Abstract
Background
We have previously identified suggestive linkage for alcohol consumption in a community based sample of Australian adults. In this companion paper, we explore the strength of genetic linkage signals for alcohol dependence symptoms.
Methods
An alcohol dependence symptom score, based on DSM-IIIR and DSM-IV criteria, was examined. Twins and their non-twin siblings (1654 males, 2518 females), aged 21–81 years, were interviewed, with 803 individuals interviewed on two occasions, approximately 10 years apart. Linkage analyses were conducted on datasets compiled to maximize data collected at either the younger or the older age. In addition, linkage was compared between full samples and truncated samples that excluded the lightest drinkers (approximately 10% of the sample).
Results
Suggestive peaks on chromosome 5p (LODs > 2.2) were found in a region previously identified in alcohol linkage studies using clinical populations. Linkage signal strength was found to vary between full and truncated samples and when samples differed only on the collection age for a sample subset.
Conclusions
The results support the finding that large community samples can be informative in the study of alcohol-related traits.
Keywords: alcohol dependence symptoms, genetic linkage analysis, community sample
INTRODUCTION
Alcohol dependence is associated with significant economic burden, both directly and via its comorbidity with other substance use disorders and psychopathology. Data collected for the Australian National Survey of Mental Health and Wellbeing in 1997 showed that 4.1% those surveyed met the criteria for DSM-IV alcohol dependence (19% meeting at least one DSM-IV alcohol abuse or dependence criterion) in the preceding 12 months (2006). Findings are similar in the United States, with prevalence for 12-month and lifetime DSM-IV alcohol dependence being 3.8% and 12.5% (Hasin et al., 2007).
It is now known that genetic factors influence variation in alcohol dependence, with heritability estimates reported to range 50–70% (Agrawal and Lynskey, 2008; Heath et al., 1997; Prescott and Kendler, 1995; Prescott and Kendler, 1999). Over the last two decades, several linkage scans have been undertaken to locate and identify the specific genes that contribute to alcohol dependence. Amongst these efforts are the Collaborative Study on Genetics of Alcoholism (COGA) (Begleiter et al., 1995; Reich et al., 1998) and the Irish Affected Sib-Pair Study of Alcohol Dependence (IASPSAD) (Prescott et al., 2006; Prescott et al., 2005), which have used linkage methods to identify loci on several chromosomes. These studies have focussed on patients with a family history of drinking problems.
In the current study we utilize data collected from a community sample of over 4,000 Australian adults. In line with our previous study (Hansell et al., 2009), which found suggestive linkage for alcohol consumption in the same hetereogeneous sample, the primary aim of the present study was to examine the viability of using linkage methods on alcohol dependence data collected from a community sample. In addition, we investigated the effects of excluding very light drinkers (approximately 10% of the distribution of consumption). This exclusion was undertaken to provide a focus on genetic factors predisposing to problem drinking. Low consumption may reflect a mixture of both biological causes (which would include protective genes such as the aldehyde dehydrogenase gene ALDH2 (Luczak et al., 2006) and which may be evident in analyses of the full distribution) and social factors (such as church attendance (Strawbridge et al., 1997)). Third, as data were collected on two occasions (10 years apart on average) for approximately 19% of our sample, we examined the effect of including data collected at either the younger or the older age. Lifetime dependence symptomatology can vary with age, either due to increasing symptoms with age, or due to incorrect recall of symptoms that were apparent at the earlier, but not the later, age.
MATERIALS AND METHODS
Sample
Data were obtained from three phases of telephone interviews conducted between 1992 and 2005, as described previously (Hansell et al., 2009). Interviews were adapted from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA, Bucholz et al., 1994). Lifetime abstainers (i.e. individuals who had never tried alcohol - 2% of those interviewed) were excluded. For 63% of our sample, self-reported ancestry for all grandparents was available. Ancestry was predominantly European with 96% having full European ancestry.
