Abstract
Acinetobacter baumannii is emerging as an important nosocomial pathogen worldwide. We report molecular epidemiology of 65 carbapenem-nonsusceptible A. baumannii isolates identified from hospitals in New York, Pennsylvania, Florida, Missouri, Nevada, and California between 2008 and 2009. All isolates were subjected to pulsed-field gel electrophoresis (PFGE). Select isolates then underwent multilocus sequence typing (MLST). While the PFGE patterns tended to cluster within each hospital, sequence types (STs) belonging to the clonal complex 92 (CC92) and the pan-European clonal lineage II (EUII; worldwide clonal lineage 2) were predominant in all hospitals. Of them, ST122 and ST208 were the most common and were found in four of the six hospitals. Isolates belonging to the pan-European clonal lineages I and III were identified in one hospital each. Carbapenemase-encoding genes blaOXA-23 and/or ISAba1-blaOXA-51-like were present among the majority of isolates. These findings suggest that carbapenem-nonsusceptible A. baumannii isolates found in U.S. hospitals constitute part of the global epidemic driven by CC92, but have unique STs other than ST92, which may be spreading by means of patient transfer between health care facilities within the United States.
INTRODUCTION
Over the last decade, Acinetobacter baumannii has emerged to become an important cause of nosocomial infections in many parts of the world (25). Of great concern is the recent, remarkable rise in the frequency of carbapenem-nonsusceptible A. baumannii. According to data from the National Healthcare Safety Network (NHSN) surveillance system, 34% of A. baumannii isolates surveyed from U.S. hospitals between 2006 and 2008 were resistant to carbapenems, along with at least three other classes of antimicrobials, including ampicillin-sulbactam, antipseudomonal penicillins, broad-spectrum cephalosporins, fluoroquinolones, and aminoglycosides (16). In another nationwide survey, nonsusceptibility to carbapenems increased from 22% in 2002 to 52% in 2008 (20). Carbapenem-nonsusceptible A. baumannii infections are difficult to manage, as their therapy relies on salvage agents such as colistin and tigecycline. However, resistance to these agents is emerging among carbapenem-nonsusceptible A. baumannii isolates (8, 24).
Three clonal lineages of A. baumannii, commonly referred to as the pan-European clonal lineages (EUI, EUII, and EUIII), have predominated in many European countries since the 1990s (7). These clonal lineages have subsequently been identified worldwide, including in the United States (14, 36). It has thus been proposed to refer to them as the “worldwide” clonal lineages (WW1, WW2, and WW3) by some investigators (14).
Various molecular typing methods are employed to study the molecular epidemiology of A. baumannii. The most commonly used technique among them is pulsed-field gel electrophoresis (PFGE), which is highly discriminatory. However, PFGE is not well suited for interlaboratory comparisons unless the procedures are meticulously standardized (29), and its interpretation may pose a challenge in nonoutbreak situations (31). Repetitive sequence-based PCR, which is now commercially available, has operating characteristics similar to those of PFGE for A. baumannii (12, 28). Multilocus sequence typing (MLST), on the other hand, compares nucleotide sequences of housekeeping genes among isolates and generates objective and portable data. Three MLST schemes are currently used. The first MLST scheme for A. baumannii was published by Bartual et al. in 2005 (2), and it has subsequently been revised and employed to study the epidemiology of the organism in many countries (11, 13, 15, 17, 21, 23, 26, 28, 30). The second MLST scheme, which shares three of the loci with the original scheme, was developed by Diancourt et al. at the Pasteur Institute (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html) (6). The third scheme utilizes PCR and mass spectrometry (9). While MLST is an expensive typing method due to the need for DNA sequencing, selective use of this technique can substantially enhance our understanding of molecular epidemiology across different hospitals and geographic areas.
The primary aim of the present study was to investigate the molecular epidemiology of contemporary carbapenem-nonsusceptible A. baumannii isolates collected from hospitals across the continental United States using MLST.
MATERIALS AND METHODS
Bacterial isolates.
