Abstract
We had previously reported that Mitsunobu-based introduction of alkyl substituents onto the imidazole N(π)-position of a key histidine residue in phosphothreonine-containing peptides can impart high binding affinity against the polo box domain of polo like kinase 1. Our current paper investigates the mechanism leading to this N(π)-alkylation and provides synthetic methodologies that permit the facile synthesis of histidine N(π)-modified peptides. These agents represent new and potentially important tools for biological studies.
Introduction
The serine/threonine specific polo-like kinase 1 (Plk1) is one of four Plks involved in cell cycle regulation. Plk1 is of particular interest as a therapeutic target, due to its ability to promote oncogenic transformation.1–3 Pharmacological inhibition of Plk1 is being approached through kinase catalytic site-directed agents.4–11 Inhibitors of Plk1 function are also being investigated that interfere with the actions of its C-terminal polo-box domains (PBDs). These latter homologous protein modules facilitate sub-cellular localization of Plk1 by recognizing and binding to phosphoserine (pSer)/phosphothreonine (pThr)-containing sequences.12,13
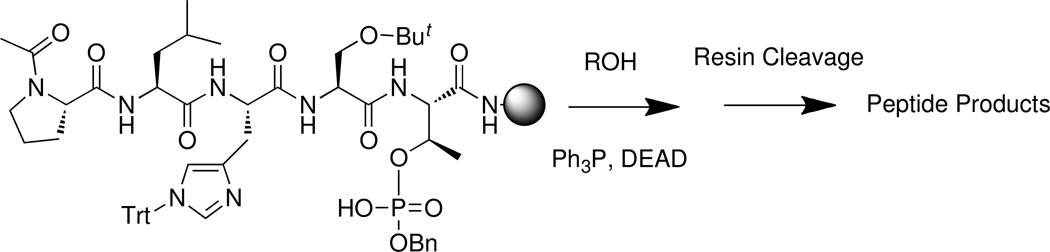
The polo-box interacting protein 1 (PBIP1) is a Plk1 substrate, which undergoes phosphorylation at its T78 residue to form a Plk1 PBD-binding site that is critical for recruiting Plk1 to the interphase and mitotic kinetochores.14 We had previously shown that the wild-type PBIP1-derived five-mer sequence, 74-PLHSpT-78 represents a minimal peptide (1) that specifically interacts with the Plk1 PBD with high affinity (Kd = 0.45 µM).15 More recently, in an effort to investigate whether reduction in the phosphoryl anionic charge could also be accomplished with retention of binding affinity, we performed synthetic experiments to esterify one of the two pThr phosphoryl hydroxyls of the parent peptide 1.16 Our approach was to conduct solid-phase Mitsunobu coupling17 of a series of alcohols with the protected resin-bound peptide, Ac- Pro-Leu-His[N(τ)Trt]-Ser(OBut)-Thr[OPO(OBn)(OH)]-amide,18 which contained a single free phosphoryl hydroxy group (Figure 1). Applications of Mitsunbu chemistries to resin-supported substrates and to the synthesis of phosphoryl esters are known.19,20
Figure 1.
Solid-phase Mistunobu reaction
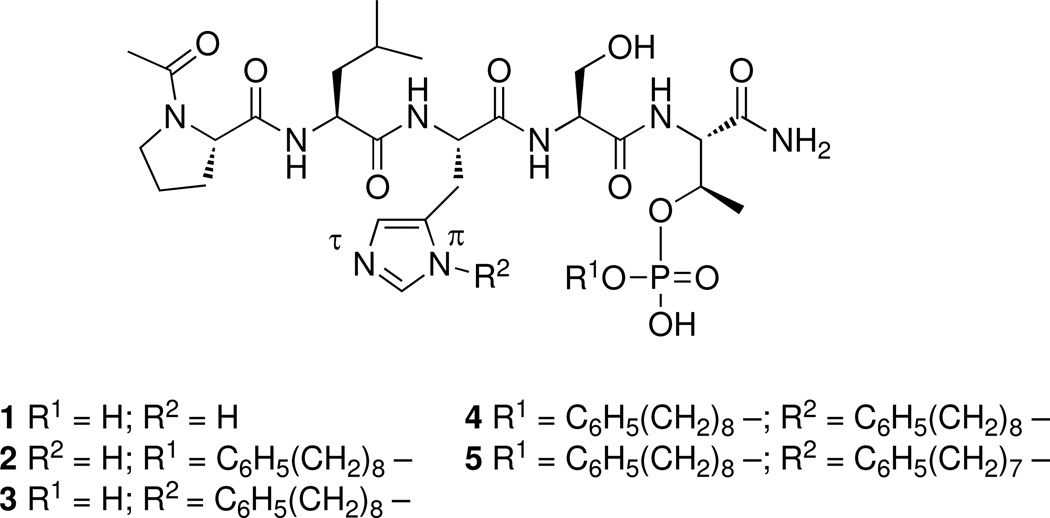
Following coupling reactions, peptides were cleaved from the resin under mild acid conditions. In addition to the expected phosphoryl esters, unanticipated synthetic peptide byproducts were isolated having the same molecular weights as the intended products. These byproducts exhibited exceptionally high affinities in Plk1 PBD binding assays and in some these affinities were approximately three orders of magnitude greater than the parent peptide (1). A Plk1 PBD co-crystal structure of the highest affinity peptide byproduct, which was obtained in small amount along with the expected major product (2) following Mitsunobu reaction with C6H5(CH2)8-OH, showed it to be the modified peptide 3, in which alkylation had occurred on the histidine imidazole N(π) group (Figure 2).16 The high affinity and unexpected nature of this histidine adduct prompted us to investigate potential mechanisms leading to its formation and to develop synthetic methodologies to prepare related peptide analogues.
