Abstract
Co-ordinated movement and controlled positioning of leucocytes is key to the development, maintenance and proper functioning of the immune system. Chemokines and their receptors play an essential role in these events by mediating directed cell migration, often referred to as chemotaxis. The chemotactic property of these molecules is also thought to contribute to an array of pathologies where inappropriate recruitment of specific chemokine receptor-expressing leucocytes is observed, including cancer and inflammatory diseases. As a result, chemokine receptors have become major targets for therapeutic intervention, and during the past 15 years much research has been devoted to understanding the regulation of their biological activity. From these studies, processes which govern the availability of functional chemokine receptors at the cell surface have emerged as playing a central role. In this review, we summarize and discuss current knowledge on the molecular mechanisms contributing to the regulation of chemokine receptor surface expression, from gene transcription and protein degradation to post-translational modifications, multimerization, intracellular transport and cross-talk.
Keywords: chemokine receptors, chemokines, regulation, immunity and infection
Chemokine receptor function and regulation
Chemokine receptors belong to the G protein-coupled receptor (GPCR) superfamily and are divided into four classes, named according to the type of chemokine (CC, CXC, CX3C or XC) with which they interact.1 Since the cloning of the interleukin-8 (CXCL8) receptor,2 a total of 10 CC, seven CXC, one CX3C and one XC receptors have been identified.1,3 There is apparent redundancy in the system, as many chemokines bind multiple receptors of one class and more than one receptor can interact with each chemokine. However, some groups have found different receptor signalling and trafficking responses to individual chemokines, suggesting that this redundancy may not be as widespread as thought previously.4,5
Chemokine receptors have a wide range of biological functions and can be grouped as constitutive or inflammatory receptors depending on whether they play a role predominantly in development and homeostasis, or in host response to inflammation and infection.6 They control the trafficking and positioning of leucocytes throughout the body by inducing directed cell movement towards the source of chemokine gradients (chemotaxis). In particular, inflammatory chemokine receptors have a significant role in host defence due to their ability to trigger leucocyte mobilization in response to chemokines secreted at sites of injury. Many chemokine receptors have been associated with various pathologies, including human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), cancer and inflammatory diseases. However, with the exception of HIV/AIDS, for which it is established that CXCR4 and CCR5 act as co-receptors for virus entry,7–10 the molecular mechanisms by which chemokine receptors contribute to diseases are poorly understood. Work has been carried out in developing drugs targeting at least 10 of the known chemokine receptors. Although antagonists for several receptors are in clinical trials,11–13 the only drug licensed to date is a CCR5 antagonist (Maraviroc) used in HIV therapy.14 As CCR5 antagonism has failed to show clinical benefit with rheumatoid arthritis, it has been suggested that multiple chemokine receptor blockade may be more effective.14,15 Consequently, much effort is currently put towards developing promiscuous antagonists to tackle the problem of redundancy/compensation,12,13 but a greater understanding of the mechanisms regulating chemokine receptor activity might also be required for the development of more efficient drugs.
The ability of cells to respond to chemokines can be modulated by mechanisms affecting either the chemokine or its receptor. Control can be exerted on the chemokine receptors to modulate the cellular levels of receptor molecules, or the presentation of functionally active receptors at the cell surface. Regulation of protein expression can be targeted at the level of gene regulation, mRNA and protein synthesis. However, these processes are too slow to be solely responsible for the changes required by individual cells to fine-tune their response according to the specific composition of the local environment.16 Therefore, tight control of the presence of functional chemokine receptors at the cell surface is essential, and can be achieved by affecting the activation state, signalling ability and/or cellular localization of the receptor. This rapid control can be mediated in response to ligand binding but also as a consequence of cross-talk from other receptors.
A considerable amount of our knowledge regarding chemokine receptor biology comes from concepts uncovered for other GPCRs. However, a few chemokine receptors such as CXCR1, CXCR2, CXCR4, CCR2 and CCR5 have received much attention in the last two decades, leading to the discovery that as part of the desensitization process, chemokine-stimulated receptors are removed from the plasma membrane by endocytosis and transported within the cell.5 Although the trafficking trend appears conserved between chemokine receptors, the mechanisms involved vary and thus cannot be considered generic. Understanding these mechanisms at the molecular and cellular levels could lead to new approaches to target chemokine receptors for disease therapy. In this review we summarize current knowledge about the various molecular mechanisms regulating the presence of functional chemokine receptors at the surface of cells.
Regulation of protein expression
Long-term regulation of chemokine receptors is achieved by controlling the cellular levels of receptor molecules through changes in gene expression, mRNA stability and protein degradation. This can lead to both up- and down-regulation of a specific receptor, as reported for CXCR4.17,18 With regard to leucocytes, the expression of chemokine receptors is tightly regulated on the different subtypes and changes through the processes of cell differentiation, activation and polarization.19–24 This regulation is particularly important for inducible chemokine receptors such as CCR2 and CCR5 helping to recruit blood neutrophils, monocytes and activated T cells to sites of infection.15,25 Host–pathogen interactions can also regulate chemokine receptor expression. For example, it was shown that bacterial lipopolysaccharide (LPS) interfered with CCL2-mediated recruitment of blood neutrophils and monocytes in vivo by down-regulating CCR2 expression.26,27 LPS was found to act in vitro by affecting CCR2 mRNA stability,28,29 as did the inflammatory cytokines interleukin-1 (IL-1), tumour necrosis factor (TNF-α) and interferon-γ (IFN-γ),29,30 but with no major effect on CCR5 transcripts. In contrast, reactive oxygen intermediates produced by phagocytes for killing pathogens increased CCR2, CCR5 and CXCR4 mRNA expression and opposed the down-regulation induced by LPS.31 Interestingly, chemokine receptor switch and modulation of mRNA expression has also been reported with Mycobacterium tuberculosis antigens and proposed to be part of a normal programme of cell co-ordination needed to contain infection.32 Enhancing protein degradation independently of, or in combination with, a transcriptional control is also an efficient way to down-regulate chemokine receptor expression, as described for CXCR1 and CXCR2 on activated neutrophils or CCR2 during monocyte differentiation.20,33 Significantly, changes in the regulation of chemokine receptor expression can contribute to pathological conditions such as Alzheimer's disease, where there is evidence for binding of the amyloid β protein to the receptor for advanced glycation end-products (RAGE) up-regulating CCR5 expression on brain endothelial cells causing T cell infiltration in the brain.34
Control of chemokine receptor functional activity
To be functionally active, cell surface chemokine receptors have to be coupled to a heterotrimeric G protein, presented in a conformation compatible with agonist binding, and ready to transmit intracellular signals. Other GPCRs are thought to reside in the plasma membrane in equilibrium between active and inactive states, depending on complex allosteric interactions and conformational changes affected by ligands as well as cell-specific parameters.35–37 This is still relatively uncharted territory for chemokine receptors but, as will be discussed in detail later, experimental findings suggest that they may be subject to similar regulation. There is evidence for conformational heterogeneity in cell surface CXCR4 and CCR5 receptor populations sometimes related, but not always, to post-translational modifications of the proteins.38–40 Indeed, sulphation and glycosylation have both been shown to influence ligand binding and signalling by CXCR4 and CCR5.39,41 The membrane environment is another factor influencing the activation state of CXCR4 and CCR5, which require cholesterol and lipid rafts for chemokine binding and signalling.42–44 However, if these parameters are important to maintain receptor integrity, whether or not they are accounting for their regulation remains unknown. One feature confirmed to impact on the functional regulation of many chemokine receptors is multimerization.
