Abstract
The primary embryonic signal in primates is chorionic gonadotropin (CG, designated hCG in humans), that is classically associated with corpus luteum rescue and progesterone production. However, research over the past decade has revealed the presence of the hCG receptor in a variety of extragonadal tissues. Additionally, discoveries of the multiple variants of hCG, namely, native hCG, hyperglycosylated hCG (hyp-hCG) and the β-subunit of the hyperglycosylated hCG (hCG-free β) has established a role for extragonadal actions of hCG. For the initiation and maintenance of pregnancy, hCG mediates multiple placental, uterine and fetal functions. Some of these include development of syncytiotrophoblast cells, mitotic growth and differentiation of the endometrium, localized suppression of the maternal immune system, modulation of uterine morphology and gene expression and coordination of intricate signal transduction between the endometrium. Recurrent pregnancy loss, preeclampsia and endometriosis are associated with altered responses of hCG, all of which have a detrimental effect on pregnancy. A role for hyp-hCG in mediating the development of both trophoblastic and non-trophoblastic tumors has also been suggested. Other significant non-gonadal applications of hCG include predicting preeclampsia, determining the risk of Down’s syndrome and gestational trophoblastic disease, along with relaxing myometrial contractility and preventing recurrent miscarriages. Presence of hCG free-β in serum of cancer patients enables its usage as a diagnostic tumor marker. Thus, the extragonadal functions of hCG encompasses a wide spectrum of applications and is an open area for continued investigation.
Keywords: Chorionic gonadotropin, Embryo implantation, Endometrium, Endometriosis, Cancer
1 The multiple variants of human chorionic gonadotropin
Gonadotropins are glycoprotein hormones primarily of pituitary or placental origin that play an integral role in regulating and maintaining the complex endocrine system. In primates and humans, the primary embryonic signal, CG/hCG, maintains a functional corpus luteum and progesterone production to prevent the sloughing of the endometrial lining in the event of conception. Typical to the structure of glycoprotein hormones, hCG is a non-covalently linked heterodimer composed of α and β subunits, structurally related to, but considerably more potent than luteinizing hormone (LH). hCG is synthesized in two stages, primarily by the cytotrophoblasts which produce the CGα subunit, followed by multiplication and differentiation of the cytotrophoblasts to form syncytyotrophoblasts, which then produce the β subunit [1]. This process of cytotrophoblastic differentiation [2] as well as the initial differentiation of the embryonic stem cells into trophoblasts [3] is regulated by hCG itself [4]. The levels of hCG become detectable 3 weeks after the last menstrual period (LMP) in a conception cycle, continue to rise exponentially to peak around week 10, then drops to one tenth the concentration and plateaus until term. Through weeks 6–7 of gestation, the primary function of hCG is the rescue of corpus luteum and the maintenance of progesterone production, following which the placenta takes over progesterone production from the corpus luteum. However, these 3–4 weeks of activity encompasses only about 10% of the gestational length, leading to the initial speculation that corpus luteum rescue and gonadal actions are not the sole function of hCG.
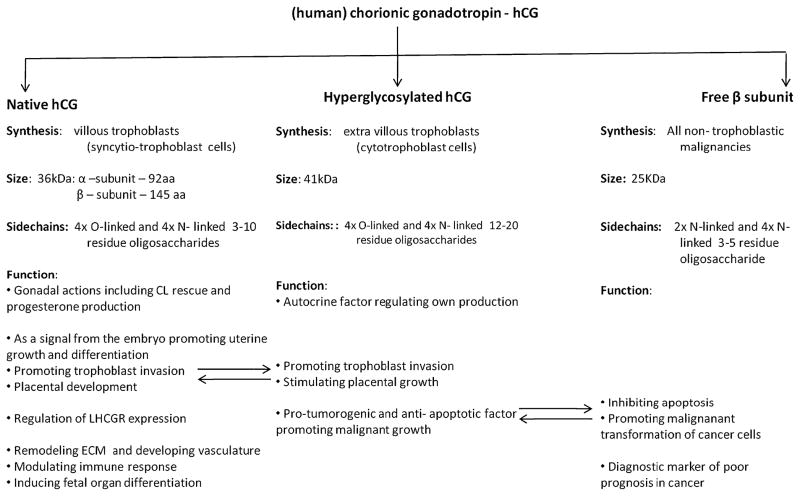
Research over the past decade has established that the trophoblasts embark upon two discrete pathways involving the villous and the extavillous trophoblast cells, in order to generate three distinct variants of hCG, all of which share the hCGβ amino acid sequence [5]. These are: native hCG, hyperglycosylated hCG and the hyperglycosylated hCG free β subunit, each having separate roles in evolution and distinct physiological functions [6] (Fig. 1). The syncytyotrophoblasts that are generated from the villous trophoblast cells produce regular hCG while the extravillous invasive trophoblasts produce hyp-hCG [5]. The peptide structure of hCG, established by Bahl et al. revealed that the regular hCG molecule comprises a 145 amino acid β-subunit and a 92 amino acid α-subunit [7]. However, 20–30% of the total molecular weight of hCG is contributed by oligosaccharide side chains, which also establishes the distinction between the different forms of hCG, for example increasing the molecular weight of hyp-hCG to 41,000 as compared to 36,000 for native hCG [8, 9]. The free β subunit of hCG represents the alternatively glycosylated monomeric variant of hCG, generated by all non-trophoblastic advanced malignancies [10].
Fig. 1.
The various isoforms of human chorionic gonadotropin are: native hCG, hyperglycosylated hCG and the hyperglycosylated hCG free β subunit. Each has separate roles in evolution and distinct physiological functions. The arrows indicate the functions where the different isoforms of hCG interact overlap
hCG signals primarily through the same seven-transmembrane G-protein coupled receptor as does LH—the LH-CG receptor (LHCGR) [11], however with a higher binding affinity. The LHCGR is an 880 kb gene comprising 10 introns and 11 exons. The greater part of the receptor including the entire carboxyl terminal consisting of the seven transmembrane helices, the three interconnecting extracellular and intracellular loops and the cytoplasmic tail is encoded by the 11th exon. The first ten exons only comprise the extracellular N-terminal exodomain consisting of numerous leucine-rich repeats involved in protein-protein interactions. Previously, the extragonadal responses of hCG appeared to evolve through the modification of gonadal function, either through enhanced or inhibited response to LH/hCG stimulation. However, the detection of the LHCGR in multiple tissues and organs of non-gonadal origin (Table 1) has led us to the concept that extragonadal gonadotropin action forms an integral function of this incredibly dynamic molecule.