A total of 65 carbapenem-nonsusceptible A. baumannii isolates were investigated. Only one isolate per patient was included. All isolates were identified from clinical specimens at hospitals across the United States between 2008 and 2009. The participating hospitals in New York, Pittsburgh, PA, and St. Louis, MO, have an ongoing collection of carbapenem-nonsusceptible A. baumannii isolates. From these hospitals, only one isolate was included for a given month. The hospitals in Jacksonville, FL, Las Vegas, NV, and Torrance, Los Angeles County, CA, collected serial carbapenem-nonsusceptible A. baumannii isolates for this study for 1 or 2 months in 2009. Only one isolate was included from each patient. The isolates were identified as A. baumannii using an automated instrument in each clinical microbiology laboratory (Microscan WalkAway [Siemens Healthcare Diagnostics, Deerfield, IL], Vitek 2 [bioMérieux, Durham, NC], or Phoenix 100 [BD, Franklin Lakes, NJ]). The identification as A. baumannii was subsequently confirmed by detection of blaOXA-51-like by PCR in the research laboratory (34). In addition, nonsusceptibility to carbapenems was confirmed by resistance to imipenem and/or meropenem using the disk diffusion method in the research laboratory located at the University of Pittsburgh.
Susceptibility testing.
Susceptibility testing was performed following the methodology and breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) (5). Disk diffusion testing was used for the following agents: ampicillin-sulbactam, piperacillin-tazobactam, ceftazidime, cefepime, imipenem, meropenem, ciprofloxacin, gentamicin, and amikacin. For tigecycline, the Food and Drug Administration (FDA) breakpoints for Enterobacteriaceae (≤14 mm, resistant; ≥19 mm, susceptible) were used. In addition, MICs were determined for imipenem and meropenem using the agar dilution method.
PFGE and MLST.
Pulsed-field gel electrophoresis (PFGE) was conducted using a CHEF DR III system (Bio-Rad, Hercules, CA) using ApaI as the restriction enzyme, as described previously (1). Bionumerics version 6.01 (Applied Maths, Sint-Martens-Latem, Belgium) was used for pattern analysis, which utilizes the unweighted-pair group method. There are currently two multilocus sequence typing (MLST) schemes for A. baumannii which are widely utilized: one described by Bartual et al. (http://pubmlst.org/abaumannii/) (2) and one developed at the Pasteur Institute (http://www.pasteur.fr/mlst) (6). The two schemes share three of the seven loci. For the present study, we primarily used the Bartual scheme with modification of the primer sequences proposed by Martinovich et al. (19). For the purpose of comparison, we then selected one isolate from each sequence type (ST) obtained by the Bartual scheme and determined the corresponding ST based on the Pasteur Institute scheme. The new alleles and STs identified through this study have been deposited into the relevant databases. The relationship among the new and existing STs was surveyed by the use of the eBURST program (http://eburst.mlst.net/) (10).
PCR and sequencing of carbapenemase-encoding genes.
Detection of blaOXA-51-like and the ISAba1/blaOXA-51-like complex was performed by PCR using primer sets and conditions described previously (18, 33). The presence of the ISAba1/blaOXA-51-like complex has been implicated in various degrees of carbapenem resistance in A. baumannii (33). PCR for the blaOXA-23, blaOXA-40, and blaOXA-58 genes, the three major groups of acquired carbapenamase-encoding genes that confer clinically relevant resistance to carbapenems, was conducted using a multiplex scheme (38). For sequencing, the full structural genes of blaOXA-23 and blaOXA-40 were amplified using primers described previously (18). Sequencing was performed on a 3730 DNA analyzer (Life Technologies, Carlsbad, CA).
RESULTS
Susceptibility of the carbapenem-nonsusceptible A. baumannii isolates.
Of a total of 67 isolates in the initial collection, two were found to be susceptible to both imipenem and meropenem in the research laboratory and were excluded. All of the remaining 65 isolates were positive for blaOXA-51-like by PCR. The numbers of isolates from each participating hospital were as follows: New York, 12; Pittsburgh, 10; St. Louis, 13; Jacksonville, 6; Las Vegas, 14; and Los Angeles, 10. The isolates were identified from various sources, including blood, sputum, bronchoalveolar lavage, wound, and urine specimens.