Figure 2.
Structures of peptides discussed in the text.
Results and Discussion
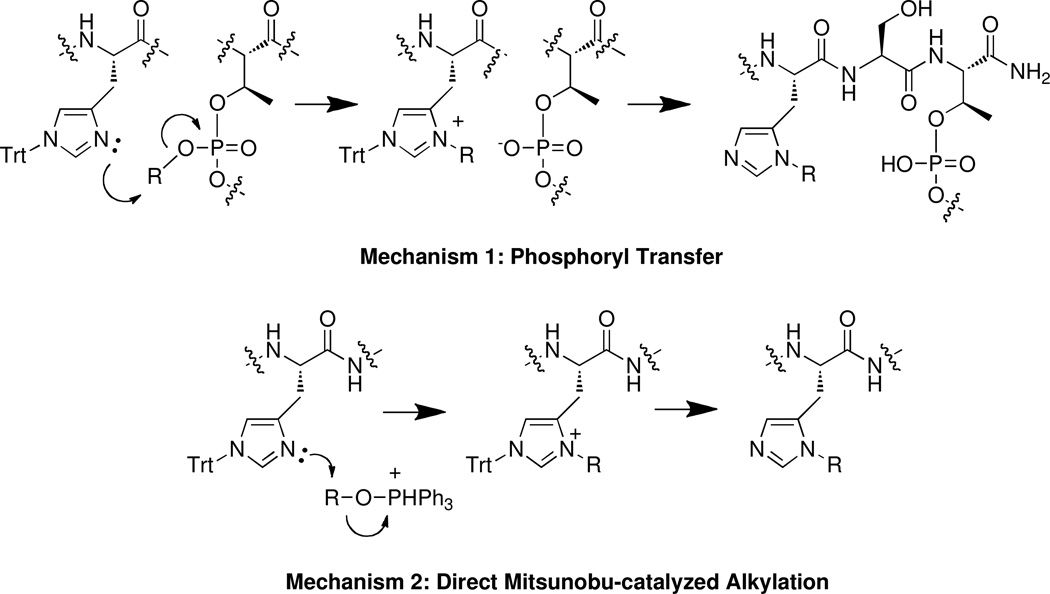
We rationalized that alkylation at the His N(π) of peptide 3 could potentially have occurred either indirectly by intramolecular alkyl transfer of the phosphoryl ester group (Mechanism 1, Figure 3), or directly through alkylation by the alcohol reactants under Mitsunobu conditions (Mechanism 2, Figure 3). There are literature precedents for both mechanisms. For example, in 1-substituted imidazoles, alkylation of the 3-nitrogen (corresponding to the N(π) position of histitine) can be effected by phosphoryl esters. However these reactions require elevated temperatures.21,22 Similarly, alkylation of imidazole by alcohols can be achieved using Mitsunobu chemistries.23–25
Figure 3.
Two possible mechanisms of histidine alkylation.
In order to examine whether phosphoryl ester-mediated transfer was involved in the formation of 3 (Mechanism 1), Mistunobu esterification of the free phosphoryl hydroxyl of resin-bound N(α)-Fmoc-O-(benzyl phosphoryl)-threonine using C6H5(CH2)8-OH was conducted prior to coupling of the N(α)-Fmoc-[N(τ)-Trt]-His residue. Following Mitsunobu phosphoryl ester formation, the resin was washed and peptide synthesis was completed in standard fashion using Fmoc protocols. A portion of the resulting finished resin was cleaved and examined by HPLC. The chromatogram showed the presence of the peptide phosphoryl ester 2 with no evidence of product 3. This indicated that N(π) alkylation did not occur by intramolecular transfer from the phosphoryl ester.
Direct Mitsunobu-induced N(π) alkylation (Mechanism 2) was examined next by again subjecting a portion of the above, fully-formed resin-bound peptide to a second Mitsunobu coupling with C6H5(CH2)8-OH prior to resin cleavage. HPLC analysis of the resulting peptide products showed that in addition to 2, a new product was obtained having mass consistent with bis-alkylated product (4, Figure 2). This indicated that histidine alkylation had occurred during the second Mitsunobu reaction. In order to unambiguously discriminate whether the additional alkyl adduct originated directly from the alcohol used in the Mitsunobu reacton or from pre-formed phosphoryl ester under Mitsunobu catalysis, the above reaction was repeated using a homologous alcohol having a shorter chain length [C6H5(CH2)7-OH)] as compared with the C6H5(CH2)8-OH) used in the second Mitsunobu reaction. This allowed differentiation between sources of the alkylating species. In this case, it was found that the mass of the bis-adduct was consistent with addition of C6H5(CH2)7 – (peptide 5, Figure 2), indicating that histidine alkylation occurred directly from the alcohol used in the second Mitsunobu reaction (Mechanism 2). We concluded from these studies that the high affinity byproduct 3 was formed by direct alkylation with C6H5(CH2)8-OH during the Mitsunobu phosphoryl esertification reaction.
