Abstract
Three reference and 45 clinical isolates of Trichosporon were analyzed by conventional phenotypic and molecular methods to determine the species and genotypes of Trichosporon isolates from China. Target loci for molecular methods included the internal transcribed spacer (ITS) region, the D1/D2 domain of the 26S rRNA gene, and the intergenic spacer 1 (IGS1) region. Identification of eight Trichosporon species was achieved, of which Trichosporon asahii was the most common. Of the sequence-based molecular methods, the one targeting the D1/D2 domain assigned 97.9% (47/48) of isolates (seven species) correctly, while tests targeting both the ITS and IGS1 regions correctly identified all 48 isolates. The commercial API 20C AUX and Vitek 2 Compact YST systems correctly identified 91.9% and 73% of isolates when their biochemical profiles were queried against those of species contained in the databases, respectively, and misidentified 63.6% and 36.4% of isolates of species that were unclaimed by the databases, respectively. The predominant genotype among T. asahii clinical isolates, genotype 4 (51.4%), is rarely found in other countries. Voriconazole and itraconazole were the most active drugs in vitro against all the Trichosporon species tested, while caspofungin and amphotericin B demonstrated poor activity.
INTRODUCTION
Trichosporon species are emerging fungal pathogens capable of causing localized or systemic mycoses. Disseminated infection typically occurs in patients with underlying hematological malignancy, those who have undergone organ transplantation, and those who are otherwise heavily immunocompromised (5, 9, 18). Such infection is often lethal (mortality rates, 42 to 80%) (4, 13, 27). Furthermore, morbidity is significant, with multiple sequelae, including respiratory and renal failure and disseminated intravascular coagulation (5, 9, 30, 35).
With the advent of modern molecular identification techniques, the nomenclature within the genus Trichosporon has been substantially revised. While the major species previously encompassed Trichosporon beigelii (10, 32, 33), today, at least 13 Trichosporon species have been reported to be human pathogens. These include T. asahii, T. asteroides, T. coremiiforme, T. cutaneum, T. dermatis, T. domesticum, T. faecale, T. inkin, T. japonicum, T. jirovecii, T. loubieri, T. montevideense, and T. mucoides (5, 25). Yet the identification of Trichosporon and closely related yeasts to species level still largely relies on phenotype-based methods, which are insensitive and time-consuming (20). Further, none of the available commercial phenotypic identification systems include all new taxonomic categories or species in their databases. Specifically, the API 20C AUX (bioMérieux, Marcy l'Etoile, France) as well as the Vitek 2 Compact YST (bioMérieux) systems are able to identify only three Trichosporon species, T. asahii, T. inkin, and T. mucoides, which may lead to erroneous species assignment (6, 11, 17, 23, 24). Species identification is important for epidemiological purposes and to better define species-specific clinical associations (5, 11). In addition, certain Trichosporon species may be more resistant to antifungal drugs (2, 5, 25).
To enable accurate species identification, a number of molecular methods have been developed, of which DNA sequencing of the internal transcribed spacer (ITS) region, the D1/D2 domain of the 26S subunit of the rRNA gene region, and the intergenic spacer 1 (IGS1) region is the most frequently used (5, 7, 12, 19, 25, 26, 28–31). The IGS1 gene region has been particularly useful in phylogenetic studies and in delineating intraspecies variation. These data are fundamental to better understanding both species distribution and genotype differences that have been reported according to geographic region, such as that observed with T. asahii (5, 12, 19, 26, 28, 29).
In contrast to data from South and North America and Japan, studies examining the species distribution, phylogenetic diversity, and antifungal susceptibilities of Trichosporon pathogens in China are few. In the present study, we studied a collection of 45 clinical Trichosporon isolates obtained from three hospitals in China along with three well-characterized reference strains. Species identification of all isolates was undertaken by sequence analysis of the ITS region, D1/D2 domain, and IGS1 region and compared with that obtained by the API 20C AUX and Vitek 2 Compact YST methods. Testing of the antifungal susceptibilities of Trichosporon isolates to five antifungal agents was also performed.
MATERIALS AND METHODS
Trichosporon strains and DNA extraction.
Forty-eight Trichosporon isolates were studied, including (i) 3 reference strains, namely, T. asahii CBS 2479 (PUMCH strain identification no. PUMCHBY15), T. cutaneum ATCC 28592 (PUMCH strain identification no. PUMCHBY28), and T. laibachii CGMCC 2.1963 (PUMCH strain identification no. PUMCHMC31) (Table 1), and (ii) 45 clinical isolates collected from three hospitals (Peking Union Medical College Hospital, Peking University First Hospital, and First Affiliated Hospital of Chinese People's Liberation Army General Hospital) in Beijing, China. Of the 45 clinical isolates, 22 strains were isolated from sputum, 12 from tissue or body fluid, 3 from urine, 2 from blood, and 6 from unknown clinical sources (Table 1). All isolates were initially identified by the API 20C AUX and Vitek 2 Compact YST systems according to the manufacturers' instructions (Table 1) and then subjected to DNA sequencing (see below). DNA was extracted from pure cultures of each of the isolates as described previously (16) and stored at −20°C before use.
