Abstract
Artificial nest experiments (ANEs) are widely used to obtain proxies of natural nest predation for testing a variety of hypotheses, from those dealing with variation in life-history strategies to those assessing the effects of habitat fragmentation on the persistence of bird populations. However, their applicability to real-world scenarios has been criticized owing to the many potential biases in comparing predation rates of artificial and natural nests. Here, we aimed to test the validity of estimates of ANEs using a novel approach. We related predation rates on artificial nests to population viability analyses in a songbird metapopulation as a way of predicting the real impact of predation events on the local populations studied. Predation intensity on artificial nests was negatively related to the species' annual population growth rate in small local populations, whereas the viability of large local populations did not seem to be influenced, even by high nest predation rates. The potential of extrapolation from ANEs to real-world scenarios is discussed, as these results suggest that artificial nest predation estimates may predict demographic processes in small structured populations.
Keywords: habitat fragmentation, nest predation, artificial nest experiment, population viability analysis
1. Introduction
Nest predation is the primary source of breeding failure in birds, influencing population dynamics, and exerting a strong selective force which shapes many life-history traits [1–3]. Artificial nest experiments (ANEs) are frequently used by avian ecologists to overcome logistical problems when monitoring natural nests and assessing predation rates. Whether nest predation rates obtained by ANEs accurately simulate natural nest predation intensity is, however, a hotly debated topic with a questioned applicability to real-world scenarios [4]. The main criticism concerns the possible relationship between measures of predation on artificial and natural nests [4], which can depend on several methodological artefacts. First and most importantly, nest predation risk can differ among artificial and natural nests because each might be predated by different species [5]. Second, the wide difference between the level of sophistication of artificial nests and the characteristics of experimental eggs has been claimed to be a major discrepancy [6,7]. Third, the absence of parental care might lead to an overestimate of predation rates at artificial nests [8]. Fourth, an increased nest density owing to a surplus of artificial nests can lead to higher predation rates [9]. Finally, investigator activity might affect predation intensity of artificial nests, although this observer effect can also be an important source of bias in natural nests [10,11].
Here, we present a novel methodological approach to evaluate estimates of predation rates obtained with artificial nests. Instead of comparing predation rates of artificial and natural nests, we assessed the relationship between predation rates on artificial nests and the population dynamics of a ground-nesting passerine known for suffering elevated predation in the breeding stage. We studied a highly fragmented metapopulation of the Dupont's lark (Chersophilus duponti) and hypothesized that, if ANEs reflect natural conditions, the estimated predation pressures should covary with indices of population dynamics. By conducting ANEs concurrently with a study of the species' population dynamics, we tested whether nest predation, estimated through ANEs, was associated with a population parameter, the intrinsic annual growth rate (λ). This approach had the dual benefit of testing the validity of artificial nests as proxies for natural ones, and also provided a means of determining how well ANEs predict the real impact of predation events on study populations. Our hypothesis is based on the assumption that stochastic predation events have a major impact on small populations [12], and that small populations inhabit small patches containing proportionally more edge habitats, which have been shown to act as population sinks, consequently having a dominant impact on overall population dynamics [13].
2. Material and methods
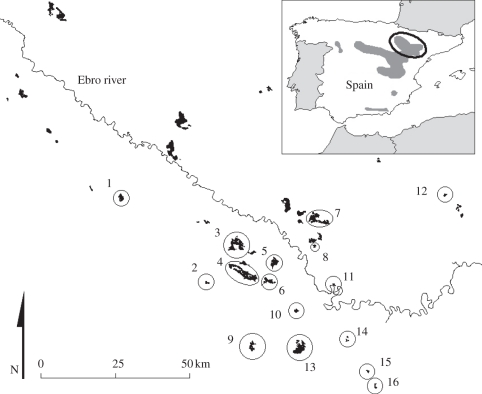
The Dupont's lark is restricted to natural and flat steppe vegetation in Europe and North Africa. The European population is limited to Spain, where habitat destruction and conversion to agriculture have led to a reduced and scattered distribution with continuously declining populations [14]. The study was carried out in 16 local populations of different size within the Ebro Valley metapopulation (see the electronic supplementary material), thus representing an optimal gradient of habitat and demographic conditions to perform this study. Ebro Valley is the second most important European area of this endangered species, albeit holding only ca 680 occupied territories [15] (figure 1).
