Abstract
Alterations in synaptic strength over short time scales, termed short-term synaptic plasticity, can gate the flow of information through neural circuits. Different information can be extracted from the same presynaptic spike train depending on the activity- and time-dependent properties of the plasticity at a given synapse. The parallel processing in the brain stem auditory pathways provides an excellent model system for investigating the functional implications of short-term plasticity in neural coding. We review recent evidence that short-term plasticity differs in different pathways with a special emphasis on the ‘intensity’ pathway. While short-term depression dominates the ‘timing’ pathway, the intensity pathway is characterized by a balance of short-term depression and facilitation that allows linear transmission of rate-coded intensity information. Target-specific regulation of presynaptic plasticity mechanisms underlies the differential expression of depression and facilitation. The potential contribution of short-term plasticity to different aspects of ‘intensity’-related information processing, such as interaural level/intensity difference coding, amplitude modulation coding, and intensity-dependent gain control coding, is discussed.
1. Introduction
1.1. Short-term synaptic plasticity
Dynamic regulation of synaptic strength on short-term time scales has a profound impact on the transmission of information through neural circuits (Abbott & Regehr, 2004). The release of neurotransmitter is a complex biochemical event, and the resulting synaptic response depends on a number of presynaptic and postsynaptic processes that occur on millisecond to second time scales (Zucker & Regehr, 2002). Specific forms of short-term plasticity have been associated with specific synaptic connections, suggesting it is precisely regulated to serve a functional role (Beierlein et al., 2003; Dittman et al., 2000; Markram et al., 2004; Reyes et al., 1998; Sun et al., 2005). Applying different plasticity to the same presynaptic spike train pattern will create different resulting effects on the postsynaptic neurons (Dittman et al., 2000). Several studies have furthermore shown that the same presynaptic element can make synapses that differ in their plasticity depending on the identity of the target postsynaptic neuron (Beierlein et al., 2003; Markram et al., 2004; Reyes et al., 1998).
The particular time- and frequency-dependence of the synaptic dynamics results in a connection that behaves like a filter for information transmission from one neuron to the next. The rich diversity of functional pathways in the auditory brain stem provides an opportunity to test the hypothesis that short-term plasticity is important for neural coding. Recent work in the brain stem nuclei of birds and mammals has provided evidence that short-term plasticity may contribute to the division of the pathways that encode temporal and intensity information for the purpose of sound localization (MacLeod & Carr, 2007; MacLeod et al., 2007). The auditory nerve bifurcates and projects to separate divisions of the cochlear nuclei where differences in the anatomy, morphology, physiology and short-term plasticity properties contribute to the parallel pathway divisions of acoustic information.
1.2 Neural pathways for the encoding of intensity
For the purposes of the study of sound localization, the ‘intensity’ pathway generally refers to those neural elements that are directly involved in interaural level difference computation. Two main acoustic cues for sound localization arise due to binaural differences in the timing and intensity (or ‘level’) of sound at each ear, called interaural time differences (ITD) and interaural level differences (ILD) (Konishi, 2003). We focus on the avian model system because it is here that the ITD/ILD division and the plasticity results are clearest, but comparisons with the mammalian system are drawn as appropriate.
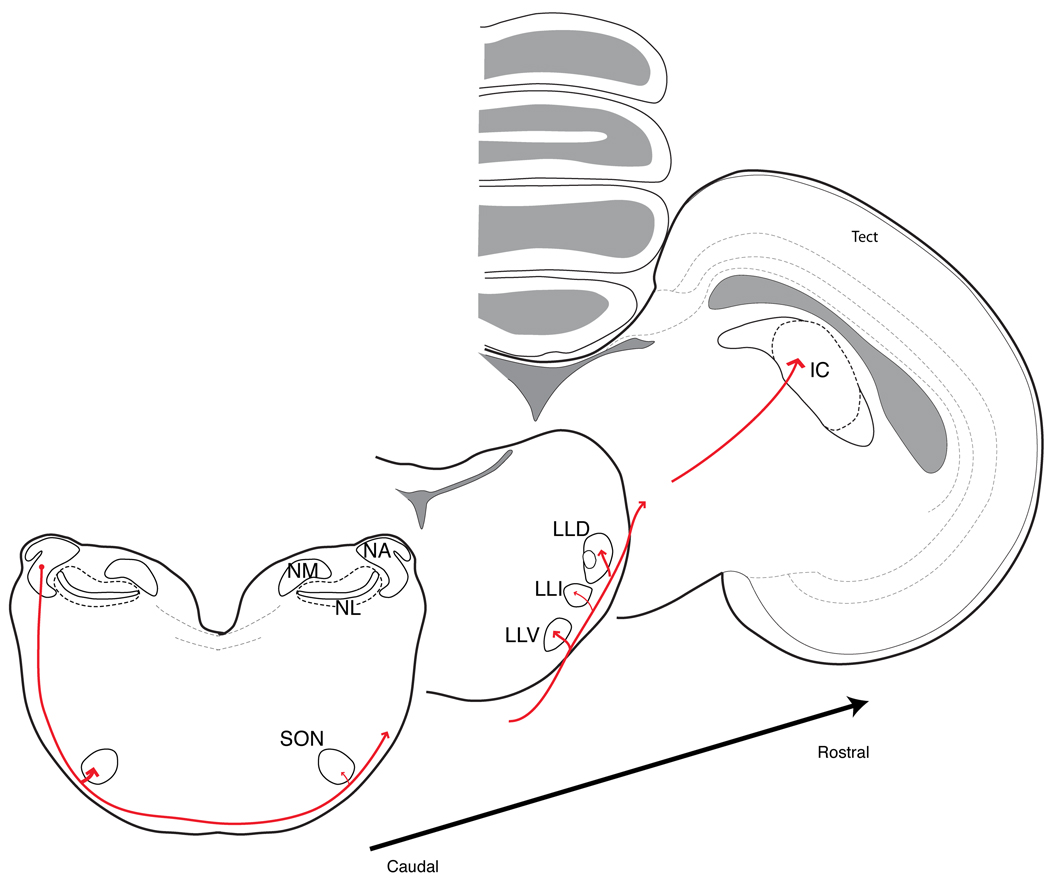
Intensity is one of the primary dimensions of sound: overall loudness is a fundamental percept, relative intensity across frequency defines the spectral content sounds, and the spectrotemporal modulations of sound amplitude defines most natural communication and environmental signals, yet these aspects of sound coding are poorly understood. The diversity of intensity related tasks is reflected in the heterogeneity of the cochlear nucleus (CN) and its several ascending projections (Fig. 1). The information for binaural ITD and ILD cues for sound localization is segregated into parallel neural pathways beginning with the cochlear nuclei. Cochlear nucleus angularis (NA) encodes intensity information to be used in the calculation of ILD, while cochlear nucleus magnocellularis (NM) encodes timing cues to be used in the calculation of ITD (Knudsen et al., 1977; Konishi et al., 1985; Köppl & Carr, 2003; Sullivan, 1985; Sullivan & Konishi, 1984; Takahashi et al., 1984). NM projects bilaterally only to nucleus laminaris (NL), creating a highly specialized circuit for the computation of ITD (Konishi, 2003). In contrast, NA neurons and response profiles have a diversity of functional types that bear a strong resemblance to those in the mammalian CN (Carr & Soares, 2002; Fukui & Ohmori, 2003; Köppl & Carr, 2003; MacLeod & Carr, 2007; Soares & Carr, 2001; Soares et al., 2002). Neurons in NA have four major projections: to the superior olivary nucleus (SON) in the brain stem, two lemniscal nuclei, and directly to the inferior colliculus (IC) in the midbrain. The anatomical evidence from multiple avian species shows that these ascending connections appear to be fairly conserved among birds despite some expectation of species variation due to differences in head size and ecology (Arends & Zeigler, 1986; Conlee & Parks, 1986; Krutzfeldt et al., 2010a, b; Takahashi & Konishi, 1988; Wild et al., 2010).
