Abstract
Our previous studies have shown that the decreased nitric oxide (NO) and increased glutamatergic mechanisms on sympathetic regulation within the paraventricular nucleus (PVN) may contribute to the elevated sympatho-excitation during chronic heart failure (CHF). In the present study, we investigated the effects of neuronal NO synthase (nNOS) gene transfer on N-methyl-D-aspartic acid (NMDA) receptor subunit NR1 in the rats with coronary ligation model of CHF. Adenovirus vectors encoding either nNOS (AdnNOS) or β-galactosidase (AdβGal) were transfected into the PVN in vivo. Five days after application of AdnNOS, the increased expression of nNOS within the PVN was confirmed by NADPH-diaphorase staining, real time PCR and Western blot. In anesthetized rats, AdnNOS treatment significantly enhanced the blunted renal sympathetic nerve activity (RSNA), blood pressure (BP) and heart rate (HR) responses to NOS inhibitor L-NMMA in the rats with CHF compared to CHF-AdβGal group. AdnNOS significantly decreased the enhanced RSNA, BP and HR responses to NMDA in the rats with CHF (RSNA: 44±2% versus 79±6%, P<0.05) compared to CHF-AdβGal group. AdnNOS transfection significantly reduced the increased NR1 receptor mRNA expression (Δ35±5%) and protein levels (Δ24±4%) within the PVN in CHF rats. Furthermore, in neuronal NG-108 cells, NR1 receptor protein expression decreased in a dose-dependent manner after AdnNOS transfection. According to our results nNOS down-regulation enhances glutamate transmission in the PVN by increasing NR1 subunit expression. This mechanism may enhance RSNA in CHF rats.
Keywords: glutamatergic, sympathetic activity, blood pressure, heart failure
Introduction
Increased sympathetic nerve activity is a characteristic symptom of chronic heart failure (CHF) (1–3). The elevated sympathetic activity induces vasoconstriction and an increase in peripheral resistance that increases cardiac afterload. In addition, peripheral vasoconstriction and sodium and water retention leads to increased cardiac preload. All of these aggravate the morbidity and raise the mortality in the CHF. Peripheral adrenergic blockade cannot completely eliminate the state of sympatho-excitation. Recent studies have suggested that altered central mechanism(s) may be responsible for these impaired reflex regulations and may contribute to the elevated neuro-humoral drive in CHF (2–4).
The paraventricular nucleus (PVN) of the hypothalamus is an important central site for the integration of sympathetic nerve activity (5). Using retrograde tracing techniques, the studies have shown that the PVN is a major source of forebrain input to the sympathetic nervous system (6, 7). In the PVN a number of neurotransmitters, excitatory and inhibitory, converge to influence its neuronal activity (5, 8). Among them are nitric oxide (NO) and glutamate. Previously we have shown that the increased activity of PVN neurons associated with CHF is due to an increase in glutamatergic mechanism and a decrease in NO mechanism within the PVN (9, 10).
The interaction of NO and the glutamate receptor is important for neuronal development and function (11, 12). The NO system acts as a negative feedback system for the excitatory glutamatergic system (13). Glutamate through N-methyl-D-aspartic acid (NMDA) receptors activates NO synthase (NOS) in neurons and induces an increase in NO production (12, 14). On the other hand, NO elicits a regulatory effect on glutamate receptor activity (15, 16). In the PVN, NO and glutamate interact to regulate neuronal function (11, 17). NO inhibits NMDA-mediated increases in the renal sympathetic nerve activity (RSNA) within the PVN. This indicates a short loop inhibition by NO of excitation by NMDA receptor activation to increase RSNA within the PVN (13). This may be an important interaction in dictating sympathetic outflow in CHF known to have altered NOS and glutamate activity in the PVN with a concomitant increase in basal sympathetic tone.
The purpose of present study was to test the effect of the interaction between NO and the NMDA-glutamate receptor in the PVN on the subsequent regulation of sympathetic nerve activity and cardiovascular responses in CHF. We hypothesize that restoration of nNOS with adenoviral gene transfer ameliorates the NMDA receptor and subsequently the excitatory mechanisms within the PVN. This approach has the potential to elucidate the mechanism(s) responsible for the increased neuro-humoral drive in the CHF state.
Methods
Animals
This study was approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee, and conformed to the guidelines for the care and use of laboratory animals of the National Institutes of Health and the American Physiological Society. Male Sprague-Dawley rats (SASCO, NE) weighing 200–220g were assigned to four groups (sham-AdβGal, CHF-AdβGal, sham-AdnNOS, and CHF-AdnNOS).
