Abstract
BACKGROUND
Chemotherapy and radiation treatments for cancer and other diseases can cause male infertility. There are currently no options to preserve the fertility of prepubertal boys who are not yet making sperm. Cryopreservation of spermatogonial stem cells (SSCs, obtained via testicular biopsy) followed by autologous transplantation back into the testes at a later date may restore fertility in these patients. However, this approach carries an inherent risk of reintroducing cancer.
METHODS
To address this aspect of SSC transplantation safety, prepubertal non-human primate testis cell suspensions were inoculated with MOLT4 T-lymphoblastic leukemia cells and subsequently sorted for cell surface markers CD90 (THY-1) and CD45.
RESULTS
Cancer cells segregated to the CD90−/CD45+ fraction and produced tumors in nude mice. Nearly all sorted DEAD box polypeptide 4-positive (VASA+) spermatogonia segregated to the CD90+/CD45− fraction. In a preliminary experiment, a purity check of the sorted putative stem cell fraction (CD90+/CD45−) revealed 0.1% contamination with cancer cells, which was sufficient to produce tumors in nude mice. We hypothesized that the contamination resulted from mis-sorting due to cell clumping and employed singlet discrimination (SD) in four subsequent experiments. Purity checks revealed no cancer cell contamination in the CD90+/CD45− fraction from three of the four SD replicates and these fractions produced no tumors when transplanted into nude mouse testes.
CONCLUSIONS
We conclude that spermatogonia can be separated from contaminating malignant cells by fluorescence-activated cell sorting prior to SSC transplantation and that post-sorting purity checks are required to confirm elimination of malignant cells.
Keywords: spermatogonial stem cells, fertility preservation, cancer survivors, reproductive risks, primate
Introduction
Chemotherapy and radiation treatments for cancer and other non-malignant disorders can cause male infertility (Wallace et al., 2005; Mitchell et al., 2009). For men and boys who are making sperm, cryobanking of seminal sperm before the initiation of treatment is possible and allows for future IVF, including ICSI. However, sperm banking is not an option for prepubertal boys who are not yet producing sperm. This is a significant problem because the overall event-free survival rate for childhood cancers is approaching 80% (Ries et al., 2007), which enables patients to look beyond cancer to a productive life after cure. Parenthood is important to cancer survivors and distress over infertility can have long-term psychological and relationship implications (Schover, 2009).
Spermatogonial stem cell (SSC) transplantation is an experimental approach that may have application for preserving and restoring fertility of prepubertal boys. SSCs are the stem cells of the testes that give rise to sperm through the process of spermatogenesis. Although prepubertal boys are not making sperm, their testes contain SSCs that are poised to initiate spermatogenesis at puberty (Ehmcke et al., 2006; Culty, 2009; Wu et al., 2009). In animal models (rodents, pigs, goats and dogs), transplantation of SSCs into the testes of infertile males can lead to restoration of spermatogenesis (Brinster and Avarbock, 1994; Ogawa et al., 2000; Shinohara et al., 2001; Nagano et al., 2001; Brinster et al., 2003; Honaramooz et al., 2003; Orwig and Schlatt, 2005; Mikkola et al., 2006; Kim et al., 2008). Stem cells from all ages, newborn to adult, are competent to produce complete spermatogenesis following transplantation into recipient testes (Shinohara et al., 2001; Ryu et al., 2003). In addition, SSCs from a variety of species can be cryopreserved and retain spermatogenic function upon thawing and transplantation (Avarbock et al., 1996; Dobrinski et al., 1999, 2000; Brinster, 2002; Kanatsu-Shinohara et al., 2003; Hermann et al., 2007, 2009). Thus, SSCs obtained via biopsy of a prepubertal human testis could theoretically be frozen prior to chemotherapy or radiation treatment and reintroduced into the testes after cure of the underlying disease. This approach has the potential to regenerate spermatogenesis and possibly natural fertility in these patients who currently have no other option to preserve their future fertility (Brinster, 2007; Hermann et al., 2007; Ginsberg et al., 2010).
