SUMMARY
Human chymotrypsin C (CTRC) plays a protective role in the pancreas by mitigating premature trypsinogen activation through degradation. Mutations that abolish activity or secretion of CTRC increase the risk for chronic pancreatitis. The aim of the present study was to determine whether human CTRC undergoes asparagine-linked (N-linked) glycosylation and to examine the role of this modification in CTRC folding and function. We abolished potential sites of N-linked glycosylation (Asn-Xaa-Ser/Thr) in human CTRC by mutating the Asn residues to Ser individually or in combination, expressed the CTRC mutants in HEK 293T cells and determined their glycosylation state using PNGase F and endo H digestion. We found that human CTRC contains a single N-linked glycan on Asn52. Elimination of N-glycosylation by mutation of Asn52 (N52S) reduced CTRC secretion about 10-fold from HEK 293T cells but had no effect on CTRC activity or inhibitor binding. Overexpression of the N52S CTRC mutant elicited endoplasmic reticulum stress in AR42J acinar cells, indicating that N-glycosylation is required for folding of human CTRC. Despite its important role, Asn52 is poorly conserved in other mammalian CTRC orthologs, including the rat which is monoglycosylated on Asn90. Introduction of the Asn90 site in a non-glycosylated human CTRC mutant restored full glycosylation but only partially rescued the secretion defect. We conclude that N-linked glycosylation of human CTRC is required for efficient folding and secretion, however, the N-linked glycan is unimportant for enzyme activity or inhibitor binding. The position of the N-linked glycan is critical for optimal folding, and it may vary among the otherwise highly homologous mammalian CTRC sequences.
Keywords: Asn-linked glycosylation, secretion defect, serine protease, pancreatic chymotrypsin, ER stress, misfolding
Chymotrypsin C (CTRC) is a digestive serine protease secreted by the acinar cells of the pancreas as an inactive zymogen (chymotrypsinogen C) which becomes activated in the duodenum after trypsin-mediated cleavage of the activation peptide [1]. The activation peptide remains attached to the enzyme through the Cys17-Cys141 disulfide link and may be further processed by CTRC to a shorter form [2]. CTRC cleaves dietary proteins and peptides after aromatic (Phe, Tyr) and aliphatic (Leu) amino acid residues with characteristically high activity on leucyl peptide bonds [3–6]. Beyond its digestive function, human CTRC also plays a role in regulating intestinal digestive enzyme levels by promoting the activation of human cationic trypsinogen and procarboxypeptidase A1 and A2 [7, 8]. Furthermore, CTRC mediates degradation of trypsinogen and trypsin in a manner that is regulated by calcium [9]. The trypsin(ogen)-degrading activity of CTRC seems to have an important protective function against intrapancreatic premature trypsin activation, indicated by the observation that loss of function variants of CTRC increase the risk for chronic pancreatitis [1, 10, 11]. CTRC mutations cause impaired catalytic activity or diminished secretion, in all likelihood due to misfolding and intracellular degradation. [10]. In addition, the A73T CTRC mutant has been shown to elicit endoplasmic reticulum (ER) stress, which may contribute to acinar cell damage through induction of apoptosis [12].
The majority of secretory proteins undergo asparagine-linked (N-linked) glycosylation during their ER transit. First, a preassembled core oligosaccharide is attached to the Asn residue of the Asn-Xaa-Ser/Thr motif (sequon) in the nascent polypeptide chain entering the ER lumen [13, 14]. The oligosaccharide promotes protein folding by increasing stability and preventing aggregation [15, 16]. The core glycan undergoes further modifications in the ER and Golgi and the resulting glycan structure provides information for quality control and intracellular trafficking within the secretory pathway [17, 18]. N-linked glycosylation can also influence the stability of folded, mature proteins and may modify protein function including catalytic activity of enzymes and ligand binding to receptors [19, 20]. Although the human and other mammalian CTRC sequences contain one or more potential N-linked glycosylation sites; whether or not these sites are actually occupied is unknown. The crystal structure of the bovine CTRC zymogen in complex with procarboxypeptidase A and proproteinase E does not show a sugar moiety attached to CTRC, suggesting that the bovine isoform is not glycosylated [21]. In the present study we examined the glycosylation state of human CTRC and characterized the role of N-glycosylation in CTRC function. We found that human CTRC undergoes glycosylation on Asn52, which is required for efficient folding and secretion but it has no impact on enzyme activity or inhibitor binding.
RESULTS
Human CTRC contains a single N-linked glycan on Asn52
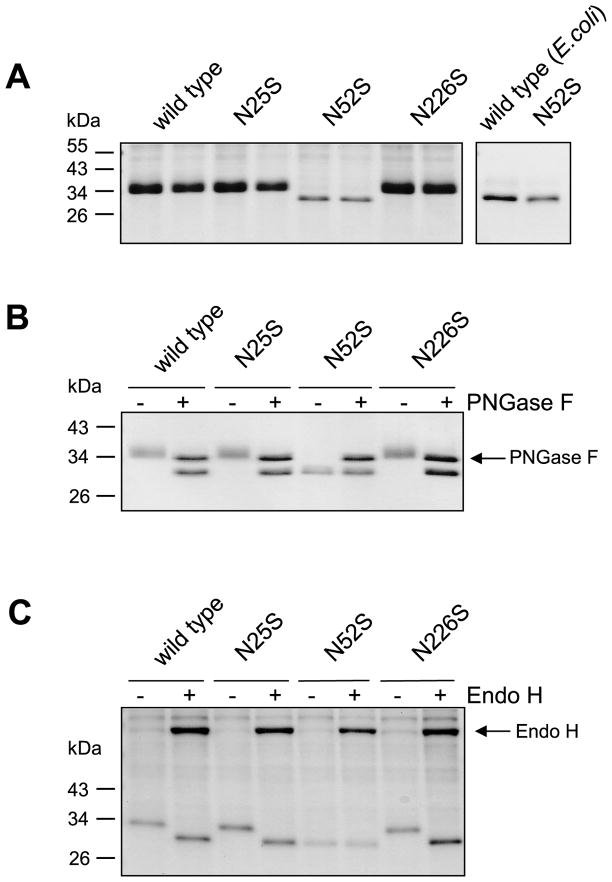
The calculated molecular mass of full length human CTRC is 28.6 kDa. However, on SDS-PAGE the CTRC zymogen purified from conditioned media of transfected HEK 293T cells migrated at around 35 kDa as a broad, diffuse band (Fig 1A), suggesting that human CTRC might be N-glycosylated. This assumption was confirmed when we treated human CTRC with PNGase F, an enzyme that cleaves the linkage between Asn and N-acetylglucosamine and releases Asn-associated glycans. As shown in Fig 1B, PNGase F markedly changed the electrophoretic behavior of human CTRC, which now migrated as a sharp band close to its predicted molecular mass. The primary amino-acid sequence of human CTRC contains three potential N-linked glycosylation sites (Asn-Xaa-Ser/Thr) at the N25, N52 and N226 positions. To examine whether these sites are occupied by glycosyl residue, we eliminated these sequons by mutating the Asn to Ser individually and in various combinations. Wild-type and mutant CTRC were expressed in HEK 293T cells and the conditioned media were analyzed by SDS-PAGE. As shown in Fig 1A, single mutants N25S and N226S were indistinguishable from wild-type CTRC on SDS-PAGE, however, mutant N52S migrated as a lower molecular mass sharp band. To generate a non-glycosylated control, we expressed human CTRC in E. coli without its signal-peptide and purified the zymogen, as described in [7]. Mutant N52S and wild-type CTRC of bacterial origin exhibited identical electrophoretic mobilities, indicating that N52S is not glycosylated (Fig 1A, right panel). Thus, it appears that human CTRC is N-glycosylated on a single site, on Asn52.
Figure 1.
