2003). Recently, prophylactic vaccines have been shown to prevent HPV infection (Campo and Roden, 2010). These vaccines induce type-specific immunity to the two most common high risk HPVs (HPV-16 and HPV-18) which account for approximately 70% of cervical cancers. However, they are not effective in women who have existing HPV infections. Due to the long latency of cervical carcinogenesis, the prophylactic vaccine is unlikely to have a major impact on the incidence of cervical cancer for many years. In addition, the vaccine may be less effective in women who are immune deficient and infected with multiple high-risk types (AIDS patients and transplant recipients) (McKenzie et al., 2010). Thus, women who have a high risk for cervical cancer would benefit from improved methods for therapy or chemoprevention that target signal pathways critical for cervical cancer development.
The epidermal growth factor receptor (EGFR) is a membrane tyrosine kinase expressed by most epithelial cells. Cervical epithelial cells secrete several EGF-like growth factors that activate the EGFR by autocrine and paracrine pathways (Woodworth et al., 1995). Ligand binding leads to receptor dimerization, tyrosine kinase activation and stimulation of intracellular signal pathways that regulate growth, survival, motility, and angiogenesis. EGFR signaling is important for normal cell function, but inappropriate activation or over expression can contribute to malignant development (Normanno et al., 2005). Over expression of the EGFR or mutations in the EGFR gene have been implicated in the pathogenesis of multiple types of human cancer. The EGFR is frequently over expressed in cervical dysplasia and cervical cancer, and patients who have high levels of EGFR in their tumors have a poor prognosis (Kersemaekers et al., 1999). The HPV-16 E6 and E7 proteins stimulate EGFR expression on epithelial cells (Akerman et al., 2001; Sizemore et al., 1998) and the HPV E5 protein increases recycling of the EGFR to the cell surface (Straight, Herman, and McCance, 1995) and alters EGF endocytic trafficking (Suprynowicz et al., 2010). Targeted disruption of the EGFR gene inhibits development of papillomas and carcinomas from HPV-immortalized epithelial cells in mice (Woodworth et al., 2000). Together, these observations suggest that activation of the EGFR is important for the HPV life cycle and progression to cervical cancer.
Inhibitors of the EGFR have been used for therapy of several types of human cancer (Chen et al., 2009). The two major classes of inhibitors are monoclonal antibodies that interfere with ligand binding and small molecule kinase inhibitors that selectively block receptor activation. Erlotinib is a small molecule inhibitor that competes reversibly with ATP for binding to the tyrosine kinase domain of the EGFR. Erlotinib is administered to patients orally and the side effects are usually well tolerated (Li and Perez-Soler, 2009). Erlotinib has been approved by the FDA for treatment of recurrent non small cell lung cancer and for first-line treatment of advanced pancreatic cancer with gemcitabine (Iyer and Bharthuar, 2010). Given the importance of EGFR signaling to the HPV life cycle, we investigated whether erlotinib had activity against HPV-infected cervical cells. We observed that erlotinib prevented immortalization of cultured human cervical epithelial cells by the complete HPV-16 genome or the E6/E7 genes. This response was associated with induction of apoptosis in cells that express E6/E7 and stimulation of senescence in surviving cells. These results suggest that the EGFR might be an effective target for chemoprevention in women who have a high risk of cervical cancer.
RESULTS
Erlotinib prevents immortalization by the complete HPV-16 genome
Primary cultures of human cervical epithelial cells were transfected with a plasmid containing the complete HPV-16 genome plus the neomycin resistance gene. After selection in medium with G418, stably transfected cells were maintained in keratinocyte serum-free medium (KSFM) until they became senescent or grew continuously. As a negative control for immortalization, cultures were transfected with the plasmid containing only the neomycin resistance gene (no HPV). Cells transfected with HPV-16 grew continuously and became immortalized (Table 1, untreated), although there was evidence of crisis (slow growth and cell lysis) in some dishes. Cells transfected with only the neo gene became senescent after 2 to 3 passages (data not shown). Senescence occurred after a total of 40–60 population doublings and 20–40 population doublings were required to establish and transfect cell cultures. Treatment with erlotinib significantly (p ≤ 0.05) decreased the percentage of cultures that contained immortal cells in 3 independent experiments using cells from different individuals (Table 1). Erlotinib inhibited immortalization in a dose-dependent manner over a range of drug concentrations between 0.1 to 1.0 μM, 0.2 μM of the drug inhibited immortalization by 50% (ID50).
Table 1.
Frequency of immortalization of human cervical cells by the complete HPV-16 genome after treatment with erlotinib.
| Erlotinib | KSFM1 | w/o EGF | ||||
|---|---|---|---|---|---|---|
| HCX-1 | HCX-2 | HCX-3 | HCX-1 | HCX-2 | HCX-3 | |
| untreated | 6/62 | 6/6 | 5/5 | 6/6 | 6/6 | 6/6 |
| 0.1 μM | 5/6 | 3/6 | 5/6 | 0/5 | 0/6 | 0/6 |
| 0.3 μM | 2/5 | 0/5 | 0/6 | 0/5 | 0/6 | 0/6 |
| 1.0 μM | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/6 |
Immortalization assays were performed in KSFM with 5 ng/ml EGF (3 left columns) or KSFM without EGF (3 right columns). Each column represents an independent experiment using cells from a different sample of cervix.
Number of wells with immortal cells/the total number of wells examined.
The serum-free medium used to culture primary cervical cells contains relatively high levels of recombinant EGF (5 ng/ml) which raised the possibility that cells maintained in this medium might become dependent on exogenous EGF for growth. Cultured cervical cells produce multiple growth factors (including the EGF-family members amphiregulin and TGF-α) that stimulate cells by autocrine and paracrine methods (Woodworth et al., 1995). Therefore, we investigated whether erlotinib prevented immortalization when cells were maintained in medium without exogenous EGF. Immortalization assays were repeated in KSFM lacking EGF and bovine pituitary extract (which contains EGF). Under these conditions, HPV-16 transfected cells grew more slowly but continued to form immortal colonies (Table 1). In 3 independent experiments, erlotinib completely prevented immortalization by HPV-16. Immortalization was prevented by low concentrations of erlotinib (0.1 μM) that were not effective when assays were performed in complete KSFM with exogenous EGF (Table 1). Because erlotinib inhibits the EGFR in a reversible manner, high concentrations of EGF might compete more effectively with the drug for receptor activation. These results suggest that paracrine or autocrine growth stimulation by endogenously produced EGF-family growth factors is important for immortalization of cervical epithelial cells, and that erlotinib prevented immortalization more effectively in the absence of high levels of recombinant EGF.
