Abstract
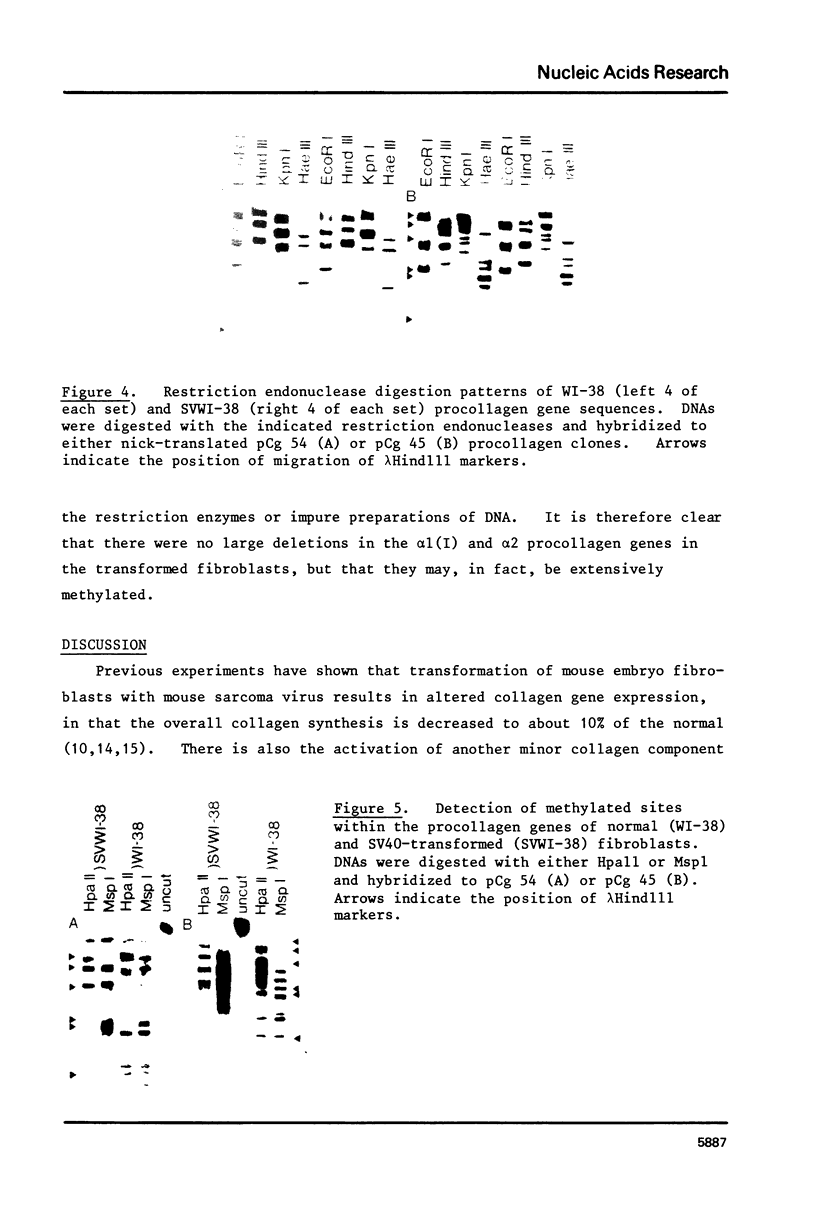
Transformation of human lung fibroblasts (WI-38) by Simian Virus 40 (SV40) resulted in a decline of 25-30% in the amount of secreted collagen. The collagen produced by the transformed fibroblasts contained no type I collagen (i.e. alpha 1(I) and alpha 2 chains), which was the major collagen component produced by untransformed fibroblasts. Measurement of the procollagen mRNA levels by dot hybridization with nick-translated procollagen-cDNA clones showed that the absence of type I collagen was due to the absence of alpha 1(I) and alpha 2 procollagen mRNAs. This result was confirmed by hybridization of cDNA to total RNA with southern blots of the procollagen clones. To clarify the mechanism by which type I procollagen gene transcription is abolished in transformed cells, the methylation patterns of the alpha 1(I) and alpha 2 procollagen genes in normal and SV40-transformed fibroblasts were compared, using the chicken alpha 1(I) and alpha 2 procollagen-cDNA clones as probes. Methylated sites were detected by means of the restriction endonuclease isoschizomers HpaII and MspI. Methylation of the procollagen alpha 1(I) and alpha 2 genes was increased in the SV40-transformed fibroblasts, concurrently with the loss of type I collagen synthesis. DNA methylation may thus contribute to altered regulation of gene expression upon cell transformation.
