Abstract
An exponential increase in the number of studies investigating how different components of the diet interact at the molecular and cellular level to determine the fate of a cell has been witnessed. In search for anticancer drugs compelling data from laboratories, epidemiologic investigations, and human clinical trials showed that flavonoids have important effects on cancer chemoprevention and chemotherapy. In many molecular mechanisms of action for prevention against cancer, flavonoids play a major role by interacting between different types of genes and enzymes. Many mechanisms of action have been identified, including carcinogen inactivation, antiproliferation, cell cycle arrest, induction of apoptosis, inhibition of angiogenesis, antioxidation, and reversal of multidrug resistance or a combination of these mechanisms. This review focuses on the anticancer activity of flavonoids as well as their molecular mechanisms, including the treatment of mammary and prostate cancer. This review also highlights some advanced derivatives of flavonoids, which play an important role against cancer.
Keywords: Apoptosis, estrogens receptor, flavonoids, mammary cancer, prostate cancer
INTRODUCTION
More than 8000 different compounds of polyphenols have been known and that can be further divided into 10 different general classes.[1] Flavonoids are part of this family & have more than 4000 varieties. They have been classified according to their molecular structure that consists of two benzene rings joined by a linear three-carbon chain and forms an oxygenated heterocycle (C6-C3-C6) and their large number arises from the various combinations of multiple hydroxyl, methoxyl, and O-glycoside group substituents on the basic benzo--pyrone (C6-C3-C6)[2] moiety.
Flavonoids are one of the common components in the human diet. They are present in foods generally as O-glycosides with sugars bound at C3 position. Average intake of all flavonoids is estimated to be 1 g/day.[3] Phenolic acids, flavonoids, stilbenes, and lignans are the most abundantly occurring polyphenols in plants out of which flavonoids and phenolic acids account for 60% and 30%, respectively, of dietary polyphenols. Major sources of polyphenols are fruits, vegetables, and seeds. Flavonoids are widely present in the genus Citrus (family Rutaceae).[4]
They exhibit properties beneficial for human health because they interact with number of cellular targets, such as anti-oxidant and free-radical scavenger activities also the anti-inflammatory, antiviral, and especially anti-cancer properties. Cancer chemoprevention by use of natural or synthetic substances and its prevention through dietary intervention has become an important issue. It may be controlled by various means, including suppression, blockage, and transformation. Suppressing agents prevent the formation of new cancers from procarcinogens, blocking agents prevent carcinogenic compounds from reaching critical initiation sites, and transformation agents facilitate the metabolism of carcinogenic components into less toxic materials or prevent their biological actions. Flavonoids can act in all the three ways.[5] Many other potential chemopreventive polyphenols may interrupt or reverse the carcinogenesis process.[6]
MAJOR MOLECULAR MECHANISM OF ACTION
Polyphenolic compounds display a remarkable spectrum of biological activities, including those that might influence the processes that are dysregulated during cancer development. This includes antiallergic, anti-inflammatory, antioxidant, antimutagenic, anticarcinogenic, and modulation of enzymatic activities.[7–9] They may therefore have beneficial health effects and can be considered as chemopreventive or therapeutic agents against cancer.[10]
Carcinogenesis is generally considered as a complex and multistep process in which distinct molecular and cellular alterations occur, & to simplify the different possible options for chemoprevention and chemotherapy in cancer development and progression, three stages have been described.
-
(i)
Initiation is a rapid phase, comprises of exposure and interaction of cells, especially DNA, with a carcinogenic agent.
-
(ii)
Promotion is relatively lengthy than the previous stage, abnormal cells persist, replicate and may originate a focus of preneoplastic cells.
-
(iii)
Progression stage is the final phase of the tumorigenesis that involves the gradual conversion of premalignant cells to neoplastic ones with an increase in invasiveness, metastasis potential, and new blood vessel formation (angiogenesis) [Figure 1].
Figure 1.

Three stages of cancer development and progression
One of the most exciting discoveries is the identification of oncogenes.[11,12] More than 40 oncogenes have been identified and their protein products have been characterized. These include protein kinases, GTP-binding proteins, and nuclear transcription factors. A unique hypothesis that the activation of transformation might act via protein phosphorylation came into existence. Protein- tyrosine kinases (PTKs) are the group of enzymes that catalyze the transfer of the phosphate of ATP to the hydroxyl group of tyrosine on many key proteins, which in turn induce the cascade of altered cell parameters, a characteristic of transformed cells.[13–17] This hypothesis has been supported by several recent findings:
Activated PTKs have been identified to be the products of approximately half of the known viral transforming genes (oncogenes) [Table 1].
The plasma-membrane receptors for several polypeptide growth factors, such as epidermal growth factor (EGF), transforming growth factor-α (TGF-α), platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-l), macrophage colony stimulating factor-1 (CSF-l), fibroblast growth factors (FGF-1 and FGF-2), nerve growth factor (NGF), and hepatocyte growth factor (HGF) are ligand-activated PTKs [Table 1].
Table 1.
Examples of protein-tyrosine kinase gene families

Biomolecular activities of flavonoids
Antioxidative effects: inactivation of oxygen radicals
Binding of electrophils
Induction of protective enzymes: phase 2 with conjugating activities (GT, GST)
Apoptosis rate increase
Cell proliferation inhibition
Lipid peroxidation inhibition
Angiogenesis inhibition
H-Donation (e.g. GSH-peroxidases)
-
DNA oxidation inhibition
GST, glutathione S-transferases; GT, glucuronosyl transferases; GSH, glutathione.
The biological properties of citrus flavonoids [5] and the effectiveness of polyphenolics in tea regarding cancer prevention and induction of apoptosis[8] have been widely studied. Flavonols from Brussels sprouts and flavones induce protective enzymes, such as conjugating enzymes, for example, uridine 5′-diphospho (UDP)-GT, GST in gut and liver.[18,19] These enzymes inactivate electrophils, free radicals, and reactive oxygen species (ROS) and thereby preventing them from becoming mutagens.[20]
Figure 1 demonstrates the potential mechanisms of inhibition of carcinogenesis by flavonoids & mainly illustrates the inhibitory effects of tea flavonoids on the main biological events that can lead to mutagens and shows how the carcinogenic neoplastic processes (initiation, promotion, and progression) are influenced.