Table 1.
Extragonadal tissues that express the lh/cg r
| Location | Reference |
|---|---|
| Uterus | Reshef et al. 1990; Han et al., 1997; Zhang et al., 2001b, Fields et al., 2004 |
| Breast/Mammary gland | Tao et al., 1997, Ziecik et al., 2001 |
| Adrenals | Pabon et al., 1996a; Apaja et al. 2005 |
| Oviduct | Lei et al., 1993; Han et al., 1996; Zhang et al., 2001 |
| Neural Retina | Thomson et al., 1998 |
| Brain | Lei et al., 1993 |
| Cervix | Lin et al., 2003, Stepien et al., 2000 |
| Fetal Tissues | Abdallah et al., 2004 |
| Skin | Pabon et al., 1996 |
| Bladder | Tao et al., 1998 |
| Placenta | Reshef et al., 1990 |
| Prostate and Seminal Vesicles | Reiter et al., 1995 |
| Sperm and Penis | Eblen et al., 2001, Kokk et al. |
| Thyroid | Frazier et al. 1990 |
| Cancers | Breast - Meduri et al., 1997, Hudelist et al., 2009 |
| Prostate – Lie et al., 2010 | |
| Ovarian – Puett et al., 2010 | |
| Adrenocortical – Galac et al., 2010 ; Costa et al., 2009 | |
| Endometrial – Nocci et al., 2008 |
2 Biological actions
2.1 Native hCG—endometrial responses and initiation of embryo implantation
Fertilization in primates, as in most species occurs in the oviduct. Around the sixth day following fertilization, the developing embryo (called a blastocyst at this stage) hatches from the zona pellucida prior to its attachment to the uterine epithelium. In addition to hCG and its different variants, as described above, the trophoblast and syncytio-trophoblast produce various hormones and cytokines e.g. interleukin −1 and insulin- like growth factor II, displaying profound effects on the endometrium [12–14]
Studies have confirmed that the LHCGR is present in the primate and human endometrium [15, 16]. However, the regulation and expression of the receptor in the endometrium appears to be highly dynamic and for the most part, is still under speculation. We have studied LHCGR expression in the baboon very closely and have reported that that the LHCGR is absent in the endometrium during the proliferative phase, while in the secretory phase, both the luminal and the glandular epithelial cells express the receptor. With the onset of pregnancy, the expression of the LHCGR is limited to the stromal cells surrounding the spiral arteries. This expression persists until day 25 of pregnancy and subsequently decreases between days 40–60 as pregnancy progresses, in a manner similar to that in humans [17]. We confirmed these results in vitro by demonstrating that decidualization (the differentiation of stromal cells into a secretory phenotype) of endometrial stromal cells, is associated with a decrease in LHCGR expression, and also showed that hCG regulates the levels of its own receptor [15].
The paracrine effects of hCG on the endometrium have been explored in detail using the in vivo baboon model of implantation [18]. Infusion of hCG into the uterine cavity of normal cycling baboons during the window of uterine receptivity induces a multitude of morphological, biochemical and molecular changes in the estrogen and progesterone primed endometrium. The luminal epithelium forms plaques and increases its overall secretory function while the stromal response is characterized by induction of α-smooth muscle actin (αSMA). Additionally we analyzed the genes that were expressed in the endometrium in response to treatment with hCG using a cDNA array [19]. Among the genes significantly influenced by CG are the leukemia inhibitory factor (LIF), an interleukin 6 class cytokine, the complement component C3, the radical scavenging enzyme superoxide dismutase 2 (SOD2), the matrix metalloproteinase 7 (MMP7) and the immunomodulatory compound glycodelin, all of which were up-regulated in response to CG. The regulator of the Wnt-signaling pathway, soluble frizzled receptor protein 4 (SFRP4) and ApolipoprotenA1 (ApoA1) were down-regulated in response to CG treatment [19, 20] which suggest that the effects of hCG on the uterine endometrium plays a role in inhibiting apoptosis and enhancing cell adhesion [21, 22]. Affymetrix microarray analysis further demonstrated that hCG synergizes with estrogen and progesterone to regulate gene expression in a human endometrial epithelial cell line (Banerjee et al., unpublished data).
The role of hCG in coordinating embryo implantation has been demonstrated by its extragonadal responses in multiple model systems. Remodeling of the extracellular matrix to facilitate trophoblast invasion requires a delicate balance between tissue degradation and restoration, mediated by CG-regulated MMPs [19, 23–25] and LIF [26–28]. The expression of LIF is mediated upstream by prokinectin which is also regulated by hCG [29]. Stimulation of isolated epithelial cells with hCG significantly increased the production of of proteins such as interleukin 11, CXCL 10, granulocyte macrophage colony-stimulating factor and FGF2 all of which have been postulated to play a role in endometrial receptivity and embryo implantation [30]. Validation of the hCG response in epithelial cells was further demonstrated by the up-regulation of two known hCG-regulated proteins, vascular endothelial growth factor and leukaemia inhibitory factor. Additionally, the process of implantation necessitates modulation and the localized suppression of the maternal immune response to prevent rejection of the fetus by the mother. hCG, a molecule with potent immunological properties [31], is known to play a critical role in regulation of the maternal immune system. This is mediated by multiple mechanisms, including in conjunction with a progesterone regulated molecule, glycodelin [18, 32, 33], the complement component C3 [19, 34] or by conferring resistance to oxidative stress via FOXO1 and SOD2 [35]. Uterine natural killer (uNK) cells play a critical role in the establishment of pregnancy The proliferation of uNK cells has been reported to be modulated via the mannose receptor (CD206) as opposed to the classical LHCG receptor [36]. This altered signaling pathway might be of significance since the endometrial LHCG receptor is rapidly down regulated following the establishment of pregnancy and the onset of decidualization [15]. Interestingly dysregulation of hCG either in the form of altered levels of the hormone or modification of the oligosaccharide chains has been suggested to play a role in preeclampsia [37]. Since deglycosylation of hCG inhibits binding to the mannose receptor, it leaves open the question whether oligosaccharide modifications to hCG may be a contributing factor to pregnancy complications that are associated with hCG dysregulation.