Transplantation of cryopreserved testis cells isolated from patients with cancer carries an inherent risk of reintroducing contaminating malignant cells back into patients. Caution is certainly warranted as Jahnukainen and coworkers have demonstrated that as few as 20 leukemic cells transplanted to a rat testis can result in the development of terminal leukemia within 21 days (Jahnukainen et al., 2001) and magnetic-activated cell sorting (MACS) did not effectively remove malignant contamination (Hou et al., 2009). However, Fujita and colleagues provided the initial proof in principle that fluorescence-activated cell sorting (FACS) can be used to remove cancer cells from testis cell suspensions of leukemic mice (Fujita et al., 2005). When the resulting cell suspension was transplanted into recipient mouse testes, all mice survived and exhibited functional donor-derived spermatogenesis (Fujita et al., 2005). Thus, there are conflicting reports about the feasibility of separating SSCs from malignant cells using immune-based approaches (Fujita et al., 2005, 2006, 2007; Geens et al., 2007a,b; Hou et al., 2009). In general, it appears that immunomagnetic (MACS)-based strategies do not effectively eliminate contaminating malignant cells (Geens et al., 2007b; Hou et al., 2009). In contrast, immunofluorescent (FACS)-based sorting, which provides better resolution and sorting fidelity, can deplete or eliminate malignant contamination in some (Fujita et al., 2005), but not all cases (Geens et al., 2007b).
The current study was designed to demonstrate, in a model that is relevant to the target patient population (prepubertal boys), that contaminated testis cell suspensions can be rendered safe for SSC transplantation back into cancer survivors. We contaminated testis cells from prepubertal monkeys with a green fluorescent protein (GFP)-labeled MOLT4 human acute lymphoblastic leukemia cell line that produces transplantable tumors at high frequency in nude mice. The GFP label provided a sensitive measure of malignant cell contamination [minimal residual disease (MRD)] before and after FACS-sorting. Using a combination positive/negative selection strategy with a spermatogonial marker (CD90, Hermann et al., 2009) and a cancer cell marker (CD45, Fujita et al., 2006), we show that cancer-free germ cells can be isolated from a heterogeneous primate testis cell suspension. The results may have implications for prepubertal boys who are most likely to benefit from a new stem cell-based technology to preserve and restore fertility after cancer treatment.
Materials and Methods
Animals
All experiments utilizing animals were approved by the Institutional Animal Care and Use Committees of the Magee-Womens Research Institute and the University of Pittsburgh and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (assurance # A3654-01).
Preparation of prepubertal rhesus macaque testis cell suspensions
Testis tissue was collected by castration from prepubertal rhesus macaques at 15–20 months of age. Cells were recovered from testicular parenchyma using a two-step enzymatic digestion procedure, as described (Hermann et al., 2007, 2009). Briefly, testis tissue was digested sequentially with collagenase type IV followed by trypsin and DNase I. Cells were cryopreserved and stored in liquid nitrogen, as described (Hermann et al., 2007, 2009). Briefly, rhesus testis cells were suspended at 40 × 106/ml in medium [minimal essential medium α (MEM α) + 10% fetal bovine serum (FBS)], aliquoted in cryovials and an equal volume of cryoprotectant-containing medium [MEMα + 20% FBS + 20% dimethylsulphoxide (DMSO)] was added drop-wise. Vials were frozen in −1°C/min controlled-rate freezing containers (Nalgene-Nunc International, Rochester, NY, USA) to −80°C and stored in liquid nitrogen. (The final cryopreserved cell concentration was 20 × 106/ml in MEMα + 15% FBS + 10% DMSO.) For this study, vials of cryopreserved cells were thawed rapidly at 37°C, excess medium (MEMα + 10% FBS) was added to the cell mixture drop-wise, then cells were washed in medium, and used for flow cytometry analysis, immunocytochemistry (ICC) and inoculated FACS sorting experiments.
MOLT4-GFP cell line
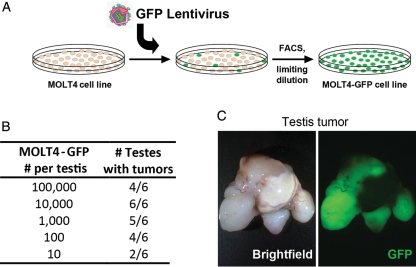
The human acute lymphoblastic leukemia cell line MOLT4 (CRL-1582) was purchased from the American Type Culture Collection (Manassas, VA, USA) and expanded per depositor recommendations. Cells were labeled with a GFP-expressing lentivirus modified from a previously described vector (Lois et al., 2002). MOLT4 cells were sorted for GFP expression using a FACSVantageSE (Becton Dickinson, Franklin Lakes, NJ, USA), expanded, and cloned by limiting dilution. Progeny of a single GFP-expressing clone, MOLT4-GFP D5, was used for all experiments in this study, including determination of tumorigenicity (see Fig. 1) and inoculation experiments with non-human primate testis cells.