Erlotinib prevents immortalization by HPV-16 E6 and E7 genes
The complete HPV-16 genome encodes 3 oncoproteins including E5, E6, and E7. The E6 and E7 proteins are sufficient for immortalization (Munger et al., 1989), but E5 increases signaling through the EGFR (Straight, Herman, and McCance, 1995) and alters epithelial growth via an EGFR dependent pathway (Genther Williams et al., 2005). Therefore, we examined whether erlotinib could prevent immortalization in the absence of E5. Cervical cells were infected with retroviruses encoding the HPV-16 E6 and E7 genes but lacking the E5 gene. All viruses contained the neomycin resistance gene (Halbert, Demers, and Galloway, 1991). As a negative control for immortalization, cultures were infected with retroviruses containing only the neo gene. Infected cultures were selected in KSFM containing G418 and stably infected cells were plated at clonal density (500 cells/60mm dish). Cultures were fed every 2 days with KSFM or KSFM containing various concentrations of erlotinib. We found that cultures infected with HPV-16 E6/E7 retrovirus grew rapidly and became immortalized (Table 2, untreated). In contrast, cultures infected with only the neo gene became senescent after 2 to 3 passages (data not shown). In 3 independent experiments using cells from different individuals, erlotinib significantly (p ≤ 0.05) reduced the percentage of cultures that contained immortal cells (Table 2). The ID50 for inhibition of immortalization by E6/E7 was 0.2 μM (the same as required for the complete HPV-16 genome). These results indicate that expression of the E5 protein was not required for erlotinib-induced prevention of immortalization. Expression of E6 and E7 is regulated by the long terminal repeat in retroviruses, whereas it is controlled by the natural HPV promoter in the long control region (LCR) in the complete genome. Thus, erlotinib blocks immortalization when E6/E7 expression is driven by either promoter.
Table 2.
Frequency of immortalization of human cervical cells by HPV-16 E6/E7 genes or by SV40 after treatment with erlotinib.
| Erlotinib | HCX4 - E6/E7 | HCX5 - E6/E7 | HCX6 - E6/E7 | HCX7 - SV40 |
|---|---|---|---|---|
| untreated | 5/51 | 6/6 | 6/6 | 6/6 |
| 0.1 μM | 4/6 | 4/6 | 5/6 | 5/6 |
| 0.3 μM | 3/6 | 2/6 | 2/6 | 4/6 |
| 1.0 μM | 0/6 | 0/6 | 0/5 | 0/5 |
| 3.0 μM | 0/4 | 0/6 | 0/6 | 0/6 |
Number of wells with immortal cells/total number of wells examined. Each column represents an independent experiment using cells from a different sample of cervix. All experiments were performed in KSFM plus 5 ng/ml EGF.
Interestingly, the effect of erlotinib on immortalization was not specific for HPV-16. Erlotinib also blocked immortalization by Simian Virus 40 (SV40) (Table 2), a DNA tumor virus that inactivates the p53 and retinoblastoma pathways similarly to HPV. To confirm that erlotinib prevented immortalization by specifically targeting the EGFR, we tested a monoclonal antibody that blocks ligand binding to the EGFR (Woodworth et al., 1995). In 2 independent experiments, this antibody inhibited immortalization by the complete HPV-16 genome or by E6/E7 retroviruses (Table 3). Similar results were obtained using various concentrations of PD153035, another small molecule kinase inhibitor that is specific for the EGFR (Table 3). In contrast, an inhibitor of platelet derived growth factor receptor, AG1295, did not prevent immortalization of cervical cells. Overall, these results indicate that erlotinib prevents immortalization of cervical epithelial cells that express HPV-16 E6/E7 genes by blocking signaling through the EGFR.
Table 3.
Frequency of immortalization after treatment with a neutralizing antibody to the EGFR, an alternate drug that specifically inhibits EGF-R kinase activation (PD153035), or an inhibitor of the platelet derived growth factor receptor (AG1295).
| anti-EGF-R | HPV16 | E6/E7-1 | |
|---|---|---|---|
| untreated | 6/61 | 6/6 | ND |
| 5 μg/ml | 0/3 | 0/6 | ND |
| 10 μg/ml | 0/3 | 0/6 | ND |
| PD153035 | HPV16 | E6/E7-1 | E6/E7-2 |
|---|---|---|---|
| untreated | 6/6 | 5/5 | 5/6 |
| 0.1 μM | 3/6 | 2/6 | 4/6 |
| 0.3 μM | 1/6 | 2/5 | 0/5 |
| 1.0 μM | 0/6 | 0/5 | 0/6 |
| AG1295 | HPV16 | E6/E7-1 | |
|---|---|---|---|
| untreated | 6/6 | 5/5 | ND |
| 1.0 μM | 5/5 | 6/6 | ND |
Number of wells with immortal cells/total number of wells examined. Each column represents an independent experiment using cells from a different sample of cervix. Cultures were transfected with the complete HPV-16 genome or infected with retroviruses encoding E6/E7. Experiments using anti-EGF-R antibody were performed in KSFM without EGF, and those using AG1295 or PD153035 used KSFM with 5 ng/ml EGF. ND= not done.
Erlotinib inhibits clonal growth of cervical cells
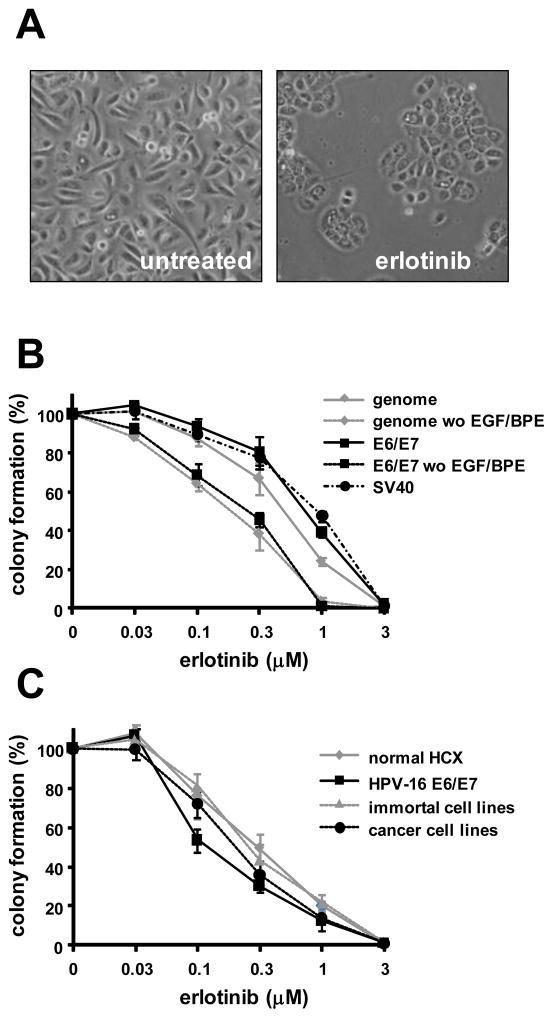
A potential mechanism for prevention of immortalization is the ability of erlotinib to block cell proliferation. We performed clonal growth assays to measure growth inhibition. Cervical cells (either E6/E7-infected, transfected with the HPV-16 genome, or transfected with SV40 DNA) were plated at clonal density and fed KSFM ± various concentrations of erlotinib for 14–21 days. Untreated cultures grew rapidly and formed colonies composed of loosely associated cells. In contrast, cultures treated with erlotinib grew slowly and contained small colonies of flat, tightly associated cells (Figure 1A). Erlotinib inhibited colony formation in a dose-dependent manner (Figure 1B) with an ID50 of 0.7 μM. Thus, the ID50 for prevention of immortalization (0.2 μM) was 3-fold lower that the ID50 for inhibition of colony formation. Erlotinib also blocked colony formation by SV40-expressing cells. Interestingly, the lowest dose of erlotinib (0.03 μM) stimulated a small but reproducible increase in colony formation. When clonal growth assays were performed without exogenous EGF in the culture medium, cells continued to grow slowly and form colonies. Erlotinib inhibited clonal growth of cells transfected with HPV-16 or E6/E7 significantly more strongly (p ≤ 0.05) in the absence of EGF (Figure 1B).
