Abstract
Immune evasion is a defining feature of the virus-host relationship. During infection, herpes simplex virus type 1 (HSV-1) utilizes multiple proteins to manipulate the host immune response. In the present study, we investigated the mechanism by which the virion host shutoff (vhs) protein blocks the activation of dendritic cells (DCs). Previously, we found that coinfection of wild-type HSV-1 with a panel of RNA viruses resulted in a block to DC activation that was attributable to vhs. These observations led us to hypothesize that the vhs-mediated inhibition was dependent on signaling through the RIG-I-like receptor (RLR) signaling pathway. By examining DCs generated from MAVS (IPS-1) knockout (KO) mice, we determined that RLR/MAVS signaling is not essential for the DC response to HSV-1. We also evaluated the requirement for the type I interferon (IFN) signaling pathway in DC activation following infection with HSV-1 and found that stimulation of DCs with wild-type HSV-1 required intact type I IFN signaling for the production of cytokines, whereas the vhs deletion (vhs−) mutant virus activated DCs without the need for exogenous IFN signaling. Comparisons of transcription factor activation in DCs infected with wild-type HSV and the vhs− mutant virus revealed that NF-κB activation was inhibited by vhs in the early phase of the infection. In contrast, IRF3 activation was not influenced by vhs. In these studies, measurement of proinflammatory cytokines and type I IFN release from the infected DCs reflected the activation status of these transcription factors. Taken together, the work presented here (i) describes a novel role for the vhs protein as an inhibitor of the early activation of NF-κB during HSV-1 infection of DCs and (ii) offers a mechanistic explanation of how this protein interferes with DC activation.
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) is a highly successful human pathogen belonging to the Alphaherpesviridae subfamily of herpesviruses. Initial exposure to virus results in lifelong infection, and it is estimated that between 60 and 80% of humans are seropositive for the virus (52). Normal HSV infections are characterized by cycling between lytic infection at epithelial surfaces and stages of latency in neuronal cells (reviewed in reference 47). The pathology of HSV infection is greatly influenced by the immune status of the host, which impacts both disease severity and the frequency of reactivation (21, 35, 42, 48, 69).
Early during primary infection of the epithelium, HSV encounters a specialized type of immune cell, the dendritic cells (DCs). DCs function as a crucial link between innate and adaptive immune responses (reviewed in reference 4). These cells survey peripheral tissues in an immature state and undergo a process referred to as maturation (or activation) upon encounter with virus-associated molecules (5, 32). DC maturation is initially characterized by the secretion of type I and III interferons (IFNs) and proinflammatory cytokines (e.g., interleukin 6 [IL-6], tumor necrosis factor alpha [TNF-α], and IL-12) and regulation of molecules necessary for migration to peripheral lymph nodes (32). En route to these secondary lymphoid organs, the DCs upregulate several costimulatory markers (CD86 and CD80) and load viral antigen onto major histocompatibility complex (MHC) molecules, which in concert serve to stimulate naïve B and T cells (4).
Numerous viral proteins are utilized by HSV to evade the host immune response at all stages of the virus life cycle (7, 9, 31, 33, 39, 63). Immunomodulatory proteins are either produced during the virus replication cycle or prepackaged in viral particles in the tegument and deposited into the cell immediately following virus envelope-host cell membrane fusion. The virion-host shutoff (vhs) protein is one such tegument-localized viral protein synthesized with late kinetics and packaged into mature virion particles (14, 25, 51, 59). Functionally, vhs is a viral RNase that is known to preferentially degrade both host and viral mRNA species (14, 44, 45, 49, 51, 59). vhs has been reported to interfere with DC activation during both productive and nonproductive HSV infection (7, 49). At present, the precise mechanism by which vhs acts to silence HSV-induced DC activation remains undefined. We have previously shown that the activation of DCs by HSV occurs through a pathway independent of Toll-like receptor (TLR) signaling and that vhs blocks this non-TLR route of viral recognition (7). Moreover, vhs can block the activation of DCs triggered during coinfection of HSV-1 with RNA viruses. One implication of this earlier study is that vhs may target the RIG-I-like receptor (RLR) family of cytosolic sensors, the pattern recognition receptors that detect these RNA viruses.
Type I IFNs (IFN-α/β) are critical antiviral factors produced during virus infection (reviewed in reference 60). An initial induction phase results in modest levels of IFN-β expression driven by activation of the transcription factors NF-κB, IRF3, and AP-1. Secreted IFN-β binds to its receptor in both an autocrine and a paracrine manner and signals through the Jak-STAT pathway to activate IRF7, leading to both IFN-α production and an amplification of the initial IFN-β signal. An important additional consequence of IFN-α/β receptor signaling is the induction of several interferon-stimulated genes (ISGs) known to inhibit virus replication. In addition to limiting virus infection, activation of the type I IFN pathway is important for DCs to undergo the maturation process (55, 60). Infection by certain viruses, such as Newcastle disease virus (NDV) and murine cytomegalovirus (MCMV), fail to mature DCs when these cells are generated from IFN receptor (IFNR) knockout (KO) mice, highlighting a crucial role for type I IFN signaling in this process (8, 18).
The replication of HSV-1 viruses in which the gene for vhs (UL41) is either mutated or absent is only moderately affected in both transformed and primary cell lines (7, 41). In contrast, in vivo infections with a vhs mutant virus are severely attenuated (26, 57, 58). There is much interest in employing vhs-null viruses as live, attenuated vaccines for HSV due in large part to this replication defect (15, 22, 23, 64). Interestingly, this growth defect is partially overcome when IFN signaling is absent, suggesting that the vhs block of the pathway is important for virus replication and pathogenesis (36). Consistent with this idea are the in vitro findings that vhs mutant infections of DCs results in increased STAT1 phosphorylation and more ISG transcripts than wild-type (WT) virus infections (10, 27, 41).
Here, we sought to better understand the early events governing the interaction between HSV-1 and DCs. To achieve this, we utilized a recombinant HSV deficient in host shutoff activity (which strongly activates DCs) as a tool to aid in understanding which molecular pathways are involved in the early activation of DCs following HSV-1 infection. In addition, we utilized this vhs-null virus to understand mechanistically how the vhs-mediated repression of DC function occurs. Our data show that the vhs protein carried in the virion and delivered into DCs blocks the early replication-independent activation of NF-κB during HSV-1 infection.
MATERIALS AND METHODS
Viruses, TLR agonists, and infection conditions.