Figure 1.
MOLT4-GFP cells produce tumors in nude mice. (A) The human acute lymphocytic leukemia (ALL) cell line MOLT4 was marked with a GFP lentivirus and clonally expanded by limiting dilution. These cells were used later in inoculation experiments to monitor malignant cell contamination of unsorted and sorted cell populations. (B) Results of tumor analyses of MOLT4-GFP cells transplanted into the interstitial space of nude mouse testes, presented as the number of injected testes that developed tumors over the total number injected. (C) Representative bright-field and epifluorescent images of a GFP+ testis tumor that was formed from MOLT4-GFP cells.
Cancer cell inoculation experiments and FACS
Prepubertal rhesus testis cells were inoculated with MOLT4-GFP cells (10% contamination) and suspended (2 × 106 cells/ml) in pre-chilled Dulbecco's phosphate-buffered saline (D-PBS) containing 10% FBS. The actual percentage of MOLT4-GFP contamination was confirmed at the time of sorting following cell staining and varied depending upon cell viabilities and recovery with an average of 9.4% MOLT4-GFP contamination over five experiments (see Supplementary data, Table SI). Cells were incubated with antibodies (CD90-APC, clone 5E10, 0.5 µg/106 cells; CD45-PE, clone TÜ116, 20 µl/106 cells; Becton Dickinson) for 20 min on ice, followed by two washes with excess cold D-PBS + FBS. Staining was compared with isotype control antibodies (Mouse immunoglobulin G (IgG)1k-APC, Mouse IgG1k-PE; Becton Dickinson) to correct for non-specific antibody binding. After the washes, stained cells were filtered through a 35 µm nylon membrane and maintained on ice in the dark until FACS analysis. Propidium iodide (0.5 µg/ml, Becton Dickinson) was added for discrimination of dead cells. Evaluation of antibody staining by flow cytometry and cell sorting (FACS) was performed using a FACSvantage SE (Becton Dickinson) equipped with 488 nm argon and 633 nm helium-neon lasers. Antibody stained cells were sorted into three fractions (i.e. Gate I: CD90+/CD45−; Gate II: CD90−/CD45−; Gate III: CD90−/CD45+). The sorted CD90+/CD45− fraction was assessed for sorting purity by re-analysis on the sorter. In experiments utilizing singlet discrimination (SD) during cell sorting, single cells (singlets) were identified as cells with forward scatter-area (FSC-A) to forward scatter-height (FSC-H) ratios of ∼1.
Immunocytochemistry
Detection of DEAD box polypeptide 4 (DDX4, VASA) in unsorted and sorted cells was performed as described previously (Hermann et al., 2009). Briefly, cells from each population were spotted onto glass slides, fixed (7:1 v/v ethanol:glacial acetic acid) and dried. Spotted cells were rehydrated, blocked for 30 min in antibody diluent [D-PBS + 0.1% Triton X-100, 5% goat serum, 3% bovine serum albumin (BSA)] and incubated with the VASA primary antibody (rabbit anti-DDX4 IgG; Abcam, Cambridge, MA), and detected by indirect immunofluorescence with goat anti-rabbit IgG AlexaFluor488 (Invitrogen, Carlsbad, CA). Samples were mounted with VectaShield mounting media containing 4(6-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA) and visualized by fluorescence microscopy. The number of VASA+ cells was counted in 5–10 random microscopic fields for each cell population (depending on the number of cells available) and the percentage expressing VASA was calculated by dividing the number of labeled cells by the total number of 4(6-diamidino-2-phenylindole) (DAPI+) cells in the same fields. Using analysis of variance (ANOVA) on nested generalized linear mixed-effects models, we tested whether the percentages of VASA-positive cells were significantly different between the unsorted fraction and either the CD90+/CD45− or CD90−/CD45− fractions using the lme4 package (Bates et al., 2011) of the statistical computing program R (R Development Core Team, 2011).