Both alcohol phenotypes and genotypes suitable for linkage analysis were available for 4172 individuals (1654 males, 2518 females) from 1690 families. The sample comprised 3085 twins (including 1465 complete pairs, of which 34 were MZ) and 1087 non-twin siblings. The number of quasi-independent sibling pairs (QISPs – i.e. twin pairs plus pairings formed between twins and their non-twin siblings) in the dataset was 3837. Genotyping was available for 1338 parents (including 483 parental pairs) and these were used to refine estimates of IBD probabilities.
Participants were aged 21 to 81 years (39.7±10.5) at the time of first interview. Further, 803 participants were interviewed on a second occasion, with a mean time between interviews of 10.4±1.8 years (range = 2–13 years). Linkage analyses were run on the full sample, first, using all first interview data (Younger dataset) and second, with data from the second interview replacing first interview data for the 803 individuals interviewed twice (Older dataset).
A detailed description of zygosity determination and genotyping for this sample can be found in Hansell et al (2009). For the 4172 participants with dependence symptom data, the number of unique autosomal markers per individual ranged from 301 to 1359 (M = 559.9±234.1).
Alcohol Dependence Symptom Score
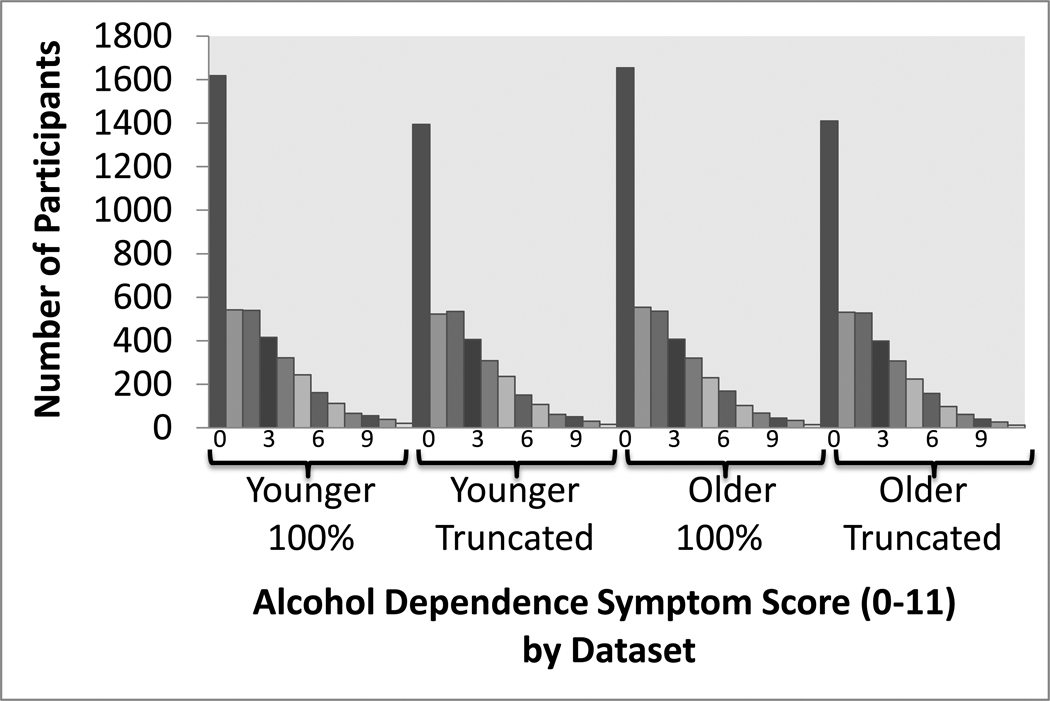
Assessment was based on lifetime prevalence. An alcohol dependence symptom score was obtained from nine items based on the substance dependence criteria from the Third Revised and Fourth Editions of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1987; 2000), as listed in Hansell et al. (2008). All items were scored Yes/No (1/0), with the exception of items 2 and 7, which were coded as three level ordinal measures (Item 2: no = 0, one unsuccessful attempt to cut down = 1, more than one unsuccessful attempt to cut down = 3, and Item 7: no = 0, a marked (but less than 50%) increase in the amount required to achieve the desired effect = 1, at least a 50% increase in the amount required = 2). Items were summed to obtain a single score. Distributions of alcohol dependence symptom scores are shown in Figure 1. Approximately 34% of the sample had positive responses for 3 or more criteria, consistent with being classified as alcohol dependent.
