Abstract
Lymphocytes such as T cells, B cells and natural killer (NK) cells form specialized contacts, called immunological synapses, with other cells in order to engage in specific intercellular communication and killing. Synapse formation is associated with the polarization of the microtubule-organizing center (MTOC) toward the contact site, which enables the directional secretion of cytokines and lytic factors. Although MTOC reorientation to the synapse is crucial for lymphocyte function, it has been difficult to study because of technical constraints. We have developed a photoactivation and imaging strategy that enables high-resolution analysis of cytoskeletal dynamics in individual T cells. Using this approach, we have demonstrated that the lipid second messenger diacylglycerol plays a crucial role in promoting MTOC reorientation by recruiting three members of the protein kinase C family to the synapse. Here, I will discuss these results along with studies from other labs, which have explored the role of polarity-inducing protein complexes after synapse formation. I will also propose a two-step model for MTOC reorientation in lymphocytes that reflects what we now know about the subject. Finally, I will consider the extent to which lymphocyte polarity resembles analogous cell polarity systems in other cell types.
Key words: polarity, T cell, microtubule, cytoskeleton, signaling, lymphocyte, chemical biology
Cell polarity is a precondition of multicellular lifestyle. Polarized cells interact with their surroundings in a fundamentally anisotropic manner, which is crucial for establishing systems, such as neuronal circuits, in which there is directional flow of information. Cell polarity is also required for asymmetric cell division, cell migration and the formation of epithelia, which together facilitate the development of complex tissues.
It is becoming increasingly clear that cell polarity also plays a central role in lymphocyte function,1,2 a fact that belies the textbook depiction of lymphocytes as featureless and spherically symmetric. While patrolling secondary lymphoid organs and peripheral tissues, lymphocytes adopt a “hand-mirror” configuration consisting of a lamellipodial leading edge followed by a stalk-like uropod (Fig. 1). In this manner, they survey the surfaces of other cells for molecular indicators of pathology. T cells and B cells bind to antigenic peptides and proteins, respectively, while NK cells sense surface markers of cellular distress. Recognition of any of these components leads to the formation of a specialized cell-cell contact between the lymphocyte and the target cell called an immunological synapse (IS),3 which is accompanied by a dramatic change in cellular morphology. First, the lymphocyte gloms onto the side of the target cell, forming a radially symmetric contact that is sealed by a dense ring of actin and integrins. Then, the MTOC or centrosome, of the lymphocyte moves to a position just beneath the interface. MTOC reorientation effectively aligns the lymphocytes' secretory apparatus with the IS, thereby enabling the release of soluble factors directionally toward the target cell.2 This is crucial from maintaining the specificity of secretory responses. For example, MTOC reorientation is the reason cytotoxic T cells and NK cells can specifically kill target cells without damaging the surrounding tissue. In addition, several recent studies have suggested that T cells undergo asymmetric cell division in response to antigenic stimulation by dendritic cells.4,5 In this context, polarization of the MTOC would presumably be important for establishing a division plane parallel to the IS.
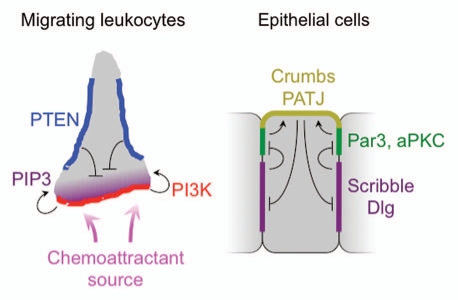
Figure 1.
Left, schematic diagram of lipid-based direction sensing in migrating leukocytes. The accumulation of PIP3 (purple) at the leading edge is maintained by the coordinated activities of PI-3 kinases (red) and lipid phosphatases (e.g., PTEN, blue), which localize to the leading and lateral edges, respectively. Right, schematic diagram summarizing the interactions between polarity complexes in polarized epithelial cells. The Par complex (green) localizes to adherens junctions, and promotes the recruitment of the Crumbs/PATJ complex (yellow) to the apical membrane. The Scrib complex (purple) accumulates on basolateral membranes, and inhibits the spreading of the Par complex. The Crumbs/PATJ complex, in turn, inhibits the spreading of the Scrib complex.
