The myocardial response to ischemia may be markedly augmented by risk factors associated with lifestyle, leading to left ventricular hypertrophy – an important contributor to cardiovascular morbidity and mortality. Left ventricular hypertrophy and hypertension impairs heart function, and may negatively affect the outcome of ischemia/reperfusion injury. However, ischemic tolerance may persist in altered hearts of hypertensive animals, and may be modified by short- or long-term adaptation to stressful conditions. This article discusses differences in the effects of long-term adaptation to crowding stress and short-term adaptation to ischemic stress, on ischemic tolerance in altered hearts of hypertensive rats.
Keywords: Adaptation, Hypertension, Hypertrophy, Myocardial ischemia, Preconditioning, Social stress
Abstract
Chronic hypertension may have a negative impact on the myocardial response to ischemia. On the other hand, intrinsic ischemic tolerance may persist even in the pathologically altered hearts of hypertensive animals, and may be modified by short- or long-term adaptation to different stressful conditions. The effects of long-term limitation of living space (ie, crowding stress [CS]) and brief ischemia-induced stress on cardiac response to ischemia/reperfusion (I/R) injury are not yet fully characterized in hypertensive subjects. The present study was designed to test the influence of chronic and acute stress on the myocardial response to I/R in spontaneously hypertensive rats (SHR) compared with their effects in normotensive counterparts. In both groups, chronic, eight-week CS was induced by caging five rats per cage in cages designed for two rats (200 cm2/rat), while controls (C) were housed four to a cage in cages designed for six animals (480 cm2/rat). Acute stress was evoked by one cycle of I/R (5 min each, ischemic preconditioning) before sustained I/R in isolated Langendorff-perfused hearts of normotensive and SHR rats. At baseline conditions, the effects of CS were manifested only as a further increase in blood pressure in SHR, and by marked limitation of coronary perfusion in normotensive animals, while no changes in heart mechanical function were observed in any of the groups. Postischemic recovery of contractile function, severity of ventricular arrhythmias and lethal injury (infarction size) were worsened in the hypertrophied hearts of C-SHR compared with normotensive C. However, myo-cardial stunning and reperfusion-induced ventricular arrhythmias were attenuated by CS in SHR, which was different from deterioration of I/R injury in the hearts of normotensive animals. In contrast, ischemic preconditioning conferred an effective protection against I/R in both groups, although the extent of anti-infarct and anti-arrhythmic effects was lower in SHR. Both forms of stress may improve the altered response to ischemia in hypertensive subjects. In contrast to short-term preconditioning stress, chronic psychosocial stress was associated with a higher risk of lethal arrhythmias and contractile failure in normotensive animals exposed to an acute ischemic challenge.
Ischemic heart disease and its most severe manifestations – acute myocardial infarction and sudden death due to lethal dysrhythmias – is one of the major causes of morbidity due to cardiovascular diseases in modern society. Myocardial response to ischemia may be markedly augmented by risk factors associated with lifestyle, including chronically elevated blood pressure, which leads to left ventricular hypertrophy (LVH) (1,2). In adults, LVH has been identified as one of the most important contributors to cardiovascular morbidity and mortality (3). This is particularly important in cardiac surgery because LVH is frequently associated with postischemic contractile dysfunction (4). Chronic hypertension and the development of LVH impairs heart function under normal conditions, and may have a negative impact on the outcome of ischemia/reperfusion (I/R) injury (1,5) due to impaired regulation of energy metabolism and homeostasis of ions, in particular, calcium handling (6). Moreover, it has been suggested that pathological conditions, such as hypertension, not only interfere with the pathophysiological mechanisms of I/R per se, but may also suppress intrinsic mechanisms of cardioprotection known as ischemic preconditioning (I-PC) (7). Acute adaptation induced by PC with brief episodes of ischemia or hypoxia and its various modifications is a particularly effective method of increasing cardiac ischemic tolerance (8) in all species including humans (9), and is applicable to bypass surgery and angioplasty (10). Although some studies have indicated that repeated, brief, stressful stimuli could evoke a short-term adaptative response that modulate cardiac susceptibility to acute oxygen deprivation (11) – even in the remodelled myocardium of hypertensive rats – it is still a matter of debate as to whether PC is a phenomenon of the ‘healthy heart’, or whether the potential of intrinsic cardioprotection also exists in diseased hearts (6,12,13).
Similar to the short-term cardioprotection induced by I-PC, enhanced resistance to ischemia develops as the result of long-term adaptation to some physiological stimuli (eg, physical exercise) or to pathological processes associated with myocardial hypoxia and hypertrophy such as long-term exposure to chronic hypoxia (14,15).
Limitation of living space represents a special form of chronic stress with a strong emotional component. Humans exposed to an acute challenging task, such as living in a crowded neighbourhood, demonstrate greater cardiovascular reactivity (ie, a higher increase in arterial blood pressure and heart rate) (16). Although psychosocial stress induced by crowding is a relatively mild form of stress, in animal models, it has been shown to impair vascular regulation that increases the risk of development of hypertension (17,18). However, little evidence is available with respect to the modulation of cardiac ischemic tolerance by chronic psychosocial stress, in particular, in hypertensive models.