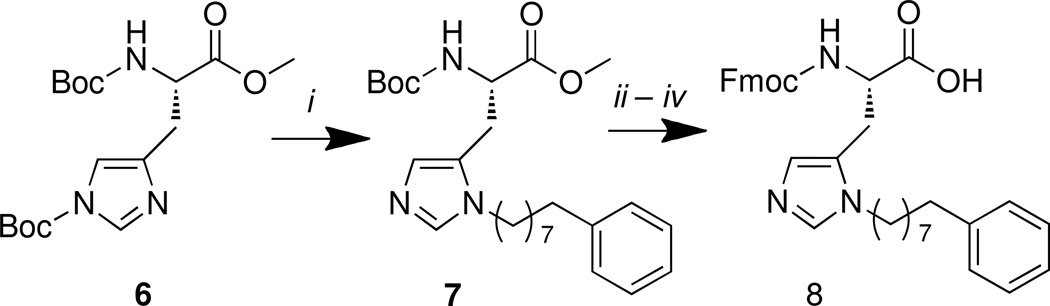
As exemplified by 3, N(π)-alkyl-histidine residues can impart exceptional Plk1 PBD-binding affinity.16 However, the adducts in the referenced study were obtained as minor reaction byproducts. In order to facilitate a broader application of N(π)-alkyl-histidine analogues, we undertook the preparation of N(α)-Fmoc-[N(π)-R]-His [8 for R = C6H5(CH2)8 –], which is suitably protected for incorporation of histidine derivatives into peptides using standard Fmoc protocols. We employed Hodges’ regiospecific synthesis of N(π)-substituted histidines, which relies on the use of N(τ)-Boc-protected histidines and alkyl triflates as the alkyting species.26 Our approach started with N(α), N(τ)-bis(tert-butoxycarbonyl)-L-histidine methyl ester (6).26 A solution of 8-phenyloctan-1-ol in CH2Cl2 at −75 °C was treated with triflic anhydride and diisopropylethylamine (DIEPA, one equivalent) to form the triflate ester in situ. This was then reacted with the N(τ)-Boc histidine derivative 6 to form the corresponding N(π)-adduct 7 in 60% yield (Scheme 1). Without purification, global deprotection was performed and the crude product was reacted with 9-fluorenylmethyl succinimidyl carbonate (Fmoc-OSu) and aqueous NaHCO3 in tetrahydrofuran to yield the final, protected amino acid analogue, 8 (83% from 7, Scheme 1).
Scheme 1.
Reagents and conditions: (i) Ph(CH2)7CH2OH (1.1 equiv.), Tf2O (1.1 equiv), DIPEA (1.1 equiv.), CH2Cl2, −75 °C – rt, 16 – 18 h, 60% yield; (ii) LiOH•H2O (2.0 equiv.), THF, H2O (v/v 4:1), 0 °C – rt, 1 h; (iii) 4 M HCl in dioxane (10 equiv.), rt, 1 h; (iv) Fmoc-OSu (1.5 equiv.), NaHCO3 (4.0 equiv.), THF – H2O (v/v 1:1), rt, 12 h (82% over three steps).
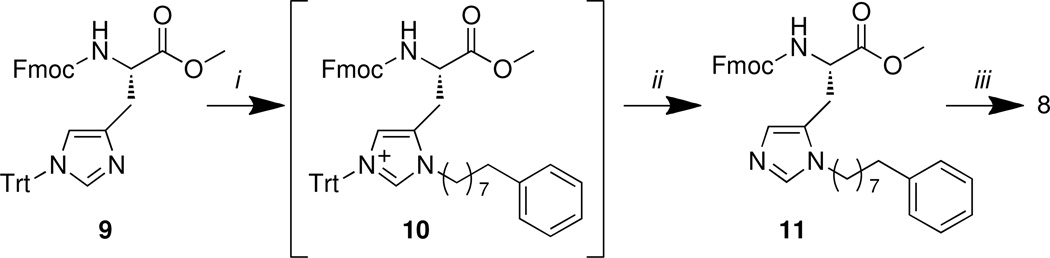
N(π)-derivatization in the presence of N(τ)-Trt protection has also previously been reported using alkyl halides as the alkylating species, however these conditions either involve elevated temperatures, prolonged reaction times or large excesses of reagent.27–29 In model reactions on N(α)-Fmoc-[N(τ)-Trt]-His methyl ester (9)30 (prepared from commercially available N(τ)-trityl-L-histidine methyl ester) we found that N(π)-alkylated product (11) could be obtained in high yield at room temperature using in situ generated alkyl triflate. The crude product from this reaction contained a mixture of the N(τ)-trityl, N(π)-alkyl bis-adduct (10) as well as the desired product (11) (Scheme 2). Treatment of this mixture with TFA gave 11 in 90% yield from 9 and demethylation under nucleophilic conditions gave 8 in good yield. This approach represents a more direct synthesis of 8 than that described above using starting material 6 (Scheme 1).
Scheme 2.
Reagents and conditions: (i) Ph(CH2)7CH2OH (1.1 equiv.), Tf2O (1.1 equiv.), DIPEA (1.1 equiv.), CH2Cl2, −75 °C – rt, 16 – 18 h; ii) TFA (10 equiv.), triisopropylsilane (1.1 eq.), CH2Cl2, rt, 2 h, (90% over two steps). (iii) LiI (6.0 equiv.), EtOAc, reflux, 20h, 80%.