Figure 1.
Erlotinib inhibits clonal growth. A. Phase contrast micrographs showing cell growth in KSFM without (left) or with erlotinib (right). B. Erlotinib inhibits colony formation in a dose-dependent manner. Cervical epithelial cells were transfected with the complete HPV-16 genome, SV40 DNA, or infected with retroviruses encoding HPV-16 E6/E7 and then treated with various doses of erlotinib for 10 days in KSFM or KSFM without added EGF and BPE. For each experiment colony number was determined by counting cells in 3 replicate dishes from 3 different donors. C. Erlotinib inhibits colony formation by cells at progressive stages leading to cancer. Three normal cervical cell strains (normal HCX), 3 strains infected with HPV-16 E6/E7 retroviruses, 3 HPV-16-immortal cell lines, and 3 cervical carcinoma cell lines were treated with erlotinib and the mean cloning efficiency was measured. Points represent percent colony formation ± standard deviation in 3 independent experiments.
We examined whether cells at progressive stages leading to cervical cancer differed in susceptibility to growth inhibition by erlotinib. We used clonal growth assays to compare cervical cells from 3 different normal individuals, 3 cell cultures infected with HPV-16 E6/E7 retroviruses, 3 different HPV-16 immortalized cell lines, and 3 different cervical carcinoma cell lines (Woodworth et al., 1995). Erlotinib inhibited colony formation by normal cervical cells as effectively as cells that expressed HPV (Figure 1C). Thus, growth inhibition by erlotinib was not specific for HPV-expressing cells. The dose of erlotinib that inhibited clonal growth by 50% (ID50) was similar for normal cells, E6/E7-infected cells and HPV-16-immortalized cell lines, but the ID50 was variable in cervical carcinoma cell lines (data not shown).
Erlotinib stimulates apoptosis in cervical cells that express HPV-16
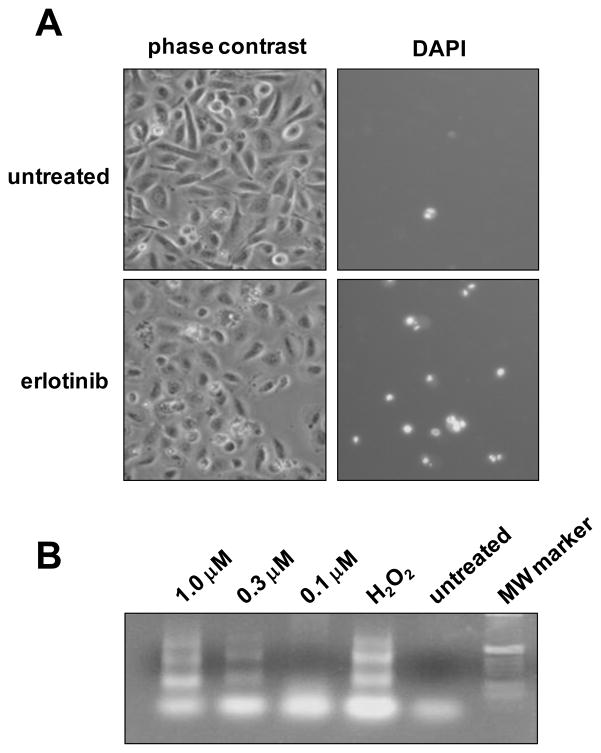
Phase contrast microscopy showed that erlotinib induced lysis of cells and nuclear fragmentation that resembled apoptosis (Figure 2A, left). To measure the extent of cell lysis, cultures were stained with DAPI, a DNA binding dye that penetrates cells that have membrane damage. Cells expressing HPV-16 E6/E7 genes were stained brightly by DAPI when treated with erlotinib (Figure 2A, right) and demonstrated nuclear condensation and fragmentation. One characteristic feature of cells undergoing apoptosis is inter nucleosomal DNA fragmentation. We validated our results using DAPI-staining with a PCR based TUNEL assay to show that inhibition of the EGF-R stimulated DNA fragmentation in a ladder-like pattern consistent with apoptosis (Figure 2B).
Figure 2.
HPV-16 E6/E7 expression sensitizes cervical cells to erlotinib-mediated apoptosis. A. Phase contrast micrographs (left) and fluorescent micrographs (right) of E6/E7-infected cells that were untreated (top) or treated with 1.0 μM erlotinib for 48 hours (bottom) and then stained by DAPI to label cells undergoing apoptosis. B. TUNEL assay of cells treated with erlotinib. Cells infected with HPV-16 E6/E7 were treated with EGFR inhibitor for 24 hours and analyzed for apoptosis using a DNA Ladder Assay PCR kit to detect inter nucleosomal DNA fragmentation. H2O2 served as a positive control for apoptosis.
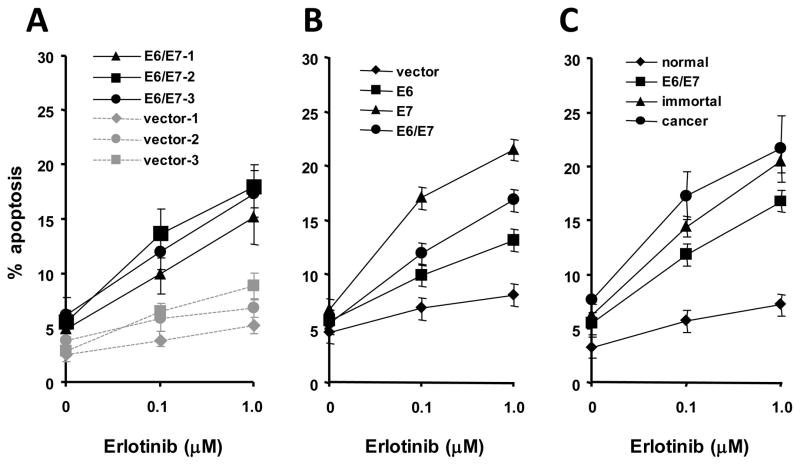
The extent of erlotinib-induced apoptosis was measured by counting the percentage of DAPI-positive cells after 48 hours (Figure 3A). Erlotinib stimulated more apoptosis (p ≤ 0.05) in 3 primary cultures of cervical cells that were infected with HPV-16 E6/E7 than in 3 cultures of cells that did not express HPV genes (Figure 3A). The percentage of dying cells continued to increase when treatment time was extended to 72 hours (data not shown). To determine which HPV-16 gene was important for sensitizing cells to apoptosis, 3 normal cervical cell cultures were infected with retroviruses encoding HPV-16 E6, E7, E6/E7 or the empty vector. In 3 independent experiments, the E7 protein sensitized cells to erlotinib-induced apoptosis most strongly whereas E6/E7 or E6 alone were less effective but statistically different (p ≤ 0.05) than the empty vector (Figure 3B). To determine whether cells at progressive stages leading to cervical cancer were sensitive to erlotinib-induced apoptosis, we compared 3 normal cervical cell cultures, 3 cultures infected with HPV-16 E6/E7 retroviruses, 3 HPV-immortal cell lines and 3 cervical carcinoma cell lines. Erlotinib stimulated apoptosis only in the cells that contained HPV-16 genes (Figure 3C, cancer, immortal and E6/E7-infected). Normal cultures were significantly less sensitive (p ≤ 0.05). Erlotinib also stimulated apoptosis in SV40-immortalized cervical cell lines by 10–15%. Overall, these results indicate that erlotinib selectively kills cervical cells that express HPV-16 or SV40 DNA. Thus, erlotinib-induced apoptosis may eliminate HPV-expressing cells before they undergo immortalization.