Fig. 1. Principal projections from nucleus angularis form the ascending ‘intensity’ pathway.
Neurons in nucleus angularis (NA) project bilaterally to the superior olivary nuclei (SON) in the brain stem, then their axons continue on via the lateral lemniscus, with ramifications most heavily in the ventral and dorsal lateral lemniscus (LLV and LLD), before continuing on to terminate in the inferior colliculus (IC). The LLD has been shown in barn owl and zebra finch to receive non-overlapping projections from NA and nucleus laminaris (NL) to anterior and posterior divisions (LLDa and LLDp, respectively). LLDp is the first site of binaural level difference comparisons. Not shown are weaker ipsilateral connections from NA to LLI and LLV. NM, nucleus magnocellularis.
Drawings representing the anatomy of the chick brain modified with permission from Puelles et al. (2007) The Chick Brain in Stereotaxic Coordinates, Academic Press, Elsevier, New York. Projections and nomenclature based on work on several avian species including pigeon (Arends and Zeigler (1986)), chick (Conlee and Parks (1986)), barn owl (Takahashi and Konishi (1988)), and zebra finch (Krutzfeldt et al. (2010a,b)).
Each of these target nuclei have different roles that are still being discerned. One of the lemniscal nuclei is the dorsal nucleus of the lateral lemniscus, posterior division (LLDp) and is the nucleus that first encodes ILD. Projections to the SON mediate descending control of an intensity-dependent gain signal via an inhibitory feedback loop to NA, NM and NL (Burger et al., 2005; Hyson, 2005; Lachica et al., 1994; Monsivais et al., 2000; Nishino et al., 2008; Pena et al., 1996; Yang et al., 1999). The function of another lemniscal target, the ventral nucleus of the lateral lemniscus (LLV), is virtually unknown, as is the role of the direct projection to IC. Auditory information is passed to the forebrain via an ascending pathway through the thalamic nucleus ovoidalis to Field L.
In the mammalian brainstem, the ‘intensity’ pathways are even more complex; for brevity the reader is referred to detailed discussions found in Cant (2003), Oertel (1999), and Tollin (2008). Avian and mammalian CN display many similarities in terms of physiological functional classes, but fewer in terms of neuronal morphology, CN organization, and ascending projections. Further comparative analyses in avian and mammalian species may eventually show how different circuitry may provide distinct solutions to similar functional problems in intensity coding, a topic of current intense discussion in the ITD pathway (Joris & Yin, 2007; McAlpine & Grothe, 2003).
2. Short-term synaptic plasticity in the auditory pathways
Short-term plasticity has distinct properties in the avian brain stem parallel pathways for timing and intensity: short-term depression dominates the ‘timing’ pathway, while the intensity pathway is characterized by a balance of short-term depression and facilitation. The specific time- and activity-dependent mechanisms in each pathway contribute to the division of these two cues into distinct information processing streams. Several good reviews on plasticity in timing pathways are readily available (Grande & Spain, 2005; Schneggenburger et al., 2002; Trussell, 1999; von Gersdorff & Borst, 2002).
2.1 In vitro studies of short-term plasticity show differences between the timing and intensity pathways
2.1.1 Cochlear nucleus: case study of plasticity and function in the avian brain stem
Auditory nerve fibers (ANF) enter the brain stem and bifurcate to terminate onto different regions of the cochlear nuclei. The synaptic terminals differ with a suite of anatomical and physiological specializations that contribute to the differentiation of timing and intensity information. Recent work has shown that short-term plasticity is another mechanism that also contributes to this differentiation. Earlier study of short-term plasticity in auditory circuits described a profound depression at ANF synapses onto NM neurons (Brenowitz & Trussell, 2001a; Brenowitz & Trussell, 2001b; Trussell, 1999; Zhang & Trussell, 1994). The large conductances at these axo-somatic endbulb terminals results in a highly reliable connection necessary to transmit precise timing information. These synapses have a strong depression profile that might be termed ‘classic’: the EPSC amplitude monotonically declines during trains of pulses delivered at a constant frequency until a steady state is reached, and the steady state amplitude declines with respect to the stimulation frequency. The depression at NM is due to vesicle depletion and partially on postsynaptic desensitization at higher frequencies.
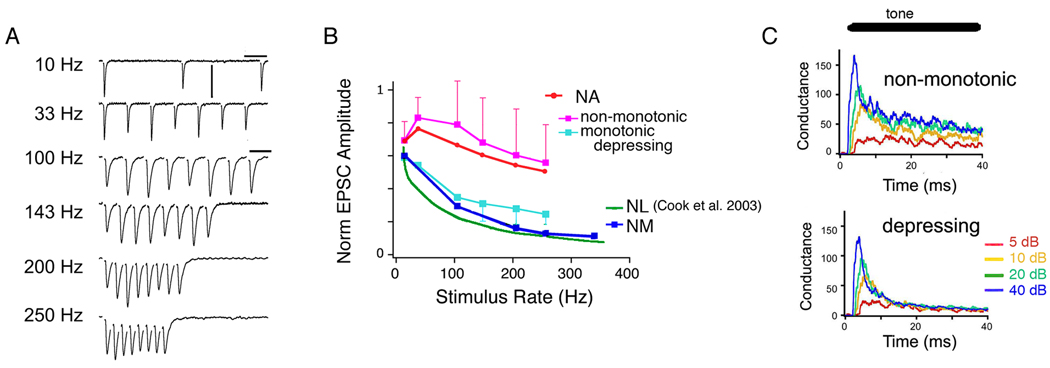
In contrast, ANF synapses onto NA are composed of many smaller inputs delivered at bouton-like synapses (Carr & Boudreau, 1991; MacLeod & Carr, 2005) and it is now known that the plasticity is markedly different from NM as well (MacLeod et al., 2007). A majority of synapses were characterized by a plasticity profile that could be termed ‘balanced’ or ‘mixed-mode’, while a minority, composing about a quarter of synapses, showed depression profiles similar to those in NM. The balanced synapses were more difficult to describe than the ‘classical’ depressing synapses, but had the following characteristics: 1) markedly less depression than in NM, especially at higher frequencies, 2) the presence of some degree of facilitation, and 3) a non-monotonic function of the steady state amplitude with train frequency (Fig. 2). These characteristics are interrelated, of course, and a key feature is the balancing presence of a moderate degree of facilitation that appears to be absent from the ‘classic’ depressing synapses in NM and NA. The net effect of this balance is to produce a consistent response amplitude at steady state over a wide range of presynaptic firing rates. The implications for information transfer can be shown by calculating the total synaptic conductance transmission at steady state for each presynaptic firing rate (MacLeod et al., 2007). A depressing synapse results in a saturating, nonlinear function with rate: beyond a certain point, increasing the presynaptic rates results in less and less increase in synaptic drive. In contrast, the flatter synaptic transfer function at balanced synapses leads to a more linear relationship of total synaptic drive with increasing presynaptic rate. Thus at many NA synapses, the linear transmission of rate is extended over a wide range of physiological firing rates.
Fig. 2. Short-term synaptic plasticity of excitatory postsynaptic currents recorded between auditory nerve afferents and NA neurons.
(A) Excitatory postsynaptic currents (EPSCs) recorded in one NA neuron showing variations in amplitude depending on stimulation rate. EPSCs were pharmacologically isolated AMPA-receptor mediated and evoked using an extracellular electrode in the medial afferent tract. Note scale is different for 10 Hz train.