CHF was produced by left coronary artery ligation, as described previously (18). Echocardiograms were performed after 6–7 weeks of ligation surgery. Rats with elevated left ventricular end-diastolic pressure (LVEDP; >15mmHg), infarct size >30% of total left ventricle wall, significant reductions in dp/dtmax and ejection fraction (>40%) were considered to be in CHF.
Adenovirus Transfection in the PVN
7–8 weeks after cardiac surgery, each rat was anesthetized. A 200nl solution (1×108pfu/ml) of adenovirus vectors encoding either nNOS (AdnNOS) or β-galactosidase (AdβGal) was injected into the PVN. With a concentration of 1×108pfu/ml, no damage to the neurons within the PVN was observed from light microscopic evaluation. These adenovius vectors were generously provided by Dr. Channon at Oxford (19, 20).
General Surgery for Hemodynamic, RSNA Measurement and Microinjection
5 days after viral injection, the rat was anesthetized with urethane (750mg/kg, i.p) and α-chloralose (70mg/kg, i.p) and instrumented for recording arterial pressure (AP) and heart rate (HR) as described previously (18). The changes in integration of the nerve discharge during the experiment were expressed as a percentage from basal value. A NOS inhibitor L-NMMA or NMDA was injected into the PVN in three doses (50, 100, 200pmol in 100nl) in random order. The responses in RSNA, MAP and HR over 30min were recorded.
NADPH-diaphorase Activity as a Marker of NOS Activity
The rat was perfused with heparinized saline followed by 4% paraformaldehyde. The brain sections in nitroblue tetrazolium solution were then placed in an oven at 37°C for 1hr. The presence of NADPH-diaphorase in the PVN was examined under a microscope. The density of the staining was evaluated by counting the number of cells that were positively stained for NADPH-diaphorase.
Micropunch of the PVN
In the other four groups of rats (sham-AdβGal, CHF-AdβGal, sham-AdnNOS, and CHF-AdnNOS), after the rats were euthanized, the brains were removed and frozen at -80°C. The PVN were punched bilaterally with a blunt needle (ID: 0.5mm). The punched tissue was put in TRI Reagent (Molecular Research Center Inc, OH) or protein extraction buffer.
Real-time RT-PCR for the Measurement of nNOS and NR1 Receptor mRNA
Total RNA extracted from the punched tissue was subjected to reverse transcription. The cDNA was amplified by real-time quantitative RT-PCR with the BioRad iCycler IQ system (Biorad Laboratories). Relative mRNA expression of nNOS or NR1 receptor was calculated using the Pfaffl equation relates expression of the target gene to expression of a reference gene (ribosomal protein L 19, rpl19).
Western Blot Assay of nNOS and NR1 Receptor Protein
The punched tissues were incubated with protein lysis buffer. Then the fractionized proteins on the gel were electrophoretically transferred onto the polyvinylidene fluoride (PVDF) membrane. The membrane was probed with primary nNOS and NR1 receptor antibodies. The signals were visualized using an enhanced chemiluminescence substrate (Pierce) and detected by digital image system (UVP BioImaging). The expression of nNOS or NR1 receptor protein was calculated as the ratio of intensity of the nNOS or NR1 receptor band relative to the intensity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) band.
Cell Culture
The NG108-15 (neuroblastoma X glioma) hybrid cells were grown in Dulbecco's Modified Eagle's medium (DMEM). The Cells were then treated with AdnNOS, in a dose-dependent manner (104~108pfu/ml, 24hrs) for 24hrs. Lysates (30–40μg) were processed to measure nNOS and NR1 receptor protein by Western blot.
Immunofluorescent Staining of NG108 cells
Adherent NG108 cells were fixed and incubated with primary nNOS and NR1 receptor. Secondary antibody consisted of Cy2-conjugated donkey anti-mouse IgG and Cy3-conjugated anti-goat was used. Labeled cells were visualized by Olympus fluorescence microscope equipped with digital camera
Statistical Analysis
Data are presented as mean±SE. Differences between groups were determined by a 2-way ANOVA followed by the Newman–Keuls test for posthoc analysis of significance (Statview II, Abacus Inc, Berkeley, CA). P<0.05 was considered statistically significant.
An expanded Methods section is available in the online Data Supplement at http://hyper.ahajournals.org.
Results
General Data
Supplement table S1 summarizes the salient morphological and hemodynamic characteristics of rats utilized in the present study. Any rats subjected to coronary artery ligation that displayed myocardial infarcts <30% of the left ventricular wall were excluded from the study (5 out of 40 rats with coronary artery ligation surgery). The infarction area in CHF group was approximately 40% of the endocardial surface. Sham rats had no observable damage to the myocardium. Heart weight was significantly greater in CHF rats than in sham rats. LVEDP was significantly elevated in CHF rats compared to sham rats. AdnNOS injections did not change the LVEDP and infracted size in both sham and CHF groups.