Both chronic social stress and acute stress induced by a brief ischemic episode are associated with modulation of sympathetic activity (18–21). Under certain conditions, sympathetic activation can trigger a protective response in the myocardium (22). Moreover, protection against different end points of I/R injury (eg, arrhythmias, stunning and infarction) conferred by I-PC has been shown to involve stimulation of beta- or alpha-1 adrenergic receptors (20,21,23,24). However, most previous studies have been performed in the hearts of healthy animals. Therefore, the present study was designed to reveal potential differences in the effects of long-term adaptation to crowding stress (CS) and short-term adaptation to ischemic stress, on ischemic tolerance in pathologically altered hearts of hypertensive rats. An additional goal was to compare the effects of both forms of stress on myocardial response to I/R in hypertensive rats versus their normotensive counterparts.
METHODS
Animals
Adult male spontaneously hypertensive rats (SHR) and their normotensive control counterparts (Wistar Kyoto rats) were fed a standard diet and tap water ad libitum, and were housed under standard conditions with a constant 12 h light/12 h dark cycle (lights on at 06:00 h), a mean (± SD) temperature of 22°±2°C. All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication 85–23, revised 1996), and approved by the Animal Health and Welfare Division of the State Veterinary and Food Administration of the Slovak Republic.
In the protocol of chronic CS, each group of animals was randomly divided into two subgroups: control group, in which groups of four rats were housed in cages designed for six (ie, living space 480 cm2/rat); and a stressed group, in which five rats were housed in cages designed for two (ie, living space 200 cm2/rat). After baseline measurement of arterial blood pressure (BP) by noninvasive tail-cuff plethysmography (ADInstruments, Germany), data regarding parameters of heart weight and body weight, as well as nitric oxide (NO) synthase (NOS) activity were recorded. The animals were subsequently entered into the CS protocol. After eight weeks, the animals were sacrificed and all measurements were repeated. In addition, transmission electron microscopy examination of heart tissue was performed in all groups of animals. Additional experiments were performed in isolated perfused hearts.
Perfusion technique
The hearts of anesthetized (sodium pentobarbitone 60 mg/kg, intraperitoneally) animals were rapidly excised and perfused at 37°C in the Langendorff mode at a constant perfusion pressure of 73 mmHg. In the hearts of hypertensive animals, perfusion pressure was proportionally adjusted to the higher blood pressure in SHR in vivo (Table 1). The perfusion solution was a modified Krebs-Henseleit buffer infused with 95% O2 and 5% CO2 (pH 7.4) containing the following: 118 mM NaCl, 3.2 mM KCl, 1.2 mM MgSO4, 25 mM NaHCO3, 1.18 mM KH2PO4, 2.5 mM CaCl2 and 5.5 mM glucose. An epicardial electrogram was registered by means of two electrodes attached to the apex of the heart and the aortic cannula. Left ventricular (LV) pressure was measured by means of a nonelastic balloon inserted into the LV cavity (water-filled to obtain end-diastolic pressure of 5 mmHg) and connected to a pressure transducer (MLP844 [ADInstruments, Germany]). LV developed pressure (LVDP [systolic minus diastolic pressure]), LV end-diastolic pressure (LVEDP), maximal rates of pressure development and fall (+[dP/dt]max and −[dP/dt]max, respectively) as the indexes of contraction and relaxation, heart rate and coronary flow were measured during a preischemic stabilization period, and were continuously recorded until the end of the experiment using PowerLab/8SP Chart 7 software (ADInstruments, Germany).
TABLE 1.
Weight and heart function characteristics of normotensive and hypertensive rats exposed to eight weeks of crowding stress
| Group parameters |
Normotensive |
Hypertensive |
||
|---|---|---|---|---|
| Control | Stressed | Control | Stressed | |
| BW, g | 390±11 | 376±11 | 339±5* | 332±6* |
| HW, g | 1.15±0.07 | 1.15±0.02 | 1.47±0.02* | 1.37±0.02* |
| LVW/BW, mg/g | 1.5±0.04 | 1.5±0.03 | 2.2±0.09* | 2.3±0.05* |
| BP, mmHg | 110.7±2.3 | 112±1.5 | 189±3.6* | 197±3.0*† |
| HR, beats/min | 307±23 | 292±4 | 316±24 | 295±4 |
| LVDP, mmHg | 71±6 | 77±8 | 86±2* | 95±8* |
| +(dP/dt)max, mmHg/s | 2156±293 | 2112±438 | 3052±270* | 2893±105* |
| −(dP/dt)max, mmHg/s | 1206±125 | 1096±1118 | 1614±56* | 1460±212* |
| CF/HW, mL/min/g | 11.8±1.2 | 8.4±0.5† | 9.4±0.1* | 8.5±0.1† |
Data presented as mean ± SEM of eight to 10 animals per group.
P<0.05 versus normotensive rats;
P<0.05 versus unstressed control rats. +(dP/dt)max Maximal rate of pressure development; −(dP/dt)max Maximal rate of pressure fall; BP Blood pressure; BW Body weight; CF/HW Specific coronary flow; HR Heart rate; HW Heart weight; LVDP Left ventricular developed pressure (LV systolic pressure – diastolic pressure); LVW/BW Relative LV weight to BW
Protocols of test ischemia
The hearts of all experimental groups were randomly assigned to the following protocols (n=8 to 10 hearts per group).