Receptor multimerization
It is now accepted that GPCRs not only operate as single entities (monomers), but can also function as multimers regulated by allosteric mechanisms.45,46 Chemokine receptors have been shown to form homomers as well as heteromers with other chemokine receptors, GPCRs or distinct types of cell surface receptors (Tables 1 and 2). Techniques commonly used to ascertain receptor–receptor interactions and demonstrate the presence of multimers in living cells include co-immunoprecipitation and fluorescence or bioluminescence resonance energy transfer (FRET or BRET; Tables 1 and 2). Note that many of the studies describing chemokine receptor multimers have been carried out on transfected cells where at least one of the interacting partners is over-expressed, and features of endogenous receptor complexes as well as their biological significance in vivo remain largely to be explored.
Table 1.
Identified chemokine receptor homomers
| Cells | |||||
|---|---|---|---|---|---|
| Receptor | Formation | Methods | Overexp. | Endogenous | References |
| CCR2 | Constitutive | BRET | HEK-293 | 133,134 | |
| Inducible | IP | HEK-293 | MM-1 | 48,135 | |
| CCR5 | Constitutive | IP, Y2H, FLIM, BRET, FRET | HeLa, HEK-293, RBLs | 57,76,78,133,136 | |
| Inducible | IP | HEK-293 L1.2 | 136–138 | ||
| CXCR1 | Constitutive | Co-IP | HEK-293 | 56 | |
| FRET | |||||
| BRET | |||||
| CXCR2 | Constitutive | IP | HEK-293 | Neurons | 56,139 |
| FRET | |||||
| BRET | |||||
| WB | |||||
| CXCR4 | Constitutive | IP | HEK-293, HEK-tsA201 | 49,65,134,140 | |
| FRET | |||||
| BRET | |||||
| Inducible | IP | MOLT4 | 47 | ||
| DARC | Constitutive | BRET | HEK-293 | 141 | |
BRET: bioluminescence resonance energy transfer; CO-IP: co-immunoprecipitation; DARC: duffy antigen receptor for chemokines; FLIM: fluorescence lifetime imaging; FRET: fluorescence resonance energy transfer; IP: immunoprecipitation; WB: Western blot; Y2H: yeast-2-hybrid.
Table 2.
Identified chemokine receptor heteromers and their functional outcomes
| Cells | ||||||
|---|---|---|---|---|---|---|
| Receptors | Formation | Methods | Overexp. | Endogenous | Co-operativity (assays) | References |
| Chemokine receptors | ||||||
| CXCR1/CXCR2 | Constitutive | Co-IP, FRET | HEK-293 | No | 55,56 | |
| BRET | ||||||
| CXCR4/CXCR7 | Constitutive | Co-IP, FRET | HEK-293 | IM-9 | Positive (Ca2+ flux) | 71 |
| CXCR4/CCR2 | Constitutive | BRET | CHO-K1 HEK-293 | Negative (binding, chemotaxis) | 74 | |
| CXCR4/CCR5 | Constitutive | Co-IP | NIH 3T3 | Positive(chemotaxis) | 63,130 | |
| CXCR4/CCR2/CCR5 | Constitutive | BRET | HEK-293 | Negative (binding, chemotaxis) | 73 | |
| CCR2/CCR5 | Inducible | Co-IP | HEK-293 | PBMCs | Positive (Ca2+ flux) | 48 |
| Constitutive | Co-IP, BRET | CHO-K1 HEK-293 | CD4+ T cells | Negative (binding) | 133 | |
| DARC/CCR5 | Constitutive | Co-IP, BRET | HEK-293 | Negative (chemotaxis, Ca2+ flux) | 141 | |
| GPCRs | ||||||
| CCR5/C5aR | Constitutive | Co-IP, BRET | RBLs HEK-293 | Negative(co-internalization) | 76 | |
| CXCR2/DOP | Constitutive | Co-IP, FRET | HEK-293 | Positive(G protein activation) | 72 | |
| BRET | ||||||
| CXCR4/DOP | Constitutive | Co-IP, FRET | HEK-293 | MM-1Monocytes | Negative(chemotaxis, adhesion, Ca2+ flux) | 142 |
| CCR5/opioid receptors | Constitutive | Co-IP | CHO | CEMx174 | Negative(chemotaxis) | 132,143 |
| Others | ||||||
| CXCR2/AMPA GluR1 | Constitutive | Co-IP | HEK-293 | Neurons | Negative (chemotaxis) | 144 |
| CXCR4/CD4 | Inducible (HIV) | Co-IP | PBMCs | N.D. | 145,146 | |
| CXCR4/TCR | Inducible | Co-IP, FRET | Jurkat T | PBMCsT cells | Positive (Ca2+ flux) | 50 |
| CXCR4/IGF-R1 | Constitutive | Co-IP | MCF-7MDA-MB-231 | Positive (chemotaxis) | 147 | |
| CXCR4/CD63 | Inducible | Co-IP | HEK-293 | N.D. | 60 | |
| CCR5/CD4 | Constitutive | FRET | HEK-293CHO K1 | N.D. | 61,64,148 | |
| BRET, Co-IP | ||||||
| Inducible (HIV) | FRET | HEK-293 | DCs | N.D. | 66 | |
AMPA GluR1: a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate-type glutamate receptor 1; BRET: bioluminescence resonance energy transfer; C5aR: complement component 5a receptor; CO-IP: co-immunoprecipitation; DARC: duffy antigen receptor for chemokines; DCs: dendritic cells; DOP: δ-opioid receptor; FRET: fluorescence resonance energy transfer; IGF-R1: insulin-like growth factor-1 receptor; N.D.: not determined; PBMCs: peripheral blood mononuclear cells.