Fig. 1.
Distributions of alcohol dependence symptom scores (derived from 9 symptoms with a maximum score of 11) are shown for full (100%) and truncated samples and for datasets that differ for a subset of 803 individuals for whom data were collected on two occasions (1st occasion data (M = 1.8 ± 2.2) were included in the Younger dataset and 2nd occasion data M = 2.2 ± 2.6) in the Older dataset).
Statistical Analyses
Pedigree-wide analyses were conducted for the 22 autosomal chromosomes using MERLIN-REGRESS and the X chromosome using MINX (Abecasis et al., 2002). Multipoint linkage analyses were examined for the Younger and Older datasets. Data for both co-twins from MZ pairs were modelled in analyses (with zygosity identified), although the MZ relationship per se does not contribute to linkage. Post-hoc linkage analyses examined the effects of excluding individuals with the least alcohol exposure by dropping approximately 10% of the sample (8.3% for the Younger dataset and 7.6% for the Older) from the lower end of the sex and age adjusted distribution of consumption. Essentially, this subset were non-drinkers during the previous 12 month period (<1 drink per week), although many reported having dependence symptoms at some time during their lives (reported symptoms ranged 0–11, M = 1.4±2.8 and 1.3±2.7 for the Younger and Older datasets respectively).
Preliminary analyses were performed using SPSS (version 15.0 for Windows, 2006). The effects of sex, age, age2, sex*age, and sex*age2 were examined using stepwise linear regression. The effects of significant covariates (p <. 05) were regressed out before linkage analyses. For the younger dataset, sex, age, age2 and their interactions (sex*age & sex*age2) were significant in both the full and truncated samples (all p-values < 0.001). In the older dataset, sex, age2 and sex*age were significant in the full sample (p = 0.001, 0.001, 0.009 respectively) while only sex*age (p < 0.001) was significant in the older dataset with the truncation. Note that age and year of birth correlated >0.90, and consequently, age was a good surrogate covariate to account for the effects of possible secular trends.
Normalized residuals (mean 0, variance 1) were used for all variables to facilitate analyses in MERLIN-REGRESS (Sham et al., 2002). There were no univariate outliers. Bivariate outliers, as defined in Benyamin et al. (2008), accounted for less than 0.4% of the sample and were excluded from linkage analyses.
RESULTS
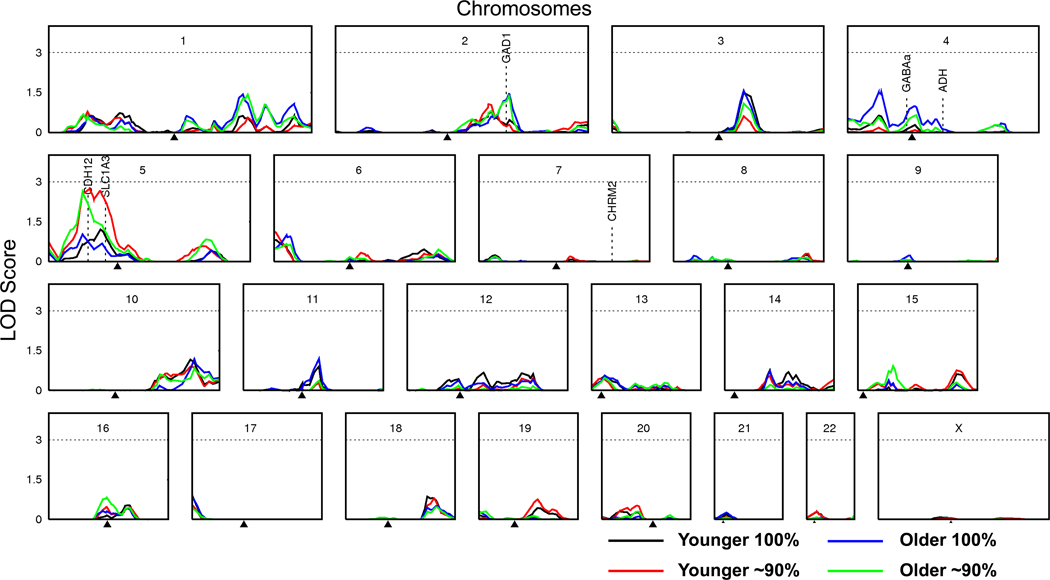
Multipoint linkage results are shown in Figure 2. LOD scores exceeding 1.5 were noted on chromosome 3 (D3S1292, 140.7cM), chromosome 4 (GATA70E01, 37.1cM), and chromosome 5 (ATAG078, 35.1cM; GATA134B03, 42.6cM; and GATA7C06, 53.8cM).
