Abstract
Methamphetamine (meth) is a potent psychostimulant known to cause neurotoxicity. Clinical reports suggest meth abuse is a risk factor for Parkinson’s disease. We investigated changes in the blood brain barrier and cerebral vasculature as a mechanism underlying this risk in rats treated acutely and trained to self-administer meth. We observed blood brain barrier leakage in rats treated acutely with meth. Hypoperfusion in the striatum was detected with acute and chronic meth treatment and associated with hypoxia. This was correlated with reductions in striatal tyrosine hydroxylase in rats trained to self-administer meth. These findings suggest a new mechanism of meth-induced neurotoxicity involving striatal vasoconstriction resulting in hypoxia and dopamine reductions leading to an increased risk for Parkinson’s disease for meth abusers.
Keywords: methamphetamine, blood brain barrier, cerebral vasculature, hypoxia inducible factor 1 alpha, Parkinson’s disease
Introduction
Methamphetamine (meth) and its active metabolite amphetamine, are indirect agonists causing excessive release of dopamine (DA) and other neurotransmitters in the striatum and other brain regions [1]. With an estimated 26 million abusers globally (U.S. DEA 2006 International Drug Enforcement Conference), the use of this potent psychostimulant is made more alarming by recent studies indicating an increased risk of developing Parkinson’s disease in patients with a history of meth abuse [2]. The nigrostriatal DA pathway is particularly vulnerable to the neurotoxic effects of meth [3] and post-mortem studies revealed that chronic meth abusers of unknown abuse intensity displayed reduced striatal DA function [4]. Chronic meth abusers detoxified for at least 11 months also displayed motor deficits [5] similar to patients with Parkinson’s disease. High dose meth treatment has also been shown to produce Parkinson’s disease-like lesions in numerous animal models [6]. Thus, preclinical studies complement the aforementioned clinical reports and show reductions in DA function in the striatum of rats following chronic meth administration [3,7], similar to the neuropathology displayed in early Parkinson’s disease [8]. Unfortunately, the mechanism(s) underlying this increased risk are unknown. It has been hypothesized that meth-induced DA efflux promotes the production of DA quinones and/or reactive oxygen species that facilitate neurotoxicity and neuroinflammation riddle [9–11]. Additionally, meth-neurotoxicity may stem from blood brain barrier dysfunction or hyperthermia [1,12].
The present study indicates a novel mechanism for meth-induced DA neuron loss and is the first to propose meth-induced hypoxia in the striatum as a potential mechanism of neurotoxicity.
Material and Methods
Animals
Male Sprague–Dawley rats (n=43, Harlan Laboratories, Indianapolis, IN) weighing 225–250 grams upon arrival were housed in pairs, handled daily, and acclimated to environmentally controlled conditions for one week prior to experimentation. They had access to food and water ad libitum throughout the study. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington DC) with protocols approved by the Rush University Institutional Animal Care and Use Committee.
Acute Meth Administration
(+) methamphetamine HCl (Sigma, St. Louis, MO) was dissolved in saline and all doses reflect the base. Rats were administered an intraperitoneal injection of 3 or 9mg/kg meth (n=12 per treatment group), or 0.9% saline (n=10). Doses were selected based on prior studies indicating that 2.5mg/kg meth induced brain and behavioral activity without overt signs of toxicity [13] while 9mg/kg altered brain and behavioral function and induced neurotoxicity [14]. Two or 24hr after meth treatment, rats were anesthetized and perfused with fluorescein isothiocyanate labeled albumin (FITC-LA) to assess acute effects of meth on blood brain barrier integrity and vascularity.
Chronic Meth Self-Administration
Rats were instrumented with jugular vein catheters as previously described [15] and trained to self-administer meth for 27 days with assessments testing meth-seeking behavior every two days between days 15–27 (n=5). Rats self-administered meth on a fixed ratio 1 schedule of reinforcement on days 1–7 and fixed ratio 5 schedule on all subsequent self-administration sessions lasting 3hrs/day. Meth was administered at 0.1mg/kg/0.1ml infusion. On average, the rats self-administered 2–3mg/kg meth over 3hr. Between days 28–37, rats were withdrawn from methamphetamine and remained in their home cages. The rats were administered an acute 1mg/kg non-contingent injection of meth on days 34 and 35 for motor assessments; rats were given no further meth after day 35 prior to perfusion.