(B) Summary of the end-of-train normalized EPSC amplitudes versus stimulus train firing rate for different brainstem nuclei. EPSC amplitudes were average for the last several EPSCs in the trains. Data redrawn from MacLeod et al. (2007), except NL curve redrawn from Cook et al. (2003).
(C) Simulated conductance poststimulus histograms showing the effect of short-term plasticity on the intensity-dependent responses.
Modified with permission from APS MacLeod et al (2007) Journal of Neurophysiology 97, 2863–74, and McMillan Publishers Ltd. Cook et al. Nature 421, 66–70, copyright (2003).
Natural auditory nerve spike trains increase in average rate with intensity, but also often have a phasic onset peak following by a tonic component in their postsynaptic time histograms (Saunders et al., 2002). To test whether balanced synapses convey intensity information contained in realistic spikes trains, simulated synaptic responses were calculated using a model of short-term plasticity. This simulation showed that the balanced synapses maintain the intensity information throughout the train, including during the tonic activity (Fig. 2C). In contrast, the same simulation run with depressing synapses virtually eliminated the intensity information contained in the tonic component. Due to the time lag for depression to occur, however, the phasic component of these responses still contained intensity information albeit only in the first 7–10 ms. Depressing synapses are good at enhancing transients and signaling changes (Markram et al., 1998), which suggests that a subset of neurons in NA could signal modulations in sound intensity. In contrast, the depression at synapses in NM may have little functional effect, due to the strength of the overall efficacy.
2.1.2 Short-term synaptic plasticity in the mammalian brain stem
Similar to the avian system, the mammalian nerve axons bifurcate and terminate with both endbulb and bouton-like synapses onto different postsynaptic target populations in the CN. The large endbulb synapses that resemble those in NM also show ‘classical’ short-term depression, including those onto spherical and globular bushy cells, and the calyx of Held at the next stage of processing, where bushy cell axons terminate onto the neurons of the medial nucleus of the trapezoid body (MNTB). Extensive studies of the mechanisms of synaptic release and short-term depression at the MTNB synapse have been made (for review, see Schneggenburger et al., 2002; von Gersdorff & Borst, 2002). Like the synapses in NM, the MNTB synapse is associated with the encoding of precise timing, strengthening the thesis that there is a correlation between short-term depression and timing. However, the very reliability of the synapse means that from the viewpoint of information processing, the depression appears to have little functional consequence in vivo (Lorteije et al., 2009), although it is argued that the high levels of spontaneous activity that occur in vivo would reduce synaptic efficacy to an amplitude range within which depression could have a functional impact (Hermann et al., 2007).
In the ventral cochlear nucleus, bouton-like synapses are made by nerve fibers onto stellate neurons, which are thought to be involved in intensity coding, as well as octopus neurons, which may have a timing role and detect highly synchronous activity. Short-term plasticity at synapses onto stellate neurons shows a much milder depression compared to those onto octopus neurons or bushy cells (Cao & Oertel, 2010; Cao et al., 2008). Experiments that measured entrainment of mammalian CN neurons to electrical stimulation also showed differences by cell type, although this could reflect both synaptic plasticity and intrinsic properties (Babalian et al., 2003). Accurately comparing the information transmission properties across species will require more data on the frequency- and activity-dependence of the plasticity in the CN, along with the isolation of the synaptic EPSC independent of the neuron intrinsic properties with voltage clamp data.
2.1.3 The role of short-term depression in the neural circuits for ITD
If differences in short-term plasticity were correlated with the division of fine-timing versus intensity coding at the level of the CN, one would predict depression to be prevalent at the ITD circuits. In birds, nucleus laminaris (NL) receives direct and bilateral input from the NM, which faithfully transmits precise timing information from either ear. The neurons of NL perform neural coincidence detection and are responsible for the calculation of interaural time differences (ITD) for sound localization (Carr & Konishi, 1990). At these excitatory synapses, strong short-term depression is found, with a firing rate dependence quite similar to that at the ANF-NM synapses (Cook et al., 2003; Kuba et al., 2002a). Similar short-term depression has been described in the mammalian medial superior olive (MSO), the analogous nucleus shown to encode ITD in mammals (Smith et al., 2000). Modeling and in vitro studies of the short-term depression in NL suggests that at this synapse the plasticity can improve the encoding of ITD by counteracting the problems that intensity changes can produce in coincidence detection (Cook et al., 2003; Kuba et al., 2002a). Studies recording ITD tuning in NL shows a high degree of invariance in the face of overall sound intensity and interaural intensity differences (Pena et al., 1996; Viete et al., 1997).
2.1.4 Synaptic plasticity and the ascending intensity pathways
At further levels of the ascending pathway, little systematic study of the short-term plasticity of synapses has been published for either birds or mammals. If balanced synapses are crucial for ILD coding, we would predict such plasticity to be found at the NA inputs to LLDp. Similar arguments could be made for the gain control feedback from the SON to the CN; in fact, inhibitory synaptic inputs from SON to NA were shown to have little depression during high frequency trains (Kuo et al., 2009). As mentioned previously, a notable exception is the extensive study of the bushy cell-MNTB synapse, the calyx of Held, which has been invaluable to the study of transmitter release. However, interesting functional effects of short-term plasticity in terms of neural coding are more likely to be found in circuits that utilize smaller synaptic connections and perform integration across multiple inputs. In section 3, we discuss different hypothetical roles for short-term plasticity in intensity coding.
2.2 Synaptic mechanisms involved in short-term depression
2.2.1 Postsynaptic glutamate receptor desensitization
Most auditory brainstem neurons, including those in the ‘intensity’ pathway, express fast excitatory neurotransmitter receptors that are distinct from many other brain regions (Gardner et al., 1999; Gardner et al., 2001; Levin et al., 1997; MacLeod & Carr, 2005; Raman et al., 1994; Sugden et al., 2002). The postsynaptic receptors are composed primarily of GluR3 and GluR4 subunits, not much GluR2, and contain the ‘flop’ splice variant, a posttranslational modification (Parks, 2000; Petralia et al., 2000). This subunit combination confers rapid kinetics, calcium-permeability, sensitivity to blockade by polyamines, and rapid desensitization in the continued presence of neurotransmitter (Dingledine et al., 1999; Geiger et al., 1995; Mosbacher et al., 1994; Parks, 2000; Sugden et al., 2002). In the timing nuclei, the rapid kinetics assist in phase-locking and coincidence detection. Blockade of desensitization with pharmacological agents such as cyclothiazide or aniracetam prolongs the time course of individual evoked EPSCs in NA and NM. In NA, desensitization has a negligible contribution to short-term depression, as tested with cyclothiazide and aniracetam (MacLeod & Horiuchi, 2011). In the mammalian VCN, cyclothiazide prolongs the EPSCs of stellate and octopus cells but has only a minor effect on short-term depression (Cao & Oertel, 2010). The failure of receptor desensitization to contribute to short-term plasticity under synaptic stimulation at bouton synapses may result from a lower release probability and small terminal size, where reuptake mechanisms may limit accumulation of neurotransmitter in the cleft (Cao et al., 2008; Carr & Boudreau, 1991; Rouiller et al., 1986; Ryugo & Parks, 2003). At the non-endbulb synapses in NL, desensitization also seems to have little effect on short-term depression (Kuba et al., 2002a). In the timing pathway, endbulb synapses release large amounts of neurotransmitter into the synaptic cleft, setting up conditions favorable to desensitization. The role of desensitization at endbulb synapses, however, is more controversial, with some studies suggesting that desensitization is present at immature synapses, greatly reduced at mature synapses, and potentially occluded by saturation, an effect that has not been explicitly excluded at intensity pathway synapses (Brenowitz & Trussell, 2001a; Otis et al., 1996; Wang & Manis, 2008; Wong et al., 2003; Yang & Xu-Friedman, 2008). These results suggest that desensitization shapes the falling phase of the EPSC but is unlikely to contribute meaningfully to short-term plasticity in the intensity pathway.