Traditionally, during peptide synthesis protection of the histidine imidazole ring is important to avoid side product formation arising from ring acylation and subsequent migration of acyl species to other regions of the peptide. Protection also minimizes intramolecular base-catalyzed racemization of activated histidine by the N(π)-group. Acid-labile N(τ)-Trt derivatization is commonly employed for this purpose in combination with N(α)-Fmoc protection.31 Analogue 8 is somewhat unique in that its synthesis, and providing potentially important biological functionality. To verify the synthetic utility of 8, we employed the reagent for the solid-phase synthesis of peptide 3, which had previously been obtained only as a minor byproduct from the synthesis of intended product 2. The successful completion of this synthesis confirmed the utility of 8 as providing a much improved route to peptides containing N(π)-modified histidine residues.
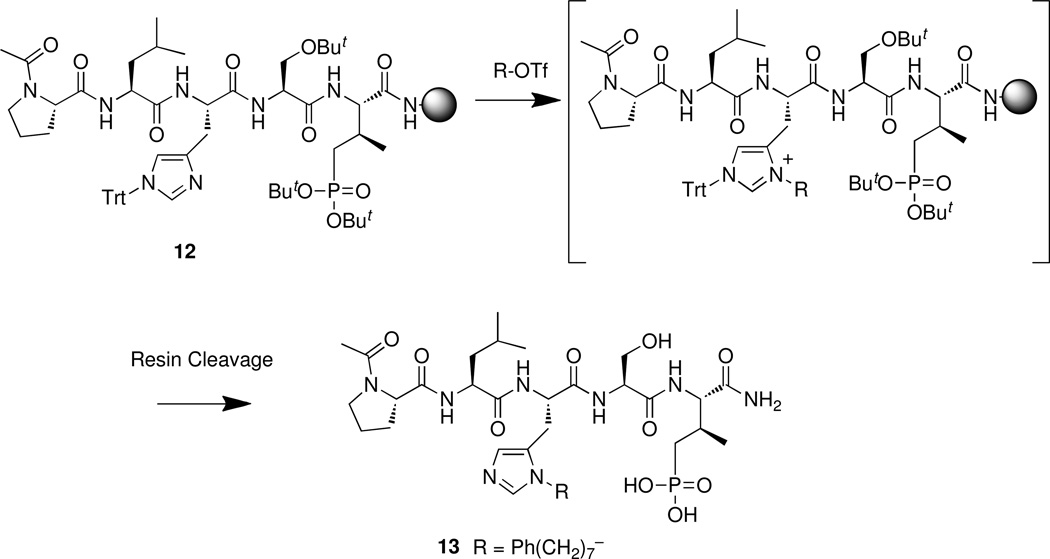
The general solid-phase methodology outline above using modified histidine analogue 8 is inherently limited because it requires that a new histidine reagent be prepared for each unique N(π)-substituent installed. A more useful approach that would allow the generation of multiple peptides containing unique N(π)-substituents, could be realized through on-resin modification of the histidyl residue in post-peptide synthesis fashion. Scheme 3 shows the application of such a protocol to the synthesis of peptide Peptide 13 is a variant of 3 having the labile pThr residue replaced by (2S,3R)-2-amino-3-methyl-4-phosphonobutyric acid (Pmab), which is a phosphatase-stable pThr mimetic32 that retains the PBD-binding affinity of parent pThr-containing peptides.15,16 A key feature of the route outlined in Scheme 3 is its use of commercially-available N(α)-Fmoc-[N(τ)-Trt]-His for the incorporation of an N(τ)-Trt-protected histidine residue. Although N(α)-Fmoc-[N(τ)-Boc]-His is also commercially available, the N(τ)-Boc group suffers from a lack of stability against prolonged or repeated exposure to piperidine used in Fmoc-based protocols.31
Scheme 3.
Post-peptide synthesis on-resin N(π)-alkylation.
In conclusion, our current paper provides evidence that formation of high affinity Plk1 PBD-binding peptide byproducts previously observed as minor components following on-resin Mitsunobu esterification, result from direct alkylation at the N(π)-position. We detail the high yield preparation of N(α)-Fmoc-N(π)-modified histidine reagents from N(α)-Boc and N(α)-Fmoc protected precursors, which permit facile Fmoc-based solid-phase synthesis of N(π)-modified peptides. We extend the utility of this chemistry by demonstrating on-resin triflate-mediated derivatization of fully formed peptides that should permit the ready preparation of libraries of N(π)-alkyl peptides. Although only a very narrow range of alcohols is specifically examined in our current report, it is anticipated that the approach should provide access to a broad range of N(π)-modified histidine analogues that may be useful in a variety of biological contexts.
Experimental Section
Synthesis N(α)-Fmoc-[N(π)-(8-phenyloctyl)]-histidine (8) from (6)
N(α), N(τ)-bis[(1,1-dimethylethoxy)carbonyl]-L-histidine Methyl Ester (6).26
Triethylamine (11.51 mL, 83 mmmol) was added to a stirred suspension of L-histidine methyl ester hydrochloride (10.0 g, 41.3 mmol) in MeOH (98 mL) and stirring was continued at room temperature until dissolution was complete (30 min). A solution of di-tert-butyl dicarbonate (18.03 g, 83 mmol) in MeOH (49 mL) was then added dropwise over 30 min and stirring was continued at room temperature (48 h). Solvent was removed in vacuo and the residue was partitioned between CH2Cl2 and H2O. The organic layer is washed with 10% citric acid, dried (MgSO4), concentrated and purified by gel column chromatography (EtOAc : hexanes; from = 4:1 to 1.5:1) to afford known 6 as a white amorphous solid (11.06 g, 72% yield).