Figure 1.
Distribution of the Dupont's lark in Spain (inset map) and of the remaining local populations (black patches) in the Ebro Valley metapopulation. See the electronic supplementary materials for characteristics of the 16 local populations where artificial nest predation experiments were conducted (circled patches).
Between 2 and 15 April 2006, we set out 334 artificial nests, the number of which being similar to the occupied territories in each local population. We designed the artificial nests to emulate natural Dupont's lark nests and selected their location according to the size, visibility and orientation measurements of Dupont's lark nests in our study area (see details in the electronic supplementary material). The artificial nests were controlled for any predation occurrence on the third, sixth and 12th day after setting out. Predated nests were considered those from which the egg disappeared or showed evident signs of predation, as well as those removed from their original nest site.
The λ values of Dupont's lark were adopted from population viability analysis (PVA) by Laiolo et al. [16], who ran conservative simulations including demographic parameter values derived directly from the local populations, explicitly acknowledging their spatial context (see the electronic supplementary material for PVA details).
We used generalized linear models (GLMs) with a normal error distribution and an identity link function in SAS 9.2 to fit λ values of Dupont's lark as dependent variable, and population size, nest predation and the interaction of both effects as independent ones.
3. Results
Our ANE detected an overall nest predation rate of 58.7 per cent, ranging between 17 and 100 per cent in the different local populations (see the electronic supplementary material). Population size alone had no effect on nest predation (binomial GLM, χ2 = 0.00, p = 0.95). The λ values of the analysed local populations from 2004 to 2006 were greater than 1 in only two populations (3 and 13), and were 0 in populations 15 and 16 (see the electronic supplementary material); the latter two went extinct in 2006.
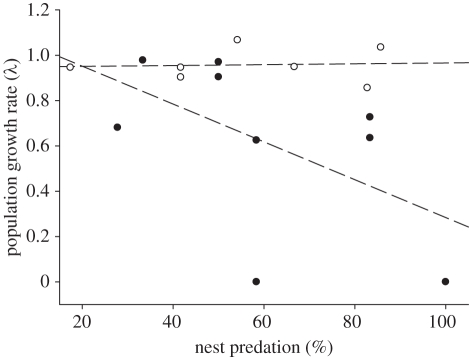
We found a significant relationship between λ and the interaction between population size and nest predation (table 1). While λ of large populations was not affected by nest predation, a negative relationship was revealed for small populations (figure 2). Interestingly, we observed a similar relationship when replacing λ by productivity (GLM, χ2 = 4.79, p < 0.05).
Table 1.
Generalized linear model showing the relationship between Dupont's lark population growth rates (λ) and the interaction of experimental nest predation and population size.
| variable | parameter estimate | s.e. | χ2 | p |
|---|---|---|---|---|
| intercept | 1.2449 | 0.2423 | 26.41 | <0.0001 |
| nest predation | −0.0117 | 0.0039 | 7.23 | 0.007 |
| population size | −0.0111 | 0.0096 | 1.28 | 0.26 |
| nest predation × population size | 0.0004 | 0.0002 | 4.72 | 0.03 |
Figure 2.
Relationship between artificial nest predation rate and the growth rate of Dupont's lark local populations. Although population size was analysed as a continuous variable (table 1), the populations were separated into small (less than 25 occupied territories) and large (greater than or equal to 25 occupied territories) ones for simplicity. The linear regressions illustrate the differential effect of nest predation on λ for the two types of patches. Filled circles, small populations; open circles, large populations.
4. Discussion
According to our prediction, predation intensity on artificial nests and Dupont's lark population dynamics were significantly associated, although the observed pattern of covariation depended on the local population status. Predation pressure on artificial nests was negatively related to the species' λ in small populations, whereas the viability of large populations did not seem to be influenced, even by high nest predation rates.
These main findings were supported by the corresponding outcome for productivity, despite the potential limitations of using the yearling to adult ratio as a surrogate for productivity. Thus, small populations appeared to be highly sensitive to decreases in productivity exerted by predators, while large populations are probably buffered by dilution effects and/or high rates of immigration resulting from conspecific attraction processes [17]. To our knowledge, this is the first study that directly relates nest predation rates obtained by an ANE with bird population dynamics, and gives an alternative perspective on the questioned validity of ANEs. Although this evidence suggests that indirect (experimental) predation estimates may produce reliable proxies of natural phenomena, we highlight the main caveat of ANEs: extrapolations to real-world scenarios have to be carefully validated.
