Abstract
Early life nutrition has substantial influences on postnatal health, with both under-and overnutrition linked with permanent metabolic changes that alter reproductive and immune function and significantly increase metabolic disease risk in offspring. Since perinatal nutrition depends in part on maternal metabolic condition, maternal diet during gestation and lactation is a risk factor for adult metabolic disease. Such developmental responses may be adaptive, but might also result from constraints on, or pathological changes to, normal physiology. The rising prevalence of both obesity and osteoporosis, and the identification of links among bone, fat, brain, and gut, suggest that obesity and osteoporosis may be related, and moreover that their roots may lie in early life. Here we focus on evidence for how maternal diet during gestation and lactation affects metabolism and skeletal acquisition in humans and in animal models. We consider the effects of overall caloric restriction, and macronutrient imbalances including high fat, high sucrose, and low protein, compared to normal diet. We then discuss potential mechanisms underlying the skeletal responses, including perinatal developmental programming via disruption of the perinatal leptin surge and/or epigenetic changes, to highlight unanswered questions and identify the most critical areas for future research.
Keywords: perinatal developmental programming, genomic imprinting, caloric restriction, high fat diet, bone
INTRODUCTION
Beginning with the observation that individuals born at low birthweight have increased risk of cardiovascular disease [1], there is increasing evidence that early life nutrition influences postnatal health. Both low birthweight [2–10] and high birthweight [11–14] are associated with reduced reproductive and immune function, and significantly higher likelihood of obesity, atherosclerosis, type II diabetes, and the metabolic syndrome in adulthood [15], suggesting that poor intrauterine nutrition might be a risk factor for adult metabolic disease [16–17] (Figure 1). For example, children of women who were pregnant during the Dutch Hunger Winter of 1944–1945 have increased incidence of obesity, hyperlipidemia, and atherosclerosis if their mothers were impacted by famine during the first trimester; higher rates of pulmonary and kidney disease if during the second trimester; and impaired glucose tolerance if during the third trimester of pregnancy [18–20]. In comparison, children of obese mothers, or those born large for gestational age, are also at increased risk of developing the metabolic syndrome compared to children born at normal birthweight from normal weight mothers, particularly if their mothers also had gestational diabetes mellitus [13].
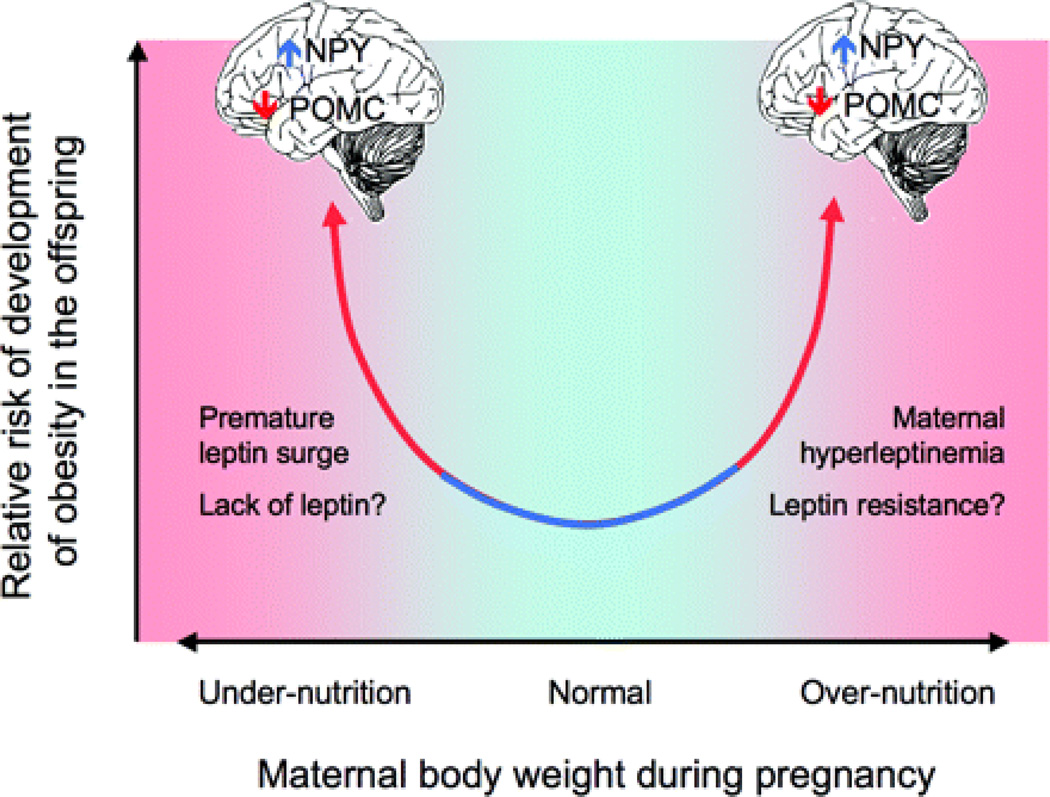
Figure 1.
The relationship between perinatal nutrition and postnatal metabolic disease may be U-shaped, with higher risk of metabolic dysregulation in both perinatal under- and overnutrition (adapted from Figure 1 in Grattan, D. R. Endocrinology 2008;149:5345–5347).
While these associations are striking, they do not explain why intrauterine nutrition would alter postnatal metabolism, nor reveal the mechanisms involved. The Barker Hypothesis [21], now known as the Developmental Origins of Adult Health and Disease model, posits that the perinatal environment induces lasting changes in physiological parameters such as hormone levels, glucose tolerance, and satiety, a phenomenon known as developmental programming. The hypothesis is that such ‘programming’ allows offspring to use maternal cues to adapt to the likely postnatal nutritional environment. For example, women born at lower birthweight have lower estrogen levels and are more susceptible to ovarian suppression during energetic stress compared to women born at higher birthweight [22], suggesting restricted perinatal energy availability results in increased sensitivity to adult energetic stress. However, when perinatal and postnatal environments are mismatched, offspring are at increased risk of metabolic disease, a model known as the Predictive Adaptive Response Hypothesis [9, 23]. For example, Hales and Barker demonstrated a strong association of low birthweight and type II diabetes risk [4], and proposed that this phenomenon resulted from offspring developing a “thrifty” phenotype in expectation of food scarcity [24]. Similarly, there appears to be a greater risk of obesity in children with low birthweight who exhibit rapid postnatal catch-up growth, compared to children who grow more slowly [25–26], although these relationships are complex [27]. Alternative models include the Maternal Capital Hypothesis, which suggests that offspring exhibit plasticity in early growth in order to match their energetic needs to maternal metabolic resources, and the Intergenerational Phenotypic Inertia Hypothesis, which posits that offspring adapt not to the transient intrauterine nutritional environment, but rather to the mother’s long-term energetic history [28–30].