Basal MAP, HR and RSNA are also presented in supplement table S1. Although the level of raw RSNA in rats with CHF trends to be higher than sham operated rats, it did not reach statistical significance. Since an increase in RSNA and norepinephrine is present in conscious rats with CHF (21, 22), it would seem possible that the anesthetics may effect the expression of the increase in RSNA. And also it is not strict to reliably compare multifiber sympathetic nerve activity recordings between groups of rats because of differences in numbers of the fibers on the electrode, and damage to the fibers being recorded. There were no statistically significant differences in basal MAP or HR between the sham and CHF groups. AdnNOS injections did not change the basal MAP, HR and RSNA in both sham and CHF groups.
Adenoviral Gene Transfer of nNOS within the PVN
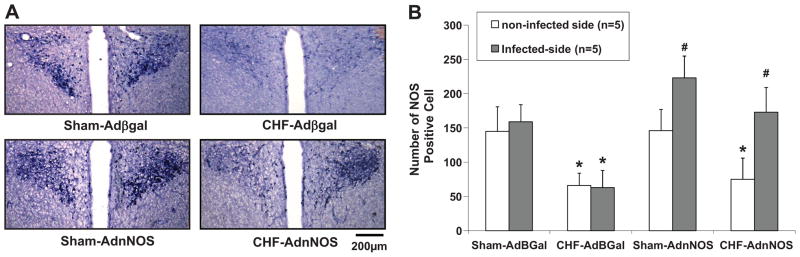
We evaluated the efficacy of AdnNOS gene transfer in the PVN by comparing the NADPH-diaphorase staining of the PVN. An example of the differences in staining of the infected versus uninfected PVN is shown in Figure 1A. There was a significant increase in the number of diaphorase-positive cells in the AdnNOS-infected PVN compared with the contralateral uninfected PVN in sham (increased 53%) and CHF (increased 136%) group (P<0.05). AdβGal-injected groups demonstrated no significant increased diaphorase labeled cells in the injected side of the PVN in both sham and CHF groups (Figure 1B).
Figure 1.
A: NADPH-diaphorase labeled neurons in the PVN of four groups of rats: sham-AdβGal, CHF-AdβGal, sham-AdnNOS, and CHF-AdnNOS. Right side of the PVN is the viral infected side. Left side is non-infected side. B: Number of NOS positive cells in the PVN in four groups of rats: sham-AdβGal, CHF-AdβGal, sham-AdnNOS, and CHF-AdnNOS. Values represent mean±SE. *P<0.05 versus sham group. #P<0.05 versus non-infected contralateral PVN.
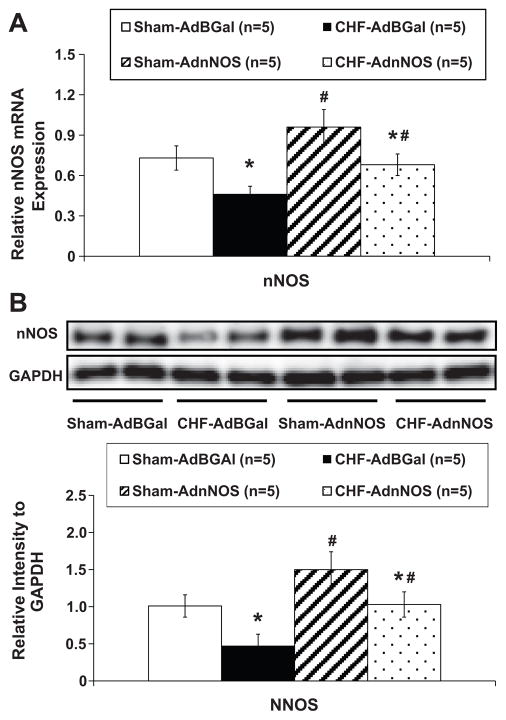
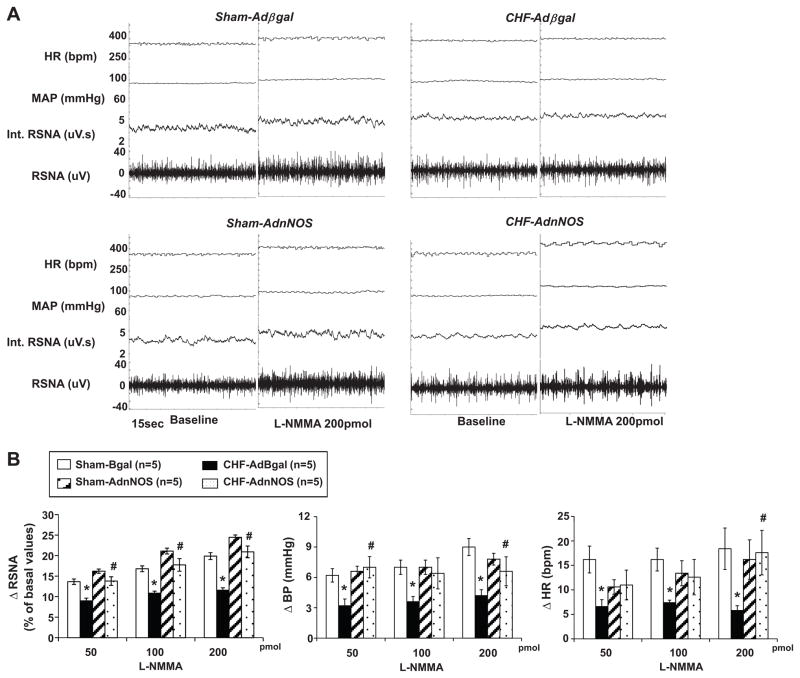