Fig. 2.
Multipoint LOD scores for alcohol dependence symptom score are shown for Younger and Older samples (identical except for a subset of 822 individuals for whom data were collected on two occasions with first occasion data included in the Younger dataset and second occasion data in the Older dataset) and for full (100%) and truncated (~90%) samples. Gene locations are shown for the GABAA and ADH gene clusters and CHRM2, all of which have been associated with alcohol phenotypes in multiple studies (Edenberg and Foroud, 2006) and GAD1, SLC1A3, and CDH12, which are candidate addiction genes found under current linkage peaks.
The highest multipoint LOD of 2.7 (p = 0.0002) was found on chromosome 5 using the dataset maximising data collected at a younger age, and with lightest drinkers dropped. A similar LOD (2.6) was found when maximising data collected at an older age. However, including light drinkers reduced maximum LOD scores in the region to 1.2 and 1.0 respectively for the younger and older samples. While none of these findings are significant by standard criteria for linkage studies, those LOD scores ≥2.2 could be considered suggestive based on an expectation of statistical evidence occurring one time at random in a genome scan (a pointwise significance level of 7 × 10−4 for sib-pair studies), as proposed by Lander and Kruglyak (1995).
DISCUSSION
We sought to identify linkage regions for an alcohol dependence symptom score in a large community sample of Australian adults. Although none of our findings achieve genomewide levels of significance, our LOD scores greater than 2.0 are in a region on chromosome 5 previously linked to alcohol-related traits (e.g. Schuckit et al., 2001). The current findings suggest that genes in this region may influence alcohol-related problems ranging from mild through to the severe problems typically found in a clinical sample. Further, our results indicate, as previously found for alcohol consumption (Hansell et al., 2009), that the linkage method is sensitive to relatively small differences in inclusion criteria. In this case, variations in participant age and drinking status led to peak variation.
Given expected error in location estimates (Atwood and Heard-Costa, 2003), our suggestive findings converge with numerous previous reports. Linkage peaks on chromosome 5, which are maximal for the younger sample (LOD 2.7) and enhanced through exclusion of the lowest alcohol users, are approximately 10–15cM from that reported for response to alcohol (Schuckit et al., 2001) and age of onset/harm avoidance/novelty seeking (Dick et al., 2002) and approximately 35cM from a region implicated in alcoholism (Hill et al., 2004).
Genes of interest in this region include SLC1A3 and CDH12. SLC1A3 (solute carrier family 1 (glial high affinity glutamate transporter), member 3; gene map locus 5p13) is a glutamatergic neurotransmission gene with expression levels found to be significantly elevated in post-mortem prefrontal cortex of alcoholic individuals compared to non-alcoholic controls (Flatscher-Bader et al., 2005; Flatscher-Bader and Wilce, 2006). CDH12 (cadherin 12, gene map locus 5p14-p13) shows increased expression in post-mortem temporal cortex of individuals with a history of alcohol abuse or dependence compared to controls (Sokolov et al., 2003) and similarly, in the orbitofrontal cortex in violent suicide victims compared to non-psychiatric controls (Thalmeier et al., 2008). Cell adhesion-related genes such as CDH12 have roles in building and maintaining synaptic structures (Benson et al., 2000), pointing to possible disturbances of synaptic neuroplasticity in individuals with alcohol dependence (Sokolov et al., 2003).