Table 1.
Identification, genotyping, and antifungal susceptibilities of 48 Trichosporon isolates
| Strain IDa | Body site of isolation | Molecular ID (IGS1 genotype)b,c | ID by API 20C AUX/Vitek 2 Compact YST | MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|---|---|
| CAS | FLC | VRC | ITC | AMB | ||||
| PUMCHBY15/CBS 2479 | Unknown | Trichosporon asahii (genotype 1) | T. asahii | 4 | 1 | ≤0.032 | 0.5 | 1 |
| PUMCHBY28/ATCC 28592 | Unknown | Trichosporon cutaneum | Unidentified | 4 | 0.125 | 0.032 | 0.125 | 0.5 |
| PUMCHMC31/CGMCC 2.1963 | Unknown | Trichosporon laibachii | Trichosporon mucoides/unidentified | 4 | 0.5 | ≤0.032 | 0.125 | 1 |
| PUMCH6Z2374 | Unknownd | Trichosporon jirovecii (genotype 1) | Cryptococcus humicola/Cryptococcus laurentii | 16 | 8 | 0.064 | 0.5 | 0.5 |
| PUMCH6Z10950 | Sputum | T. jirovecii (genotype 2) | C. humicola/C. laurentii | 16 | 1 | 0.032 | 0.25 | 2 |
| PUMCHBY27 | Blood | Trichosporon japonicum (genotype 1) | T. asahii | 8 | 4 | 0.064 | 0.25 | 8 |
| PUMCHBY11 | Hand | T. japonicum (genotype 2) | Unidentified | 16 | 1 | 0.032 | 0.25 | 4 |
| PUMCHBY23 | Blood | Trichosporon inkin | T. inkin/unidentified | 8 | 1 | 0.032 | 0.125 | 0.5 |
| PUMCHNJ32 | Unknownd | Trichosporon domesticum | Unidentified | 8 | 1 | 0.032 | 0.125 | 0.25 |
| PUMCHBY20 | Urine | T. domesticum | Unidentified/T. asahii | >16 | 4 | 0.125 | 0.5 | 0.5 |
| PUMCHBY21 | Sputum | T. domesticum | T. inkin/unidentified | >16 | 2 | 0.064 | 0.25 | 0.5 |
| PUMCHBY22 | Sputum | T. domesticum | T. inkin/unidentified | 16 | 4 | 0.064 | 0.25 | 0.5 |
| PUMCHBY24 | Abscess | Trichosporon dermatis | T. mucoides/unidentified | 16 | 32 | 0.5 | 0.5 | 1 |
| PUMCH30404 | Sputum | T. asahii (genotype 1) | T. asahii | 8 | 8 | 0.064 | 1 | 1 |
| PUMCH7Z7552 | Liver drain | T. asahii (genotype 1) | T. asahii | 8 | 2 | 0.125 | 1 | 1 |
| PUMCH30407 | Sputum | T. asahii (genotype 1) | T. asahii | 8 | 2 | 0.064 | 1 | 1 |
| PUMCHBY16 | Urine | T. asahii (genotype 1) | T. asahii | 8 | 4 | ≤0.032 | 1 | 1 |
| PUMCHBY17 | Sputum | T. asahii (genotype 1) | T. asahii | 4 | 2 | 0.064 | 0.25 | 1 |
| PUMCHBY18 | Unknownd | T. asahii (genotype 1) | T. asahii/unidentified | 4 | 1 | ≤0.032 | 0.25 | 1 |
| PUMCHBY25 | Skin (face) | T. asahii (genotype 1) | Unidentified | >16 | 32 | 0.25 | 2 | 0.25 |
| PUMCHBY26 | Tissue (face) | T. asahii (genotype 1) | Unidentified/C. laurentii | >16 | 16 | 0.064 | 0.25 | 0.064 |
| PUMCHBY29 | Liver | T. asahii (genotype 1) | T. asahii | 16 | 16 | 0.5 | 1 | ≤0.032 |
| PUMCH8W2360 | Catheter tip | T. asahii (genotype 3) | T. asahii | 8 | 4 | 0.064 | 1 | 1 |
| PUMCH7R7615 | Sputum | T. asahii (genotype 3) | T. asahii | 8 | 1 | 0.064 | 1 | 1 |
| PUMCH30406 | Sputum | T. asahii (genotype 3) | T. asahii | 8 | 1 | 0.5 | 1 | 1 |
| PUMCH5Z6443 | Lung | T. asahii (genotype 3) | T. asahii | 8 | 4 | 0.5 | 1 | 1 |
| PUMCH6Z8369 | Unknownd | T. asahii (genotype 3) | T. asahii | 4 | 1 | 0.125 | 1 | 1 |
| PUMCH6W5203 | Unknownd | T. asahii (genotype 3) | T. asahii | 8 | 2 | 1 | 1 | 1 |
| PUMCH8W2883 | Urine | T. asahii (genotype 3) | T. asahii/C. laurentii | 8 | 4 | 0.125 | 0.25 | 1 |
| PUMCH5Z6527 | Sputum | T. asahii (genotype 4) | T. asahii | 16 | 2 | 0.064 | 0.5 | 1 |
| PUMCH6Z6579 | Sputum | T. asahii (genotype 4) | T. asahii | 16 | 1 | 0.125 | 0.5 | 1 |
| PUMCH6Z2782 | Nasal secretions | T. asahii (genotype 4) | T. asahii | 8 | 1 | ≤0.032 | 0.5 | 1 |
| PUMCH6Z10766 | Unknownd | T. asahii (genotype 4) | T. asahii | 2 | 1 | 0.064 | 2 | 1 |
| PUMCH6Z9690 | Feces | T. asahii (genotype 4) | T. asahii | 8 | 1 | 0.064 | 1 | 1 |