Early work has indicated that chemokine receptor dimerization was ligand-induced, as described for CXCR4 homodimers and CXCR4/CCR5 or CCR2/CCR5 heterodimers.47–51 However, the current view is that chemokine receptor dimers are constitutively formed (Tables 1 and 2), and ligand binding stabilizes or reorganizes pre-existing complexes.52–54 CXCR1 and CXCR2 exemplify this: a recent study revealed that CXCL8 binding stabilizes homodimers but alters heterodimers.55 In fact, dimers are thought to assemble during biosynthesis prior to arriving at the cell surface, as shown for CXCR1/CXCR2 heterodimers56 or for CCR5 homomers.57 Other factors, such as the type of molecules complexed with the chemokine receptor or the cellular background, could affect where and how dimers form. For example, CXCR4 and the T cell receptor (TCR) only dimerize at the surface of T cells following CXCL12 stimulation,50 while CXCR4 interacts with the tetraspannin CD63 in the biosynthetic pathway of B cells.58–60 For CCR5, there are reports of constitutive intracellular interactions with CD4 in a monocytic cell-line61 and stable cell surface CCR5/CD4 heteromers complexed with or without CXCR4 on transfected cells or blood-derived dendritic cells.62–64 Another study described co-localized but independent monomeric CCR5 and CD4 molecules interacting upon binding of HIV-gp120 at the surface of transfected cells.65–67 Pathogen-induced interaction has also been established for CXCR4 and the Toll-like receptor 2 (TLR-2).68
Importantly, multimerization impacts on the cell's biological response to chemokine exposure. Cross-talk within homomers or heteromers enables regulation of chemokine receptors in response to stimuli other than their own ligands. This process, called receptor or ligand-binding co-operativity, is known to occur within all types of GPCR dimers.69 Positive binding co-operativity has been shown for the constitutive CXCR4/CXCR7 dimer in which CXCR7, a chemokine receptor unable to trigger G protein signalling,70 enhances CXCR4-mediated signals following CXCL12 stimulation.71 Positive co-operativity has also been described for the CXCR2/δ-opioid receptor (DOR) heterodimer, but in that case it is antagonism of CXCR2 that enhances the DOR response to ligand.72 Nevertheless, dimers of chemokine receptors have been shown more often to exhibit negative binding co-operativity, where binding of an agonist to one receptor inhibits ligand binding to the other.53 Antagonist binding to one chemokine receptor has also been shown to cross-inhibit the other chemokine receptor in the pair, both in vitro and in vivo.73,74 Although a few publications have shown that binding co-operativity within a dimer can involve co-internalization of receptors,75,76 it is not considered to be the rule. As for other GPCRs, it is thought that both negative and positive co-operativity are mediated through allosteric changes in receptor conformation following ligand binding.45,53,77 A ‘cigar bundle’ model has been proposed recently for chemokine receptors whereby clusters of dimers are packed at the cell surface, with the potential for allosteric cross-talk between neighbouring dimers to affect more distant receptors in a domino effect.54
The physiological relevance of chemokine receptor oligomerization was highlighted initially with CCR5, when a naturally occurring truncation (Δ32) of this receptor leading to retention of wild-type CCR5/CCR5Δ32 heterodimers in the endoplasmic reticulum was found to confer resistance to HIV-1 infection.78,79 More recently, it was shown using blood cells from CCR5Δ32-expressing individuals that CCR2/CXCR4/CCR5 heteromers accounted for a negative ligand-binding co-operativity, which inhibited leucocyte recruitment in vitro and in vivo.73 Overall, multimerization is emerging as an additional level of regulation providing cell and tissue specificity to fine-tune chemokine receptor activity in vivo.
Chemokine receptor desensitization
Chemokine receptors are coupled to heterotrimeric G proteins and undergo conformational changes following ligand binding. The G protein dissociates into guanosine triphosphate (GTP)-bound Gα and the Gβ/γ complex, which activate second messengers and stimulate effector proteins leading to intracellular signalling.80 It has emerged that GPCRs can also elicit G protein-independent signals through interaction with the scaffolding proteins β-arrestins, linking activated receptors to various signalling pathways that act independently of, in synergy with or in opposition to, G protein-mediated signals.81 However, β-arrestins are best known for their pivotal role in the regulation of GPCR signals via the process of desensitization, a feedback mechanism protecting cells from overstimulation. In this section we consider what is called homologous desensitization only affecting agonist-activated receptors (Fig. 1).82 Briefly, following agonist binding, signalling receptors become rapidly phophorylated on their cytoplasmic tail, usually by one member of the G protein receptor kinase (GRK) family, which uncouples the G protein from the receptor and prevents further activation. Phosphorylated receptors interact with one of the β-arrestins acting as a scaffold targeting receptors for internalization, leading to a permanent or transient loss of cell surface receptors due to degradation or subsequent recycling of internalized molecules, respectively.5 The ability of a chemokine receptor to interact with β-arrestins can influence its fate in multiple ways. First, the strength and stability of receptor/β-arrestins interactions seem critical in determining whether or not an agonist-activated chemokine receptor is internalized, as described for CCR7 and CCR2.83–85 Secondly, the affinity of these interactions can influence the destiny of receptors once internalized. Indeed, GPCRs that rapidly recycle (Class A) preferentially bind β-arrestin 2 with low affinity and dissociate from it upon internalization, whereas those that slowly recycle or are degraded (Class B) bind both β-arrestins with high affinity and remain β-arrestin-bound inside the cell.86 To date, only class B chemokine receptors have been described, with evidence for β-arrestins binding to agonist-treated CXCR4, CCR2 and CCR5 in internal compartments87–89 (see Fig. 2).
Figure 1.
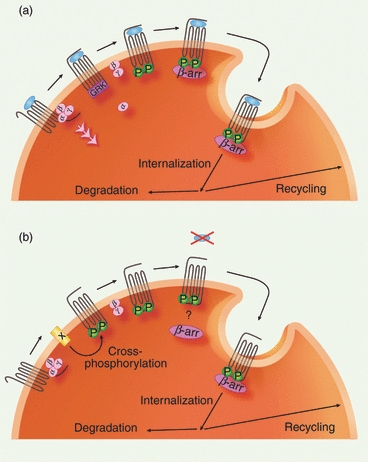
Agonist-dependent (a) and independent (b, heterologous) chemokine receptor desensitization. (a) Following agonist binding and G protein mediated signalling, the chemokine receptor cytoplasmic tail is rapidly phosphorylated, usually by a G protein receptor kinase (GRK); this uncouples the G protein, which dissociates into guanosine triphosphate (GTP)-bound Gα and the Gβγ complex, and enables interaction with a β-arrestin, which acts as a scaffold targeting the receptor for internalization. Once internalized, the receptor follows recycling or degradation pathways. (b) Receptor X mediates cross-phosphorylation of the chemokine receptor, which may involve protein kinase C (PKC), leading to inhibition of chemokine-induced signalling and in some cases internalization of the receptor.
Figure 2.
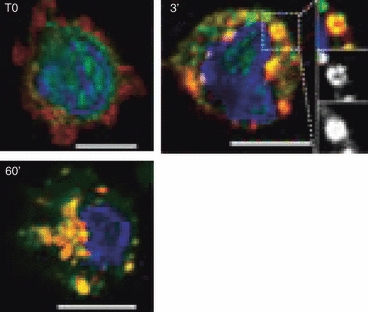
Intracellular transport of β-arrestin-bound CCR5 receptors following CCL5-treatment. Isolated human blood monocytes were treated with 100 nm CCL5 for the indicated time-period. Cells were fixed and permeabilized before labelling for CCR5 (red) and β-arrestins (green), as described previously.88 Scale bar 5 μm.