Minor linkage on chromosome 2 (LOD 1.5) is approximately 15–30cM from that reported for alcoholism (Hill et al., 2004), rank-transformed alcohol dependence symptom count in the IASPSAD sample (Kendler et al., 2006), heavy alcohol use in the Heart Study (Wyszynski et al., 2003), severity of alcohol use in Mission Indians (Ehlers et al., 2004), and maximum drinks in COGA (Saccone et al., 2005). Further, it is about 40–50cM from regions implicated in alcohol dependence (Reich, 1996), conduct disorder (Dick et al., 2004), and more recently, an alcohol dependence symptom score (Agrawal et al., 2008), in the COGA sample. In the current analyses, linkage was maximal in the older sample and sample truncation did not alter the result.
This region of chromosome 2 is home to GAD1 (glutamate decarboxylase 1, gene map locus 2q31), which catalyzes the conversion of glutamic acid to gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the vertebral central nervous system. It has been associated with alcohol dependence in Han Taiwanese males (Loh et al., 2006). In the IASPSAD sample, no association was found with dependence, but significant associations were found with initial sensitivity to alcohol and to age at onset of dependence (Kuo et al., 2008). Kuo et al. (2008) proposed that the underlying pathophysiology regulated by GAD1 may be more directly related to the component processes of dependence, than to the disorder itself.
A small linkage peak (LOD 1.6) on chromosome 3 was located in the same region as linkage found previously in the same sample for alcohol consumption (LOD 2.7) (Hansell et al., 2009). In addition, a minor peak (LOD 1.0) was observed in the region encompassing the gamma-aminobutyric acid receptor A subunit 2 (GABRA2) gene. This gene and others in its cluster on chromosome 4 (GABRA4, GABRB1, GABRAG1) have been found to be associated with alcohol dependence (as well as other substance use disorders and psychopathology) across several independent studies (Covault et al., 2004; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005; Lind et al., 2008; Matthews et al., 2007; Soyka et al., 2008).
Some limitations need to be considered. First, these results were not genomewide significant. However, the likelihood that these are false positives is somewhat diminished as the results replicate linkage found in these regions by previous studies. Further, quantitative trait loci of small effect are to be expected given that an alcohol dependence symptom score is likely to be influenced by many genes of small effect, as has been found for numerous traits in genomewide association studies (Wray et al., 2007). Larger linkage signals, as have been reported previously, may simply reflect the high variance of effect size estimates found with smaller samples. Linkage differences related to sample variation were found in the current study, but are not sufficiently large for meaningful interpretation. A further limitation is that the component samples were collected for heterogeneous purposes and may not be entirely representative of the adult Australian population. Notwithstanding these limitations, our analyses demonstrate the utility of a community sample, results of which are easily generalizable, for linkage analyses. The chromosomal regions identified provide a focus for future gene-mapping efforts.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participating families, the project coordinators and interviewers under the supervision of Dixie Statham, the data managers under the supervision of John Pearson and David Smyth, and laboratory personnel under the supervision of Megan Campbell and Anjali Henders. For genome scans of twins and siblings, we acknowledge and thank the Mammalian Genotyping Service, Marshfield WI (Director: Dr James Weber) for genotyping under a grant to Dr Daniel O’Connor; Drs Eline Slagboom and Dorret Boomsma for the Leiden genome scan; Dr Peter Reed for the Gemini genome scan; and Dr Jeff Hall for the Sequana genome scan. National Institute of Health grants have supported this work, including grants to Drs Andrew Heath (AA07728, AA10248, AA11998, AA13321), Pamela Madden (DA12854), Nick Martin (AA13326), Michele Pergardia (DA019951), Richard Todd (AA13320), and John Whitfield (AA014041). Dr Arpana Agrawal receives support from DA023668 and ABMRF/The Foundation for Alcohol Research and Dr Katherine Morley from a National Health and Medical Research Council Public Health Fellowship (520452). With the exception of ABMRF, all funding is government sourced. Funding bodies played no role in the collection or analysis of data nor in the writing of this paper.