| PUMCH7Z102 | Sputum | T. asahii (genotype 4) | Unidentified/T. asahii | 16 | 2 | 0.064 | 1 | 1 |
| PUMCH30401 | Sputum | T. asahii (genotype 4) | T. asahii | 2 | 0.5 | 0.064 | 0.25 | 1 |
| PUMCH30402 | Sputum | T. asahii (genotype 4) | T. asahii | 1 | 2 | 0.064 | 0.25 | 1 |
| PUMCH30403 | Sputum | T. asahii (genotype 4) | T. asahii/unidentified | 4 | 1 | 0.064 | 1 | 1 |
| PUMCH30405 | Sputum | T. asahii (genotype 4) | T. asahii/unidentified | 8 | 2 | 0.064 | 1 | 1 |
| PUMCH30408 | Sputum | T. asahii (genotype 4) | T. asahii/unidentified | 8 | 2 | 0.064 | 0.5 | 1 |
| PUMCH30409 | Sputum | T. asahii (genotype 4) | T. asahii | 8 | 2 | 0.064 | 1 | 1 |
| PUMCH30410 | Sputum | T. asahii (genotype 4) | T. asahii | 8 | 2 | 0.064 | 1 | 1 |
| PUMCHBY12 | Sputum | T. asahii (genotype 4) | T. asahii/unidentified | >16 | 8 | 0.064 | 0.25 | 0.5 |
| PUMCHBY13 | Sputum | T. asahii (genotype 4) | T. asahii/unidentified | 8 | 1 | 0.064 | 0.5 | 1 |
| PUMCHBY14 | Sputum | T. asahii (genotype 4) | T. asahii | 2 | 1 | ≤0.032 | 0.25 | 1 |
| PUMCHBY19 | Abdominal fluid | T. asahii (genotype 4) | T. asahii | 4 | 1 | ≤0.032 | 0.5 | 1 |
| PUMCH8R5548 | Sputum | T. asahii (genotype 4) | T. asahii | 8 | 2 | 0.064 | 1 | 1 |
| PUMCHJS30 | Pleural fluid | T. asahii (genotype 6) | T. asahii | 8 | 0.5 | 0.064 | 1 | 1 |
ID, identification.
By sequence analysis of the ITS region, D1/D2 domain, and IGS1 region (for detailed information, see Table S1 in the supplemental material).
These isolates were from clinical specimens, but sites of isolation were not available.
PCR and DNA sequencing.
Amplification of the ITS region, D1/D2 domain, and IGS1 region was performed as previously described with primer pairs ITS1/ITS4, F63/R635, and 26SF/5SR, respectively (7, 29). A total of 8 μl of amplified PCR products was visualized on a 2% agarose gel after staining with SYBR safe DNA gel stain (Invitrogen, Mt. Waverley, VIC, Australia). The PCR products were then sequenced by Tsingke Co. Ltd. (Beijing, People's Republic of China) with the DNA analyzer ABI 3730XL system (Applied Biosystems, Foster City, CA) in both directions using the PCR amplification primers.
Sequencing-based species identification and phylogenetic analysis.
All DNA sequence chromatograms were checked manually to ensure high-quality sequences. For species identification, sequences of the ITS region, D1/D2 domain, and IGS1 region obtained in this study were queried against sequences of type strains for all Trichosporon species in the Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Center database using pairwise sequence alignment with the BioloMICSNet software (http://www.cbs.knaw.nl/collections/BioloMICSSequences.aspx). The sequence of each target gene region and concatenated sequences of the ITS region and D1/D2 domain were then aligned using Clustal W software (36) and adjusted manually to form the consensus sequences for all 48 isolates. Phylogenetic trees were computed with MEGA, version 4 (Molecular Evolutionary Genetic Analysis software, version 4.0.2; http://www.megasoftware.net) using maximum-parsimony analysis (34), with all positions containing gaps and missing data eliminated from the data set. ITS region, D1/D2 domain, and IGS1 region sequences from the whole-genome sequence of Cryptococcus neoformans var. neoformans strain B-3501A (15) were used as outgroups to generate the maximum-parsimony trees (MPTs). The IGS1 region was further targeted to determine the genotypes of the T. asahii isolates as described previously (26, 28, 29).