2.2.2 Activity dependent recovery from depression in the intensity pathway
Presynaptic vesicle depletion is generally credited as the main contributor to short-term depression at auditory brain stem synapses, with postsynaptic desensitization playing a minor role. Recovery from depletion is especially crucial at auditory synapses to maintain responsiveness under wide range of high physiological firing rates, suggesting activity-dependent processes are required. Two observations suggest an activity-dependent component to the recovery from depression. First, at several auditory brain stem synapses studied, recovery curves after stimuli that evoke strong depression exhibit a double exponential trajectory, suggesting multiple processes in the recovery. Second, comparing the recovery following strong versus weak stimulation, the faster component of recovery following strong stimulation causes a larger degree of recovery sooner than that following the weak, resulting in a ‘crossing over’ of their recovery time courses (Brenowitz & Trussell, 2001b; Griesinger et al., 2005; Wang & Kaczmarek, 1998; Wang & Manis, 2008; Yang & Xu-Friedman, 2008). When end-bulb synapses in NM and bouton synapses in NA were directly compared, they showed very similar recovery curves following strong stimulation, including a rapid component (τfast ~40–60 ms) and a slow component (τslow ~3–4 seconds)(MacLeod & Horiuchi, 2011). This suggests that there are common activity-dependent mechanisms that occur at both types of terminals. At NA synapses, recovery curves following weaker stimulation were more complex and the rapid component was not readily apparent, suggesting it was either not present or occluded by some other component. The leading hypothesis to explain the activity-dependence relies on a calcium feedback mechanism accelerating the replenishment process (Dittman et al., 2000; Fuhrmann et al., 2002; Hosoi et al., 2007). Manipulations of calcium levels have been shown to slow down the rapid recovery, such as a reduction in extracellular calcium (Dittman & Regehr, 1998; Wang & Manis, 2008), calcium chelation in the presynaptic terminals (Dittman & Regehr, 1998; Yang & Xu-Friedman, 2008) or the use of calmodulin inhibitors (Hosoi et al., 2007; Sakaba & Neher, 2001; Wang & Manis, 2008).
2.3 Synaptic mechanism of short-term facilitation
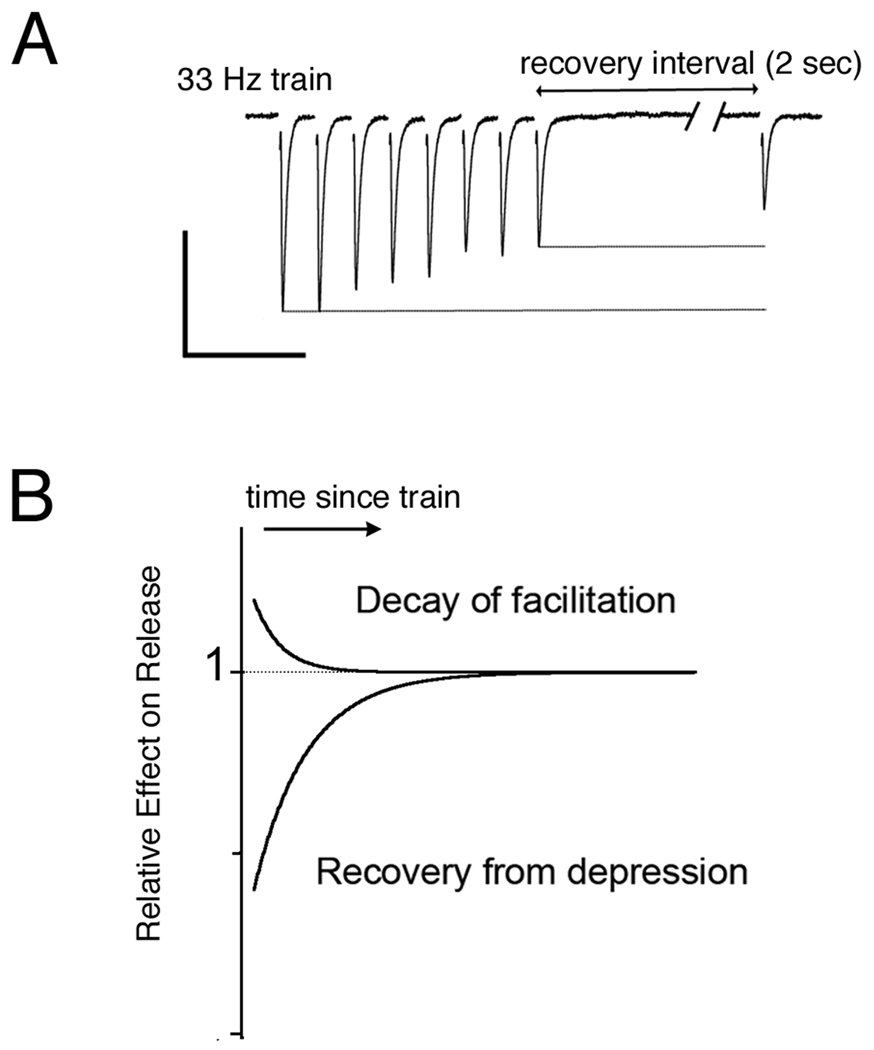
One difference in the synaptic plasticity that was readily apparent between the timing and intensity cochlear nuclei in the avian system was the presence of short-term facilitation at synapses in the intensity pathway. Net facilitation was observed directly in some recordings from NA neurons (Fig. 2). Competing effects of facilitation and depression were also observed when an EPSC elicited after a recovery period was more depressed than the EPSCs during a train; this effect could be explained if the facilitation built up during the train decayed faster than the vesicle depletion recovered (Fig. 3). It is not yet clear why short-term facilitation appears more prevalent at ANF terminals in NA than in NM. If both types of synapses have the same mechanisms for facilitation, perhaps the ‘balanced’ synapses are simply starting with a lower baseline release probability, required for enhanced release probability since high release probabilities result in a ceiling effect. Release probability at NM synapses has been estimated to be as high as ~0.7 in young chicks and falling to ~0.5 with maturation (E18 versus P2–3)(Brenowitz & Trussell, 2001a). In contrast, the plasticity at MNTB synapses is skewed toward depression despite a release probability that has been estimated to be quite low, ~0.3 or less (n.b., obtained with lower bath calcium (2 mM) than in NM study (3 mM) (Iwasaki & Takahashi, 2001; Taschenberger et al., 2002). In both cases, reducing release probability reduces depression (Brenowitz & Trussell, 2001b) and sometimes reveals facilitation (Wong et al., 2003).
Fig. 3. Facilitation and depression both contribute to synaptic amplitudes.
(A) EPSCs evoked during a train and then upon a recovery test pulse delivered with a 2 second delay. The ‘recovery’ EPSC was smaller than at any time during the train. Scale bars: 100 pA vertical, 100 ms horizontal.
(B) A hypothetical explanation for this effect is schematically shown. Two mechanisms at work, one has that enhances the EPSC, facilitation, and one that decreases the EPSC, depression. The net effect on the train EPSCs depends on the relative strength of the two during activity, and the recovery EPSC depends on the relative decay of the effects with time.
Modified with permission from APS MacLeod et al (2007) Journal of Neurophysiology 97, 2863–74.