KOS 1.1, referred to in this study as KOS, was the wild-type strain of HSV-1 used in all experiments. The vhs Δsma mutant, referred to in this study as vhs−, was generously provided by G. Sullivan Read and has been previously described (45). It contains a deletion of the 588-bp region between SmaI restriction enzyme sites in the UL41 gene. Importantly, this vhs− deletion virus does not incorporate the mutant vhs polypeptide into virions (45). All viruses were propagated and quantified on Vero cells as previously described (3, 37). For experiments requiring UV-inactivated viruses, viral stocks were exposed to UV irradiation at 10 cm from a germicidal lamp (UVP Multiple-Ray 8-WUV lamp [60 Hz]; Fisher) for 4 min. This amount of UV irradiation has previously been shown to prevent viral transcription and replication without inactivating the function of a representative tegument protein, VP16 (50). To confirm UV inactivation, transcript levels for viral immediate-early gene products were undetectable compared to non-UV-inactivated stocks via quantitative real-time (qRT)-PCR. Further, no virus growth was observed during Vero cell plaque assay. Recombinant NDV B1 was generated from the B1 Hitchner avian vaccine strain as previously described (40). Sendai virus (SeV)-HD (strain Cantel) and SeV-LD (strain 52) were grown in 10-day-old embryonated eggs as previously described (68). CpG oligodeoxynucleotide was obtained from Coley Pharmaceutical Group and utilized at a concentration of 6 μg/ml. Lipopolysaccharide (LPS) was obtained from Alexis Biochemicals and utilized at a concentration of 300 ng/ml. For all experiments, HSV-1 (both wild-type and vhs− mutant viruses) was added to DCs at a multiplicity of infection (MOI) of 5 (PFU/cell). RNA viruses were added at an MOI of 1.5. For cotreatment of HSV-1-infected cells with TLR agonists and coinfections with RNA viruses, following HSV-1 treatment, the TLR agonist (or RNA virus) was added to the cells immediately. Previously, we found that mock Vero cell lysate preparations did not activate DCs (7). In this study, where noted, noninfected cells received serum-free medium in parallel with cells that were treated with virus diluted in serum-free medium.
Mice.
Wild-type C57BL/6 and Sv129 mice were obtained from Taconic Farms (Germantown, NY). Type I IFNR KO mice were obtained from B&K Universal on the Sv129 genetic background, STAT1 KO mice were obtained from Taconic Farms on the Sv129 background, and MAVS (IPS-1) KO mice were generously provided by Kate Fitzgerald (University of Massachusetts) on the C57BL/6 background with the permission of Zhijian Chen (University of Texas Southwestern). All mice were bred and maintained in our facility consistent with regulations for animal care standard protocols as described by the Mount Sinai School of Medicine IACUC.
Cells.
Vero cells were grown in tissue culture medium (Dulbecco's modified Eagle's medium [Mediatech]) with 10% fetal calf serum (HyClone), 50 μg/ml gentamicin (Invitrogen). All cells were grown at 37°C at 5 to 7% CO2.
Murine bone marrow-derived dendritic cells (BM-DCs) were cultured from bone marrow-derived precursor cells as previously reported (29). Briefly, bone marrow was extracted from mouse femurs and tibias. Red blood cells were lysed with ammonium chloride buffer. MHC class II-expressing cells and lymphocytes were removed using a cocktail of antibodies and magnetic-bead separation. The purified precursor cells were cultured in 24-well dishes in RPMI medium containing 10% fetal bovine serum (FBS), 50 μg/ml gentamicin, 2 mM glutamine, 1 mM sodium pyruvate (NaPy), and 50 U/ml recombinant granulocyte macrophage colony-stimulating factor (PreproTech EC) at 1 × 106 cells/ml.
Capture ELISAs.
Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-6 (DuoSet ELISA development systems) were obtained from R&D Systems. ELISAs for mouse IL-12p40 were conducted using capture and secondary antibodies from BD Pharmingen. ELISA kits for IFN-β were obtained from PBL. Manufacturer instructions were followed for all ELISA methods used. Plates were read on an ELISA reader from Bioteck Instruments.
Western blotting and antibodies.
Protein extracts were generated from infected cell cultures by lysing cells in an NP-40-based lysis buffer containing protease and phosphatase inhibitors and 0.5 M EDTA (Thermo Scientific). Denatured samples were resolved on 10% Tris-Bis gels, transferred to polyvinylidene difluoride (PVDF) membranes, and immunoblotted with antibodies to ΙκΒ-α, phospho-ΙκB-α (Ser32/36), IRF3, and phospho-IRF3 (Ser396) (all obtained from Cell Signaling). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody was obtained from Sigma. Mouse monoclonal and rabbit polyclonal secondary antibodies (horseradish peroxidase [HRP] linked) were obtained from Jackson Immunologicals. Lumi-light Western blotting substrate was used for HRP detection (Roche).
Flow cytometry and antibodies.
Phycoerythrin (PE)-CD86, fluorescein isothiocyanate (FITC)-CD80, and PE-MHC II antibodies were obtained from BD Pharmingen. Cells were stained with antibodies and subjected to flow cytometry using the FC500 flow cytometer from Beckman Coulter. Data were analyzed with Flowjo software.
qRT-PCR.
RNA isolation was conducted on infected and noninfected cell cultures at the indicated time points using the commercially available Absolutely RNA Microprep Kit from Stratagene. RNA was concentrated via precipitation with ammonium acetate. An equal amount of RNA was reverse transcribed to generate the cDNA template to be used in qRT-PCRs using oligo(dT) (Roche) and Affinity Script Reverse Transcriptase (Stratagene). cDNA was diluted 50-fold in water. PCRs were conducted in triplicate using specific primers, Platinum Taq Polymerase (Roche), and SYBR green (Roche). The following primer sequences were used: IFN-λ (IL-28a), sense, 5′-AGGTCTGGGAGAACATGACTG-3′, and antisense, 5′-CTGTGGCCTGAAGCTGTGTA-3′; IRF7, sense, 5′-GGGCTGCAGTGGCTGAACGA-3′, and antisense, 5′-GCAGGTTAACTCCACTAGGT-3′; Mx1, sense, 5′-CAACTGGAATCCTCCTGGAA-3′, and antisense, 5′-GGCTCTCCTCAGAGGTATCA-3′; IFN-β, sense, 5′-AGATGTCCTCAACTGCTCTC-3′, and antisense, 5′-AGATTCACTACCAGTCCCAG-3′; IL-6, sense, 5′-ACAGAAGGAGTGGCTAAGGA-3′, and antisense, 5′-CGCACTAGGTTTGCCGAGTA-3′; TNF-α, sense, 5′-TCACTGGAGCCTCGAATGTC-3′, and antisense, 5′-GTGAGGAAGGCTGTGCATTG-3′; α-tubulin, sense, 5′-TGCCTTTGTGCACTGGTATG-3′, and antisense, 5′-CTGGAGCAGTTTGACGACAC-3′; rps11 sense, 5′-CGTGACGAAGATGAAGATGC-3′, and antisense, 5′-GCACATTGAATCGCACGTC-3′.