Preventing carcinogen metabolic activation
One of the most important mechanism by which flavonoids can exert their effects is through their interaction with phase I metabolizing enzymes (eg, cytochrome P450), which metabolically activate a large number of procarcinogens to reactivate intermediates that can interact with cellular nucleophiles and ultimately trigger carcinogenesis. Flavonoids inhibit the activities of certain P450 isozymes, such as CYP1A1 and CYP1A2,[21,22] thus they are likely to have a protective role against the induction of cellular damage by the activation of carcinogens. Another mechanism of action is the induction of phase II metabolizing enzymes (eg, GST, quinone reductase, and UDP-GT)[23,24] by which carcinogens are detoxified & eliminated from the body. This helps in explaining the chemopreventive effects of flavonoids against carcinogenesis [Figure 2].
Figure 2.
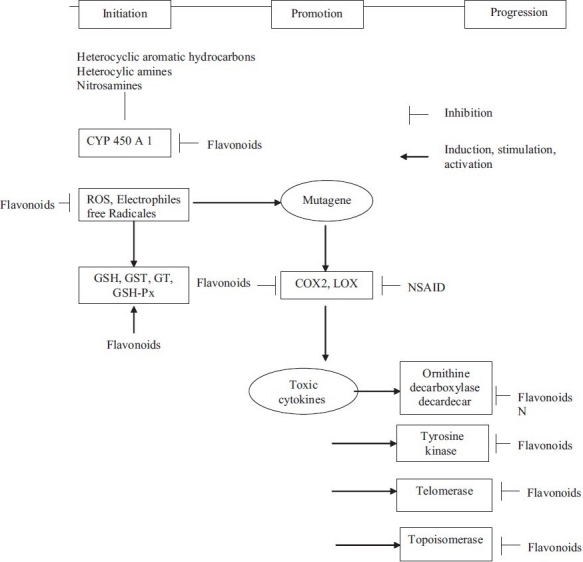
Hypothesis of inhibition of carcinogenesis by flavonoids- LOX, Lipoxygenase; COX, Cyclooxygenase; Px, Peroxidase; ROS, Reactive oxygen species; GSH, Glutathione; GST, Glutathione S-transferases; GT, UDP-Glucuronosyl transferases; Px, Peroxidase; NSAID, Nonsteroidal antiinflammatory drugs
Antiproliferation
The molecular mechanism of antiproliferation may involve the inhibition of the prooxidant process that causes tumor promotion. Growth promoting oxidants and ROS are the major catalysts of the tumor promotion and progression stages. Flavonoids are effective in inhibiting xanthine oxidase,[25] COX or LOX55,[26] and therefore inhibit tumor cell proliferation.
In addition, the mechanism of inhibition of polyamine biosynthesis can contribute to the antiproliferative activities of flavonoids. Ornithine decarboxylase is a rate-limiting enzyme in polyamine biosynthesis & is correlated with the rate of DNA synthesis and cell proliferation in several tissues. Several experiments show that flavonoids can inhibit ornithine decarboxylase induced by tumor promoters causing a subsequent decrease in polyamine and inhibition of DNA and protein synthesis.[27–29]
Cell cycle arrest
Perturbations in cell cycle progression may account for the anticarcinogenic effects of flavonoids. Due to mitogenic signals, cells enter into a series of regulated steps allowing traverse of the cell cycle, and cyclin-dependent kinases (CDKs) are recognized as key regulators of cell cycle progression. Alteration and deregulation of CDK activity are pathogenic hallmarks of neoplasia. Various types of cancers are associated with hyper activation of CDKs due to mutation of CDK genes or CDK inhibitor genes. Therefore, inhibitors or modulators are of great interest as novel therapeutic agents in cancer.[30,31] Checkpoints at both G1/S and G2/M of the cell cycle in cultured cancer cell lines have been found to be perturbed by flavonoids, such as silymarin, genistein, quercetin, daidzein, luteolin, kaempferol, apigenin, and epigallocatechin 3-gallate.[32–34] Studies from various laboratories have revealed that flavopiridol could induce cell cycle arrest during either G1 or G2/M by inhibiting all CDKs.[30,35]
Induction of apoptosis
The significant anticancer properties observed in flavonoids may be due to frank apoptosis.[36–39] Apoptosis is an active form of cell death that plays an essential role in the development and survival by eliminating damaged or unwanted cells. It is tightly regulated by a set of genes that promote apoptosis cell survival and is mediated through a highly organized network of interacting protease and their inhibitors in response to noxious stimuli from either inside or outside of the cell. Dysregulation of apoptosis plays a critical role in oncogenesis. Flavonoids have shown to induce apoptosis in some cancer cell lines, while sparing normal cells. The molecular mechanisms by which flavonoids induce apoptosis have not been clarified. Several mechanisms may be involved, including inhibition of DNA topoisomerase I/II activity,[40–42] decrease of ROS,[43] regulation of heat shock protein expression,[44] modulation of signaling pathways,[37] downregulation of nuclear transcription factor kappa B (NF-κB), activation of endonuclease, and suppression of Mcl-1 protein.[38,43,45,46]
In vitro studies of flavonoids
In vitro, flavonoids modify the activity of enzymatic systems in mammals (eg, kinases, phospholipases, ATPase, lipooxygenases, cyclooxygenases, and phosphodiesterases). A correlation has been observed in some cases between the flavonoid structure and its enzymatic activity.[47–51] Much of these effects can be attributed to the abilities of flavonoids to interact with the nucleotide binding sites of regulatory enzymes.
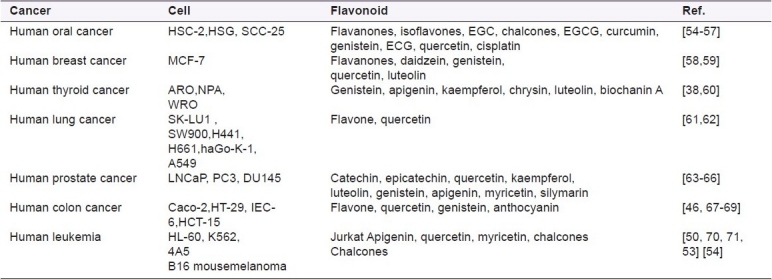
Many researchers have conducted in vitro studies on the potential anticancer activity of flavonoids in diverse cell systems. The report on the inhibitory properties of flavonoids against carcinogenesis are summarized in Table 2. Hirano and co-workers examined anticancer efficacy of 28 flavonoids on human acute myeloid leukemia cell line HL-60 and differences between antiproliferative activity and cytotoxicity of these compounds with those of four clinical anticancer agents. Out of 28 flavonoids, 8 showed considerable suppressive effects on HL-60 cell growth with IC50s ranging from 10-940 ng/mL. Among these compounds, genistein, honokiol, machilin A, matairesinol, and arctigenin had the strongest effects with IC50s less than 100 ng/ml, which were almost equivalent to the effects of current anti-cancer agents. The flavonoid genistein and the lignans, however, showed little or no cytotoxicity against HL-60 cells as assessed by dye exclusion tests (LC50s>2,900ng/ml), whereas the regular anti-cancer agents had potent cytotoxicity[52,53] and more than 30 flavonoids were screened for their effects on cell proliferation and potential cytotoxicity in human colon cancer cell lines Caco-2 and HT-29. There was no obvious structure activity relationship in the antiproliferative effects either on the basis of sub-classes with respect to type or position of substituents within a class.[53]
Table 2.