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) document M27-A3 and M27-S3 broth microdilution method (21, 22). The following antifungal drugs were tested at the indicated concentration ranges: amphotericin B (AMB; North China Pharmaceutical Group Corporation, Shijiazhuang, China), 0.032 to 16 μg/ml; caspofungin (CAS; Merck Research Laboratories, Rahway, NJ), 0.032 to 16 μg/ml; fluconazole (FLC; Pfizer Incorporated, New York, NY), 0.125 to 64 μg/ml; itraconazole (ITC; National Institute for Food and Drug Control, Beijing, China), 0.032 to 16 μg/ml; and voriconazole (VRC; Pfizer Incorporated), 0.032 to 16 μg/ml. The microdilution plates were incubated at 35°C, except for those for T. cutaneum ATCC 28592 (strain PUMCHBY28), where plates were incubated at 28°C as described previously (14). All plates were observed for visible growth at 48 h. Microdilution wells were scored with the aid of a reading mirror, and the growth in each well compared was with that of the growth-control (drug-free) well (21). Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as the quality control strains.
Nucleotide sequence accession numbers.
New data for sequences within the ITS region, D1/D2 domain, and IGS1 region generated in this study have been deposited in the GenBank database with accession numbers EU863532 to EU863579 (for ITS region), EU882088 to EU882103 (for D1/D2 domain), and JF302977 to JF303024 (for IGS1 region).
RESULTS
Sequencing-based identification of Trichosporon species.
All 48 Trichosporon isolates were identified by querying their unknown ITS, D1/D2, and IGS1 sequences against sequences of Trichosporon type strains in the CBS Fungal Biodiversity Center database, and results are summarized in Table S1 in the supplemental material.
The three loci correctly identified the reference strains to species level (Table 1; see Table S1 in the supplemental material). Among 45 clinical isolates, six species were identified. Trichosporon asahii was the most common species (35 isolates, 77.8%), followed by T. domesticum (4 isolates, 8.9%), T. japonicum and T. jirovecii (2 isolates each, 4.4%), and T. dermatis and T. inkin (1 isolate each, 2.2%) (Table 1; see Table S1 in the supplemental material). Sequencing of both the ITS and IGS1 regions successfully identified all 45 clinical isolates. D1/D2 domain sequences correctly assigned 44 of 45 (97.8%) strains to species level, with the exception of strain PUMCHBY24, which was identified as T. mucoides but as T. dermatis by ITS and IGS1 sequencing; T. dermatis was assigned the correct species for this strain (see Table S1 in the supplemental material).
Comparison of phenotype-based and molecular identification.
Identification results for 48 Trichosporon isolates tested with the Vitek 2 Compact YST and API 20C AUX systems are summarized in Tables 1 and 2. As the databases of both the API 20C AUX and Vitek 2 Compact YST systems contain only three Trichosporon species (T. asahii, T. inkin, and T. mucoides), isolates in the present study were further subgrouped as “species claimed by databases” (37 isolates, two species) and “species unclaimed by databases” (11 isolates, six species) for analysis (Table 2).
Table 2.
Performance of the commercial biochemical methods in the present studya
| Species by molecular ID | No. (%) of isolates |
||||||
|---|---|---|---|---|---|---|---|
| Total | API 20C AUX |
Vitek 2 Compact YST |
|||||
| C | U | M | C | U | M | ||
| T. asahiib | 36 | 33 (91.7) | 3 (8.3) | 0 | 27 (75.0) | 7 (19.4) | 2 (5.6) |
| T. inkinb | 1 | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 |
| T. laibachiic | 1 | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
| T. cutaneumc | 1 | 0 | 1 (100) | 0 | 0 | 1 (100) | 0 |
| T. dermatisc | 1 | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
| T. domesticumc | 4 | 0 | 2 (50) | 2 (50) | 0 | 3 (75) | 1 (25) |
| T. japonicumc | 2 | 0 | 1 (50) | 1 (50) | 0 | 1 (50) | 1 (50) |
| T. jiroveciic | 2 | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) |
| Total species claimed by databases | 37 | 34 (91.9) | 3 (8.1) | 0 | 27 (73.0) | 8 (21.6) | 2 (5.4) |
| Total species unclaimed by databases | 11 | 0 | 4 (36.4) | 7 (63.6) | 0 | 7 (63.6) | 4 (36.4) |
| Total | 48 | 34 (70.8) | 7 (14.6) | 7 (14.6) | 27 (56.3) | 15 (31.2) | 6 (12.5) |
Abbreviations: ID, identification; C, isolates that were correctly identified; U, isolates that were unidentified; M, isolates that were misidentified.
Species claimed by databases of API 20C AUX and Vitek 2 Compact YST systems.
Species not claimed by databases of API 20C AUX and Vitek 2 Compact YST systems.
Compared with the results obtained by our three-locus sequencing scheme, the API 20C AUX system correctly identified 34 of 37 (91.9%) isolates among species claimed by databases, with the remaining three isolates unidentified (with their biochemical profiles yielding either “low discrimination” or “no identification”) and no isolates misidentified, while among species unclaimed by databases, 4 of 11 isolates were correctly shown to be unidentified but 7 were misidentified (Tables 1 and 2). The ability of the Vitek 2 Compact YST system to identify Trichosporon spp. among species claimed by databases was even lower: only 27 T. asahii isolates (75.0% of 36 T. asahii isolates studied) were correctly identified. However, among species unclaimed by databases, 7 of 11 isolates were correctly shown to be unidentified but 4 isolates were misidentified (Tables 1 and 2).