N-linked glycosylation of human CTRC. Conditioned media from transfected HEK 293T cells were analyzed by 12% SDS-PAGE and Coomassie blue staining. A. Electrophoretic mobility of wild-type CTRC and mutants N25S, N52S, and N226S. Samples were run in duplicate (left panel). Non-glycosylated CTRC produced in E. coli was used as control (right panel). B. PNGase F digestion of wild-type and mutant CTRC. C. Endo H digestion of wild-type and mutant CTRC produced in kifunensine treated cells.
To demonstrate that the observed gel shift of the N52S CTRC mutant is caused by the lack of N-glycosylation, we treated wild-type CTRC and all three mutants with PNGase F. After enzymatic deglycosylation, wild type CTRC and mutants N25S and N226S co-migrated with mutant N52S on SDS-PAGE (Fig 1B). Importantly, PNGase F had no effect on the mobility of mutant N52S. To confirm these results using a different glycosidase, we treated human CTRC with endoglycosidase H (endo H), which cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. As expected with proteins that are processed by Golgi mannosidases, the N-glycans of human CTRC were resistant to endo H treatment (not shown). Therefore, we expressed and purified CTRC in HEK 293T cells treated with the mannosidase inhibitor kifunensine. CTRC produced by this method was fully deglycosylated by endo H. As seen with PNGase F, wild-type, N25S and N226S CTRC exhibited faster mobility after endo H treatment, whereas mutant N52S was unaffected (Fig 1C).
Analysis of double mutants N25S,N52S, N25S,N226S, N52S,N226S and the triple mutant N25S,N52S,N226S confirmed our conclusion that human CTRC was N-glycosylated on Asn52 only. Thus, double-mutants N25S,N52S, N52S,N226S and the triple mutant N25S,N52S,N226S behaved exactly as the single mutant N52S, whereas double mutant N25S,N226S was N-glycosylated as wild-type CTRC (Supplementary Figure S1).
N-linked glycosylation of CTRC is required for efficient secretion but not for zymogen activation, enzyme activity or inhibitor binding
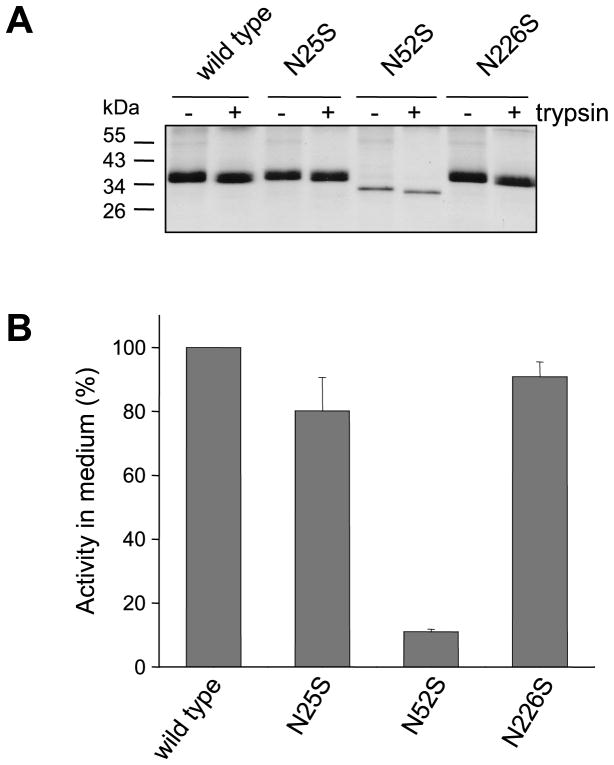
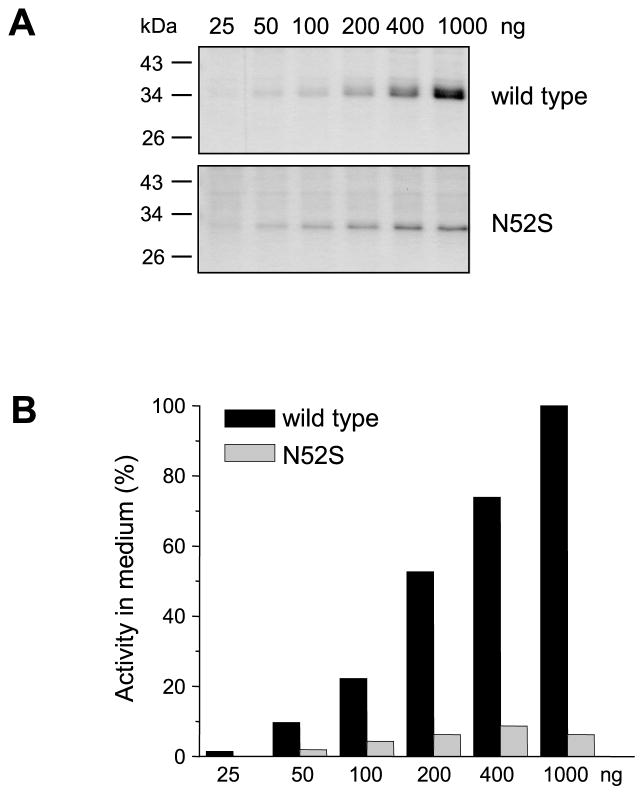
Inspection of Coomassie-stained gels indicated that the N52S single mutant or double and triple mutants containing this mutation were present in significantly lower amounts than wild-type CTRC in the conditioned media of transfected HEK 293T cells. Measurement of chymotrypsin activity after trypsin-mediated activation of wild-type and mutant CTRC zymogens confirmed that the N52S mutation diminished CTRC secretion at least 10-fold (Fig 2). To examine how this apparent secretion defect depends on the overall expression levels, we transfected HEK 293T cells with increasing concentrations of expression plasmids for wild-type CTRC and mutant N52S. Expression of wild-type CTRC increased proportionally with higher plasmid concentrations, as judged by SDS-PAGE analysis (Fig 3A) and activity measurements (Fig 3B). In contrast, mutant N52S exhibited smaller increments in expression over the same plasmid concentration range. Accordingly, more dramatic differences in expression between wild-type CTRC and mutant N52S were apparent at higher plasmid concentrations.
Figure 2.
Activation of wild-type CTRC and mutants N25S, N52S and N226S by trypsin. A. Conditioned media (200 μL) were incubated with 100 nM human cationic trypsin in 0.1 M Tris-HCl (pH 8.0) and 10 mM CaCl2 (final concentrations) at 37 °C for 1 h in 250 μL final volume. Proteins were precipitated with 10% trichloroacetic acid (final concentration) and analyzed by 12% SDS-PAGE and Coomassie blue staining. B. CTRC activity in the conditioned medium was measured with the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate as described in Experimental Procedures. Activity was expressed as percentage of wild-type CTRC activity. Error bars indicate SEM (n=3).
Figure 3.
Effect of plasmid concentration on CTRC secretion from transfected HEK 293T cells. Transfections were performed with increasing concentrations of wild-type and N52S CTRC expression plasmids (from 25 ng to 1000 ng). Total plasmid DNA in the transfections was kept constant at 1000 ng by adding empty pcDNA3.1(−) vector. A. Conditioned media were analyzed by 12% SDS-PAGE and Coomassie blue staining. B. CTRC activity in the conditioned medium was measured with the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate as described in Experimental Procedures. Activity was expressed as percentage of CTRC activity measured in the conditioned media of cells transfected with 1000 ng wild-type CTRC plasmid.