Anticancer activities of flavonoids in various cancer cell lines
Flavonoids showed inhibition of carcinogenesis in vitro but substantial evidences indicate that they can also do so in vivo.[72,73] Studies on animals and investigations using different cellular models suggest that certain flavonoids could inhibit tumor initiation as well as tumor progression.[27–29,74] Siess and co-workers investigated the effects of feeding rats with flavone, flavanone, tangeretin, and quercetin on two steps of aflatoxin B1(AFB1)-induced hepatocarcinogenesis (initiation and promotion) and found that flavone, flavanone, and tangeretin administered through the initiation period, decreased the number of γ-glutamyl transpeptidase-preneoplastic foci. Therefore flavanone acts as an anti-initiator as well as an anti-promoter.[75] Lung tumorigenesis is prevented by catechin enriched tea and is demonstrated in A/J mice.[76] Two weeks before the 4-(methylnitrosamine)-1-(3-pyridyl)-1 butanone (NNK) treatment, decaffeinated green or black tea is given to mice for 3 or 15 weeks. This markedly reduced the number of tumors formed in the mice. In those mice adenomas have developed at 16 weeks after the NNK injection; and, the progression of adenomas to adenocarcinomas was significantly inhibited by administration of black tea. These experiments infer that tea has broad inhibitory activity against lung carcinogenesis and it is effective when administered during the initiation, promotion, or progression stages of carcinogenesis. Moreover, there is evidence for the suppression of tumor invasion and metastasis by flavonoids.
Catechins, a group of flavonoid molecules, in vitro[77] inhibit the invasion of mouse MO4 cells into embryonic chick heart fragments. A polymethoxy flavonoid, nobiletin from Citrus depressa inhibits the tumor invasion activity of human fibrosarcoma HT-108 cells in the Matrigel model, by the suppression of expression of matrix metalloproteases (MMPs) and augmenting of tissue inhibitors of metalloproteinases.[78] Quercetin and apigenin inhibited melanoma cell (B16-BL6) growth and metastatic potential in syngenetic mice, in vitro.[60] They were found to significantly decrease the invasion of B16-BL6 cells. Also apigenin significantly decreased the invasion of lymphatic vessel of intestinal adenocarcinomas that are induced by azoxymethane and are of cancer peritoneal metastasis, enhanced by bombesin in male Wistar rats. The inhibitory effect of apigenin on cancer metastasis may be through the inhibition of phosphorylation of mitogen-activated protein kinase (MAPK).[79]
TREATMENT OF DIFFERENT TYPES OF CANCER BY FLAVONOIDS
In Western countries, breast cancer is one of the most common causes of death in women and prostate cancer is the second most common cause of death in men. In China, Japan, and other Asian countries, where diets include relatively high concentrations of soy isoflavones, death due to cancer is comparatively rare.[80]
Phytoestrogens are plant-derived chemicals that bind to the estrogen receptor (ER) and induce various estrogenic and antiestrogenic responses.[81] The extensively studied class of phytoestrogens are the isoflavones. High concentrations of the isoflavones genistein and diadzein are present in legumes and ingestion of these substances may reduce the risk of cancer, particularly in the breast and prostate.
Experimentally, thoroughly studied soy isoflavone is genistein. It is clear, however, that in vitro micromolar concentrations of genistein can inhibit the growth of a wide variety of cancer cells.[81] In ER-positive cells, growth inhibitors compete with estradiol for receptor binding, and translocation of the hormone-receptor complex takes place in the nucleus and ultimately, reduce the stimulation of a variety of downstream effects.[82]
Soy containing isoflavones are among the most versatile biopharmaceuticals known. Genistein, daidzein, and glycitein are the main isoflavones found in soy foods [Figure 3]. Isoflavones, one of the major class of phytoestrogens, are structurally similar to estrogens,[83] binds to ERs, and hence have estrogenic and anti-estrogenic activities and their own growth-inhibitory effects are independent of ER.[84,85] Isoflavones and their metabolites are considered to reduce the risk of cancer and to have potent anticarcinogenic activities[81,86] by direct inhibition of PTK,[87] inhibition of DNA-topoisomerase II,[88] inhibition of angiogenesis,[89] antiproliferation, and cell cycle arrest,[90] and induction of apoptosis.[91]
Figure 3.
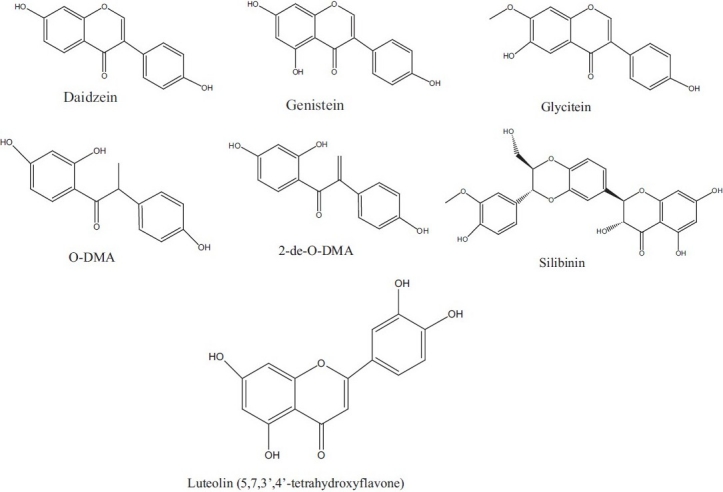
Some of the isoflavones found in soy foods
Two major types of cancer and their treatment using flavonoids are discussed in the following sections.
MAMMARY TUMOR
Evidences support that estrogens are involved in mammary carcinomas. Researchers have found that in ER-positive and ER-negative mammary cell lines of women affected with breast cancer, the tumor-suppressing gene pRb2/p13 binds to a specific region on the ER gene alpha and forms molecular complexes recruiting and interacting with several proteins. They discovered that ER-negative cells that are able to silent the expression of the ER pRb2/p13 form a specific molecular complex recruiting a different sequence of proteins than in the ER-positive cells. Our hypothesis is that the sequence of epigenetic events for establishing and maintaining a silence state of ER gene alpha during the breast cancer progression is mediated by pRb2/p13 in association with specific proteins that modify the DNA structure through specific mechanisms.[92]
Estradiol, the most potent endogenous estrogen, is biosynthesized from androgens by cytochrome P450 enzyme complex called aromatase. Some flavonoids have been reported as potent aromatase inhibitors.[93–95] Therefore, flavonoids are considered as potential agents against breast cancer by inhibiting aromatase activity.