N(α)-[(1,1-Dimethylethoxy)carbonyl]-N(π)-(8-phenyloctyl)-L-histidine Methyl Ester (7)
To a stirred solution of triflic anhydride (1.51 mL, 8.93 mmol) in CH2Cl2 (18 mL) under nitrogen at −75 °C, was added a solution of 8-phenyloctan-1-ol (1.88 mL, 8.93 mmol) and diisopropylethylamine (DIEPA) (1.56 mL, 8.93 mmol) in CH2Cl2 (12.0 mL) dropwise over 10 min. Stirring was continued at −75 °C (20 min), then a solution of 6 (3.0 g, 8.12 mmol) in CH2Cl2 (21 mL) was added dropwise and the mixture was allowed to gradually warm to room temperature over a period of 16 – 18 h. The mixture was poured into a solution of aqueous NaHCO3 and stirred vigorously (30 min). The organic layer was diluted with CH2Cl2 and washed with aqueous NaHCO3 and brine, then dried (Na2SO4) and concentrated to a gum. Purification by silica gel flash chromatography (CHCl3 : MeOH; from 100:1 to 100:2) provided 7 as colorless gum (2.23 g, 60% yield). [α]D21 23.99 (c 1.045, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.43 (s, 1H), 7.30-7.24 (m, 2H), 7.20-7.14 (m, 3H), 6.78 (s, 1H), 5.18 (d, J = 8 Hz, 1H), 4.54 (q, J = 4 Hz, 1H), 3.82 (t, J = 8 Hz, 2H), 3.74 (s, 3H), 3.16-2.96 (m, 2H), 2.60 (t, J = 8 Hz, 2H), 1.83 (brs, 1H), 1.77-1.65 (m, 2H), 1.65-1.55 (m, 2H), 1.43 (s, 9H), 1.31 (brs, 8H); 13C NMR (100 MHz, CDCl3) δ 171.7, 155.0, 142.7, 137.4, 128.3, 128.2, 128.0, 125.8, 125.5, 80.2, 53.0, 52.5, 44.7, 35.9. 31.3, 30.9, 29.3, 29.1, 29.0, 28.2, 26.9, 26.6; IR νmax 2929, 2361, 1705, 1164 cm−1; HR-ESI MS calcd for C26H40N3O4 (M+H)+: 458.3013, found: 458.3023.
N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N(π)-(8-phenyloctyl)-L-histidine (8)
To a solution of 7 (0.7 g, 1.53 mmol) in dissolved in THF (10 mL) and H2O (2.5 mL) at 0 °C was added LiOH•H2O (128 mg, 3.06 mmol) and the mixture was stirred at 0 °C (1.5 h). The solution was acidified to pH 1 – 2 (aqueous HCl), then THF was removed in vacuo and the residue was extracted (EtOAc) and the organic layer was dried and concentrated in vacuo. The residue was dissolved in a solution of 4M HCl in dioxane (4 mL) and EtOAc (4 mL) and stirred at room temperature (1 h), then concentrated in vacuo. The residue was dissolved in solution of THF (7 mL) and H2O (7 mL) and 9-fluorenylmethyl-succinimidyl carbonate (Fmoc-OSu) (774 mg, 2.30 mmol) and NaHCO3 (514 mg, 6.12 mmol) were added and the mixture was stirred at room temperature (overnight). The mixture was neutralized by the addition of saturated NH4Cl, extracted (EtOAc) and the organic layer was dried and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography first by elutiom with (CHCl3: MeOH; from 100:1 to 100:3) to remove byproduct and then (CHCl3: MeOH; from 10:1 to 3:1) to provide the desire product 8 as a white wax (716 mg, 83% yield from 7). [α]D21 38.78 (c 0.90, CHCl3); 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1H), 7.89 (d, J = 8 Hz, 2H), 7.85 (d, J = 8 Hz, 1H), 7.67 (d, J = 8 Hz, 2H), 7.41 (t, J = 8 Hz, 2H), 7.32 (t, J = 8 Hz, 2H), 7.25 (t, J = 8 Hz, 2H), 7.20 – 7.10 (m, 3H), 7.8 (s, 1H), 4.35 – 4.15 (m, 4H), 4.10 – 3.90 (m, 2H), 3.20 - 2.90 (m, 2H), 2.60 (s, 1H), 2.55 – 2.47 (m, 2H), 1.75 – 1.60 (m, 2H), 1.60 – 1.45 (m, 2H), 1.23 (brs, 9H); 13C NMR (100 MHz, DMSO-d6) δ 172.4, 155.9, 143.7, 142.2, 140.7, 135.9, 129.1, 128.20, 128.16, 127.6, 127.1, 125.5, 125.2, 121.8, 120.1, 65.7, 53.0, 46.6, 45.0, 35.1, 30.9, 29.8, 28.7, 28.5, 28.4, 25.8, 25.2; IR νmax 2930, 2361, 1713, 1452, 1255 cm−1; HR-ESI MS calcd for C35H40N3O4 (M+H)+: 566.3013, found: 566.3022.