Another possibility is that the molecular mechanisms involved in facilitation may differ between synapses in NA and NM. First, residual calcium signals may differ. Facilitation is often attributed to residual calcium buildup in the presynaptic terminal following spiking activity (Atluri & Regehr, 1996; Felmy et al., 2003; Zucker, 1999). Calcium binding proteins are highly expressed in the auditory brain stem (Caillard et al., 2000; Kubke et al., 1999; Muller et al., 2007). Differential expression of these proteins could affect the time course of the presynaptic calcium signal and facilitation. Second, calcium influx itself may differ depending on the presence of calcium channels with activity-dependent facilitation (Cuttle et al., 1998). P/Q-type calcium channels show facilitation, but N-type channels do not, and altering their expression alters plasticity (Cuttle et al., 1998; Inchauspe et al., 2007; Inchauspe et al., 2004). Exocytosis at ANF synapses onto NM neurons is mediated mostly by N-type calcium channels (ω-conotoxin GVIA-sensitive) (Lu, 2009; Sivaramakrishnan & Laurent, 1995). If P/Q channels are more prevalent at synapses in NA, this could contribute to greater facilitation. Third, the sensitivity of the release mechanisms to residual calcium may differ. Presynaptic proteins involved in vesicle release, such as neuronal calcium sensor-1, could increase short-term facilitation without affecting baseline release either indirectly by affecting calcium channel facilitation (Tsujimoto et al., 2002) or directly (Sippy et al., 2003).
2.4 Development and short-term synaptic plasticity
For a functional interpretation of the importance of short-term synaptic plasticity effects in vivo, an important caveat is the fact that most of the synaptic data is derived from in vitro slice preparations from relatively young animals. Where developmental effects on plasticity have been investigated or comparable data from older animals is available, there appears to be an overall trend toward weaker short-term depression, less desensitization, and more rapid recovery (Brenowitz & Trussell, 2001a; Taschenberger & von Gersdorff, 2000; Wang & Manis, 2008). These studies have focused on the timing pathways, where depression is the dominant factor, and leaves open the question of age-related effects on facilitation at synapses with mixed depression and facilitation such as in the avian nucleus angularis. If both depression and facilitation diminish with time, then the synaptic transfer function becomes much flatter, leading to highly linear transmission. If depression decreases, but facilitation remains the same or increases, then the synaptic transfer function would change, but it is difficult to predict the effects on transmission without more information on the time- and frequency-dependence of the plasticity. Facilitation could be affected by changes in the expression of calcium-binding proteins, which tend to increase with age (Kubke et al., 1999). Furthermore, changes in the intrinsic properties of the neurons of the auditory nuclei will alter how they integrate their synaptic inputs (Fukui et al., 2003; Kuba et al., 2002b; Taschenberger & von Gersdorff, 2000).
3. Function role for short-term plasticity in intensity coding
3.1 Interaural level difference
The signal for ILD processing starts with a rate code at the level of the ANF. Auditory nerve fibers signal changes in sound intensity with a rate code. Increasing sound level results in monotonic increases in firing with a roughly sigmoidal relationship. In order to compare level from the two ears, the level signal must be maintained up to the site of binaural comparison. In the avian brain, neurons in NA receive direct input from the ANF and send an excitatory projection to LLDp (see Fig. 1), the first site of binaural intensity coding. LLDp neurons are excited by contralateral sound and inhibited by ipsilateral sound and are tuned sigmoidally to interaural level difference (Adolphs, 1993; Manley et al., 1988; Mogdans & Knudsen, 1994; Moiseff & Konishi, 1983; Takahashi & Konishi, 1988). The source of the inhibition is the contralateral LLDp. A similar arrangement of excitatory input from one ear and disynaptic, inhibitory input from the opposite ear can be found in the mammalian LSO (Tollin et al., 2008). In order to make the correct binaural computation, one would expect the intensity information contained in the rate must be transmitted as linearly as possible. Otherwise, the apparent ILD would be reduced due to compression of the coding of the louder sound stimulus by short-term depression in the presence of non-zero ILDs. The balanced synapses found in NA maintain most of the intensity information in natural spike trains. We would predict similar synaptic plasticity at the excitatory inputs to LLDp, as well as at the contralateral inhibitory inputs, if they are providing a simple sign inversion or ‘subtraction’ of the intensity signal from the two ears.
3.2 Inhibitory gain control loop via SON
One of the major outputs from NA is a bilateral projection to the superior olivary nucleus (SON), with the heaviest projection to the ipsilateral SON (Conlee & Parks, 1986; Takahashi & Konishi, 1988). SON neurons provide a major source of inhibitory feedback to NA, NL and NM and form a reciprocal inhibitory loop with the contralateral SON (Burger et al., 2005; Lachica et al., 1994; Yang et al., 1999). The projections from NA to SON carry intensity information and therefore indirectly contribute to the gain control of activity in the ITD pathway (Nishino et al., 2008). The contralateral projections between SON have been proposed to create an ILD-dependent feedback signal that improves the ITD response by reducing the louder NM input to NL and favoring coincident inputs (Burger et al., 2005; Fujita & Konishi, 1991; Pena et al., 1996; Viete et al., 1997). To generate a signal proportional to sound intensity, one would expect the excitatory synaptic inputs to SON from NA, as well as inhibitory synaptic feedback currents, to transmit rate information linearly, as found at ANF-NA synapses. While the synaptic dynamics of the inhibitory inputs to NM and NL have not been described in detail, analysis of 100 Hz trains of IPSCs in NA show little depression (Kuo et al., 2009).
3.3 Amplitude modulation coding
The neural representation of amplitude modulation in the brain is marked by progressive change in the coding scheme along the ascending pathway, starting with a nearly entirely temporal coding scheme at the auditory nerve and ending with a selectively rate-tuned coding scheme for particular best modulation frequencies at the level of the midbrain (Frisina, 2001; Joris et al., 2004). Neurons the mammalian cochlear nucleus have been found to have an intermediate coding scheme with components of rate and temporal coding. Most studies of AM have focused on the mammalian brain, but a similar progression in AM representation can be observed between avian auditory nerve and inferior colliculus in songbirds, although data is lacking in avian CN (Gleich & Klump, 1995; Woolley & Casseday, 2005).
In the mammalian CN, several studies have shown that synchronization is enhanced in a subset of CN neurons, leading to higher gains in the temporal modulation transfer function, a shift from low pass to band pass functions, and improved ability to encode AM at high intensities (Frisina et al., 1985; Frisina et al., 1990a; Frisina et al., 1990b; Moller, 1973; Moller, 1976; Rhode, 1994; Rhode & Greenberg, 1994; Wang & Sachs, 1992). While the mechanisms responsible for these changes are not fully understood, changes in the temporal patterning and overall firing rate (due to changes in modulation frequency and average sound intensity, respectively) could potentially interact with short-term synaptic plasticity present at the auditory nerve to cochlear nucleus connection.
A partial solution to the improved encoding of AM at high intensities in the CN over ANF probably lies in the fact that CN neurons probably receive input from ANFs that have a range of response thresholds and dynamic ranges. At low intensities, low threshold ANFs provide a modulated firing rate signal. As intensity increases, higher-threshold neurons are providing modulated input, but the low threshold neurons saturate their firing rates. At the top of their firing rate range, low threshold fibers no longer modulate. Thus at high intensities, CN neurons receive a high firing rate unmodulated signal and a lower firing rate modulated signal.