Investigation of seven metabolites of isoflavones for their growth-inhibitory effects and later compared with the isoflavones genistein, daidzein, and glycitein present on human breast cancer MCF-7 and MDA-MB- 468 cells. The novel metabolite 2-de-O-DMA exhibited a potent growth inhibitory effect on human breast ER-positive MCF-7 cells and ER-negative MDA-MB-468 cells. This metabolite was further examined on other human breast cancer SK-BR-3 (ER-negative), human breast noncancer MCF-10A (ER-negative), human prostate cancer LNCaP [androgen receptor (AR)-positive], and DU145 (AR-negative) cell lines. Hence this study shows that the novel metabolite 2-de-O-DMA is still able to inhibit the proliferation of MCF-10A (ER-negative), SK-BR-3 (ER-negative), LNCaP, and DU145 cells.[84–91]
Epidemiologic studies have showed that populations with high isoflavone intake through soy consumption have low rates of breast, prostate, and colon cancer. The isoflavone polyphenol genistein in soybean is considered to be a potent chemopreventive agent against cancer.[96]
PROSTATE CANCER
Prostate cancer (PCA) is considered as one of the major concerns in the field of cancer therapy. PCA is an aging disease and oxidative stress & is a major factor in the promotion/progression of malignancy.[97] Furthermore, activation of many kinases involved in NF-κB pathway is dependent on oxidative stress.[98,99] ROS cause prolonged NF-κB DNA binding activity and antioxidants have shown to diminish this activity.[100] Based on the above study, one approach to control PCA growth and progression can be inhibition of constitutive NF-κB activation, however, limited efforts have been made in this direction. Some flavonoids play an important role in preventing PCA by various modes of action.
Silibinin is a flavonolignan present in milk thistle seeds. It is a promising chemopreventive agent against human PCA without showing any apparent toxic side effects.[101] Silibinin has shown strong anticancer efficacy against both androgen-dependent and -independent advanced human PCA cells.[102,103] Silibinin inhibits TGF expression-, secretion, and down-regulates EGFR-Erk1/2 activation in both LNCaP and DU145 cells, which contributes to the growth inhibitory effects in these cell lines.[103]
Recently, at pharmacologically achievable silibinin concentrations (0.02-20 μM) observed increased insulin-like growth factor-binding protein 3 (IGFBP-3) accumulation in PC-3 cell conditioned medium and a dose-dependent increase of IGFBP-3 mRNA abundance with a 9-fold increase over baseline at 20 μM silibinin.[104] Silibinin also showed, effect on membrane signaling related to erbB1 activation in human PCA LNCaP and DU145 cells.[105] Activation of NF-κB by cytokines is mediated by signal transduction cascades, leading to activation of the IκB kinases, IKKα and IKKβ. Silibinin inhibited the constitutive activation of NF-κB and IKKα in PC-3 and DU-145 cells, and blocked stimulated activation of NF-κB in LNCaP cells.[106] Lastly, the results clearly indicate that silibinin effectively sensitizes DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptosis, possibly via an increase in G2M arrest suggesting that studies done in vivo are needed in pre-clinical PCA models of such combinations, which might provide scientific base in developing more effective treatment against human PCA.
Luteolin (30,40,5,7-tetrahydroxyflavone) has an antiproliferation property that acts via arresting cell cycle and apoptosis in many human cancer cells, including PCA cells.[107,108] Studies showed that luteolin inhibits the expression of AR and growth in LNCaP human PCA cells and xenografted mice. The reduction in AR levels by luteolin involve a transcriptional or post translational mechanism. Moreover, it suggests that luteolin suppresses the association between AR and heat-shock protein 9(hsp90) and induces AR protein degradation through a proteasome-mediated pathway.[109] These results indicate that AR is critical for PCA cell growth and survival and that it is a potential molecular target for luteolin-mediated anticancer therapy.
Quercetin exerts the strongest expression of MMP-2 and -9 through the inhibition of protein kinases. Quercetin inhibits the secretion of MMP-2 also in tumor cells and thereby reduces the potential for metastasis.[110–112] Quercetin also acts as a preventive agent against cancer invasion. By targeting specific genes that regulate the expression of MMPs can be advantageous in the treatment of PCA.
Nutritional science contributes substantially also. Identification of biomarkers plays an important role in this effort through the use of new and emerging technologies. At the same time, gene expression profiling and proteomics provide novel insights into cancer-related traits. Early detection is the desired strategy for reducing cancer-related morbidity and mortality, and collaborative effort between academic and industry leaders brings expert solutions for cancer.
ADVANCED DEVELOPMENT OF ANTICANCER AGENT FROM NATURAL FLAVONOIDS
In the search for anticancer drugs from botanical sources, plant extracts were fractionated by various means and were then in vitro tested for anticancer properties. Here, we discuss flavonoid compounds that display their anticancer activity in recent time, including those that are able to influence processes that are dysregulated during cancer development.
