Abstract
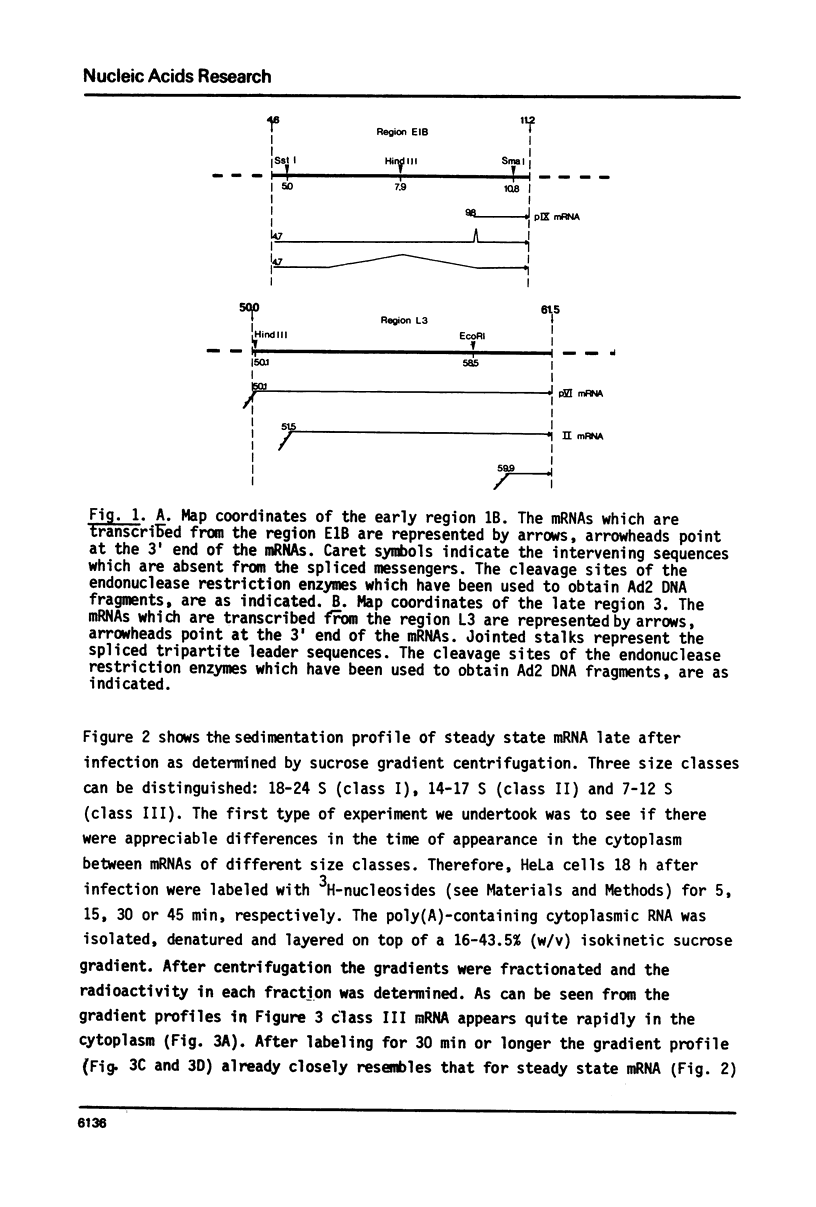
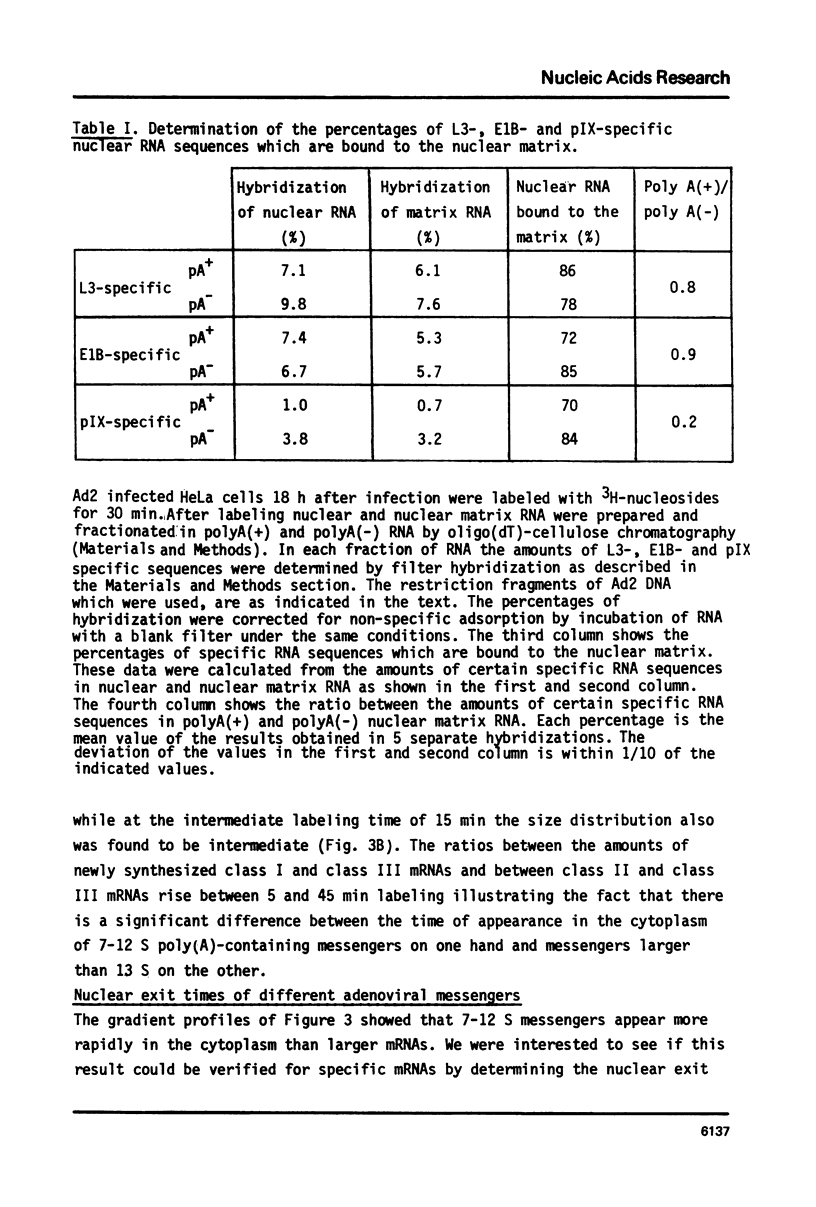
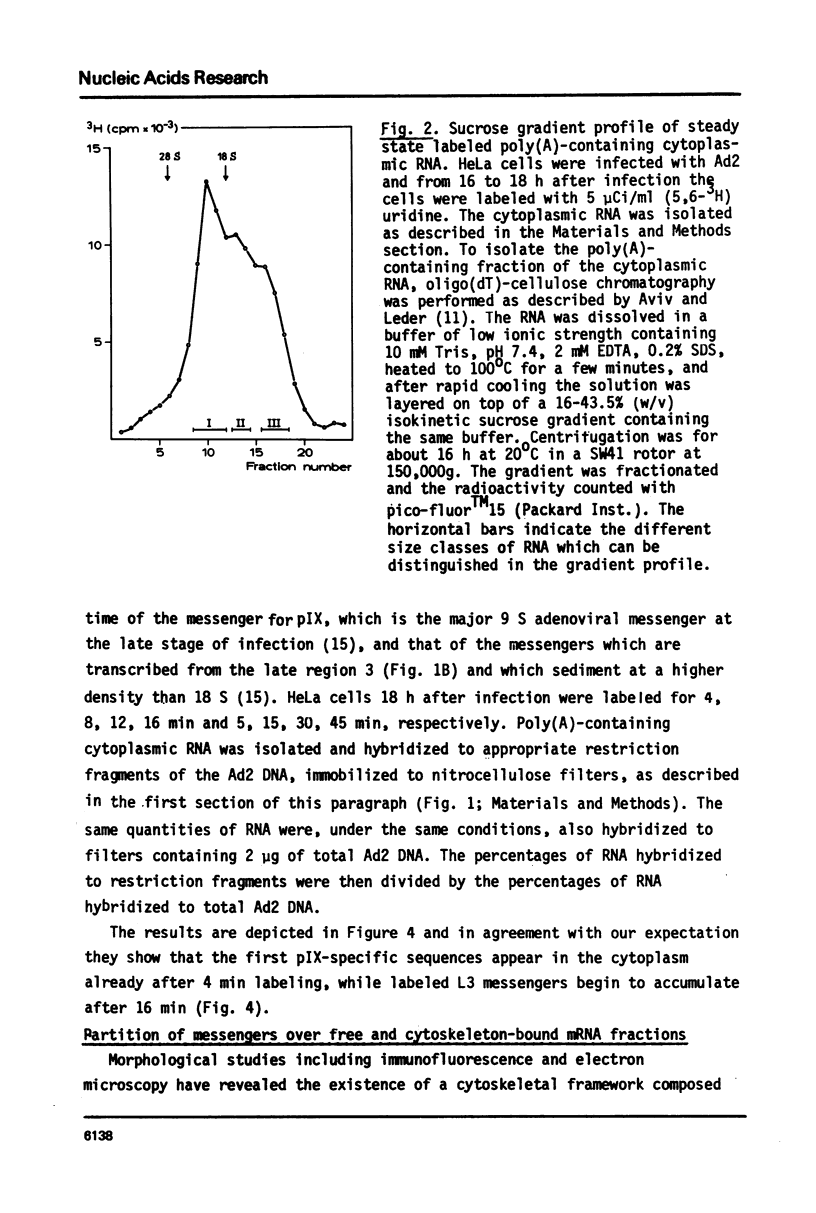
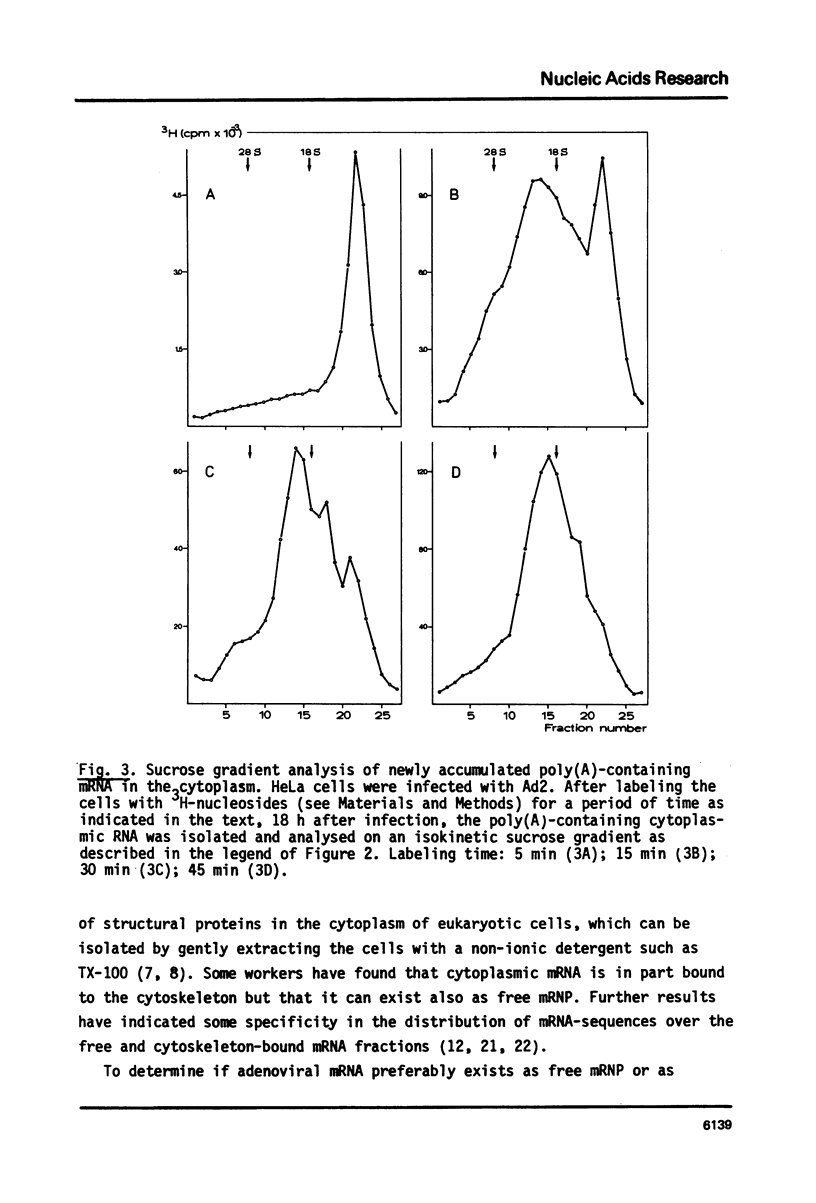
In the nucleus of HeLa cells late after infection with adenovirus type 2 mRNA-sequences which are processed via RNA splicing are attached to the nuclear matrix (Mariman et al., 1982). Although the mRNA, which codes for polypeptide IX, is not formed via splicing, about 70% of the non-polyadenylated pre-mRNA and the polyadenylated pIX mRNA are bound to the matrix structure, indicating that polyadenylation is performed while the RNA is associated with the matrix. Binding to the nuclear matrix seems to be a common property of all mRNA-sequences in the nucleus. At the late stage of infection most of the newly synthesized mRNAs which appear in the cytoplasm are viral specific (Beltz & Flint, 1979). Kinetic analysis of the newly synthesized poly(A)-containing mRNA on sucrose gradients reveals that 7-12 S messengers appear more rapidly in the cytoplasm than messengers larger than 13 S. More specifically, the nuclear exit time of the pIX-mRNA, which is the major 9 S adenoviral messenger late after infection, was determined to be about 4 min, while messengers transcribed from the late region 3 need more than 16 min to arrive in the cytoplasm. In the cytoplasm about 70% of the mRNA is bound to the cytoskeletal framework, while 30% remains as free mRNP. Analysis of the mRNA in both fractions reveals that L3-, E1B- and pIX-specific polyadenylated mRNA preferably exist as cytoskeleton-bound mRNA. However, significant differences occur in the partition of specific messengers over free and cytoskeletal RNA fractions.