After a 20 min stabilization period, the hearts were subjected to 25 min of global ischemia followed by 40 min of reperfusion by clamping and unclamping of aortic inflow for the evaluation of postischemic contractile dysfunction (myocardial stunning) and reperfusion-induced tachyarrhythmias. Recovery of LVDP at the end of reperfusion (percentage of pre-ischemic values) and ventricular arrhythmias occurring during the first 10 min of reperfusion served as the end points of injury. Arrhythmias were quantified in accordance with The Lamberth Conventions (25). Data collection was focused on the incidence of the most severe arrhythmias, sustained ventricular fibrillation (SVF, duration >2 min) and duration of ventricular tachycardia (VT).
After a 20 min stabilization period, the hearts were subjected to 30 min of global ischemia followed by 2 h of reperfusion for the determination of infarct size (IS) as the primary end point of injury.
I-PC protocol
In an acute ischemic stress setting, after a 20 min stabilization period, the hearts of normotensive and hypertensive animals were subjected to one 5 min cycle of ischemia and 5 min of reperfusion before 30 min of global ischemia, followed by 2 h reperfusion for the evaluation of LVDP recovery and IS.
IS determination
The size of the infarcted area and the area at risk size were delineated by staining with 2,3,5-triphenyltetrazolium chloride and determined by a computerized planimetric method as previously described (26). Because the area at risk represents the entire area of the LV in the global ischemia protocol, IS was expressed as a percentage of LV size.
Transmission electron microscopy examination of qualitative structural alterations
In parallel subsets of experiments, perfusion fixation of the hearts with 4% buffered paraformaldehyde was performed by retrograde perfusion via the ascending aorta at a constant pressure of 80 mmHg for 3 min. Small (1 mm3) LV heart tissue samples (n=5 per group) were postfixed in buffered glutaraldehyde and OsO4, dehydrated in a series of ethanol and propylene oxide infiltration, and finally embedded in Epon 812 (Electron Microscopy Sciences, USA) and routinely processed for transmission electron microscopy as described elsewhere (27).
NOS activity
NOS activity was measured in the crude heart tissue homogenates by determination of 3HL-citrulline formation from 3HL-arginine (Amersham, United Kingdom), as described previously (28), and expressed as pmol/min/mg of protein.
Statistical evaluation
The data were expressed as mean ± SEM. One-way ANOVA and subsequent Student-Newman-Keuls test were used for comparing differences in normally distributed variables between the groups. Variables with nonparametric distribution were compared using Fisher’s exact test or Mann-Whitney test using GraphPad Prism version 5.00 (GraphPad Software, USA) for Windows (Microsoft Corporation, USA). Differences were considered to be statistically significant at P<0.05.
RESULTS
Effect of hypertension and crowding on weight parameters and baseline myocardial function
In both hypertensive groups, lower body weight, increased heart weight, LVH and enhanced BP were observed compared with normotensive groups. There were no differences in spontaneous heart rate between the groups. On the other hand, parameters of contractile function (LVDP, dP/dtmax) were increased in the hearts of SHR. Furthermore, coronary perfusion of the myocardium (specific coronary flow) was markedly decreased in these hearts (Table 1).
The effect of CS was observed only in hypertensive animals, in which CS further increased BP compared with unstressed SHR. The mechanical function of the heart was not affected by CS in any of the groups. In contrast, coronary perfusion of the myocardium was reduced in both stressed groups – normotensive and hypertensive – compared with their respective controls. Moreover, this effect of CS was significantly more pronounced in the hearts of normotensive animals (Table 1).
Effect of hypertension and CS on myocardial ultrastructure
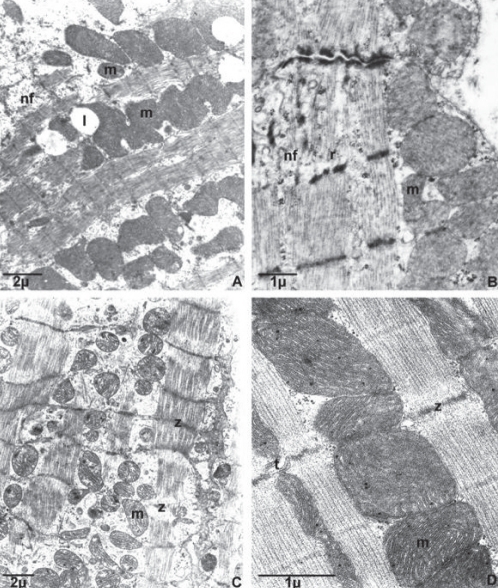
Examination of subcellular structures revealed all of the characteristics of normal ultrastructure in the hearts of normotensive, unstressed control animals. The ultrastructure of myocardial muscle of hypertensive animals showed signs of hypertrophy (Figure 1A). Mitochondria were more numerous, smaller and darker, with fusions of mitochondria also present. In addition, signs of neofibrilogenesis (the creation of new myofilaments) and an increased number of ribosomes were frequently viewed (Figure 1B).
Figure 1).
Effect of eight-week crowding stress on the myocardial ultrastructure of normotensive and hypertensive rats. Transmission electron microscopy examination of qualitative structural alterations. A and B Myocardial ultrastructure of unstressed hypertensive rats. C Myocardial ultrastructure of normotensive stressed rats. D Myocardial ultrastructure of hypertensive stressed rats (higher magnification). l Lipid droplet; m Mitochondria; nf Neofibrilogenesis; r Ribosome; t T tubule; z Z line. Samples were obtained from five different hearts per group
In the myocardium of normotensive animals exposed to stress, foci of hypercontractions and mostly reversibly altered mitochondria representing the manifestations of ischemia-like changes were observed (Figure 1C). Myocardial ultrastructure in the stressed animals of the SHR group did not appreciably differ from the ultrastructure of the unstressed SHR group (Figure 1D).