Reactions were run on 384-well plates using the Roche Lightcycler; normalizations were conducted using the levels of the α-tubulin and rps11 housekeeping genes.
Statistical analysis.
Statistical analysis was performed using a t test and paired two-sample analysis.
RESULTS
Cytokine mRNA is not degraded during cotreatment of wild-type HSV-infected cells with TLR agonists.
Initially, we addressed the impact of the potential vhs RNase function in the repression of DC activation during HSV-1 infection. We have previously reported that coinfection of HSV-infected cells with RNA viruses (SeV and NDV) resulted in a significant vhs-dependent block to cytokine secretion (7). In contrast, when HSV-1-infected cells were treated with TLR agonists, the presence or absence of the vhs protein had no effect on the accumulation of proinflammatory cytokines in the supernatants (7), suggesting that mRNA for these cytokines is not a target for the nuclease activity of vhs. We began this study by utilizing this coinfection model of HSV-1 infection and directly measured the mRNA levels for proinflammatory cytokines and type I IFN.
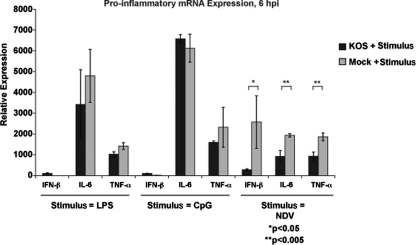
We infected BM-DCs with wild-type HSV-1 and then immediately added LPS, CpG, or NDV to the cells. The transcription of cytokines and type I IFN was measured by qRT-PCR (Fig. 1). We compared this level of mRNA expression to the level induced when the DCs were solely treated with either the TLR agonists or NDV (gray bars). Consistent with our previous findings looking at protein secretion, the mRNA expression for proinflammatory genes was not significantly altered during TLR agonist treatment of HSV-infected cells (compared to TLR treatment alone). We did, however, observe a significant decrease in IFN-β and cytokine mRNA expression during coinfection of HSV with NDV. These results highlight the sensitivity of the qRT-PCR assay to detect changes in cytokine and IFN-β mRNA expression. Moreover, these findings demonstrate that these select mRNA transcripts are not universally targeted for degradation by vhs and suggest that vhs may act by an as yet undescribed mechanism to inhibit DC activation. Based on the ability of wild-type HSV-1 to block NDV-induced proinflammatory cytokine mRNA expression, we next systematically evaluated what role known cellular signaling pathways critical for DC activation in response to NDV play during HSV-1 infection.
Fig. 1.
Coinfection of DCs with HSV-1 and an RNA virus, but not cotreatment with TLR agonists, significantly impairs proinflammatory gene mRNA expression. BM-DCs were generated from C57BL/6 mice and infected with wild-type HSV (KOS) at an MOI of 5 and then immediately treated with LPS (300 ng/ml), CpG (6 μg/ml), or NDV (MOI = 1.5). mRNA expression for IFN-β, IL-6, and TNF-α was measured by qRT-PCR at 6 h p.i. (black bars). In parallel, BM-DCs were also solely treated with LPS, CpG, or NDV and assayed for the same genes (gray bars). The error bars represent the differences between triplicate assays. *, P < 0.05; **, P < 0.005.
Activation of DCs by HSV-1 and subsequent inhibition by vhs are MAVS independent.
DC activation by RNA viruses is largely governed by members of the RLR family of cytosolic proteins (reviewed in reference 61). These receptors recognize the RNA of these viruses and signal through the mitochondrial adaptor protein MAVS to activate downstream transcription factors necessary for the expression of proinflammatory cytokines and IFNs. Based on the capacity for HSV to interfere with the activation of DCs in response to RNA virus infection (NDV and SeV [Fig. 1 and reference 7]), we investigated the role of MAVS in the DC response to HSV.
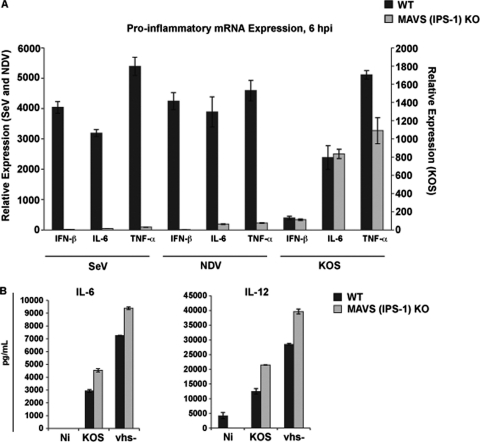
We generated BM-derived DCs from MAVS KO mice and infected them with SeV, NDV, and wild-type HSV-1. In contrast to SeV and NDV, HSV-1 could induce the expression of cytokines and type I IFN independently of MAVS (Fig. 2A). Further, when we compared wild-type HSV-1 to the vhs− mutant virus, which fails to incorporate the mutated vhs polypeptide into virion particles (45), we observed enhanced cytokine production for the vhs− mutant in both wild-type DCs and MAVS KO DCs (Fig. 2B). The data shown in Fig. 2 eliminate MAVS and, by extension, the RLR signaling pathway as critical for the activated DC phenotype observed during in vitro wild-type HSV or vhs− virus infection.
Fig. 2.
The HSV-1 vhs protein modulates an RLR-independent pathway to proinflammatory release during HSV-1 infection. (A) BM-DCs were generated from C57BL/6 control and MAVS KO mice and infected with wild-type HSV (KOS) (MOI = 5), SeV (MOI = 1.5), and NDV (MOI = 1.5). mRNA expression for IFN-β, IL-6, and TNF-α was measured by qRT-PCR at 6 h p.i. The error bars represent the differences between triplicate assays. (B) At 24 h p.i., control and (MAVS) IPS-1 KO DCs infected with KOS and vhs− viruses at an MOI of 5 were harvested, and the levels of IL-6 and IL-12p40 were measured from infected cell supernatants by ELISA. The error bars represent the differences between duplicate assays.
vhs blocks a pathway to proinflammatory cytokine release independently of type I IFN signaling.