We have recently shown that in the pathological condition of endometriosis, which essentially represents the presence of endometrial glands and stroma outside the uterine cavity, the endometrial response to hCG is altered [38]. Using the baboon model of endometriosis, we treated animals with experimentally induced or spontaneous endometriosis with hCG during the window of uterine receptivity and analyzed alterations in gene expression using microarray analysis. We confirmed abnormal responses of known hCG-regulated genes. APOA1, SFRP4, and pregnancy associated plasma protein A (PAPPA) which are normally down regulated by hCG were up-regulated by hCG in animals with endometriosis. These studies suggest that the induction of endometriosis results in altered responses to hCG. Some endometrial transcripts, which are normally regulated by hCG, show attenuated responses, whereas others respond in the opposite manner to that seen in normal animals. Cytoskeletal reorganization as manifested by the expression of αSMA is a prerequisite for decidualization and this process is impaired as a consequence of endometriosis [38, 39]. The decidual response to embryonic signals is also impaired in other instances. Decidual cells can be sensors of embryo quality since when co-cultured with defective or arresting embryos the decidualizing cells respond by shutting down the production of key implantation mediators and immunomodulators [40]. In a recent study of decidual cells obtained from women with recurrent pregnancy loss, prolactin production was attenuated and prokineticin-1 production was prolonged and enhanced. This was also associated with a complete dysregulation of both proteins in response to treatment with hCG [41]. Therefore, these altered responses prevent the acquisition of the full endometrial molecular repertoire necessary for implantation.
To study the intricate signaling pathways that were induced by hCG in the endometrium, we studied hCG signaling in an endometrial cell line and compared these responses to a gonadal cell line [42]. Interestingly, we discovered that the signal transduction pathway that is activated by hCG via its receptor in epithelial cells is distinct from hCG signaling in the gonads. While gonadal hCG signaling by the LHCGR has been shown by multiple groups to be primarily mediated through a cAMP-PKA pathway [43–45], we have shown a novel cAMP-PKA independent and PI3K-Akt dependent MAPK pathway is activated in the endometrium by hCG, [42, 46]. We believe that since the epithelium is the first area of contact for hCG, this epithelial response to hCG serves as the initial trigger for implantation. We further speculate that this alternative pathway may be a mechanism employed within the endometrium to prevent rapid receptor desensitization [47] in the presence of high concentrations of ligand that would be present during implantation and early embryonic development. Thus this pathway would ensure that hCG continues to modulate the signaling via the endometrial receptor to develop a receptive endometrium that is essential for embryo implantation. hCG induced phosphorylation of ERK1/2, activates the nuclear transcription factor, Elk1, in a PI3K-MAPK-dependent manner which in turn regulates the expression of the microsomal enzyme PGE2 synthase (mPTGES), a terminal prostanoid synthase responsible for PGE2 synthesis. Subsequently, PGE2 via PTGER2 induces expression of CXCR4 the chemokine receptor which is an important regulator of early events associated with pregnancy. These data suggest that early embryonic signals may regulate fetal-maternal crosstalk in the human endometrium by inducing CXCR4 expression via the PGE2-PTGER2-mediated induction of the EGFR, PI3K and ERK1/2 pathways [48]. hCG also has a significant influence on the stromal compartment of the endometrium, which undergoes a shift from proliferation to differentiation (decidualization) in response to pregnancy. CG mediates this by the induction of αSMA [18] as a consequence of stromal integrin binding to secreted ECM proteins [49, 50]. This change in cytoskeletal architecture is also critical to prevent these cells from undergoing apoptosis that usually occurs at the end of each non-conception cycle [22]. Decidualization requires the disruption of actin filaments but the apoptotic response is now inhibited by prolactin and insulin-like growth factor binding protein-1, which are the major proteins associated with stromal cell transformation into the decidual phenotype [22, 51]. The role of hCG in preventing stromal cell apoptosis has been well documented [21] and we have further shown that hCG induces the induction of anti-apoptotic genes e.g. Bcl2. Finally, we propose that all these responses that enable stromal cell cells to undergo decidualization may be regulated by Notch 1 which is induced by hCG and can transcriptionally regulate αSMA. Inhibition of Notch1 in stromal cells prevents them from undergoing decidualization. This has been further validated in a mouse model in which tissue specific ablation of Notch 1 in the uterus results in an inhibition of decidualization and an increase in apoptosis (Afshar et al., 2011—manuscripts submitted).
2.2 Hyperglycosylated hCG—role in trophoblast invasion and placentation
Cole and colleagues initially reported that the hCG molecules from choriocarcinoma (cancer of the cytotrophoblast cells) patients bore oligosaccharide side chains that were significantly different from those of normal patients [52], paving the way for exploring the biological role of the hyperglycosylated variant of hCG. In a normal pregnancy, hyp –hCG accounts for ~90% of the total hCG during the peak hCG production in the week following implantation [53]. In normal women, hyp-hCG is an autocrine factor produced by the cytotrophoblast cells, regulating its own production and also promoting trophoblast invasion. Muller’s group has suggested it’s becoming established as a marker for early trophoblast invasion [54]. Evidence for the invasive properties of hyp-hCG came from a series of in vitro and in vivo experiments. Using a Matrigel preparation of choriocarcinoma cells, followed by treatment with hyp-or regular hCG, Cole et al. observed that hyp-hCG preparations led to significant penetration of cells as compared to the native hCG. This suggested a possible role of hyp-hCG, acting as an autocrine factor, on the choriocarcinoma cells, assisting their invasion and thus promoting malignancy [55]. They further elucidated the tumor-promoting characteristics of hyp-hCG by demonstrating that tumors developed in nude mice transplanted with choriocarcinoma cells regressed rapidly using a specific antibody against hyp-hCG [55]. Similar anti-apoptotic characteristics of hyp-hCG were also established by Hamada et al. [56].