Figure 3.
Erlotinib stimulates apoptosis in cells that contain HPV-16 DNA. A. Three primary cultures of normal cervical cells were infected with retroviruses encoding HPV-16 E6/E7 or vector only (control) and apoptosis induced by erlotinib (1.0 μM for 48 hours) was measured. B. HPV-16 E7 is most effective in sensitizing cells to apoptosis. Three cultures from different individuals were infected with retroviruses encoding HPV-16 E6, E7, E6/E7 or the empty vector (control), and treated with erlotinib. C. Erlotinib stimulates apoptosis in HPV-containing cells at progressive stages leading to cancer. Three normal cervical cultures, 3 cultures infected with HPV-16 E6/E7 retroviruses, 3 HPV-immortal cell lines and 3 cervical carcinoma cell lines) were examined for susceptibility to erlotinib-induced apoptosis. Percent apoptosis (600 cells counted) = # DAPI-stained cells/600 × 100. The percentage of apoptotic cells was determined in triplicate dishes. Points represent the mean ± standard deviation of the 3 experiments
Erlotinib induces premature senescence in a subpopulation of cells
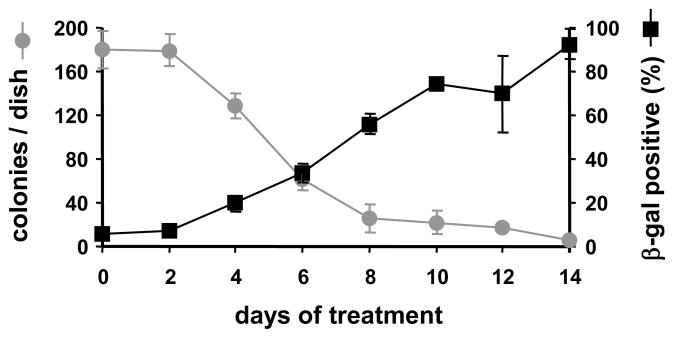
Normal cervical epithelial cells grow for 2 to 3 passages before they undergo replicative senescence. Erlotinib competes with ATP for binding to the tyrosine kinase domain of the EGFR, and it blocks EGFR activation in a competitive and potentially reversible manner. We performed clonal growth assays to test whether erlotinib inhibited growth reversibly or irreversibly. Cervical cells were infected with HPV-16 E6/E7 retroviruses and plated at clonal density. Cultures were treated continuously with erlotinib for specific intervals (0, 2, 4, 6, 8, 10, 12 or 14 days). Subsequently, cells from each treatment interval were rinsed with fresh medium and allowed to form colonies in KSFM without erlotinib for an additional 10 days. The ability to form colonies decreased strongly after cells were treated with erlotinib for 6–8 days (Figure 4). During this time, a large percentage of surviving cells became positive for β-galactosidase (Figure 4), a marker for cell senescence. These results indicate that (1) erlotinib induced irreversible growth inhibition after 6–8 days, and (2) a subpopulation of E6/E7-infected cells underwent senescence rather than apoptosis.
Figure 4.
Erlotinib stimulates premature senescence in cells that fail to undergo apoptosis. Cells were infected with retroviruses encoding HPV-16 E6 and E7 and cultured in KSFM with 1.0 μM erlotinib for up to 14 days. At intervals of 2 days, triplicate cultures were removed from the drug and fed with KSFM for an additional 10 days to allow colony formation. Triplicate cultures were stained for β-galactosidase. Symbols represent the mean and bars indicate ± standard deviation.
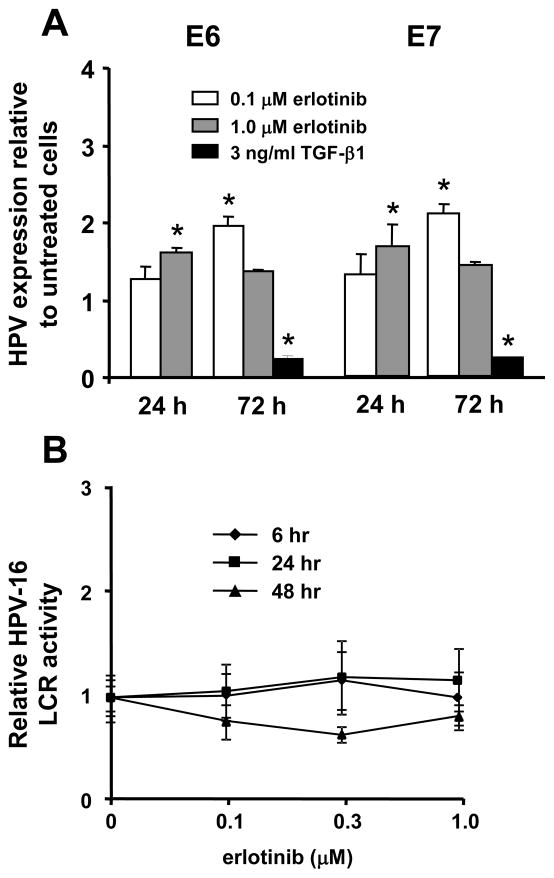
Erlotinib does not inhibit expression of HPV-16 E6 and E7 RNAs
Continued expression of the HPV-16 E6 and E7 genes is required to maintain cell growth and the transformed phenotype of cervical carcinoma cells (von Knebel Doeberitz et al., 1992). We examined whether erlotinib might block growth and immortalization by inhibiting expression of E6 or E7 RNAs. Cervical cells were transfected with the complete HPV-16 genome and then treated with either 0.1 or 1.0 μM erlotinib for 24 or 72 hours. RNA was extracted and analyzed for expression of E6 and E7 using real time PCR. As a negative control, cells were treated with 3 ng/ml TGF-β1 which is known to inhibit E6 and E7 expression (Woodworth, Notario, and DiPaolo, 1990). In two independent experiments, erlotinib significantly stimulated levels of E6 and E7 RNAs (Figure 5A). In contrast, TGF-β1 significantly inhibited (p ≤ 0.05) E6 and E7 RNAs. We used a reporter gene assay to check our results. Normal cervical cells were transiently transfected with a plasmid containing the HPV-16 long control region (LCR), the natural HPV promoter, linked to the firefly luciferase gene (Khan et al., 1997). Erlotinib did not significantly alter HPV promoter activity in two independent experiments (Figure 5B).
