Summary
Polypeptide growth factors bind to the extracellular domains of cell surface receptors, triggering activation of receptor-intrinsic or receptor-associated protein kinases. Although this central thesis is widely accepted, one family of proteins, the fibroblast growth factors (FGFs), have for more than a decade attracted a research “counter-culture” looking for direct FGF actions inside cells. Goldfarb discusses how the search for alternative signaling pathways is moving mainstream with the help of two recent publications reporting specific intracellular targets for FGF and FGF-like proteins.
Following the original purification and cloning of acidic FGF (FGF-1) and basic FGF (FGF-2) more than 15 years ago, additional vertebrate fibroblast growth factors (FGFs) encoded by 22 genes have been identified on the basis of their 25 to 55% sequence homology to the prototypic FGFs within a core domain of 120 to 130 residues (1, 2). Most of these proteins are known to bind the ectodomains of FGF receptors, promoting receptor dimerization and receptor tyrosine kinase activation (3), which in turn induces and sustains the activation of the SHP2/RAS/ERK signaling pathway, as well as other pathways (Fig. 1). Genetic studies in mice, frogs, flies, and worms along with studies in cultured fibroblasts, myoblasts, and neurons have demonstrated that receptor-mediated induction of the SHP2/RAS/ERK pathway is a central, evolutionarily conserved mechanism by which FGFs elicit a broad spectrum of biological activities, including cell growth, differentiation, and morphogenesis (4–15).
Fig. 1.
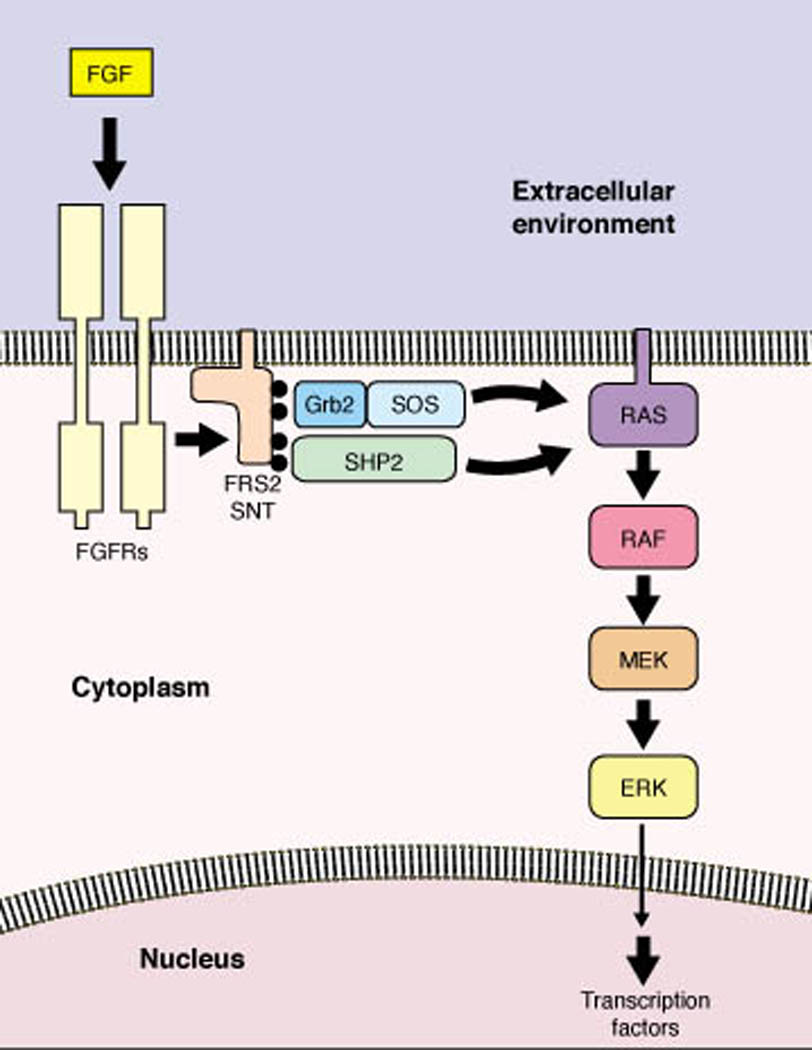
FGF receptors signal through the evolutionarily conserved SHP2/RAS/ERK pathway to mediate biological responses. FGF receptor activation induces FRS2(SNT) tyrosine phosphorylation (black circles), which in turn induces recruitment of Grb2/SOS and SHP2. These initial events promote the sustained activation of RAS and the ERK kinase cascade leading to changes in gene transcription (4–7, 10–15, 56). Drosophila does not have FRS2(SNT) proteins, but may use a different protein, DOF, as the transducer from receptor to RAS/ERK activation (8, 9). Activation of phosphatidylinositol-3´ kinase, STAT-1, and Src tyrosine kinase by FGF receptors also contribute to certain FGF-induced biological responses (57–60).