-
(a)
Flavone 8-acetic acid (FAA) represents a novel chemical structure undergoing clinical trials as an anticancer drug. Most unusual property of FAA is its ability to reduce tumour blood flow dramatically, which may provide the appropriate conditions for reactive chemistry to occur which distinguish it from a conventional cytotoxic compound, particularly in the response of solid murine tumors.[113] Its clinical use has a number of pharmacologic drawbacks, including low dose potency and dose-dependent pharmacokinetics. So in search for better analogs of FAA-, several derivatives of FAA were synthesized that showed potent antitumor effect, such as xanthenone-4-acetic acid (XAA) and its 5,6-dimethyl derivative (5,6-MeXAA) that displayed very effective pharmacokinetic properties.[114,115] 5,6-MeXAA ( NSC 640488) was 14-fold more potent than the investigational chemotherapeutic drug flavone-8-acetic acid (NSC 347512) in stimulating tumouricidal activity in cultures of resident murine peritoneal macrophages.[116] 5,6-MeXAA, an another derivative of FAA is a small molecule of flavonoid class that has an antitumor activity due to its ability to induce high local levels of tumor necrosis factor (TNF) that disrupts established blood vessels within tumors.[117,118] 5,6-MeXAA and FAA have shown potential antitumour activity in several bioreductive drugs by inhibiting enzyme DT-diaphorase (NAD(P)-H Quinone oxidoreductase EC 1.6.99.2) with respect to NADH, Ki values of 75 and 20 μM, respectively.[118,119] Also fluorine derivative of FAA bearing a fluorine atom at the 7th position is a most active compound showing remarkable activities in murine cells, but not confirmed in human models.[120]
-
(b)
Flavopiridol, the first CDK inhibitor tested on human, demonstrated clear effects on cell cycle progression, induced differentiation, and apoptosis depending on the relation between transcription factor E2F1 and RB.[121,122] Flavopiridol undergoes Phase II single-agent trials and Phase I combination trials (with paclitaxel and cisplatin). The drug showed in vivo antitumor activity against a variety of tumor xenografts.[123,124]
-
(c)
A new flavone glycoside, chrysoeriol 7-O-(2′′-O-6′′′-O-acetyl-β-D-glucopyranosyl-β-D-glucopyranoside (1), along with 14 known compounds (2-15) was isolated from Carduus crispus Linn. plant. The antitumor activity of compound (1), (4) and (5) was also tested.[125]
-
(d)
Nobiletin (5,6,7,8,3′′,4′′-hexamethoxyflavone)-, a citrus flavonoid-, extracted from Citrus depressa Hayata, was examined for its antitumor activity on human gastric cancer cell lines. Cell-cycle analysis revealed that nobiletin acted on these cells through several ways, namely, by direct cytotoxicity, induction of apoptosis, and modulation of cell cycle.[126]
-
(e)
The flavonoid silybin and its bioavailable derivative IdB 1016 (silipide) enhance the antitumor activity of cisplatin (CDDP), the most commonly used drug in the treatment of gynecologic malignancies [Figure 4].[127]
-
(f)
Natural flavone diosmetin showed inhibition of proliferation of breast adenocarcinoma MDA-MB 468 and normal breast MCF-10A cells and was found that this compound is selective for the cancer cells with slight toxicity in the normal breast cells.[128]
-
(g)
Quercetin 3-O-amino acid-esters, a new type of quercetin derivatives have been successfully prepared for the first time. Different from quercetin, the novel compounds show higher selectivity as inhibitors against Src tyrosine kinase (IC50) than against EGFR tyrosine kinase.[129]
-
(h)
The modified derivative of flavone, such as trans-bis-(3-aminoflavone-kappa2 N,-O)bis (perchlorato kappa O) copper(II), showed potential antitumor properties.[130]
-
(i)
Myricetin-3-O-(L-rhamnopyranoside and quercetin-3-O-lactopyranoside isolated from Byrsonima crassa, Davilla elliptica, and Mouriri showed antitumor and anti-inflammatory activities.[131]
Figure 4.

Chemical structure of advanced anticancerous flavonoids
In the past, many attempts have been made to obtain anticancerous plant originated flavonoids and the efforts will further be continued until a satisfactory treatment becomes available. In this regard, a number of medicinal plants having anticonvulsant potential are reviewed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56:317–33. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodek P, Trefil P, Stiborova M. Flavonoids- Potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139:1–21. doi: 10.1016/s0009-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhnau J. The flavonoids.A class of semi-essential food components:their role in human nutrition. World Rev Nutr Diet. 1976;24:117–91. [PubMed] [Google Scholar]
- 4.Obdulio BG, Julian C, Francisco RM, Ana O, Jose AD. Uses and properties of Citrus flavonoids. J Agric Food Chem. 1997;45:4505–15. [Google Scholar]
- 5.Manthey JA, Guthrie N, Grohmann K. Biological properties of Citrus flavonoids Pertaining to cancer and inflammation. Curr Med Chem. 2001;8:135–53. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 7.Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 8.Galati G, Teng S, Moridani MY, Chan TS, O Brien PJ. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol Drug Interact. 2000;17:311–49. doi: 10.1515/dmdi.2000.17.1-4.311. [DOI] [PubMed] [Google Scholar]
- 9.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 10.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention Flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–77. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg RA. New York: Cold Spring Harbor; 1989. Oncogenes and the Molecular Origins of cancer.: Cold Spring Harbor Laboratory. [Google Scholar]
- 12.Pimentel E. 2nd ed. Florida: CRC Press; 1989. Oncogenes. [Google Scholar]
- 13.Hunter T, Cooper A. The Enzymes. In: Boyer PD, Krebs EG, editors. Vol. 17. Florida: Academic Press; 1987. pp. 191–246. [Google Scholar]
- 14.Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Ann Rcv Biorhnn. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- 15.Yarden T, Ullrich A. Growth factor receptor tyrosine kinases. Am Rcv Biorhnn. 1988;57:443–78. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 16.Geahlen RL, Harrison ML. Peptides and Protein Phosphorylation. In: Kemp BE, editor. Florida: CRC Press; 1989. pp. 239–53. [Google Scholar]
- 17.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–12. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 18.Nijhoff WA, Groen GM, Peters WH. Induction of rat hepatic and intestinal glutathione S-transferases and glutathione by dietary naturally occuring anticarcinogens. Int J Oncol. 1993;3:1131–9. doi: 10.3892/ijo.3.6.1131. [DOI] [PubMed] [Google Scholar]
- 19.Vander LE, Roelofs HM, Nagengast FM, Peters WH. Induction of rat hepatic and intestinal UDP-glucuronosyl transferases by naturally occurring dietary anticarcinogens. Carcinogenesis. 2003;24:1651–6. doi: 10.1093/carcin/bgg117. [DOI] [PubMed] [Google Scholar]