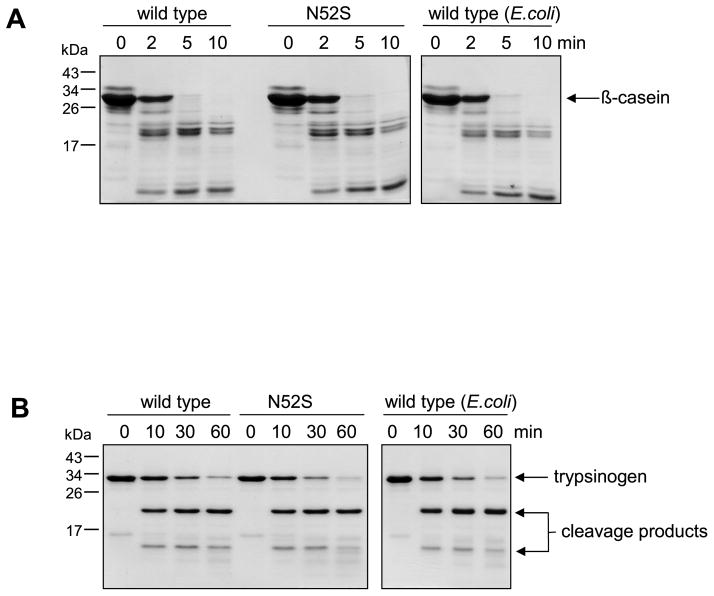
The experiment in Fig 2A also indicates that N-glycosylation does not affect trypsin-mediated activation of CTRC zymogen, as judged by the trypsin-induced mobility shift of wild-type and mutant CTRC. Trypsin cleaves the Arg29-Val30 activating peptide bond in CTRC, which results in a small but detectable increase in mobility on reducing SDS-PAGE, because under reducing conditions the cleaved but disulfide-linked activation peptide is released from CTRC. The effect of N-glycosylation on zymogen activation was also studied by comparing the activation kinetics of intact and enzymatically deglycosylated wild-type CTRC using activity measurements. As shown in Supplementary Fig S2, no appreciable difference was detected between the activation time courses. Catalytic activity of glycosylated wild-type CTRC, the N52S mutant and non-glycosylated wild-type CTRC produced in E. coli was essentially identical when measured on the small peptide substrate Suc-Ala-Ala-Pro-Phe-p-nitroanilide (Table 1), by digestion of bovine β-casein (Fig 4A) or by cleavage of the Leu81-Glu82 peptide bond in human cationic trypsinogen (Fig 4B), a physiological substrate of CTRC. Binding of the Schistocerca gregaria proteinase inhibitor-2 (SGPI-2) to wild-type and N52S mutant CTRC was also comparable, exhibiting equilibrium dissociation constants of 3.9 nM and 2.7 nM, respectively [cf. ref 22]. Taken together, these observations indicate that N-glycosylation of CTRC is important for efficient secretion of CTRC but the glycosyl residue on Asn52 has no effect on the trypsin-mediated activation of CTRC or the function of active CTRC.
Table 1.
Kinetic parameters of wild-type human CTRC and mutants N52S and E92T on the synthetic substrate Suc-Ala-Ala-Pro-Phe-p-nitroanilide, measured at 22 °C in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2 and 0.05% Tween 20. CTRC was expressed in HEK 293T cells or in E. coli, as indicated. Note that the E92T mutant was created in the background of the non-glycosylated triple mutant N25S,N52S,N226S.
| KM (μM) | kcat (s−1) | kcat/KM (M−1 s−1) | |
|---|---|---|---|
| wild type | 13.8 ± 1.0 | 16.1 ± 0.2 | 1.2 × 106 |
| wild type (E. coli) | 13.8 ± 2.9 | 14.1 ± 0.6 | 1.0 × 106 |
| N52S | 13.6 ± 1.7 | 16.8 ± 0.4 | 1.2 × 106 |
| E92T | 19.3 ± 1.5 | 17.8 ± 0.3 | 0.9 × 106 |
Figure 4.
Digestion of bovine β-casein (A) and human cationic trypsinogen (B) with wild-type and mutant N52S CTRC produced in HEK 293T cells and wild-type CTRC produced in E.coli. At the indicated times samples were precipitated with 10% trichloroacetic acid (final concentration) and analyzed by 15% SDS-PAGE and Coomassie blue staining. A. Casein at 0.2 mg/mL concentration was incubated with 5 nM CTRC at 37 °C in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2 (final concentrations) in 100 μL final volume. B. Human cationic trypsinogen was incubated at 2 μM concentration with 20 nM CTRC at 37 °C in 0.1 M Tris-HCl (pH 8.0), 0.14 mM CaCl2, and 20 nM human SPINK1 trypsin inhibitor in 100 μL final volume. Note that for these experiments a stable trypsinogen mutant was used in which the activation site was destroyed by a K23Q mutation.
The non-glycosylated N52S CTRC mutant causes ER stress in rat acinar AR42J cells
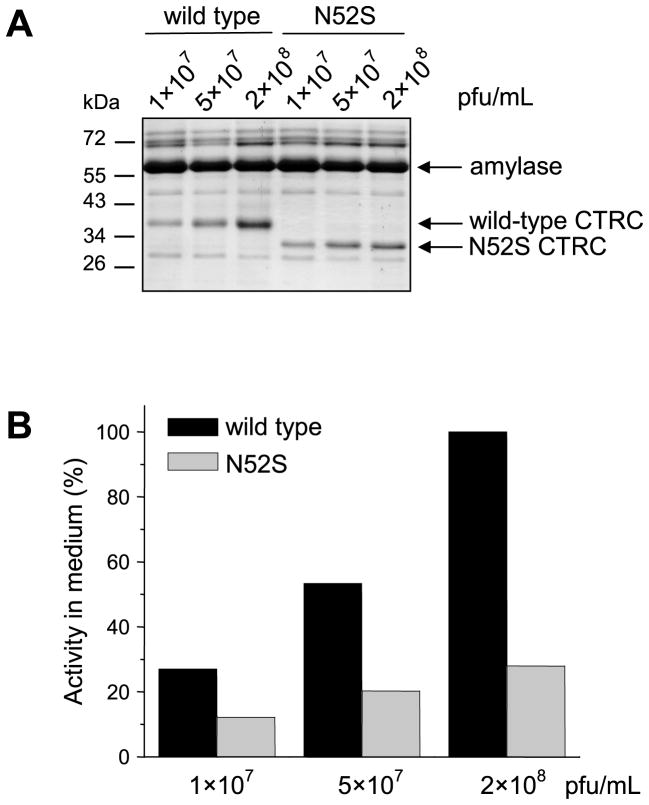
To examine the effect of N-glycosylation on the secretion of CTRC in a cell line that more closely resembles pancreatic acinar cells, we used AR42J cells, which originate from a rat pancreatic acinar tumor [23, 24]. Since these cells are poorly transfected by plasmid-based methods, we constructed adenoviral vectors carrying wild-type CTRC or mutant N52S. Dexamethasone-differentiated AR42J cells were then transfected with increasing concentrations of recombinant adenovirus and conditioned media were analyzed by SDS-PAGE (Fig 5A) and activity measurements (Fig 5B). Consistent with our previous observation with HEK 293T cells, secretion of the non-glycosylated N52S mutant from AR42J cells was reduced relative to wild-type CTRC and this difference was much more pronounced at higher expression levels. On the other hand, the secretion defect was less severe in AR42J cells (~3-fold) than in HEK 293T cells (~10-fold), probably due to the more robust ER and secretion apparatus of pancreatic acinar cells.
Figure 5.
Secretion of wild-type CTRC and mutant N52S from AR42J cells transfected with recombinant adenovirus. Cells were infected for 24 h with the indicated concentrations of adenovirus, expressed in plaque forming units (pfu) per mL. A. Conditioned media were analyzed by 12% SDS-PAGE and Coomassie blue staining. Note that AR42J cells do not express endogenous rat CTRC to a detectable level. The secretion of high levels of amylase is a well-know property of this cell line. B. CTRC activity in the conditioned medium was measured with the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate, as described in Experimental Procedures. Activity was expressed as percentage of CTRC activity measured in the conditioned medium of cells infected with 2×108 pfu/mL wild-type CTRC adenovirus.