Although there is some support for each of these hypotheses, the idea that the metabolic changes induced by perinatal developmental programming are adaptive is itself a hypothesis. As Ellison and Jasienska [31] point out, such developmental responses may be adaptations, but might also result from constraints on, or pathological changes to, normal physiology. Thus the notion that a given trait is an adaptation is a hypothesis that must be tested against the alternative hypotheses that the trait arose through pathology or constraint. The most rigorous approach for discriminating among these alternatives is to develop falsifiable predictions for each hypothesis—adaptation, pathology, or constraint—that can be evaluated against the data [31]. As an example, we might expect different patterns of changes in offspring body size, bone mass, and body composition in response to maternal caloric restriction depending on whether this response is an adaptation to expected postnatal energy restriction, the result of a constraint on intrauterine energy that is released after birth, or the product of permanent pathological disruption of growth processes (Table 1).
Whether the changes are adaptive or not, it is clear that early life nutrition alters postnatal metabolism, particularly adiposity and glucose tolerance. Recent interest has focused on the possibility that the perinatal environment also affects skeletal health [32]. The rising prevalence of both obesity and osteoporosis, and the identification of common mechanisms linking skeletal and metabolic homeostasis, suggest that obesity and osteoporosis may be related disorders, and moreover that their roots may lie in early life (Figure 2). Recent studies in mice demonstrate that interconnections of bone and fat, as well as brain and gut, play a major role in postnatal glucose homeostasis, fat mass and bone mass, raising the possibility that these mechanisms also affect human metabolism [33–34]. Osteoblasts and adipocytes derive from the same population of mesenchymal stem cells (MSCs), such that increased commitment of MSCs to the adipocytic over the osteoblastic lineage might shift the balance between fat mass and bone mass [35–37]. Osteoblast-derived uncarboxylated osteocalcin increases insulin sensitivity and reduces fat mass in mice; the adipokine leptin suppresses this effect by favoring carboxylation of osteoblast-derived osteocalcin, decreasing insulin sensitivity and insulin secretion [38–39]. Leptin also reduces murine bone mass by inhibiting the anabolic effects of brain-derived serotonin, and by increasing sympathetic tone via beta-adrenergic receptors on osteoblasts [40–42]. Fat-derived peroxisome proliferator-activated receptor (Ppar)-gamma increases marrow fat and decreases bone mass [43], while the protein LDL-receptor related protein 5 (LRP5), which plays a crucial role in bone remodeling, increases bone mass by suppressing production of gut-derived serotonin in rodent models [44]. Thus an increase in adiposity has the potential to decrease bone mass, at least in animal models.
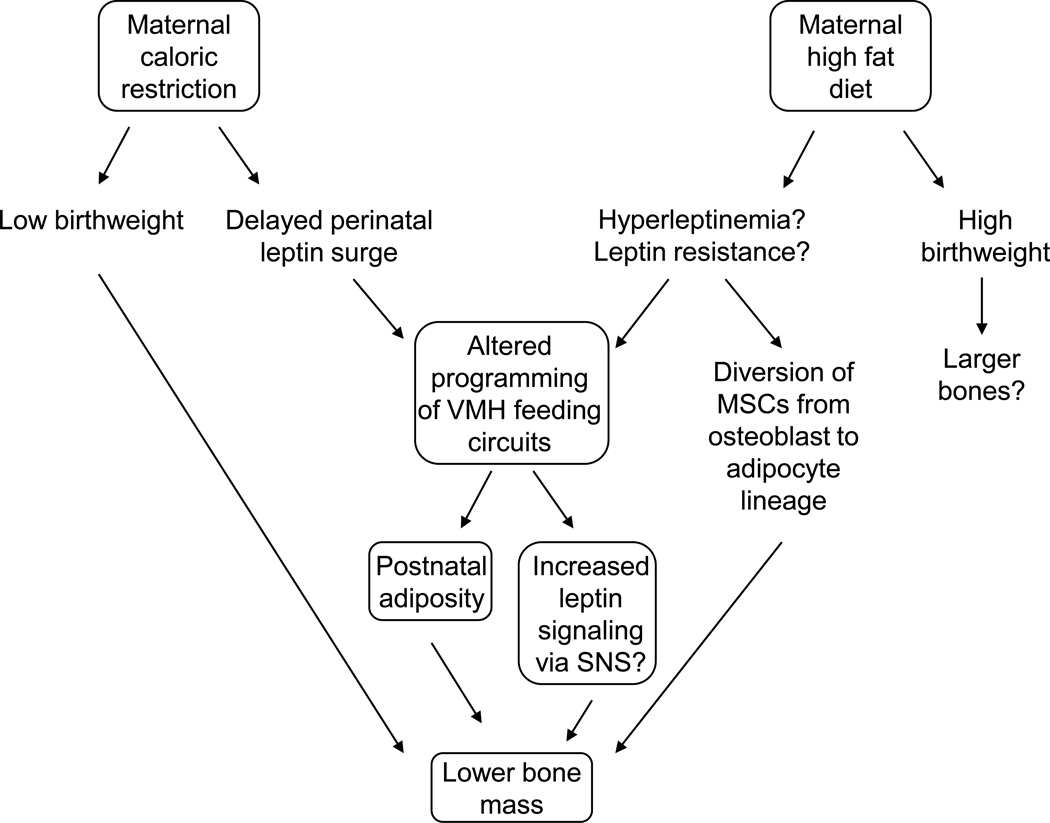
Figure 2.
Maternal caloric restriction and maternal high fat diet may induce similar changes in offspring bone mass via altered programming of ventromedial hypothalamic feeding circuits, postnatal adiposity, and potentially increased sympathetic nervous system activity that induces bone loss. Changes in peripheral leptin may also divert endosteal mesenchymal stem cells from the osteoblast to the adipocyte lineage, increasing marrow fat and decreasing bone mass.
How might maternal diet and body composition affect these interactions between offspring bone and fat mass? It is clear that during postnatal life, specific micronutrients, such as Vitamin D and calcium, are essential for proper skeletal development and bone mass acquisition [45–46]. However, the perinatal influences of these micronutrients on skeletal health are more difficult to establish. In addition to micronutrient intake, mothers may be overnourished or undernourished before pregnancy, and may overeat or undereat relative to their metabolic needs during gestation and lactation. Even when overall caloric intake is appropriate, the proportion of macronutrients—carbohydrate, protein, and fat—may not be optimal, and maternal diets high in fat or sucrose, or low in protein, have all been associated with offspring metabolic abnormalities [47–49]. However, less is known about the effects of dietary macronutrient profile or overall caloric availability (sufficient or insufficient) on offspring bone mass. The timing of exposure to diet also appears to be critical. For example, caloric restriction in adults may be beneficial to skeletal health, while perinatal or early postnatal caloric restriction is deleterious. Here we focus on evidence for how maternal diet during gestation and lactation affects metabolism and skeletal acquisition in humans and in animal models. We consider the effects of overall caloric restriction, macronutrient imbalances including high fat, high sucrose/maternal diabetes, and low protein, and low Vitamin D, compared to normal diet. We then consider potential mechanisms to highlight unanswered questions and identify the most critical areas for future research.