Effect of hypertension and CS on NOS activity
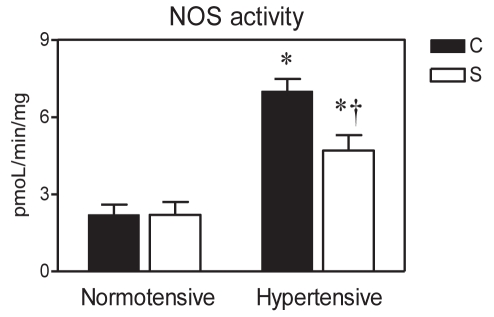
The activity of NOS was markedly elevated in the myocardium of SHR compared with normotensive controls (P<0.05). In contrast, in the stressed hypertensive group, NOS activity was significantly lower than in the respective control group, while no effect of CS on NOS activity was observed in the normotensive animals (Figure 2).
Figure 2).
Effect of eight-week crowding stress on myocardial nitric oxide synthase (NOS) activity in normotensive and hypertensive rats. Data presented as mean ± SEM of four to five hearts per group. C Unstressed normotensive and hypertensive controls; S Stressed rats. *P<0.05 versus normotensive rats; †P<0.05 versus respective unstressed control rats
Effect of hypertension on myocardial ischemic tolerance
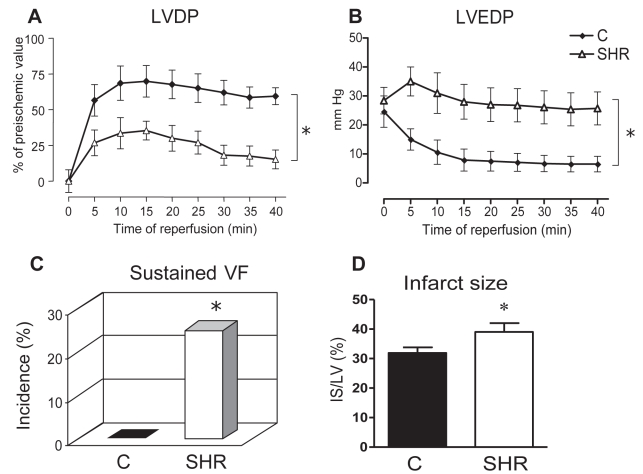
In both experimental protocols, testing of myocardial ischemic tolerance demonstrated significantly lower recovery of systolic and diastolic function in the hearts of the SHR group (LVDP 15±6% of pre-ischemic value; LVEDP 26±6 mmHg versus 60±6% and 12±3 mmHg, respectively, in normotensive animals [P<0.05]) (Figures 3A and 3B). In a second protocol of more severe I/R focused on lethal injury, in which myocardial stunning was more pronounced, deterioration of LVDP recovery in hypertensive rats compared with the normotensive rats was also obvious (Figure 4A).
Figure 3).
Myocardial ischemic tolerance in isolated Langendorff-perfused hearts of spontaneously hypertensive (SHR) and normotensive Wistar Kyoto control (C) rats subjected to 25 min global ischemia and 40 min reperfusion (A to C) or 30 min ischemia and 2 h reperfusion (D). A, B Timecourse of postischaemic recovery of left ventricular developed pressure (LVDP) and left ventricular end-diastolic pressure (LVEDP) expressed as a percentage of preischemic values and mmHg, respectively. C Incidence of sustained ventricular fibrillation (VF) expressed as a percentage. D Size of myocardial infarction. Infarct size (IS) area is expressed as a percentage of the area at risk (ie, IS area/LV area). Data presented as mean ± SEM of eight to 10 hearts per group. *P<0.05 versus normotensive control group
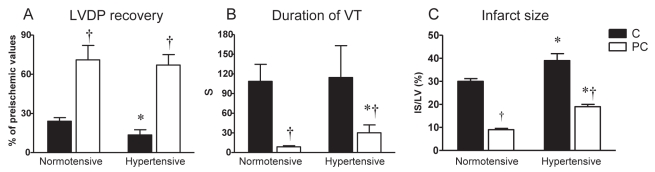
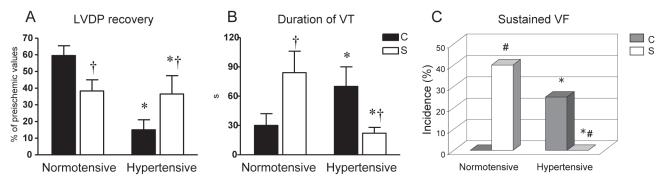
Figure 4).
Effect of acute ischemic preconditioning on postischemic recovery of left ventricular diastolic pressure (LVDP) (A), duration of ventricular tachycardia (VT) (B) and size of infarction (C) in Langendorff-perfused rat hearts subjected to 30 min of global ischemia and 2 h reperfusion. C Nonpreconditioned normotensive and hypertensive controls; PC Preconditioned hearts. Data presented as means ± SEM of eight to 10 hearts per group. *P<0.05 versus normotensive rats; †P<0.05 versus respective nonpreconditioned control rats
In addition, the hearts of the hypertensive group exhibited longer duration of VT (84±22 s versus 30±12 s in the normotensive group [P<0.05]), and while lethal SVF did not occur in the hearts of the normotensive group, it was observed in 25% of the SHR hearts (Figure 3C). Similarly, myocardial IS was significantly larger in the SHR group (IS/LV 39±3.0% versus 30±1.2% in the normotensive group [P<0.05] (Figure 3D).