Synthesis N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl-[N(π)-(8-phenyloctyl)]-histidine (8) from (9)
N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N(τ)-(triphenylmethyl)-L-histidine Methyl Ester (9)
To a solution of N(τ)-(triphenylmethyl)-L-histidine methyl ester hydrochloride (0.8 g, 1.78 mmol) and NaHCO3 (0.45 g, 5.3 mmol) in THF : H2O (v/v 1:1) at 0 °C, was added Fmoc-OSu (0.9 g, 2.67 mmol) and the mixture was allowed to come to room temperature and stirred (overnight). The organic layer was collected, the aqueous layer was extracted (EtOAc) and the combined organic layers were washed with H2O, brine and dried (Na2SO4). Concentration in vacuo provided a residue, which was purified by silica gel column chromatography (EtOAc : petroleum ether; from 1 : 10 to 1 : 4) to yield known 930 as white foam (84% yield). [α]D23 9.55 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 2H), 7.64 (t, J = 8.0 Hz, 2H), 7.44 (s, 1H), 7.40 (t, J = 8.0 Hz, 2H), 7.37 - 7.32 (m, 9H), 7.30 (t, J = 8.0 Hz, 2H), 7.16 – 7.10 (m, 6H), 6.60 (s, 1H), 6.55 (d, J = 8.0 Hz, 1H), 4.68 – 4.63 (m, 1H), 4.40 – 4.24 (m, 3H), 3.65 (s, 3H), 3.14 – 3.17 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 172.3, 156.4, 144.3, 144.1, 142.5, 141.4, 139.0, 136.6, 129.9, 128.26, 128.25, 127.8, 127.2, 125.6, 125.5, 120.1, 119.8, 75.5, 67.4, 54.5, 52.4, 47.4, 30.3; IR νmax 2361, 1719, 1492, 1445, 1248 cm−1; HR-ESI MS calcd for C41H35N3O4Na (M+Na)+: 656.2525, found: 656.2538.
N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N(τ)-(8-phenyloctyl)-L-histidine Methyl Ester (11)
To a stirred solution of triflic anhydride (0.101 mL, 0.60 mmol) in CH2Cl2 (3.8 mL) under nitrogen at −75 °C was added a solution of 8-phenyloctan-1-ol (124 mg, 0.60 mmol) and DIEA (0.104 mL, 0.60 mmol) in CH2Cl2 (2.6 mL) dropwise over 10 min at −75 °C. Stirring was continued at −75 °C (20 min), then and a solution of 9 (0.34 g, 0.54 mmol) in CH2Cl2 (4.5 mL) was added dropwise and mixture was allowed to gradually warm to room temperature and stirred (16 – 18 h). The mixture was poured into aqueous NaHCO3 and stirred vigorously (30 min), then extracted with CH2Cl2 and the organic extract washed with aqueous NaHCO3 and brine, dried (Na2SO4) and concentrated in vacuo. To a solution of the resulting gum in CH2Cl2 (4.2 mL) was added trifluoroacetic acid (0.42 mL, 5.4 mmol) and triisopropylsilane (TIS) (0.12 mL, 0.60 mmol) and the mixture was stirred at room temperature until reaction was complete as shown by TLC (2 h). Solvent was removed in vacuo and the residue was purified by silica gel column chromatography (CH2Cl2 : MeOH; from 100:1 to 20:1) to provide 11 a white foam (90% yield from 9).
[α]D22.4 1.35 (c 1.01, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.62 (s, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 8.0 Hz, 2H), 7.39 (t, J = 8.0 Hz, 3H), 7.40 -7.23 (m, 4H), 7.22 – 7.10 (m, 4H), 6.05 (d, J = 8.0 Hz, 1H), 4.56 (d, J =4.0 Hz, 1H), 4.38 (d, J = 8.0 Hz, 2H), 4.18 (d, J = 8.0 Hz, 2H), 4.06 – 4.01 (m, 2H), 3.77 (s, 3H), 3.25 – 3.14 (m, 2H), 2.58 (t, J = 8.0 Hz, 2H), 1.81 – 1.73 (m 2H,), 1.63 – 1.53 (m, 2H), 1.28 (s, 8H); 13C NMR (100 MHz, CDCl3) δ 170.6, 156.2, 143.79, 143.74, 142.9, 141.5, 134.9, 129.8, 128.6, 128.4, 128.0, 127.4, 125.8, 125.2, 120.2, 119.1, 67.5, 53.3, 53.1, 47.2, 36.1, 31.5, 30.2, 29.4, 29.3, 29.0, 26.5, 26.3; IR νmax 2928, 2361, 1718, 1166, 1028 cm−; HR-ESI MS calcd for C36H42N3O4 (M+H)+: 580.3175, found: 580.3178.
N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N(τ)-(8-phenyloctyl)-L-histidine (8)
Lithium iodide (1.13 g, 8.45 mmol) was added to a stirred solution of 11 (0.82 g, 1.41 mmol) in dry EtOAc (28 mL) under nitrogen and the mixture was heated at reflux (20 h). The mixture was brought to room temperature and the reaction quenched by the addition of 10% aqueous HCl and extracted (EtOAc). The organic extract was dried (Na2SO4), concentrated in vacuo and the residue was purified by silica gel flash chromatography (CH2Cl2 : MeOH; from 95:5 to 80:20) to provide 8 as a colorless gum(80% yield).