MATERNAL BODY COMPOSITION, DIET, AND OFFSPRING BONE MASS
Several studies support the hypothesis that the perinatal environment, as reflected by birthweight, influences postnatal bone mass and/or bone size in humans. For example, in young adults, birthweight is positively associated with bone size and bone mineral content, after controlling for current height, weight, and age [50–51]. In the Hertfordshire cohort study, birthweight is positively associated with adult spine and hip BMC, although the effect is modest, explaining only 1–4% of variation in the adult bone properties [52]. Femoral growth velocity in late gestation, presumably related to maternal nutrition, explains about 10% of the variation in whole body bone area (excluding the head) at 4 yrs of age in the Southampton Women’s Study cohort [53]. However, association does not prove causation. Birthweight depends in part on maternal diet and energy availability during gestation, but this association may be confounded by genetics, placental function, smoking, and socioeconomic status. Thus it is instructive to consider twin studies, in which genetic and environmental factors are likely similar within pairs. For example, in female monozygous twins, Antoniades et al. [54] report a significant association of birthweight with adult BMD at weight-bearing sites (spine and hip), but not the non-weight bearing forearm.
Undernutrition
Fetal undernutrition, resulting from maternal caloric restriction or from placental abnormalities that restrict fetal blood supply, is linked to decreased bone mass. Low maternal fat stores and vigorous maternal exercise are also associated with lower neonatal bone mass in humans [5], and infants born small for gestational age (SGA) have lower bone turnover markers vs. larger infants [55]. These differences persist into adulthood, as individuals who were born at very low birthweight have lower BMD at the forearm [56] and at the lumbar spine and femoral neck [57] compared to adults born at higher birthweights. However, as with the general association between birthweight and adult bone mass described above, the effect of low birthweight on bone mass may reflect postnatal conditions and/or reduced adult body mass. For example, adults born prematurely have lower BMD vs. adults born at full term, but their BMD is appropriate for their shorter stature [58]. Similarly, low birthweight is associated with early puberty in humans [59], which may decrease adult size and bone mass by prematurely ending skeletal growth.
In animal models, whereas it is clear that maternal calorie restriction or fetal growth restriction increases the likelihood of postnatal hyperphagia, obesity, and insulin resistance [49], the skeletal effects are less well established. Maternal calorie restriction in C57Bl/6J and A/J mice leads to reduced whole body BMC in B6 but not A/J offspring at 6 months of age [60], suggesting strain differences in sensitivity to maternal diet. Rats exposed to intrauterine growth restriction via uterine artery ligation are shorter, with narrower bones, lower whole body and femoral BMC, and lower bone strength in adulthood [61–62]. However, more research is needed on the effects of maternal calorie restriction on offspring skeletal acquisition and maintenance, given prior evidence that calorie restriction has substantially different effects across the lifespan. For example, in mice, calorie restriction impairs skeletal acquisition if initiated at weaning, but causes fewer skeletal deficits and may actually reduce age-related bone loss if initiated in adulthood [63–65]. In addition, more work is needed to define optimal approaches to maximize the catch-up in skeletal acquisition following early life caloric restriction. In humans with stunted childhood growth [66] or anorexia nervosa [67], adult skeletal deficits remain despite refeeding, suggesting that energetic deficits during critical windows of skeletal ontogeny may not be reversible.
Overnutrition
In humans, fetal overnutrition due to maternal high fat diet and/or preexisting obesity, or from maternal preexisting or gestational diabetes, is also associated with skeletal changes, although the effect of large size for gestational age (LGA) on bone properties may depend on the root cause, and on whether overnutrition continues postnatally. Human LGA infants generally have higher total body BMC and BMD vs. non-LGA infants, even relative to their higher body mass [68–70]. In contrast, offspring of diabetic mothers, who are frequently born LGA, have reduced total body BMD vs. controls [71], although bone turnover markers in amniotic fluid, fetal and maternal blood are normal [55, 72]. From 5–18 yrs of age, children of mothers with type 1 diabetes tend to be both taller and heavier vs. controls, with higher total body fat mass; however, both BMC and total body bone area are higher, such that BMD does not differ from controls [73]. Thus although high maternal glucose appears to increase offspring size at birth and continuing into childhood, fetal bone turnover is normal, and childhood bone mass appears appropriate for body mass. In comparison, LGA resulting from maternal obesity or high fat diet may be more deleterious. There is a negative association between maternal fat mass and offspring BMD in childhood [74], and between maternal fat intake in the 3rd trimester and offspring femoral neck and lumbar spine BMD at age 16 [75]. Further, mothers consuming a “healthy” diet in pregnancy (low intake of sugar, fat, and processed foods) have offspring with up to 6% greater BMD at 9 yrs of age, compared to mothers consuming more processed foods [76].
In animal models, maternal diabetes in rats is associated with reduced calcium content and delayed skeletal mineralization vs. controls [77]. Maternal high fat diet is frequently used to induce metabolic syndrome in rodent offspring [78–79], although there are conflicting data on whether maternal obesity must be present prior to pregnancy in order to induce a metabolic phenotype [80], or whether its effects are similar whether the diet is long term or confined to pregnancy and lactation [47]. In mice, offspring of dams exposed to high fat diet are shorter, with lower total body bone mass at fetal day 19 [81]. Offspring of mothers fed a high fat diet that are weaned onto the same high fat diet have higher marrow adiposity, greater body mass, and shorter femurs with larger cross-sectional dimensions at 30 wks of age [82]. However, it is unclear whether these differences would remain after adjustment for their higher body weight. Altogether, whereas studies in animals consistently show that maternal high fat diet leads to increased risk of metabolic disturbances in offspring, the effects on skeletal health are less clear.
Micronutrient intake
Maternal micronutrient intake may also impact offspring bone health, although the data are difficult to interpret for several reasons. First, the definition of “adequate” maternal vitamin D levels during pregnancy remains contentious [83]. Second, while some studies report an association of infant vitamin D levels and BMC [84–85], others do not, even in extreme vitamin D deficiency. For example, in the absence of vitamin D supplements, total body BMC is higher in summer-born than in winter-born Korean infants [86]; in the US, where vitamin D supplementation is more common, BMC is actually higher in winter-born vs. summer-born infants [87–89]. On the other hand, infants with 1-alpha-hydroxylase deficiency or vitamin D receptor mutations, who cannot synthesize or bind vitamin D, have normal skeletal phenotypes at birth and can be treated with calcium, bypassing vitamin D entirely and suggesting it is not essential for normal bone mineralization [90]. Thus, although it has been reported that offspring of mothers with 25-hydroxyvitamin D levels <25 nM/L during pregnancy have lower total body and lumbar spine BMC at 9 years of age, compared to offspring of mothers with vitamin D levels >50 nM/L during pregnancy [91], it is not clear that this association involves developmental programming.