MTOC reorientation to the IS was first characterized in T cells close to 30 years ago.6,7 It has been difficult to study, however because the process occurs so quickly (<5 minutes) and because lymphocytes are so small. In the intervening years, however, considerable progress has been made toward understanding cell polarity in more tractable systems such as fibroblasts, astrocytes and epithelial cells.8,9 Studies in these cell types have indentified a number of distinct protein complexes that accumulate in a polarized manner within defined regions of the plasma membrane (Fig. 1). The mutual inhibition of some complexes by others acts to establish and stabilize the polarized state. Interestingly, migrating fibroblasts, astrocytes and neurons reorient their MTOC toward the leading edge of the cell, and it has been tempting to speculate that the machinery used for MTOC polarization in these systems is shared by lymphocytes. It is important to note, however, that in adherent cell types polarity is established slowly, over a period of hours, and that it can persist for days or even longer. Lymphocyte polarity, by contrast, is highly dynamic and often transient. Hence, it is not unreasonable to expect that distinct molecular mechanisms are at work during MTOC polarization to the IS.
Our lab uses a combination of photochemistry and single cell imaging to examine lymphocyte signaling and cytoskeletal dynamics with high spatial and temporal resolution. Below, I will discuss recent progress we have made toward understanding the molecular mechanisms that drive MTOC reorientation to the IS in T cells. I will then attempt to place this work in the context of what is known about polarity in other cell types, and speculate about the extent to which molecular pathways and design concepts derived from other systems can be used to guide future studies in lymphocytes.
T Cell Receptor Photoactivation provides Spatiotemporal Control
Our approach is based on a “photoactivatable” peptide-major histocompatibility complex (pMHC) reagent that binds to its cognate T cell receptor (TCR) only after irradiation with ultraviolet (UV) light (Fig. 2).10,11 T cells expressing the 5C.C7 TCR bind specifically to a peptide derived from moth cytochrome C (MCC, a.a. 88–103) in the context of the mouse class II MHC protein I-Ek. We attached a photocleavable ortho-nitrophenylethyl urethane (NPE) group to a lysine residue in the MCC peptide that is crucial for TCR recognition. I-Ek bearing this peptide does not bind to the 5C.C7 TCR. Upon UV irradiation, however, the NPE group detaches, allowing TCR stimulation to occur.
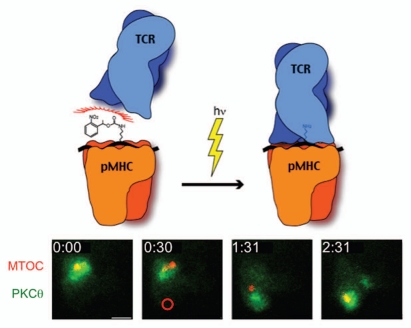
Figure 2.
Photoactivation of the TCR induces MTOC reorientation. Above, schematic diagram of the photoactivation strategy, which involves UV-induced cleavage of an NPE group attached to a central lysine in the MCC peptide. Below, a timelapse montage from a TCR photoactivation experiment showing a T cell expressing GFP-labeled PKCθ and RFP-labeled α-tubulin (to visualize the MTOC). Time (in min) is shown in the top left corner of each image. The region of UV irradiation, which was applied at the 30 s timepoint, is indicated by a red circle. Scale bar = 5 µm.