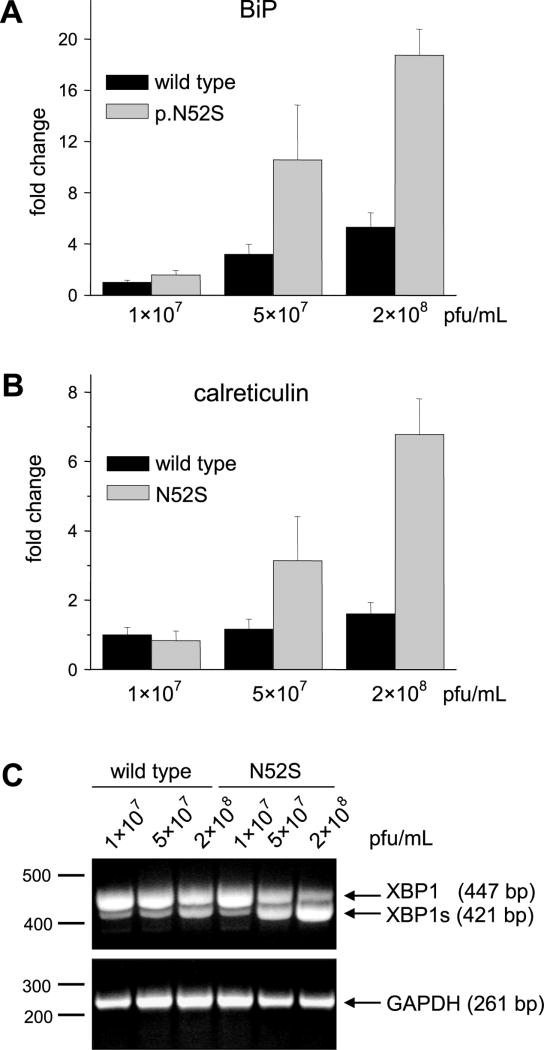
The reduced secretion of the N52S mutant suggested that this non-glycosylated CTRC variant might not fold properly resulting in intracellular retention and degradation. If this was the case, markers of ER stress should be elevated in cells expressing the N52S mutant [25]. To test this hypothesis, we measured mRNA levels of the chaperones immunoglobulin binding protein (BiP) and calreticulin in cell lysates by quantitative real-time RT-PCR. We found that both chaperones were significantly upregulated in cells expressing the N52S mutant, relative to cells transfected with wild type CTRC (Figs 6A and 6B). ER stress was more pronounced at higher CTRC expression levels; i.e. when higher adenovirus concentrations were used.
Figure 6.
ER stress markers in AR42J cells expressing wild-type CTRC or N52S mutant. Cells were infected for 24 h with the indicated concentrations of adenovirus, given in plaque forming units (pfu) per mL. A, B. Quantitative real-time PCR measurement of BiP (A) and calreticulin (B) mRNA with TaqMan probes, as described in Experimental Procedures. Expression levels were normalized to the RPII internal control gene. Data were expressed as fold changes relative to levels measured in cells transfected with 107 pfu/mL wild-type CTRC adenovirus. Error bars represent standard deviation (n=3). C. XBP1 splicing assessed by RT-PCR and agarose gel electrophoresis with ethidium bromide staining. XBP1s, spliced form. GAPDH was measured as control for sample integrity and equal loading.
During ER stress the membrane-embedded inositol-requiring enzyme 1 (IRE1) becomes activated and catalyzes the cytoplasmic splicing of the X-box binding protein-1 (XBP1) mRNA [25]. IRE1 removes a 26-nucleotide intron from the XBP1 mRNA to create a shorter, spliced variant, which encodes a potent transcriptional activator of the unfolded protein response. We used RT-PCR to measure the extent of XBP1 splicing in AR42J cells expressing wild-type CTRC or the N52S mutant. Fig 6C demonstrates that the N52S mutant caused a significant increase in spliced XBP1 compared to the lower levels observed in cells expressing wild-type CTRC. The results demonstrate that expression of non-glycosylated human CTRC elicits ER stress in AR42J cells, in all likelihood due to misfolding.
Rat CTRC is N-glycosylated on Asn90
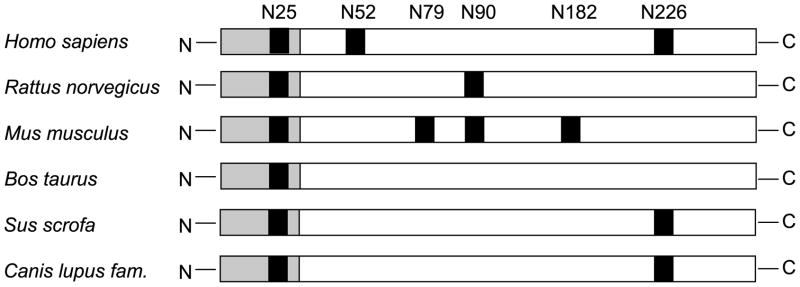
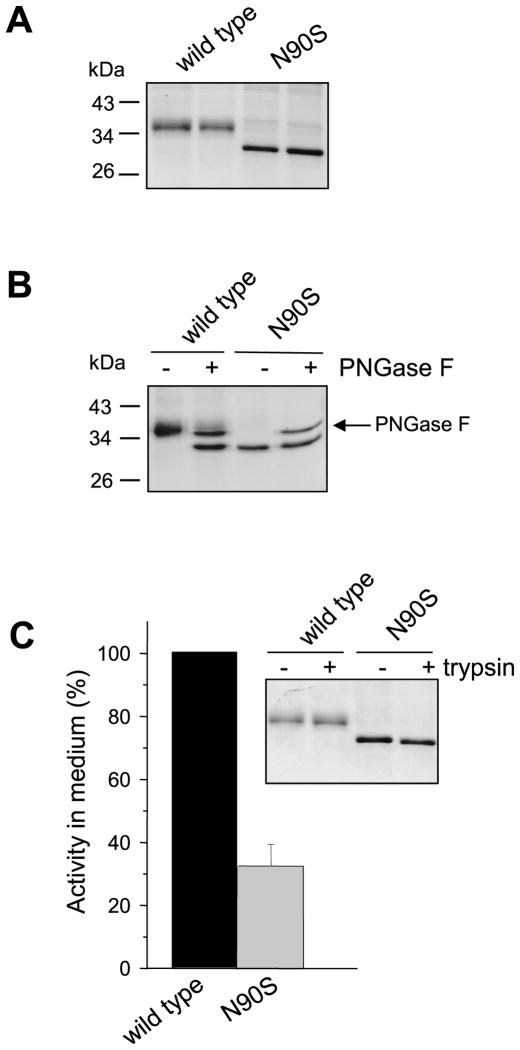
Despite its apparently important role in folding, the Asn52 N-glycosylation site is not conserved among the otherwise highly homologous mammalian CTRC sequences (Fig 7). Thus, rat CTRC contains potential N-glycosylation sites at Asn25 and Asn90, whereas mouse CTRC contains sequons at Asn25, Asn79, Asn90 and Asn182. Only the Asn25 site is present in bovine CTRC, while pig and dog CTRC contain sequons at Asn25 and Asn226. Interestingly, the only sequon conserved in all CTRC sequences is Asn25 within the activation peptide, however, this site is not glycosylated in human CTRC (this work) or in the published crystal structure of bovine CTRC. To examine the role of N-glycosylation of CTRC from another species, we mutated Asn90 to Ser (N90S) in rat CTRC. Elimination of this potential glycosylation site caused a mobility shift of rat CTRC on SDS-PAGE (Fig 8A). The mobility shift of the N90S mutant was caused by the loss of the N-glycan, because after deglycosylation with PNGase F wild-type rat CTRC co-migrated with the N90S mutant (Fig 8B). Furthermore, PNGase F treatment had no effect on the mobility of the N90S mutant. The N90S mutant showed about 3-fold reduced secretion levels relative to wild-type rat CTRC, as judged by chymotrypsin activity measurements of conditioned media after trypsin-mediated activation (Fig 8C). This difference was not readily apparent when Coomassie stained gels were inspected, presumably due to the increased staining intensity of the non-glycosylated N90S band (Fig 8A). Because of this discrepancy we carried out additional control experiments to confirm that the difference in chymotrypsin activity indeed reflected levels of CTRC secretion. First, we verified that the activated CTRC molecules are not prone to degradation either by trypsin or autocatalytically (Fig 8C inset). Second, we performed active site titration using the chymotrypsin inhibitor SGPI-2 and found that levels of N90S in the conditioned medium were indeed about 3-fold lower relative to wild-type rat CTRC (62.4 nM versus 175 nM). We conclude that rat CTRC is monoglycosylated on Asn90, which plays a similar, albeit lesser, role in facilitating CTRC folding and secretion as N-glycosylation of human CTRC on Asn52.