The associations between postnatal bone health and intrauterine exposure to other micronutrients are similarly modest. For calcium, a study in the Gambia reported no difference in whole body BMC in the first year of life in infants of supplemented vs. non-supplemented women [92]. However, in Indian women, maternal dietary calcium intake in pregnancy is associated with higher offspring BMD at 6 years of age [93]. Finally, in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, maternal magnesium, potassium, and folate consumption in pregnancy are positively associated with bone properties at 9 years of age, although only the latter remains significant after body size adjustment [94].
To summarize, these studies demonstrate that perturbations of maternal caloric intake and/or dietary macronutrient composition in gestation and lactation have lasting effects on offspring skeletal acquisition and maintenance, particularly in animal models. However, the extent to which these changes occur via perinatal developmental programming or via discordant perinatal vs. postnatal somatic growth remains to be determined, particularly in humans.. Nonetheless, when the human and animal data are taken together, what is striking is that any imbalance in maternal diet—too few calories, too much fat, too little protein—seems to alter postnatal bone mass and microarchitecture. Perinatal exposure to calorie restriction or low protein diet is deleterious to both cortical and trabecular bone, while exposure to high fat diet appears to have complex positive and negative effects on different aspects of trabecular and cortical microarchitecture, though more data are needed.
MECHANISMS THAT MAY UNDERLIE THE SKELETAL RESPONSE TO ALTERED PERINATAL NUTRITION
The findings reviewed above demonstrate the need for additional studies in humans to test the hypothesis that the perinatal environment sensitizes the fetus to the expected postnatal nutritional environment [95], which, for the skeleton, might involve a reduction in bone mass that decreases energy requirements but increases osteoporosis risk [96]. The question is whether the mechanisms involve direct perinatal developmental programming of bone, as has been shown for metabolic phenotypes, and/or involve other mechanisms or secondary effects of perinatal programming of other tissues that then influence skeletal metabolism. Given the limited number of studies addressing this question, particularly for bone, any effort to identify mechanisms is necessarily speculative and relies heavily on data from animal studies. Furthermore, there are many potential factors that could underlie the effects of perinatal diet on skeletal acquisition, including changes in the growth hormone/insulin-like growth factor (IGF-1) axis [97–98], or alterations in glucocorticoid levels, such as cortisol [99]. Two other strong candidate hormonal mediators of bone-diet interactions are the adipokine leptin, which may affect bone mass directly [100–102], via hypothalamic-mediated β-adrenergic signaling [42, 103–104], or via suppression of brain-derived serotonin production [41]; and osteocalcin, which has recently been identified as a bone-derived hormone that controls energy metabolism [105–106]. Some data demonstrate that osteocalcin is produced by adipocytes as well as by osteoblasts, suggesting both fat and bone may contribute to glucose homeostasis via osteocalcin production [107]. There may also be direct effects of nutrition on bone cell number, proliferation, and growth rate [32, 108–109]. Here we focus on two potential mechanisms that are currently under investigation: perinatal developmental programming of postnatal leptin levels, and epigenetic changes due to genomic imprinting or DNA methylation.
Perinatal developmental programming of hormone levels
Although the mechanisms involved in developmental programming of metabolism are incompletely understood, one hypothesis is that maternal undernutrition or overnutrition disrupts the perinatal leptin surge, altering hypothalamic development and postnatal regulation of food intake and metabolism [110–111]. In rodents, the perinatal leptin surge promotes neuronal connections between the arcuate and paraventricular nuclei, particularly neuropeptide Y (Npy) and agouti-related protein (AgRP) projections [112–113], and sensitizes hypothalamic neurons to postnatal leptin [114–116]. Maternal caloric restriction in rodents triggers this leptin surge prematurely, resulting in postnatal obesity, peripheral leptin resistance, and impaired leptin transport to the brain [117–119]. Similarly, delay and inhibition of the perinatal leptin surge in a rat model of intrauterine growth restriction causes hypothalamic disorganization [120]. Neonatal leptin treatment rescues postnatal metabolism in mouse pups exposed to maternal calorie restriction, but causes obesity and metabolic problems despite isocaloric intake in offspring exposed to maternal normal diet [121], whereas leptin antagonist treatment in early postnatal life mimics the effects of maternal caloric restriction, causing hyperleptinemia and obesity [122]. Similarly, maternal caloric restriction triggers insulin resistance and obesity in postnatally ad libitum-fed mice, but not in leptin-treated or postnatally calorie-restricted mice [49, 114].
In maternal overnutrition, the mechanisms involved in developmental programming are less clear. In rodent models, it has been suggested that neonatal hyperleptinemia alters leptin receptor expression and contributes to selective leptin resistance in the arcuate nucleus of the hypothalamus, as is seen in postnatal hyperleptinemia [15]. High fat diet has also been shown to promote the growth of neurons expressing orexigenic peptides in rats [123], which could increase fat mass and consequently leptin levels. Increased fat intake by rat dams during late pregnancy and lactation is associated with higher offspring fat mass and leptin levels at weaning, but lower leptin levels than controls thereafter [124].
Unfortunately, the existence and timing of a perinatal leptin surge in humans remain unclear, although it appears that fetal leptin levels are high just prior to birth and fall in the first week of life. What is clear is that perinatal undernutrition or overnutrition alters postnatal leptin levels. Infants who are born large for gestational age (LGA) have higher than normal leptin levels [125–126], which persist into childhood [127]. Conversely, children born small for gestational age (SGA) have abnormally low leptin levels at birth [128–129] and in childhood [130]. Further, breast milk leptin levels vary with maternal diet, and it has even been suggested that this variation—and the absence of leptin in infant formula—may contribute to the protective effect of breastfeeding on postnatal obesity [131–132]. However, the possible ramifications of leptin levels on skeletal acquisition are unclear. While some studies report a positive association between neonatal skeletal size, bone mineral density and umbilical cord blood leptin levels [133], others find no such association [69].