Two findings have driven some investigators to consider additional mechanisms of FGF action. First, FGF ligand/receptor complexes are internalized, leading to the accumulation of receptor, ligand, or both in intracellular compartments, including perinuclear vesicles and the nucleus proper (16–20). It has been suggested that receptors deliver FGFs to internal sites, where they can interact with other targets to trigger biological responses (17, 21). Although several lines of indirect evidence have been put forth to support this model, no postuptake FGF target proteins have been identified to date. It has also been suggested that FGF receptors translocate to the nucleus to promote FGF-mediated biological responses (22, 23). Evidence for this model is also indirect and not yet substantiated by identification of nuclear protein targets for receptors.
Second, some FGFs have poor or no classical secretion signal motifs and reside largely in the cytoplasm or nucleus of the producing cell. These include FGF-1, FGF-2, higher molecular weight (HMW) variants of FGF-2 and FGF-3, and the four fibroblast growth factor homologous factors (FHFs), which were also designated as FGF-11 through FGF-14 (24–29). In somes cases, intracellular localization of FGFs may provide stores of ligand to be released for extracellular activity by cell death or by nonclassical secretory mechanisms (30). But in the case of HMW FGF-3 and the FHFs, novel intracellular protein targets have been identified which may account for specialized biochemical and biological functions for these factors (31, 32).
Murine Fgf3 messenger RNAs are translated into two protein isoforms through initiation at an AUG methionine codon or an upstream, in-frame, CUG leucine codon (26). The CUG codon is, in fact, the preferential site of translation initiation on Fgf3 mRNA transcribed from one of the gene’s two promoters. The methionine-initiated isoform has a NH2-terminal hydrophobic leader which directs the protein into the secretory pathway, whereas the leucine-initiated HMW isoform is partitioned into two pathways: it can employ either the aforementioned, recessed hydrophobic signal for secretion or, alternatively, utilize multiple nuclear localization sequences to traffick to the nucleus (33) (Fig. 2). Much of the nuclear FGF-3 is concentrated in nucleoli, and this localization is governed by motifs in the central and COOH-terminal portions of the protein (34). Whereas transfection-mediated expression of secreted FGF-3 in fibroblasts or epithelial cells promotes DNA synthesis and cell proliferation through activation of FGF receptors, engineered exclusive expression of the nucleolar HMW FGF-3 strongly inhibits the G1/S transition and proliferation (34). Human Fgf3 mRNA bears CUG codons in-frame and upstream of the classical initiator AUG, and the nuclear and nucleolar localization motifs are present, suggesting that the complex biosynthesis and trafficking of FGF-3 is evolutionarily conserved.
Fig. 2.