Fluorescein isothiocyanate labeled albumin (FITC-LA) Intracardial Perfusions
At the designated kill times, rats were anesthetized with 400mg/mL/kg chloral hydrate and perfused with FITC-LA (MW=68–70 KDa, Sigma, St. Louis, MO) to assess vascular leakage and perfusion [16].
Immunohistochemistry
Forty µm sections were labeled overnight with antibodies for the marker for DAergic neurons, (tyrosine hydroxylase-immunoreactive (TH-ir; mouse vs. rat TH [1:10,000]; Immunostar, Hudson, WI)) and for the marker of hypoxic injury (hypoxia inducible factor 1α (HIF1α; rabbit vs. rat HIF1α [1:100]; Millipore, Temecula, CA)) followed by incubation with species appropriate secondary antibodies (1:200; Vector Labs, Burlingame, CA). TH-ir and HIF1α were visualized using a peroxidase-conjugated avidin–biotin complex and 3, 3-diaminobenzidine.
Optical Density Measurements
Optical density was assessed using ImageJ (NIH). Photomicrographs of FITC-LA taken at 10X magnification of the prefrontal cortex, striatum, and nucleus accumbens shell were selected based on anatomical landmarks. A 200 µm2 box was traced around each region to measure optical density. Selected optical densities were measured in the prefrontal cortex and nucleus accumbens shell. An observer blinded to treatment identified 2 photomicrographs from these regions appearing to have the most leakage for each rat treated with 3 or 9mg/kg meth. Optical densities were measured for the selected sections as well as the serial sections immediately rostral and caudal and then averaged. Optical density averages were compared to yoked sections from rats treated with saline or trained to self-administer meth. For TH-ir, striatal optical density was measured in photomicrographs taken at 2.5X magnification. A 16 mm2 field from the center of the striatum was measured. Additionally, optical density of two 1 mm2 fields, from the anterior commissure and lateral septum in the same section were used to determine background staining to normalize for procedural variability. Averaged background was then subtracted from the averaged value of the striatal field [17].
Stereological Assessments
An Olympus BX60 microscope with a computer-controlled motorized stage, high sensitivity HV-C20 CCD video camera (Hitachi, Japan) and StereoInvestigator software version 5.1 (MicroBrightField, Colchester, VT) was used to estimate TH-ir cell counts and volume within the substantia nigra pars compacta in equidistant serial sections. The number of TH-ir positive cells was counted within the thickness of the tissue section under an Olympus 100× objective using the unbiased three-dimensional counting. Six sections containing the substantia nigra were quantified per animal and approximately 20% of the region was quantified using a 200 × 200 µm counting frame. The total number of TH-ir positive cells and the volume of tissue evaluated were divided to obtain a density measurement expressed as total number/mm3.
Statistics
Optical density data is presented as mean ±SEM. A one-way analysis of variance (ANOVA) was used to analyze the differences in optical density measurements in the prefrontal cortex, striatum, and nucleus accumbens shell of rats treated with saline, 3 mg/kg meth, 9 mg/kg meth, and rats trained to self-administer meth. When appropriate, a Newman–Keuls post hoc analysis was used. For all analyses, α = 0.05. Data were analyzed using SPSS Statistics (IBM; Somers, NY).