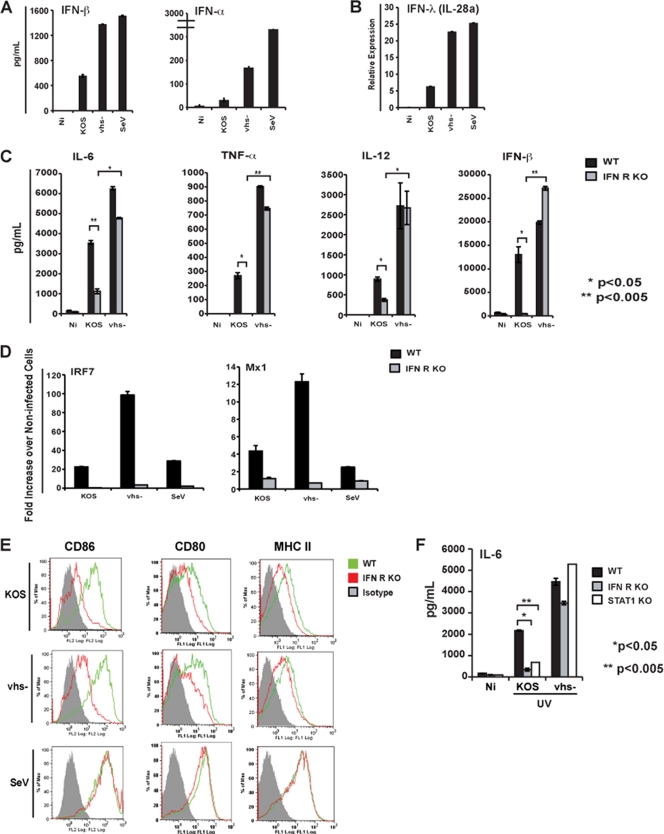
vhs has been reported to negatively regulate the type I IFN signaling pathway by blocking the phosphorylation of STAT1, as well as interfering with the expression of ISGs (10, 27, 41). These prior observations led us to hypothesize that, through its potential inhibition of type I IFN signaling, vhs might exert its block to the activation of DCs observed during HSV infection. This would also explain the phenotype observed during HSV-1 coinfection with NDV (Fig. 1), as type I IFN signaling is required for this RNA virus to trigger DC activation (18, 67, 68). Moreover, the presence of elevated secretion of IFN-α/β in the vhs− mutant-infected DC cultures compared to wild-type-infected DC cultures (Fig. 3A) would fit with a model where inhibition of type I IFN signaling by vhs impairs the amplification phase of type I IFNs.
Fig. 3.
HSV-induced DC maturation has type I IFN signaling-dependent and -independent requirements. (A) BM-DCs were generated from C57BL/6 mice and infected with the following: wild-type HSV-1 (strain KOS) (MOI = 5), vhs− mutant virus (MOI = 5), and SeV-HD (strain Cantel) (MOI = 5). At 6 h p.i., cells were harvested, and the quantities of IFN-β and IFN-α in the supernatants were measured by ELISA. The error bars represent the differences between duplicate assays. (B) At 12 h p.i., RNA was extracted from infected cells, reverse transcribed, and assayed for IFN-λ (IL-28a) by qRT-PCR. The error bars represent the differences between triplicate assays. (C) BM-DCs were generated from Sv129 control and type I IFNR KO mice and infected with the following: KOS (MOI = 5) and vhs− virus (MOI = 5). At 24 h p.i., cells were harvested, and the quantities of IL-6, TNF-α, IL-12p40, and IFN-β in the supernatants were measured by ELISA. The error bars represent the differences between duplicate assays. (D) At 6 h p.i., RNA was extracted from infected cells, reverse transcribed, and assayed for IRF7 and Mx1 by qRT-PCR. The error bars represent the differences between triplicate assays. (E) At 24 h p.i., infected cells were assayed for cell surface expression of CD86, CD80, and MHC II by flow cytometry. (F) BM-DCs were generated from Sv129, IFNR KO, and STAT1 KO mice and infected with the following: UV-inactivated KOS and vhs− viruses at an MOI of 5. At 12 h p.i., IL-6 was measured by ELISA. The error bars represent the differences between duplicate assays. *, P < 0.05; **, P < 0.005. NI, not infected.
To evaluate the role of IFN signaling in HSV-1-induced DC activation, we infected BM-derived DCs generated from mice genetically deficient in the type I IFN receptor (IFNR KO mice). As a control, confirmation of this deficiency is shown in Fig. 3D by the failure of these cells to induce expression of the IRF7 and Mx1 ISGs following virus infection. As shown in Fig. 3C, the production of cytokines associated with DC activation was robustly induced in the vhs−-infected IFNR KO DC cultures to levels similar to that observed in control B6 BM-DCs. In contrast to the vhs− virus-infected cells, wild-type HSV infection required type I IFN signaling for the production of the same cytokines. Importantly, vhs exerted its inhibitory function in the absence of exogenous IFN signaling.
We also measured the cell surface expression of costimulatory molecules (CD80 and CD86) and MHC II. In contrast to cytokines, all surface markers tested required type I IFN signaling during HSV infection (both wild-type and vhs− mutant viruses) for optimal expression (Fig. 3E). Finally, we investigated the role of virus replication in HSV-induced DC activation in the absence of type I IFN signaling. Upon infection of all WT and KO BM-DCs with UV-inactivated HSV, we observed once again that wild-type HSV requires signaling through the type I IFN receptor, whereas the vhs mutant virus does not (Fig. 3F). These results demonstrate that the vhs protein packaged within virion particles is responsible for inhibition of an IFN signaling-independent pathway to cytokine release during HSV infection. Moreover, activation of this initial DC response is also independent of virus replication.
vhs blocks activation of NF-κB during HSV-1 infection of DCs.
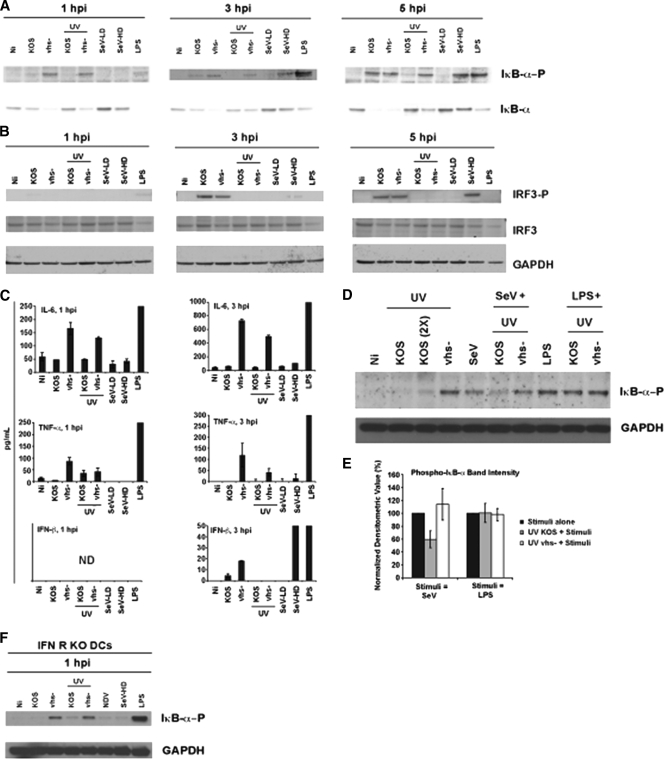
To address which cellular signaling pathway was the target of vhs in the type I IFN signaling-independent pathway to cytokine release, we focused our attention on the activation of transcription factors associated with cytokine and type I IFN production during viral infection of DCs. We assessed NF-κB activation by assessment of the phosphorylation state of ΙκΒ-α by Western blotting during an early time course of infection of BM-DCs. As shown in Fig. 4A, more phosphorylated ΙκΒ-α was detected in vhs− virus-infected cultures at 1 and 3 h postinfection (p.i.) for both live and UV-inactivated HSV treatments. These results are significant, as they imply that an early (<1 h p.i.) block of NF-κB exists during HSV infection.