Effect of CS on ischemic tolerance in hypertensive and normotensive rat hearts
In the hearts of SHR, CS led to a better restoration of heart function after I/R challenge than in the unstressed SHR controls. This was manifested as a mitigation of contractile dysfunction (LVDP recovery 37±11% versus C-SHR [P<0.05]) and by a total suppression of SVF (Figures 5A and 5C). Moreover, susceptibility to VT was also reduced in the stressed SHR group (Figure 5B), and its total duration was shorter than in C-SHR.
Figure 5).
Effect of eight weeks of crowding stress on postischemic recovery of left ventricular diastolic pressure (LVDP) (A), duration of ventricular tachycardia (VT) (B) and incidence of sustained ventricular fibrillation (VF) (C) in Langendorff-perfused rat hearts. C Unstressed normotensive and hypertensive control rats; S Stressed rats. Data presented as mean ± SEM of eight to 10 hearts per group. *P<0.05 versus normotensive rats; †P<0.05 versus respective unstressed control rats
In contrast, CS impaired the postischemic restoration of mechanical function and exacerbated reperfusion-induced arrhythmias in the hearts of normotensive rats (Figure 5). Thus, LVDP in the stressed normotensive group reached 33±6% of its preischemic value, duration of VT was prolonged to 70±20 s (both P<0.05 versus unstressed controls [Figures 5A and 5B]), with SVF occurring in 40% of these hearts (Figure 5C).
Effect of I-PC on ischemic tolerance in hypertensive and normotensive rat hearts
I-PC markedly improved recovery of LVDP in hypertensive and normotensive groups (both P<0.05 versus respective nonpreconditioned controls [Figure 4A]). Similarly, the duration of VT and IS were significantly lower in the preconditioned hearts of hypertensive and normotensive rats compared with their respective nonpreconditioned controls (Figures 4B and 4C). However, both the duration of VT and the extent of lethal changes in the preconditioned hearts of the SHR group were higher than in the preconditioned hearts of normotensive rats.
DISCUSSION
Our results, showing impaired postischemic contractile recovery in the hypertrophied hearts of SHR rats (Table 1, Figures 1 and 3), are in agreement with the data of Snoeckx et al (29), who, in a model of global I/R injury, showed that isolated working hearts from adult SHR, demonstrated substantially worsened recovery of myocardial function on reperfusion than the hearts from normotensive control rats. One of the reasons for the compromised functional recovery in the remodelled myocardium could be the fact that capillary proliferation in the pressure-induced LVH heart fails to match the increase in myocyte size, resulting in lower capillary density and increased capillary-to-cell O2 diffusion distance leading to insufficient O2 supply even under normal conditions (1). Moreover, abnormalities of the coronary vasculature, such as narrowed arteriolar lumens and increased minimal coronary vascular resistance, might also contribute to the loss of coronary flow reserve and O2 limitation in LVH at baseline (30). More specifically, cellular O2 deprivation upon reperfusion has been shown to correlate with depressed postischemic functional and energetic recovery in SHR (31). Consistent with these data, our study demonstrated a reduced coronary perfusion of the myocardium of SHR (Table 1) and ultrastructure of the myocardium (Figures 1A and 1B), which showed features of hypertrophy (32) already present at baseline conditions that could account for the impaired functional recovery of these hearts on reperfusion and acceleration of lethal injury (Figures 3A, 3B and 3D).
In another model of hypertension and LVH induced by aortic constriction (33), it was also demonstrated that these rats exposed to acute myocardial ischemia experienced an increased postoperative mortality rate due to more frequent occurrence of lethal arrhythmias. The incidence of reperfusion-induced arrhythmias is dependent to a major extent on the generation of reactive oxygen species (ROS) (34). It is well acknowledged that chronic hypertension is associated with changes in oxidative stress and the redox state of the myocardium (35,36). In addition, increased formation of NO in the myocardium can also exert detrimental effects contributing to the pathophysiology of myocardial dysfunction on I/R (37). We observed elevated NOS activity in the myocardium of SHR (Figure 2); hence, enhanced generation of NO and its deleterious effects due to production of toxic metabolites (38,39) may also be considered. Thus, increased production of ROS (40), along with abnormal intracellular calcium homeostasis due to decreased gene and protein expression of sarcoplasmic reticulum Ca2+-ATPase in the myocardium of hypertensive animals (41), may have a negative impact on the recovery of heart function under conditions of acute ischemic challenge (42) and might underlie the enhanced arrhythmogenesis in the SHR hearts shown in the present study (Figure 3C).
Effect of CS on the response to I/R in the myocardium of hypertensive rats
Paradoxically, the hearts of hypertensive animals exposed to chronic CS did not exhibit any additional deterioration in their response to I/R injury. On the contrary, the degree of myocardial stunning, in addition to the duration and severity of reperfusion-induced tachyarrhythmias, were attenuated in these hearts compared with the unstressed SHR controls (Figure 4). In contrast, CS diminished the restoration of mechanical function and exacerbated reperfusion-induced arrhythmias in the hearts of normotensive animals (Figure 5).