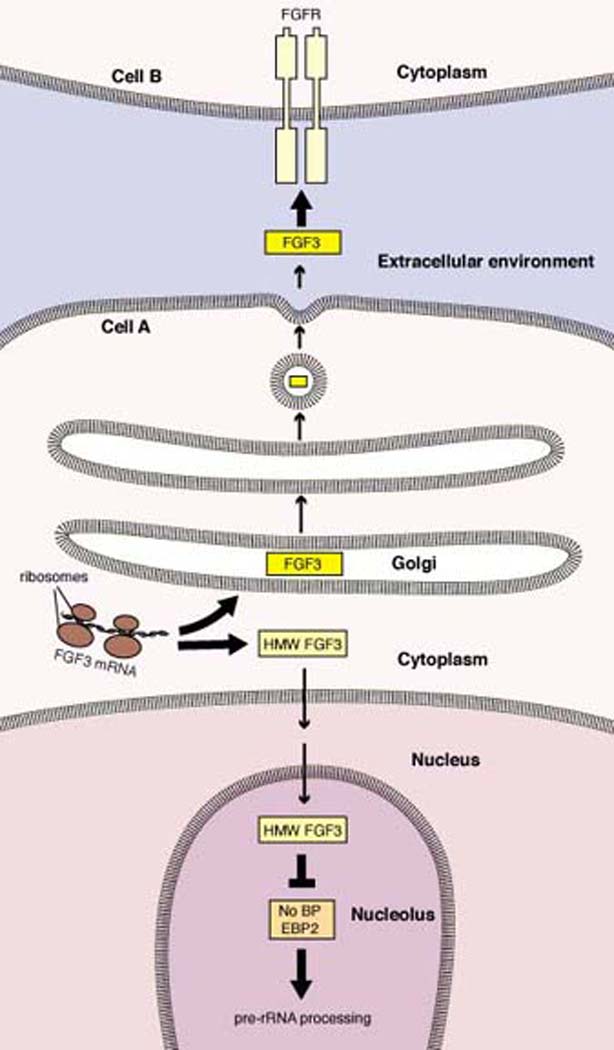
Model for intracellular HMW FGF-3 function. The competing actions of multiple intrinsic trafficking motifs direct HMW FGF-3 either into the secretory pathway or to the nucleus (26, 33). Nuclear HMW FGF-3 probably achieves its final nucleolar localization through interaction with NoBP, and FGF-3 may act as an inhibitor of NoBP proliferative activity (31). While the biochemical function of NoBP is uncertain, the homologous protein in yeast promotes maturation of ribosomal RNA precursors (37).
Reimers et al. recently reported the identification of a 39 kD nucleolar protein, NoBP (Nucleolar Binding Protein), as an FGF-3-interacting protein (31). Human NoBP, isolated in a yeast two-hybrid screen using FGF-3 as the bait, is identical to the previously described Epstein Barr Virus Nuclear Antigen Binding Protein-2 (EBP2) (35) and nucleolar p40 (36). NoBP overexpression in fibroblasts increases the rate of cell proliferation, and induces growth in serum-poor medium (31). Although the mechanism by which NoBP influences growth is uncertain, the function of a highly related yeast protein, Ebp2p, has been characterized. Ebp2p also localizes to nucleoli and is required for ribosome biosynthesis and cell growth (37). Specifically, Ebp2p promotes exonucleolytic processing of yeast 27S-A pre-rRNA to 27S-B pre-rRNA, an essential step in the synthesis of 23S and 5.5S rRNAs. The 200 amino acid segment of yeast Ebp2p necessary for rRNA processing is the region bearing sequence homology to human NoBP (37). These findings suggest that mammalian NoBP may enhance ribosome synthesis to support cell growth (Fig. 2).
FGF-3 appears to localize to nucleoli through its strong affinity for NoBP. FGF-3’s nucleolar localization in vivo and interaction with NoBP in the yeast two-hybrid assay both require sequences in the FGF core homology region together with the unique COOH-terminal tail of the growth factor (31). The ability of nucleolar FGF-3 to inhibit cell growth may result from its ability to bind and inactivate NoBP, since NoBP overexpression counteracts the growth suppressive effect of nucleolar FGF-3 in mammary epithelial cells (31) (Fig. 2). For what benefit would a cell which synthesizes and secretes growth-inducing FGF-3 also produce an intracellular growth-suppressive isoform? As suggested by Reimers et al., nucleolar FGF-3 may be designed to inhibit the autocrine activity of secreted FGF-3, restricting the growth factor to paracrine stimulation.
Reimers et al. paint a preliminary picture of intracellular FGF-3 action, and several fundamental questions must still be answered. Most importantly, does endogenously expressed nucleolar FGF-3 in cells and tissues function analogously to the transfected, overexpressed protein? Does nucleolar FGF-3 inhibit ribosome synthesis through NoBP? What is the structural basis for FGF-3/NoBP interaction? These questions notwithstanding, FGF-3 has now been shown to mediate distinct signals through interactions with extracellular and intracellular binding partners.