REFERENCES
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genetics. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Hinrichs A, Dunn G, Bertelsen S, Dick D, Saccone SF, Saccone NL, Grucza R, Wang JC, Cloninger CR, Edenberg H, Foroud T, Hesselbrock V, Kramer J, Bucholz K, Kuperman S, Nurnberger J, Porjesz B, Schuckit M, Goate A, Bierut L. Linkage scan for quantitative traits identifies new regions of interest for substance dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Drug and Alcohol Dependence. 2008;93:12–20. doi: 10.1016/j.drugalcdep.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey M. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103(7):1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd revised ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Atwood LD, Heard-Costa NL. Limits of fine-mapping a quantitative trait. Genetic Epidemiology. 2003;24:99–106. doi: 10.1002/gepi.10225. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li TK, Schuckit M, Edenberg H, Rice JP. The collaborative study on the genetics of alcoholism. Alcohol Health and Research World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Schnapp LM, Shapiro L, Huntley GW. Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends in Cell Biology. 2000;10:473–482. doi: 10.1016/s0962-8924(00)01838-9. [DOI] [PubMed] [Google Scholar]
- Benyamin B, Perola M, Cornes BK, Madden PAF, Palotie A, Nyholt DR, Montgomery GW, Peltonen L, Martin NG, Visscher PM. Within-family outliers: segregating alleles or environmental effects? A linkage analysis of height from 5815 sibling pairs. European Journal of Human Genetics. 2008;16:516–524. doi: 10.1038/sj.ejhg.5201992. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelerntner J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, Porjesz B, Bucholz K, Goate A, Nurnberger J, Foroud T. A genome-wide screen for genes influencing conduct disorder. Molecular Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- Dick DM, Nurnberger J, Jr, Edenberg HJ, Goate A, Crowe R, Rice J, Bucholz KK, Kramer J, Schuckit MA, Smith TL, Porjesz B, Begleiter H, Hesselbrock V, Foroud T. Suggestive linkage on chromosome 1 for a quantitative alcohol-related phenotype. Alcoholism, Clinical and Experimental Research. 2002;26(10):1453–1460. doi: 10.1097/01.ALC.0000034037.10333.FD. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li T-K, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha-2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addiction Biology. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2004;129B:110–115. doi: 10.1002/ajmg.b.30057. [DOI] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatric Genetics. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, Wilce PA. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. Journal of Neurochemistry. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Wilce PA. Chronic smoking and alcoholism change expression of selective genes in the human prefrontal cortex. Alcoholism, Clinical and Experimental Research. 2006;30(5):908–915. doi: 10.1111/j.1530-0277.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Hansell NK, Agrawal A, Whitfield JB, Morley KI, Gordon SD, Lind PA, Pergadia ML, Montgomery GW, Madden PAF, Todd RD, Heath AC, Martin NG. Can we identify genes for alcohol consumption in samples ascertained for heterogeneous purposes? Alcoholism, Clinical and Experimental Research. 2009 doi: 10.1111/j.1530-0277.2008.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell NK, Agrawal A, Whitfield JB, Morley KI, Zhu G, Lind PA, Pergadia ML, Madden PAF, Todd RD, Heath AC, Martin NG. Long-term stability and heritability of telephone interview measures of alcohol consumption and dependence. Twin Research and Human Genetics. 2008;11(3):287–305. doi: 10.1375/twin.11.3.287. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Stratham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kuo P-H, Kalsi G, Prescott CA, Hodgkinson CA, Goldman D, Alexander J, Van den Oord EJ, Chen X, Sullivan PF, Patterson DG, Walsh D, Kendler KS, Riley BP. Associations of glutamate decarboxylase genes with initial sensitivity and age-at-onset of alcohol dependence in the Irish Affected Sib Pair Study of Alcohol Dependence. Drug and Alcohol Dependence. 