How might programming of leptin circuits in the brain affect postnatal bone mass? Leptin has complex effects on trabecular and cortical bone, both directly and via the hypothalamus. In wildtype mice, intracerebroventricular (ICV) leptin infusion increases sympathetic tone via binding to hypothalamic receptors, which then activates β-adrenergic receptors in osteoblasts, stimulating bone resorption and decreasing bone formation [101, 103–104]. However, a recent study reports that ICV leptin increases bone formation in the leptin-deficient ob/ob mouse [134]. In addition, there is some evidence that leptin has peripheral anabolic effects, promoting commitment of human-derived mesenchymal stem cell lines (MSCs) to bone vs. fat lineages, and increasing periosteal bone formation and inhibiting ovariectomy-induced bone loss in rodent models [100, 102, 135]. However, a subsequent study in mice found that leptin receptor (Lepr) deletion in osteoblasts did not alter skeletal phenotype, suggesting leptin has no direct effect on osteoblasts [136]. Accordingly, there are at least two mechanisms by which perinatal programming could affect postnatal skeletal development by altered leptin levels: 1) via direct effects on osteoblast progenitors and mature osteoblasts, and/or 2) via alterations in leptin-induced, hypothalamic-mediated β-adrenergic signaling in bone. In addition to these potential direct mechanisms, there are likely indirect effects of leptin on bone homeostasis via modulation of other central or peripheral hormones, such as osteocalcin and adiponectin [137]. However, it is not clear whether more leptin is always better for bone. Injections of leptin in pregnant rats, which should signal greater energy availability, actually reduce offspring postnatal skeletal growth and cortical bone mass, as well as adiposity [138]. Furthermore, one recent study has challenged the notion that perinatal leptin is primarily responsible for postnatal metabolic dysregulation. In ob/ob mice, which lack leptin, pups exposed to maternal calorie restriction followed by catch-up growth have higher body mass than controls, demonstrating that programming induced by maternal diet does not occur through leptin alone [139].
While the concept of perinatal developmental programming of bone is an intriguing one, more data are needed to establish whether the skeletal changes induced by maternal diet involve programming of leptin or other hormones, and to understand how interactions of leptin levels in the perinatal, lactational, and post-weaning time frames affect postnatal skeletal acquisition and maintenance.
Epigenetic changes
Epigenetic changes in gene expression, such as altered DNA methylation, have been implicated in a wide range of human diseases, from cancer [140] to allergy and asthma [141] to the function of specific genes including the glucocorticoid receptor gene [142]. Given the enormous potential of this mechanism to explain how perinatal influences might cause lifelong changes in offspring physiology, interest in potential epigenetic effects of maternal diet on offspring bone mass is high. Although this research is in its early stages, there are some tantalizing hints. For example, perinatal famine exposure, as in the Dutch Hunger Winter, has been associated with altered methylation of the IGF2 gene in adulthood [143], which could also influence bone mass.
Diet has also been shown to alter postnatal transcriptional activity of multiple genes that may affect bone mass. In rats, offspring of mothers exposed to high fat diet have altered postnatal expression of the leptin receptor (ObRb), pro-opiomelanocortin (Pomc), neuropeptide Y (Npy), and signal transducer and activator of transcription 3 (Stat3) [144–145]. Perinatal calorie restriction combined with postnatal high fat diet in rats is also associated with significant changes in Pomc, AgRP, Npy, and ObRb expression, along with high leptin and insulin levels [146]. Offspring of mothers exposed to high fat diet also have increased preference for sugary and fatty foods, and show DNA hypomethylation in genes associated with reward, including opioids and dopamine [147]. At the other extreme of energy availability, offspring from calorie-restricted rats have altered expression of genes involved in lipid metabolism and glucose homoeostasis, including higher expression of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), lower expression of peroxisome proliferator-activated receptor (Ppar)-alpha, the glucocorticoid receptor, and phosphoenolpyruvate carboxykinase (Pepck), and exhibit increased methylation of the PPARalpha promoter and glucocorticoid receptor genes, but neonatal leptin treatment normalizes both gene expression and metabolic phenotype [148].
Among the most intriguing observations is that high fat diet-induced changes can persist for multiple generations via epigenetic mechanisms. Four generations of mice continuously raised on high fat diet exhibit progressively increasing fat mass despite no changes in food intake, along with changes in gene expression including colony-stimulating factor 3 (also known as granulocyte colony-stimulating factor) and Nocturnin [149]. Both male and female mice exposed to maternal high fat and/or overfeeding during lactation can transmit reduced insulin sensitivity and increased body length to their offspring through epigenetic inheritance [150–151].
These studies provide a template for understanding how perinatal diet might influence offspring metabolism, and illustrate the importance of epigenetic mechanisms in moderating between the individual and the environment. The great challenges going forward will be to determine whether epigenetic regulation has direct effects on offspring bone mass and microarchitecture, and whether deleterious epigenetic changes might be modified postnatally to reduce disease risk.
UNANSWERED QUESTIONS AND FUTURE DIRECTIONS
Due to the growing evidence that perintatal programming influences metabolic and cardiovascular disease risk in humans, there is tremendous interest in understanding how the perinatal environment, including maternal diet and other factors such as smoking, exercise, and body mass, influence postnatal skeletal acquisition and maintenance. Studies in animal models demonstrate that both undernutrition and overnutrition, as well as low protein intake, are deleterious to offspring bone mass. In humans, birthweight, as a surrogate for maternal perinatal nutrition, is linked to adult bone mass, but the proportion of variance in bone mass explained by birthweight tends to be small. It is also unclear whether this association results from perinatal developmental programming of postnatal skeletal acquisition, or simply reflects the normal relationship between skeletal size and bone mass. However, while perinatal developmental programming of the skeleton in humans remains to be definitively established, there is some evidence for an influence of developmental programming of bone in animal models. Further studies are needed to determine whether the effects of perinatal programming on the skeleton are direct effects on bone cells and/or bone metabolism, or are indirect effects due to alterations in metabolism and energy utilization.
Maternal perinatal diet may influence offspring bone mass via perinatal developmental programming of leptin levels, epigenetic mechanisms such as DNA methylation or other types of genomic imprinting, or other mechanisms such as direct effects on bone cell differentiation, proliferation or gene expression. More data on the nature of a perinatal leptin surge in humans, as well as a better understanding of how the perinatal leptin surge influences bone mass, would be particularly helpful. Another key area for future research is the observation that the effects of maternal diet vary in gestation versus lactation, particularly in terms of neuronal development and programming of feeding circuits. For example, maternal calorie restriction in rats during gestation only, followed by rapid catch-up growth in lactation, is associated with obesity and hyperleptinemia, while offspring of mothers whose calorie restriction continued through lactation do not exhibit these abnormalities [152]. Understanding the relative importance of diet during gestation vs. lactation in establishing postnatal bone mass may shed light on the mechanisms involved.
Finally, the hypothesis that perinatal developmental programming of skeletal phenotypes is adaptive should be evaluated in a rigorous hypothesis testing framework that considers pathology and constraint as alternatives to adaptation [31]. Once the mechanisms underlying these developmental responses have been established, the next goal will be to develop strategies to improve bone mass and reduce osteoporosis risk in affected individuals.