Results
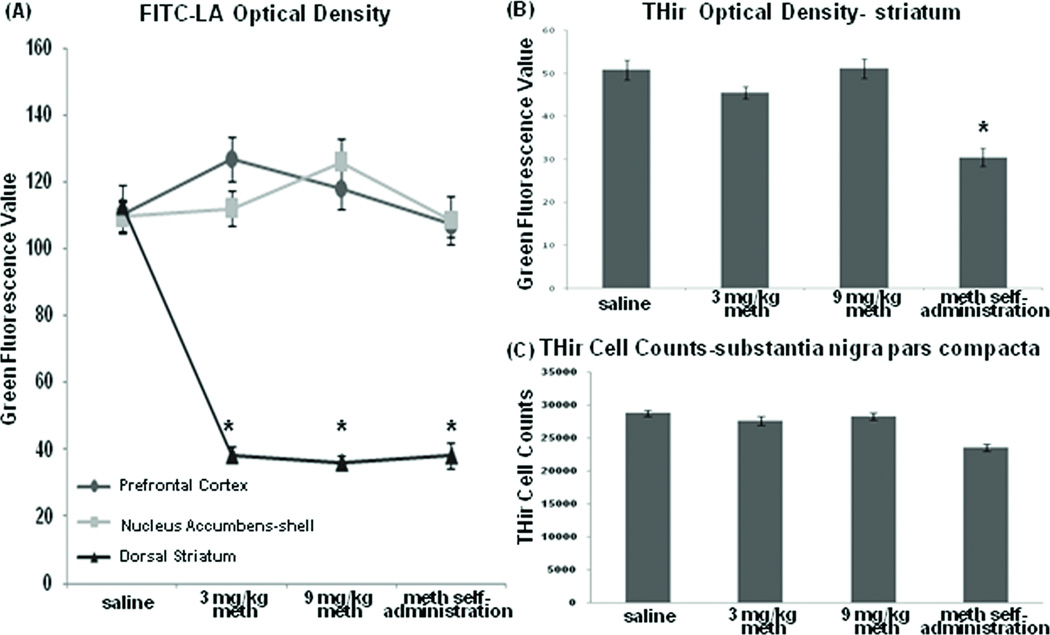
FITC-LA imaging (Fig. 1A) and fluorescence intensity measurements (Fig.2A) indicated that FITC-LA leakage did not occur throughout all brain regions, but appeared as punctate areas of FITC-LA leakage in the prefrontal cortex and nucleus accumbens shell of rats acutely treated with meth and perfused at 2 and 24hr post-meth; no leakage was observed in rats trained to self-administer meth (Fig. 1A). FITC-LA leakage was also observed within the circumventricular areas of the hypothalamus where the blood brain barrier is absent (data not shown; [18]).
Figure 1.
Effects of meth on fluorescein isothiocyanate-labeled albumin (FITC-LA), hypoxia inducible factor 1 alpha (HIF1α), and tyrosine hydroxylase- immunoreactive (TH-ir) immunostaining in the nucleus accumbens shell and striatum of rats. (A) Blood brain barrier disruption 24hr following acute 3 and 9mg/kg meth shown by FITC-LA in the nucleus accumbens shell. Ten days following meth self-administration, no leakage was observed (scale bar 250µm). (B) Hypoperfusion in the dorsal striatum shown by FITC-LA in rats treated with acute 3 or 9mg/kg meth and 10 days after self-administration (scale bar 250µm). (C) HIF1α immunostaining in the dorsal striatum shows increased staining in rats treated with acute 3 and 9mg/kg meth and 10 days after self-administration when compared with saline controls (scale bar 250µm). (D) TH-ir immunostaining in the striatum shows reductions in TH-ir immunostaining only in rats sacrificed 10 days after meth self-administration (scale bar 500µm). All photomicrographs were taken at the level where the corpus callosum hits the midline. Nucleus accumbens shell (NAc-s); Striatum (Str); dorsal striatum (dStr).
Figure 2.
Quantification of fluorescein isothiocyanate -labeled albumin (FITC-LA) and tyrosine hydroxylase-immunoreactive (TH-ir) immunostaining. (A) Group means ± SEM of FITC-LA optical density measurements in the prefrontal cortex, nucleus accumbens-shell, and dorsal striatum. While there is no significant difference in the means between treatment groups in the prefrontal cortex or nucleus accumbens shell, but there are significant reductions in FITC-LA within the striatum of all meth-treated rats when compared with saline controls. (B) Group means ± SEM of TH-ir optical density measurements in the striatum show significant reductions in TH-ir in rats sacrificed 10 days after the discontinuation of meth self-administration. (C) Group means ± SEM of stereological cell counts of TH-ir positive cells in the substantia nigra pars compacta. * p < 0.05 compared with saline.
While FITC-LA produced a normal vascular pattern in the dorsal striatum and all neighboring areas (e.g., parietal cortex) in saline-treated rats, FITC-LA was overtly reduced in the dorsal striatum in rats acutely treated with meth and perfused at both 2 and 24hr post-treatment as well as in rats trained to self-administer meth (Fig. 1B). The absence of FITC-LA filled large vessels in the dorsal striatum was distinct from that seen in adjacent structures including the parietal cortex and nucleus accumbens where FITC-LA appeared to fill the vasculature normally. Quantification of fluorescence intensity in the dorsal striatum revealed a significant decrease in FITC-LA within the dorsal striatum of meth-treated animals compared with saline controls (Figure 2A; F (3,106) =221.119; p< 0.05).