Full text
PDF










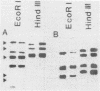
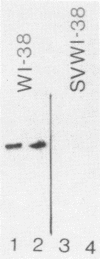
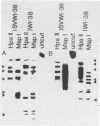
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Bateman J. F., Peterkofsky B. Mechanisms of Kirsten murine sarcoma virus transformation-induced changes in the collagen phenotype and synthetic rate of BALB 3T3 cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6028–6032. doi: 10.1073/pnas.78.10.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Dackowski W., Morrison S. L. Two alpha heavy chain disease proteins with different genomic deletions demonstrate that nonexpressed alpha heavy chain genes contain methylated bases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7091–7095. doi: 10.1073/pnas.78.11.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Diala E. S., Plent M. M., Coalson D. W., Hoffman R. M. DNA methylation in normal and SV40-transformed human fibroblasts. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1379–1384. doi: 10.1016/s0006-291x(81)80164-7. [DOI] [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Collagen synthesis as a marker for cell type in mouse 3T3 lines. Cell. 1977 May;11(1):169–172. doi: 10.1016/0092-8674(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Green H., Goldberg B., Todaro G. J. Differentiated cell types and the regulation of collagen synthesis. Nature. 1966 Nov 5;212(5062):631–633. doi: 10.1038/212631b0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. Rigid control of synthesis of collagen types I and III by cells in culture. Nature. 1977 Jul 14;268(5616):152–154. doi: 10.1038/268152a0. [DOI] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Specific changes in the collagen phenotype of BALB 3T3 cells as a result of transformation by sarcoma viruses or a chemical carcinogen. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2933–2937. doi: 10.1073/pnas.74.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Nagai Y. Separation of the alpha chains of type I and III collagens by SDS-polyacrylamide gel electrophoresis. J Biochem. 1979 Aug;86(2):453–459. doi: 10.1093/oxfordjournals.jbchem.a132544. [DOI] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Ishimoto N., Temin H. M., Strominger J. L. Studies of carcinogenesis by avian sarcoma viruses. II. Virus-induced increase in hyaluronic acid synthetase in chicken fibroblasts. J Biol Chem. 1966 May 10;241(9):2052–2057. [PubMed] [Google Scholar]
- Jones R. E., DeFeo D., Piatigorsky J. Transcription and site-specific hypomethylation of the delta-crystallin genes in the embryonic chicken lens. J Biol Chem. 1981 Aug 10;256(15):8172–8176. [PubMed] [Google Scholar]
- Kalgsbrun M. The decreased synthesis of chondroitin sulfate-containing extracellular proteoglycans by SV40 transformed Balb/c 3T3 cells. Biochim Biophys Acta. 1976 Nov 18;451(1):170–183. [PubMed] [Google Scholar]
- Kamine J., Rubin H. Coordinate control of collagen synthesis and cell growth in chick embryo fibroblasts and the effect of viral transformation on collagen synthesis. J Cell Physiol. 1977 Jul;92(1):1–11. doi: 10.1002/jcp.1040920102. [DOI] [PubMed] [Google Scholar]
- Krieg T., Aumailley M., Dessau W., Wiestner M., Müller P. Synthesis of collagen by human fibroblasts and their SV40 transformants. Exp Cell Res. 1980 Jan;125(1):23–30. doi: 10.1016/0014-4827(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Boedtker H. Construction and characterization of pro alpha 1 collagen complementary deoxyribonucleic acid clones. Biochemistry. 1979 Jul 10;18(14):3146–3152. doi: 10.2196/47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaud L., Reynaud C. A., Therwath A., Scherrer K. Modification of the methylation pattern in the vicinity of the chicken globin genes in avian erythroblastosis virus transformed cells. Nucleic Acids Res. 1981 Apr 24;9(8):1841–1851. doi: 10.1093/nar/9.8.