Susceptibility testing results of the study isolates are shown in Table 1. All isolates were nonsusceptible to meropenem, whereas 16 isolates (25%) were susceptible to imipenem by the disk diffusion method. All 5 isolates that were intermediate to meropenem were susceptible to imipenem. Most isolates were nonsusceptible to β-lactams other than carbapenems, including piperacillin-tazobactam, ceftazidime, and cefepime. Of the β-lactams, susceptibility to ampicillin-sulbactam was relatively conserved, with 51% of the isolates being susceptible and another 26% intermediate. All isolates were susceptible to tigecycline when using the breakpoint for Enterobacteriaceae defined by the FDA. The MIC50 and MIC90 of imipenem were 16 and 64 μg/ml, respectively, whereas MIC50 and MIC90 of meropenem were 32 and 128 μg/ml, respectively (Table 2).
Table 1.
Susceptibility of 65 carbapenem-nonsusceptible A. baumannii isolates determined by the disk diffusion method
| Antimicrobial | Susceptibility; no. (%) of isolates: |
||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Ampicillin-sulbactam | 33 (50.8) | 17 (26.2) | 15 (23.0) |
| Piperacillin-tazobactam | 1 (1.5) | 0 (0) | 64 (98.5) |
| Ceftazidime | 3 (4.6) | 3 (4.6) | 59 (90.8) |
| Cefepime | 2 (3.1) | 17 (26.1) | 46 (70.8) |
| Imipenem | 16 (24.6) | 8 (12.3) | 41 (63.1) |
| Meropenem | 0 (0) | 5 (7.7) | 60 (92.3) |
| Ciprofloxacin | 0 (0) | 0 (0) | 65 (100) |
| Gentamicin | 13 (20.0) | 0 (0) | 52 (80.0) |
| Amikacin | 16 (24.6) | 5 (7.7) | 44 (67.7) |
| Tigecycline | 65 (100) | 0 (0) | 0 (0) |
Table 2.
MICs of imipenem and meropenem for the 65 study isolates determined by the agar dilution method
| Antimicrobial | No. of isolates with MIC (μg/ml) of: |
MIC (μg/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >128 | 50% | 90% | |
| Imipenem | 1 | 3 | 14 | 7 | 23 | 10 | 6 | 1 | 0 | 16 | 64 |
| Meropenem | 0 | 1 | 1 | 10 | 7 | 28 | 8 | 7 | 3 | 32 | 128 |
50% and 90%, MIC50 and MIC90, respectively.
Molecular typing.
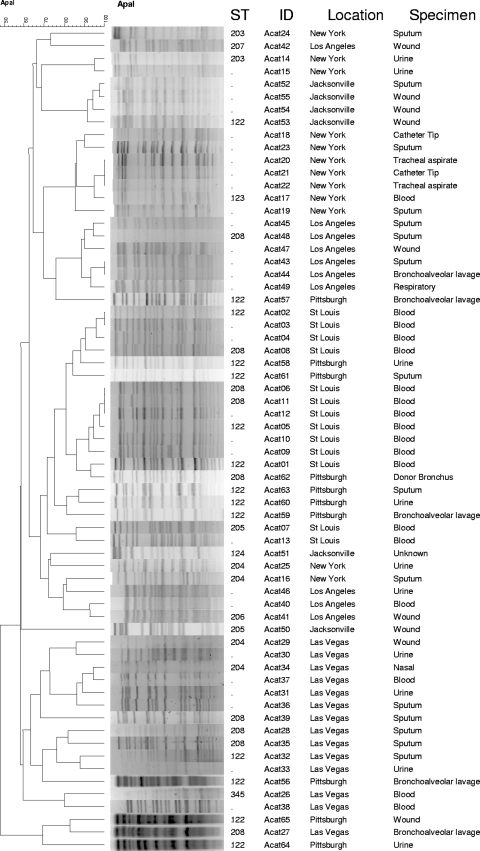
The results of PFGE and MLST are summarized in Fig. 1, along with the information on the specimen sources and the hospital locations.
Fig. 1.
PFGE and MLST results from the carbapenem-nonsusceptible study isolates. STs are based on the Bartual scheme.
PFGE.
PFGE was performed on all 65 isolates. Using a cutoff of 80% similarity, the isolates were grouped into 24 clusters (Fig. 1). The largest cluster contained 13 isolates from St. Louis and Pittsburgh. The second and third largest clusters had 7 and 6 isolates from New York and Los Angeles, respectively. Only one other cluster contained isolates from more than one hospital (one isolate each from Pittsburgh and Las Vegas).
MLST. (i) Bartual scheme.