FHF coding sequences were originally identified in cDNA database searches, with the encoded proteins bearing sufficient sequence homology to FGFs to originally warrant their assignment as new growth factors (FGF-11 through FGF-14) (27–29). They are most prominently expressed in developing and mature neurons in the central and peripheral nervous systems. However, FHFs have not displayed biological activities ascribed to FGFs, nor have they been shown to bind or activate the known FGF receptors. Furthermore, FHFs lack secretion signal sequences and have been detected within the cytoplasm of brain and dorsal root ganglia neurons (27, 38), as well as within the cytoplasm and nucleus of FHF-transfected cultured cells (27, 29).
Schoorlemmer and Goldfarb (32) have now demonstrated that the mitogen-activated protein (MAP) kinase scaffold protein Islet Brain-2 (IB-2) is an intracellular target for FHF action. FHFs interacted with IB-2 in yeast two-hybrid assays, and FHF/IB-2 complexes were seen in transfected mammalian cells. More importantly, endogenous FHF-1/IB-2 complexes were detected in lysates of adult rat cerebellum and untransfected rat insulinoma cells. FHF/IB-2 interaction is highly specific, because intracellular FGF-1 did not complex with IB-2, nor did FHFs bind the related scaffold protein JIP-1b(IB-1). FHF interaction with IB-2 requires the FGF core homology sequence together with the COOH-terminal extension unique to FHFs (32).
IB-2 and the related JIP-1b(IB-1) each assemble a signaling module by bringing together multiple protein kinases. JIP-1b(IB-1) interacts with leucine zipper/mixed lineage kinases DLK and MLK-3, the MAP kinase kinase MKK-7, and the c-Jun NH2-terminal kinase (JNK) family of MAP kinases (39). These kinases are known to form the phosphorylation/activation cascade DLK(or MLK-3)/MKK-7/JNKs. In cotransfection experiments, JIP-1b(IB-1) facilitates JNK activation by MKK-7 or, indirectly, by MLK-3 (39). It is likely that JIP-1b(IB-1) helps assemble these kinases into a module for activation by still unknown biochemical stimuli. IB-2 was also reported to interact with the same kinases as does JIP-1b(IB-1) (40). However, Schoorlemmer and Goldfarb failed to detect substantial interaction of IB-2 with JNKs. Rather, IB-2 interacted with a different MAP kinase, p38δ, in transfected cells, and this interaction was strongly potentiated by FHFs. These findings suggest that FHFs are cofactors for IB-2 in its assembly of the kinase cascade DLK(or MLK-3)/MKK-7/p38δ (32).
The biological functions of these scaffold-assembled signaling modules are unknown. However, IB-2 and JIP-1b(IB-1) also associate with both the light chain of the molecular motor kinesin and with specific transmembrane cell-surface proteins, including amyloid precursor protein and the lipoprotein receptor ApoER2 (41–44). Furthermore, interference with kinesin function blocks the accumulation of JIP-1b(IB-1), DLK, and the ApoER2 receptor at distal processes of cultured neurons (43). These findings suggest that the MAP kinase scaffold proteins are molecular bridges between a microtubule motor and specific secretory vesicle-associated receptor cargoes (Fig. 3). An intriguing possibility is that the kinases assembled on JIP-1b(IB-1) and on IB-2 (with the help of FHFs) may regulate steps in the process of vesicle transport.
Fig. 3.