We also evaluated the efficacy of AdnNOS gene transfer in the PVN by comparing nNOS mRNA and protein levels of the PVN. There was a significant increase in the mRNA (Figure 2A) and intensity of the protein bands of nNOS (Figure 2B) in the AdnNOS-infected PVN compared with the AdβGal-infected sham and CHF group (P<0.05). AdβGal demonstrated no significant increased nNOS mRNA and protein expression in the injected side of the PVN in both sham and CHF groups (Figure 2). AdnNOS treatment significantly enhanced the blunted RSNA, AP and HR responses to NOS inhibitor L-NMMA in the rats with CHF compared to CHF-AdβGal group (Figure 3).
Figure 2.
A. Mean data of relative mRNA expression of nNOS to rpl19 mRNA in the punched PVN tissues measured by real-time RT-PCR. B. Example of visualized bands of nNOS and GAPDH protein; Mean data of band densities of nNOS normalized by GAPDH. *P<0.05 versus sham group. #P<0.05 versus AdβGal injected group.
Figure 3.
A. Segments of original recordings from individual rats from each experimental group showing response to RSNA, integral of RSNA (int. RSNA), MAP and HR to the microinjections of L-NMMA (200pmol) into the PVN. B. The mean data of changes in RSNA, MAP and HR after microinjections of L-NMMA into the PVN in four experimental groups of rats. *P<0.05 versus group of sham. #P<0.05 versus AdβGal injected group.
Effects of Microinjection of NMDA into the PVN on RSNA, AP and HR
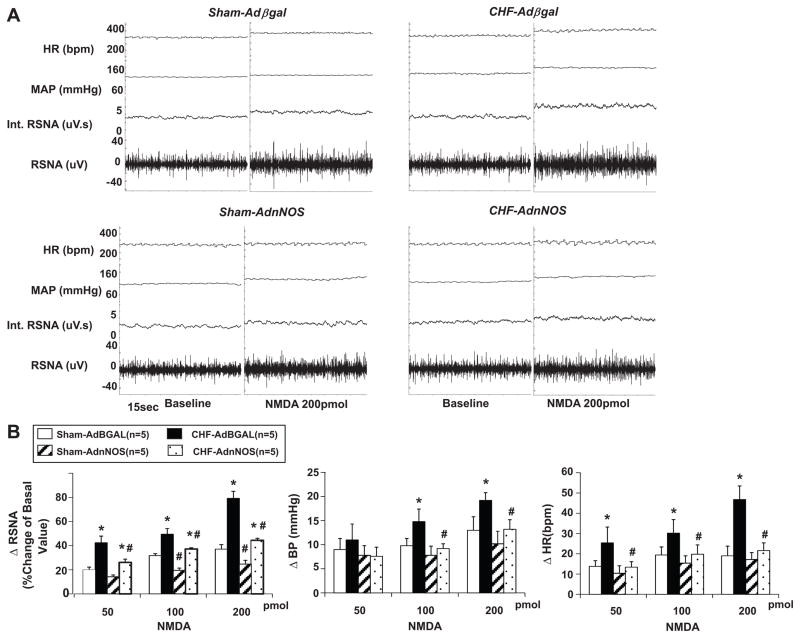
RSNA, MAP and HR responses to microinjection of NMDA (200pmol) into the PVN were significantly potentiated (RSNA: 79±6% versus 37±4%; MAP: 19.2±1.6mmHg versus 13±2.8mmHg; HR: 46.8±6.7bpm versus 19.0±4.8bpm, P<0.05) in the rats with CHF compared with sham rats. AdnNOS significantly decreased the enhanced RSNA, MAP and HR responses to NMDA in the rats with CHF (RSNA: 44±2% versus 79±6%; MAP: 13.2±2.0mmHg versus 19.2±1.6mmHg; HR: 21.6±3.8bpm versus 46.8±6.7bpm, P<0.05) compared to CHF-AdβGal group (Figure 4B). In sham-AdnNOS group, AdnNOS injection also decreased RSNA significantly. However, there were no significant changes of MAP and HR in sham group after viral transfer. AdβGal demonstrated no significant effects on the response of RSNA, MAP and HR to the NMDA in both sham and CHF groups (Figure 4).