Phylogenetic analysis of IGS1 region and genotyping.
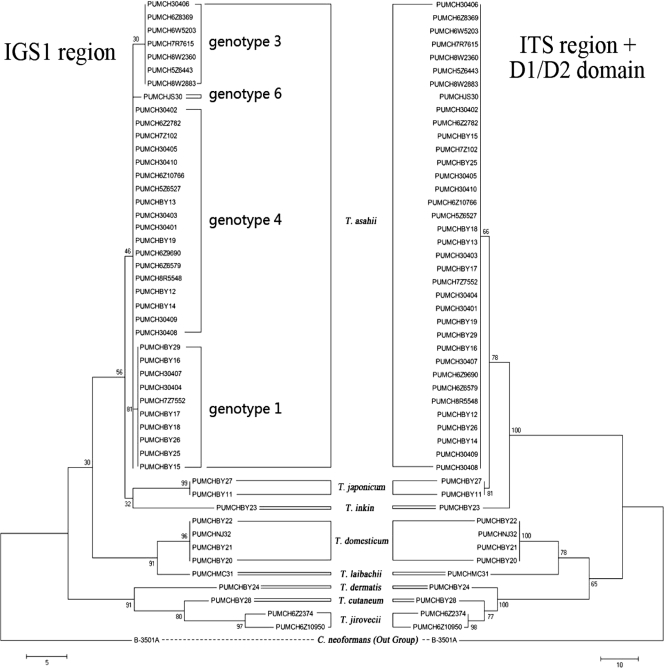
Phylogenetic analysis was performed on the basis of the results of sequence analysis of the ITS region (595 bp), D1/D2 domain (663 bp), and IGS1 region (1,110 bp) as well as by using concatenated sequences of the ITS region and D1/D2 domain (Table 3). On the basis of the number of parsimony informative characters (PICs) per bp (Table 3), the IGS1 region was more phylogenetically informative than either the ITS region or D1/D2 domain. MPTs were generated for the IGS 1 region (Fig. 1, left MPT) and ITS region plus D1/D2 domain (Fig. 1, right MPT) rooted by the outgroup C. neoformans var. neoformans strain B-3501A.
Table 3.
Tree statistics for the three genes studieda
| Genetic locus | Length (bp) | No. of PICs | No. of PICs/bp | No. of MPTs | MPT length (steps) | CI | RI | No. of sequence types/genotypes identified |
|---|---|---|---|---|---|---|---|---|
| ITS | 595 | 50 | 0.08 | 981 | 232 | 0.71 | 0.93 | 10 |
| D1/D2 | 663 | 54 | 0.08 | 605 | 153 | 0.82 | 0.93 | 8 |
| ITS + D1/D2 | 1,258 | 104 | 0.08 | 300 | 203 | 0.83 | 0.92 | 10 |
| IGS1 | 1,110 | 182 | 0.16 | 213 | 110 | 0.77 | 0.84 | 13 |
Abbreviations: CI, consistency index; RI, retention index.
Fig. 1.
MPTs by comparison of sequences of the IGS1 region and the ITS region plus D1/D2 domain of the 26S rRNA gene. ITS region, D1/D2 domain, and IGS1 region sequences from the whole-genome sequence of Cryptococcus neoformans var. neoformans strain B-3501A were used as outgroups. Eight Trichosporon species identified in the present study are indicated; and four T. asahii IGS1 genotypes are marked in the IGS1 MPT (left tree) as well. Scale bars indicate branch lengths corresponding to 5 nucleotide changes for the IGS1 region (bottom left) and 10 nucleotide changes for the ITS region plus D1/D2 domain (bottom right).
Among the 48 isolates, the IGS1 region recognized 13 sequence types/genotypes (Table 3). Genotypes of T. asahii isolates were assigned as described by Sugita et al. (28) and Rodriguez-Tudela et al. (26). Trichosporon asahii reference strain PUMCHBY15/CBS 2479 was correctly classified as genotype 1 (5). The remaining 35 clinical T. asahii isolates belonged to four genotypes, namely, genotype 1 (9 isolates, 25.7%), genotype 3 (7 isolates, 20.0%), genotype 4 (18 isolates, 51.4%), and genotype 6 (1 isolate, 2.9%) (Table 1 and Fig. 1). Four T. domesticum isolates studied had a single IGS1 sequence type, while two sequence types were found for both T. japonicum strains and two T. jirovecii strains (Table 1; see Table S1 in the supplemental material).
Antifungal susceptibility testing.