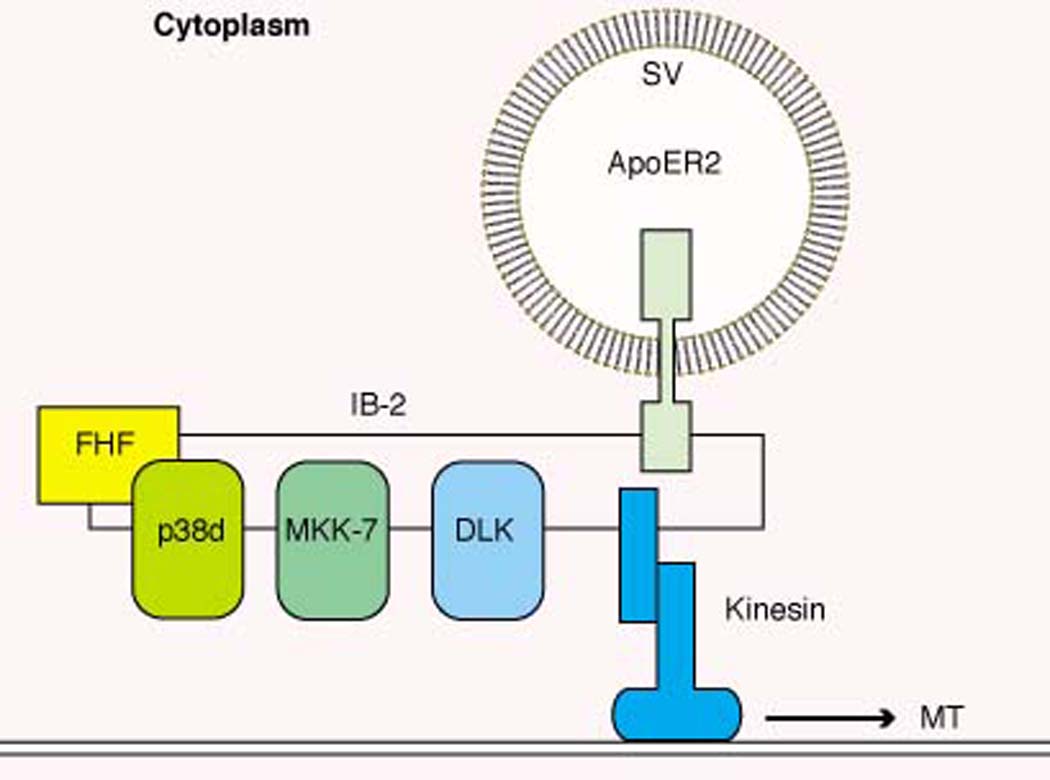
Model for intracellular FHF function. FHFs bind to the MAP kinase scaffold protein IB-2 in neural and endocrine cells (32). IB-2 binds the kinases DLK and MKK-7 (40), and FHFs may serve as cofactors for recruiting a MAP kinase, p38δ (32). IB-2 and the related scaffold JIP-1b(IB-1) also associate with the light chain of the microtubule (MT) motor protein kinesin (43) and with specific transmembrane proteins on secretory vesicles (SV), including amyloid precursor protein and the lipoprotein receptor ApoER2 (41–44). The assembly of all these proteins into a single complex is hypothetical.
In summary, although most members of the FGF family function by binding and activating FGF receptor tyrosine kinases, some members interact with and modulate intracellular proteins. This category now includes FGF-3, which has dual function as extracellular ligand for receptors and as nucleolar binding partner for NoBP, and the FHFs, which may act exclusively inside cells.
Mechanistically, how can FGFs and FHFs interact with such an unrelated set of targets? The solved structures of several FGFs have revealed a 12-stranded β-trefoil fold adopted by the core homology domain, thereby suggesting that all proteins in the FGF family have similar tertiary structures (45–47). The FGF β-trefoil promotes FGF receptor dimerization by simultaneously interacting with binding surfaces on two different receptor ectodomains (48, 49). The putative β-trefoil folds are also required for FGF-3/NoBP and FHF/IB-2 interactions (31, 32). Are there structural similarities in the assembly of these complexes compared to that formed by FGFs with receptors, or has evolution merely adopted the common FGF fold for a diverse set of protein-protein interactions? The answer lies with future structural solutions of the new FGF-target complexes.