Fig. 4.
Virion-associated vhs blocks the early replication-independent activation of NF-κB during HSV infection of DCs. BM-DCs were generated from C57BL/6 mice and infected with the following: live and UV-inactivated KOS and vhs− virus (MOI = 5), SeV-LD (LD particle) (MOI = 1.5), SeV-HD (HD particle) (MOI = 1.5), and LPS (300 ng/ml). (A and B) Infected cells were harvested at 1, 3, and 5 h p.i. Denatured protein extracts were resolved by gel electrophoresis and immunoblotted for phospho- and total ΙκΒ-α (A) or phospho- and total IRF3 and GAPDH (B). (C) Infected cell supernatants were assayed for IL-6, TNF-α, and IFN-β by ELISA at the indicated time points. The error bars represent the differences between duplicate assays. (D) BM-DCs were generated from C57BL/6 mice and infected with the following: UV-inactivated KOS virus (MOI = 5), UV-inactivated KOS virus (2×, MOI = 10), UV vhs− virus and SeV-HD (MOI = 1.5), and LPS-treated virus (300 ng/ml). Where noted, UV-inactivated KOS and UV-inactivated vhs− virus-infected cells were also coinfected with SeV or cotreated with LPS. SeV and LPS were added sequentially immediately after HSV. Cells were harvested at 3 h p.i., and denatured protein extracts were resolved by gel electrophoresis. Expression of phospho-ΙκΒ-α and GAPDH was determined by immunoblotting. (E) The intensities of the phospho-ΙκΒ-α bands from three independent experiments were determined by densitometry using the AlphaImager3000. Values were normalized to 100% and graphed. The error bars represent triplicate readings from each of the three experiments. (F) BM-DCs were generated from IFNR KO mice and infected with the following: live and UV-inactivated KOS and vhs− viruses (MOI = 5), NDV (MOI = 1.5), SeV-HD (MOI = 1.5), and LPS-treated virus (300 ng/ml). Cells were harvested at 1 h p.i., and denatured protein extracts were resolved by gel electrophoresis. The expression levels of phospho-IκB-α and GAPDH were determined by immunoblotting.
With wild-type HSV, little to no phosphorylated ΙκΒ-α was detected at 1 and 3 h p.i., while significant activation was observed at 5 h p.i. This pattern of NF-κB activation during wild-type HSV replication is consistent with all previous reports (16, 17, 66). Importantly, no phosphorylated ΙκΒ-α was observed with UV-treated HSV at any time point, confirming the requirement for viral replication of this wild-type HSV-induced activation of NF-κB.
We also looked at the activation of IRF3 (also measured by its phosphorylation status). IRF3 phosphorylation was induced with later kinetics than ΙκΒ-α, was not influenced by vhs, and had a requirement for replication during HSV infection of DCs (Fig. 4B). As controls, we used LPS treatment or infection of DCs with two different preparations of SeV containing either a low (LD) or high (HD) concentration of defective interfering particles.
Phosphorylation of ΙκΒ-α results in proteasome-mediated degradation of the protein, leading to the release of the p65 and p50 NF-κB subunits, which translocate to the nucleus and turn on NF-κB-dependent genes (65). The observed total levels of ΙκΒ-α are consistent compared with its phosphorylation status throughout the time course of infection (Fig. 4A). Furthermore, the levels of NF-κB-dependent genes (IL-6 and TNF-α genes) are increased for live and UV-inactivated vhs− virus-infected cultures relative to wild-type HSV-infected cultures (Fig. 4C). IFN-β (whose production requires both NF-κB and IRF3 activation) is also consistent with the activation of these proteins: undetectable at 1 h p.i., higher for vhs− virus-infected cultures at 3 h p.i., and not induced for UV-inactivated HSV treatments. Taking the results shown in Fig. 4 together, we conclude that the virion-associated form of the vhs protein targets the early replication-independent activation of NF-κB during HSV infection of DCs.
Using the RNA virus coinfection model, we examined ΙκΒ-α phosphorylation during HSV infection in the presence of SeV or a TLR agonist (LPS). To eliminate the potential contribution of de novo-synthesized viral gene products in the antagonism during coinfection, we designed these experiments using only UV-inactivated HSV. As shown in Fig. 4D, we observed a decrease in the intensity of the band for phospho-ΙκΒ-α during coinfection of UV light-irradiated wild-type HSV with SeV, but not during cotreatment of UV wild-type HSV with LPS. This decrease was quantitated over multiple experiments using densitometry, normalized, and graphed (Fig. 4E). The implication of these results is that virion-supplied vhs is able to block NF-κB activation triggered by signaling through the (SeV-induced) RLR pathway.
DISCUSSION
We initiated these studies to determine the mechanism through which the HSV-1 vhs protein blocks dendritic cell activation upon infection. Our key findings are as follows: (i) vhs did not degrade certain proinflammatory gene mRNAs under experimental conditions where HSV-1-infected cells were treated with TLR agonists; (ii) DC activation by wild-type HSV-1 and the vhs− mutant virus occurs by an RLR/MAVS-independent mechanism; (iii) while the vhs effect on DC cytokine responses is independent of the type I IFN receptor, the IFN receptor-dependent ISG response remains intact during wild-type HSV-1 infection; and most importantly, (iv) a novel activity of vhs was identified that targets an immediate activation of NF-κB during productive HSV infection.