To permit deep penetration of the trophoblast into the myometrium, it has been proposed that hyp-hCG interacts with native hCG to coordinate the invasive activities of the trophoblastic villi. The extravillous cytotrophoblast cells are the main invasive cells of the placenta, forming fingerlike projections or villi, as a result of rapid cellular proliferation [57]. The trophoblastic villi produce hyp-hCG, which multiplies the proliferative response exponentially, invading the villi through the decidua and driving the embryo deep into the myometrium, thus anchoring it [58]. The gestational villous tissue, by the 7th week of gestation is primarily composed of syncytiotrophoblast cells, below which are the villous trophoblast cells awaiting fusion to form more syncytiotrophoblast cells. This action is aided and augmented by the action of native hCG, which brings about angiogenesis and elaborate transformation of myometrial spiral arteries and ensures blood supply to the villous area after around 10 weeks of gestation [2]. Subsequently, the endometrium transforms into the decidua. The vascularization of the endometrium and decidua is primarily maintained by hCG via vascular endothelial growth factor (VEGF) [59], the intrauterine concentration of which increases in a time dependent manner [60]. Using a microdialysis system, Licht et al. demonstrated that hCG infusion into the uterine cavity during the secretory phase of the menstrual cycle directly modulated VEGF expression [27]. Thus, hemochorial placentation is the end result of a close interplay between hCG hormonal activity involving VEGF induction and hyp-hCG driven deep myometrial invasion, supplemented by development and expansion of spiral arteries. Low levels of hyp-hCG and inadequate interaction between the native and hyp-forms of hCG also appear to be associated with clinical preeclampsia, which is marked by insufficient trophoblast invasion and poor spiral artery remodeling [61]. However, in must be kept in mind that studies on the role of hyp-hCG has been the focus of a very few laboratories and by and large, not been extensively validated suggesting that and significant more research is required to understand if this molecule has biological and functional significance.
2.3 Free hyperglycosylated β subunit—role in malignancy and cellular transformation
The hyperglycosylated free β subunit of hCG is the alternatively gylcosylated monomeric variant of hCG made by all non-trophoblastic advanced malignancies [6]. Excesses of the free β-subunit are produced in hydatiform moles, choriocarcinomas and other cancers. Acevedo et al analyzed human cancer cells of different types and origins and claimed that synthesis and expression of the free β subunit of hCG is a common biochemical denominator of cancer [62]. They demonstrated the presence of the β subunit in metastatic malignancies in vivo [63] as well as in cultured cancer cells in vitro [64]. Other groups have confirmed this is cervical cancer and other cancers [65–67].
The free β subunit has been shown to play a specific role in inducing malignancy and transformation of cells. Using multiple models including bladder carcinoma [68] and other tumor cell lines [69], the direct effect of the β-subunit on stimulation of malignant cell growth has been demonstrated. When cancer cells regress and dedifferentiate, they express cytotrophoblast proteins, and by default, the hyperglycosylated free β-subunit is expressed, which then promotes cancer growth [62, 70]. Both hyp-hCG and the β-subunit has been shown to act through the TGFβ receptor, and it is hypothesized that this occurs via similar pathways [6]. The application of hCG-β as a prognostic marker of poor prognosis in cancer is being examined in multiple tumor paradigms including cervical, vaginal, endometrial and bladder [65, 71–73]. These studies collectively indicate that the expression of the free β-subunit invokes a negative outcome in human malignancies.
This has directed efforts towards using the different free β-derivatives as vaccines in treatment of non-gestational malignancies [74]. Immunotherapies using a synthetic vaccine targeting β-hCG are being explored and have been associated with longer overall survival rates [75].
3 Diagnostic and therapeutic applications of hCG
Assays for hCG and its variants are widely used clinically in pregnancy detection, prediction of anomalous and pre-term pregnancies, managing gestational trophoblastic diseases and are very useful in management of testicular malignancies and other germ cell malignancies. Additionally, the ease of use of hCG, its relatively nontoxic nature, and scalability without proportional increase in cost, lends itself as a very promising molecule that could be utilized as a therapeutic agent [4].
3.1 Pregnancy tests
The invention of radioimmunoassays led to large scale quantitative determinations of hCG in the late 1960s and hence the development of the hCG assays [76]. Currently there are two clear categories of hCG tests: intact hCG tests (detecting only the dimer) and the total hCG test (detecting the dimer, the hyperglycosylated variant, as well as its free β-subunit) [77]. As mentioned above, serum hCG in early pregnancy is primarily hyp-hCG (week 3–4 of gestation), which is when most women test for pregnancy. hCG changes in its nature as pregnancy progresses, becoming primarily native hCG (~98% of total hCG in the third trimester).
Hyp-hCG has several unique advantages as a pregnancy test [78], particularly in IVF pregnancies, during which native hCG is administered to induce ovulation. It is only when the exogenous hCG has been cleared from the system, that endogenous hCG can be detected and the native hCG test provides an accurate result. Using hyp-hCG enables pregnancy to be detected around 6–7 days following embryo transfer [79]. Further, hyp-hCG tests have also demonstrated advantages in assessing pregnancy outcomes and failing term pregnancies as discussed below.
3.2 Miscarriages and pre term birth
Implantation failures, both spontaneous and biochemical, account for approximately 41–45% of human pregnancy failures, leading to this to be a significant concern in maternal and neonatal healthcare. Traditionally, discriminatory hCG concentrations during early pregnancy were considered the best indicators of pregnancies at risk for adverse outcomes [80], however high false positives lead to these not being highly reliable tests. The relationship of hyp-hCG to implantation defects and miscarriages had been first demonstrated by Sasaki et al. [81]. Currently there is sufficient evidence to believe that high ratio of native hCG vs. hyp-hCG during early pregnancy is considered the best indication of miscarriages or abortions [82–84].
Over twenty years ago, Rao and colleagues had shown the presence of the LHCGR in the myometrium, [16] but its functionality remained far from clear for a long time. The role of hCG on myometrial quiescence has of late generated sufficient interest primarily to consider it as a potential treatment option for recurrent miscarriages [85] or pre-term birth [86]. Rao and colleagues showed the capability of hCG to inhibit prostaglandin-induced preterm delivery in a murine model [87]. Recent physiological evidence has shown a dose dependent decrease in myometrial contractility with increasing doses of hCG [88]. However clinical trials have not yet been able to demonstrate any significant decrease in miscarriage rates upon hCG administration and many of these postulated functions remain to be independently validated in appropriate and well-designed clinical trials [89–91].