Chemokine receptors can be internalized via clathrin- or caveolin-dependent endocytosis, although other independent pathways have also been reported.5 Interestingly, CCR2 and CCR5 have been shown to follow both clathrin-dependent and caveolin-mediated pathways and the route of endocytosis could be cell-type dependent.42,90–93 The intracellular path followed by a chemokine receptor determines the fate of this receptor, i.e. being sent for degradation (down-regulation) or being sequestered intracellularly before returning to the cell surface (resensitization). Receptors can follow one path exclusively, such as CCR5 or CXCR3 sent for recycling or degradation, respectively.94–98 Alternatively, they can enter either pathway depending on the cell-type and duration of ligand treatment, as reported for CXCR2 and CXCR4.99–101 Note that the agonist itself can impact upon the fate of a receptor. For instance, with CCR5, any agonist-stimulated receptors seem to follow the recycling route but the distribution of receptors along the pathway could be agonist-specific (Fig. 3). Following internalization, CCR5 receptors treated with the natural chemokine CCL5 [regulated upon activation normal T cell expressed and secreted (RANTES)] are located in recycling endosomes (RE) before re-accumulating in the plasma membrane.95 In contrast, they keep cycling back from the cell surface to the RE after exposure to the chemically modified aminooxypentane (AOP)-RANTES,95 become trapped in the trans-Golgi network (TGN) after passage through RE with Nα-(n-nonanoyl)-des-Ser1-[l-thioproline2, l-α-cyclohexyl-glycine3] PSC-RANTES,102 and appear to bypass the RE to accumulate in the TGN with methionine MET-RANTES.103
Figure 3.
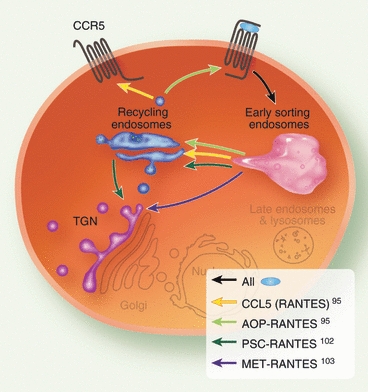
Different trafficking routes proposed for agonist-treated CCR5. Following agonist-stimulation, internalized CCR5 receptors are transported through the early endocytic pathway towards recycling and avoiding degradation. However, there are suggestions that the route followed by CCR5 may be ligand-dependent, as summarized here for the chemokine CCL5 and three of its derivatives.
Sorting of internalized chemokine receptors to the recycling or degradative pathways requires complex interactions with the machinery mediating movement of molecules between intracellular compartments. Endocytic adaptors recognize specific determinants in the cytoplasmic domains of the receptors, mainly small sorting motifs and post-translational modifications.5,104 Two of these determinants, the PDZ ligand motif and ubiquitination, have received much interest recently, and were shown to support recycling or degradation of chemokine receptors, respectively. At least 12 chemokine receptors have been identified as containing potential PDZ ligand motifs in their extreme C-terminal cytoplasmic tail.5 The PDZ ligand motifs are presumed to interact with PDZ domain containing proteins of the sorting machinery, but only a few of these interactions have been unveiled. CCR5 post-endocytic sorting to the recycling pathway is dependent on its PDZ ligand motif,94 which has been shown to interact with a protein implicated in receptor recycling called EBP50/NHERF-1.105 For CXCR2 that can be both recycled following short ligand exposure and degraded following more prolonged ligand treatment,99 the PDZ ligand motif serves to delay degradation by preventing lysosomal sorting, due probably to interaction with an as yet unknown PDZ-containing protein.106 Ubiquitination has emerged as an important modification for sending the chemokine receptor CXCR4107 and other GPCRs104 to degradation. For CXCR4, CXCL12 stimulation leads to ubiquitination of cell surface receptors as well as ubiquitin-dependent endocytosis and trafficking of ubiquitinated CXCR4 to lysosomes.108,109 However, ubiquitination does not seem to be required for the degradation of all chemokine receptors.98,106
Cross-talk and heterologous regulation
In addition to co-operativity within chemokine receptor multimers, various examples for regulation by indirect cross-talk with other receptors, without evidence of physical interactions but occurring through interconnectivity of cellular signalling networks, have been described.53 Note that such regulation can be bidirectional, although here we consider only cases of cross-talk towards chemokine receptors. The cross-talk can be targeted at the receptor itself, the heterotrimeric G protein it is coupled to or downstream signalling components, resulting in trans-inhibition or -activation of chemokine receptor activity.
Trans-inhibition results from a negative pathway of cross-talk leading to desensitization of chemokine receptors or the down-regulation of their expression, as discussed in an earlier section. Here we are considering agonist-independent (heterologous) desensitization involving inactivation and/or down-modulation of cell surface chemokine receptors. As for the other mechanisms of regulation presented in this review, the pathways of heterologous desensitization are undoubtedly receptor- and cell-type dependent. Heterologous desensitization often implies rapid signalling inactivation of surface chemokine receptors, inhibiting chemokine-induced intracellular calcium mobilization. It happens whether the cross-talk comes from another chemokine receptor such as for CXCR1 and CXCR2 with CCR5 in transfected cells,110 or CXCR4 with CCR5 in human pre-B and -T cells,111,112 another GPCR as for CXCR1 with the N-formyl peptide (FPR) and C5a receptors,113 or an unrelated surface receptor such as the TcR with CXCR4 in immortalized cell lines.114 In many reports, the inactivation has been linked to rapid cross-phosphorylation of the chemokine receptor, with some studies identifying protein kinase C (PKC) as the point of convergence between the different receptor pathways.110,113,115,116 Alternatively, receptor inactivation can result from indirect effects as reported for CXCR4 either in pre-B cells, where CD24 altered its distribution in membrane lipid rafts by changing cholesterol levels,117 or in leukaemia cells, where an oncoprotein has been shown to highjack kinases of the CXCR4-dependent calcium pathway.118 Signalling inactivation can be, but is not always, followed by the down-modulation of cell surface chemokine receptors.116,119,120 Conversely, heterologous down-modulation can occur without prior desensitization of chemokine-mediated signalling, as we uncovered with the cross-regulation of CC chemokine receptors 1, 2 and 5 by TLR-2 on human blood monocytes.88 In this instance, we found that activation of TLR-2 triggered relatively slow phosphorylation and removal of cell surface CCR5 molecules by activating the machinery used to support chemokine-dependent endocytosis.88
Cross-talk can also lead to trans-activation of chemokine receptors and a potentiation of their functional activity, but few studies have been able to identify the mechanisms involved.53 Potentiation of calcium signalling has been reported for CXCR2 upon co-stimulation of another GPCR, the PY2 nucleotide receptor, and suggested ligand-induced synergy between the two receptors.121 Activation of the neurokinin 1 receptor has also been shown to potentiate the effect of CXCL8 on human neutrophils and was proposed to have a priming effect on CXCR1 and CXCR2.122 The chemokinetic effect of cytokines is thought to prime cells to increase their migratory response to chemokines, as found with IL-5-enhancing eosinophil chemotaxis in response to CCL11.123 Furthermore, potentiation and synergy between different chemokine receptors has been involved in the migration of primary cells. For example, CXCL8 has been shown to increase monocyte migration towards CCL2 and CCL7,124 while CCL2 and CCL7 can stimulate neutrophil chemotaxis towards CXCL8.125 An intriguing finding came from the study of cross-talk between CCR1 and the high-affinity IgE receptor FcεRI in transfected cells, whereby engagement of FcεRI inhibited CCL3-mediated chemotaxis but engagement of both CCR1 and FcεRI had a synergistic effect on cell degranulation.126 This would suggest that receptor cross-talk can take place at multiple levels and could have a relatively complex bearing on cell response to chemokine stimulation.