Figure 7.
Schematic representation of potential Asn-linked glycosylation sites (shown in black) in various mammalian CTRC sequences. The activation peptide is highlighted in gray.
Figure 8.
N-linked glycosylation of rat CTRC. Conditioned media from transfected HEK 293T cells were analyzed by 12% SDS-PAGE and Coomassie blue staining. A. Electrophoretic mobility of wild-type CTRC and mutant N90S. Samples were run in duplicate. B. PNGase F digestion of wild-type and N90S mutant rat CTRC. C. CTRC activity of wild-type CTRC and mutant N90S. Enzyme activity in the conditioned medium was measured after activation with trypsin, using the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate, as described in Experimental Procedures. Activity was expressed as percentage of wild-type CTRC activity. Error bar indicates SEM (n=3). Inset: SDS-PAGE analysis of activation with human cationic trypsin. See legend to Fig 2 for reaction conditions.
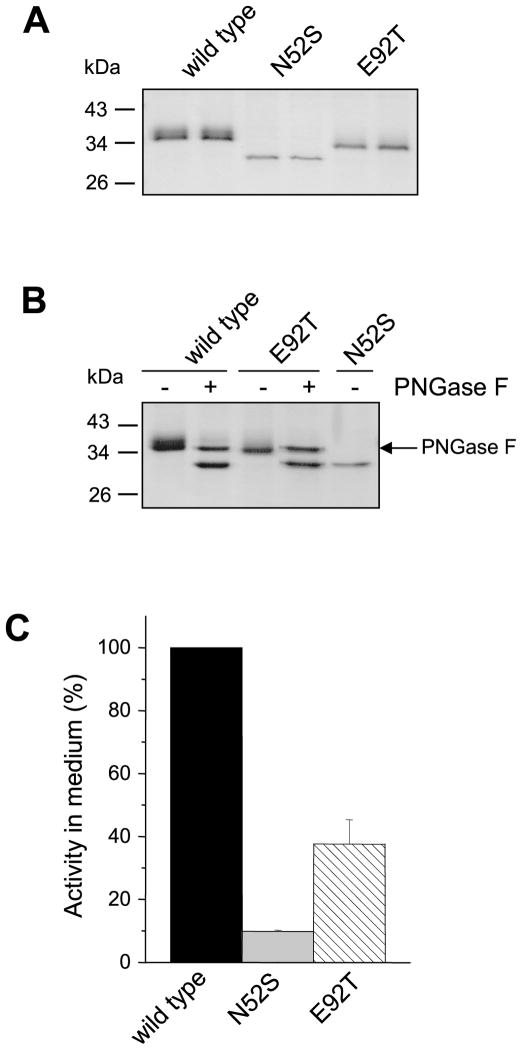
Introduction of the rat N-glycosylation site partially rescues the secretion defect of non-glycosylated human CTRC mutant
To determine whether N-glycosylation per se, or its position, is important for folding, we introduced the Asn90 rat N-glycosylation site into the human non-glycosylated CTRC triple mutant N25S,N52S,N226S by replacing Glu92 with Thr (E92T) to create the sequon Asn90-Leu91-Thr92. SDS-PAGE analysis of conditioned media from HEK 293T cells transfected with the E92T human CTRC mutant indicated that the introduced rat N-glycosylation site was fully glycosylated (Fig 9A). This was evidenced by the slower mobility of the E92T mutant relative to the N25S,N52S,N226S triple mutant. This mobility difference was abolished by PNGase F treatment of the E92T mutant, confirming glycosylation of Asn90 (Fig 9B). As seen on the Commassie-stained gels, and confirmed by activity measurements, glycosylation on Asn90 partially rescued the secretion defect of the non-glycosylated human CTRC mutant. Thus, relative to wild-type CTRC, the activity of the N25S,N52S,N226S mutant was 10-fold lower in the conditioned media, whereas the activity of the E92T mutant was only 4-fold reduced, i.e. secretion was restored to about 40% of wild-type levels (Fig 9C). In a control experiment using purified CTRC proteins, we confirmed that the E92T mutant cleaved the small peptide substrate Suc-Ala-Ala-Pro-Phe-p-nitroanilide (Table 1) and digested bovine s-casein (not shown) comparably to wild-type CTRC, indicating that glycosylation on Asn90 does not influence CTRC function.
Figure 9.
Effect of an engineered glycosylation site on the glycosylation and secretion of a non-glycosylated human CTRC mutant. The Asn90-Leu91-Thr92 sequon from rat CTRC was reconstructed by mutation E92T in the non-glycosylated human CTRC triple mutant N25S,N52S,N226S. A. HEK 293T cells were transfected and after 48 h incubation conditioned media was collected. Aliquots (200 μL) were precipitated with 10% trichloroacetic acid (final concentration) and analyzed by 12% SDS-PAGE and Coomassie blue staining. B. PNGase F digestion of wild-type and mutant CTRC. C. CTRC activity in the conditioned medium was measured with the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate, as described in Experimental Procedures. Activity was expressed as percentage of wild-type CTRC activity. Error bars indicate SEM (n=3).
Introduction of rat or human glycosylation site has no effect on secretion of bovine CTRC
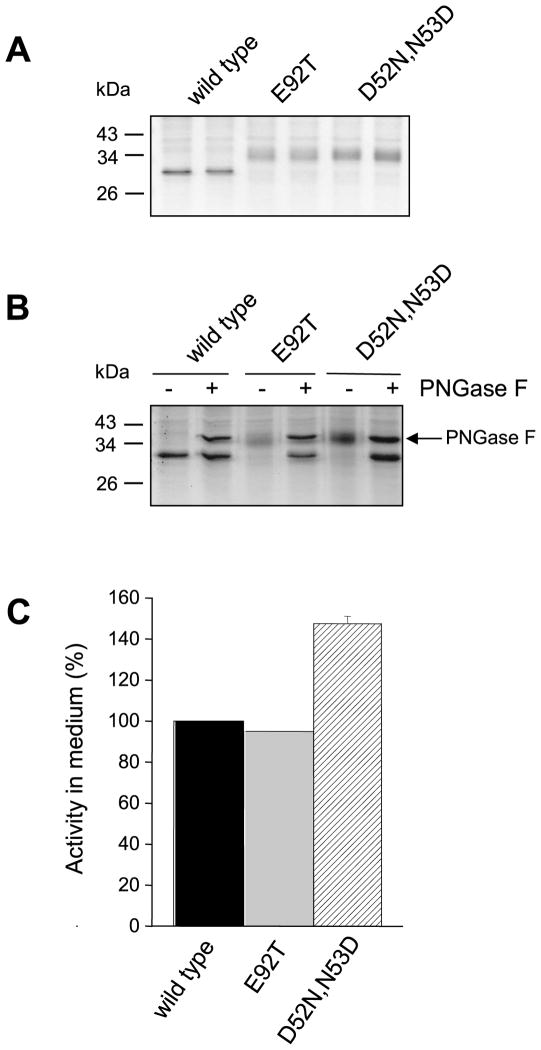
Bovine CTRC contains one potential N-linked glycosylation site at Asn25 within the activation peptide (Fig 7) but this site is not occupied and bovine CTRC is not glycosylated at all [21]. On SDS-PAGE bovine CTRC ran as a sharp band and exhibited a lower apparent molecular mass than human CTRC. Furthermore, PNGase F digestion had no effect on the mobility of bovine CTRC, confirming the absence of N-glycosylation (Fig 10A). To test whether N-glycosylation might improve secretion of bovine CTRC, we reconstructed the rat Asn90-Leu91-Thr92 sequon or the human Asn52-Asp53-Thr54 sequon in bovine CTRC by introducing mutations E92T or D52N,N53D, respectively. SDS-PAGE analysis of conditioned media from HEK 293T cells transfected with the mutant bovine CTRC constructs indicated that both the Asn52 and Asn90 sites underwent full glycosylation, because both mutants migrated as diffuse bands with higher apparent molecular masses than wild-type bovine CTRC (Fig 10A). PNGase F digestion confirmed that the higher molecular mass of the mutants was caused by N-glycosylation (Fig 10B). Surprisingly, however, none of the newly introduced N-glycosylation sites increased the secretion of the bovine CTRC to a significant extent, as determined by the CTRC activity of the conditioned media (Fig 10C). Thus, bovine CTRC mutant E92T glycosylated on Asn90 was secreted as well as wild-type bovine CTRC, whereas mutant D52N,N53D N-glycosyated on Asn52 exhibited a modest 1.5-fold increase in secretion.