HSV-1 and NF-κB share an interesting relationship. Activation of NF-κB has been shown to occur in two distinct phases (2). UV-inactivated virions have been shown to activate the NF-κB signaling pathway at early times postinfection (2, 30, 53). Mechanistically, this has been proposed to occur via either HSV glycoprotein D (gD) interacting at the cell surface with the receptor for HSV-1, the herpesvirus entry mediator (HVEM), or via tegument protein UL37-mediated TRAF6 activation (28, 30). Importantly for the work presented in this study, the viral glycoproteins have been shown to induce cytokine secretion and costimulatory marker upregulation in DCs (46). However, this report also showed that a gD mutant that fails to bind to HVEM and nectin-1 still activated the DCs. Interestingly, the gD mutant did not activate the DCs to the same degree as wild-type gD (46). One possible explanation for this phenotype is that in DCs a synergistic activation involving both the gD-HVEM/nectin-1 interaction and UL37 may be required for complete NF-κB activation and, ultimately, DC activation. Alternatively, there may be cell type specificity in what determines activation of NF-κB in DCs whereby UL37 alone mediates activation, not the gD-HVEM interaction. A second wave of NF-κB activation occurs around the time of the onset of virus replication, and this has been reported to play a role in cell survival during HSV-1 infection (16, 17). Interestingly, one report describes how HSV-1 hijacks this pathway for its own benefit, directing NF-κB subunits to the immediate-early viral promoter of ICP0 to enhance its transcription (2). Since HSV-1 relies on activated NF-κB for efficient virus replication and to interfere with apoptosis, it is also placed in a potentially problematic situation where an immune response will also be initiated as a consequence. How the virus reconciles this may be critical for efficient host pathogenesis—a sufficient amount to limit apoptosis and aid in viral gene expression yet not so much as to generate large toxic quantities of proinflammatory cytokines (IL-6 and TNF-α). Our work suggests that the vhs protein contained in the virion particle helps to maintain an appropriate balance of cellular levels of activated NF-κB in the early phase of infection.
Important insights into how HSV-1 regulates transcription factor activation in DCs were obtained by comparing infections with live and UV-inactivated wild-type and vhs− mutant viruses (Fig. 4). Our data demonstrate (i) that the biphasic induction of NF-κB occurs in DCs and (ii) that vhs inhibits the early replication-independent induction (Fig. 4A). Biphasic induction of NF-κB refers to the fact that while NF-κB activation is detected with wild-type HSV-1 only between 3 and 5 h p.i. (16), there is also an earlier activation, which can be slightly detected under certain conditions (2, 17, 30, 53). It is this immediate activation of NF-κB that vhs prevents. These results highlight the value in our study of using the vhs− mutant virus as a tool to better understand the early molecular pathways involved in DC cytokine production: a clear view of the dual nature of NF-κB activation (replication-dependent versus -independent triggering) is observed that otherwise would not be simply by analyzing wild-type HSV treatments via Western blotting.
An important question that remains is why vhs blocks the replication-independent activation of NF-κB but not the replication-dependent triggering. vhs may be important in the early hours (between 0 and 5 h p.i.) of the infection for limiting the host response to HSV-1 (blocking NF-κB activation and thus preventing a robust induction of proinflammatory cytokines). Later in the infection, when NF-κB plays an opposite role in limiting the host response (through its antiapoptotic function [16, 17]), vhs blocking the response would seem counterproductive. The fact that we observed more activated NF-κB during infection with the vhs− mutant virus in DCs fits well with previous observations that there was less background apoptosis during infection with vhs− mutants in other cell types (3). The data in Fig. 4 clearly show equivalent NF-κB activation, as measured by the accumulation of phospho-IκB-α, between live wild-type and vhs− mutant viruses at 5 h p.i. The virion form of vhs may become inactivated (in its capacity to block NF-κB signaling) early during the course of infection (sometime between 3 and 5 h p.i.). This inactivation phenomenon has been described for its nuclease function (54, 56). It is also a possibility that by 5 h into the infection, the stimulation of the DCs by replicating virus is so strong that vhs can no longer effectively block NF-κB signaling. Moreover, by this time, de novo synthesis of other viral proteins with immune antagonistic functions will also be under way.
Alternatively, as different signaling pathways presumably contribute to the two different waves of NF-κB activation, vhs may specifically target one but not the other. This could potentially explain our results when we coinfected HSV with RNA viruses or cotreated with TLR agonists (Fig. 1 and 4). At least one report describes different roles for some of the signaling components involved in NF-κB and IRF activation when comparing TLR to RLR activation of cells (24). Recently, the HSV virulence factor γ(1)34.5 was described to interfere with NF-κB activation by impairing the function of the upstream IκB kinase (IKK) when DCs are triggered by TLR agonists (20). Experiments to identify putative binding partners for vhs and to address precisely where in the NF-κB signaling pathway vhs may be acting are required to fully decipher the mechanism underlying this new functionality for vhs.
The presence of phosphorylated IRF3 in DCs infected with live virus (but not UV-inactivated virus) during our early time course experiments was unexpected (Fig. 4B). Reports of other nonimmune cell types tested show that IRF3 functionality as a transcriptional regulator is impaired following wild-type HSV infection by the immediate-early protein ICP0 (33, 34, 39). Specifically, in fibroblasts, infection with wild-type HSV resulted in no detection of phospho-IRF3 (39). We observed the opposite in DCs infected with HSV-1; the wild type showed accumulation of the phosphoprotein, whereas UV-inactivated viruses did not. We speculate that the differential activation of IRF3 in different cell types (e.g., DCs compared to fibroblasts) following HSV infection may be due to the relationship between the pathways of virus detection in these different cell types (TLR versus non-TLR signaling pathways) and ICP0. Alternatively, the functionality, localization, and behavior of ICP0 might be different or compromised in DCs relative to other cell types, which may impact its ability to interfere with IRF3 activation.
vhs, which has homology to several other nonviral endonucleases, was initially believed to indiscriminately degrade all mRNA molecules in infected cells in a global fashion (13). However, multiple reports point to the possibility that there may be some selectivity as to which mRNA substrates vhs degrades (7, 11, 12). Furthermore, some cell types (neurons and dendritic cells) appear to show some resistance to vhs-mediated RNA degradation (7, 38). When we stimulated wild-type HSV-infected DCs with either LPS or CpG, we failed to detect any significant differences in the mRNA expression of certain proinflammatory genes. If vhs had an affinity for these mRNA molecules (with respect to nuclease-driven degradation), the nature of what drives their expression would presumably be irrelevant, and there would be at least some measurable decrease in their cellular expression levels. However, to definitively examine the contribution of the nuclease function of vhs to the block to DC activation, experiments comparing wild-type HSV-1 to point mutants that have inactivated the nuclease motif of vhs are required. In contrast to TLR costimulation, coinfecting DCs with wild-type HSV and NDV showed an impairment in accumulation of these same proinflammatory cytokine mRNAs. It is tempting to speculate that NDV-specific factors might act to modulate vhs activity. Alternatively, these results also raise the intriguing possibility that HSV might actually affect 5′-triphosphate-containing RNA (5′-ppp-RNA) or double-stranded RNA (dsRNA), targets of RIG-I and MDA5, respectively. Such an effect might explain the mechanism by which HSV blunts cytokine expression during NDV/SeV coinfection with HSV. This could be an alternative explanation for (or a factor contributing to) the results (Fig. 4D) when we observed a decrease in levels of phospho-IκB-α during wild-type HSV-1 and SeV coinfection.