Figure 4.
A. Segments of original recordings from individual rats from each experimental group showing response to RSNA, integral of RSNA (int. RSNA), MAP and HR to the microinjections of NMDA (200pmol) into the PVN. B. The mean data of changes in RSNA, MAP and HR after microinjections of NMDA into the PVN in four experimental groups of rats. *P<0.05 versus group of sham. #P<0.05 versus AdβGal injected group.
Measurements of NR1 Receptor Expression in the PVN
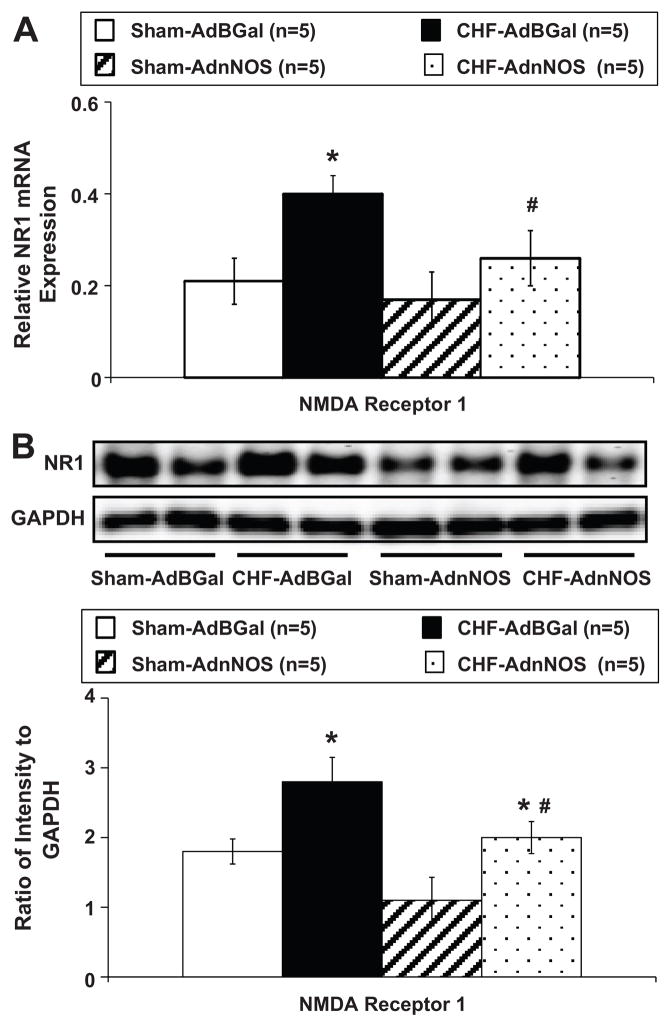
Result of real-time RT-PCR experiments indicated that NR1 receptor mRNA expression in the punched PVN tissues from the CHF rats was significantly increased compared with sham rats (Figure 5A). However, in the CHF-AdnNOS group, relative NR1 receptor expression was significantly lower than CHF-AdβGal and not different from sham-AdβGal or sham-AdnNOS group. Consistent with these results, western blot showed that NR1 receptor protein levels were also significantly higher in CHF rats compared with sham rats (Figure 5B). In the CHF-AdnNOS group, relative NR1 receptor expression was significantly lower than CHF-AdβGal and not different from sham-AdβGal or sham-AdnNOS group. Sample gels showing NR1 receptor and GADPH protein in the four experimental groups are presented in Figure 5B.
Figure 5.
A. Mean data of relative mRNA expression of NR1 receptor to rpl19 mRNA in the punched PVN tissues measured by real-time RT-PCR. *P<0.05 versus sham group. #P<0.05 versus AdβGal injected group. B. Example of visualized bands of NR1 receptor and GAPDH protein; Mean data of band densities of NR1 receptor normalized by GAPDH. *P<0.05 versus sham group. #P<0.05 versus AdβGal injected group.