- 20.Steele VE, Kelloff GJ, Balentine D, Boone CW, Mehta R, Bagheri D, et al. Comparative chemopreventive mechanisms of green tea, black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000;21:67–3. doi: 10.1093/carcin/21.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Marchand LL, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 22.Tsyrlov IB, Mikhailenko VM, Gelboin HV. Isozyme- and species-specific susceptibility of cDNA-expressed CYP1A P-450s to different flavonoids. Biochim Biophys Acta. 1994;1205:325–35. doi: 10.1016/0167-4838(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 23.Bu-Abbas A, Clifford MN, Walker R, Ioannides C. Contribution of caffeine and flavanols in the induction of hepatic Phase II activities by green tea. Food Chem Toxicol. 1998;36:617–21. doi: 10.1016/s0278-6915(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 24.Sun XY, Plouzek CA, Henry JP, Wang TT, Phang JM. Increased UDP-glucuronosyl transferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 1998;58:2379–84. [PubMed] [Google Scholar]
- 25.Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13:2165–70. [PubMed] [Google Scholar]
- 26.Mutoh M, Takahashi M, Fukuda K, Komatsu H, Enya T, Matsushima HY, et al. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: Structure-activity relationship. Jpn J Cancer Res. 2000;91:686–91. doi: 10.1111/j.1349-7006.2000.tb01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M, Satoh K, et al. Chemoprevention of azoxymethane-induced rat colon carcinogenesis By the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–65. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A, et al. Chemoprevention of 4- nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997;57:246–52. [PubMed] [Google Scholar]
- 29.Makita H, Tanaka T, Fujitsuka H, Tatematsu N, Satoh K, Hara A, et al. Chemoprevention of 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis by the dietary flavonoids chalcone, 2-hydroxychalcone, and quercetin. Cancer Res. 1996;56:4904–9. [PubMed] [Google Scholar]
- 30.Senderowicz AM. Flavopiridol. The first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs. 1999;17:313–20. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 31.Senderowicz AM. Development of cyclin-dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia. 2001;15:1–9. doi: 10.1038/sj.leu.2401994. [DOI] [PubMed] [Google Scholar]
- 32.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: Induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998;4:1055–64. [PubMed] [Google Scholar]
- 33.Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, et al. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–44. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 34.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61:1205–15. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang HK. The therapeutic potential of flavonoids. Expert Opin Invest Drugs. 2000;9:2103–19. doi: 10.1517/13543784.9.9.2103. [DOI] [PubMed] [Google Scholar]
- 36.Sakagami H, Jiang Y, Kusama K, Atsumi T, Ueha T, Toguchi M, et al. Induction of apoptosis by flavones, flavonols (3-hydroxyflavones) and isoprenoid-substituted flavonoids in human oral tumor cell lines. Anticancer Res. 2000;20:271–7. [PubMed] [Google Scholar]
- 37.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of Growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–303. [PubMed] [Google Scholar]
- 38.Wenzel U, Kuntz S, Brendel MD, Daniel H, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000;60:3823–31. [PubMed] [Google Scholar]
- 39.De Vincenzo R, Ferlini C, Distefano M, Gaggini C, Riva A, Bombardelli E, et al. In vitro evaluation of newly developed chalcone analogues in human cancer cells. Cancer Chemother Pharmacol. 2000;46:305–12. doi: 10.1007/s002800000160. [DOI] [PubMed] [Google Scholar]
- 40.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related Flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL- 60 cells. Eur J Cancer. 1999;35:1517–25. [PubMed] [Google Scholar]
- 41.Bailly C. Topoisomerase I poisons and suppressors as anticancer drugs. Curr Med Chem. 2000;7:39–58. doi: 10.2174/0929867003375489. [DOI] [PubMed] [Google Scholar]
- 42.Sukardiman, Darwanto A, Tanjung M, Darmadi MO. Cytotoxic mechanism of flavonoid from Temu Kunci (Kaempferia pandurata) in cell culture of human mammary carcinoma. Clin Hemorheol Microcirc. 2000;23:185–90. [PubMed] [Google Scholar]
- 43.Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol. 2002;63:225–36. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 44.Rong Y, Yang EB, Zhang K, Mack P. Quercetin-induced apoptosis in the monoblastoid cell line U937 in vitro and the regulation of heat shock proteins expression. Anticancer Res. 2000;20:4339–45. [PubMed] [Google Scholar]
- 45.Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 2000;64:1813–20. doi: 10.1271/bbb.64.1813. [DOI] [PubMed] [Google Scholar]
- 46.Konig A, Schwartz GK, Mohammad RM, Al-Katib A, Gabrilove JL. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B-cell leukemia lines. Blood. 1997;90:4307–12. [PubMed] [Google Scholar]
- 47.Horak E, Smith K, Bromley L, LeJeune S, Greenall M, Lane D, et al. Oncogene. 1991;6:2277–84. [PubMed] [Google Scholar]
- 48.Gross ME, Zorbas MA, Danels YJ, Garcia R, Wick GE, Olive M, et al. Cuncer Ru. 1991;51:1452. [PubMed] [Google Scholar]
- 49.Chen SC, Chou CK, Wong FH, Chang CM, Hu CP. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and autocrine stimulation in human esophageal carcinoma cells. Cancer Res. 1991;51:1898–903. [PubMed] [Google Scholar]
- 50.Petrides PE, Bock S, Bovens J, Hofmann R, Jakse G. Modulation of pro-epidermal growth factor, pro-transforming growth factor alpha and epidermal growth factor receptor gene expression in human renal carcinomas. Cancer Res. 1990;50:3934–9. [PubMed] [Google Scholar]
- 51.Weidner M, Peter S, Strokmeyer T, Hussnatter R, Ackermann R, Sies H. Inverse relationship of epidermal growth factor receptor and HER2/neu gene expression in human renal cell carcinoma. Cancer Res. 1990;50:4504–9. [PubMed] [Google Scholar]
- 52.Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061–9. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]
- 53.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–42. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 54.Shi YQ, Fukai T, Sakagami H, Chang WJ, Yang PQ, Wang FP, et al. Cytotoxic flavonoids with isoprenoid groups from Morus mongolica. J Nat Prod. 2001;64:181–8. doi: 10.1021/np000317c. [DOI] [PubMed] [Google Scholar]
- 55.Fukai T, Sakagami H, Toguchi M, Takayama F, Iwakura I, Atsumi T, et al. Cytotoxic activity of low molecular weight polyphenols against human oral tumor cell lines. Anticancer Res. 2000;20:2525–36. [PubMed] [Google Scholar]
- 56.Elattar TM, Virji AS. Effect of tea polyphenols on growth of oral squamous carcinoma cells in vitro. Anticancer Res. 2000;20:3459–65. [PubMed] [Google Scholar]
- 57.Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000;20:1733–38. [PubMed] [Google Scholar]
- 58.Pouget C, Lauthier F, Simon A, Fagnere C, Basly JP, Delage C, Chulia AJ. Flavonoids: Structural requirements for antiproliferative activity on breast cancer cells. Bioorg Med Chem Lett. 2001;11:3095–7. doi: 10.1016/s0960-894x(01)00617-5. [DOI] [PubMed] [Google Scholar]