At least one isolate from each cluster was selected for MLST. Overall, 36 of the 65 isolates were typed by MLST under the Bartual scheme. The STs identified included ST122 to -124, which we recently reported in isolates from Pittsburgh (32), as well as ST203 to -208 and ST345, which are new STs that were identified in the present study. The alleles and STs described in this study are summarized in Table 3.
Table 3.
Sequence types and alleles of the carbapenem-nonsusceptible study isolates and select reference strains
| ST by Bartual scheme | MLST by Bartual scheme |
ST by Pasteur Institute scheme | MLST by Pasteur Institute scheme |
City(ies) or reference strain | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele |
Clonal complex | Allele |
Clonal complex | |||||||||||||||
| gltA | gyrB | gdhB | recA | cpn60 | gpi | rpoD | cpn60 | fusA | gltA | pyrG | recA | rpiB | rpoB | |||||
| 122 | 1 | 3 | 61 | 2 | 2 | 7 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | Pittsburgh, St. Louis, Las Vegas, Jacksonville |
| 123 | 1 | 35 | 61 | 2 | 2 | 3 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | New York |
| 124 | 1 | 17 | 60 | 10 | 28 | 66 | 32 | 113 | 79 | 26 | 2 | 2 | 2 | 29 | 4 | 5 | Singleton | Jacksonville |
| 203 | 41 | 1 | 72 | 32 | 1 | 98 | 6 | Singleton | 124 | 3 | 3 | 2 | 2 | 28 | 1 | 3 | 3 | New York |
| 204 | 1 | 17 | 61 | 2 | 2 | 99 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | New York, Las Vegas |
| 205 | 1 | 17 | 60 | 10 | 28 | 99 | 32 | 113 | 79 | 26 | 2 | 2 | 2 | 29 | 4 | 5 | Singleton | St. Louis, Jacksonville |
| 206 | 1 | 17 | 72 | 2 | 2 | 99 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | Los Angeles |
| 207 | 10 | 53 | 84 | 11 | 4 | 100 | 5 | Singleton | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | Los Angeles |
| 208 | 1 | 3 | 61 | 2 | 2 | 97 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | Pittsburgh, St. Louis, Los Angeles, Las Vegas |
| 345 | 46 | 12 | 122 | 1 | 16 | 141 | 50 | Singleton | 123 | 5 | 2 | 3 | 2 | 3 | 1 | 5 | Singleton | Las Vegas |
| 231 | 10 | 12 | 88 | 11 | 4 | 98 | 5 | Singleton | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | AYE |
| 207 | 10 | 53 | 84 | 11 | 4 | 100 | 5 | Singleton | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | AB0057 |
| 12 | 8 | 4 | 4 | 4 | 4 | 5 | 5 | Singleton | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | RUH875 |
| 1 | 12 | 61 | 2 | 2 | 97 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ACICU | |
| 6 | 1 | 4 | 3 | 2 | 2 | 3 | 3 | 92 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | RUH134 |
| 112 | 1 | 12 | 56 | 36 | 1 | 61 | 26 | Singleton | 77 | 3 | 2 | 2 | 2 | 3 | 4 | 28 | Singleton | ATCC 17978 |
ST122 was the most commonly observed ST, accounting for 14 of 36 isolates that were typed by MLST, followed by ST208, which was identified in 9 isolates. Both ST122 and -208 were found in four of the six hospitals. These STs belong to EUII and clonal complex 92 (CC92). CC92 is the most prevalent clonal complex worldwide, encompassing over 50 STs that have been identified from many countries, including some from the United States (11, 13, 15, 17, 21, 23, 28, 32, 37). The next most common ST was ST204 (4 isolates), which along with ST206 (1 isolate) comprised isolates from three hospitals and also belonged to CC92. Three isolates belonged to CC113, which is commonly reported from Argentina and Brazil (http://pubmlst.org/abaumannii/) (12a). They included ST124 (1 isolate) and ST205 (2 isolates) from two hospitals. There were three singleton STs, ST203, ST207, and ST345. ST203 was identified from New York and belonged to EUIII. ST207 was identified from Los Angeles as a DLV of STs within CC109, which belongs to EUI. CC109 has been identified worldwide, including Europe, East Asia, and South America (http://pubmlst.org/abaumannii/). ST345, which was found in an isolate from Las Vegas, could not be categorized according to any of the three major pan-European clonal lineages based on MLST.