For most of our experiments, photoactivatable pMHC is immobilized on a glass coverslip along with a protein to promote T cell adhesion (typically ICAM-1 or an antibody against a T cell surface marker). Primary T cells expressing the 5C.C7 TCR together with fluorescent signaling probes (usually a GFP or RFP-labeled signaling proteins) are then attached to the coverslip and imaged (Fig. 2). During the imaging experiment, a source of focused UV light is used to generate a micron-sized region of activated pMHC beneath the T cell. Signaling and cytoskeletal responses are then monitored using either epifluorescence or total internal reflection fluorescence (TIRF) microscopy. The MTOC typically reorients to the position of UV stimulation in less than two minutes.11,12 The ability to control TCR stimulation spatially and temporally and to follow responses in real time has enabled us to dissect molecular mechanisms with unprecedented resolution. Using this approach, it is possible to distinguish events that occur within five seconds of each other. Thus, a very fine order of operations can be established, greatly facilitating the interpretation of loss-of-function experiments and other perturbation studies.
Diacylglycerol Couples Early TCR Signaling to Cytoskeletal Remodeling
It has been known for some time that MTOC reorientation to the IS depends on TCR stimulation.13 Indeed, the response can distinguish between antigen-presenting cells containing different amounts of agonist pMHC, polarizing preferentially toward the cell with more antigen.11,14 Accordingly, proteins involved in early TCR signaling, including the Src kinase Lck, the Syk kinase Zap70, and the scaffolding proteins LAT and SLP76, were all shown to be required for MTOC reorientation.15,16 However, these molecules are important for all aspects of the TCR signaling response, and knowing that they are involved in MTOC reorientation sheds little light on the molecular mechanisms that couple early TCR signaling specifically to cytoskeletal remodeling.
One of the most important effector enzymes recruited to the LAT-SLP76 complex by TCR signaling is phospholipase C-γ (PLC-γ), which hydrolyzes phosphatidyl-inositol bis-phosphate (PIP2) to yield two second messengers, inositol tris-phosphate (IP3) and diacylglycerol (DAG). IP3 stimulates the influx of calcium (Ca2+) into the cytoplasm, while DAG recruits proteins to the plasma membrane that contain “typical” C1 domains. Because DAG accumulates specifically in the IS after TCR stimulation, we investigated whether it might play an instructive role in guiding the polarization of the MTOC.12 Using the C1 domains of protein kinase C-δ (PKCδ) as a biosensor for DAG, we were able to show in TCR photoactivation experiments that DAG accumulates at the site of TCR stimulation ′10 s prior to MTOC reorientation. A small molecule inhibitor of PLC-γ blocked the polarization response, consistent with a role for localized DAG in this process. Stimulation of unpolarized DAG-dependent signaling with phorbol myristate acetate (PMA) completely disrupted MTOC reorientation. Furthermore, inhibition of DAG kinases (DGKs), which convert DAG into phosphatidic acid, destabilized synaptic DAG accumulation and impaired MTOC recruitment to the IS. In contrast, blocking Ca2+ signaling with extracellular and intracellular chelators had no effect on polarization. Hence, it is DAG signaling, and not Ca2+, that plays the operative role in this pathway downstream of PLC-γ.
To further explore the mechanisms by which DAG influences the MTOC, we focused next on the PKC family of enzymes, which have been implicated in polarity induction in multiple cell types. PKCs can be divided into three subfamilies based on their regulatory properties.17 Classical PKCs (cPKCs) require both DAG and Ca2+ for activation, novel PKCs (nPKCs) require DAG but not Ca2+, and atypical PKCs (aPKCs) require neither DAG nor Ca2+. Because DAG, but not Ca2+, is necessary for MTOC reorientation,12 we chose to investigate the nPKC isoforms, of which there are four: PKCδ, PKCε, PKCη and PKCθ. Of these, PKCθ was known to be involved in TCR signaling, having been implicated previously in transcriptional activation and the upregulation of integrin-mediated adhesion.18,19 Less was known about the other three proteins. Indeed, some reports suggested that PKCε and PKCη played no part in the TCR signaling network.20,21
Using our photoactivation and imaging approach, we demonstrated that PKCε, PKCη, and PKCθ, but not PKCδ, are recruited to the IS in an ordered cascade (Fig. 3).22 Approximately 15 seconds before MTOC reorientation, PKCε and PKCη accumulate in a broad region of membrane centered at the site of TCR stimulation. PKCθ is recruited ∼5 seconds later, and it occupies a more restricted zone that is fully contained within the region of PKCε and PKCη accumulation. To explore the functional relevance of these three enzymes, we employed siRNA knockdown and also made use of available knockout mice. In this manner, we showed that PKCθ is required for optimal MTOC reorientation, and that PKCε and PKCη function redundantly with each other to promote PKCí recruitment and subsequent cytoskeletal polarization. Redundancy between PKCε and PKCη is consistent with their observed similarities in recruitment pattern, and possibly explains why PKCε knockout mice display no obvious defect in T cell activation.20
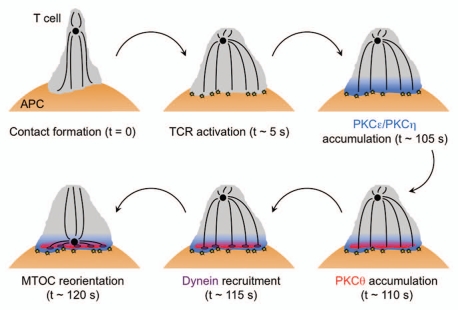
Figure 3.