Interestingly, a relatively mild stress (ie, CS) led to more prominent changes in the myocardial ultrastructure of the hearts of normotensive rats (Figure 1C), whereas in the hypertensive animals, subcellular structures were minimally affected by CS, and myocardial ultrastructure remained practically unchanged compared with that in the already remodelled myocardium of unstressed SHR rats (Figures 1B and D). In addition, CS caused significantly more pronounced dysregulation of coronary microcirculation and altered myocardial perfusion in normotensive rats than in the hearts of hypertensive rats that experienced limitation of coronary perfusion before CS (Table 1). Thus, it might be assumed that in the adaptive phase of hypertrophy, the myocardium of SHR was already adjusted to chronic O2 deprivation and could, therefore, better tolerate additional stressful conditions, such as CS, than the nonadapted myocardium of normotensive rats.
Furthermore, the development of cross-adaptation caused by two concurrent pathologies (43,44) cannot be excluded. Several stressful factors (eg, ROS [45]) and changes in cell volume (46) may play a dual role in the mechanism of I/R injury and, aside from the deleterious effects, induce short- or long-term adaptive processes (14,47) that enhance the resistance of the heart to subsequent ischemia. These factors might also account for an improved response to an acute ischemic challenge in the hearts of stressed SHR. In addition, CS suppressed enhanced cardiac activity of NOS in hypertensive rats (Figure 2). It is, therefore, conceivable that apparently lower production of NO and limited generation of toxic reactive nitrogen species might be involved in the mechanisms of enhanced resistance to I/R in SHR exposed to chronic stress.
On the other hand, CS did not modify the activity of NOS, and hence the production of NO, in the myocardium of normotensive rats in the present study (Figure 2). Moreover, as shown recently, myocardial ROS production was not increased in these hearts (48). Thus, it is plausible that the stressed myocardium of normotensive animals might be less adapted to the deleterious effects of oxidative stress, and the latter might be of particular importance under conditions of acute ischemic challenge. However, to the best of our knowledge, no study aimed at exploring the impact of chronic psychosocial stress on myocardial response to I/R in hypertensive versus normotensive subjects has been conducted.
Effect of PC in the myocardium of hypertensive rats
Adaptive mechanisms can be blunted by pathologies related to lifestyle; some authors consider PC as a ‘healthy heart’ phenomenon, the efficiency of which may be abolished by concomitant pathological conditions (6,13). However, the effect of risk factors has not been unequivocally proven. Although hypertensive rats are generally more sensitive to I/R (1), the hearts of SHR rats exposed to osmotic PC (5) and the hearts of female SHR rats (49) have been shown to be more tolerant to I/R. Moreover, other studies demonstrated the persistance of the cardioprotective effect of I-PC in remodelled myocardium (11) and even in aged hypertensive animals (50). In concert, the results of our study demonstrate that the hearts of hypertensive rats could be effectively preconditioned against myocardial stunning, reperfusion-induced arrhythmias and against irreversible injury (Figure 5), although the degree of anti-infarct and anti-arrhythmic protection was lower than in the preconditioned hearts of normotensive rats. These results suggest that the potential of intrinsic cardioprotection is retained even in the pathologically altered myocardium, and that there might be possibilities to restore reduced myocardial ischemic tolerance in these hearts, albeit using a preconditioning stimulus of higher intensity.
CONCLUSIONS
Cardiac tolerance to ischemia is impaired in hypertensive rats. However, the response to I/R is improved in rats exposed to chronic CS and to acute ischemic stress evoked by PC. On the other hand, CS represents a higher risk of lethal arrhythmias and contractile failure in the hearts of normotensive individuals exposed to an acute ischemic challenge. Triggering of more complex endogenous adaptive mechanisms under the above conditions cannot be ruled out and requires additional investigation.