Several findings suggest the existence of additional intracellular targets for FGFs and FHFs. First, as is the case for FGF-3, HMW FGF-2 isoforms display nuclear and nucleolar localization (24, 25). Furthermore, transfection-mediated overexpression of HMW FGF-2 enhances proliferation of fibroblasts under serum-poor conditions in a manner seemingly independent of FGF receptor engagement (50, 51). A candidate binding partner for FGF-2, termed FIF (FGF2-interacting factor), has recently been characterized (52). FIF is a nuclear protein that complexes with HMW FGF-2 in vivo and does not interact with other FGFs. FIF’s biological and biochemical functions are unknown, and it remains to be determined whether FIF is a mediator of HMW FGF-2 biological activity. Second, Let-756, one of the two FGFs in the nematode C. elegans, partially localizes to the nuclei and nucleoli of expressing cells in vivo (53), suggesting that this FGF may also have an additional intracellular function. Third, the FHFs are expressed during embryogenesis in several tissues where IB-2 is not, including craniofacial and limb bud mesenchyme, cardiac myocytes, nephrogenic tubules, and connective tissue (28, 54, 55). If these additional sites of expression are physiologically significant, FHFs may be acting there through other uncharacterized target proteins.
REFERENCES
- 1.Coulier F, Pontarotti P, Roubin R, Hartung H, Goldfarb M, Birnbaum D. Of worms and men: an evolutionary perspective on the fibroblast growth factor (FGF) and FGF receptor families. J. Mol. Evol. 1997;44:43–56. doi: 10.1007/pl00006120. [DOI] [PubMed] [Google Scholar]
- 2.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3) doi: 10.1186/gb-2001-2-3-reviews3005. reviews3005.1-3005.12 http://genomebiology.com/2001/2/3/reviews/3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 4.Tang TL, Freeman RM, O’Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 5.Saxton TM, Henkemeyer M, Gasea S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- 7.Sundaram M, Yochem J, Han M. A Ras-mediated signal transduction pathway is involved in the control of sex myoblast migration in Caenorhabditis elegans. Development. 1996;122:2823–2833. doi: 10.1242/dev.122.9.2823. [DOI] [PubMed] [Google Scholar]
- 8.Vincent S, Wilson R, Coelho C, Affolter M, Leptin M. The Drosophila protein Dof is specifically required for FGF signaling. Mol. Cell. 1998;2:515–525. doi: 10.1016/s1097-2765(00)80151-3. [DOI] [PubMed] [Google Scholar]
- 9.Michelson AM, Gisselbrecht S, Buff E, Skeath JB. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development. 1998;125:4379–4389. doi: 10.1242/dev.125.22.4379. [DOI] [PubMed] [Google Scholar]
- 10.Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szebenyi G, Fallon J. Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- 12.Schutzman JL, Borland CZ, Newman JC, Robinson MK, Kokel M, Stern MJ. The C. elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in FGF signal transduction. Mol. Cell. Biol. doi: 10.1128/MCB.21.23.8104-8116.2001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusakabe M, Masuyama N, Hanafusa H, Nishida E. Xenopus FRS2 is involved in early embryogenesis in cooperation with the Src family kinase Laloo. EMBO Rep. 2001;2:727–735. doi: 10.1093/embo-reports/kve152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8578–8583. doi: 10.1073/pnas.161259898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hama J, Xu H, Goldfarb M, Weinstein D. SNT-1/FRS2α physically interacts with Laloo and mediates mesoderm induction by fibroblast growth factor. Mech. Dev. doi: 10.1016/s0925-4773(01)00524-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson IA, Johnson EMJ. Fibroblast growth factor receptor-bearing neurons in the CNS: identification by receptor-mediated retrograde transport. J. Comp. Biol. 1991;313:693–706. doi: 10.1002/cne.903130412. [DOI] [PubMed] [Google Scholar]
- 17.Wiedlocha A, Falnes PO, Madshus IH, Sandvig K, Olsnes S. Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell. 1994;76:1039–1051. doi: 10.1016/0092-8674(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 18.Wiedlocha A, Falnes PO, Rapak A, Klingenberg O, Munoz R, Olsnes S. Translocation of cytosol of exogenous, CAAX-tagged acidic fibroblast growth factor. J. Biol. Chem. 1995;270:30680–30685. doi: 10.1074/jbc.270.51.30680. [DOI] [PubMed] [Google Scholar]