Xenotransplant tumor assay
Nude mice (NCr nu/nu; Taconic, Germantown, NY) at 6 weeks of age were used as recipients for tumor analysis. These mice are T-cell deficient, which facilitates tumor cell engraftment. Approximately 10 µl of donor testis cell suspension (MOLT4-GFP, and unsorted/sorted cell fractions from inoculation experiments) in Hank's buffered salt solution at 5 × 106 cells/ml were injected into recipient testes via the efferent ducts essentially as described (Ogawa et al., 1997), except that cells were introduced into the interstitial space in the present study, rather than the seminiferous tubules. The testicular interstitial space provides an ideal environment for efficient tumor formation (unpublished data), and thus, maximizes the sensitivity for evaluating malignant potential. The number of nude mice transplanted for each fraction was dependent on the number of cells available before and after FACS sorting. Animals were palpated regularly to check for testicular and abdominal tumors and were evaluated at necropsy by 4 months after transplantation. The presence or the absence of tumors was assessed upon gross dissection of recipient mice and confirmed by epifluorescent microscopy for GFP (see Fig. 1).
Results
To initiate inoculation studies, we labeled the MOLT4 human acute lymphoblastic leukemia cell line with a GFP-expressing lentivirus and clonally expanded a single derivative (Fig. 1A). This approach facilitated evaluation of MOLT4 contamination (MRD) in testis cell suspensions irrespective of cell surface phenotype and provided definitive determination of tumor origin in subsequent analyses of recipient nude mice. The MOLT4-GFP cell line formed GFP+ solid tumors in the interstitial space of nude mouse testes with as few as 10 cells transplanted (Fig. 1B and C), confirming the sensitivity of the tumor formation assay for detecting malignant contamination.
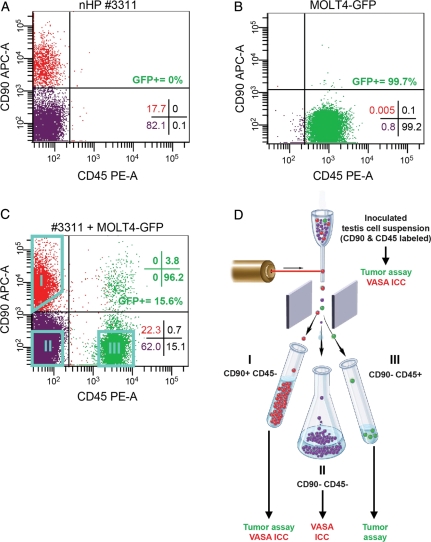
THY-1 cell surface antigen (THY-1 or CD90) is an established marker of transplantable SSCs in mice and rats and our recent results using a primate-to-nude mouse xenotransplant assay showed that spermatogonial colonizing activity segregates to the THY-1+ fraction in rhesus macaque testes (Ryu et al., 2004; Kubota et al., 2003; Hermann et al., 2009). Fujita and coworkers demonstrated that the pan leukocytic marker Protein tyrosine phosphatase receptor type C (PTPRC, CD45) marks MOLT4 cells (Fujita et al., 2006). Flow cytometry analysis for CD90 and CD45 staining in prepubertal rhesus testis cells or MOLT4-GFP cells confirmed that each exhibits a distinct phenotypic profile that could be exploited to sort spermatogonia from MOLT4 cancer cells (Fig. 2A and B). The CD90+ population of rhesus testis cells (putative SSC-containing fraction) varied in proportion between animals (8.5–31.7%; Table I), while very few rhesus testis cells were CD45+ (Fig. 2A). In comparison, nearly all MOLT4-GFP cells were CD45+ and of those that were CD45−, nearly all were also CD90− (Fig. 2B). On average, we found that only 1 in 3311 (0.0302%) MOLT4-GFP cells exhibited a CD90+/CD45− phenotype (Table I). Thus, we hypothesized that the CD90+/CD45− phenotype could be exploited using FACS to separate spermatogonia from contaminating malignant MOLT4-GFP cells. To begin utilizing this phenotypic information, testis cell suspensions previously isolated and frozen from prepubertal rhesus macaques were inoculated with 10% MOLT4-GFP cells (Supplementary data, Table SI), stained with CD90 and CD45 antibodies, evaluated by flow cytometry (Fig. 2C) and used for sorting experiments (Fig. 2D). We sorted three fractions from each inoculated cell suspension, CD90+/CD45− (Gate I, putative stem cell fraction), CD90−/CD45− (Gate II) and CD90−/CD45+ (Gate III; putative MOLT4 fraction) (Supplementary data, Table SI) and determined spermatogonial and malignant cell content.
Figure 2.