We only determined ST for approximately half of the study isolates. If we assume that isolates from the same PFGE cluster belong to the same clonal complex, then 55 of the 65 isolates would be assigned to CC92.
(ii) Pasteur Institute scheme.
To examine how the two MLST schemes compare, we then conducted MLST based on the Pasteur Institute scheme for isolates representing each ST based on the Bartual scheme. The five STs representing CC92 under the latter scheme were all assigned to ST2 under the Pasteur Institute scheme (Table 3).
Carbapenemase-encoding genes.
Twenty-seven of the 65 isolates (42%) were positive for blaOXA-23 by PCR. Thirteen of them were subjected to sequencing of the entire gene, and they were all consistent with OXA-23, underscoring the homogeneity of this enzyme. blaOXA-23-positive isolates were found from all six hospitals and in multiple STs (ST122 and ST204 to -208). Nine isolates (14%) were positive for blaOXA-40. Seven of them were from ST123 isolates from New York and were found to encode OXA-72 upon sequencing, which is a single-amino-acid variant of OXA-40 (32). The other two isolates were from Pittsburgh (ST122) and Jacksonville (ST124) and encoded OXA-40. None of the isolates was positive for blaOXA-58. Forty-two isolates (65%) were positive for the ISAba1/blaOXA-51-like complex, which may contribute to carbapenem resistance (33). Two isolates were negative for any of the carbapenemase genes mentioned above.
DISCUSSION
It is increasingly recognized that A. baumannii is clonal in nature and that a large part of the global epidemic of multidrug-resistant A. baumannii is driven by strains that belong to EUII, in particular those defined as CC92 by MLST (11, 15, 17, 21, 23, 28). In the United States, an outbreak of multidrug-resistant A. baumannii from a hospital in Houston that took place between 2005 and 2006 was caused by CC92 strains (30). A survey of bacteremic isolates collected from 52 U.S. hospitals between 1998 and 2004 also showed the preponderance of CC92 (37). Carbapenem resistance is becoming more and more common among A. baumannii isolates in U.S. hospitals in recent years (16, 20). It poses a substantial clinical challenge as therapeutic options are extremely limited for these organisms. This study was conducted to better understand the molecular epidemiology of carbapenem-nonsusceptible A. baumannii in U.S. hospitals, with the aim of identifying predominant clonal lineages currently circulating in this country.
Our analysis revealed CC92 to be the most prevalent clonal complex, identified in isolates from all 6 participating hospitals. Interestingly, however, ST92, the predicted founder of CC92 and reported from a number of European and East Asian countries as well as Australia, was not found in this study. Instead, ST122, ST123, ST204, ST206, and ST208 were identified as CC92 STs, with predominance of ST122 and ST208, both of which were found in 4 of the 6 participating hospitals. While ST123 was only found in the hospital in New York in this study, we previously reported this ST from an isolate in Pittsburgh (32). To our knowledge, these STs have not been identified outside the United States. Furthermore, they share a novel gdhB allele encoding quinoprotein glucose dehydrogenase B (allele 61), with the exception of ST206. Taken together, these findings may suggest that the current epidemic of carbapenem-nonsusceptible CC92 strains in the United States represents clonal expansion of progenitor strains, which was likely facilitated by patient transfer, rather than repeated importation of CC92 strains from overseas. The other clonal complex identified in this study was CC113 (ST124 and ST205). Only two singletons were identified from the other pan-European lineages: ST203 from New York (EUIII) and ST207 from Los Angeles (EUI), further underscoring the clonal nature of carbapenem-nonsusceptible A. baumannii isolates currently circulating in the United States.
While MLST is a powerful molecular typing methodology, its cost could be forbidding for routine use. We opted to first group isolates by PFGE, which is more discriminatory than MLST, and then subject representative isolates from each pulse type to MLST. As can be seen in Fig. 1, the isolates from the same hospital tended to cluster together. There were also some instances where isolates from different hospitals could be categorized as the same pulse type (e.g., ST122 isolates from Pittsburgh and St. Louis), but the PFGE patterns were generally diverse for isolates from different hospitals, even when the STs were identical. This finding is in line with the operating characteristics of these two typing methods, where PFGE is well suited for studying isolates from a defined temporal and spatial epidemiologic setting, whereas MLST has an advantage in defining clonal lineages of isolates from larger geographic areas over time.