The reduction in FITC-LA in the dorsal striatum seen in rats 10 days after the meth self-administration suggested that meth-induced hypoperfusion was long-lived and may lead to ischemic hypoxia. Consequently, hypoxia was assessed using HIF1α, an oxygen-dependent transcription factor that is upregulated as early as 4hr after hypoxic injury [19]. An apparent increase in HIF1α immunostaining was observed in the dorsal striatum of rats treated acutely with meth and perfused 24hr, but not 2hr, after meth injection as well as in rats that self-administered meth (Fig. 1C). HIF1α immunostaining appeared normal in neighboring brain regions including the cortex and was similar to the low level of staining observed in saline controls.
Quantification of the density of TH-ir, a standard marker for DA neurons and their process terminals, revealed a significant decrease in striatal TH-ir in rats trained to self-administer meth (Fig. 2B; F(3,32) =14.777; p<0.05). Stereological assessment of TH-ir positive cell bodies in the substantia nigra pars compacta, the source of striatal DA terminals, resulted in an 18% decrease in cell counts in rats withdrawn from meth self-administration compared with controls (Fig. 2C; F(3,30) =10.964). No differences in TH-ir cell counts were observed in rats acutely administered meth compared to saline controls.
Discussion
The present study revealed effects of meth on the blood brain barrier, cerebral vasculature, hypoxia and the DA system. FITC-LA leakage, a measure of blood brain barrier dysfunction, was observed in the prefrontal cortex and nucleus accumbens shell of rats treated acutely with 3 and 9mg/kg meth and perfused at 2 and 24hr post-meth similar to the findings of Kiyatkin and colleagues [12]. Rats trained to self-administer meth and sacrificed 10 days later did not exhibit blood brain barrier leakage. The varying effects of acute and chronic meth treatment may be a consequence of the higher acute doses compared with the 2–3 mg/kg of meth self-administered over three hours or withdrawal from self-administration for 10 days and subsequent repair of the damaged blood brain barrier.
While assessing blood brain barrier dysfunction using FITC-LA, we were surprised to discover a significant reduction in perfusion of the dorsal striatum following both acute and chronic meth administration. We repeated the acute treatment studies multiple times while monitoring cardiac perfusion pressure to ensure that it was similar to systemic blood pressure (monitored in the tail) and consistently observed the same hypoperfusion suggesting that the outcome was not technique-based. We then observed the same results in rats trained to self-administer meth and perfused 10 days after the last self-administration session suggesting the hypoperfusion was long-lived. While the cortex and dorsal striatum are both supplied by the middle cerebral artery, only the striatal region exhibited severe vasoconstriction with meth administration resulting in an upregulation of HIF1α in the striatum and not the cortex. This suggests that meth may interact with brain parenchyma to alter regional vascularity in the striatum via the neurovascular unit [20]. Thus, the striatal neurovascular unit may release vasoactive factors not produced in other brain regions resulting in local vasoconstriction due to meth exposure.
A possible mechanistic link between meth-induced hypoxia and loss of DA terminals in the striatum involves the upregulation of HIF1α observed in all meth-treated rats. HIF1α is upregulated in the striatum at 24hr following acute meth treatment and may potentiate persistent hypoperfusion in the striatum through its interactions with hypoxia response elements of genes for vasoregulatory factors, including endothelin-1, a potent vasoconstrictor [21]. Increased endothelin-1 release from the cerebral vasculature into the striatum may result in further vasoconstriction and persistent hypoperfusion, as observed in rats sacrificed 10 days following the discontinuation of meth self-administration. Interestingly, studies show that increased endothelin-1 activity at striatal endothelin-1 type B receptors located on DA terminals during hypoxia results in impaired DA release and potential DA terminal toxicity [22,23]. Furthermore, endothelin-1 is regulated by DA [23] and excess DA released by the action of meth within the striatum may increase striatal endothelin-1 levels leading to a shift in the vasoregulatory balance towards vasoconstriction and increased endothelin-1 activity at striatal endothelin-1 type B receptors on DA terminals that can lead to DA terminal damage.
The persistent hypoperfusion profile seen in the striatum of rats trained to self-administer meth concurs with clinical reports that meth abusers free from drug for 8–24 months similarly show decreased regional cerebral blood flow in the putamen, cerebral cortex, and right anterior cingulated cortex [24,25]. Although Chung and colleagues observed a similar effect in the striatum of patients [25], we did not observe reduced perfusion in the cortex or cingulated cortex. This may reflect differences in perfusion across species, differences between assessing cerebral blood flow formally and our simple assessment of perfusion only, or species differences in the effects of meth in the cortex.