- 59.Han D, Tachibana H, Yamada K. Inhibition of environmental estrogen-induced proliferation of human breast carcinoma MCF-7 cells by flavonoids. In vitro Cell Dev Biol Anim. 2001;37:275–82. doi: 10.1007/BF02577543. [DOI] [PubMed] [Google Scholar]
- 60.Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9:369–76. doi: 10.1089/thy.1999.9.369. [DOI] [PubMed] [Google Scholar]
- 61.Bai F, Matsui T, Ohtani-Fujita N, Matsukawa Y, Ding Y, Sakai T. Promoter activation and following induction of the p21/WAF1 gene by flavone is involved in G1 phase arrest in A549 lung adenocarcinoma cells. FEBS Lett. 1998;437:61–4. doi: 10.1016/s0014-5793(98)01198-3. [DOI] [PubMed] [Google Scholar]
- 62.Caltagirone S, Ranelletti FO, Rinelli A, Maggiano N, Colasante A, Musiani P, et al. Interaction with type II estrogen binding sites and antiproliferative activity of tamoxifen and quercetin in human non-small-cell lung cancer. Am J Respir Cell Mol Biol. 1997;17:51–9. doi: 10.1165/ajrcmb.17.1.2728. [DOI] [PubMed] [Google Scholar]
- 63.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids Suppress Androgen independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–22. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- 64.Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate. 2001;46:98–107. doi: 10.1002/1097-0045(20010201)46:2<98::aid-pros1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 65.Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, et al. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37:223–33. doi: 10.1207/S15327914NC372_16. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000;60:1051–9. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 67.Kamei H, Hashimoto Y, Koide T, Kojima T, Hasegawa M. Anti-tumor effect of methanol extracts from red and white wines. Cancer Biother Radiopharm. 1998;13:447–52. doi: 10.1089/cbr.1998.13.447. [DOI] [PubMed] [Google Scholar]
- 68.Kuo SM, Morehouse HF, Lin CP. Effect of antiproliferative flavonoids on ascorbic acid accumulation in human colon adenocarcinoma cells. Cancer Lett. 1997;116:131–7. doi: 10.1016/s0304-3835(97)00183-3. [DOI] [PubMed] [Google Scholar]
- 69.Kuo SM. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996;110:41–8. doi: 10.1016/s0304-3835(96)04458-8. [DOI] [PubMed] [Google Scholar]
- 70.Chung HS, Chang LC, Lee SK, Shamon LA, van Breemen RB, Mehta RG, et al. Flavonoid constituents of Chorizanthe diffusa with potential cancer chemopreventive activity. J Agric Food Chem. 1999;47:36–41. doi: 10.1021/jf980784o. [DOI] [PubMed] [Google Scholar]
- 71.Csokay B, Prajda N, Weber G, Olah E. Molecular mechanisms in the antiproliferative action of quercetin. Life Sci. 1997;60:2157–63. doi: 10.1016/s0024-3205(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 72.Kamei H, Koide T, Kojimam T, Hasegawa M, Terabe K, Umeda T, et al. Flavonoid-mediated tumor growth suppression demonstrated by in vivo study. Cancer Biother Radiopharm. 1996;11:193–6. doi: 10.1089/cbr.1996.11.193. [DOI] [PubMed] [Google Scholar]
- 73.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 74.Yang M, Tanaka T, Hirose Y, Deguchi T, Mori H, Kawada Y. Chemopreventive effects of diosmin and hesperidin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary-bladder carcinogenesis in male ICR mice. Int J Cancer. 1997;73:719–24. doi: 10.1002/(sici)1097-0215(19971127)73:5<719::aid-ijc18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 75.Siess MH, Le Bon AM, Canivenc-Lavier MC, Suschetet M. Mechanisms involved in the chemoprevention of flavonoids. Biofactors. 2000;12:193–9. doi: 10.1002/biof.5520120131. [DOI] [PubMed] [Google Scholar]
- 76.Yang CS, Chung JY, Yang GY, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S–8S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 77.Bracke M, Vyncke B, Opdenakker G, Foidart JM, De Pestel G, Mareel M. Effect of catechins and citrus flavonoids on invasion in vitro. Clin Exp Metastasis. 1991;9:13–25. doi: 10.1007/BF01831706. [DOI] [PubMed] [Google Scholar]
- 78.Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, et al. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080cells. Cancer Res. 2002;62:1025–9. [PubMed] [Google Scholar]
- 79.Tatsuta M, Iishi H, Baba M, Yano H, Murata K, Mukai M, et al. Suppression by apigenin of peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 2000;18:657–62. doi: 10.1023/a:1013133803806. [DOI] [PubMed] [Google Scholar]
- 80.Parkin DM, Pisani P, Ferlay J. Estimates of the world-wide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–41. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 81.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–31. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 82.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45:399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 83.Setchell KD, Adlercreutz H. Mammalian lignans and phytoestrogens: Recent studies on their formation, metabolism and biological role in health and disease. In: Rowland IR, editor. Role of the Gut Flora in Toxicity and Cancer. London: London Academic; 1988. pp. 315–45. [Google Scholar]
- 84.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–7. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 85.Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer. Cell Growth Differ. 1996;7:1345–51. [PubMed] [Google Scholar]
- 86.Wei H, Bowen R, Cai Q, Barnes S, Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 1995;208:124–30. doi: 10.3181/00379727-208-43844. [DOI] [PubMed] [Google Scholar]
- 87.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 88.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–7. [PubMed] [Google Scholar]
- 89.Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary Ingested isoflavone, inhibits cell proliferation and in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:6902–94. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Katsuhiko M, et al. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993;53:1328–31. [PubMed] [Google Scholar]
- 91.Constantinou AI, Kamath N, Murley JS. Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur J Cancer. 1998;34:1927–34. doi: 10.1016/s0959-8049(98)00198-1. [DOI] [PubMed] [Google Scholar]
- 92.Hong X, Schevzov G, Gunning P, Williams HM, Silink MA. Comparative Study of Growth-Inhibitory Effects of Isoflavones and Their Metabolites on Human Breast and Prostate Cancer Cell Lines. Nutr Cancer. 2002;42:224–32. doi: 10.1207/S15327914NC422_12. [DOI] [PubMed] [Google Scholar]
- 93.Brueggemeier RW. Aromatase, aromatase inhibitors, and breast cancer. Am J Ther. 2001;8:333–44. doi: 10.1097/00045391-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Pouget C, Fagnere C, Basly JP, Besson AE, Champavier Y, Habrioux G, et al. Synthesis and aromatase inhibitory activity of flavanones. Pharm Res. 2002;19:286–91. doi: 10.1023/a:1014490817731. [DOI] [PubMed] [Google Scholar]
- 95.Jeong HJ, Shin YG, Kim IH, Pezzuto JM. Inhibition of aromatase activity by flavonoids. Arch Pharm Res. 1999;22:309–12. doi: 10.1007/BF02976369. [DOI] [PubMed] [Google Scholar]