Figure 10.
Effect of engineered glycosylation sites on the glycosylation and secretion of bovine CTRC. The Asn90-Leu91-Thr92 sequon from rat CTRC and the Asn52-Asp53-Thr54 sequon from human CTRC were reconstructed in bovine CTRC by mutations E92T and D52N,N53D, respectively. A. Electrophoretic mobility of wild-type bovine CTRC and mutants E92T and D52N,N53D. Samples were run in duplicate. B. PNGase F digestion of wild-type and mutant CTRC. C. CTRC activity in the conditioned medium. Measurements were performed with the Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate, as described in Experimental Procedures. Activity was expressed as percentage of wild-type CTRC activity. Error bars indicate SEM (n=3).
DISCUSSION
In the present study we demonstrated that human CTRC undergoes N-linked glycosylation on a single site, on amino-acid residue Asn52. Mutation of Asn52 (N52S) prevented glycosylation of CTRC and significantly reduced its secretion from HEK 293T and AR42J cells. In both cell types the extent of the secretion defect of the non-glycosylated CTRC mutant was proportional with the expression levels, suggesting that this mutant is poorly folded in the secretory pathway. Consistent with this interpretation, overexpression of the N52S mutant elicited ER stress in AR42J acinar cells. Taken together, these observations indicate that N-glycosylation is required for efficient folding of human CTRC. Glycosylation can facilitate CTRC folding by several, mutually non-exclusive, mechanisms. (1) N-glycans mediate association with lectin chaperones calnexin and calreticulin in the ER, which bind to the glycosyl residue of newly synthesized glycoproteins after the two terminal glucose residues are removed by glucosidase I and II [17, 18]. Both lectins form a complex with ERp57 thiol oxidoreductase which facilitates the correct formation of disulfide bonds [17, 18]. (2) Intracellular trafficking and sorting of CTRC may be also dependent on N-glycosylation which would allow binding to the ERGIC-53 or VIP36 mannose-specific lectins or other potential cargo receptors involved in ER exit and the ER-to-Golgi traffic of glycoproteins [26–28]. (3) The glycosyl residue may have a direct effect on CTRC folding. The large polar oligosaccharide unit can increase solubility of nascent proteins, prevent their aggregation and direct proper folding by orienting the surrounding polypeptide segments toward the surface of protein domain. Glycosylation can also increase overall stability by reducing protein flexibility [15, 16, 29].
N-glycosylation can affect enzyme activity, substrate specificity or binding affinity, when the oligosaccharide chain is in close proximity to the active site of the enzyme and can sterically influence substrate accessibility [19, 20, 30, 31]. Interestingly, in human CTRC Asn52 is located in a surface loop that participates in substrate binding. This loop carries Lys51 and Arg56, which recently were found to be determinants of the P4′ acidic specificity of human CTRC [22]. Together with Arg80, these basic amino-acid residues form a positively charged cluster which interacts with the negatively charged Glu or Asp amino acid residues in the P4′ position of substrates or inhibitors. Notwithstanding its proximity to these important specificity determinants, we found no evidence that the N-glycan on Asn52 would have any effect whatsoever on substrate or inhibitor binding or catalysis. The normal function of secreted non-glycosylated CTRC also indicates that it retained the ability to fold properly, although with less efficiency than its glycosylated wild-type counterpart. This finding is in agreement with previous studies suggesting that N-glycosylation facilitates the folding process but it is usually not essential for maintaining the structure of the folded, mature protein [15, 16].
Despite its critical role, Asn52 is not conserved among the otherwise highly homologous mammalian CTRC sequences which have potential N-linked glycosylation sites at different positions (see Fig 7). We found that rat CTRC is N-glycosylated on Asn90 and this modification is required for the efficient secretion of the rat proenzyme, although to a lesser degree than glycosylation of Asn52 in human CTRC. To examine the importance of the position of the N-glycan, we introduced the rat Asn90 glycosylation site into a non-glycosylated human CTRC mutant. The newly created site was fully glycosylated and it partially restored the secretion of the CTRC mutant to about 40% of wild-type levels. There are at least three possible explanations why the engineered Asn90 glycosylation site could not fully rescue the secretion defect of the non-glycosylated human CTRC mutant. (i) The correct position of the glycosyl residue on Asn52 may be important due to its local stabilizing effects. As mentioned above, glycosyl residues can locally influence the folding process by increasing the stability of the surrounding protein segments and can directly interact with the neighboring amino acids [16]. (ii) Alternatively, the location of the N-linked glycan may influence the correct chaperone binding of the nascent human CTRC. It has been demonstrated that if the initial glycosyl residue is located within about 50 amino acids of the protein N terminus, it associates with calnexin and calreticulin and this binding prevents interaction with Bip. However, if the glycosyl residue is found more toward the C terminus, the glycoproteins interact first with Bip before associating with the calnexin/calreticulin system [32]. Therefore, the glycosyl residue on Asn52 may direct human CTRC to interact with calnexin/calreticulin, while shifting the N-glycan to Asn90 may promote BiP binding, resulting in altered folding efficiency. (iii) Finally, the sequence context of the N-glycosylation site may influence chaperone binding and N-glycosylated Asn52 and Asn90 may function optimally only within their respective, native sequence environment.
In contrast to human and rat CTRC, we found that bovine CTRC was not glycosylated and introduction of the human Asn52 or rat Asn90 sequons did not increase its secretion from HEK 293T cells even though both sites were fully occupied by N-glycans. Unlike human or rat CTRC, bovine CTRC forms a physiological ternary complex with procarboxypeptidase A (proCPA) and proproteinase E, or a binary complex with proCPA [33, 34]. The crystal structure of the ternary complex has been determined and no glycosylation was observed on the CTRC moiety [21]. Therefore, it seems reasonable to speculate that folding of bovine CTRC may be facilitated by complex formation with proCPA rather than by N-glycosylation.
In summary, in the present study we showed that human CTRC contains a single N-linked glycan on Asn52, which is important for efficient folding and secretion of the proenzyme but it does not affect enzyme activity or inhibitor binding. The position of the N-linked glycan is critical for optimal folding, and it may vary among the otherwise highly homologous mammalian CTRC sequences.