Schematic diagram showing the molecular events leading to MTOC reorientation toward the T cell IS. The MTOC is shown as a black circle. TCR activation is indicated by yellow stars and dynein by purple ovals. APC, antigen presenting cell.
Precisely how DAG and the nPKCs influence the molecular machinery that actually moves the MTOC remains unknown. It is generally thought that MTOC reorientation is mediated by cytoplasmic dynein, the preeminent minus end-directed microtubule motor. Dynein participates in MTOC positioning in multiple cell types,8 and we and others have observed that it accumulates at the IS in response to TCR stimulation.12,23,24 Dynein recruitment occurs 5–10 seconds after DAG first appears, and it requires a stable DAG gradient (Fig. 3).12 Taken together, these observations suggest that dynein operates downstream of DAG in this pathway. How DAG and the nPKCs are linked to dynein is unclear, and is an area of active research. There are a number of intriguing candidate molecules for this role, including the scaffolding protein ADAP, which binds to both dynein and SLP76, and the formin mDia, which regulates actin and microtubule polarization in multiple cell types. Both proteins have been implicated in T cell MTOC reorientation,23,25 and it will be important to decipher how they function in relation to the DAG-dependent pathway we have characterized.
Polarity Complexes Stabilize the Polarized State
Cell polarity in adherent cell types depends on a number of evolutionarily conserved protein complexes.9 Among the best studied are the Par (for partitioning defective) complex, consisting of the adaptor proteins Par3 and Par6 together with aPKC; and the Scrib complex, consisting of the adaptor proteins Scribble, Discs-large (Dlg), and Lethal giant larvae (Lgl). The components of these complexes contain numerous protein-protein interaction domains, enabling them to associate with specific cell surface proteins and cytoskeletal structures. In this manner, they organize distinct membrane domains that subsequently become polarized to different parts of the cell surface due to the mutual inhibition of each other's growth. In polarized epithelial cells, for example, the Scrib complex accumulates on the basolateral surface, while the Par complex associates with the adherens junctions separating the apical and basolateral domains (Fig. 1). Disruption of either complex leads to a breakdown in cell polarity.
The observation that synaptically polarized lymphocytes, like epithelial cells, partition their membranes into distinct domains has led a number of labs to investigate the roles of polarity complexes during IS formation in T cells. Immunocytochemical studies have demonstrated that Par3 and phosphorylated PKCζ accumulate at the IS while Scribble and Dlg localize to the back of the cell.26,27 Synaptic recruitment of Par3 is consistent with other work showing that the kinase Par1b, which inhibits Par3 function, dissociates from the plasma membrane in response to TCR stimulation.28 Interestingly, polarized accumulation of the Par and Scrib complexes was only observed after 30 minutes of conjugation, well beyond the time required for MTOC reorientation to the IS (Fig. 4). This temporal discordance suggests that Par and Scrib components may not be involved in the initial polarization event. Nevertheless, functional experiments have indicated that they are required for T cell polarity at some level. Pharmacological inhibition or siRNA knockdown of PKCζ impaired MTOC reorientation,26 as did expression of dominant negative forms of Par1b.28 Knockdown of Scribble also disrupted MTOC localization to the IS, although this result may have been secondary to a profound adhesion defect observed in these T cells.27 Importantly, the position of the MTOC was scored at relatively late timepoints (>20 minutes after TCR stimulation) in all of these studies, leaving open the possibility that initial polarization did occur.