General Solid-Phase Peptide Synthesis
Protected amino acids used were: Fmoc-Thr(PO(OBzl)OH)-OH; Fmoc-Ser(OtBu); N(τ)-Trt-N(α)-Fmoc-His; Fmoc-Leu and Fmoc-Pro (purchased from Novabiochem). Peptides were synthesized on NovaSyn®TGR resin (Novabiochem, cat. no. 01-64-0060) using standard Fmoc solidphase protocols in N-Methyl-2-pyrrolidone (NMP). 1-O-Benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU) (5.0 eq.), hydroxybenzotriazole (HOBT) (5.0 eq.) and N,N-diisopropylethylamine (DIPEA) (10.0 eq.) were used as coupling reagents. Amino terminal acetylation was achieved using 1-acetylimidazole. Finished resins were washed with N,N-dimethylforamide (DMF), MeOH, CH2Cl2 and Et2O and then dried under vacuum (overnight), then cleaved by treatment with a solution of TFA : H2O : TIS (95:2.5:2.5) (4 h). The resin was removed by filtration, and the filtrate was concentrated under vacuum, then precipitated with ether, and the precipitate was washed with ether. The resulting solid was dissolved in 50% aqueous MeCN (5 mL) and purified by reverse phase preparative HPLC using a Phenomenex C18 column (21 mm 3 250 mm, cat. no: 00G-4436-P0) with a linear gradient from 0% aqueous MeCN (0.1% TFA) to 65% MeCN (0.1% TFA) over 30 min at a flow rate of 10.0 mL/min (detection at 220 nm). Lyophilization provided products as white powders. For the synthesis of Pmab-containing peptides, (2S,3R)-4-[di-(tert-butyl)-oxyphosphinyl]-N-Fmoc-L-valine32 was used in place Fmoc-Thr(PO(OBzl)OH)-OH.
Synthesis of Peptide 3 using N(α)-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N(τ)-(8-phenyloctyl)-L-histidine (8)
The synthesis of peptide 3 was accomplished according to the procedures listed under General Solid-Phase Synthesis, except that reagent 8 was used in place of N(τ)-Trt-N(α)-Fmoc-His.
On Resin N(π)-Alkylation Using Mitsunobu Reaction Conditions
Peptides 2 and 3 were prepared as previously reported.16 For the synthesis of peptides 4 and 5, resin-bound Fmoc-Thr[(OP(OBn)OH]-amide, prepared as indicated above, (400 mg, 0.1 mmol) was swelled in CH2Cl2 (15 min) and then treated with triphenylphosphine (262 mg, 1.0 mmol), diethyl azidodicarboxylate (DEAD) (0.46 mL, 40% solution in toluene, 1.0 mmol) and 8-phenyloctan-1-ol (206 mg, 1.0 mmol) in dry CH2Cl2 at room temperature (2 h). Coupling of the remaining peptide residues was conducted as described above in General Solid-Phase Synthesis. The resulting resin bond Ac-Pro-Leu-[(τ)-Trt-His]-Ser(OtBu)-Thr[(OP(OBn)(O(CH2)8Ph)]-amide was subjected to Mitsunobu coupling using triphenylphosphine (262 mg, 1.0 mmol), DEAD (0.46 mL, 40% solution in toluene, 1.0 mmol) and appropriate alchohol [8-phenyloctan-1-ol (206 mg, 1.0 mmol) for peptide 4 and 7-phenylheptan-1-ol (192 mg, 1.0 mmol) for peptide 5] in dry CH2Cl2 at room temperature (2 h). The resins were then washed (CH2Cl2), dried under vacuum (2 h) and cleaved by treatment with [TFA : H2O : TIS (95:2.5:2.5) (4 h)]. Peptides 4 and 5 were following procedures indictated in General Solid-Phase Synthesis. HPLC and mass spectral data are provided in Table 1.
Table 1.
Peptide ESI MS spectral data and HPLC retention times.
| No | Expect (M+H)+ | Observed (M+H)+ | Retention (min) | HPLCa |
|---|---|---|---|---|
| 216 | 863.4 | 863.4 | 25.2 | II |
| 316 | 863.4 | 863.4 | 24.3 | II |
| 4 | 1051.6 | 1051.5 | 29.8 | I |
| 5 | 1037.6 | 1037.4 | 29.2 | I |
| 1316 | 861.5 | 861.4 | 23.3 | I |
HPLC Method: λ= 220 nm, flow rate: 10 mL/min Solvent A: H2O, 0.1% TFA; Solvent B: MeCN, 0.1% TFA; Gradients as indicated below:
| Time | 0 | 30 | 30.1 | 35 |
| B% | 0 | 100 | 100 | 100 |
| Time | 0 | 30 | 30.1 | 35 |
| B% | 0 | 90 | 100 | 100 |
Synthesis of Peptide 13 by On-resin N(π)-alkylation
To a stirred solution of triflic anhydride (42 µL, 0.25 mmol) in CH2Cl2 (3 mL) under nitrogen at −75 °C was added a solution of 8-phenyloctan-1-ol (52 mg, 0.25 mmol) and DIEA (45 µL, 0.26 mmol) in CH2Cl2 (2.0 mL) dropwise over 10 min. Stirring was continued at −75 °C (20 min), then the mixture was warmed to room temperature and added to peptide resin 1216 (200 mg, 0.05 mmol) that had been swelled in CH2Cl2 for 15 min. Coupling was conducted at room temperature (12 h), then the resin was washed (CH2Cl2 and Et2O), dried under vacuum (2 h) and cleaved (TFA : H2O : TIS; 95:2.5:2.5) (4 h). Peptide 13 was obtained following work up and HPLC purification as indicated in General Solid-Phase Synthesis. HPLC and mass spectral data are provided in Table 1.
Supplementary Material
Acknowledgements
This work was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research, NCI-Frederick and the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Supporting information: 1H and 13C NMR spectra of 7, 8, 9, and 11, including 1D NOESY assignment of N(π)-alkylation in 11 and HPLC charts of peptide 2, 3, 4, 5, 13. This material is available free of charge via the Internet at http://pubs.acs.org/.