The susceptibilities of 48 isolates representing eight species (on the basis of the molecular identification result) to five antifungal agents are shown in Table 1. MIC ranges (and geometric mean [GM] MICs) were 0.125 to 32 μg/ml (1.09 μg/ml) for AMB, 1 to 16 μg/ml (9.15 μg/ml) for CAS, 0.125 to 8 μg/ml (3.97 μg/ml) for FLC, 0.125 to 2 μg/ml (0.66 μg/ml) for ITC, and 0.032 to 1 μg/ml (0.12 μg/ml) for VRC (Table 1). CAS MICs for all Trichosporon isolates tested were consistently high (≥2 μg/ml for all strains tested except one T. asahii isolate, for which the CAS MIC was 1 μg/ml). Two T. japonicum isolates exhibited AMB MICs of ≥4 μg/ml, while all T. asahii isolates had AMB MICs of ≤1 μg/ml (GM MIC, 0.91 μg/ml). With regard to the azoles, FLC MICs for 27 Trichosporon isolates were ≥2 μg/ml. GM MICs of FLC were 3.97 μg/ml for all tested isolates and 3.67 μg/ml for the most common species, T. asahii. ITC and VRC were the most potent agents in vitro against all Trichosporon spp., particularly VRC, with GM MICs of 0.12 μg/ml.
DISCUSSION
Trichosporon species are medically important yeast pathogens, but their prevalence and species distribution have not been well studied in China. A recent study of approximately 800 isolates causing invasive yeast infections in China has estimated that 1% of all such infections are due to Trichosporon spp. (China Hospital Invasive Fungal Surveillance Net [CHIF-NET], 2009-2010, unpublished data). In the present study, we note that commercial biochemical-based methods may be limited in their capacity to identify Trichosporon species, even those that are claimed to be included within their databases. We confirmed that both the ITS and IGS1 sequences are suitable targets for accurate species identification by sequencing (with 100% sensitivity) (29, 30; this study). Further, the IGS1 locus also provided discriminatory information with regard to strain variation within the predominant species, T. asahii, encountered in China.
To date, the identification of Trichosporon yeasts in the clinical mycology laboratory has largely relied on morphological, physiological, and biochemical characteristics (20). Specifically, API test strips have been long considered the “gold standard” for clinical diagnosis (3, 20) in many clinical microbiology laboratories in China. However, neither system can assign Trichosporon isolates to species level among species unclaimed by databases (misidentification of 63.6% by API 20C AUX system and 36.4% by Vitek 2 Compact YST system, respectively; Table 2). The present study also showed that among Trichosporon species claimed by the databases of commercial systems, not all isolates were correctly identified (no identification for 8.1% and 21.6% with API 20C AUX and Vitek 2 Compact YST, respectively, and 5.4% misidentification for Vitek 2 Compact YST; Table 2).
Our results confirmed the findings of others (5, 11, 25, 29) that molecular methods have good potential to provide reliable, accurate species identification. Earlier studies exploited sequence variation within the ITS region and D1/D2 domain between species for species assignment (7, 25, 30, 31). However, as Trichosporon species are phylogenetically very closely related to each other, several reports indicated that sequence analyses of the ITS and D1/D2 regions were unable to unambiguously distinguish between all species (5, 11, 28, 29). In the present study, the ITS region identified all 48 isolates studied, and the D1/D2 domain identified 47 of 48, though for certain Trichosporon species, distinction by ITS region and D1/D2 domain was based on differences of only a few nucleotide base pairs or even a single nucleotide base pair. For example, the ITS sequences of two T. japonicum isolates in the present study were 100% (541/541) identical to the ITS sequence of T. japonicum CBS 8641T (see Table S1 in the supplemental material), with a 1-bp difference compared to the ITS sequence of T. asteroides CBS 2481T (540/541, 99.8%) and only a 3-bp difference compared to the ITS sequence of T. asahii CBS 2479T (538/541, 99.4%). Thus, among closely related Trichosporon spp., such as T. japonicum and T. asteroides, if a cutoff value of less than 100% was employed by sequencing of the ITS region and D1/D2 domain, there could be a risk of misidentification, and sequencing of an additional locus, e.g., the IGS1 region, should be considered. The IGS1 region was characterized by a greater degree of nucleotide polymorphism than either the ITS region or D1/D2 domain (5, 26, 28, 29); the present study showed that IGS1 sequences with sequence identities as low as 97.1% could still belong to the same species (as demonstrated for T. asahii genotypes 1, 3, and 6; see Table S1 in the supplemental material) and IGS1 sequences with sequence identities even as low as 90.9% could still belong to the same species (as demonstrated for T. jirovecii genotype 2 and T. jirovecii CBS 6864T; see Table S1 in the supplemental material). Thus, when using the IGS1 region for species identification, a lower cutoff value should be employed.
Of interest, one of the two T. japonicum isolates studied (PUMCHBY27) was identified as T. asahii by commercial identification systems (Table 1). In the first reported isolation of T. japonicum from a clinical specimen, the isolate was likewise misidentified as T. asahii (1). We were also unable to discriminate between T. dermatis and T. mucoides by D1/D2 sequencing (Table 1; see Table S1 in the supplemental material). This is in keeping with the observations of others reporting on the potential misidentification of T. dermatis as T. mucoides by ITS sequencing (5, 11). Since these two species have different propensities to cause superficial versus invasive infections (5, 11), accurate species identification is important for clinical diagnosis and in fungal surveillance.