EXPERIMENTAL PROCEDURES
Plasmid construction and mutagenesis
Construction of the human CTRC expression plasmid in the pcDNA3.1(−) vector was described previously [9]. The coding DNA for rat CTRC was PCR amplified from total cDNA prepared from AR42J cells using the following primers. Rat CTRC XhoI sense, 5′-AAA TTT CTC GAG GAA CAC CTG AAC CAT GTT GGG AAT TAC -3′ (where the XhoI site is underlined and the initiator codon is emboldened) and Rat CTRC HindIII antisense, 5′-TGG AAG AAG CTT TAT TGC TGT TGG GAG -3′ (where the HindIII site is underlined). The PCR product was digested with XhoI and HindIII and subcloned into the pcDNA3.1(−) vector. The coding DNA for bovine CTRC was custom synthesized (Genscript, Piscataway, NJ) and cloned into the pcDNA3.1(−) vector using XhoI and EcoRI restriction sites. CTRC mutants were generated by overlap extension PCR mutagenesis and ligated into pcDNA3.1(−) expression plasmid. Recombinant adenovirus containing the N52S human CTRC mutant with a GluGlu epitope tag at the C terminus was made by Viraquest, Inc. (North Liberty, Iowa), as described previously for wild-type human CTRC [12].
Cell culture and transfection
HEK 293T cells were cultured in 6-well tissue culture plates (106 cells per well) in DMEM supplemented with 10% fetal bovine serum, 4 mM glutamine and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Transfections were carried out in a total volume of 2 mL DMEM using 10 μL Lipofectamine 2000 (Invitrogen) and 4 μg expression plasmid, unless indicated otherwise. After overnight incubation, cells were washed and the transfection media was replaced with 2 mL OptiMEM. The conditioned OptiMEM media were harvested after 48 h incubation. Where indicated, transfections and incubations were performed in the presence of 5 μM kifunensine (final concentration in medium). AR42J rat pancreatic acinar cells (ATCC #CRL-1492) were maintained in DMEM supplemented with 20% fetal bovine serum, 4 mM glutamine and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 Prior to transfection cells were plated in 6-well plates (106 cells per well) and were grown in he presence of 100 nM concentration of dexamethasone for 48 h to induce differentiation [24]. Adenoviral infections were performed using 107, 5×107, and 2×108 pfu/mL final adenovirus concentrations in a total volume of 1 mL OptiMEM in the presence of dexamethasone (100nM final concentration). Conditioned media were harvested after 24 h incubation.
SDS-polyacrylamide gel electophoresis (SDS-PAGE) of conditioned media
Proteins in the conditioned media of HEK 293T cells (200 μL) or AR42J cells (100 μL) were precipitated with 10% trichloroacetic acid (final concentration), resuspended in 15 μL Laemmli sample buffer containing 100 mM dithiothreitol, heat-denatured at 95 °C for 5 min and electrophoresed on 12% SDS-polyacrylamide gels. Gels were stained with Coomassie Blue.
Deglycosylation of CTRC
Peptide:N-Glycosidase F (PNGase F) digestion: 200 μL conditioned media were precipitated with 10% trichloroacetic acid (final concentration), resuspended in 20 μL 1× G7 reaction buffer (50 mM sodium phosphate (pH 7.5)) and incubated at 37 °C for 3 h with 0.5 μL (250 units) PNGase F (New England Biolabs, Ipswich, MA). Reactions were terminated by adding 20 μL Laemmli sample buffer with 100 mM dithiothreitol followed by heat denaturation at 95 °C for 5 min. Endoglycosidase H (Endo H) digestion: 200 μL conditioned media were supplemented with 20 μL 10× G5 reaction buffer (50 mM sodium citrate (pH 5.5), final concentration) and incubated at 37 °C for 3 h with 1 μL (500 units) Endo H. For these experiments, a recombinant protein fusion of Endo H and maltose binding protein (Endo Hf, New England Biolabs, catalog# P0703S) was used. Reactions were terminated by adding 10% trichloroacetic acid (final concentration). Samples were analyzed by 12% SDS-PAGE and Coomassie blue staining.
Reverse transcriptase (RT)-PCR analysis and real-time PCR
RNA was isolated from transfected cells using the RNAqueous kit (Ambion, Austin, TX) and 1 μg RNA was reverse-transcribed with M-MLV reverse transcriptase (Ambion) in 20 μL volume.
To assay splicing of X-box binding protein-1 (XBP1), 1 μL cDNA was used as template in a 50 μL PCR reaction containing HotStar DNA Polymerase (Qiagen), XBP1 sense primer: 5′–GCT TGT GAT TGA GAA CCA GG–3′; and XBP1 antisense primer: 5′–AGG CTT GGT GTA TAC ATG G–3′. These primers amplify both the spliced and unspliced forms of XBP1 cDNA. As a reference control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was amplified using GAPDH sense primer: 5′–GTC TAC TGG CGT CTT CAC CA–3′ and GAPDH antisense primer: 5′–GTG GCA GTG ATG GCA TGG AC– 3′. PCR products were electrophoresed on 1.5 % agarose gels and stained with ethidium bromide.
To determine immunoglobulin-binding protein (BiP) and calreticulin mRNA expression, quantitative real-time PCR assays were performed in 25 μL final volume containing 0.2 μL cDNA, 1× ABI PCR master mix, gene-specific TaqMan primers and FAM-labeled probe (Applied Biosystems). The following TaqMan primer sets were used: rat RNA polymerase II (RPII): Rn00580118_m1; rat BiP: Rn00565250_m1; rat calreticulin: Rn00574451_m1. Amplification and signal detection were performed using an ABI 7500 Real-Time PCR System (Applied Biosystems). Denaturation at 95 °C for 10 min was followed by 40 thermocycles (65 °C, 15 sec and 60 °C, 1 min). Reactions were performed in triplicate using RNase-free water as negative control. Gene expression was quantitated using the comparative CT method (ΔΔCT method). Threshold cycle (CT) values were determined using the 7500 System Sequence Detection Software 1.3. Expression levels of target genes were first normalized to the RNA polymerase II (RPII) internal control gene (ΔCT) and then to expression levels measured in cells infected with 107 pfu/mL wild-type CTRC adenovirus (ΔΔCT ). Results were expressed as fold changes calculated with the formula 2−ΔΔCT.
Expression and purification of CTRC
HEK 293T cells were cultured in 75 cm2 flasks to 95% confluence. Transfections were carried out in a total volume of 20 mL DMEM using 75 μL Lipofectamine 2000 (Invitrogen) and 30 μg wild-type or mutant CTRC plasmid. After overnight incubation, cells were washed and the transfection media was replaced with 20 mL OptiMEM. The conditioned media were harvested after 48 h incubation, replaced with 20 mL fresh OptiMEM and harvested again at 96 h. Conditioned medium (40–60 mL pooled from 2–3 parallel transfections) was directly applied to a 2 mL ecotin column equilibrated with 20 mM Tris-HCl (pH 8.0) and 0.2 M NaCl and the column was washed with the same buffer. CTRC zymogen was eluted with 50 mM HCl and the acid was neutralized by adding Tris-HCl (pH 8.0) to 0.1 M final concentration. CTRC was activated with human cationic trypsin and CTRC concentrations were determined by active site titration with ecotin, as described previously [22].
Measurement of CTRC activity
Conditioned media (37 μL from HEK 293T cells or 12 μL from AR42J cells) were incubated with 100 nM concentration of human cationic trypsin at 37 °C for 1 h in 0.1 M Tris-HCl (pH 8.0) and 10 mM CaCl2 in 50 μL final volume. CTRC activity was measured by adding 150 μL Suc-Ala-Ala-Pro-Phe-p-nitroanilide substrate (0.15 mM final concentration) dissolved in 0.1 M Tris-HCl (pH 8.0) and 1 mM CaCl2 at 22 °C. One-minute time courses of p-nitroaniline release were followed at 405 nm in a Spectramax Plus 384 microplate reader (Molecular Devices). Enzyme kinetic analysis of purified CTRC was performed in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2 and 0.05% Tween 20, using 10 nM final enzyme concentration.
Inhibitor binding assay
Binding of the Schistocerca gregaria proteinase inhibitor-2 (SGPI-2) to CTRC was characterized by determining the KD value of the reaction in equilibrium, as described previously [22].
Supplementary Material
Acknowledgments
These studies were supported by NIH grants R01 DK082412, R01 DK082412-S2 (ARRA) and R01 DK058088 (to M.S.-T.) and a scholarship from the Rosztoczy Foundation (to M.B.). Richárd Szmola is gratefully acknowledged for initiating this project.