References and notes
- 1.Strebhardt K, Ullrich A. Nature Rev. Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 2.Eckerdt F, Yuan J. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 3.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 4.Goh KC, Wang H, Yu N, Zhou Y, Zheng Y, Lim Z, Sangthongpitag K, Fang L, Du M, Wang X, Jefferson AB, Rose J, Shamoon B, Reinhard C, Carte B, Entzeroth M, Ni B, Taylor ML, Stuenkel W. Drug Dev. Res. 2004;62:349–361. [Google Scholar]
- 5.McInnes C, Mezna M, Fischer PM. Curr. Top. Med. Chem. 2005;5:181–197. doi: 10.2174/1568026053507660. [DOI] [PubMed] [Google Scholar]
- 6.Gumireddy K, Reddy MVR, Cosenza SC, Nathan RB, Baker SJ, Papathi N, Jiang J, Holland J, Reddy EP. Cancer Cell. 2005;7:275–286. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Lansing TJ, McConnell RT, Duckett DR, Spehar GM, Knick VB, Hassler DF, Noro N, Furuta M, Emmitte KA, Gilmer TM, Mook RA, Cheung M. Mol. Cancer Ther. 2007;6:450–459. doi: 10.1158/1535-7163.MCT-06-0543. [DOI] [PubMed] [Google Scholar]
- 8.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters J-M. Curr. Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Lu L-Y, Yu X. Cell Div. 2009;4 doi: 10.1186/1747-1028-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reindl W, Yuan J, Kraemer A, Strebhardt K, Berg T. ChemBioChem. 2009;10:1145–1148. doi: 10.1002/cbic.200900059. [DOI] [PubMed] [Google Scholar]
- 11.Strebhardt K. Nat. Rev. Drug Discov. 2010;9:643–659. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 12.Elia AEH, Cantley LC, Yaffe MB. Science (Washington, DC, U. S.) 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 13.Cheng K-Y, Lowe ED, Sinclair J, Nigg EA, Johnson LN. EMBO J. 2003;22:5757–5768. doi: 10.1093/emboj/cdg558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YH, Park J-E, Yu L-R, Soung N-K, Yun S-M, Bang JK, Seong Y-S, Yu H, Garfield S, Veenstra TD, Lee KS. Mol. Cell. 2006;24:409–422. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Yun S-M, Moulaei T, Lim D, Bang Jeong K, Park J-E, Shenoy Shilpa R, Liu F, Kang Young H, Liao C, Soung N-K, Lee S, Yoon D-Y, Lim Y, Lee D-H, Otaka A, Appella E, McMahon JB, Nicklaus MC, Burke TR, Jr, Yaffe MB, Wlodawer A, Lee Kyung S. Nat. Struct. Mol. Biol. 2009;16:876–882. doi: 10.1038/nsmb.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Park J-E, Qian W-J, Lim D, Gräber M, Berg T, Yaffe MB, Lee KS, Burke TR., Jr Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat. Chem. Biol. 2011;7:81–91. doi: 10.1038/nchembio.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsunobu O. Synthesis. 1981:1–28. [Google Scholar]
- 18.Designation of imidazole nitrogens is according to IUPAC nomenclature.
- 19.Ma X, Shi R, Zhang B, Yan B. J. Comb. Chem. 2009;11:438–445. doi: 10.1021/cc900004m. [DOI] [PubMed] [Google Scholar]
- 20.Khaled A, Gravier-Pelletier C, Le MY. Tetrahedron: Asymm. 2007;18:2121–2124. [Google Scholar]
- 21.Kuhlmann E, Himmler S, Giebelhaus H. Green Chem. 2007;9:233–242. [Google Scholar]
- 22.Vijayaraghavan R, Surianarayanan M, Armel V, MacFarlane DR, Sridhar VP. Chem. Commun. (Cambridge, U. K.) 2009:6297–6299. doi: 10.1039/b911568d. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou KC, Cao G-Q, Pfefferkorn JA. Angew. Chem. Int. Ed. Engl. 2000;39:739–743. [PubMed] [Google Scholar]
- 24.Kim EJ, Ko SY, Dziadulewicz EK. Tetrahedron Lett. 2005;46:631–633. [Google Scholar]
- 25.Petit S, Azzouz R, Fruit C, Bischoff L, Marsais F. Tetrahedron Lett. 2008;49:3663–3665. [Google Scholar]
- 26.Hodges JC. Synthesis. 1987:20–24. [Google Scholar]
- 27.Jones JH, Rathbone DL, Wyatt PB. Synthesis. 1987:1110–1113. [Google Scholar]
- 28.Kimbonguila AM, Boucida S, Guibe F, Loffet A. Tetrahedron. 1997;53:12525–12538. [Google Scholar]
- 29.Kim BM, Park JS, Cho JH. Tetrahedron Lett. 2000;41:10031–10034. [Google Scholar]
- 30.Himes RA, Park GY, Barry AN, Blackburn NJ, Karlin KD. J. Am. Chem. Soc. 2007;129:5352–5353. doi: 10.1021/ja0708013. [DOI] [PubMed] [Google Scholar]
- 31.Sieber P, Riniker B. Tetrahedron Lett. 1987;28:6031–6034. [Google Scholar]
- 32.Liu F, Park J-E, Lee KS, Burke TR., Jr Tetrahedron. 2009;65:9673–9679. doi: 10.1016/j.tet.2009.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.