A major finding of the present study is that, in contrast to the other two regions analyzed, examination of sequence variation within the IGS1 region enabled the accurate identification of all 48 study isolates, consistent with previous findings (5, 11, 12, 19, 26, 28, 29). Indeed, eight species were identified among the 48 test strains, including six species among 45 clinical isolates, supporting previous reports that the sequence polymorphisms within the IGS1 region provide powerful and discriminatory information for distinguishing between phylogenetically closely related species and that the IGS1 region is the preferred target for sequence-based identification of Trichosporon species (1, 5, 25, 28, 29).
Further, IGS1 sequence analysis also shows great potential as an epidemiological tool. For instance, the geographic distribution of different genotypes of T. asahii isolates by IGS1 sequencing has been described by a number of studies (5, 12, 19, 26, 28, 29). In the present study, among 35 T. asahii Chinese isolates, the most predominant genotype was genotype 4 (51.4%), previously only rarely found (approximately 1.0 to 4.5%) in Japan, South America, and Turkey (5, 12, 29). Other genotypes observed in this study were genotype 1 (25.7%), genotype 3 (20.0%), and genotype 6 (2.9%). Of note, IGS1 genotype 1 is reported to be the most predominant in Japan as well as in South America and Europe (57% to 87%) (5, 12, 26, 28, 29), and genotype 3 is reported to be common in the United States (about 60%) (29).
Consistent with the findings of others (5, 12, 19), MICs to CAS for all isolates were high (GM MIC, 9.15 μg/ml; Table 1). This in vitro finding is consistent with the increasing number of reports of disseminated Trichosporon infection in patients receiving echinocandin antifungal treatment (8). In contrast, VRC (GM MIC, 0.12 μg/ml) and ITC (GM MIC, 0.66 μg/ml) were the most active anti-Trichosporon drugs (Table 1) (5, 12, 19, 25). With the exception of two strains of T. japonicum and one strain of T. jirovecii that had AMB MICs of ≥2 μg/ml, all the other clinical isolates (including all T. asahii isolates studied) had low MICs to AMB (Table 1); these findings are in contrast to the low susceptibility to AMB for T. asahii isolates reported by others (2, 5, 12, 25). FLC was less active than VRC and ITC (MIC range, 0.125 to 8 μg/ml; GM MIC, 3.97 μg/ml), with the GM MIC being higher than that reported in a Brazilian study (1.1 μg/ml), although it was lower than that in a study from Turkey (12.5 μg/ml) (5, 12). This suggests that where possible, antifungal susceptibility testing should be performed on all clinical isolates not only to guide therapy but also to document local epidemiological trends.
Limitations of the present study include the small number of isolates available for study and the fact that all clinical isolates were collected from hospitals in Beijing. Further collection and study of Trichosporon isolates in China are ongoing on the basis of the nationwide surveillance program CHIF-NET, which seeks to describe the clinical and molecular epidemiology of a range of yeast pathogens.
In conclusion, the present study provided accurate species identification of a Chinese collection of Trichosporon isolates by combining the use of ITS, D1/D2, and IGS1 sequencing. Eight species were identified, including six species among clinical isolates. Phenotypic methods for Trichosporon species identification, including API 20C AUX and Vitek 2 Compact YST, were not as accurate as molecular methods. Phylogenetic analysis and genotyping by IGS1 sequencing showed a significant subgeographic difference among Trichosporon clinical isolates in China, with genotype 4 being the predominant genotype among T. asahii isolates. VRC was the most active drug in vitro against all Trichosporon species, while CAS demonstrated poor activity.
Supplementary Material
ACKNOWLEDGMENT
This study was funded by the Ministry of Health of the People's Republic of China public service sector fund 200802026.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 7 September 2011.
REFERENCES
- 1. Agirbasli H., et al. 2008. Two possible cases of Trichosporon infections in bone-marrow-transplanted children: the first case of T. japonicum isolated from clinical specimens. Jpn. J. Infect. Dis. 61:130–132 [PubMed] [Google Scholar]
- 2. Araujo Ribeiro M., Alastruey-Izquierdo A., Gomez-Lopez A., Rodriguez-Tudela J. L., Cuenca-Estrella M. 2008. Molecular identification and susceptibility testing of Trichosporon isolates from a Brazilian hospital. Rev. Iberoam. Micol. 25:221–225 [PubMed] [Google Scholar]
- 3. Aubertine C. L., Rivera M., Rohan S. M., Larone D. H. 2006. Comparative study of the new colorimetric VITEK 2 yeast identification card versus the older fluorometric card and of CHROMagar Candida as a source medium with the new card. J. Clin. Microbiol. 44:227–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassetti M., et al. 2004. Trichosporon asahii infection treated with caspofungin combined with liposomal amphotericin B. J. Antimicrob. Chemother. 54:575–577 [DOI] [PubMed] [Google Scholar]