nNOS and NR1 Receptor Expression in NG108 Cell Line
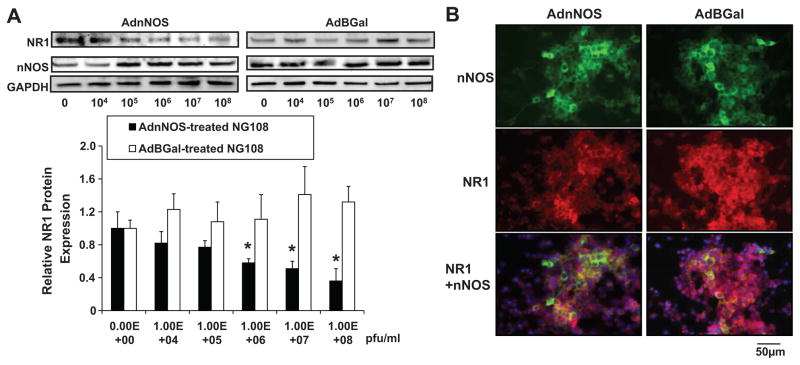
To determine the effects of over-expression of nNOS on regulation of NR1 receptor expression in vitro, the neuronal NG108 cells were treated with AdnNOS or AdβGal. The changes in nNOS and NR1 receptor protein expression were analyzed by Western blot after 24hrs viral transfection (Figure 6A). NR1 receptor protein expression was down-regulated significantly even at the lower concentrations of AdnNOS compared to the control (without virus). The highest dose showed an approximately 50% decrease in NR1 receptor protein expression compared to the control dose.
Figure 6.
A. Protein expression of NR1 receptor and nNOS measured by Western blot in AdnNOS-treated NG108 cell line. *P<0.05 versus non-treated group. B. Immunofluorescent photomicrographs in AdnNOS/AdβGal-treated NG108 cell stained for NR1 receptor and nNOS. Blue spot shows the nucleus stained by 4', 6-diamidino-2-phenylindole (DAPI).
Using immunofluorescent staining, nNOS and NR1 receptor interaction and their subcellular localization were studied in the NG108 cells (Figure 6B). The intensity of nNOS staining was increased while NR1 receptor staining was decreased with AdnNOS treatment. AdnNOS-treated cell shows more nNOS and NR1 receptor colocalization in cytoplasm as compared to the control (AdβGal-treated cells).
Discussion
In the present study, we observed that, in rats with CHF, AdnNOS normalizes the potentiated increase in RSNA, AP and HR in response to microinjection of NMDA into the PVN, accompanying with down-regulated NR1 receptor message and protein in the PVN. The results indicate that the effects of endogenous nNOS on the glutamatergic mechanism within the PVN may play an important role in the altered balance and tone of sympathetic outflow in the CHF condition.
The AdnNOS and AdβGal used in the present study were constructed in Dr. Channon’s laboratory at Oxford (19, 20). Their findings demonstrate AdnNOS to be a versatile and efficient tool for nNOS gene transfer in vascular cells and gene therapy (19, 20). They also demonstrated that up-regulation of nNOS via gene transfer provided a novel method for increasing cardiac vagal function (23). We have demonstrated the efficacy of adenoviral gene transfer of nNOS into cells of the PVN of rats (24, 25) by using same AdnNOS. It should be noted that this viral upregulation of nNOS is not specifically targeted to just preautonomic neurons in the PVN. As a consequence this upregulation occurs in other neuronal types as well as the glia and vascular cells in the PVN. Since we were examining the effects on RSNA we are attributing the changes to effects on the preautonomic neurons in the PVN. AdnNOS infects cells in the PVN and leads to a functional effect on RSNA mediated by the PVN. The results provide a novel approach to restore neuronal levels of NOS, thus providing a potentially important candidate gene for cardiovascular gene therapy in diseases states, such as CHF and hypertension lacking central nNOS.
The PVN is one of major central nervous sites that directly controls sympathetic outflow (5). CHF is characterized by elevated systemic sympathetic activity and salt and water retention (26), both involve the function of the PVN. We have found significantly increased hexokinase activity, an index of neuronal activity, in the parvocellular PVN and magnocellular PVN of rats with CHF compared to control rat (27). A study has confirmed this finding in the same model, using Fos related-antigen (FRA)-like activity at 2 and 4 weeks after coronary occlusion (28). This has also been confirmed more recently by Felder and colleagues (29) by direct recording of increased firing of neurons within the PVN in rats with myocardial infarction.
Inhibitory mechanism of sympathetic regulation within the PVN via NO (30) was reduced, while the excitatory mechanism regulated NMDA NR1 receptor was enhanced (9) in CHF rats. These alterations may induce an imbalance of the inhibitory and excitatory mechanisms in this area and influence sympathetic outflow. We have shown that there is an interaction between glutamatergic system and the NO system within the PVN (13). It appears that the NO system acts as a negative feedback system for the excitatory glutamatergic system. Since both the NO and NMDA systems are altered in CHF it remains to be examined whether the link between the glutamatergic system and the NO system is altered in CHF leading to sympatho-excitation.