The impact of receptor cross-talk on how immune cells adapt their behaviour to specific situations is undeniable. Combinations of chemokines, cytokines and growth factors act synergistically to amplify inflammatory responses, and this is thought to be due to integration of multiple signalling pathways.123,124 Cross-talk initiated from non-chemokine receptors is also emerging as an important and complex phenomenon used to enhance or modulate innate immune responses to pathogens. Synergy between CCR2 and FPR agonists has recently been shown to co-operate with TLR-4 for production of the inflammatory chemokine CXCL8 upon LPS stimulation, which in turn synergizes with CCL2 to mediate CXCR1/CXCR2-dependent chemotaxis of human monocytic cells.127 In addition, heterologous desensitization between TLR-2 and the CC chemokine receptors 1, 2 and 5 or CXCR2 has been shown to take place in vivo, affecting the migration and homing of mouse monocytes and neutrophils.128,129 Furthermore, synergy and cross-talk may have therapeutic implications, as illustrated with some HIV-related studies. Synergy between CXCR4 and CCR5 was recently shown to enhance human monocyte and T cell chemotaxis and to completely block infection by a dual tropic HIV-1 strain,130 while cross-desensitization of CCR5 by the opioid receptor specifically decreased the susceptibility of peripheral blood mononuclear cells (PBMCs) and macrophages to HIV-1 R5 viruses.131 However, it remains to be ascertained whether these are pure cross-talk situations or involve receptor multimerization.63,132
Conclusion
Advances in our understanding of chemokine receptor biology have highlighted the fact that a controlled regulation of their activity is probably more important than their activation per se, certainly in the context of the immune system for both homeostasis and inflammatory responses. It is becoming apparent that individual receptors are subject to different mechanisms of regulation depending upon the type of cells on which they are expressed, the cell differentiation and activation status, as well as the microenvironment. We have learnt that some of the molecular mechanisms involved in the regulation are shared among chemokine receptors while others are purely receptor-specific, with either transient or permanent consequences on cell responsiveness to chemokine stimulation. Overall, we can conclude that the complexity of the regulation process confers specificity to what is an apparently redundant chemokine/chemokine receptor system. Nevertheless, much more research is needed to appreciate the ins and outs of this regulation, evaluate the true relevance of individual mechanisms in vivo and establish how the chemokine system integrates with the rest of the immunoregulatory machinery.
Acknowledgments
We thank Professor Paul Kaye from the CII for constructive discussions and critically reading the manuscript. The Signoret laboratory was supported by grants from the Biotechnology and Biological Sciences Research Council and The Royal Society (London, UK). L.D.B. is the recipient of a BBSRC studentship.
Disclosures
The authors declare having no conflicts of interest.
References
- 1.IUIS/WHO subcommittee on chemokine nomenclature Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21:48–9. [Google Scholar]
- 2.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–3. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 3.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–63. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
- 4.Zidar DA. Endogenous ligand bias by chemokines: implications at the front lines of infection and leukocyte trafficking. Endocr Metab Immune Disord Drug Targets. 2011;11:120–31. doi: 10.2174/187153011795564160. [DOI] [PubMed] [Google Scholar]
- 5.Borroni EM, Mantovani A, Locati M, Bonecchi R. Chemokine receptors intracellular trafficking. Pharmacol Ther. 2010;127:1–8. doi: 10.1016/j.pharmthera.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Johnson Z, Schwarz M, Power CA, Wells TN, Proudfoot AE. Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol. 2005;26:268–74. doi: 10.1016/j.it.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 11.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 12.Pease JE, Horuk R. Chemokine receptor antagonists: part 2. Exp Opin Ther Pat. 2009;19:199–221. doi: 10.1517/13543770802641353. [DOI] [PubMed] [Google Scholar]
- 13.Pease JE, Horuk R. Chemokine receptor antagonists: part 1. Exp Opin Ther Pat. 2009;19:39–58. doi: 10.1517/13543770802641346. [DOI] [PubMed] [Google Scholar]
- 14.Westby M, van der Ryst E. CCR5 antagonists: host-targeted antiviral agents for the treatment of HIV infection, 4 years on. Antivir Chem Chemother. 2010;20:179–92. doi: 10.3851/IMP1507. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol. 2010;88:41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 16.Weber C, Koenen RR. Fine-tuning leukocyte responses: towards a chemokine ‘interactome’. Trends Immunol. 2006;27:268–73. doi: 10.1016/j.it.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta SA, Christopherson KW, Bhat-Nakshatri P, Goulet RJ, Jr, Broxmeyer HE, Kopelovich L, Nakshatri H. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene. 2007;26:3329–37. doi: 10.1038/sj.onc.1210120. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Kremmer E, Palermo B, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–45. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94:875–83. [PubMed] [Google Scholar]
- 21.Patel L, Charlton SJ, Chambers JK, Macphee CH. Expression and functional analysis of chemokine receptors in human peripheral blood leukocyte populations. Cytokine. 2001;14:27–36. doi: 10.1006/cyto.2000.0851. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Sebastiani S, Allavena P, Albanesi C, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 25.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 26.Zhou Y, Yang Y, Warr G, Bravo R. LPS down-regulates the expression of chemokine receptor CCR2 in mice and abolishes macrophage infiltration in acute inflammation. J Leukoc Biol. 1999;65:265–9. doi: 10.1002/jlb.65.2.265. [DOI] [PubMed] [Google Scholar]
- 27.Maus U, von Grote K, Kuziel WA, et al. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med. 2002;166:268–73. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Rahimpour R, Ran L, et al. Regulation of CCR2 chemokine receptor mRNA stability. J Leukoc Biol. 1997;62:653–60. doi: 10.1002/jlb.62.5.653. [DOI] [PubMed] [Google Scholar]
- 29.Sica A, Saccani A, Borsatti A, et al. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–74. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, Sozzani S, Mantovani A. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-gamma. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 31.Saccani A, Saccani S, Orlando S, Sironi M, Bernasconi S, Ghezzi P, Mantovani A, Sica A. Redox regulation of chemokine receptor expression. Proc Natl Acad Sci USA. 2000;97:2761–6. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arias MA, Pantoja AE, Jaramillo G, Paris SC, Shattock RJ, Garcia LF, Griffin GE. Chemokine receptor expression and modulation by Mycobacterium tuberculosis antigens on mononuclear cells from human lymphoid tissues. Immunology. 2006;118:171–84. doi: 10.1111/j.1365-2567.2006.02352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doroshenko T, Chaly Y, Savitskiy V, Maslakova O, Portyanko A, Gorudko I, Voitenok NN. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood. 2002;100:2668–71. doi: 10.1182/blood.100.7.2668. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Shang DS, Zhao WD, et al. Amyloid beta interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood–brain barrier. J Immunol. 2009;182:5778–88. doi: 10.4049/jimmunol.0803013. [DOI] [PubMed] [Google Scholar]