Acknowledgments
The authors are grateful to Dezider Pancza Dipl Ing, Mrs Iveta Blažíčková and Iveta Formánková for their excellent technical assistance. This study was supported by grants VEGA SR 2/0054/11, APVV-LPP-0393-09, APVV 0538-07 and APVV 0523-10. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg. 2003;75:S678–84. doi: 10.1016/s0003-4975(02)04692-1. [DOI] [PubMed] [Google Scholar]
- 2.Perreault S, Dragomir A, Roy L, et al. Adherence level of antihypertensive agents in coronary artery disease. Br J Clin Pharmacol. 2010;69:74–84. doi: 10.1111/j.1365-2125.2009.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho KK, Levy D, Kannel WB, Pinsky JL. The epidemiology of heart failure: The Framingham study. J Am Coll Cardiol. 1993;22:6–13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 4.Orsinelli DA, Aurigemma GP, Battista S, Krendel S, Gaasch WH. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis: A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol. 1993;22:1679–83. doi: 10.1016/0735-1097(93)90595-r. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Azuma M, Maeda K, Kajimoto N, Higashino H. Impaired heart function and noradrenaline release after ischaemia in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2000;27:664–70. doi: 10.1046/j.1440-1681.2000.03325.x. [DOI] [PubMed] [Google Scholar]
- 6.Galinanes M, Fowler AG. Role of clinical pathologies in myocardial injury following ischaemia and reperfusion. Cardiovasc Res. 2004;61:512–21. doi: 10.1016/j.cardiores.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Balakumar P, Singh H, Singh M, Anand-Srivastava MB. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res. 2009;60:18–23. doi: 10.1016/j.phrs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 9.Vohra HA, Galinanes MJ. Myocardial preconditioning against ischemia-induced apoptosis and necrosis in man. Surg Res. 2006;134:138–44. doi: 10.1016/j.jss.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Loubani M, Hassouna A, Galinanes M. Delayed preconditioning of the human myocardium: Signal transduction and clinical implications. Cardiovasc Res. 2004;61:600–9. doi: 10.1016/j.cardiores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Speechly-Dick ME, Baxter GF, Yellon DM. Ischaemic preconditioning protects hypertrophied myocardium. Cardiovasc Res. 1994;28:1025–9. doi: 10.1093/cvr/28.7.1025. [DOI] [PubMed] [Google Scholar]
- 12.Adameová A, Kuželová M, Andelová E, et al. Hypercholesterolemia abrogates an increased resistance of diabetic rat hearts to ischemia-reperfusion injury. Mol Cell Biochem. 2007;295:129–36. doi: 10.1007/s11010-006-9282-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 14.Kolár F, Jezková J, Balková P, et al. Role of oxidative stress in PKC-delta upregulation and cardioprotection induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2007;292:H224–30. doi: 10.1152/ajpheart.00689.2006. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy J, Lochner A, Opie LH, Sack MN, Essop MF. PKCɛ promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition. J Cell Physiol. 2011;226:2457–68. doi: 10.1002/jcp.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming I, Baum A, Davidson LM, Rectanus E, McArdle S. Chronic stress as a factor in physiologic reactivity to challenge. Health Psychol. 1987;6:221–37. doi: 10.1037//0278-6133.6.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Bernatova I, Csizmadiova Z. Effect of chronic social stress on nitric oxide synthesis and vascular function in rats with family history of hypertension. Life Sci. 2006;78:1726–32. doi: 10.1016/j.lfs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Toot JD, Reho JJ, Novak J, Dunphy G, Ely DL, Ramirez RJ. Colony social stress differentially alters blood pressure and resistance-sized mesenteric artery reactivity in SHR/y and WKY male rats. Stress. 2011;14:33–41. doi: 10.3109/10253890.2010.491876. [DOI] [PubMed] [Google Scholar]
- 19.Dronjak S, Gavrilović L, Filipović D, Radojcic MB. Immobilization and cold stress affect sympathoadrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–15. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Imani A, Faghihi M, Sadr SS, Keshavarz M, Niaraki SS. Noradrenaline reduces ischemia-induced arrhythmia in anesthetized rats: Involvement of alpha1-adrenoceptors and mitochondrial K ATP channels. J Cardiovasc Electrophysiol. 2008;19:309–15. doi: 10.1111/j.1540-8167.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- 21.Lochner A, Marais E, Genade S, Huisamen B, du Toit EF, Moolman JA. Protection of the ischaemic heart: Investigations into the phenomenon of ischaemic preconditioning. Cardiovasc J Afr. 2009;20:43–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Akita Y, Otani H, Matsuhisa S, et al. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol. 2007;292:H2051–9. doi: 10.1152/ajpheart.01102.2006. [DOI] [PubMed] [Google Scholar]
- 23.Bankwala Z, Hale SL, Kloner RA. α-Adrenoceptor stimulation with exogenous norepinephrine or release of endogenous catecholamines mimicks ischemic preconditioning. Circulation. 1994;90:1023–8. doi: 10.1161/01.cir.90.2.1023. [DOI] [PubMed] [Google Scholar]
- 24.Ravingerová T, Pancza D, Ziegelhoffer A, Styk J. Preconditioning modulates susceptibility to ischemia-induced arrhythmias in the rat heart: The role of alpha-adrenergic stimulation and K(ATP) channels. Physiol Res. 2002;2:109–19. [PubMed] [Google Scholar]
- 25.Walker MJ, Curtis MJ, Hearse DJ, et al. The Lamberth conventions: Guidelines for study of arrhythmia in ischemia, infarction and reperfusion. Cardiovasc Res. 1988;22:447–55. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 26.Ravingerová T, Matejíková J, Pancza D, Kolář F. Reduced susceptibility to ischemia-induced arrhythmias in the preconditioned rat heart is independent of PI3-kinase/Akt. Physiol Res. 2009;58:443–7. doi: 10.33549/physiolres.931743. [DOI] [PubMed] [Google Scholar]