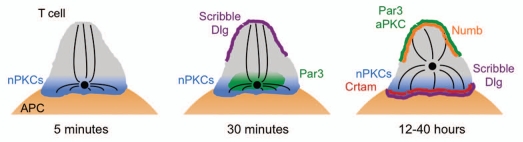
Figure 4.
Schematic diagram showing the accumulation of polarity complexes as the T cell IS matures over a period of hours. The MTOC is shown as a black circle. APC, antigen presenting cell.
When taken together with the delayed recruitment behavior of Par and Scrib components, these results suggest that polarity complexes may be important for long-term maintenance of the polarized state. Hence, MTOC reorientation to the IS can be divided into two stages: a direction-sensing phase driven by DAG and nPKCs, followed by a stabilization phase that requires the Par and Scrib complexes (Fig. 4). It will be interesting to determine how and when activated T cells transition from the first to the second phase of polarization. It is conceivable that sustained DAG and PKC signaling at the IS could induce the recruitment of polarity complexes. It is also possible that the MTOC, after moving to the IS, could itself trigger the requisite signaling events. The centrosome contains a large number of unique signaling proteins, and the close apposition of these proteins with plasma membrane components at the IS could profoundly affect local signaling dynamics.
Separating MTOC polarization into two distinct steps would presumably allow the transition between these steps to be regulated. In this manner, synapse stability could be tailored to serve specific biological functions. One might imagine that highly stable synapses would be required for targeted cytokine-mediated communication over a period of hours, or to prepare cells for asymmetric division. In contrast, serial killing by cytotoxic lymphocytes, which combines directional secretion of cytolytic factors with rapid movement between target cells, would perhaps be best served by transient direction sensing without subsequent stabilization. Further studies will be required to test these ideas. Clearly, however, close analysis of synaptic polarity has reinforced the concept of the IS as a structure that evolves in time to suite the needs of the lymphocyte and its partner.
In that regard, it is interesting to note that, in the hours that follow the initial recruitment of Par and Scrib complexes in conjugated T cells, synaptic polarity appears to undergo a dramatic inversion (Fig. 4). Twelve hours after TCR stimulation, Scribble and Dlg are now localized to the IS.5 The transmembrane protein Crtam, which is thought to be important for promoting late stage cytokine responses, becomes incorporated into the Scrib complex at this stage.29 Strikingly, synaptic accumulation of Scribble and Dlg is associated with the movement of Par3 and aPKC to the back of the cell.4,5 The reasons for this inversion are not known, but they could have something to do with aligning the cell for asymmetric division. Indeed, the PKCζ substrate Numb, which is involved in asymmetric cell division in multiple systems, also accumulates at the back of the cell with the Par complex.5 This late stage reconfiguration of polarity proteins is correlated with a relaxing of MTOC polarization toward the IS, which may be a requisite step for mitosis. Importantly, PKCθ still localizes to the IS at this time,5 indicating that synaptic direction sensing has not broken down. Thus, late stage (>10 h) conjugated T cells adopt an entirely distinct form of cell polarity that may be designed to meet the needs of asymmetric cell division. Future studies will no doubt delve more deeply into the functional relevance of polarity complexes for this process.
Concluding Remarks
In this post-genomic era, the sheer bulk of biomedical research is such that we are never at a loss for seemingly analogous systems in other cell types that we can use as templates for our own studies. Extension by analogy is indeed a very productive scientific approach. The Par and Scrib complexes, for example, were first characterized in developmental models and adherent cell lines, and the knowledge gleaned from those studies has served as a foundation for more recent work in lymphocytes. It is important to note, however, that whereas polarity complexes drive MTOC reorientation in adherent cells, they appear to act as stabilizers of the polarized state in lymphocytes. Hence, when pursuing biological analogies we must always be sensitive to the possibility of cell type-specific differences, especially when comparing cells as structurally distinct as lymphocytes, fibroblasts and neurons.