Dendritic localization of inputs have been proposed to solve this problem by weighting the inputs of low threshold and higher threshold differently (Hewitt et al., 1992; Wang & Sachs, 1992; Winslow et al., 1987). The dendritic theory proposes that high-threshold ANFs terminate closer to the cell body or spike initiation zone, and low-threshold inputs terminate on more distal dendrites, which could shift the ‘weighting’ toward the high-threshold inputs under high activity conditions. However, analysis of the endings of low spontaneous rate afferents versus high spontaneous rate afferents failed to find any dichotomy in their innervation, and indeed both types terminate on the cell bodies of stellate cells (Rouiller et al., 1986).
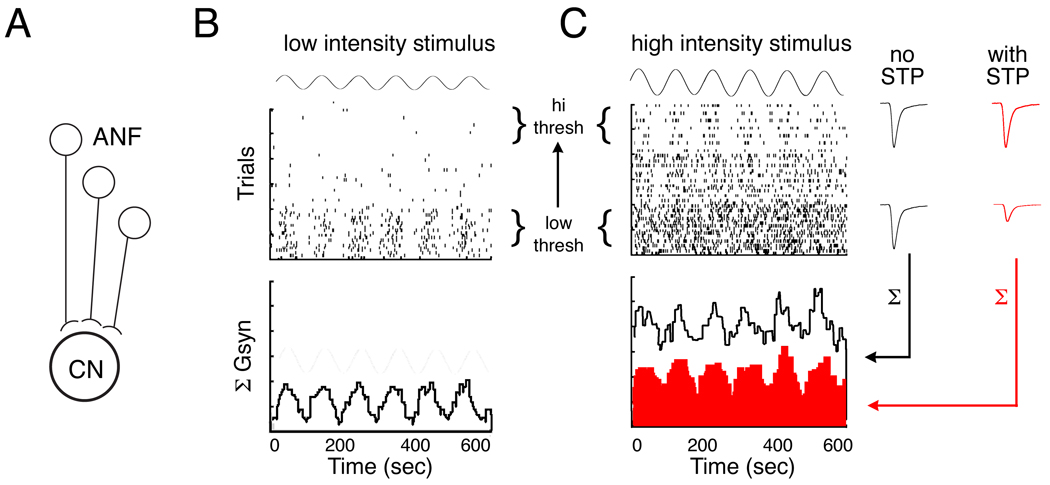
Previous modeling studied of short-term depression suggests that it is capable of several effects that would be useful for auditory signal analysis. One of the properties of short-term depression is to perform an input-specific type of gain control (Abbott et al., 1997). This type of gain control could be useful for enhancing the dynamic range for AM at higher intensities as illustrated in Figure 4. Short-term depression dynamically adjust the gain on high-firing rate inputs to equalize inputs of differing firing rates, (Abbott et al., 1997). This creates a synapse-specific weighting of inputs that is directly proportional to the afferent firing rate itself. AM coding could also be enhanced by a mixture of facilitation and depression which could lead to rate filtering and contribute to a conversion from low-pass to band-pass filtering (Chance et al., 1998).
Fig. 4. Hypothetical role for short-term depression in AM coding.
(A) Cartoon shows the convergence of ANFs of different thresholds onto a postsynaptic CN neuron.
Top graphs in B and C show hypothetical raster plots of the spike times of the ANFs in response to an AM stimulus. Bottom graphs show the conductance histograms as the CN neurons summed input from the ANFs.
(B) With an AM stimulus of low average intensity, the firing rate modulation is only contained in the low threshold fibers, and the conductance input has a large AC:DC ratio.
(C) With an AM stimulus of high average intensity, the high threshold fibers modulate their firing, but low threshold fire continuously. Far right: EPSC amplitudes of low versus high threshold inputs in the absence (black) and presence (red) of short-term plasticity (STP) during high intensity stimulation. Without STP, the conductance input has larger DC component along with the AC component that carries the AM information. With STP, the EPSC amplitudes of the high frequency trains are relatively scaled down, reducing the DC component (red histogram).
3.5 Short-term plasticity in vivo
Most of the data supporting a role for short-term plasticity in neural coding comes from slice studies in vitro. It is critically important to directly assess the role of short-term synaptic plasticity in sensory function in vivo, but very few studies directly assess plasticity in the intact brain. Short-term depression may be important for somatosensory adaptation in cortical circuits (Chung et al., 2002), but in the visual cortical circuits the evidence is more mixed (Boudreau & Ferster, 2005; Carandini et al., 2002; Chance et al., 1998; Usrey et al., 2000). In the brain stem auditory timing pathways a cross-correlation analysis between auditory nerve fibers and CN neurons in vivo was used to examine changes in synaptic strength at short interspike intervals and found no evidence for enhancement or depression that might be the result of short-term plasticity (Lorteije et al., 2009; Young & Sachs, 2008). However, extracellular methods may be limited in their ability to distinguish synaptic function from other circuit or intrinsic mechanisms, and demonstration of the role of short-term plasticity may depend on the use of direct electrical stimulation in vivo coupled with intracellular recordings.
4. Conclusions
The differential regulation at different components of the intensity circuits is an indication that the mechanisms that underlie the plasticity must be specifically regulated and target-dependent. The auditory nerve fibers bifurcate to form radically different morphological axonal endings and distinct synaptic mechanisms with similarities (glutamate receptor subunit composition, vesicle replenishment kinetics) but also some critical differences (basal release probability, tendency to facilitate, postsynaptic desensitization). At these circuits short-term synaptic depression could perform a role complementary to inhibitory feedback. Short-term plasticity works in a synapse-specific way to differentially influence each individual input based on its own presynaptic activity. In contrast the inhibitory feedback likely integrates input across a broad population, and delivers a universal suppression signal. The variation in short-term plasticity observed in different circuits within the auditory pathways suggests that a broader analysis of these synapse will lead to important discoveries illuminating the role of short-term synaptic plasticity in neural coding.