Acknowledgments
Funding for this project was provided by NIH F32HD060419, T32DK007028, and RC1AR058389.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rich-Edwards JW, Kleinman K, Michels KB, Stampfer MJ, Manson JE, Rexrode KM, Hibert EN, Willett WC. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. Bmj. 2005;330:1115. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 6.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ozanne SE, Fernandez-Twinn D, Hales CN. Fetal growth and adult diseases. Semin Perinatol. 2004;28:81–87. doi: 10.1053/j.semperi.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Buhrer C, Harder T, Plagemann A, Vetter K, Kordonouri O. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28:1745–1750. doi: 10.2337/diacare.28.7.1745. [DOI] [PubMed] [Google Scholar]
- 11.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? Bmj. 1994;308:942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Bmj. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Liang L, Junfen FU, Lizhong DU. Metabolic syndrome in obese children born large for gestational age. Indian J Pediatr. 2007;74:561–565. doi: 10.1007/s12098-007-0108-9. [DOI] [PubMed] [Google Scholar]
- 15.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- 16.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010;83:325–331. doi: 10.1095/biolreprod.110.084517. [DOI] [PubMed] [Google Scholar]
- 17.Castro LC, Avina RL. Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol. 2002;14:601–606. doi: 10.1097/00001703-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 20.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4:293–298. doi: 10.1375/1369052012605. [DOI] [PubMed] [Google Scholar]
- 21.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the Predictive Adaptive Response hypothesis. Proc Natl Acad Sci U S A. 2006;103:12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–59. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 25.Claris O, Beltrand J, Levy-Marchal C. Consequences of intrauterine growth and early neonatal catch-up growth. Semin Perinatol. 2010;34:207–210. doi: 10.1053/j.semperi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 28.Wells JC. The thrifty phenotype: An adaptation in growth or metabolism? Am J Hum Biol. 2011;23:65–75. doi: 10.1002/ajhb.21100. [DOI] [PubMed] [Google Scholar]
- 29.Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 30.Wells JC. Environmental quality, developmental plasticity and the thrifty phenotype: a review of evolutionary models. Evol Bioinform Online. 2007;3:109–120. [PMC free article] [PubMed] [Google Scholar]
- 31.Ellison PT, Jasienska G. Constraint, pathology, and adaptation: how can we tell them apart? Am J Hum Biol. 2007;19:622–630. doi: 10.1002/ajhb.20662. [DOI] [PubMed] [Google Scholar]
- 32.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 33.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 34.Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 35.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Lecka-Czernik B, Suva LJ. Resolving the Two "Bony" Faces of PPAR-gamma. PPAR Res. 2006;2006:27489. doi: 10.1155/PPAR/2006/27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, Chua SC, Jr, Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karsenty G, Yadav VK. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med. 2011;62:323–331. doi: 10.1146/annurev-med-090710-133426. [DOI] [PubMed] [Google Scholar]
- 41.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 43.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greer FR, Krebs NF. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578–585. doi: 10.1542/peds.2005-2822. [DOI] [PubMed] [Google Scholar]
- 46.Gordon CM, Bachrach LK, Carpenter TO, Karsenty G, Rauch F. Bone health in children and adolescents: a symposium at the annual meeting of the Pediatric Academic Societies/Lawson Wilkins Pediatric Endocrine Society, May 2003. Curr Probl Pediatr Adolesc Health Care. 2004;34:226–242. doi: 10.1016/j.cppeds.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 50.de Bono S, Schoenmakers I, Ceesay M, Mendy M, Laskey MA, Cole TJ, Prentice A. Birth weight predicts bone size in young adulthood at cortical sites in men and trabecular sites in women from The Gambia. Bone. 2010;46:1316–1321. doi: 10.1016/j.bone.2010.01.381. [DOI] [PubMed] [Google Scholar]
- 51.Schlussel MM, de Castro JA, Kac G, da Silva AA, Cardoso VC, Bettiol H, Barbieri MA. Birth weight and bone mass in young adults from Brazil. Bone. 2010;46:957–963. doi: 10.1016/j.bone.2010.01.365. [DOI] [PubMed] [Google Scholar]
- 52.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 53.Harvey NC, Mahon PA, Robinson SM, Nisbet CE, Javaid MK, Crozier SR, Inskip HM, Godfrey KM, Arden NK, Dennison EM, Cooper C. Different indices of fetal growth predict bone size and volumetric density at 4 years of age. J Bone Miner Res. 2010;25:920–927. doi: 10.1359/jbmr.091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford) 2003;42:791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 55.Harrast SD, Kalkwarf HJ. Effects of gestational age, maternal diabetes, and intrauterine growth retardation on markers of fetal bone turnover in amniotic fluid. Calcif Tissue Int. 1998;62:205–208. doi: 10.1007/s002239900418. [DOI] [PubMed] [Google Scholar]
- 56.Fricke O, Semler O, Stabrey A, Tutlewski B, Remer T, Herkenrath P, Schoenau E. High and low birth weight and its implication for growth and bone development in childhood and adolescence. J Pediatr Endocrinol Metab. 2009;22:19–30. doi: 10.1515/jpem.2009.22.1.19. [DOI] [PubMed] [Google Scholar]
- 57.Hovi P, Andersson S, Jarvenpaa AL, Eriksson JG, Strang-Karlsson S, Kajantie E, Makitie O. Decreased bone mineral density in adults born with very low birth weight: a cohort study. PLoS Med. 2009;6:e1000135. doi: 10.1371/journal.pmed.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalziel SR, Fenwick S, Cundy T, Parag V, Beck TJ, Rodgers A, Harding JE. Peak bone mass after exposure to antenatal betamethasone and prematurity: follow-up of a randomized controlled trial. J Bone Miner Res. 2006;21:1175–1186. doi: 10.1359/jbmr.060516. [DOI] [PubMed] [Google Scholar]
- 59.Ibanez L, de Zegher F. Puberty and prenatal growth. Mol Cell Endocrinol. 2006;254–255:22–25. doi: 10.1016/j.mce.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Knight BS, Pennell CE, Adamson SL, Lye SJ. The impact of murine strain and sex on postnatal development after maternal dietary restriction during pregnancy. J Physiol. 2007;581:873–881. doi: 10.1113/jphysiol.2006.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romano T, Wark JD, Wlodek ME. Calcium supplementation does not rescue the programmed adult bone deficits associated with perinatal growth restriction. Bone. 2010;47:1054–1063. doi: 10.1016/j.bone.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Engelbregt MJ, van Weissenbruch MM, Lips P, van Lingen A, Roos JC, Delemarre-van de Waal HA. Body composition and bone measurements in intra-uterine growth retarded and early postnatally undernourished male and female rats at the age of 6 months: comparison with puberty. Bone. 2004;34:180–186. doi: 10.1016/j.bone.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–878. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 65.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–641. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- 66.Coly AN, Milet J, Diallo A, Ndiaye T, Benefice E, Simondon F, Wade S, Simondon KB. Preschool stunting, adolescent migration, catch-up growth, and adult height in young senegalese men and women of rural origin. J Nutr. 2006;136:2412–2420. doi: 10.1093/jn/136.9.2412. [DOI] [PubMed] [Google Scholar]