We must also guard against focusing on one analogy to the exclusion of others. Our work has demonstrated that MTOC reorientation in T cells is guided by a gradient of a lipid second messenger, DAG, and that perturbing the enzymes responsible for maintaining this gradient, PLCγ and the DGKs, disrupts polarization. This mechanism is remarkably similar to lipid-based direction sensing during leukocyte migration,30 which is based on a polarized phosphatidyl-inositol tris-phosphate (PIP3) gradient that is generated by the coordinated activity of PI-3 kinases and lipid phosphatases (Fig. 1). In retrospect, the similarities between DAG-dependent MTOC polarization and PIP3-dependent direction sensing represents an excellent example of biological analogy, but it was one that we missed while pursuing other hypotheses that were, more often than not, based on different analogies.
Moving forward, it is probably worth remembering that biological analogies are most useful as conceptual, rather than absolute guides. The study of T cell MTOC reorientation and leukocyte chemotaxis has demonstrated that lipid-based direction sensing is a robust and rapid way to establish polarity in structurally plastic cell types. The actual molecules involved in each system, however, are not the same, nor should we expect them to be. Indeed, there are often compelling reasons for them to be different. The use of DAG during IS formation, for example, enables T cells to establish a new type of polarity that is chemically orthogonal to the migratory, “hand-mirror” morphology potentiated by PIP3. In future studies of conceptually similar systems, we should keep principles such as lipid-based direction sensing in mind, but be open to the possibility that unexpected players could emerge in the important roles.
Acknowledgments
I apologize to those whose work I did not cite due to space limitations. Supported by the US National Institutes of Health (R01-AI087644) and the Cancer Research Institute.
References
- 1.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 2.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:2311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 5.Oliaro J, Van Ham V, Sacirbegovic F, Pasam A, Bomzon Z, Pham K, et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J Immunol. 2010;185:367–375. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 9.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMond AL, Starr T, Dustin ML, Groves JT. Control of antigen presentation with a photoreleasable agonist peptide. J Am Chem Soc. 2006;128:15354–15355. doi: 10.1021/ja065304l. [DOI] [PubMed] [Google Scholar]
- 11.Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 13.Sedwick CE, Morgan MM, Jusino L, Cannon JL, Miller J, Burkhardt JK. TCR, LFA-1 and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J Immunol. 1999;162:1367–1375. [PubMed] [Google Scholar]
- 14.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Kuhne MR, Lin J, Yablonski D, Mollenauer MN, Ehrlich LI, Huppa J, et al. Linker for activation of T cells, zeta-associated protein-70 and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 16.Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it's all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 19.Letschka T, Kollmann V, Pfeifhofer-Obermair C, Lutz-Nicoladoni C, Obermair GJ, Fresser F, et al. PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008;112:4617–4627. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- 20.Gruber T, Thuille N, Hermann-Kleiter N, Leitges M, Baier G. Protein kinase Cepsilon is dispensable for TCR/CD3-signaling. Mol Immunol. 2005;42:305–310. doi: 10.1016/j.molimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 22.Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Cofreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordon-Alonso M, Sung CH, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertrand F, Esquerre M, Petit AE, Rodrigues M, Duchez S, Delon J, et al. Activation of the ancestral polarity regulator protein kinase C zeta at the immunological synapse drives polarization of Th cell secretory machinery toward APCs. J Immunol. 2010;185:2887–2894. doi: 10.4049/jimmunol.1000739. [DOI] [PubMed] [Google Scholar]
- 27.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Hou KK, Piwnica-Worms H, Shaw AS. The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J Immunol. 2009;183:1215–1221. doi: 10.4049/jimmunol.0803887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]