- 19.Maher PA. Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J. Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stachowiak EK, Maher PA, Tucholski J, Mordechai E, Joy A, Moggett J, Coons S, Stachowiak MK. Nuclear accumulation of fibroblast growth factor receptors in human glial cells--association with cell proliferation. Oncogene. 1997;14:2201–2211. doi: 10.1038/sj.onc.1201057. [DOI] [PubMed] [Google Scholar]
- 21.Wiedlocha A, Falnes PO, Rapak A, Munoz R, Klingenberg O, Olsnes S. Stimulation of proliferation of a human osteosarcoma cell line by exogenous acidic fibroblast growth factor requires both activation of receptor tyrosine kinase and growth factor internalization. Mol. Cell. Biol. 1996;16:270–280. doi: 10.1128/mcb.16.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng H, Moffett J, Myers J, Fang X, Stachowiak EK, Maher P, Kratz E, Hines J, Fluharty SJ, Mizukoshi E, Bloom DC, Stachowiak MK. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol. Biol. Cell. 2001;12:449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J. Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bugler B, Amalric F, Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol. Cell. Biol. 1991;11:573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quarto N, Finger FP, Rifkin DB. The NH2-terminal extension of high molecular weight bFGF is a nuclear targeting signal. J. Cell Physiol. 1991;147:311–318. doi: 10.1002/jcp.1041470217. [DOI] [PubMed] [Google Scholar]
- 26.Acland P, Dixon M, Peters G, Dickson C. Subcellular fate of the Int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990;343:662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- 27.Smallwood PM, Munoz-Sanjuan I, Tong P, Macke JP, Hendry SHC, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. Fibroblast growth factor (FGF) homologous factors: New members of the FGF family implicated in nervous system development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9850–9857. doi: 10.1073/pnas.93.18.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartung H, Feldman B, Lovec H, Coulier F, Birnbaum D, Goldfarb M. Murine FGF-12 and FGF-13: expression in embryonic nervous system, connective tissue and heart. Mech. Dev. 1997;64:31–39. doi: 10.1016/s0925-4773(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, McEwen DG, Ornitz DM. Subcellular and developmental expression of alternatively spliced forms of fiborlbast growth factor 14. Mech. Dev. 1999;88:1–5. doi: 10.1016/s0925-4773(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 30.Friesel R, Maciag T. Fibroblast growth factor prototype release and fibroblast growth factor receptor signaling. Thromb. Haemos. 1999;82:748–754. [PubMed] [Google Scholar]
- 31.Reimers K, Antoine M, Zapatka M, Blecken V, Dickson C, Kiefer P. NoBP, a nuclear fibrblast growth factor 3 binding protein, is cell cycle regulated and promotes cell growth. Mol. Cell. Biol. 2001;21:4996–5007. doi: 10.1128/MCB.21.15.4996-5007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoorlemmer J, Goldfarb M. Fibroblast growth factor homologous factors are intracellular signalling proteins. Curr. Biol. 2001;11:793–797. doi: 10.1016/s0960-9822(01)00232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiefer P, Acland P, Pappin D, Peters G, Dickson C. Competition between nuclear-localization and secretory signals determines the subcellular fate of a single CUG-initiated form of FGF3. EMBO J. 1994;13:4126–4136. doi: 10.1002/j.1460-2075.1994.tb06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiefer P, Dickson C. Nucleolar association of fibroblast growth factor 3 via specific sequence motifs has inhibitory effects on cell growth. Mol. Cell. Biol. 1995;15:4364–4374. doi: 10.1128/mcb.15.8.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shire K, Ceccarelli DF, Avolio-Hunter TM, Frappier L. EBP2, a human protein that interacts with sequences of the Epstein-Barr virus nuclear antigen 1 important for plasmid maintenance. J. Virol. 1999;73:2587–2595. doi: 10.1128/jvi.73.4.2587-2595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee A, Freeman JW, Busch H. Identification and partial characterization of a Mr 40,000 nucleolar antigen associated with cell proliferation. Cancer Res. 1987;47:1123–1129. [PubMed] [Google Scholar]