In the present study, AdnNOS significantly decreased the enhanced RSNA, BP and HR responses to NMDA in the rats with CHF. These observations support the contention that an over-expression of nNOS within the PVN may be responsible for the increased suppression of sympathetic outflow. The endogenous NO-mediated effect in the PVN of AdnNOS-treated rats is more effective in suppressing RSNA compared to AdβGal-treated rats. Meanwhile, we also found that over-expression of nNOS into the PVN suppressed the sympathetic activity response to NMDA in sham rats, but less than in CHF rats. The explanation for this is that AdnNOS transfer is more effective on sympatho-inhibition in rats with CHF lacking central nNOS. Over-expression of NOS in the central nervous system attenuated sympatho-excitation in CHF state. Using the CHF mice model, Sakai et al (31) transferred adenoviral vectors encoding either endothelial NOS (AdeNOS) or AdβGal into the nucleus tractus solitarius (NTS) to examine the effect of increased NO production in the NTS on the enhanced sympathetic drive in CHF. After the gene transfer, they found urinary norepinephrine excretion was reduced in AdeNOS-transfected CHF mice and over-expression of eNOS in the NTS attenuates the enhanced sympathetic drive in this model.
NMDA NR1 receptor mRNA expression and protein level in the PVN are significantly increased in CHF (9) which may contribute to the elevated sympatho-excitation during CHF. In CHF state, many central and peripheral humoral factors are significantly altered. The up-regulation of NMDA receptor and the subsequent increase in glutamate activity within the PVN may be part of the compensatory responses. NO has been recognized as a factor eliciting a wide range regulation of gene expression (32). We have shown that the numbers of NOS-positive cells in the PVN was decreased in rats with CHF (30). In the present study, we found that NO may elicit an inhibitory action on the NMDA NR1 receptor to produce an inhibition of sympathetic nerve activity in CHF states. In an isolated cell culture system, incubation of AdnNOS with NG108 cells, that have been known to have both nNOS and NR1 receptors (33, 34), causes a dose dependent decrease in expression of NR1 receptor protein. These data are consistent with the idea that increased nNOS in rats with CHF may have caused the decrease in NR1 receptor protein expression within the PVN of rats with CHF.
Bains et al (11, 35), using whole cell recording from the hypothalamic slice preparation in rats, observed that application of NO increased NMDA-induced inhibitory postsynaptic potentials. Conversely, blocking NO with NG-nitro-L-arginine methyl ester (L-NAME) elicited more pronounced NMDA-induced depolarization but no accompanying increase in IPSP. These results provide evidence suggesting that there is a negative feedback mechanism between the NO and glutamate systems within the PVN. Our results suggest that there is an ongoing endogenous inhibitory NO mechanism that opposes the excitatory NMDA mechanism in the PVN. With regard to the effect of NO on NMDA receptors, some recent studies (15) have shown that, as a radical molecule, NO and its derivative can redox glutamate-NMDA receptors and reduce the activity of the receptors, which are believed to be one of mechanisms of modulation of NO on the nervous system. The chemical reactions of NO are largely dictated by its redox state. Recent data suggest that NO can react with critical sulfhydryl group(s) of the NMDA receptor to down-regulate its activity (36).
Although there is no direct evidence showing a neuronal intracellular signaling pathway in affecting expression of NMDA NR1 receptors by NO, NO signaling in cGMP-dependent way or through nitrosative conformational changes regulates various transcription factors, such as CREB, c-fos, c-jun and zinc-finger transcription factors, such as ERG-1, and NF-kappaB (37). An active AP1, SP1, NF-kappaB egr-1, and phosphorylated CREB binding sites were identified in NR1 promoter by computer analysis and DNA-protein binding assay (38). A role of these transcription factors downstream of PKG in regulation of NO mediated NR1 expression remains to be determined.
Perspectives
We have demonstrated that induction of nNOS within the PVN of rats with CHF not only ameliorates NO mediated inhibition via the PVN in CHF, but also improves glutamate-mediated sympatho-excitation that is observed in rats with CHF. The ability of AdnNOS to cause an increase in nNOS within the PVN appears to be primarily responsible for the down-regulation of NR1 receptors and consequent attenuation of enhanced responses to glatamatergic tone. The findings underscore the importance of targeting nNOS within the central sites such as PVN to attenuate the sympatho-exitation and subsequent cardiovascular risk in conditions with enhanced sympatho-excitation such as CHF and hypertension.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health Program Project Grant HL62222.
Footnotes
Disclosures
None.