- 35.Vauquelin G, Van Liefde I. G protein-coupled receptors: a count of 1001 conformations. Fundam Clin Pharmacol. 2005;19:45–56. doi: 10.1111/j.1472-8206.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 36.Gilchrist A. Modulating G-protein-coupled receptors: from traditional pharmacology to allosterics. Trends Pharmacol Sci. 2007;28:431–7. doi: 10.1016/j.tips.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Wess J, Han SJ, Kim SK, Jacobson KA, Li JH. Conformational changes involved in G-protein-coupled-receptor activation. Trends Pharmacol Sci. 2008;29:616–25. doi: 10.1016/j.tips.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baribaud F, Edwards TG, Sharron M, et al. Antigenically distinct conformations of CXCR4. J Virol. 2001;75:8957–67. doi: 10.1128/JVI.75.19.8957-8967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sloane AJ, Raso V, Dimitrov DS, et al. Marked structural and functional heterogeneity in CXCR4: separation of HIV-1 and SDF-1alpha responses. Immunol Cell Biol. 2005;83:129–43. doi: 10.1111/j.1440-1711.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee B, Sharron M, Blanpain C, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–26. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 41.Farzan M, Mirzabekov T, Kolchinsky P, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 42.Signoret N, Hewlett L, Wavre S, Pelchen-Matthews A, Oppermann M, Marsh M. Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent. Mol Biol Cell. 2005;16:902–17. doi: 10.1091/mbc.E04-08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen DH, Taub DD. Inhibition of chemokine receptor function by membrane cholesterol oxidation. Exp Cell Res. 2003;291:36–45. doi: 10.1016/s0014-4827(03)00345-8. [DOI] [PubMed] [Google Scholar]
- 44.Cardaba CM, Kerr JS, Mueller A. CCR5 internalisation and signalling have different dependence on membrane lipid raft integrity. Cell Signal. 2008;20:1687–94. doi: 10.1016/j.cellsig.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–25. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuxe K, Marcellino D, Leo G, Agnati LF. Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis. Curr Opin Pharmacol. 2010;10:14–22. doi: 10.1016/j.coph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–710. [PubMed] [Google Scholar]
- 48.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, Jones DR, Toran JL, Martinez AC. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 50.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–24. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Isik N, Hereld D, Jin T. Fluorescence resonance energy transfer imaging reveals that chemokine-binding modulates heterodimers of CXCR4 and CCR5 receptors. PLoS ONE. 2008;3:e3424. doi: 10.1371/journal.pone.0003424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Norcross M. Dimerization of chemokine receptors in living cells: key to receptor function and novel targets for therapy. Drug Discov Today. 2008;14:625–32. doi: 10.1016/j.drudis.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Salanga CL, O'Hayre M, Handel T. Modulation of chemokine receptor activity through dimerization and crosstalk. Cell Mol Life Sci. 2009;66:1370–86. doi: 10.1007/s00018-008-8666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thelen M, Munoz LM, Rodriguez-Frade JM, Mellado M. Chemokine receptor oligomerization: functional considerations. Curr Opin Pharmacol. 2010;10:38–43. doi: 10.1016/j.coph.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Martinez Munoz L, Lucas P, Navarro G, Checa AI, Franco R, Martinez AC, Rodriguez-Frade JM, Mellado M. Dynamic regulation of CXCR1 and CXCR2 homo- and heterodimers. J Immunol. 2009;183:7337–46. doi: 10.4049/jimmunol.0901802. [DOI] [PubMed] [Google Scholar]
- 56.Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280:28663–74. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- 57.Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, Labbe-Jullie C, Bouvier M, Marullo S. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem. 2002;277:34666–73. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida N, Kitayama D, Arima M, et al. CXCR4 expression on activated B cells is downregulated by CD63 and IL-21. J Immunol. 2011;186:2800–8. doi: 10.4049/jimmunol.1003401. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida T, Ebina H, Koyanagi Y. N-linked glycan-dependent interaction of CD63 with CXCR4 at the Golgi apparatus induces downregulation of CXCR4. Microbiol Immunol. 2009;53:629–35. doi: 10.1111/j.1348-0421.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida T, Kawano Y, Sato K, et al. A CD63 mutant inhibits T-cell tropic human immunodeficiency virus type 1 entry by disrupting CXCR4 trafficking to the plasma membrane. Traffic. 2008;9:540–58. doi: 10.1111/j.1600-0854.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 61.Achour L, Scott MG, Shirvani H, Thuret A, Bismuth G, Labbe-Jullie C, Marullo S. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113:1938–47. doi: 10.1182/blood-2008-02-141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao X, Kinter A, Broder CC, Dimitrov DS. Interactions of CCR5 and CXCR4 with CD4 and gp120 in human blood monocyte-derived dendritic cells. Exp Mol Pathol. 2000;68:133–8. doi: 10.1006/exmp.1999.2300. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Alvarez R, Roderiquez G, Guan E, Norcross MA. Constitutive association of cell surface CCR5 and CXCR4 in the presence of CD4. J Cell Biochem. 2004;93:753–60. doi: 10.1002/jcb.20161. [DOI] [PubMed] [Google Scholar]
- 64.Baker AM, Sauliere A, Gaibelet G, et al. CD4 interacts constitutively with multiple CCR5 at the plasma membrane of living cells. A fluorescence recovery after photobleaching at variable radii approach. J Biol Chem. 2007;282:35163–8. doi: 10.1074/jbc.M705617200. [DOI] [PubMed] [Google Scholar]
- 65.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–85. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 66.Yi L, Fang J, Isik N, Chim J, Jin T. HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J Biol Chem. 2006;281:35446–53. doi: 10.1074/jbc.M607302200. [DOI] [PubMed] [Google Scholar]
- 67.Steffens CM, Hope TJ. Localization of CD4 and CCR5 in living cells. J Virol. 2003;77:4985–91. doi: 10.1128/JVI.77.8.4985-4991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci USA. 2008;105:13532–7. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–4. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sierro F, Biben C, Martinez-Munoz L, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245–56. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael JY. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–9. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sohy D, Parmentier M, Springael JY. Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem. 2007;282:30062–9. doi: 10.1074/jbc.M705302200. [DOI] [PubMed] [Google Scholar]