- 37.Huber MD, Dworet JH, Shire K, Frappier L, McAlear MA. The budding yeast homology of the human EBNA1-binding protein 2 (Ebp2p) is an essential nucleolar protein required for pre-rRNA processing. J. Biol. Chem. 2000;275:28764–28773. doi: 10.1074/jbc.M000594200. [DOI] [PubMed] [Google Scholar]
- 38.Goldfarb M, Schoorlemmer J. unpublished data. [Google Scholar]
- 39.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 42.Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider WJ, Nimpf J. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J. Biol. Chem. 2000;275:25625–25632. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- 43.Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda S, Yasukawa T, Homma Y, Ito Y, Nikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, Kouyama K, Yamamoto T, Kyriakis JM, Nishimoto I. c-Jun N-terminal kinase (JNK)-Interacting Protein-1b/Islet-Brain-1 scaffolds Alzheimer's amyloid precursor protein with JNK. J. Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X, Komiya H, Chirino A, Faham S, Fox GM, Arakawa T, Hsu BT, Rees DC. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991;251:90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]
- 46.Osslund TD, Syed R, Singer E, Hsu EW, Nybo R, Chen BL, Harvey T, Arakawa T, Narhi LO, Chirino A, Morris CF. Correlation between the 1.6 Å crystal structure and mutational analysis of keratinocyte growth factor. Protein Sci. 1998;7:1681–1690. doi: 10.1002/pro.5560070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plotnikov AN, Eliseenkova AV, Ibrahimi OA, Shriver Z, Sasisekharan R, Lemmon MA, Mohammadi M. Crystal structure of fibroblast growth factor 9 reveals regions implicated in dimerization and autoinhibition. J. Biol. Chem. 2001;276:4322–4329. doi: 10.1074/jbc.M006502200. [DOI] [PubMed] [Google Scholar]
- 48.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 49.Stauber DJ, DiGabriele AD, Hendrickson WA. Structural interactions of fibroblast growth factor receptor with its ligands. Proc. Natl. Acad. Sci. U.S.A. 2000;97:49–54. doi: 10.1073/pnas.97.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bikfalvi A, Klein S, Pintucci G, Quarto N, Mignatti P, Rifkin DB. Differential modulation of cell phenotype by different molecular weight forms of basic fibroblast growth factor: possible intracellular signaling by the high molecular weight forms. J. Cell. Biol. 1995;129:233–243. doi: 10.1083/jcb.129.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnaud E, Touriol C, Boutonnet C, Gensac MC, Vagner S, Prats H, Prats AC. A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol. Cell. Biol. 1999;19:505–514. doi: 10.1128/mcb.19.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Berghe L, Laurell H, Huez I, Zanibellato C, Prats H, Bugler B. FIF [Fibroblast Growth Factor-2 (FGF-2)-Interacting-Factor], a nuclear putatively antiapoptotic factor, interacts specifically with FGF-2. Mol. Endocrinol. 2000;14:1709–1724. doi: 10.1210/mend.14.11.0556. [DOI] [PubMed] [Google Scholar]
- 53.Roubin R, Birnbaum D. unpublished data. [Google Scholar]
- 54.Munoz-Sanjuan I, Simandl BK, Fallon JF, Nathans J. Expression of chicken fibroblast growth factor homologous factor (FHF)-1 and of differentially spliced isoforms of FHF-2 during development and involvement of FHF-2 in chicken limb development. Development. 1999;126:409–421. doi: 10.1242/dev.126.2.409. [DOI] [PubMed] [Google Scholar]
- 55.Munoz-Sanjuan I, Cooper MK, Beachy PA, Fallon JF, Nathans J. Expression and regulation of chicken fibroblast growth factor homologous factor (FHF)-4 during craniofacial morphogenesis. Dev. Dyn. 2001;220:238–245. doi: 10.1002/1097-0177(20010301)220:3<238::AID-DVDY1104>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 56.Xu H, Goldfarb M. Multiple effector domains within SNT-1 coordinate ERK activation and neuronal differentiation of PC12 cells. J. Biol. Chem. 2001;276:13049–13056. doi: 10.1074/jbc.M009925200. [DOI] [PubMed] [Google Scholar]
- 57.Carballada R, Yasuo H, Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. doi: 10.1242/dev.128.1.35. [DOI] [PubMed] [Google Scholar]
- 58.Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6074–6079. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Huang C, Zhan X. Src is required for cell migration and shape changes induced by fibroblast growth factor 1. Oncogene. 1999;18:6700–6706. doi: 10.1038/sj.onc.1203050. [DOI] [PubMed] [Google Scholar]