It has been suggested that two MLST loci used in the Bartual scheme (gyrB and gpi) are prone to recombination (13). In our comparison of the two MLST schemes, ST2 under the Pasteur Institute scheme was represented by 5 STs under the Bartual scheme. This variation resulted from the presence of 4, 3, and 2 alleles at the gpi, gyrB, and gdhB loci in the Bartual scheme, respectively. We did not have enough STs in EUI and EUIII to make a useful comparison.
As expected, blaOXA-23 was the most common carbapenemase-encoding gene, which was found across different clonal complexes of carbapenem-nonsusceptible isolates from all hospitals. Globally, blaOXA-23 is the most prevalent acquired carbapenemase-encoding gene that is associated with carbapenem resistance (21). blaOXA-40 and its variant blaOXA-72 were found in isolates from three hospitals. The finding of isolates with blaOXA-23 and blaOXA-72 from the hospital in New York was noteworthy, since a previous comprehensive study describing resistance mechanisms of 40 multidrug-resistant A. baumannii isolates collected from hospitals in the area between 2001 and 2006 did not reveal the presence of acquired OXA-type carbapenemase-encoding genes (4). It is thus possible that an ST204 strain with blaOXA-23 and an ST123 strain with blaOXA-72 were introduced in the area between the two study periods.
While the primary scope of this study was molecular epidemiology, we also found that susceptibility to ampicillin-sulbactam was maintained in 33 of 65 isolates, all of which were nonsusceptible to at least one carbapenem tested. They included 12 blaOXA-23-positive isolates, 2 blaOXA-40-positive isolates, and 7 blaOXA-72-positive isolates. Sulbactam, the β-lactamase inhibitor component of this formulation, is known to have intrinsic activity against A. baumannii, which is believed to be due to its high affinity to certain penicillin-binding proteins (35). Several clinical studies have suggested that ampicillin-sulbactam may be clinically efficacious in the management of infections due to carbapenem-resistant A. baumannii (3, 22). While susceptibility to tigecycline was maintained well, we included only the initial isolate from each patient for this study. Since resistance to tigecycline may develop in subsequent isolates after exposure to this agent (24, 27), our results may underestimate the prevalence of nonsusceptibility on a per case basis.
The limitation of our study was that the isolates were collected in a relatively brief time period at some of the participating hospitals, which may not represent the overall epidemiology of carbapenem-nonsusceptible A. baumannii at those hospitals. Based on our findings, we plan to conduct a longitudinal surveillance study to further elucidate the epidemiology of this organism in the United States.
In conclusion, STs representing CC92 appear to be predominant among carbapenem-nonsusceptible A. baumannii isolates in U.S. hospitals, suggesting that they constitute part of the global epidemic driven by this clonal complex belonging to EUII. However, the finding that STs in CC92 were not ST92 but predominantly ST122 and ST208, which are thus far unique to the United States, suggests that these organisms may be spreading through transfer of colonized patients between health care facilities within the country.
ACKNOWLEDGMENTS
We thank Lenie Dijkshoorn for provision of the pan-European clonal lineage reference strains. We are also grateful to the curators of the MLST databases for their assistance (Sergio Bartual, Hilmar Wisplinghoff, and Laure Diancourt).
This study was funded by the National Institute of Allergy and Infectious Diseases (K22AI80584) and the Pennsylvania Department of Health (grant no. 4100047864).