- 67.Misra M. Long-term skeletal effects of eating disorders with onset in adolescence. Ann N Y Acad Sci. 2008;1135:212–218. doi: 10.1196/annals.1429.002. [DOI] [PubMed] [Google Scholar]
- 68.Hammami M, Walters JC, Hockman EM, Koo WW. Disproportionate alterations in body composition of large for gestational age neonates. J Pediatr. 2001;138:817–821. doi: 10.1067/mpd.2001.114018. [DOI] [PubMed] [Google Scholar]
- 69.Akcakus M, Kurtoglu S, Koklu E, Kula M, Koklu S. The relationship between birth weight leptin and bone mineral status in newborn infants. Neonatology. 2007;91:101–106. doi: 10.1159/000097126. [DOI] [PubMed] [Google Scholar]
- 70.Koklu E, Akcakus M, Narin F, Saraymen R. The relationship between birth weight, oxidative stress and bone mineral status in newborn infants. J Paediatr Child Health. 2007;43:667–672. doi: 10.1111/j.1440-1754.2007.01184.x. [DOI] [PubMed] [Google Scholar]
- 71.Mimouni F, Steichen JJ, Tsang RC, Hertzberg V, Miodovnik M. Decreased bone mineral content in infants of diabetic mothers. Am J Perinatol. 1988;5:339–343. doi: 10.1055/s-2007-999720. [DOI] [PubMed] [Google Scholar]
- 72.Ogueh O, Khastgir G, Studd J, Jones J, Alaghband-Zadeh J, Johnson MR. Maternal and fetal plasma levels of markers of bone metabolism in gestational diabetic pregnancies. Early Hum Dev. 1998;53:155–161. doi: 10.1016/s0378-3782(98)00048-6. [DOI] [PubMed] [Google Scholar]
- 73.Mughal MZ, Eelloo J, Roberts SA, Maresh M, Ward KA, Ashby R, Sibley CP, Adams JE. Body composition and bone status of children born to mothers with type 1 diabetes mellitus. Arch Dis Child. 2010;95:281–285. doi: 10.1136/adc.2008.151555. [DOI] [PubMed] [Google Scholar]
- 74.Jones G, Riley MD, Dwyer T. Maternal diet during pregnancy is associated with bone mineral density in children: a longitudinal study. Eur J Clin Nutr. 2000;54:749–756. doi: 10.1038/sj.ejcn.1601082. [DOI] [PubMed] [Google Scholar]
- 75.Yin J, Dwyer T, Riley M, Cochrane J, Jones G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur J Clin Nutr. 2010;64:131–137. doi: 10.1038/ejcn.2009.117. [DOI] [PubMed] [Google Scholar]
- 76.Cole ZA, Gale CR, Javaid MK, Robinson SM, Law C, Boucher BJ, Crozier SR, Godfrey KM, Dennison EM, Cooper C. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J Bone Miner Res. 2009;24:663–668. doi: 10.1359/jbmr.081212. [DOI] [PubMed] [Google Scholar]
- 77.Verhaeghe J, van Bree R, van Herck E, Rummens K, Vercruysse L, Bouillon R, Pijnenborg R. Pathogenesis of fetal hypomineralization in diabetic rats: evidence for delayed bone maturation. Pediatr Res. 1999;45:209–217. doi: 10.1203/00006450-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 79.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang C, Oest ME, Jones JC, Prater MR. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res B Dev Reprod Toxicol. 2009;86:362–369. doi: 10.1002/bdrb.20204. [DOI] [PubMed] [Google Scholar]
- 82.Lanham SA, Roberts C, Hollingworth T, Sreekumar R, Elahi MM, Cagampang FR, Hanson MA, Oreffo RO. Maternal high-fat diet: effects on offspring bone structure. Osteoporos Int. 2010;21:1703–1714. doi: 10.1007/s00198-009-1118-4. [DOI] [PubMed] [Google Scholar]
- 83.Hewison M, Adams JS. Vitamin D insufficiency and skeletal development in utero. J Bone Miner Res. 2010;25:11–13. doi: 10.1002/jbmr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Viljakainen HT, Korhonen T, Hytinantti T, Laitinen EK, Andersson S, Makitie O, Lamberg-Allardt C. Maternal vitamin D status affects bone growth in early childhood-a prospective cohort study. Osteoporos Int. 2011;22:883–891. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, Andersson S, Laitinen K, Lamberg-Allardt C. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 86.Namgung R, Tsang RC, Lee C, Han DG, Ho ML, Sierra RI. Low total body bone mineral content and high bone resorption in Korean winter-born versus summer-born newborn infants. J Pediatr. 1998;132:421–425. doi: 10.1016/s0022-3476(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 87.Namgung R, Mimouni F, Campaigne BN, Ho ML, Tsang RC. Low bone mineral content in summer-born compared with winter-born infants. J Pediatr Gastroenterol Nutr. 1992;15:285–288. doi: 10.1097/00005176-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Namgung R, Tsang RC, Specker BL, Sierra RI, Ho ML. Reduced serum osteocalcin and 1,25-dihydroxyvitamin D concentrations and low bone mineral content in small for gestational age infants: evidence of decreased bone formation rates. J Pediatr. 1993;122:269–275. doi: 10.1016/s0022-3476(06)80132-0. [DOI] [PubMed] [Google Scholar]
- 89.Namgung R, Tsang RC, Specker BL, Sierra RI, Ho ML. Low bone mineral content and high serum osteocalcin and 1,25-dihydroxyvitamin D in summer- versus winter-born newborn infants: an early fetal effect? J Pediatr Gastroenterol Nutr. 1994;19:220–227. doi: 10.1097/00005176-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 90.Malloy PJ, Feldman D. Genetic disorders and defects in vitamin d action. Endocrinol Metab Clin North Am. 2010;39:333–346. doi: 10.1016/j.ecl.2010.02.004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper CA. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 92.Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83:657–666. doi: 10.1093/ajcn.83.3.657. [DOI] [PubMed] [Google Scholar]
- 93.Ganpule A, Yajnik CS, Fall CH, Rao S, Fisher DJ, Kanade A, Cooper C, Naik S, Joshi N, Lubree H, Deshpande V, Joglekar C. Bone mass in Indian children--relationships to maternal nutritional status and diet during pregnancy: the Pune Maternal Nutrition Study. J Clin Endocrinol Metab. 2006;91:2994–3001. doi: 10.1210/jc.2005-2431. [DOI] [PubMed] [Google Scholar]
- 94.Tobias JH, Steer CD, Emmett PM, Tonkin RJ, Cooper C, Ness AR. Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos Int. 2005;16:1731–1741. doi: 10.1007/s00198-005-1912-6. [DOI] [PubMed] [Google Scholar]
- 95.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 96.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 97.Fall C, Hindmarsh P, Dennison E, Kellingray S, Barker D, Cooper C. Programming of growth hormone secretion and bone mineral density in elderly men: a hypothesis. J Clin Endocrinol Metab. 1998;83:135–139. doi: 10.1210/jcem.83.1.4487. [DOI] [PubMed] [Google Scholar]
- 98.Rosen CJ. IGF-I and osteoporosis. Clin Lab Med. 2000;20:591–602. [PubMed] [Google Scholar]