The experimental design concepts of internal and external validity help to characterize the problems with artificial nest methodology [4]. We tried to minimize the potential shortcomings of ANEs, consequently maximizing the measurements' accuracy within the experiment (i.e. the internal validity). The structure and location of the artificial nests were carefully simulated according to local natural nests of the species, thus avoiding problems of ‘wicker basket’ nests [7]. Artificial nests containing quail eggs have been claimed to inaccurately simulate natural predation intensities owing to a varying presence of small-mouthed mammals unable to break these eggs [6]. In Spanish steppe habitat, however, red fox (Vulpes vulpes) and feral dogs have been identified as principal nest predators of ground-nesting larks, and even most secondary predators (snakes, lizards, hedgehog Erinaceus europaeus and southern grey shrike Lanius meridionalis) are big enough to break quail eggs [18]. The wide range of artificial nest predation rates (95% CI: 47.1% and 70.3%) was comparable with the range of natural nest predation rates registered for ground-nesting larks in Spanish steppe habitats. Rates varied between 44 and 98 per cent when considering six different species, whereas the only two existing studies on the Dupont's lark revealed rates of 46 (n = 28) and 84 per cent (n = 24), respectively [18].
To what extent can the results of our ANE be generalized to contexts other than the experimental population (i.e. external validity)? We do not expect that ANE predation rates provide exact estimates of real nest predation rates. Nevertheless, and contrary to doubts posed about the interpretation of ANEs, the association with population viability supports their use as indices of relative predation pressure, at least for open cup ground-nesting passerines living in shrub steppes. These and similar habitats with low structural complexity (e.g. grasslands), where all species use similar microhabitats for nesting, may provide a privileged scenario for ANEs, and generate a close match between predation on artificial and natural nests. By contrast, maximizing external validity of ANEs may be more difficult in more complex habitats (e.g. forests), with different nesting guilds within the passerine community, and a greater number of potentially confounding variables to take into account. Furthermore, inconsistent temporal and species effects have been found when comparing predation rates of artificial and real nests even if the general trends of predation were similar for both nest types throughout separate seasons [19]. Hence, we strongly encourage further research designed to shed light on the interplay between predation intensity (on both artificial and natural nests in different habitats) and demographic data.
Direct measurement of reproductive success by the usual protocols is in general difficult, costly and often involves invasive methods with a potential negative impact, especially for sensitive and threatened species [20]. According to the outcomes of the study, ANE seems indeed to represent a valid tool for the Dupont's lark, a species with elusive behaviour, low local population sizes and delicate conservation status. In our particular case, the contribution of predation to compromising the viability of small local populations may have dramatic consequences for the metapopulation persistence, as indicated by the overall negative trend and frequent recent episodes of local extinction [14,15]. Compared with time-demanding and costly predator surveys, carefully designed and evaluated ANEs may therefore be an affordable, efficient and non-invasive way to gather information about the impact of nest predation on threatened species with extremely low population sizes.
Acknowledgements
M.V. was supported by pre- and post-doctoral fellowships (I3P-CISC/MICINN), and with P.L. by a PIE project (CSIC). Funds were provided by Excellence Project RNM1274, Junta of Andalucía. We thank G. D. Fairhurst, and three anonymous referees for comments on the article.