It is possible then that persistent hypoperfusion leads to hypoxia which is supported by our findings that increased HIF1α immunostaining was observed only in the dorsal striatum of meth-treated rats and not in saline controls or in the neighboring regions of the meth-treated rats displaying normal vasculature with FITC-LA. Meth-induced hypoxia in the dorsal striatum would render this structure vulnerable to further neuronal damage. Accordingly, we observed significant reductions in striatal TH-ir terminal density in rats with a history of meth self-administration. Thus, meth exposure resulting in reductions of TH-ir is an indication of potential DA terminals and neuron damage within the nigrostriatal pathway, and could thus increase the risk for Parkinson’s disease development.
Conclusion
We provide evidence that meth exposure leads to striatal hypoperfusion and increased production of HIF1α, that the hypoperfusion and hypoxia produced by chronic self-administration lasts at least 10 days, and these changes are associated with reductions in TH-ir-positive terminals in the striatum. These data provide new insights into meth-induced neurotoxicity and provide a potential mechanism underlying meth abuse as a risk factor for Parkinson’s disease development later in life.
Acknowledgments
This research was supported by the following grants from the National Institutes of Health: NS052414, DA15760, and DA024923, and support from the Kenneth Douglas Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Document Classification:
Cellular, molecular and developmental neuroscience: Neuropharmacology and neurotoxicology
References
- 1.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010 doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- 3.Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102 Suppl 1:44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve markers in human, chronic methamphetamine users. Nature Medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Thrash B, Karuppagounder SS, Uthayathas S, Suppiramaniam V, Dhanasekaran M. Neurotoxic effects of methamphetamine. Neurochem Res. 2010;35:171–179. doi: 10.1007/s11064-009-0042-5. [DOI] [PubMed] [Google Scholar]
- 7.Prudencio C, Abrantes B, Lopes I, Tavares MA. Structural and functional cellular alterations underlying the toxicity of methamphetamine in rat retina and prefrontal cortex. Ann N Y Acad Sci. 2002;965:522–528. doi: 10.1111/j.1749-6632.2002.tb04193.x. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 9.Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimm Pharm. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci. 2007;26:1242–1253. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- 13.McDaid J, Graham MP, Napier TC. Methamphetamine-induced sensitization differentially alters pCREB and DFosB throughtout the limbic circuit of the mammalian brain. Molecular Pharmacology. 2006;70:2064–2074. doi: 10.1124/mol.106.023051. [DOI] [PubMed] [Google Scholar]
- 14.Sharma HS, Kiyatkin EA. Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy. J Chem Neuroanat. 2009;37:18–32. doi: 10.1016/j.jchemneu.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biol Psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, et al. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakoori K, Murphy NP. Reduced degeneration of dopaminergic terminals and accentuated astrocyte activation by high dose methamphetamine administration in nociceptin receptor knock out mice. Neurosci Lett. 2010;469:309–313. doi: 10.1016/j.neulet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111:291–314. doi: 10.1111/j.1471-4159.2009.06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Wu J, Keep RF, Hua Y, Hoff JT, Xi G. Hypoxia-inducible factor-1alpha accumulation in the brain after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22:689–696. doi: 10.1097/00004647-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 21.Belaidi E, Joyeux-Faure M, Ribuot C, Launois SH, Levy P, Godin-Ribuot D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol. 2009;53:1309–1317. doi: 10.1016/j.jacc.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Van den Buuse M, Webber KM. Endothelin and dopamine release. Prog Neurobiol. 2000;60:385–405. doi: 10.1016/s0301-0082(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 23.Kohzuma M, Kataoka Y, Koizumi S, Shibaguchi H, Nakashima MN, Yamashita K, et al. ETB receptor involvement in stimulatory and neurotoxic action of endothelin on dopamine neurones. Neuroreport. 1994;5:2653–2656. doi: 10.1097/00001756-199412000-00062. [DOI] [PubMed] [Google Scholar]
- 24.Iyo M, Sekine Y, Mori N. Neuromechanism of developing methamphetamine psychosis: a neuroimaging study. Ann N Y Acad Sci. 2004;1025:288–295. doi: 10.1196/annals.1316.036. [DOI] [PubMed] [Google Scholar]
- 25.Chung YA, Peterson BS, Yoon SJ, Cho SN, Chai S, Jeong J, et al. In vivo evidence for long-term CNS toxicity, associated with chronic binge use of methamphetamine. Drug Alcohol Depend. 2010;111:155–160. doi: 10.1016/j.drugalcdep.2010.04.005. [DOI] [PubMed] [Google Scholar]