3.3 Gestational trophoblastic diseases
Gestational trophoblastic diseases (GTDs) are anomalies characterized by abnormal proliferation of trophoblasts or abnormal formation of the placenta. As described in section 2.2, hyp-hCG has been proposed to play a role in cell proliferation and the invasion of the trophoblast leading to hemochorial placentation and embryo implantation in humans. At the other extreme, uncontrolled and unregulated proliferation and invasion is responsible for the development of cancer.
A typical case of molar pregnancy involves the implantation of a non-viable fertilized ovum (e.g. with an inactive haploid set), thereby leading to an abnormal pathological condition. The mass of trophoblast tissue leads to the production of extremely high levels of hCG and the formation of a hydatiform mole [92], which presents an extremely high risk of persistence or invasion of the uterus and other organs. The presence of hyp-hCG is the primary driving force of gestational trophoblastic neoplasms and choriocarnomas, which are diseases characteristic only to humans [93]. Choriocarcinomas are known to originate when residual deeply implanted extravillous cytotrophoblast cells transform and lose their ability to fuse into syncytiotrophoblast, producing one of the most malignant diseases in humans [92]. Hyp-hCG is currently the best marker of GTDs, with levels reaching >20% of the total hCG in invasive moles and >60% of the total hCG in case of choriocarcinomas [94]. The levels of hyp-hCG allow GTDs to be classified into aggressive, minimally aggressive or quiescent. The presence of high hyp-hCG levels demonstrates a highly aggressive form of GTD (40% of total hCG defined as minimally invasive and resistant to chemotherapy, while 60% or greater being classified as highly invasive and chemotherapy responsive). Further, quiescent GTD represents highly differentiated syncytiotrophoblast cells with no significant cytotrophoblast cells, and hence no detectable hyp-hCG [95].
3.4 Down’s syndrome
Screening of Down’s syndrome pregnancies was the first application determined for hyp-hCG [96]. Trisomy 21 or Down’s syndrome is the most common of the genetically abnormal pregnancies. It was shown to be associated with twice the hCG levels during pregnancy [97] as well as a significant increase in the expression of the LHCGR receptor in the trophoblast [98].
Other markers included unconjugated estriol and pregnancy-associated plasma protein A, leading to the development of the ‘triple test’ [99]. It was traditionally diagnosed using amniocentesis, performed at 14–26 weeks of gestation. Diagnosis at such a late stage, coupled with high rates of false negatives led to high parental risks when choosing to abort the fetus. Evain-Brion’s group discovered that defective fusion of trophoblast cells and lack of the formation of adequate numbers of syncytiotrophoblast cells leads to a high hyp-hCG to native hCG ratio in pregnancies with Down’s Syndrome [100, 101]. These studies laid the foundation for using chorionic villous sampling for the diagnosis of Down’s syndrome. Chorionic villous sampling can be done as early as 9–11 weeks of gestation, leading to termination decisions as early as 12 weeks of gestation. Further, the increased specificity of the hyp-hCG test led to an 80% detection rate. Currently, hyp-hCG testing has been proved to be the most significant inclusion to screening for Down’s syndrome as revealed from inter-laboratory testing comparisons [102], thus having a significant impact on improving maternal and neonatal health.
4 Conclusions
The field of hCG biology has undergone dramatic changes over the past decade, from when it has emerged from an embryonic hormone produced for maintaining the corpus luteum and sustaining progesterone production, to a complex family of related molecules that have a multitude of functions in different biological systems: both physiological and pathological. A dimer with numerous unique oligosaccharide side chains, it performs varying functions in gonadal and non-gonadal tissues. Discoveries of its functions as well as the development of specific immunoassays have permitted the use of hCG in a variety of therapeutic and diagnostic applications but much remains to be resolved with regards to its extragonadal actions. Recent studies have clearly demonstrated a role of native hCG on endometrial function both in vivo and in vitro and these responses have been validated by several independent laboratories and suggest an important role of hCG in early embryo-maternal cross talk. Less well defined are the functions of the other variants of hCG and the independent validation of their potential biological function.
Contributor Information
Prajna Banerjee, Department of Medicine/Oncology, Stanford University, Stanford, CA 94305, USA.
Asgerally T. Fazleabas, Email: asgi@hc.msu.edu, Department of Obstetrics and Gynecology, and Reproductive Biology, College of Medicine, Michigan State University, Grand Rapids, MI 49503, USA
References
- 1.Muyan M, Boime I. Secretion of chorionic gonadotropin from human trophoblasts. Placenta. 1997;18(4):237–41. doi: 10.1016/s0143-4004(97)80056-2. [DOI] [PubMed] [Google Scholar]
- 2.Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132(3):1387–95. doi: 10.1210/endo.132.3.7679981. [DOI] [PubMed] [Google Scholar]
- 3.Gallego MJ, Porayette P, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Res Ther. 2010;1(4):28. doi: 10.1186/scrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao CV, Lei ZM. The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol Cell Endocrinol. 2007;269(1–2):2–8. doi: 10.1016/j.mce.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, et al. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28(2–3):175–84. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahl OP, Carlsen RB, Bellisario R, Swaminathan N. Human chorionic gonadotropin: amino acid sequence of the alpha and beta subunits. Biochem Biophys Res Commun. 1972;48(2):416–22. doi: 10.1016/s0006-291x(72)80067-6. [DOI] [PubMed] [Google Scholar]
- 8.Morgan FJ, Birken S, Canfield RE. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975;250(13):5247–58. [PubMed] [Google Scholar]
- 9.Elliott MM, Kardana A, Lustbader JW, Cole LA. Carbohydrate and peptide structure of the alpha- and beta-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7(1):15–32. doi: 10.1007/BF02778058. [DOI] [PubMed] [Google Scholar]