Figure 5.
Erlotinib increases expression of HPV-16 E6/E7 RNAs in cervical cells. A. Real time PCR analysis of HPV-16 E6 and E7 RNAs in cells that were transfected with HPV-16 and then treated with erlotinib at 0.1 or 1.0 μM for 24 or 72 hours. Cells were treated with 3ng/ml TGF-β1 as a positive control. Bars represent the mean of two independent experiments ± standard error, and asterisks are values that differ statistically (p ≤ 0.05) from controls. B. Reporter gene assay to measure activity of HPV-16 LCR in cervical cells after treatment with various concentrations of erlotinib for 6, 24 or 48 hours. Points represent the mean of 3 wells ± standard error.
We also examined whether erlotinib altered splicing of the E6/E7 transcript to yield more E6*/E7 RNA, which is known to influence relative expression of E6 and E7 proteins (Rosenberger et al., 2010). HPV-16 transfected cervical cells expressed mainly the E6/E7 transcript and treatment with 1.0 μM erlotinib did not increase the expression of E6*/E7 RNA (data not shown). Overall, these results suggest that erlotinib does not inhibit immortalization by decreasing HPV-16 E6 and E7 RNA expression or splicing.
DISCUSSION
The EGFR is frequently over expressed in cervical dysplasias and cancers, and high level expression is associated with a poor prognosis (Kersemaekers et al., 1999). The HPV-16 oncoproteins E5, E6 and E7 directly stimulate signaling via the EGFR (Akerman et al., 2001; Sizemore et al., 1998; Straight, Herman, and McCance, 1995) suggesting that this pathway is important for the HPV life cycle. We found that erlotinib, a kinase inhibitor specific for the EGFR, prevented immortalization of cultured human cervical epithelial cells by the complete HPV-16 genome. Inhibition of immortalization did not depend on the presence of the E5 oncoprotein because erlotinib also prevented immortalization by E6/E7 genes alone. We found that erlotinib inhibited clonal growth, stimulated apoptosis, and induced premature senescence in E6/E7-expressing cervical cells. The concentrations of erlotinib that inhibited immortalization and stimulated apoptosis in vitro were comparable to the steady state plasma concentrations of erlotinib in treated patients (Smith, 2005). Persistent infection with high risk HPVs and subsequent immortalization of cervical cells are important early events in cervical carcinogenesis. Our results indicate that blocking EGFR function prevents immortalization by HPV-16 E6 and E7 genes in vitro. Thus, erlotinib or other EGFR inhibitors may represent a novel and noninvasive method for chemoprevention or therapy of premalignant cervical disease.
Our results suggest that erlotinib stimulates apoptosis in cells that express HPV-16. The drug induced apoptosis in cervical carcinoma cell lines, HPV-16-immortalized cervical cell lines, and HPV-16- or SV-40-transfected normal cell cultures. In contrast, erlotinib did not stimulate apoptosis in normal cervical epithelial cells. These results are consistent with work showing that inhibition of the EGFR by tyrphostin AG1478 stimulated apoptosis of HPV-16 immortalized foreskin keratinocytes (Ben-Bassat et al., 1999). Our results extend this finding to cervical cells and demonstrate that the E7 protein is primarily responsible for sensitizing cells to apoptosis in response to EGFR inhibition. Erlotinib-induced apoptosis may be important for preventing cell immortalization. Prior to immortalization by HPV-16, cells enter a stage of crisis where they develop genetic instability and may undergo death. Our results indicate that erlotinib sensitizes HPV-16 expressing cells to apoptosis which might prevent progression through crisis and immortalization.
Erlotinib is a reversible inhibitor of the EGFR that competes with ATP for binding to the tyrosine kinase domain of the receptor. Erlotinib is known to induce p27KIP1 up-regulation and growth arrest in the G1 phase of the cell cycle (Ling et al., 2007). We found that short term treatment with erlotinib inhibited growth of HPV-16 expressing cells in a reversible manner. Cells resumed colony formation after removal of the drug. However, chronic exposure to erlotinib induced irreversible growth arrest and premature senescence in a proportion of cells that did not undergo apoptosis. Erlotinib-treated cells lost the ability to form colonies and expressed the senescence marker β-galactosidase after 6 days of continuous treatment. Previous studies have shown that EGFR inhibitors stimulate premature senescence in vivo and in vitro, and it has been hypothesized that senescence is an important anti-tumor mechanism (Hotta et al., 2007). In this regard, inhibition of the EGFR is known to suppress the activity of telomerase in cancer cells (Budiyanto et al., 2003). We observed that a few cells escaped senescence and became immortalized in the presence of erlotinib. These immortal cell lines had increased resistance to the drug (data not shown).
Continued expression of the HPV-16 E6 and E7 proteins is required to maintain growth of HPV-immortalized cells and cervical carcinoma cell lines (von Knebel Doeberitz et al., 1992). We found that erlotinib did not decrease expression of E6 and E7 RNAs in transfected cervical epithelial cells. In fact, erlotinib significantly stimulated expression of E6 and E7 RNAs. The biological significance of this finding is unclear. Erlotinib did not alter expression from the HPV-16 LCR in reporter gene assays. We also found that erlotinib inhibited immortalization by SV40, suggesting that the drug did not prevent immortalization by specifically targeting HPV gene expression. Recently, others have reported that inhibition of the EGFR by 30.0 μM AG1478 altered splicing of E6/E7 RNAs to favor production of the E6*/E7 transcript, which decreased levels of E6 RNA and increased levels of p53 in keratinocytes (Rosenberger et al., 2010). We did not see differences in splicing in our experiments. Thus, inhibition of the EGFR may interfere with HPV-16 E6/E7 expression in some cell systems, but this effect was not critical for prevention of immortalization by HPV-16 in cultured cervical cells.
Clinical trials for chemoprevention of cervical cancer by several natural products have not yielded promising results (Sasieni, 2006). In contrast, the HPV prophylactic vaccine prevents infection by HPV-16 and -18 (Campo and Roden, 2010), and presumably will reduce cancer caused by these two high risk types (approximately 70% of cervical cancers). Due to the long latency of cervical carcinogenesis, the prophylactic vaccine is not likely to have a major impact on the overall incidence of cervical cancer for many years. In addition, the vaccine may be less effective in high risk populations that are immune compromised, such as AIDs patients or transplant recipients (Palefsky, 2009). Most importantly, the vaccine does not help women who already have HPV infections. Thus, HPV-infected women who have a high risk for cervical cancer would benefit from improved methods of chemoprevention or therapy that target signal pathways critical for cervical cancer development. Immortalization by HPV is a relevant target, and our results suggest that erlotinib might have a role in individuals with high risk for cervical cancer or other HPV-associated malignancies. Treatment with erlotinib causes side effects that are manageable, including skin rash and diarrhea (Iyer and Bharthuar, 2010; Li and Perez-Soler, 2009), and serious effects occur rarely. Currently, erlotinib is under evaluation in a chemoprevention trial (EPOC) in a population at high risk for head and neck cancer (William et al., 2009).