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Adams-Haduch J. M., et al. 2008. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob. Agents Chemother. 52:3837–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartual S. G., et al. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 43:4382–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Betrosian A. P., et al. 2007. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand. J. Infect. Dis. 39:38–43 [DOI] [PubMed] [Google Scholar]
- 4. Bratu S., et al. 2008. Correlation of antimicrobial resistance with β-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York City. Antimicrob. Agents Chemother. 52:2999–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6. Diancourt L., et al. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dijkshoorn L., et al. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doi Y., et al. 2009. Extensively drug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 15:980–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ecker J. A., et al. 2006. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J. Clin. Microbiol. 44:2921–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feil E. J., et al. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Y., et al. 2010. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J. Antimicrob. Chemother. 65:644–650 [DOI] [PubMed] [Google Scholar]
- 12. Grisold A. J., et al. 2010. Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J. Infect. 60:44–51 [DOI] [PubMed] [Google Scholar]
- 12a. Grosso F., et al. 2011. OXA-23-producing Acinetobacter baumannii: a new hotspot of diversity in Rio de Janeiro? J. Antimicrob. Chemother. 66:62–65 [DOI] [PubMed] [Google Scholar]
- 13. Hamouda A., et al. 2010. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of blaOXA-51-like genes. J. Clin. Microbiol. 48:2476–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins P. G., et al. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:233–238 [DOI] [PubMed] [Google Scholar]
- 15. Ho P. L., et al. 2010. Epidemiology and clonality of multidrug-resistant Acinetobacter baumannii from a healthcare region in Hong Kong. J. Hosp. Infect. 74:358–364 [DOI] [PubMed] [Google Scholar]
- 16. Kallen A. J., et al. 2010. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect. Control Hosp. Epidemiol. 31:528–531 [DOI] [PubMed] [Google Scholar]
- 17. Lee Y., et al. 2010. Carbapenem-non-susceptible Acinetobacter baumannii of sequence type 92 or its single-locus variants with a G428T substitution in zone 2 of the rpoB gene. J. Antimicrob. Chemother. 66:66–72 [DOI] [PubMed] [Google Scholar]
- 18. Marti S., et al. 2008. Characterization of the carbapenem-hydrolyzing oxacillinase OXA-58 in an Acinetobacter genospecies 3 clinical isolate. Antimicrob. Agents Chemother. 52:2955–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinovich A., et al. Multilocus sequence typing of Acinetobacter strains from Russia and Belarus that produce acquired OXA carbapenemases, abstr. C2-626. Abstr.. 49th Intersci. Conf. Antimicrob.; 2009. Agents Chemother. [Google Scholar]
- 20. Mera R. M., et al. 2010. Acinetobacter baumannii 2002–2008: increase of carbapenem-associated multiclass resistance in the United States. Microb. Drug Resist. 16:209–215 [DOI] [PubMed] [Google Scholar]
- 21. Mugnier P. D., et al. 2010. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 16:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliveira M. S., et al. 2008. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J. Antimicrob. Chemother. 61:1369–1375 [DOI] [PubMed] [Google Scholar]
- 23. Park Y. K., et al. 2010. A single clone of Acinetobacter baumannii, ST22, is responsible for high antimicrobial resistance rates of Acinetobacter spp. isolates that cause bacteremia and urinary tract infections in Korea. Microb. Drug Resist. 16:143–149 [DOI] [PubMed] [Google Scholar]
- 24. Peleg A. Y., et al. 2007. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J. Antimicrob. Chemother. 59:128–131 [DOI] [PubMed] [Google Scholar]
- 25. Peleg A. Y., et al. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez F., et al. 2010. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J. Antimicrob. Chemother. 65:1807–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reid G. E., et al. 2007. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy 27:1198–1201 [DOI] [PubMed] [Google Scholar]
- 28. Runnegar N., et al. 2010. Molecular epidemiology of multi-resistant Acinetobacter baumannii in a single institution over a ten year period. J. Clin. Microbiol. 48:4051–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seifert H., et al. 2005. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 43:4328–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shelburne S. A., III, et al. 2008. Sequential outbreaks of infections by distinct Acinetobacter baumannii strains in a public teaching hospital in Houston, Texas. J. Clin. Microbiol. 46:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh A., et al. 2006. Application of molecular techniques to the study of hospital infection. Clin. Microbiol. Rev. 19:512–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian G. B., et al. 2010. Identification of diverse OXA-40-group carbapenemases including a novel variant, OXA-160, from Acinetobacter baumannii in Pennsylvania. Antimicrob. Agents Chemother. 55:429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turton J. F., et al. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72–77 [DOI] [PubMed] [Google Scholar]
- 34. Turton J. F., et al. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44:2974–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Urban C., et al. 1995. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible Acinetobacter baumannii. FEMS Microbiol. Lett. 125:193–198 [Google Scholar]
- 36. van Dessel H., et al. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 155:105–112 [DOI] [PubMed] [Google Scholar]
- 37.Wisplinghoff H., et al. MLST-based molecular epidemiology of clinical Acinetobacter baumannii bloodstream isolates from 52 hospitals in the US, abstr. C2-590. Abstr.. 50th Intersci. Conf. Antimicrob.; 2010. Agents Chemother. [Google Scholar]
- 38. Woodford N., et al. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351–353 [DOI] [PubMed] [Google Scholar]