2008 doi: 10.1016/j.drugalcdep.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelerntner J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcoholism, Clinical and Experimental Research. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lind P, MacGregor S, Agrawal A, Heath AC, Martin NG, Whitfield JB. The role of GABRA2 in alcohol dependence, smoking and illicit drug use in an Australian population sample. Alcoholism, Clinical and Experimental Research. 2008;32(10):1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EW, Lane HY, Chen CH, Chang PS, Ku LW, Wang KHT, Cheng ATA. Glutamate decarboxylase genes and alcoholism in Han Taiwanese men. Alcoholism, Clinical and Experimental Research. 2006;30:1817–1823. doi: 10.1111/j.1530-0277.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychological Bulletin. 2006;132:607–612. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. Journal of Studies on Alcohol and Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and Environmental Influences on Alcohol and Tobacco Dependence among Women. NIAAA Res Monogr. 1995;vol 30:59–87. [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Sullivan PF, Kuo PH, Webb BT, Vittum J, Patterson DG, Thiselton DL, Myers JM, Devitt M, Halberstadt LJ, Robinson VP, Neale MC, van den Oord EJ, Walsh D, Riley BP, Kendler KS. Genomewide linkage study in the Irish affected sib pair study of alcohol dependence: evidence for a susceptibility region for symptoms of alcohol dependence on chromosome 4. Molecular Psychiatry. 2006;11:603–611. doi: 10.1038/sj.mp.4001811. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Sullivan PF, Myers JM, Patterson DG, Devitt M, Halberstadt LJ, Walsh D, Kendler KS. The Irish Affected Sib Pair Study of Alcohol Dependence: study methodology and validation of diagnosis by interview and family history. Alcoholism, Clinical and Experimental Research. 2005;29:417–429. doi: 10.1097/01.alc.0000156085.50418.07. [DOI] [PubMed] [Google Scholar]
- Reich T. A genomic survey of alcohol dependence and related phenotypes: results from the Collaborative Study on the Genetics of Alcoholism (COGA) Alcoholism, Clinical and Experimental Research. 1996;20:133A–137A. doi: 10.1111/j.1530-0277.1996.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics. 1998;81(3):207–215. [PubMed] [Google Scholar]
- Saccone SF, Saccone NL, Neuman RJ, Rice JP. Genetic analysis of the maximum drinks phenotype. BMC Genetics. 2005;6 Suppl 1:S124. doi: 10.1186/1471-2156-6-S1-S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcoholism, Clinical and Experimental Research. 2001;25(3):323–329. [PubMed] [Google Scholar]
- Sham PC, Purcell S, Cherny SS, Abecasis GR. Powerful regression-based quantitative-trait linkage analysis of general pedigrees. American Journal of Human Genetics. 2002;71:238–253. doi: 10.1086/341560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov BP, Jiang L, Trivedi NS, Aston C. Transcription profiling reveals mitochondrial, ubiquitin and signaling systems abnormalities in postmortem brains from subjects with a history of alcohol abuse or dependence. Journal of Neuroscience Research. 2003;72:756–767. doi: 10.1002/jnr.10631. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. Journal of Psychiatric Research. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Frequent attendance at religious services and mortality over 28 years. American Journal of Public Health. 1997;87(6):957–967. doi: 10.2105/ajph.87.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmeier A, Dickmann M, Giegling I, Schneider B, Hartmann AM, Maurer K, Schnabel A, Kauert G, Moller HJ, Rujescu D. Gene expression profiling of post-mortem orbitofrontal cortex in violent suicide victims. International Journal of Neuropsychopharmacology. 2008;11(2):217–228. doi: 10.1017/S1461145707007894. [DOI] [PubMed] [Google Scholar]
- Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research. 2007;17:1520–1528. doi: 10.1101/gr.6665407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski DF, Panhuysen CI, Ma Q, Yip AG, Wilcox M, Erlich P, Farrer LA. Genome-wide screen for heavy alcohol consumption. BMC Genetics. 2003;4 Suppl 1:S106. doi: 10.1186/1471-2156-4-S1-S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.