Erlotinib stimulated apoptosis and inhibited clonal growth of cultured cervical carcinoma cells, although the effectiveness of inhibition (ID50) varied in different lines. Erlotinib has been approved by the FDA for treatment of recurrent non small cell lung cancer and for first-line treatment of advanced pancreatic cancer with gemcitabine (Iyer and Bharthuar, 2010). Therefore, it is reasonable to test its toxicity (Nogueira-Rodrigues et al., 2008; Perez Rodrigo et al., 2009) and therapeutic activity for cervical cancer. Recently, erlotinib was shown to be ineffective as mono therapy for patients with recurrent cervical cancer in a phase II trial (Schilder et al., 2009). Similar results were reported for gefitinib (Goncalves et al., 2008), another EGFR kinase inhibitor. Several studies have shown that patients who harbor mutations in the EGFR gene have a high rate of response to erlotinib (Lynch et al., 2004), but these mutations appear to be rare in cervical cancers and dysplasias (Arias-Pulido et al., 2008). In the future, EGFR inhibitors may be most effective therapeutically in patients who are preselected for genetic susceptibility (Carter, Kelly, and Giaccone, 2009; Vaidya, Parnes, and Seiden, 2005), or when they are used in combination with other targeted therapies (Gossage and Eisen, 2010; Johnson and Brown, 2010).
CONCLUSIONS
We show that erlotinib, a specific inhibitor of EGFR tyrosine kinase activity, prevented immortalization of human cervical epithelial cells by HPV-16. Erlotinib sensitized HPV-16-expressing cells to apoptosis and induced senescence in a subpopulation of surviving cells. These results indicate that EGFR inhibitors may be useful for chemoprevention in women who have a high risk for developing cervical cancer.
MATERIALS AND METHODS
Cervical cell culture
Cervical tissue was purchased from the Cooperative Human Tissue Network. Specimens were removed after hysterectomy for nonmalignant disease. Tissues were collected in RPMI medium with 10% fetal bovine serum plus antibiotics and used within 24 hours. Cervical epithelial cells were isolated using a two step enzymatic digestion (Woodworth and Simpson, 1993). Briefly, each tissue was digested for 16 hours at 4°C in dispase (BD Biosciences) and the epithelium was removed by scraping. The epithelial sheet was cut into 1mm2 pieces and digested in 0.25% trypsin at 37°C for 10 minutes. Trypsin was neutralized by addition of fetal bovine serum and cells were collected by centrifugation at 2000×g. Normal epithelial cells, HPV-16 immortalized cells (Woodworth et al., 1990) and cervical carcinoma cell lines (Woodworth et al., 1995) were cultured in Keratinocyte Serum-Free Medium (KSFM, Invitrogen) or KSFM lacking EGF and bovine pituitary extract.
Transfection or retrovirus infection with HPV-16 genes
HPV does not undergo efficient productive infection in vitro, therefore, we introduced HPV genes into cultured cervical cells by transfection of the HPV-16 genome or infection with recombinant HPV-16 retroviruses (Halbert, Demers, and Galloway, 1991). Secondary cultures were transfected with a plasmid containing the complete HPV-16 genome plus the neomycin resistance gene (Woodworth et al., 1988) using lipofectamine (Invitrogen). Transfected cultures were split into 6 × 60mm dishes and selected for 48 hours in KSFM containing 100μg/ml G418. These cultures were used directly for immortalization and clonal growth assays. For retrovirus infection, secondary cultures were infected with high-titer retroviruses (Woodworth et al., 1992) encoding HPV-16 E6, E7, or E6/E7 genes inserted into the vector pLXSN, which contains the neomycin resistance gene (Halbert, Demers, and Galloway, 1991). Infection was performed for 3 hours in medium with 10 ng/ml polybrene with rocking every 15 minutes. Subsequently, medium was changed and cells grew for 24 hours before cultures were split 1:3. After 24 hours, infected cells were selected by growth for 2 days in KSFM containing 200μg/ml G418 and used immediately for experiments.
Immortalization assay
Immortalization assays were performed after transfection with the complete HPV-16 genome or infection with HPV-16 E6/E7-containing retroviruses. For retrovirus-based assays, cells were plated at clonal density (500 cells/60 mm dish) in 6 × 60mm dishes for each experimental group. After 24 hours, cultures were treated with KSFM containing erlotinib (OSI Pharmaceuticals) at doses of 0.03, 0.1, 0.3, 1.0 or 3.0 μM. As controls, cultures were treated with 0.1% DMSO (used to dissolve erlotinib), AG1295 (BD Biosciences) a specific inhibitor of platelet derived growth factor receptor that does not inhibit EGFR tyrosine kinase activity, PD153035 (EMD4 Biosciences) a specific inhibitor of the EGFR, or with a monoclonal antibody that blocks binding to the EGFR (LA1, Millipore). Experiments to verify inhibition of the EGFR using an anti phospho EGFR antibody in Western blots were performed previously (Woodworth et al., 2005). Cells infected with retroviruses containing only the pLXSN vector (lacking HPV-16 genes) served as the negative control for immortalization. Transfection-based assays were performed on cultures after transfection with a plasmid containing the complete of the HPV-16 genome plus the neomycin resistance gene (Woodworth et al., 1988). After selection for stably transfected cells using G418, cultures were fed with fresh KSFM containing erlotinib every two days. Immortalization was measured by pooling the colonies in each dish and subculturing cells continually in the presence or absence of erlotinib. Cervical cells became senescent 2 or 3 passages after introduction of HPV-16 genes (approx. 40–60 population doublings). About 30–40 population doublings were required to isolate primary cultures and transfect/infect with HPV-16 genes. Cells transfected with the neomycin resistance gene alone served as a negative control for immortalization. Cells transfected with a plasmid encoding the Simian Virus-40 (SV40) genome (Woodworth, Secott, and Isom, 1986) served as a control for determining specificity of drug effects for HPV-16.
Clonal growth assay
Cells were plated as described in immortalization assays. Cultures were treated with erlotinib continuously for 14 (retrovirus-infected) or 21 days (transfected). After colonies became visible, dishes were fixed and stained with Giemsa to identify colonies. A colony was defined as 50 or more cells.
Apoptosis assay
Cells were plated at a density of 5 × 105 cells/60mm dish and then treated with erlotinib for 24 or 48 hours at concentrations ranging from 0.03 to 1.0 μM. A DNA Ladder Assay PCR kit (Maxim Biotech, Inc) was used to detect fragmentation between nucleosomes as described (Iglesias et al., 1998). To determine the percentage of apoptotic cells, cultures were stained with DAPI (1 μg/ml) and examined under UV light. Apoptotic cells were identified by condensed and fragmented nuclei whereas viable cells excluded the dye and did not stain. The percentage of apoptotic nuclei was determined by counting 600 cells from randomly selected fields and 3 replicate dishes were examined for each experimental group. The percentage of apoptotic cells was calculated as the number of cells that stained blue/total cells counted × 100.