- 99.Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- 100.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 101.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 102.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 103.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 104.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 105.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010;95:3502–3506. doi: 10.1210/jc.2009-2557. [DOI] [PubMed] [Google Scholar]
- 108.Javaid MK, Cooper C. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab. 2002;16:349–367. doi: 10.1053/beem.2002.0199. [DOI] [PubMed] [Google Scholar]
- 109.Sayer AA, Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005;81:735–744. doi: 10.1016/j.earlhumdev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Bouret SG, Simerly RB. Development of leptin-sensitive circuits. J Neuroendocrinol. 2007;19:575–582. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 111.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94:79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 113.Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301. doi: 10.1111/j.1399-0004.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 114.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 115.Carlo AS, Pyrski M, Loudes C, Faivre-Baumann A, Epelbaum J, Williams LM, Meyerhof W. Leptin sensitivity in the developing rat hypothalamus. Endocrinology. 2007;148:6073–6082. doi: 10.1210/en.2007-0822. [DOI] [PubMed] [Google Scholar]
- 116.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 117.Krechowec SO, Vickers M, Gertler A, Breier BH. Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol. 2006;189:355–363. doi: 10.1677/joe.1.06679. [DOI] [PubMed] [Google Scholar]
- 118.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Mogami H, Ogawa Y, Fujii S. Neonatal exposure to leptin augments diet-induced obesity in leptin-deficient Ob/Ob mice. Obesity (Silver Spring) 2008;16:1289–1295. doi: 10.1038/oby.2008.57. [DOI] [PubMed] [Google Scholar]
- 119.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Coupe B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 121.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 122.Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153–1160. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- 123.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trottier G, Koski KG, Brun T, Toufexis DJ, Richard D, Walker CD. Increased fat intake during lactation modifies hypothalamic-pituitary-adrenal responsiveness in developing rat pups: a possible role for leptin. Endocrinology. 1998;139:3704–3711. doi: 10.1210/endo.139.9.6208. [DOI] [PubMed] [Google Scholar]
- 125.Wolf HJ, Ebenbichler CF, Huter O, Bodner J, Lechleitner M, Foger B, Patsch JR, Desoye G. Fetal leptin and insulin levels only correlate inlarge-for-gestational age infants. Eur J Endocrinol. 2000;142:623–629. doi: 10.1530/eje.0.1420623. [DOI] [PubMed] [Google Scholar]
- 126.Yang SW, Kim SY. The relationship of the levels of leptin, insulin-like growth factor-I and insulin in cord blood with birth size, ponderal index, and gender difference. J Pediatr Endocrinol Metab. 2000;13:289–296. doi: 10.1515/jpem.2000.13.3.289. [DOI] [PubMed] [Google Scholar]
- 127.Giapros V, Evagelidou E, Challa A, Kiortsis D, Drougia A, Andronikou S. Serum adiponectin and leptin levels and insulin resistance in children born large for gestational age are affected by the degree of overweight. Clin Endocrinol (Oxf) 2007;66:353–359. doi: 10.1111/j.1365-2265.2006.02736.x. [DOI] [PubMed] [Google Scholar]
- 128.Harigaya A, Nagashima K, Nako Y, Morikawa A. Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab. 1997;82:3281–3284. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- 129.Strocchio L, Bozzola E, Cerbo RM, Meazza C, Travaglino P, Pagani S, Laarej K, Stronati M, Bozzola M. [Changes in circulating levels of adiponectin and leptin in children during the first two years of life] Minerva Pediatr. 2007;59:739–744. [PubMed] [Google Scholar]
- 130.Albertsson-Wikland K, Boguszewski M, Karlberg J. Children born small-for-gestational age: postnatal growth and hormonal status. Horm Res. 1998;49 Suppl 2:7–13. [PubMed] [Google Scholar]
- 131.Savino F, Costamagna M, Prino A, Oggero R, Silvestro L. Leptin levels in breast-fed and formula-fed infants. Acta Paediatr. 2002;91:897–902. doi: 10.1080/080352502760272551. [DOI] [PubMed] [Google Scholar]
- 132.Pico C, Jilkova ZM, Kus V, Palou A, Kopecky J. Perinatal programming of body weight control by leptin: putative roles of AMP kinase and muscle thermogenesis. Am J Clin Nutr. 2011 doi: 10.3945/ajcn.110.000752. [DOI] [PubMed] [Google Scholar]
- 133.Javaid MK, Godfrey KM, Taylor P, Robinson SM, Crozier SR, Dennison EM, Robinson JS, Breier BR, Arden NK, Cooper C. Umbilical cord leptin predicts neonatal bone mass. Calcif Tissue Int. 2005;76:341–347. doi: 10.1007/s00223-004-1128-3. [DOI] [PubMed] [Google Scholar]
- 134.Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, She JX, Della-Fera MA, Baile CA. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011 doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 135.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 136.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gravenstein KS, Napora JK, Short RG, Ramachandran R, Carlson OD, Metter EJ, Ferrucci L, Egan JM, Chia CW. Cross-sectional evidence of a signaling pathway from bone homeostasis to glucose metabolism. J Clin Endocrinol Metab. 2011;96:E884–E890. doi: 10.1210/jc.2010-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nilsson C, Swolin-Eide D, Ohlsson C, Eriksson E, Ho HP, Bjorntorp P, Holmang A. Reductions in adipose tissue and skeletal growth in rat adult offspring after prenatal leptin exposure. J Endocrinol. 2003;176:13–21. doi: 10.1677/joe.0.1760013. [DOI] [PubMed] [Google Scholar]
- 139.Cottrell EC, Martin-Gronert MS, Fernandez-Twinn DS, Luan J, Berends LM, Ozanne SE. Leptin-independent programming of adult body weight and adiposity in mice. Endocrinology. 2011;152:476–482. doi: 10.1210/en.2010-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 141.Bousquet J, Jacot W, Yssel H, Vignola AM, Humbert M. Epigenetic inheritance of fetal genes in allergic asthma. Allergy. 2004;59:138–147. doi: 10.1046/j.1398-9995.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 142.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 143.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 145.Page KC, Malik RE, Ripple JA, Anday EK. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1049–R1057. doi: 10.1152/ajpregu.90585.2008. [DOI] [PubMed] [Google Scholar]
- 146.Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol. 2007;193:31–37. doi: 10.1677/joe.1.07017. [DOI] [PubMed] [Google Scholar]
- 147.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci U S A. 2007;104:12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, Mohsen-Kanson T, Amri EZ, Ailhaud G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010;51:2352–2361. doi: 10.1194/jlr.M006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pentinat T, Ramon-Krauel M, Cebria J, Diaz R, Jimenez-Chillaron JC. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology. 2010;151:5617–5623. doi: 10.1210/en.2010-0684. [DOI] [PubMed] [Google Scholar]
- 152.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]