- 96.Ullah MF, Shamim U, Hanif S, Azmi AS, Hadi SM. Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol Nutr Food Res. 2009;53:1376–85. doi: 10.1002/mnfr.200800547. [DOI] [PubMed] [Google Scholar]
- 97.Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–37. [PubMed] [Google Scholar]
- 99.Stancovski I, Baltimore D. NF-êB Activation: The IêB Kinase Revealed. Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 100.Ripple MO, Henry WF, Schwarze SR, Wilding G, Weindruch R. Effect of antioxidants on androgen-induced AP-1 and NF-kappa B DNA-binding activity in prostate carcinoma cells. J Natl Cancer Inst. 1999;91:1227–32. doi: 10.1093/jnci/91.14.1227. [DOI] [PubMed] [Google Scholar]
- 101.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. Bio Drugs. 2001;15:465–89. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 102.Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat 3 and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28:1463–70. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 103.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells implications for prostate cancer intervention. Proc Natl Acad Sci USA. 1999;96:7490–5. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like Growth factor-binding protein 3 expression and inhibits proliferation of androgen- independent prostate cancer cells. Cancer Res. 2000;60:5617–20. [PubMed] [Google Scholar]
- 105.Sharma Y, Agarwal C, Singh AK, Agarwal R. Inhibitory effect of silibinin on ligand binding to erbB1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Mol Carcinog. 2001;30:224–36. doi: 10.1002/mc.1032. [DOI] [PubMed] [Google Scholar]
- 106.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18:7389–94. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 107.Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;26:560–3. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]
- 108.Lim DY, Jeong Y, Tyner AL, Park JHY. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 109.Chiu FL, Lin JK. Down regulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2007;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 110.Aalinkeel R, Nair MPN, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–21. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 111.Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit Expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NF kappa B. Carcinogenesis. 2004;25:987–95. doi: 10.1093/carcin/bgh095. [DOI] [PubMed] [Google Scholar]
- 112.Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol Cell Biochem. 2006;287:109–16. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- 113.Cummings J, Smyth JF. Flavone 8-acetic acid: our current understanding of its mechanism of action in solid tumours. Cancer Chemother Pharmacol. 1989;24:269–72. doi: 10.1007/BF00304756. [DOI] [PubMed] [Google Scholar]
- 114.McKeage MJ, Kestell P, Denny WA, Baguley BC. Plasma pharmacokinetics of the antitumour agents 5,6-dimethylxanthenone-4-acetic acid, xanthenone-4-acetic acid and flavone-8-acetic acid in mice. Cancer Chemother Pharmacol. 1991;28:409–13. doi: 10.1007/BF00685815. [DOI] [PubMed] [Google Scholar]
- 115.Aitken RA, Bibby MC, Cooper PA, Double JA, Laws AL, Ritchie RB, et al. Synthesis and antitumour activity of new derivatives of flavone-8-acetic acid (FAA). Part 4: Variation of the basic structure. Arch Pharm (Weinheim) 2000;333:181–8. doi: 10.1002/1521-4184(20006)333:6<181::aid-ardp181>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 116.Ching LM, Joseph WR, Baguley BC. Stimulation of macrophage tumouricidal activity by 5,6-dimethyl-xanthenone-4-acetic acid, a potent analogue of the antitumour agent flavone-8-acetic acid. Biochem Pharmacol. 1992;44:192–5. doi: 10.1016/0006-2952(92)90058-q. [DOI] [PubMed] [Google Scholar]
- 117.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, et al. Activation of Tumor-Associated Macrophages by the Vascular Disrupting Agent 5,6- Dimethylxan -thenone-4-Acetic Acid Induces an Effective CD8+ T- Cell Mediated Antitumor Immune Response in Murine Models of Lung Cancer and Mesothelioma. Cancer Res. 2005;65:11752–61. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 118.Phillips RM. Inhibition of DT-diaphorase (NAD (P) H: quinone oxidoreductase, EC 1.6.99.2) by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavone-8-acetic acid (FAA): implications for bioreductive drug development. Biochem Pharmacol. 1999;58:303–10. doi: 10.1016/s0006-2952(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 119.Baguley BC, Cole G, Thomsen LL, Li Z. Serotonin involvement in the antitumour and Host effects of flavone-8-acetic acid and 5,6-dimethylxanthenone-4-acetic acid. Cancer Chemother Pharmacol. 1993;33:77–81. doi: 10.1007/BF00686027. [DOI] [PubMed] [Google Scholar]
- 120.Carrara M, Zampiron A, Barbera M, Caputo A, Bisi A, Gobbi S, et al. Mono or di- fluorinated analogues of flavone-8-acetic acid: synthesis and in vitro biological activity. Anticancer Res. 2005;25:1179–85. [PubMed] [Google Scholar]
- 121.Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs. 1999;17:313–20. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 122.Mayer F, Mueller S, Malenke E, Kuczyk M, Hartmann JT, Bokemeyer C. Induction of apoptosis by flavopiridol unrelated to cell cycle arrest in germ cell tumour derived cell lines. Invest New Drugs. 2005;23:205–11. doi: 10.1007/s10637-005-6728-x. [DOI] [PubMed] [Google Scholar]
- 123.Kelland LR. Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opin Investig Drugs. 2000;9:2903–11. doi: 10.1517/13543784.9.12.2903. [DOI] [PubMed] [Google Scholar]
- 124.Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9:2103–19. doi: 10.1517/13543784.9.9.2103. [DOI] [PubMed] [Google Scholar]
- 125.Xie WD, Li PL, Jia ZJ. A new flavone glycoside and other constituents from Carduus crispus. Pharmazie. 2005;60:233–6. [PubMed] [Google Scholar]
- 126.Yoshimizu N, Otani Y, Saikawa Y, Kubota T, Yoshida M, Furukawa T, et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment Pharmacol Ther. 2004;20:95S–101S. doi: 10.1111/j.1365-2036.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- 127.Giacomelli S, Gallo D, Apollonio P, Ferlini C, Distefano M, Morazzoni P, et al. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–59. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 128.Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21:1525–8. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 129.Huang H, Jia Q, Ma J, Qin G, Chen Y, Xi Y, et al. Discovering novel quercetin-3-O- amino acid-esters as a new class of Src tyrosine kinase inhibitors. Eur J Med Chem. 2009;44:1982–8. doi: 10.1016/j.ejmech.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rybarczyk PAJ, Ma³ecka M, Glinka £, Ochocki J. Trans-bis (3-aminoflavone- kappa 2N, O) bis (perchlorato-kappaO) copper (II), a new potential antitumour agent. Acta Crystallogr C. 2007;63:410–2. doi: 10.1107/S010827010703747X. [DOI] [PubMed] [Google Scholar]
- 131.Carli CB, De Matos DC, Lopes FC, Maia DC, Dias MB, Sannomiya M, et al. Isolated flavonoids against mammary tumour cells LM2. Z Naturforsch C. 2009;64:32–6. doi: 10.1515/znc-2009-1-206. [DOI] [PubMed] [Google Scholar]