- 10.Valmu L, Alfthan H, Hotakainen K, Birken S, Stenman UH. Site-specific glycan analysis of human chorionic gonadotropin beta-subunit from malignancies and pregnancy by liquid chromatography–electrospray mass spectrometry. Glycobiology. 2006;16(12):1207–18. doi: 10.1093/glycob/cwl034. [DOI] [PubMed] [Google Scholar]
- 11.McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, et al. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science (New York, NY) 1989;245(4917):494–9. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- 12.Cameo P, Srisuparp S, Strakova Z, Fazleabas AT. Chorionic gonadotropin and uterine dialogue in the primate. Reprod Biol Endocrinol. 2004;2:50. doi: 10.1186/1477-7827-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment–a review. Placenta. 2004;25 (Suppl A):S26–31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, et al. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146 (9):4097–104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 15.Cameo P, Szmidt M, Strakova Z, Mavrogianis P, Sharpe-Timms KL, Fazleabas AT. Decidualization regulates the expression of the endometrial chorionic gonadotropin receptor in the primate. Biol Reprod. 2006;75(5):681–9. doi: 10.1095/biolreprod.106.051805. [DOI] [PubMed] [Google Scholar]
- 16.Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab. 1990;70(2):421–30. doi: 10.1210/jcem-70-2-421. [DOI] [PubMed] [Google Scholar]
- 17.Licht P, von Wolff M, Berkholz A, Wildt L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil Steril. 2003;79 (Suppl 1):718–23. doi: 10.1016/s0015-0282(02)04822-7. [DOI] [PubMed] [Google Scholar]
- 18.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A. 1999;96(5):2543–8. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, et al. Identification of Novel Genes Regulated by Chorionic Gonadotropin in Baboon Endometrium during the Window of Implantation. Endocrinology. 2007;148(2):618–26. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- 20.Brosens I, Derwig I, Brosens J, Fusi L, Benagiano G, Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod. 2010;25(3):569–74. doi: 10.1093/humrep/dep474. [DOI] [PubMed] [Google Scholar]
- 21.Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab. 2005;90(4):2351–6. doi: 10.1210/jc.2004-2130. [DOI] [PubMed] [Google Scholar]
- 22.Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147(9):4112–21. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- 23.Yanaihara A, Otsuka Y, Iwasaki S, Koide K, Aida T, Okai T. Comparison in gene expression of secretory human endometrium using laser microdissection. Reprod Biol Endocrinol. 2004;2:66. doi: 10.1186/1477-7827-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, et al. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94(3):946–53. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knofler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149(3):979–87. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 27.Licht P, Russu V, Lehmeyer S, Wildt L. Molecular aspects of direct LH/hCG effects on human endometrium–lessons from intrauterine microdialysis in the human female in vivo. Reprod Biol. 2001;1(1):10–9. [PubMed] [Google Scholar]
- 28.Yue ZP, Yang ZM, Wei P, Li SJ, Wang HB, Tan JH, et al. Leukemia inhibitory factor, leukemia inhibitory factor receptor, and glycoprotein 130 in rhesus monkey uterus during menstrual cycle and early pregnancy. Biol Reprod. 2000;63(2):508–12. doi: 10.1095/biolreprod63.2.508. [DOI] [PubMed] [Google Scholar]
- 29.Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, et al. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. Faseb J. 2009;23(7):2165–75. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paiva P, Hannan NJ, Hincks C, Meehan KL, Pruysers E, Dimitriadis E, et al. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod. 2011;26(5):1153–62. doi: 10.1093/humrep/der027. [DOI] [PubMed] [Google Scholar]
- 31.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier D’Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod immunol. 2010;85(1):93–8. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Karande AA, Mukhopadhyay D, Jayachandran R, Sundarraj S, Alok A. Mechanism of the immunomodulatory activity of glycodelin. Indian J Physiol Pharmacol. 2005;49(3):271–83. [PubMed] [Google Scholar]
- 33.Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23(4):401–30. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 34.Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 35.Kajihara T, Uchino S, Suzuki M, Itakura A, Brosens JJ, Ishihara O. Human chorionic gonadotropin confers resistance to oxidative stress-induced apoptosis in decidualizing human endometrial stromal cells. Fertil Steril. 2011 doi: 10.1016/j.fertnstert.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150(6):2882–8. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norris W, Nevers T, Sharma S, Kalkunte S. Review: hCG, preeclampsia and regulatory T cells. Placenta. 2011;32 (Suppl 2):S182–5. doi: 10.1016/j.placenta.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwin JR, Hastings JM, Jackson KS, Mavrogianis PA, Sharkey AM, Fazleabas AT. The endometrial response to chorionic gonadotropin is blunted in a baboon model of endometriosis. Endocrinology. 2010;151(10):4982–93. doi: 10.1210/en.2010-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85(3):564–72. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5(4):e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5(4):e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee P, Sapru K, Strakova Z, Fazleabas AT. Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epithelial cell line: implications for endometrial responses for embryo implantation. Endocrinology. 2009;150(9):4326–37. doi: 10.1210/en.2009-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol. 2010;24(9):1782–93. doi: 10.1210/me.2010-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol. 2007;193(1):53–63. doi: 10.1677/JOE-06-0201. [DOI] [PubMed] [Google Scholar]
- 45.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18 (9):1351–9. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srisuparp S, Strakova Z, Brudney A, Mukherjee S, Reierstad S, Hunzicker-Dunn M, et al. Signal transduction pathways activated by chorionic gonadotropin in the primate endometrial epithelial cells. Biol Reprod. 2003;68(2):457–64. doi: 10.1095/biolreprod.102.007625. [DOI] [PubMed] [Google Scholar]