Abbreviations
- NA
nucleus angularis
- NM
nucleus magnocellularis
- NL
nucleus laminaris
- SON
superior olivary nucleus
- LLDa
dorsal nucleus of the lateral lemniscus, anterior
- LLDp
dorsal nucleus of the lateral lemniscus, posterior
- LLV
ventral nucleus of the lateral lemniscus
- IC
inferior colliculus (also referred to as MLD in avian species)
- MLD
nucleus mesencephalicus lateralis (also referred to as IC)
- ILD
interaural level difference
- ITD
interaural time difference
- CN
cochlear nucleus
- AVCN
anterior ventral cochlear nucleus
- DCN
dorsal cochlear nucleus
- MNTB
medial nucleus of the trapezoid body
- LSO
lateral superior olive
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartate
- GABA
gamma-aminobutyric acid
- EPSC
excitatory postsynaptic current
- EPSP
excitatory postsynaptic potential
- IPSP
inhibitory postsynaptic potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Bilateral inhibition generates neuronal response tuned to interaural level differences in the auditory brainstem of the barn owl. J Neurosci. 1993;13:3647–3668. doi: 10.1523/JNEUROSCI.13-09-03647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends JJ, Zeigler HP. Anatomical identification of an auditory pathway from a nucleus of the lateral lemniscal system to the frontal telencephalon (nucleus basalis) of the pigeon. Brain Res. 1986;398:375–381. doi: 10.1016/0006-8993(86)91499-x. [DOI] [PubMed] [Google Scholar]
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalian AL, Ryugo DK, Rouiller EM. Discharge properties of identified cochlear nucleus neurons and auditory nerve fibers in response to repetitive electrical stimulation of the auditory nerve. Exp Brain Res. 2003;153:452–460. doi: 10.1007/s00221-003-1619-x. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci. 2005;25:7179–7190. doi: 10.1523/JNEUROSCI.1445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci. 2001a;21:9487–9498. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Minimizing synaptic depression by control of release probability. J Neurosci. 2001b;21:1857–1867. doi: 10.1523/JNEUROSCI.21-06-01857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol. 2005;481:6–18. doi: 10.1002/cne.20334. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength and short-term plasticity. J Neurophysiol. 2010;104:2308–2320. doi: 10.1152/jn.00451.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, McGinley MJ, Oertel D. Connections and synaptic function in the posteroventral cochlear nucleus of deaf jerker mice. J Comp Neurol. 2008;510:297–308. doi: 10.1002/cne.21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci. 2002;22:10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Central projections of auditory nerve fibers in the barn owl. J Comp Neurol. 1991;314:306–3018. doi: 10.1002/cne.903140208. [DOI] [PubMed] [Google Scholar]
- Carr CE, Soares D. Evolutionary convergence and shared computational principles in the auditory system. Brain Behav Evol. 2002;59:294–311. doi: 10.1159/000063565. [DOI] [PubMed] [Google Scholar]
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci. 1998;18:4785–4799. doi: 10.1523/JNEUROSCI.18-12-04785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contribute to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Parks TN. Origin of ascending auditory projections to the nucleus mesencephalicus lateralis pars dorsalis in the chicken. Brain Res. 1986;367:96–113. doi: 10.1016/0006-8993(86)91583-0. [DOI] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature. 2003;421:66–70. doi: 10.1038/nature01248. [DOI] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol. 1998;512(Pt 3):723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001;158:1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Smith RL, Chamberlain SC. Differential encoding of rapid changes in sound amplitude by second-order auditory neurons. Exp Brain Res. 1985;60:417–422. doi: 10.1007/BF00235939. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Smith RL, Chamberlain SC. Encoding of amplitude modulation in the gerbil cochlear nucleus: I. A hierarchy of enhancement. Hear Res. 1990a;44:99–122. doi: 10.1016/0378-5955(90)90074-y. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Smith RL, Chamberlain SC. Encoding of amplitude modulation in the gerbil cochlear nucleus: II. Possible neural mechanisms. Hear Res. 1990b;44:123–141. doi: 10.1016/0378-5955(90)90075-z. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Segev I, Markram H, Tsodyks M. Coding of temporal information by activity-dependent synapses. J Neurophysiol. 2002;87:140–148. doi: 10.1152/jn.00258.2001. [DOI] [PubMed] [Google Scholar]
- Fujita I, Konishi M. The role of GABAergic inhibition in processing of interaural time difference in the owl's auditory system. J Neurosci. 1991;11:722–739. doi: 10.1523/JNEUROSCI.11-03-00722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I, Ohmori H. Developmental changes in membrane excitability and morphology of neurons in the nucleus angularis of the chicken. J Physiol. 2003;548:219–232. doi: 10.1113/jphysiol.2002.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. J Neurosci. 1999;19:8721–8729. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci. 2001;21:7428–7437. doi: 10.1523/JNEUROSCI.21-18-07428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gleich O, Klump GM. Temporal modulation transfer functions in the European starling (Sturnus vulgaris): II. Responses of auditory-nerve fibres. Hearing Research. 1995;82:81–92. doi: 10.1016/0378-5955(94)00168-p. [DOI] [PubMed] [Google Scholar]
- Grande LA, Spain WJ. Synaptic depression as a timing device. Physiology. 2005;20:201–210. doi: 10.1152/physiol.00006.2005. [DOI] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435:212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol. 2007;98:807–820. doi: 10.1152/jn.00355.2007. [DOI] [PubMed] [Google Scholar]
- Hewitt MJ, Meddis R, Shackleton TM. A computer model of a cochlear-nucleus stellate cell: responses to amplitude-modulated and pure-tone stimuli. J Acoust Soc Am. 1992;91:2096–2109. doi: 10.1121/1.403696. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Sakaba T, Neher E. Quantitative analysis of calcium-dependent vesicle recruitment and its functional role at the calyx of Held synapse. J Neurosci. 2007;27:14286–14298. doi: 10.1523/JNEUROSCI.4122-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson RL. The analysis of interaural time differences in the chick brain stem. Physiol Behav. 2005;86:297–305. doi: 10.1016/j.physbeh.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe CG, Forsythe ID, Uchitel OD. Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels. J Physiol. 2007;584:835–851. doi: 10.1113/jphysiol.2007.139683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of held presynaptic terminal. J Neurosci. 2004;24:10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–871. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci. 2007;30:70–78. doi: 10.1016/j.tins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Konishi M, Pettigrew JD. Receptive fields of auditory neurons in the owl. Science. 1977;198:1278–1280. doi: 10.1126/science.929202. [DOI] [PubMed] [Google Scholar]
- Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- Konishi M, Sullivan WE, Takahashi T. The owl's cochlear nuclei process different sound localization cues. J Acoust Soc Am. 1985;78:360–364. doi: 10.1121/1.392499. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Computational diversity in the cochlear nucleus angularis of the barn owl. J Neurophysiol. 2003;89:2313–2329. doi: 10.1152/jn.00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. I. Projections of nucleus angularis and nucleus laminaris to the auditory torus. J Comp Neurol. 2010a;518:2109–2134. doi: 10.1002/cne.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010b;518:2135–2148. doi: 10.1002/cne.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Synaptic depression improves coincidence detection in the nucleus laminaris in brainstem slices of the chick embryo. Eur J Neurosci. 2002a;15:984–990. doi: 10.1046/j.1460-9568.2002.01933.x. [DOI] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Development of membrane conductance improves coincidence detection in the nucleus laminaris of the chicken. J Physiol. 2002b;540:529–542. doi: 10.1113/jphysiol.2001.013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Bradley LA, Trussell LO. Heterogeneous kinetics and pharmacology of synaptic inhibition in the chick auditory brainstem. J Neurosci. 2009;29:9625–9634. doi: 10.1523/JNEUROSCI.0103-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Gauger B, Basu L, Wagner H, Carr CE. Development of calretinin immunoreactivity in the brainstem auditory nuclei of the barn owl (Tyto alba) J Comp Neurol. 1999;415:189–203. doi: 10.1002/(sici)1096-9861(19991213)415:2<189::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lachica EA, Rubsamen R, Rubel EW. GABAergic terminals in nucleus magnocellularis and laminaris originate from the superior olivary nucleus. J Comp Neurol. 1994;348:403–418. doi: 10.1002/cne.903480307. [DOI] [PubMed] [Google Scholar]