- 75.Contento RL, Molon B, Boularan C, Pozzan T, Manes S, Marullo S, Viola A. CXCR4-CCR5: a couple modulating T cell functions. Proc Natl Acad Sci USA. 2008;105:10101–6. doi: 10.1073/pnas.0804286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huttenrauch F, Pollok-Kopp B, Oppermann M. G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem. 2005;280:37503–15. doi: 10.1074/jbc.M500535200. [DOI] [PubMed] [Google Scholar]
- 77.Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:615–20. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J Biol Chem. 1997;272:30603–6. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- 79.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 80.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–35. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 81.DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23:621–9. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008;153(Suppl 1):S379–88. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Byers MA, Calloway PA, Shannon L, Cunningham HD, Smith S, Li F, Fassold BC, Vines CM. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J Immunol. 2008;181:4723–32. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–54. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berchiche YA, Gravel S, Pelletier ME, St-Onge G, Heveker N. Different effects of the different natural CC chemokine receptor 2b ligands on beta-arrestin recruitment, Galphai signaling, and receptor internalization. Mol Pharmacol. 2011;79:488–98. doi: 10.1124/mol.110.068486. [DOI] [PubMed] [Google Scholar]
- 86.Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21:2529–41. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox JM, Letellier E, Oliphant CJ, Signoret N. TLR2-dependent pathway of heterologous down-modulation for the CC chemokine receptors 1, 2, and 5 in human blood monocytes. Blood. 2011;117:1851–60. doi: 10.1182/blood-2010-05-287474. [DOI] [PubMed] [Google Scholar]
- 89.Minsaas L, Planaguma J, Madziva M, Krakstad BF, Masia-Balague M, Katz AA, Aragay AM. Filamin a binds to CCR2B and regulates its internalization. PLoS ONE. 2010;5:e12212. doi: 10.1371/journal.pone.0012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res. 2002;70:219–31. doi: 10.1002/jnr.10372. [DOI] [PubMed] [Google Scholar]
- 91.Ge S, Pachter JS. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine monocyte chemoattractant protein-1 by human astrocytes. J Biol Chem. 2004;279:6688–95. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- 92.Garcia Lopez MA, Aguado Martinez A, Lamaze C, Martinez AC, Fischer T. Inhibition of dynamin prevents CCL2-mediated endocytosis of CCR2 and activation of ERK1/2. Cell Signal. 2009;21:1748–57. doi: 10.1016/j.cellsig.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Venkatesan S, Rose JJ, Lodge R, Murphy PM, Foley JF. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol Biol Cell. 2003;14:3305–24. doi: 10.1091/mbc.E02-11-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delhaye M, Gravot A, Ayinde D, Niedergang F, Alizon M, Brelot A. Identification of a postendocytic sorting sequence in CCR5. Mol Pharmacol. 2007;72:1497–507. doi: 10.1124/mol.107.038422. [DOI] [PubMed] [Google Scholar]
- 95.Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–94. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mack M, Luckow B, Nelson PJ, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–24. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mueller A, Strange PG. Mechanisms of internalization and recycling of the chemokine receptor, CCR5. Eur J Biochem. 2004;271:243–52. doi: 10.1046/j.1432-1033.2003.03918.x. [DOI] [PubMed] [Google Scholar]
- 98.Meiser A, Mueller A, Wise EL, et al. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J Immunol. 2008;180:6713–24. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101:2115–24. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem. 1998;273:15883–6. doi: 10.1074/jbc.273.26.15883. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y, Foudi A, Geay JF, et al. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells. 2004;22:1015–29. doi: 10.1634/stemcells.22-6-1015. [DOI] [PubMed] [Google Scholar]
- 102.Escola JM, Kuenzi G, Gaertner H, Foti M, Hartley O. CC chemokine receptor 5 (CCR5) desensitization: cycling receptors accumulate in the trans-Golgi network. J Biol Chem. 2010;285:41772–80. doi: 10.1074/jbc.M110.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiss DL, Longden J, Fechner GA, Avery VM. The functional antagonist Met-RANTES: a modified agonist that induces differential CCR5 trafficking. Cell Mol Biol Lett. 2009;14:537–47. doi: 10.2478/s11658-009-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–29. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hammad MM, Kuang YQ, Yan R, Allen H, Dupre DJ. Na+/H+ exchanger regulatory factor-1 is involved in chemokine receptor homodimer CCR5 internalization and signal transduction but does not affect CXCR4 homodimer or CXCR4-CCR5 heterodimer. J Biol Chem. 2010;285:34653–64. doi: 10.1074/jbc.M110.106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baugher PJ, Richmond A. The carboxyl-terminal PDZ ligand motif of chemokine receptor CXCR2 modulates post-endocytic sorting and cellular chemotaxis. J Biol Chem. 2008;283:30868–78. doi: 10.1074/jbc.M804054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–12. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 108.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–22. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 109.Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation. J Biol Chem. 2009;284:5742–52. doi: 10.1074/jbc.M808507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nasser MW, Marjoram RJ, Brown SL, Richardson RM. Cross-desensitization among CXCR1, CXCR2, and CCR5: role of protein kinase C-epsilon. J Immunol. 2005;174:6927–33. doi: 10.4049/jimmunol.174.11.6927. [DOI] [PubMed] [Google Scholar]
- 111.Honczarenko M, Le Y, Glodek AM, Majka M, Campbell JJ, Ratajczak MZ, Silberstein LE. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood. 2002;100:2321–9. doi: 10.1182/blood-2002-01-0248. [DOI] [PubMed] [Google Scholar]
- 112.Hecht I, Cahalon L, Hershkoviz R, Lahat A, Franitza S, Lider O. Heterologous desensitization of T cell functions by CCR5 and CXCR4 ligands: inhibition of cellular signaling, adhesion and chemotaxis. Int Immunol. 2003;15:29–38. doi: 10.1093/intimm/dxg002. [DOI] [PubMed] [Google Scholar]
- 113.Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–6. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- 114.Schneider OD, Weiss AA, Miller WE. Pertussis toxin signals through the TCR to initiate cross-desensitization of the chemokine receptor CXCR4. J Immunol. 2009;182:5730–9. doi: 10.4049/jimmunol.0803114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Richardson RM, Pridgen BC, Haribabu B, Snyderman R. Regulation of the human chemokine receptor CCR1. Cross-regulation by CXCR1 and CXCR2. J Biol Chem. 2000;275:9201–8. doi: 10.1074/jbc.275.13.9201. [DOI] [PubMed] [Google Scholar]