- 47.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122–6. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 48.Sales KJ, Grant V, Catalano RD, Jabbour HN. Chorionic gonadotrophin regulates CXCR4 expression in human endometrium via E-series prostanoid receptor 2 signalling to PI3K-ERK1/2: implications for fetal-maternal crosstalk for embryo implantation. Mol Hum Reprod. 2011;17(1):22–32. doi: 10.1093/molehr/gaq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fazleabas AT, Donnelly KM, Hild-Petito S, Hausermann HM, Verhage HG. Secretory proteins of the baboon (Papio anubis) endometrium: regulation during the menstrual cycle and early pregnancy. Hum Reprod Update. 1997;3(6):553–9. doi: 10.1093/humupd/3.6.553. [DOI] [PubMed] [Google Scholar]
- 50.Fazleabas AT, Donnelly KM, Mavrogianis PA, Verhage HG. Secretory and morphological changes in the baboon (Papio anubis) uterus and placenta during early pregnancy. Biol Reprod. 1993;49(4):695–704. doi: 10.1095/biolreprod49.4.695. [DOI] [PubMed] [Google Scholar]
- 51.Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology. 1999;140(2):997–1004. doi: 10.1210/endo.140.2.6474. [DOI] [PubMed] [Google Scholar]
- 52.Cole LA. The O-linked oligosaccharide structures are striking different on pregnancy and choriocarcinoma HCG. J Clin Endocrinol Metab. 1987;65(4):811–3. doi: 10.1210/jcem-65-4-811. [DOI] [PubMed] [Google Scholar]
- 53.Kovalevskaya G, Kakuma T, Schlatterer J, O’Connor JF. Hyperglycosylated HCG expression in pregnancy: cellular origin and clinical applications. Mol Cell Endocrinol. 2007;260–262:237–43. doi: 10.1016/j.mce.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, et al. Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocrinol Metab. 2010;95 (10):E240–4. doi: 10.1210/jc.2010-0138. [DOI] [PubMed] [Google Scholar]
- 55.Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol. 2006;102(2):145–50. doi: 10.1016/j.ygyno.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 56.Hamada AL, Nakabayashi K, Sato A, Kiyoshi K, Takamatsu Y, Laoag-Fernandez JB, et al. Transfection of antisense chorionic gonadotropin beta gene into choriocarcinoma cells suppresses the cell proliferation and induces apoptosis. J Clin Endocrinol Metab. 2005;90(8):4873–9. doi: 10.1210/jc.2004-2458. [DOI] [PubMed] [Google Scholar]
- 57.Tarrade A, Lai Kuen R, Malassine A, Tricottet V, Blain P, Vidaud M, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Laboratory investigation. J Tech Methods Pathol. 2001;81(9):1199–211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 58.Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56. doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V, Persico MG, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56(2):489–94. doi: 10.1095/biolreprod56.2.489. [DOI] [PubMed] [Google Scholar]
- 60.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81(8):3112–8. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 61.Cole LA, Khanlian SA, Kohorn EI. Evolution of the human brain, chorionic gonadotropin and hemochorial implantation of the placenta: insights into origins of pregnancy failures, preeclampsia and choriocarcinoma. J Reprod Med. 2008;53 (8):549–57. [PubMed] [Google Scholar]
- 62.Acevedo HF, Tong JY, Hartsock RJ. Human chorionic gonadotropin-beta subunit gene expression in cultured human fetal and cancer cells of different types and origins. Cancer. 1995;76(8):1467–75. doi: 10.1002/1097-0142(19951015)76:8<1467::aid-cncr2820760826>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 63.Acevedo HF, Hartsock RJ. Metastatic phenotype correlates with high expression of membrane-associated complete beta-human chorionic gonadotropin in vivo. Cancer. 1996;78(11):2388–99. doi: 10.1002/(sici)1097-0142(19961201)78:11<2388::aid-cncr18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.Acevedo HF, Hartsock RJ, Maroon JC. Detection of membrane-associated human chorionic gonadotropin and its subunits on human cultured cancer cells of the nervous system. Cancer Detect Prev. 1997;21(4):295–303. [PubMed] [Google Scholar]
- 65.Li D, Wen X, Ghali L, Al-Shalabi FM, Docherty SM, Purkis P, et al. hCG beta expression by cervical squamous carcinoma–in vivo histological association with tumour invasion and apoptosis. Histopathology. 2008;53(2):147–55. doi: 10.1111/j.1365-2559.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- 66.Iles RK, Lee CL, Oliver RT, Chard T. Composition of intact hormone and free subunits in the human chorionic gonadotrophin-like material found in serum and urine of patients with carcinoma of the bladder. Clin Endocrinol (Oxf) 1990;33 (3):355–64. doi: 10.1111/j.1365-2265.1990.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 67.Iles RK, Purkis PE, Whitehead PC, Oliver RT, Leigh I, Chard T. Expression of beta human chorionic gonadotrophin by non-trophoblastic non-endocrine ‘normal’ and malignant epithelial cells. Br J Cancer. 1990;61(5):663–6. doi: 10.1038/bjc.1990.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000;82 (9):1553–6. doi: 10.1054/bjoc.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cosgrove DE, Campain JA, Cox GS. Chorionic gonadotropin synthesis by human tumor cell lines: examination of subunit accumulation, steady-state levels of mRNA, and gene structure. Biochim Biophys Acta. 1989;1007(1):44–54. doi: 10.1016/0167-4781(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 70.Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007;260–262:264–70. doi: 10.1016/j.mce.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Cole LA, Khanlian SA, Muller CY, Giddings A, Kohorn E, Berkowitz R. Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free beta-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol Oncol. 2006;102 (2):160–4. doi: 10.1016/j.ygyno.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 72.Cole LA, Tanaka A, Kim GS, Park SY, Koh MW, Schwartz PE, et al. Beta-core fragment (beta-core/UGF/UGP), a tumor marker: a 7-year report. Gynecol Oncol. 1996;60(2):264–70. doi: 10.1006/gyno.1996.0036. [DOI] [PubMed] [Google Scholar]
- 73.Moutzouris G, Yannopoulos D, Barbatis C, Zaharof A, Theodorou C. Is beta-human chorionic gonadotrophin production by transitional cell carcinoma of the bladder a marker of aggressive disease and resistance to radiotherapy? Br J Urol. 1993;72(6):907–9. doi: 10.1111/j.1464-410x.1993.tb16294.x. [DOI] [PubMed] [Google Scholar]