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Monson J. M., McCarthy B. J. Identification of a Balb/c mouse pro alpha 1(I) procollagen gene: evidence for insertions or deletions in gene coding sequences. DNA. 1981;1(1):59–69. doi: 10.1089/dna.1.1981.1.59. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Natzle J., Friedman J., McCarthy B. J. Expression and novel structure of a collagen gene in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1761–1765. doi: 10.1073/pnas.79.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzle J. E., Monson J. M., McCarthy B. J. Cytogenetic location and expression of collagen-like genes in Drosophila. Nature. 1982 Mar 25;296(5855):368–371. doi: 10.1038/296368a0. [DOI] [PubMed] [Google Scholar]
- Niwa O., Sugahara T. 5-Azacytidine induction of mouse endogenous type C virus and suppression of DNA methylation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6290–6294. doi: 10.1073/pnas.78.10.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Fitschen W. Globin gene expression in MSV-transformed fibroblasts. Experientia. 1979 Oct 15;35(10):1312–1313. doi: 10.1007/BF01963978. [DOI] [PubMed] [Google Scholar]
- Parker I., Fitschen W. Histone mRNA metabolism during the mouse fibroblast cell cycle. Cell Differ. 1980 Feb;9(1):23–30. doi: 10.1016/0045-6039(80)90004-4. [DOI] [PubMed] [Google Scholar]
- Parker I., Fitschen W. Procollagen mRNA metabolism during the fibroblast cell cycle and its synthesis in transformed cells. Nucleic Acids Res. 1980 Jun 25;8(12):2823–2833. doi: 10.1093/nar/8.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Soo W. J., Bissell M. J. The uncoupled regulation of fibronectin and collagen synthesis in Rous sarcoma virus transformed avian tendon cells. J Biol Chem. 1979 Dec 10;254(23):11763–11766. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. Immunoglobulin heavy chain genes: demethylation accompanies class switching. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7497–7501. doi: 10.1073/pnas.78.12.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Saito H., Uzman B. G. Production and secretion of chondroitin sulfates and dermatan sulfate by established mammalian cell lines. Biochem Biophys Res Commun. 1971 May 21;43(4):723–728. doi: 10.1016/0006-291x(71)90675-9. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Smith R., Kiehn D., Bornstein P. Correlation of collagen synthesis and procollagen messenger RNA levels with transformation in rat embryo fibroblasts. Cancer Res. 1981 Mar;41(3):830–838. [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., Niles R. Characterization of collagen synthesized by normal and chemically transformed rat liver epithelial cell lines. Biochemistry. 1980 Apr 29;19(9):1820–1825. doi: 10.1021/bi00550a014. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein G. H. Differences in DNA-binding proteins isolate from normal and transformed human cells. Exp Cell Res. 1976 Apr;99(1):115–125. doi: 10.1016/0014-4827(76)90686-8. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., Thomson J. A. Chromosomal proteins in transformed and neoplastic cells: a review. Cancer Res. 1978 May;38(5):1181–1201. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Cheah K. S., Grosveld F. G., Dahl H. H., Solomon E., Flavell R. A. Isolation and characterization of a human collagen alpha 1(I)-like gene from a cosmid library. Nucleic Acids Res. 1982 Mar 25;10(6):1981–1994. doi: 10.1093/nar/10.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M., Koshland M. E. Expression of the J chain gene during B cell differentiation is inversely correlated with DNA methylation. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4907–4911. doi: 10.1073/pnas.78.8.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Groffen J., Flavell R. A. A novel type of secondary modification of two CCGG residues in the human gamma delta beta-globin gene locus. Nucleic Acids Res. 1980 Oct 24;8(20):4563–4574. doi: 10.1093/nar/8.20.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]