Real time RT-PCR
RNA was extracted from three different primary cultures transfected with the complete HPV-16 genome using Trizol (Invitrogen). Cellular DNA was digested with Turbo DNA-Free (Ambion, Inc) and RNA was reverse transcribed using Superscript First Strand kit (Invitrogen). No RT controls were included to confirm absence of cell DNA. Real-Time PCR was performed using an iCycler IQ (Bio-Rad) using triplicate reactions (50μl) containing 25μl of 2x SYBR-Green Supermix, 0.4μM of each forward and reverse primer, and 1 μl cDNA. HPV-16 E6 primers were: forward GACCCAGAAAGTTACCACAG and reverse GCAACAAGACATACATCGAC. HPV-16 E7 primers were: forward ATGACAGCTCAGAGGAG and reverse TCATAGTGTGCCCATTAACAG. Beta-actin primers were: forward GGACTTCGAGCAAGAGATGG and reverse AGCACTGTGTTGGCGTACAG. Reactions were performed for 40 cycles and melting temperature analysis was conducted on each sample to check specificity of product. Relative expression was calculated using the ΔΔCt method. Three housekeeping genes (β-actin, GAPDH and keratin 14) were examined for normalization and β-actin was chosen because erlotinib did not alter expression. Potential effects or erlotinib on splicing of E6/E7 RNA were measured PCR using primers for E6/E6* ACTGCAATGTTTCAGGACCCA and TCAGGACACAGTGGCTTTT (Rosenberger et al., 2010) and the cDNA samples used for RT-PCR.
Dual luciferase reporter gene assay
Cells were grown in 12 well plates and cotransfected with lipofectamine (Invitrogen) for 3 hours using 0.5 μg of the NF-kB responsive firefly luciferase reporter gene (Clontech), and 0.25 μg of a renilla luciferase reporter gene (Clontech). The latter promoter has weak activity and served as an internal control for transfection efficiency. Transfected cultures were maintained in KSFM for 24 hours prior to measuring NF-kB activity using a dual luciferase assay (Promega). Each transfection was performed in triplicate, and transfection experiments were repeated 3 times using cells from different donors. Positive and negative controls for NF-kB activation included cotransfection with a p65 expression vector or a vector containing the IKBα dominant negative mutant, respectively.
Senescence assay
Cervical cells were infected with HPV-16 E6/E7 retroviruses and plated for clonal growth assays as described. Cultures were treated with a 1 μM erlotinib for various times ranging from 0–14 days. Six cultures were removed every two days and returned to normal KSFM without drug for an additional 10 days to examine whether growth inhibition was reversible. Growth was assessed by measuring colony forming ability in 3 dishes and senescence was determined in the 3 remaining dishes using a β-galactosidase assay kit (Cell Signaling Technology).
Statistical analysis
One way analysis of variance (ANOVA) was used to compare the means of multiple experimental groups when data was distributed normally, and the Kruskal-Wallis test was used for comparisons when the distribution was not normal. Tukey’s multiple comparison test was used to compare differences between each pair of means. Regression analysis was used to predict ID50 values from the results of clonal growth and apoptosis experiments.
HIGHLIGHTS.
We examined the effect on EGF-R inhibition in HPV-infected cervical cells.
Erlotinib prevented immortalization of cells by HPV-16.
Erlotinib stimulated apoptosis in cells that expressed HPV-16.
Erlotinib induced senescence in cells that did not undergo apoptosis. > The EGF-R may be a potential target for chemoprevention
Acknowledgments
We thank Dr. Lucia Pirisi for providing the HPV LCR reporter gene and Dr. Harriet Isom for the SV40 expression vector. Human cervical tissue was obtained from the Cooperative Human Tissue Network. We acknowledge OSI Oncology, a wholly owned subsidiary of Astellas Pharma, Inc. for providing erlotinib for these studies. This work was supported by National Cancer Institute award 1R15CA126855-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerman GS, Tolleson WH, Brown KL, Zyzak LL, Mourateva E, Engin TS, Basaraba A, Coker AL, Creek KE, Pirisi L. Human papillomavirus type 16 E6 and E7 cooperate to increase epidermal growth factor receptor (EGFR) mRNA levels, overcoming mechanisms by which excessive EGFR signaling shortens the life span of normal human keratinocytes. Cancer Res. 2001;61(9):3837–43. [PubMed] [Google Scholar]
- Arbyn M, Ronco G, Cuzick J, Wentzensen N, Castle PE. How to evaluate emerging technologies in cervical cancer screening? Int J Cancer. 2009;125(11):2489–96. doi: 10.1002/ijc.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Pulido H, Joste N, Chavez A, Muller CY, Dai D, Smith HO, Verschraegen CF. Absence of epidermal growth factor receptor mutations in cervical cancer. Int J Gynecol Cancer. 2008;18(4):749–54. doi: 10.1111/j.1525-1438.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H, Rosenbaum-Mitrani S, Hartzstark Z, Levitzki R, Chaouat M, Shlomai Z, Klein BY, Kleinberger-Doron N, Gazit A, Tsvieli R, Levitzki A. Tyrphostins that suppress the growth of human papilloma virus 16-immortalized human keratinocytes. J Pharmacol Exp Ther. 1999;290(3):1442–57. [PubMed] [Google Scholar]
- Budiyanto A, Bito T, Kunisada M, Ashida M, Ichihashi M, Ueda M. Inhibition of the epidermal growth factor receptor suppresses telomerase activity in HSC-1 human cutaneous squamous cell carcinoma cells. J Invest Dermatol. 2003;121(5):1088–94. doi: 10.1046/j.1523-1747.2003.12529.x. [DOI] [PubMed] [Google Scholar]
- Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010;84(3):1214–20. doi: 10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CA, Kelly RJ, Giaccone G. Small-molecule inhibitors of the human epidermal receptor family. Expert Opin Investig Drugs. 2009;18(12):1829–42. doi: 10.1517/13543780903373343. [DOI] [PubMed] [Google Scholar]
- Chen HX, Cleck JN, Coelho R, Dancey JE. Epidermal growth factor receptor inhibitors: current status and future directions. Curr Probl Cancer. 2009;33(4):245–94. doi: 10.1016/j.currproblcancer.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Genther Williams SM, Disbrow GL, Schlegel R, Lee D, Threadgill DW, Lambert PF. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 2005;65(15):6534–42. doi: 10.1158/0008-5472.CAN-05-0083. [DOI] [PubMed] [Google Scholar]
- Goncalves A, Fabbro M, Lhomme C, Gladieff L, Extra JM, Floquet A, Chaigneau L, Carrasco AT, Viens P. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008;108(1):42–6. doi: 10.1016/j.ygyno.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Gossage L, Eisen T. Targeting multiple kinase pathways: a change in paradigm. Clin Cancer Res. 2010;16(7):1973–8. doi: 10.1158/1078-0432.CCR-09-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65(1):473–8. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8(12):3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K, Tabata M, Kiura K, Kozuki T, Hisamoto A, Katayama H, Takigawa N, Fujimoto N, Fujiwara K, Ueoka H, Tanimoto M. Gefitinib induces premature senescence in non-small cell lung cancer cells with or without EGFR gene mutation. Oncol Rep. 2007;17(2):313–7. [PubMed] [Google Scholar]