In DCs, NDV is recognized by members of the RLR family of cytosolic proteins, and maturation requires type I IFN signaling. Based on the repression of immune gene transcription during NDV coinfection (Fig. 1), we evaluated both of those signaling pathways in the response to HSV-1. Analysis of cytokine and type I IFN mRNA expression following infection of DCs prepared from MAVS KO mice with wild-type HSV revealed that the RLR/MAVS-dependent signaling pathways are not essential for their production by DCs in vitro (Fig. 2A). Moreover, vhs− virus infection resulted in higher cytokine release than wild-type HSV infection for both control and MAVS KO DCs (Fig. 2B). Recently, the characterization of MAVS-independent, RIG-I-dependent immune responses to RNA viruses has been reported (43). However, these authors show that the induction of type I IFN requires MAVS, and this signaling pathway driving NF-κB activation was observed during RNA virus infection and not triggered by synthetic DNA (43). Presently, it is unknown whether this pathway contributes to the NF-κB response in DCs following infection with a DNA virus, such as HSV-1. The DNA-sensing pathway mediated by IFI16 and STING and involved in HSV detection in various immune cell types is the likely receptor recognizing replication-competent HSV here in the 3- to 5-h p.i. window (19, 62). Importantly, the data from Fig. 2 show that the RNA polymerase III-dependent RIG-I signaling pathway is not necessary for the DC response to HSV (1, 6).
We chose to evaluate the role of the type I IFN signaling pathway in DC infections by comparing wild-type HSV to the vhs mutant virus for several reasons. Our hypothesis was that by interfering with key components of this signaling cascade (STAT1 phosphorylation [41] and ISG expression [10, 27]), the vhs protein was exerting its block on the activation of DCs during HSV-1 infection. The vhs mutant stimulated DCs with or without type I IFN signaling equally, indicating that unimpeded IFN signaling was not responsible for the enhanced activation of the DCs achieved with the vhs mutant virus (Fig. 3C). Suppression of the IFN signaling pathway alone could not fully explain how vhs blocks DC activation during HSV infection. Our data showing that vhs can block the activation of NF-κB (Fig. 4) clarify how the vhs− mutant virus can activate the DCs even when the cells lack components of the type I IFN signaling pathway (Fig. 4F). We generated a model describing the insights into the modulation of DC activation by the vhs protein during HSV-1 infection gained from this study (Fig. 5).
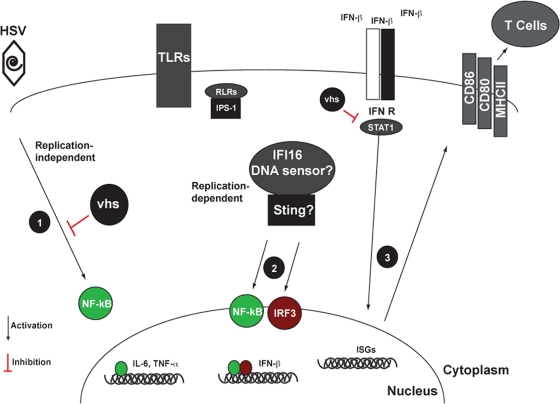
Fig. 5.
Model describing the modulation of HSV-1-induced DC maturation by the vhs protein. HSV-1 infection of DCs begins with binding of the virus to the necessary receptors expressed on the surface of the cell. The binding of HSV-1 gD to the HVEM receptor has been reported to drive NF-κB activation in response to UV-inactivated virus (step 1). A second mechanistic explanation for the early replication-independent activation of NF-κB has been reported to occur through the virion-associated protein UL37. Whatever the nature of the stimulus, the virion-associated form of vhs can block this signaling event during the first 3 h of the infection. Between 3 and 5 h p.i., a second wave of NF-κB signaling begins. IRF3 is also activated and detectable by 3 h p.i. We have shown that replication-competent HSV-1 is not detected by either TLRs (7) or RLRs/MAVS during infection. A recent report described a DNA sensor for HSV-1 in DCs. If IFI16, present in DCs, detects viral DNA, this would potentially result in the activation of IRF3 and an additional wave of NF-κB (step 2) (62). The release of IFN-β by 3 h p.i. results in signaling through the type I IFN receptor, and this has important implications for the establishment of the antiviral state in the infected cell. The surface expression of costimulatory markers and MHC molecules during HSV-1 infection is dependent on type I IFN signaling (step 3). However, stimulation with the vhs− virus circumvents the requirement for type I IFN signaling for the production of proinflammatory cytokines. We propose that the increase in the stoichiometric availability of NF-κB subunit proteins early during infection with the vhs− virus can explain this phenotype.
In summary, the work presented here reveals a novel function for the vhs protein of HSV-1 contained in the virion as an early inhibitor of the NF-κB signaling pathway in dendritic cells. In addition, the power of using a potently stimulatory virus in understanding the complex interplay between HSV and various immune signaling pathways is also highlighted throughout the study. These findings contribute to our understanding of immune antagonism by HSV while potentially having implications for future DC-based vaccine design and gene therapies.