Footnotes
In the present study we showed that human CTRC contains a single N-linked glycan on Asn52, which is important for efficient folding and secretion of the proenzyme but it does not affect enzyme activity or inhibitor binding. The position of the N-linked glycan is critical for optimal folding, and it may vary among the otherwise highly homologous mammalian CTRC sequences.
References
- 1.Zhou J, Sahin-Tóth M. Chymotrypsin C (CTRC) mutations in chronic pancreatitis. J Gastroenterol Hepatol. 2011;26:1238–1246. doi: 10.1111/j.1440-1746.2011.06791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Yuan D, Xu R, Chi CW. Purification, cDNA cloning, and recombinant expression of chymotrypsin C from porcine pancreas. Acta Biochim Biophys Sin (Shanghai) 2011;43:568–575. doi: 10.1093/abbs/gmr047. [DOI] [PubMed] [Google Scholar]
- 3.Folk JE, Schirmer EW. Chymotrypsin C. I. Isolation of the zymogen and the active enzyme: preliminary structure and specificity studies. J Biol Chem. 1965;240:181–192. [PubMed] [Google Scholar]
- 4.Folk JE, Cole PW. Chymotrypsin C. II. Enzymatic specificity toward several polypeptides. J Biol Chem. 1965;240:193–197. [PubMed] [Google Scholar]
- 5.Keil-Dlouha V, Puigserver A, Marie A, Keil B. On subunit II of bovine procarboxypeptidase A. Enzymatic specificity after tryptic activation. Biochim Biophys Acta. 1972;276:531–535. doi: 10.1016/0005-2744(72)91013-3. [DOI] [PubMed] [Google Scholar]
- 6.Iio-Akama K, Sasamoto H, Miyazawa K, Miura S, Tobita T. Active forms of chymotrypsin C isolated from autolyzed porcine pancreas glands. Biochim Biophys Acta. 1985;831:249–256. doi: 10.1016/0167-4838(85)90042-1. [DOI] [PubMed] [Google Scholar]
- 7.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–11886. doi: 10.1074/jbc.M600124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szmola R, Bence M, Carpentieri A, Szabó A, Costello CE, Samuelson J, Sahin-Tóth M. Chymotrypsin C is a co-activator of human pancreatic procarboxypeptidases A1 and A2. J Biol Chem. 2011;286:1819–1827. doi: 10.1074/jbc.M110.187369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szmola R, Sahin-Tóth M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht’s enzyme Y. Proc Natl Acad Sci USA. 2007;104:11227–11232. doi: 10.1073/pnas.0703714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosendahl J, Witt H, Szmola R, Bhatia E, Ózsvári B, Landt O, Schulz HU, Gress TM, Pfützer R, Löhr M, Kovács P, Blüher M, Stumvoll M, Choudhuri G, Hegyi P, te Morsche RH, Drenth JP, Truninger K, Macek M, Jr, Puhl G, Witt U, Schmidt H, Büning C, Ockenga J, Kage A, Groneberg DA, Nickel R, Berg T, Wiedenmann B, Bödeker H, Keim V, Mössner J, Teich N, Sahin-Töth M. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2008;40:78–82. doi: 10.1038/ng.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson E, Chen JM, Scotet V, Le Maréchal C, Férec C. Association of rare chymotrypsinogen C (CTRC) gene variations in patients with idiopathic chronic pancreatitis. Hum Genet. 2008;123:83–91. doi: 10.1007/s00439-007-0459-3. [DOI] [PubMed] [Google Scholar]
- 12.Szmola R, Sahin-Tóth M. Pancreatitis-associated chymotrypsinogen C (CTRC) mutant elicits endoplasmic reticulum stress in pancreatic acinar cells. Gut. 2010;59:365–372. doi: 10.1136/gut.2009.198903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbara I, Keith WR. Conformational implications of asparagine-linked glycosylation. Proc Natl Acad Sci USA. 1995;92:97–101. doi: 10.1073/pnas.92.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bause E. Structural requirements of N-glycosylation of proteins. Biochem J. 1983;209:331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shental-Bechor D, Levy Y. Folding of glycoproteins: toward understanding the biophysics of the glycosylation code. Curr Opin Struct Biol. 2009;19:524–533. doi: 10.1016/j.sbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 17.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 18.Lederkremer GZ. Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol. 2009;19:515–523. doi: 10.1016/j.sbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Skropeta D. The effect of individual N-glycans on enzyme activity. Bioorg Med Chem. 2009;17:2645–2653. doi: 10.1016/j.bmc.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Takacs Z, Wilhelmsen KC, Sorota S. Snake alpha-neurotoxin binding site on the Egyptian cobra (Naja haje) nicotinic acetylcholine receptor is conserved. Mol Biol Evol. 2001;18:1800–1809. doi: 10.1093/oxfordjournals.molbev.a003967. [DOI] [PubMed] [Google Scholar]
- 21.Gomis-Rüth FX, Gómez M, Bode W, Huber R, Avilés FX. The three-dimensional structure of the native ternary complex of bovine pancreatic procarboxypeptidase A with proproteinase E and chymotrypsinogen C. EMBO J. 1995;14:4387–4394. doi: 10.1002/j.1460-2075.1995.tb00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabó A, Héja D, Szakács D, Zboray K, Kékesi KA, Radisky ES, Sahin-Tóth M, Pál G. High affinity small protein inhibitors of human chymotrypsin C (CTRC) selected by phage display reveal unusual preference for P4′ acidic residues. J Biol Chem. 2011;286:22535–22545. doi: 10.1074/jbc.M111.235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessop NW, Hay RJ. Characteristics of two rat pancreatic exocrine cell lines derived from transplantable tumors. In Vitro. 1980;16:212. (Abstract) [Google Scholar]
- 24.Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985;100:1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ron D, Walter P. Signal integration in endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita K, Hara-Kuge S, Ohkura T. Intracellular lectins associated with N-linked glycoprotein traffic. Biochim Biophys Acta. 1999;1473:147–160. doi: 10.1016/s0304-4165(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 27.Schrag JD, Procopio DO, Cygler M, Thomas DY, Bergeron JJM. Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci. 2003;28:49–57. doi: 10.1016/s0968-0004(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 28.Baines AC, Zhang B. Receptor-mediated protein transport in the early secretory pathway. Trends Biochem Sci. 2007;32:381–388. doi: 10.1016/j.tibs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol. 2009;184:847–862. doi: 10.1083/jcb.200808124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller GC, Long CJ, Bojilova ED, Marchadier D, Badellino KO, Blanchard N, Fuki IV, Glick JM, Rader DJ. Role of N-linked glycosylation in the secretion and activity of endothelial lipase. J Lipid Res. 2004;45:2080–2087. doi: 10.1194/jlr.M400162-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Bieberich E, Tencomnao T, Kapitonov D, Yu RK. Effect of N-glycosylation on turnover and subcellular distribution of N-acetylgalactosaminyltransferase I and sialyltransferase II in neuroblastoma cells. J Neurochem. 2000;74:2359–2364. doi: 10.1046/j.1471-4159.2000.0742359.x. [DOI] [PubMed] [Google Scholar]
- 32.Molinari M, Helenius A. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science. 2000;288:331–333. doi: 10.1126/science.288.5464.331. [DOI] [PubMed] [Google Scholar]
- 33.Brown JR, Cox DJ, Greenshields RN, Walsh KA, Yamasaki M, Neurath H. The chemical structure and enzymatic functions of bovine procarboxypeptidase A. Proc Natl Acad Sci USA. 1961;47:1554–1560. doi: 10.1073/pnas.47.10.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerfelec B, Chapus C, Puigserver A. Existence of ternary complexes of procarboxypeptidase A in the pancreas of some ruminant species. Eur J Biochem. 1985;151:515–319. doi: 10.1111/j.1432-1033.1985.tb09132.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.