- 5. Chagas-Neto T. C., Chaves G. M., Melo A. S., Colombo A. L. 2009. Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J. Clin. Microbiol. 47:1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciardo D. E., Schar G., Bottger E. C., Altwegg M., Bosshard P. P. 2006. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J. Clin. Microbiol. 44:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz M. R., Fell J. W. 2004. High-throughput detection of pathogenic yeasts of the genus Trichosporon. J. Clin. Microbiol. 42:3696–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fera M. T., La Camera E., De Sarro A. 2009. New triazoles and echinocandins: mode of action, in vitro activity and mechanisms of resistance. Expert Rev. Anti Infect. Ther. 7:981–998 [DOI] [PubMed] [Google Scholar]
- 9. Girmenia C., et al. 2005. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 43:1818–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gueho E., et al. 1992. Contributions to a revision of the genus Trichosporon. Antonie Van Leeuwenhoek 61:289–316 [DOI] [PubMed] [Google Scholar]
- 11. Gunn S. R., et al. 2006. Use of DNA sequencing analysis to confirm fungemia due to Trichosporon dermatis in a pediatric patient. J. Clin. Microbiol. 44:1175–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalkanci A., et al. 2010. Molecular identification, genotyping, and drug susceptibility of the basidiomycetous yeast pathogen Trichosporon isolated from Turkish patients. Med. Mycol. 48:141–146 [DOI] [PubMed] [Google Scholar]
- 13. Kontoyiannis D. P., et al. 2004. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand. J. Infect. Dis. 36:564–569 [DOI] [PubMed] [Google Scholar]
- 14. Li H. M., Du H. T., Liu W., Wan Z., Li R. Y. 2005. Microbiological characteristics of medically important Trichosporon species. Mycopathologia 160:217–225 [DOI] [PubMed] [Google Scholar]
- 15. Loftus B. J., et al. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makimura K., Murayama S. Y., Yamaguchi H. 1994. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 40:358–364 [DOI] [PubMed] [Google Scholar]
- 17. Massonet C., et al. 2004. Comparison of VITEK 2 with ITS2-fragment length polymorphism analysis for identification of yeast species. J. Clin. Microbiol. 42:2209–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsue K., Uryu H., Koseki M., Asada N., Takeuchi M. 2006. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin. Infect. Dis. 42:753–757 [DOI] [PubMed] [Google Scholar]
- 19. Mekha N., et al. 2010. Genotyping and antifungal drug susceptibility of the pathogenic yeast Trichosporon asahii isolated from Thai patients. Mycopathologia 169:67–70 [DOI] [PubMed] [Google Scholar]
- 20. Nakasone I., Kinjo T., Yamane N., Kisanuki K., Shiohira C. M. 2007. Laboratory-based evaluation of the colorimetric VITEK-2 Compact system for species identification and of the Advanced Expert System for detection of antimicrobial resistances: VITEK-2 Compact system identification and antimicrobial susceptibility testing. Diagn. Microbiol. Infect. Dis. 58:191–198 [DOI] [PubMed] [Google Scholar]
- 21. NCCLS/CLSI 2009. Reference method for broth dilution antifungal susceptibility testing of yeasts: informational supplement, M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22. NCCLS/CLSI 2009. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed., M27-S3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23. Pincus D. H., Orenga S., Chatellier S. 2007. Yeast identification—past, present, and future methods. Med. Mycol. 45:97–121 [DOI] [PubMed] [Google Scholar]
- 24. Ramani R., Gromadzki S., Pincus D. H., Salkin I. F., Chaturvedi V. 1998. Efficacy of API 20C and ID 32C systems for identification of common and rare clinical yeast isolates. J. Clin. Microbiol. 36:3396–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez-Tudela J. L., et al. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 49:4026–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez-Tudela J. L., et al. 2007. Genotype distribution of clinical isolates of Trichosporon asahii based on sequencing of intergenic spacer 1. Diagn. Microbiol. Infect. Dis. 58:435–440 [DOI] [PubMed] [Google Scholar]
- 27. Ruan S. Y., Chien J. Y., Hsueh P. R. 2009. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin. Infect. Dis. 49:e11–e17 [DOI] [PubMed] [Google Scholar]
- 28. Sugita T., Ikeda R., Nishikawa A. 2004. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J. Clin. Microbiol. 42:5467–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sugita T., Nakajima M., Ikeda R., Matsushima T., Shinoda T. 2002. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J. Clin. Microbiol. 40:1826–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugita T., Nishikawa A., Ikeda R., Shinoda T. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 37:1985–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugita T., Nishikawa A., Shinoda T. 1998. Identification of Trichosporon asahii by PCR based on sequences of the internal transcribed spacer regions. J. Clin. Microbiol. 36:2742–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugita T., Nishikawa A., Shinoda T. 1994. Reclassification of Trichosporon cutaneum by DNA relatedness by the spectrophotometric method and the chemiluminometric method. J. Gen. Appl. Microbiol. 40:397–408 [Google Scholar]
- 33. Sugita T., Nishikawa A., Shinoda T., Yoshida K., Ando M. 1995. A new species, Trichosporon domesticum, isolated from the house of a summer-type hypersensitivity pneumonitis patient in Japan. J. Gen. Appl. Microbiol. 41:429–436 [Google Scholar]
- 34. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 35. Tashiro T., et al. 1994. Disseminated Trichosporon beigelii infection in patients with malignant diseases: immunohistochemical study and review. Eur. J. Clin. Microbiol. Infect. Dis. 13:218–224 [DOI] [PubMed] [Google Scholar]
- 36. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.