- 27.Slezák J, Tribulova N, Ravingerova T, Singal PK. Myocardial heterogeneity and regional variations in response to injury. Lab Invest. 1992;67:322–30. [PubMed] [Google Scholar]
- 28.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–5. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snoeckx LH, van der Vusse GJ, Coumans WA, Willemsen PH, van der Nagel T, Reneman RS. Myocardial function in normal and spontaneously hypertensive rats during reperfusion after a period of global ischaemia. Cardiovasc Res. 1986;20:67–75. doi: 10.1093/cvr/20.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Bache RJ. Effects of hypertrophy on the coronary circulation. Prog Cardiovasc Dis. 1988;30:403–40. doi: 10.1016/0033-0620(88)90005-9. [DOI] [PubMed] [Google Scholar]
- 31.Chung Y. Oxygen reperfusion is limited in the postischemic hypertrophic myocardium. Am J Physiol Heart Circ Physiol. 2006;290:H2075–84. doi: 10.1152/ajpheart.00619.2005. [DOI] [PubMed] [Google Scholar]
- 32.Slezák J, Zlatoš L, Randhawa A, Singal PK. Age-dependent differences in cardiac growth response to workload. In: Nagano M, Takeda N, Dhalla NS, editors. The cardiomyopathic heart. New York: Raven Press; 1994. pp. 354–60. [Google Scholar]
- 33.Linz W, Wiemer G, Schmits HL, Ulmer W, Ruppert D, Schölkens BA. ACE inhibition decreases postoperative mortality in rats with left ventricular hypertrophy and myocardial infarction. Clin Exp Hypertens. 1996;18:691–712. doi: 10.3109/10641969609081775. [DOI] [PubMed] [Google Scholar]
- 34.Ravingerová T, Slezák J, Tribulová J, Džurba A, Uhrík B, Ziegelhoffer A. Reactive oxygen species contribute to high incidence of reperfusion-induced arrhythmias in isolated rat heart. Life Sci. 1999;18/19:1927–31. doi: 10.1016/s0024-3205(99)00449-x. [DOI] [PubMed] [Google Scholar]
- 35.Pechánová O, Zicha J, Paulis L, et al. The effect of N-acetylcysteine and melatonin in adult spontaneously hypertensive rats with established hypertension. Eur J Pharmacol. 2007;561:129–36. doi: 10.1016/j.ejphar.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Simko F, Pechanova O. Remodelling of the heart and vessels in experimental hypertension: Advances in protection. J Hypertens. 2010;28(Suppl 1):S1–6. doi: 10.1097/01.hjh.0000388487.43460.db. [DOI] [PubMed] [Google Scholar]
- 37.Berges A, Van Nassauw L, Bosmans J, Timmermans JP, Vrints C. Role of nitric oxide and oxidative stress in ischemic myocardial injury and preconditioning. Acta Cardiol. 2003;58:119–32. doi: 10.2143/AC.58.2.2005264. [DOI] [PubMed] [Google Scholar]
- 38.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–43. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andelová E, Barteková M, Pancza D, Styk J, Ravingerová T. The role of NO in ischemia/reperfusion injury in isolated rat heart. Gen Physiol Biophys. 2005;24:411–26. [PubMed] [Google Scholar]
- 40.Sahna E, Deniz E, Bay-Karabulut A, Burma O. Melatonin protects myocardium from ischemia-reperfusion injury in hypertensive rats: Role of myeloperoxidase activity. Clin Exp Hypertens. 2008;30:673–81. doi: 10.1080/10641960802251966. [DOI] [PubMed] [Google Scholar]
- 41.Kang L, Fang Q, Hu SJ. Regulation of phospholamban and sarcoplasmic reticulum Ca2+-ATPase by atorvastatin: Implication for cardiac hypertrophy. Arch Pharm Res. 2007;30:596–602. doi: 10.1007/BF02977654. [DOI] [PubMed] [Google Scholar]
- 42.Wei GZ, Zhou JJ, Wang B, et al. Diastolic Ca2+ overload caused by Na+/Ca2+ exchanger during the first minutes of reperfusion results in continued myocardial stunning. Eur J Pharmacol. 2007;572:1–11. doi: 10.1016/j.ejphar.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 43.Lunt HC, Barwood MJ, Corbett J, Tipton MJ. ‘Cross-adaptation’: habituation to short repeated cold-water immersions affects the response to acute hypoxia in humans. J Physiol. 2010;588(Pt 18):3605–13. doi: 10.1113/jphysiol.2010.193458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning XH, Chen SH. Mild hypothermic cross adaptation resists hypoxic injury in hearts: A brief review. Chin J Physiol. 2006;49:213–22. [PubMed] [Google Scholar]
- 45.Morihira M, Hasebe N, Baljinnyam E, et al. Ischemic preconditioning enhances scavenging activity of reactive oxygen species and diminishes transmural difference of infarct size. Am J Physiol Heart Circ Physiol. 2006;290:H577–83. doi: 10.1152/ajpheart.00817.2004. [DOI] [PubMed] [Google Scholar]
- 46.Ganote CE, Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol. 2003;35:749–59. doi: 10.1016/s0022-2828(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 47.Matejíková J, Kucharská J, Pintérová M, Pancza D, Ravingerová T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: Involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol Res. 2009;58:9–19. doi: 10.33549/physiolres.931317. [DOI] [PubMed] [Google Scholar]
- 48.Puzserova A, Bernatova I. Chronic social stress increases nitric oxide-dependent vasorelaxation in normotensive rats. Interdiscip Toxicol. 2010;3:109–17. doi: 10.2478/v10102-010-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Besík J, Szárszoi O, Kunes J, et al. Tolerance to acute ischemia in adult male and female spontaneously hypertensive rats. Physiol Res. 2007;56:267–74. doi: 10.33549/physiolres.930998. [DOI] [PubMed] [Google Scholar]
- 50.Dai W, Simkhovich BZ, Kloner RA. Ischemic preconditioning maintains cardioprotection in aging normotensive and spontaneously hypertensive rats. Exp Gerontol. 2009;44:344–9. doi: 10.1016/j.exger.2009.02.005. [DOI] [PubMed] [Google Scholar]