ACKNOWLEDGMENTS
This study was funded by grants AI48582 and AI38873, provided to John A. Blaho by the United States Public Health Service National Institutes of Health (NIH); AI07647, provided to Christopher R. Cotter by the United States Public Health Service National Institutes of Health; AI07647 and CA88796 provided to Marie L. Nguyen by the United States Public Health Service National Institutes of Health; R21AI077007 and RO1AI041111 provided to Thomas M. Moran by the National Institute of Allergy and Infectious Diseases; and NIH grants AI083284 and AI080917 to Carolina B. López.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Ablasser A., et al. 2009. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amici C., et al. 2006. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 281:7110–7117 [DOI] [PubMed] [Google Scholar]
- 3. Aubert M., O'Toole J., Blaho J. A. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359–10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banchereau J., et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 5. Cella M., Sallusto F., Lanzavecchia A. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10–16 [DOI] [PubMed] [Google Scholar]
- 6. Chiu Y. H., Macmillan J. B., Chen Z. J. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cotter C. R., et al. 2010. The virion host shut-off (vhs) protein blocks a TLR-independent pathway of herpes simplex virus type 1 recognition in human and mouse dendritic cells. PLoS One 5:e8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalod M., et al. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197:885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eidson K. M., Hobbs W. E., Manning B. J., Carlson P., DeLuca N. A. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisemann J., Muhl-Zurbes P., Steinkasserer A., Kummer M. 2007. Infection of mature dendritic cells with herpes simplex virus type 1 interferes with the interferon signaling pathway. Immunobiology 212:877–886 [DOI] [PubMed] [Google Scholar]
- 11. Esclatine A., Taddeo B., Evans L., Roizman B. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. U. S. A. 101:3603–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esclatine A., Taddeo B., Roizman B. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. U. S. A. 101:18165–18170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Everly D. N., Jr., Feng P., Mian I. S., Read G. S. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560–8571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fenwick M. L., McMenamin M. M. 1984. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 65:1225–1228 [DOI] [PubMed] [Google Scholar]
- 15. Geiss B. J., Smith T. J., Leib D. A., Morrison L. A. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 74:11137–11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodkin M. L., Ting A. T., Blaho J. A. 2003. NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 77:7261–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregory D., Hargett D., Holmes D., Money E., Bachenheimer S. L. 2004. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J. Virol. 78:13582–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Honda K., et al. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. U. S. A. 100:10872–10877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishikawa H., Barber G. N. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin H., Yan Z., Ma Y., Cao Y., He B. 2011. A herpesvirus virulence factor inhibits dendritic cell maturation through protein phosphatase 1 and Ikappa B kinase. J. Virol. 85:3397–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin L., et al. 2005. A herpes simplex virus type 1 mutant expressing a baculovirus inhibitor of apoptosis gene in place of latency-associated transcript has a wild-type reactivation phenotype in the mouse. J. Virol. 79:12286–12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keadle T. L., et al. 2002. Therapeutic vaccination with vhs(-) herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 83:2361–2365 [DOI] [PubMed] [Google Scholar]
- 23. Keadle T. L., Morrison L. A., Morris J. L., Pepose J. S., Stuart P. M. 2002. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 76:3615–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konno H., et al. 2009. TRAF6 establishes innate immune responses by activating NF-kappaB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One 4:e5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwong A. D., Kruper J. A., Frenkel N. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leib D. A., et al. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin R., Noyce R. S., Collins S. E., Everett R. D., Mossman K. L. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X., Fitzgerald K., Kurt-Jones E., Finberg R., Knipe D. M. 2008. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 105:11335–11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. López C. B., Garcia-Sastre A., Williams B. R., Moran T. M. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126–1136 [DOI] [PubMed] [Google Scholar]
- 30. Medici M. A., et al. 2003. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J. Biol. Chem. 278:36059–36067 [DOI] [PubMed] [Google Scholar]
- 31. Melchjorsen J., Siren J., Julkunen I., Paludan S. R., Matikainen S. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87:1099–1108 [DOI] [PubMed] [Google Scholar]
- 32. Mellman I., Steinman R. M. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255–258 [DOI] [PubMed] [Google Scholar]
- 33. Melroe G. T., DeLuca N. A., Knipe D. M. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melroe G. T., Silva L., Schaffer P. A., Knipe D. M. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miles D. H., Willcox M. D., Athmanathan S. 2004. Ocular and neuronal cell apoptosis during HSV-1 infection: a review. Curr. Eye Res. 29:79–90 [DOI] [PubMed] [Google Scholar]
- 36. Murphy J. A., Duerst R. J., Smith T. J., Morrison L. A. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen M. L., Kraft R. M., Blaho J. A. 2005. African green monkey kidney Vero cells require de novo protein synthesis for efficient herpes simplex virus 1-dependent apoptosis. Virology 336:274–290 [DOI] [PubMed] [Google Scholar]
- 38. Nichol P. F., Chang J. Y., Johnson E. M., Jr., Olivo P. D. 1994. Infection of sympathetic and sensory neurones with herpes simplex virus does not elicit a shut-off of cellular protein synthesis: implications for viral latency and herpes vectors. Neurobiol. Dis. 1:83–94 [DOI] [PubMed] [Google Scholar]
- 39. Paladino P., Collins S. E., Mossman K. L. 2010. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS One 5:e10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park M. S., et al. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pasieka T. J., et al. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perng G. C., et al. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poeck H., et al. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 11:63–69 [DOI] [PubMed] [Google Scholar]
- 44. Read G. S., Frenkel N. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Read G. S., Karr B. M., Knight K. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reske A., Pollara G., Krummenacher C., Katz D. R., Chain B. M. 2008. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J. Immunol. 180:7525–7536 [DOI] [PubMed] [Google Scholar]
- 47. Roizman B., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2602 In Knipe D. M., et al. (ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 48. Sabri F., Granath F., Hjalmarsson A., Aurelius E., Skoldenberg B. 2006. Modulation of sFas indicates apoptosis in human herpes simplex encephalitis. J. Neuroimmunol. 171:171–176 [DOI] [PubMed] [Google Scholar]
- 49. Samady L., et al. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs- HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanfilippo C. M., Chirimuuta F. N., Blaho J. A. 2004. Herpes simplex virus type 1 immediate-early gene expression is required for the induction of apoptosis in human epithelial HEp-2 cells. J. Virol. 78:224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schek N., Bachenheimer S. L. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schillinger J. A., et al. 2004. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex. Transm. Dis. 31:753–760 [DOI] [PubMed] [Google Scholar]
- 53. Sciortino M. T., et al. 2008. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem. Pharmacol. 76:1522–1532 [DOI] [PubMed] [Google Scholar]
- 54. Smibert C. A., Popova B., Xiao P., Capone J. P., Smiley J. R. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stetson D. B., Medzhitov R. 2006. Type I interferons in host defense. Immunity 25:373–381 [DOI] [PubMed] [Google Scholar]
- 56. Strand S. S., Leib D. A. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J. Virol. 78:13562–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Strelow L., Smith T., Leib D. 1997. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology 231:28–34 [DOI] [PubMed] [Google Scholar]
- 58. Strelow L. I., Leib D. A. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779–6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strom T., Frenkel N. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tailor P., Tamura T., Ozato K. 2006. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 16:134–140 [DOI] [PubMed] [Google Scholar]
- 61. Takeuchi O., Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820 [DOI] [PubMed] [Google Scholar]
- 62. Unterholzner L., et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verpooten D., Ma Y., Hou S., Yan Z., He B. 2009. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walker J., Leib D. A. 1998. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 16:1–5 [DOI] [PubMed] [Google Scholar]
- 65. Wan F., Lenardo M. J. 2010. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 20:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yedowitz J. C., Blaho J. A. 2005. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology 342:297–310 [DOI] [PubMed] [Google Scholar]
- 67. Yount J. S., Gitlin L., Moran T. M., Lopez C. B. 2008. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai Virus defective interfering particles. J. Immunol. 180:4910–4918 [DOI] [PubMed] [Google Scholar]
- 68. Yount J. S., Kraus T. A., Horvath C. M., Moran T. M., Lopez C. B. 2006. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J. Immunol. 177:4503–4513 [DOI] [PubMed] [Google Scholar]
- 69. Zheng X., et al. 2001. Increased severity of HSV-1 keratitis and mortality in mice lacking the 2-5A-dependent RNase L gene. Invest. Ophthalmol. Vis. Sci. 42:120–126 [PubMed] [Google Scholar]