- 74.Delves PJ, Iles RK, Roitt IM, Lund T. Designing a new generation of anti-hCG vaccines for cancer therapy. Mol Cell Endocrinol. 2007;260–262:276–81. doi: 10.1016/j.mce.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 75.Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colo-rectal cancer: antibody response is associated with improved survival. Clin Cancer Res. 2002;8(7):2044–51. [PubMed] [Google Scholar]
- 76.Arends J. Radioimmunoassay of urinary human chorionic gonadotrophin. Acta Endocrinol (Copenh) 1971;66(4):611–26. doi: 10.1530/acta.0.0660611. [DOI] [PubMed] [Google Scholar]
- 77.Cole LA. Human chorionic gonadotropin and associated molecules. Expert Rev Mol Diagn. 2009;9(1):51–73. doi: 10.1586/14737159.9.1.51. [DOI] [PubMed] [Google Scholar]
- 78.Cole LA, Khanlian SA, Sutton JM, Davies S, Stephens ND. Hyperglycosylated hCG (invasive trophoblast antigen, ITA) a key antigen for early pregnancy detection. Clin Biochem. 2003;36(8):647–55. doi: 10.1016/s0009-9120(03)00108-5. [DOI] [PubMed] [Google Scholar]
- 79.Butler SA, Khanlian SA, Cole LA. Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices. Clin Chem. 2001;47(12):2131–6. [PubMed] [Google Scholar]
- 80.Bjercke S, Tanbo T, Dale PO, Morkrid L, Abyholm T. Human chorionic gonadotrophin concentrations in early pregnancy after in-vitro fertilization. Hum Reprod. 1999;14(6):1642–6. doi: 10.1093/humrep/14.6.1642. [DOI] [PubMed] [Google Scholar]
- 81.Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. 2008;89(6):1781–6. doi: 10.1016/j.fertnstert.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Kovalevskaya G, Birken S, Kakuma T, Ozaki N, Sauer M, Lindheim S, et al. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: preliminary characterization of the hyperglycosylated hCG epitope. J Endocrinol. 2002;172 (3):497–506. doi: 10.1677/joe.0.1720497. [DOI] [PubMed] [Google Scholar]
- 83.Birken S, Kovalevskaya G, O’Connor J. Immunochemical measurement of early pregnancy isoforms of HCG: potential applications to fertility research, prenatal diagnosis, and cancer. Arch Med Res. 2001;32(6):635–43. doi: 10.1016/s0188-4409(01)00329-0. [DOI] [PubMed] [Google Scholar]
- 84.O’Connor JF, Ellish N, Kakuma T, Schlatterer J, Kovalevskaya G. Differential urinary gonadotrophin profiles in early pregnancy and early pregnancy loss. Prenat Diagn. 1998;18(12):1232–40. doi: 10.1002/(sici)1097-0223(199812)18:12<1232::aid-pd439>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 85.Carp HJ. Recurrent miscarriage and hCG supplementation: a review and metaanalysis. Gynecol Endocrinol. 2010;26 (10):712–6. doi: 10.3109/09513590.2010.488779. [DOI] [PubMed] [Google Scholar]
- 86.Ticconi C, Piccione E, Belmonte A, Rao ChV. HCG–A new kid on the block in prematurity prevention. J Matern Fetal Neonatal Med. 2006;19(11):687–92. doi: 10.1080/14767050600921315. [DOI] [PubMed] [Google Scholar]
- 87.Kurtzman JT, Spinnato JA, Goldsmith LJ, Zimmerman MJ, Klem M, Lei ZM, et al. Human chorionic gonadotropin exhibits potent inhibition of preterm delivery in a small animal model. Am J Obstet Gynecol. 1999;181(4):853–7. doi: 10.1016/s0002-9378(99)70313-3. [DOI] [PubMed] [Google Scholar]
- 88.Angioni S, Spedicato M, Rizzo A, Cosola C, Mutinati M, Minoia G, et al. In vitro activity of human chorionic gonadotropin (hCG) on myometrium contractility. Gynecol Endocrinol. 2011 doi: 10.3109/09513590.2010.488780. [DOI] [PubMed] [Google Scholar]
- 89.Scott JR, Pattison N. Human chorionic gonadotrophin for recurrent miscarriage. Cochrane Database Syst Rev. 2000;(2):CD000101. doi: 10.1002/14651858.CD000101. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi NS. Treatment options for threatened miscarriage. Maturitas. 2009;65 (Suppl 1):S35–41. doi: 10.1016/j.maturitas.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Devaseelan P, Fogarty PP, Regan L. Human chorionic gonadotrophin for threatened miscarriage. Cochrane Database Syst Rev. 2010;(5):CD007422. doi: 10.1002/14651858.CD007422.pub2. [DOI] [PubMed] [Google Scholar]
- 92.Muller CY, Cole LA. The quagmire of hCG and hCG testing in gynecologic oncology. Gynecol Oncol. 2009;112(3):663–72. doi: 10.1016/j.ygyno.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Cole LA. hCG and hyperglycosylated hCG in the establishment and evolution of hemochorial placentation. J Reprod Immunol. 2009;82(2):112–8. doi: 10.1016/j.jri.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Cole LA, Butler SA, Khanlian SA, Giddings A, Muller CY, Seckl MJ, et al. Gestational trophoblastic diseases: 2. Hyperglycosylated hCG as a reliable marker of active neoplasia. Gynecol Oncol. 2006;102(2):151–9. doi: 10.1016/j.ygyno.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 95.Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–29. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 96.Cole LA, Shahabi S, Oz UA, Bahado-Singh RO, Mahoney MJ. Hyperglycosylated human chorionic gonadotropin (invasive trophoblast antigen) immunoassay: a new basis for gestational Down syndrome screening. Clin Chem. 1999;45(12):2109–19. [PubMed] [Google Scholar]
- 97.Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenat Diagn. 1987;7(9):623–30. doi: 10.1002/pd.1970070904. [DOI] [PubMed] [Google Scholar]
- 98.Jauniaux E, Bao S, Eblen A, Li X, Lei ZM, Meuris S, et al. HCG concentration and receptor gene expression in placental tissue from trisomy 18 and 21. Mol Hum Reprod. 2000;6(1):5–10. doi: 10.1093/molehr/6.1.5. [DOI] [PubMed] [Google Scholar]
- 99.Reynolds T. The triple test as a screening technique for Down syndrome: reliability and relevance. Int J Womens Health. 2010;2:83–8. doi: 10.2147/ijwh.s8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frendo JL, Vidaud M, Guibourdenche J, Luton D, Muller F, Bellet D, et al. Defect of villous cytotrophoblast differentiation into syncytiotrophoblast in Down’s syndrome. J Clin Endocrinol Metab. 2000;85(10):3700–7. doi: 10.1210/jcem.85.10.6915. [DOI] [PubMed] [Google Scholar]
- 101.Massin N, Frendo JL, Guibourdenche J, Luton D, Giovangrandi Y, Muller F, et al. Defect of syncytiotrophoblast formation and human chorionic gonadotropin expression in Down’s syndrome. Placenta. 2001;22 (Suppl A):S93–7. doi: 10.1053/plac.2001.0658. [DOI] [PubMed] [Google Scholar]
- 102.Palomaki GE, Knight GJ, Lambert-Messerlian G, Canick JA, Haddow JE. Four years’ experience with an interlaboratory comparison program involving first-trimester markers of Down syndrome. Arch Pathol Lab Med. 2010;134(11):1685–91. doi: 10.5858/2009-0670-OAR.1. [DOI] [PubMed] [Google Scholar]