References
- 1.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 3.Patel KP. Neural regulation in experimental heart failure. Bailliere's Clin Neurol. 1997;6:283–296. [PubMed] [Google Scholar]
- 4.Patel KP, Zhang K. Neurohumoral activation in heart failure: Role of paraventricular nucleus. Clin Exp Pharmacol Physiol. 1996;23:722–726. doi: 10.1111/j.1440-1681.1996.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 5.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 6.Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of the projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 7.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurons in the rat hypothalamic paraventricular nucleus. J Physiol (Lond) 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanson LW, Sawchenko PE. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 9.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93(10):990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 11.Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol (Lond) 1997;499:733–746. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 13.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- 14.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu ZQ. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and l-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS, Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO- Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 16.Lei SB, Jackson MF, Jia ZP, Roder J, Bai DL, Orser BA, MacDonald JF. Cyclic GMP-dependent feedback inhibitin of AMPA receptors is independent of PKG. Nature Neurosci. 2000;3:559–565. doi: 10.1038/75729. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson AV, Latchford KJ. Local circuitry regulates the excitability of rat neurohypophysial neurons. Exp Physiol. 2000;85 (Suppl):153S–161S. doi: 10.1111/j.1469-445x.2000.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 19.Channon KM, Blazing MA, Shetty GA, Potts KE, George SE. Adenoviral gene transfer of nitric oxide synthase: High level expression in human vascular cells. Cardiovasc Res. 1996;32:962–972. [PubMed] [Google Scholar]
- 20.West NE, Qian H, Guzik TJ, Black E, Cai S, George SE, Channon KM. Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodeling: effects on vascular smooth muscle cell differentiation and superoxide production. Circulation. 2001;104(13):1526–1532. doi: 10.1161/hc3801.095693. [DOI] [PubMed] [Google Scholar]
- 21.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1189–R1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- 22.Patel KP, Zhang K, Carmines PK. Norepinephrine turnover in peripheral tissues of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2000;278:R556–R562. doi: 10.1152/ajpregu.2000.278.3.R556. [DOI] [PubMed] [Google Scholar]
- 23.Mohan RM, Heaton DA, Danson EJ, Krishnan SP, Cai S, Channon KM, Peterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91(12):1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- 24.Li YF, Roy SK, Channon KM, Zucker IH, Patel KP. Effect of in vivo gene transfer of nNOS in the PVN on renal nerve discharge in rats. American Journal of Physiology Heart Circ Physiology. 2002;282:H594–H601. doi: 10.1152/ajpheart.00503.2001. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Wang Y, Channon KM, Schultz HD, Zucker IH, Patel KP. Manipulation of neuronal nitric oxide synthase within the paraventricular nucleus using adenovirus and antisense technology. Methods Mol Med. 2005;112:59–79. doi: 10.1385/1-59259-879-x:059. [DOI] [PubMed] [Google Scholar]
- 26.Marenzi G, Lauri G, Guazzi M, Assanelli E, Grazi M, Famoso G, Agostoni P. Cardiac and renal dysfunction in chronic heart failure: Relation to neurohumoral activation and prognosis. Am J Med Sci. 2001;321:359–366. doi: 10.1097/00000441-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- 28.Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283(1):H423–433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Li Y-F, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within the paraventricular nucleus of rats with heart failure. Am J Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 31.Sakai K, Hirooka Y, Shigematsu H, Kishi T, Ito K, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of eNOS in brain stem reduces enhanced sympathetic drive in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289(9):H2159–2166. doi: 10.1152/ajpheart.00408.2005. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 33.Grant MK, Cuadra AE, El-Fakahany EE. Endogenous expression of nNOS protein in several neuronal cell lines. Life Sci. 2002;71(7):813–817. doi: 10.1016/s0024-3205(02)01732-0. [DOI] [PubMed] [Google Scholar]
- 34.Maruoka H, Kitaoka S, Tohnai N, Inaki Y, Tanabe T. Study about the inhibition of L-cysteine derivatives of nucleic acid bases in protein production. Nucleic Acids Res Suppl. 2001;1:97–8. doi: 10.1093/nass/1.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Bains JS, Ferguson AV. Hyperpolarizing after-potentials regulate generation of long-duration plateau depolarizations in rat paraventricular nucleus neurons. Eur J Neurosci. 1998;10:1412–1421. doi: 10.1046/j.1460-9568.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, Tong G, Chen HS, Lipton SA. Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron. 2007;53(1):53–64. doi: 10.1016/j.neuron.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contestabile A. Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog Neurobiol. 2008;84(4):317–328. doi: 10.1016/j.pneurobio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Bai G, Hoffman PW. Biology of the NMDA Receptor. Chapter 5. CRC Press; 2009. Transcriptional Regulation of NMDA Receptor Expression. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.