Full text
PDF




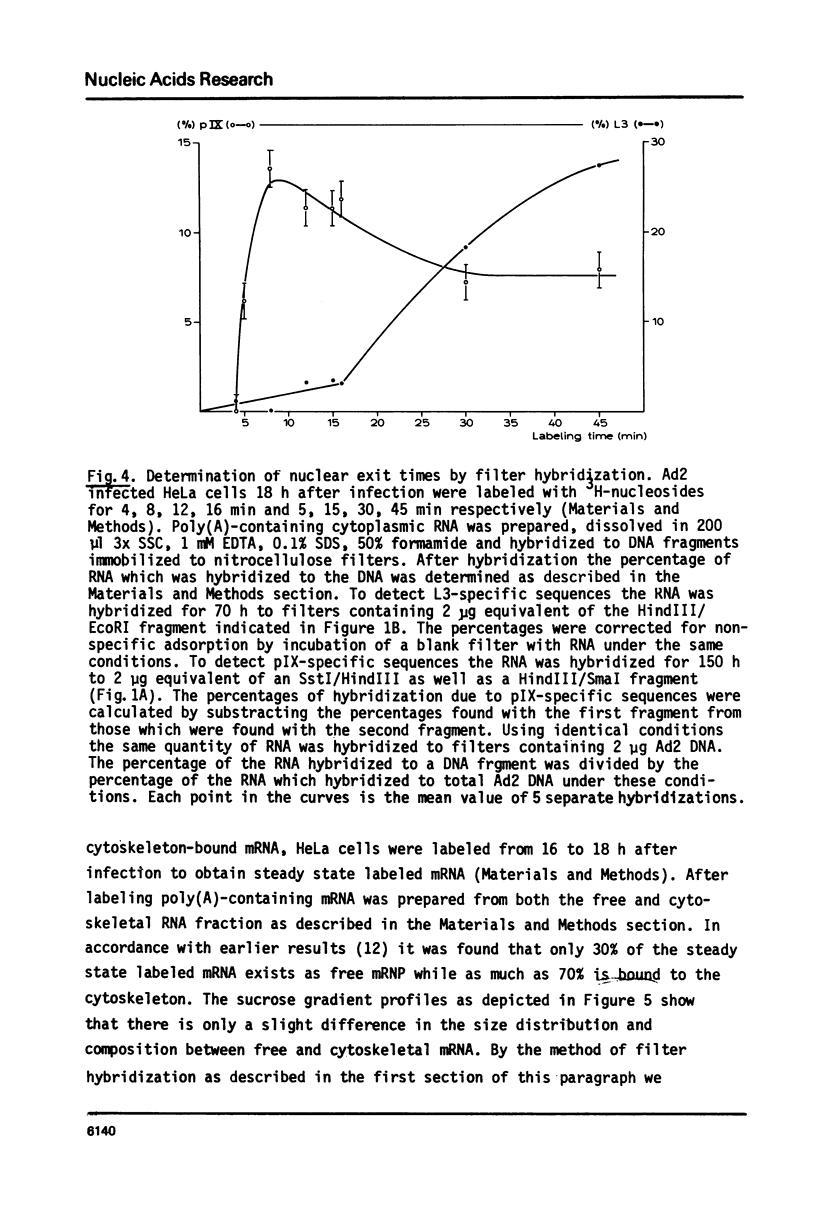
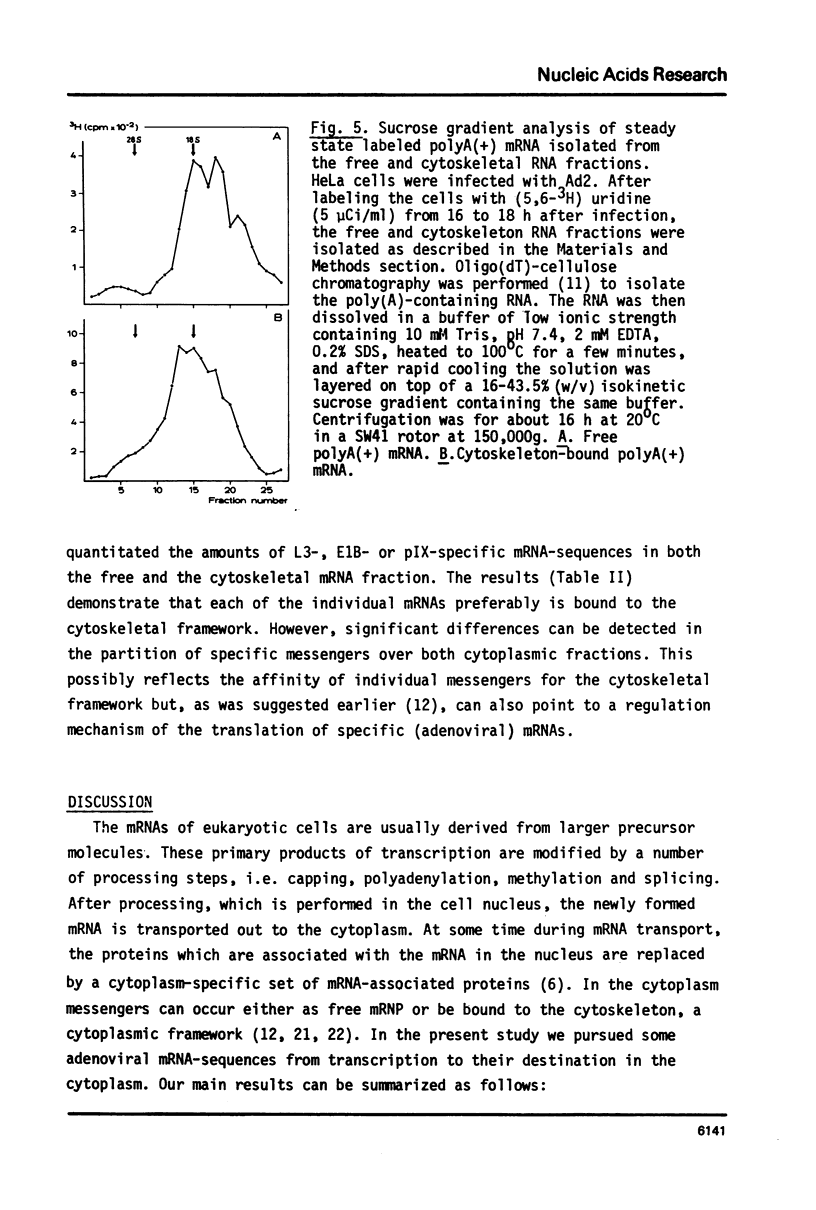




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Horowitz M., Skolnik H., Abulafia R., Laub O., Aloni Y. The metabolism of SV40 RNA is associated with the cytoskeletal framework. Virology. 1981 Jun;111(2):475–487. doi: 10.1016/0042-6822(81)90350-0. [DOI] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburgh A. J., Ross J. Processing of the mouse beta-globin mRNA precursor: at least two cleavage-ligation reactions are necessary to excise the larger intervening sequence. Cell. 1979 Aug;17(4):915–921. doi: 10.1016/0092-8674(79)90331-3. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Schodhetman G., Perry R. P. Early appearance of histone messenger RNA in polyribosomes of cultured L cells. J Mol Biol. 1972 Feb 14;63(3):591–596. doi: 10.1016/0022-2836(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., Riemen T., van Venrooij W. J. Specificity in the interaction of hnRNA and mRNA with proteins as revealed by in vivo cross linking. FEBS Lett. 1981 Aug 3;130(2):223–226. doi: 10.1016/0014-5793(81)81125-8. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]