- 116.Le Y, Wetzel MA, Shen W, Gong W, Rogers TJ, Henderson EE, Wang JM. Desensitization of chemokine receptor CCR5 in dendritic cells at the early stage of differentiation by activation of formyl peptide receptors. Clin Immunol. 2001;99:365–72. doi: 10.1006/clim.2001.5021. [DOI] [PubMed] [Google Scholar]
- 117.Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119(Pt 2):314–25. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 118.Ptasznik A, Urbanowska E, Chinta S, et al. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196:667–78. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. J Biol Chem. 2003;278:15867–73. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- 120.Finley MJ, Chen X, Bardi G, Davey P, Geller EB, Zhang L, Adler MW, Rogers TJ. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the kappa-opioid receptor. J Neuroimmunol. 2008;197:114–23. doi: 10.1016/j.jneuroim.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Werry TD, Christie MI, Dainty IA, Wilkinson GF, Willars GB. Ca(2+) signalling by recombinant human CXCR2 chemokine receptors is potentiated by P2Y nucleotide receptors in HEK cells. Br J Pharmacol. 2002;135:1199–208. doi: 10.1038/sj.bjp.0704566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dianzani C, Lombardi G, Collino M, Ferrara C, Cassone MC, Fantozzi R. Priming effects of substance P on calcium changes evoked by interleukin-8 in human neutrophils. J Leukoc Biol. 2001;69:1013–8. [PubMed] [Google Scholar]
- 123.Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005;16:561–80. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Gouwy M, Struyf S, Noppen S, Schutyser E, Springael JY, Parmentier M, Proost P, Van Damme J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol Pharmacol. 2008;74:485–95. doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- 125.Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol. 2004;76:185–94. doi: 10.1189/jlb.1003479. [DOI] [PubMed] [Google Scholar]
- 126.Toda M, Dawson M, Nakamura T, Munro PM, Richardson RM, Bailly M, Ono SJ. Impact of engagement of FcepsilonRI and CC chemokine receptor 1 on mast cell activation and motility. J Biol Chem. 2004;279:48443–8. doi: 10.1074/jbc.M408725200. [DOI] [PubMed] [Google Scholar]
- 127.Gouwy M, Struyf S, Verbeke H, Put W, Proost P, Opdenakker G, Van Damme J. CC chemokine ligand-2 synergizes with the nonchemokine G protein-coupled receptor ligand fMLP in monocyte chemotaxis, and it cooperates with the TLR ligand LPS via induction of CXCL8. J Leukoc Biol. 2009;86:671–80. doi: 10.1189/jlb.1008638. [DOI] [PubMed] [Google Scholar]
- 128.McKimmie CS, Moore M, Fraser AR, et al. A TLR2 ligand suppresses inflammation by modulation of chemokine receptors and redirection of leukocyte migration. Blood. 2009;113:4224–31. doi: 10.1182/blood-2008-08-174698. [DOI] [PubMed] [Google Scholar]
- 129.Alves-Filho JC, Freitas A, Souto FO, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci USA. 2009;106:4018–23. doi: 10.1073/pnas.0900196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gouwy M, Struyf S, Berghmans N, Vanormelingen C, Schols D, Van Damme J. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur J Immunol. 2011;41:963–73. doi: 10.1002/eji.201041178. [DOI] [PubMed] [Google Scholar]
- 131.Szabo I, Wetzel MA, Zhang N, et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74:1074–82. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- 132.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–86. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 133.El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol. 2005;67:460–9. doi: 10.1124/mol.104.003624. [DOI] [PubMed] [Google Scholar]
- 134.Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 135.Rodriguez-Frade JM, Vila-Coro AJ, de Ana AM, Albar JP, Martinez AC, Mellado M. The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc Natl Acad Sci USA. 1999;96:3628–33. doi: 10.1073/pnas.96.7.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hernanz-Falcon P, Rodriguez-Frade JM, Serrano A, et al. Identification of amino acid residues crucial for chemokine receptor dimerization. Nat Immunol. 2004;5:216–23. doi: 10.1038/ni1027. [DOI] [PubMed] [Google Scholar]
- 137.Vila-Coro AJ, Mellado M, Martin de Ana A, Lucas P, del Real G, Martinez AC, Rodriguez-Frade JM. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc Natl Acad Sci USA. 2000;97:3388–93. doi: 10.1073/pnas.050457797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodriguez-Frade JM, Vila-Coro AJ, Martin A, et al. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J Cell Biol. 1999;144:755–65. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trettel F, Di Bartolomeo S, Lauro C, Catalano M, Ciotti MT, Limatola C. Ligand-independent CXCR2 dimerization. J Biol Chem. 2003;278:40980–8. doi: 10.1074/jbc.M306815200. [DOI] [PubMed] [Google Scholar]
- 140.Wang J, He L, Combs CA, Roderiquez G, Norcross MA. Dimerization of CXCR4 in living malignant cells: control of cell migration by a synthetic peptide that reduces homologous CXCR4 interactions. Mol Cancer Ther. 2006;5:2474–83. doi: 10.1158/1535-7163.MCT-05-0261. [DOI] [PubMed] [Google Scholar]
- 141.Chakera A, Seeber RM, John AE, Eidne KA, Greaves DR. The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol Pharmacol. 2008;73:1362–70. doi: 10.1124/mol.107.040915. [DOI] [PubMed] [Google Scholar]
- 142.Pello OM, Martinez-Munoz L, Parrillas V, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–49. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- 143.Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- 144.Limatola C, Di Bartolomeo S, Trettel F, Lauro C, Ciotti MT, Mercanti D, Castellani L, Eusebi F. Expression of AMPA-type glutamate receptors in HEK cells and cerebellar granule neurons impairs CXCL2-mediated chemotaxis. J Neuroimmunol. 2003;134:61–71. doi: 10.1016/s0165-5728(02)00401-0. [DOI] [PubMed] [Google Scholar]
- 145.Lee S, Lapham CK, Chen H, et al. Coreceptor competition for association with CD4 may change the susceptibility of human cells to infection with T-tropic and macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 2000;74:5016–23. doi: 10.1128/jvi.74.11.5016-5023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lapham CK, Ouyang J, Chandrasekhar B, Nguyen NY, Dimitrov DS, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–5. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 147.Akekawatchai C, Holland JD, Kochetkova M, Wallace JC, McColl SR. Transactivation of CXCR4 by the insulin-like growth factor-1 receptor (IGF-1R) in human MDA-MB-231 breast cancer epithelial cells. J Biol Chem. 2005;280:39701–8. doi: 10.1074/jbc.M509829200. [DOI] [PubMed] [Google Scholar]
- 148.Gaibelet G, Planchenault T, Mazeres S, Dumas F, Arenzana-Seisdedos F, Lopez A, Lagane B, Bachelerie F. CD4 and CCR5 constitutively interact at the plasma membrane of living cells: a confocal fluorescence resonance energy transfer-based approach. J Biol Chem. 2006;281:37921–9. doi: 10.1074/jbc.M607103200. [DOI] [PubMed] [Google Scholar]