References
- 1.Ricklefs R. E. 1969. An analysis of nesting mortality in birds. Smithson. Contrib. Zool. 9, 1–48 [Google Scholar]
- 2.Saether B. E., Bakke Ø. 2000. Avian life history variation and the contribution of demographic traits to the population growth rate. Ecology 81, 642–653 10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2) [DOI] [Google Scholar]
- 3.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 4.Moore R. P., Robinson W. D. 2004. Artificial bird nests external validity and bias in ecological field studies. Ecology 85, 1562–1567 10.1890/03-0088 (doi:10.1890/03-0088) [DOI] [Google Scholar]
- 5.Thompson F. R., III, Burhans D. E. 2004. Differences in predators of artificial and real songbird nests: evidence of bias in artificial nest studies. Conserv. Biol. 18, 373–380 10.1111/j.1523-1739.2004.00167.x (doi:10.1111/j.1523-1739.2004.00167.x) [DOI] [Google Scholar]
- 6.Haskell D. G. 1995. A reevaluation of the effects of forest fragmentation on rates of bird-nest predation. Conserv. Biol. 9, 1316–1318 10.1046/j.1523-1739.1995.9051312.x-i1 (doi:10.1046/j.1523-1739.1995.9051312.x-i1) [DOI] [PubMed] [Google Scholar]
- 7.Rangen S. A., Clark R. G., Hobson K. A. 2000. Visual and olfactory attributes of artificial nests. Auk 117, 136–146 10.1642/0004-8038(2000)117[0136%3AVAOAOA]2.0.CO%3B2 (doi:10.1642/0004-8038(2000)117[0136%3AVAOAOA]2.0.CO%3B2) [DOI] [Google Scholar]
- 8.Cresswell W. 1997. Nest predation: the relative effects of nest characteristics, clutch size and parental behaviour. Anim. Behav. 53, 93–103 10.1006/anbe.1996.0281 (doi:10.1006/anbe.1996.0281) [DOI] [Google Scholar]
- 9.Martin T. E. 1988. On the advantage of being different: nest predation and the coexistence of bird species. Proc. Natl Acad. Sci. USA 85, 2196–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major R. E. 1990. The effect of human observers on the intensity of nest predation. Ibis 132, 608–612 10.1111/j.1474-919X.1990.tb00285.x (doi:10.1111/j.1474-919X.1990.tb00285.x) [DOI] [Google Scholar]
- 11.Rotella J. J., Taper M. L., Hansen A. J. 2000. Correcting nesting-success estimates for observer effects: maximum-likelihood estimates of daily survival rates with reduced bias. Auk 117, 92–109 10.1642/0004-8038(2000)117[0092:CNSEF]2.0.CO;2 (doi:10.1642/0004-8038(2000)117[0092:CNSEF]2.0.CO;2) [DOI] [Google Scholar]
- 12.Caughley G. 1994. Directions in conservation biology. J. Anim. Ecol. 63, 215–244 10.2307/5542 (doi:10.2307/5542) [DOI] [Google Scholar]
- 13.Woodroffe R., Ginsberg J. R. 1998. Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128 10.1126/science.280.5372.2126 (doi:10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]
- 14.Tella J. L., Vögeli M., Serrano D., Carrete M. 2005. Current status of the threatened Dupont's lark in Spain: overestimation, decline, and extinction of local populations. Oryx 39, 90–94 10.1017/S0030605305000165 (doi:10.1017/S0030605305000165) [DOI] [Google Scholar]
- 15.Vögeli M., Serrano D., Pacios F., Tella J. L. 2010. The relative importance of patch habitat quality and landscape attributes on a declining steppe-bird metapopulation. Biol. Conserv. 143, 1057–1067 10.1016/j.biocon.2009.12.040 (doi:10.1016/j.biocon.2009.12.040) [DOI] [Google Scholar]
- 16.Laiolo P., Vögeli M., Serrano D., Tella J. L. 2008. Song diversity predicts the viability of fragmented bird populations. PLoS ONE 3, e1822. 10.1371/journal.pone.0001822 (doi:10.1371/journal.pone.0001822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laiolo P., Tella J. L. 2008. Social determinants of songbird vocal activity and implications for the persistence of small populations. Anim. Conserv. 11, 433–441 10.1111/j.1469-1795.2008.00202.x (doi:10.1111/j.1469-1795.2008.00202.x) [DOI] [Google Scholar]
- 18.Suárez F., Hervás I., Herranz J. M. 2009. Las alondras de España peninsular. Madrid, Spain: Organismo Autónomo Parques Nacionales [Google Scholar]
- 19.Weidinger K. 2001. How well do predation rates on artificial nests estimate predation on natural passerine nests? Ibis 143, 632–641 10.1111/j.1474-919X.2001.tb04891.x (doi:10.1111/j.1474-919X.2001.tb04891.x) [DOI] [Google Scholar]
- 20.Greenwood J. J. D. 1996. In ecological census techniques. In Basic techniques (ed. Sutherland W. J.), pp. 11–110 Cambridge, UK: Cambridge University Press [Google Scholar]