- Iglesias M, Yen K, Gaiotti D, Hildesheim A, Stoler MH, Woodworth CD. Human papillomavirus type 16 E7 protein sensitizes cervical keratinocytes to apoptosis and release of interleukin-1alpha. Oncogene. 1998;17(10):1195–205. doi: 10.1038/sj.onc.1202054. [DOI] [PubMed] [Google Scholar]
- Iyer R, Bharthuar A. A review of erlotinib--an oral, selective epidermal growth factor receptor tyrosine kinase inhibitor. Expert Opin Pharmacother. 2010;11(2):311–20. doi: 10.1517/14656560903551283. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Brown PH. Drug development for cancer chemoprevention: focus on molecular targets. Semin Oncol. 2010;37(4):345–58. doi: 10.1053/j.seminoncol.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Kersemaekers AM, Fleuren GJ, Kenter GG, Van den Broek LJ, Uljee SM, Hermans J, Van de Vijver MJ. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999;5(3):577–86. [PubMed] [Google Scholar]
- Khan MA, Canhoto AJ, Housley PR, Creek KE, Pirisi L. Glucocorticoids stimulate growth of human papillomavirus type 16 (HPV16)-immortalized human keratinocytes and support HPV16-mediated immortalization without affecting the levels of HPV16 E6/E7 mRNA. Exp Cell Res. 1997;236(1):304–10. doi: 10.1006/excr.1997.3729. [DOI] [PubMed] [Google Scholar]
- Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107–19. doi: 10.1007/s11523-009-0114-0. [DOI] [PubMed] [Google Scholar]
- Ling YH, Li T, Yuan Z, Haigentz M, Jr, Weber TK, Perez-Soler R. Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small-cell lung cancer cell lines. Mol Pharmacol. 2007;72(2):248–58. doi: 10.1124/mol.107.034827. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- McKenzie ND, Kobetz EN, Hnatyszyn J, Twiggs LB, Lucci JA., 3rd Women with HIV are more commonly infected with non-16 and -18 high-risk HPV types. Gynecol Oncol. 2010;116(3):572–7. doi: 10.1016/j.ygyno.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Rodrigues A, do Carmo CC, Viegas C, Erlich F, Camisao C, Fontao K, Lima R, Herchenhorn D, Martins RG, Moralez GM, Small IA, Ferreira CG. Phase I trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Clin Cancer Res. 2008;14(19):6324–9. doi: 10.1158/1078-0432.CCR-07-5112. [DOI] [PubMed] [Google Scholar]
- Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6(3):243–57. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4(1):52–6. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Rodrigo I, Albornoz Lopez R, Soto Rojas M, Fernandez Garcia I, Torres Degayon V. Effectiveness and safety of erlotinib in 2 patients with carcinoma of the cervix. Farm Hosp. 2009;33(2):96–9. [PubMed] [Google Scholar]
- Rosenberger S, De-Castro Arce J, Langbein L, Steenbergen RD, Rosl F. Alternative splicing of human papillomavirus type-16 E6/E6* early mRNA is coupled to EGF signaling via Erk1/2 activation. Proc Natl Acad Sci U S A. 2010;107(15):7006–11. doi: 10.1073/pnas.1002620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasieni P. Chemoprevention of cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):295–305. doi: 10.1016/j.bpobgyn.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schiffman MH, Brinton LA. The epidemiology of cervical carcinogenesis. Cancer. 1995;76(10 Suppl):1888–901. doi: 10.1002/1097-0142(19951115)76:10+<1888::aid-cncr2820761305>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schilder RJ, Sill MW, Lee YC, Mannel R. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19(5):929–33. doi: 10.1111/IGC.0b013e3181a83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore N, Choo CK, Eckert RL, Rorke EA. Transcriptional regulation of the EGF receptor promoter by HPV16 and retinoic acid in human ectocervical epithelial cells. Exp Cell Res. 1998;244(1):349–56. doi: 10.1006/excr.1998.4179. [DOI] [PubMed] [Google Scholar]
- Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clin Ther. 2005;27(10):1513–34. doi: 10.1016/j.clinthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Straight SW, Herman B, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J Virol. 1995;69(5):3185–92. doi: 10.1128/jvi.69.5.3185-3192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz FA, Krawczyk E, Hebert JD, Sudarshan SR, Simic V, Kamonjoh CM, Schlegel R. The human papillomavirus type 16 E5 oncoprotein inhibits epidermal growth factor trafficking independently of endosome acidification. J Virol. 2010;84(20):10619–29. doi: 10.1128/JVI.00831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AP, Parnes AD, Seiden MV. Rationale and clinical experience with epidermal growth factor receptor inhibitors in gynecologic malignancies. Curr Treat Options Oncol. 2005;6(2):103–14. doi: 10.1007/s11864-005-0018-x. [DOI] [PubMed] [Google Scholar]
- Vlastos AT, Schottenfeld D, Follen M. Biomarkers and their use in cervical cancer chemoprevention. Crit Rev Oncol Hematol. 2003;46(3):261–73. doi: 10.1016/s1040-8428(02)00107-5. [DOI] [PubMed] [Google Scholar]
- von Knebel Doeberitz M, Rittmuller C, zur Hausen H, Durst M. Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6-E7 anti-sense RNA. Int J Cancer. 1992;51(5):831–4. doi: 10.1002/ijc.2910510527. [DOI] [PubMed] [Google Scholar]
- William WN, Jr, Heymach JV, Kim ES, Lippman SM. Molecular targets for cancer chemoprevention. Nat Rev Drug Discov. 2009;8(3):213–25. doi: 10.1038/nrd2663. [DOI] [PubMed] [Google Scholar]
- Woodworth C, Secott T, Isom HC. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986;46(8):4018–26. [PubMed] [Google Scholar]
- Woodworth CD, Bowden PE, Doniger J, Pirisi L, Barnes W, Lancaster WD, DiPaolo JA. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 1988;48(16):4620–8. [PubMed] [Google Scholar]
- Woodworth CD, Cheng S, Simpson S, Hamacher L, Chow LT, Broker TR, DiPaolo JA. Recombinant retroviruses encoding human papillomavirus type 18 E6 and E7 genes stimulate proliferation and delay differentiation of human keratinocytes early after infection. Oncogene. 1992;7(4):619–26. [PubMed] [Google Scholar]
- Woodworth CD, Gaiotti D, Michael E, Hansen L, Nees M. Targeted disruption of the epidermal growth factor receptor inhibits development of papillomas and carcinomas from human papillomavirus-immortalized keratinocytes. Cancer Res. 2000;60(16):4397–402. [PubMed] [Google Scholar]
- Woodworth CD, McMullin E, Iglesias M, Plowman GD. Interleukin 1 alpha and tumor necrosis factor alpha stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells. Proc Natl Acad Sci U S A. 1995;92(7):2840–4. doi: 10.1073/pnas.92.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4(4):650–8. doi: 10.1158/1535-7163.MCT-04-0238. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Notario V, DiPaolo JA. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J Virol. 1990;64(10):4767–75. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth CD, Simpson S. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am J Pathol. 1993;142(5):1544–55. [PMC free article] [PubMed] [Google Scholar]
- Woodworth CD, Waggoner S, Barnes W, Stoler MH, DiPaolo JA. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990;50(12):3709–15. [PubMed] [Google Scholar]