- Levin MD, Kubke MF, Schneider M, Wenthold R, Carr CE. Localization of AMPA-selective glutamate receptors in the auditory brainstem of the barn owl. J Comp Neurol. 1997;378:239–253. doi: 10.1002/(sici)1096-9861(19970210)378:2<239::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci. 2009;29:13770–13784. doi: 10.1523/JNEUROSCI.3285-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Regulation of glutamatergic and GABAergic neurotransmission in the chick nucleus laminaris: role of N-type calcium channels. Neuroscience. 2009;164:1009–1019. doi: 10.1016/j.neuroscience.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod KM, Carr CE. Synaptic physiology in the cochlear nucleus angularis of the chick. J Neurophysiol. 2005;93:2520–2529. doi: 10.1152/jn.00898.2004. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Carr CE. Beyond timing in the auditory brainstem: intensity coding in the avian cochlear nucleus angularis. Prog Brain Res. 2007;165:123–133. doi: 10.1016/S0079-6123(06)65008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod KM, Horiuchi TK. A rapid form of activity-dependent recovery from short-term synaptic depression in the intensity pathway of the auditory brainstem. Biological Cybernetics. 2011 doi: 10.1007/s00422-011-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod KM, Horiuchi TK, Carr CE. A role for short-term synaptic facilitation and depression in the processing of intensity information in the auditory brain stem. J Neurophysiol. 2007;97:2863–2874. doi: 10.1152/jn.01030.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Koppl C, Konishi M. A neural map of interaural intensity differences in the brain stem of the barn owl. J Neurosci. 1988;8:2665–2676. doi: 10.1523/JNEUROSCI.08-08-02665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gupta A, Uziel A, Wang Y, Tsodyks M. Information processing with frequency-dependent synaptic connections. Neurobiol Learn Mem. 1998;70:101–112. doi: 10.1006/nlme.1998.3841. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Grothe B. Sound localization and delay lines--do mammals fit the model? Trends Neurosci. 2003;26:347–350. doi: 10.1016/S0166-2236(03)00140-1. [DOI] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Representation of interaural level difference in the VLVp, the first site of binaural comparison in the barn owl's auditory system. Hear Res. 1994;74:148–164. doi: 10.1016/0378-5955(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Binaural characteristics of units in the owl's brainstem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci. 1983;3:2553–2562. doi: 10.1523/JNEUROSCI.03-12-02553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR. Coding of amplitude moduled sounds inthe cochlear nucelus of the rat. In: Moller AR, editor. Basic Mechanisms of Hearing. New York: Academic Press; 1973. pp. 593–617. [Google Scholar]
- Moller AR. Dynamic properties of primary auditory fibers compared with cells in the cochlear nucleus. Acta physiologica Scandinavica. 1976;98:157–167. doi: 10.1111/j.1748-1716.1976.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Monsivais P, Yang L, Rubel EW. GABAergic inhibition in nucleus magnocellularis: implications for phase locking in the avian auditory brainstem. J Neurosci. 2000;20:2954–2963. doi: 10.1523/JNEUROSCI.20-08-02954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27:2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci. 2008;28:7153–7164. doi: 10.1523/JNEUROSCI.4398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN. The AMPA receptors of auditory neurons. Hear Res. 2000;147:77–91. doi: 10.1016/s0378-5955(00)00122-2. [DOI] [PubMed] [Google Scholar]
- Pena JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci. 1996;16:7046–7054. doi: 10.1523/JNEUROSCI.16-21-07046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res. 2000;147:59–69. doi: 10.1016/s0378-5955(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Raman IM, Zhang S, Trussell LO. Pathway-specific variants of AMPA receptors and their contribution to neuronal signaling. J Neurosci. 1994;14:4998–5010. doi: 10.1523/JNEUROSCI.14-08-04998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Temporal coding of 200% amplitude modulated signals in the ventral cochlear nucleus of cat. Hear Res. 1994;77:43–68. doi: 10.1016/0378-5955(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Greenberg S. Encoding of amplitude modulation in the cochlear nucleus of the cat. J Neurophysiol. 1994;71:1797–1825. doi: 10.1152/jn.1994.71.5.1797. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Cronin-Schreiber R, Fekete DM, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats: an analysis of terminal morphology. J Comp Neurol. 1986;249:261–278. doi: 10.1002/cne.902490210. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Parks TN. Primary innervation of the avian and mammalian cochlear nucleus. Brain Res Bull. 2003;60:435–456. doi: 10.1016/s0361-9230(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Saunders J, Ventetuolo C, Plontke S, Weiss B. Coding of sound intensity in the chick cochlear nerve. J Neurophysiol. 2002;88:2887–2898. doi: 10.1152/jn.00381.2002. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Sippy T, Cruz-Martin A, Jeromin A, Schweizer FE. Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor-1. Nat Neurosci. 2003;6:1031–1038. doi: 10.1038/nn1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Laurent G. Pharmacological characterization of presynaptic calcium currents underlying glutamatergic transmission in the avian auditory brainstem. J Neurosci. 1995;15:6576–6585. doi: 10.1523/JNEUROSCI.15-10-06576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol 529 Pt. 2000;3:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D, Carr CE. The cytoarchitecture of the nucleus angularis of the barn owl (Tyto alba) J Comp Neurol. 2001;429:192–205. doi: 10.1002/1096-9861(20000108)429:2<192::aid-cne2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Soares D, Chitwood RA, Hyson RL, Carr CE. Intrinsic neuronal properties of the chick nucleus angularis. J Neurophysiol. 2002;88:152–162. doi: 10.1152/jn.2002.88.1.152. [DOI] [PubMed] [Google Scholar]
- Sugden SG, Zirpel L, Dietrich CJ, Parks TN. Development of the specialized AMPA receptors of auditory neurons. J Neurobiol. 2002;52:189–202. doi: 10.1002/neu.10078. [DOI] [PubMed] [Google Scholar]
- Sullivan WE. Classification of response patterns in cochlear nucleus in the barn owl: correlation with functional response properties. J Neurophysiol. 1985;53:201–216. doi: 10.1152/jn.1985.53.1.201. [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the barn owl. J Neurosci. 1984;4:1787–1799. doi: 10.1523/JNEUROSCI.04-07-01787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Lyons SA, Dobrunz LE. Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats. J Physiol. 2005;568:815–840. doi: 10.1113/jphysiol.2005.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Moiseff A, Konishi M. Time and intensity cues are processed independently in the auditory system of the owl. J Neurosci. 1984;4:1781–1786. doi: 10.1523/JNEUROSCI.04-07-01781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol. 1988;274:212–238. doi: 10.1002/cne.902740207. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing Synaptic Architecture and Efficiency for High-Frequency Transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K, Tsai JJ. Interaural level difference discrimination thresholds for single neurons in the lateral superior olive. J Neurosci. 2008;28:4848–4860. doi: 10.1523/JNEUROSCI.5421-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alonso JM, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J Neurosci. 2000;20:5461–5467. doi: 10.1523/JNEUROSCI.20-14-05461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viete S, Pena JL, Konishi M. Effects of interaural intensity difference on the processing of interaural time difference in the owl's nucleus laminaris. J Neurosci. 1997;17:1815–1824. doi: 10.1523/JNEUROSCI.17-05-01815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wang X, Sachs MB. Coding of envelope modulation in the auditory nerve and anteroventral cochlear nucleus. Philos Trans R Soc Lond B Biol Sci. 1992;336:399–402. doi: 10.1098/rstb.1992.0074. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol. 2008;100:1255–1264. doi: 10.1152/jn.90715.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Krutzfeldt NO, Kubke MF. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. III. Projections of the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2149–2167. doi: 10.1002/cne.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RL, Barta PE, Sachs MB. Rate coding in the auditory-nerve. In: Yost WA, Watson CS, editors. Auditory processing of complex signals. Hillsdalte: Lawrence Erlbaum Associates; 1987. pp. 212–224. [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci. 2003;23:4868–4877. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Casseday JH. Processing of modulated sounds in the zebra finch auditory midbrain: responses to noise, frequency sweeps, and sinusoidal amplitude modulations. J Neurophysiol. 2005;94:1143–1157. doi: 10.1152/jn.01064.2004. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol. 2008;99:2510–2521. doi: 10.1152/jn.01293.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Monsivais P, Rubel EW. The superior olivary nucleus and its influence on nucleus laminaris: a source of inhibitory feedback for coincidence detection in the avian auditory brainstem. J Neurosci. 1999;19:2313–2325. doi: 10.1523/JNEUROSCI.19-06-02313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Auditory nerve inputs to cochlear nucleus neurons studied with cross-correlation. Neuroscience. 2008;154:127–138. doi: 10.1016/j.neuroscience.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol. 1994;480:123–136. doi: 10.1113/jphysiol.1994.sp020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]