Cell surface phenotyping and FACS-based separation of malignant MOLT4-GFP cells and non-human primate spermatogonia. Flow cytometry scatter plots show the staining with antibodies against CD90 (THY-1; spermatogonial marker) and CD45 (pan leukocytic marker) in (A) Prepubertal rhesus macaque testis cells and (B) MOLT4-GFP cells, revealing distinctly different phenotypes. (C) Prepubertal rhesus testis cell suspensions were combined with MOLT4-GFP cells and stained for CD90 and CD45. This flow cytometry scatter plot shows a representative staining profile for CD90 and CD45 prior to FACS. (D) For subsequent sorting experiments, inoculated testis cell suspensions that were stained for CD90 and CD45 were sorted by FACS into three fractions: CD90+/CD45− (Gate I), CD90−/CD45− (Gate II) and CD90−/CD45+ (Gate III). Sort gates are shown as aqua polygons. Fractions were tested for tumorigenicity by xenotransplantation and germ cell content by ICC for the protein VASA, as indicated. Quadrant statistics in A–C present the percentage of viable cells that fall within the noted quadrant. Green quadrant statistics in C show the phenotypic distribution of GFP+ cells. The depiction of FACS in (D) was adapted with permission from the NIH Stem Cell Resource Figure E.i.2. [http://stemcells.nih.gov/info/scireport/appendixe.asp; © 2001 Terese Winslow (assisted by Lydia Kibiuk and Caitlin Duckwall)].
Table I.
The CD90+ CD45− population of prepubertal rhesus macaque testis cells and MOLT4-GFP cancer cells.
| Experiment | Animal | % Total testis cells, CD90+ CD45−a | % MOLT4-GFP. CD90+ CD45−b | # MOLT4-GFP, CD90+ CD45−c |
|---|---|---|---|---|
| 1 | 3311 | 14.8 | 0.005 | 1 in 20 000 |
| 2 | 3310 | 31.7 | 0.029 | 1 in 3448 |
| 3 | M310 | 11.3 | 0.025 | 1 in 4000 |
| 4 | M220 | 8.5 | 0.039 | 1 in 2564 |
| 5 | M307 | 10.7 | 0.053 | 1 in 1887 |
| Avg ± SEM | 15.4 ± 4.7 | 0.0302 ± 0.009 | 1 in 3311 |
aPercentage of viable total testis cells with the phenotype CD90+/CD45− as determined by flow cytometry.
bPercentage of viable MOLT4-GFP cells with the phenotype CD90+/CD45− in five individual flow cytometry experiments performed in parallel with testis cells.
cRelative proportion of viable MOLT4-GFP with the CD90+/CD45− phenotype.
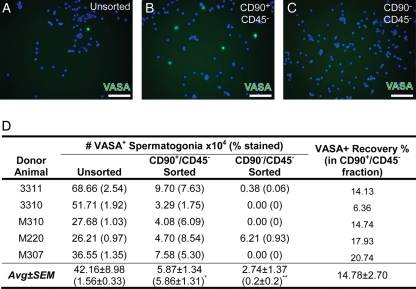
VASA ICC was used to track germ cells through the FACS experiments. We previously confirmed that VASA is a pan-germ cell marker in rhesus macaque testes (Hermann et al., 2007, 2009). The germ cell complement in prepubertal primate testes is primarily limited to undifferentiated Adark and Apale spermatogonia with some B-spermatogonia in very low numbers (Ramaswamy et al., 2000; Simorangkir et al., 2005; Ehmcke et al., 2011). As expected, nearly all VASA+ spermatogonia segregated to the CD90+/CD45− fraction (Fig. 3). Compared to the Unsorted fraction, VASA+ spermatogonia were significantly enriched in the CD90+/CD45− fraction (P = 2.2 × 10−16, Fig. 3A, B and D) and significantly depleted in the CD90−/CD45− fraction (P = 6.17 × 10−8; Fig. 3A, C and D). On average, however, only 14.78 ± 2.7% of starting VASA+ spermatogonia in the Unsorted fraction were recovered in the CD90+/CD45− fraction after sorting due to cell loss at each step of cell processing (e.g. cell staining, centrifugation and sorting; Fig. 3D). Thus, this positive (CD90)/negative (CD45) sorting strategy successfully segregated spermatogonia from the inoculated testis cell suspensions, with a cost of marked spermatogonial loss.
Figure 3.
Two-way FACS separation segregates non-human primate spermatogonia from inoculated testis cell suspensions into the CD90+/CD45− fraction. (A) Unsorted prepubertal non-human primate testis cells and the sorted (B) CD90+/CD45− and (C) CD90−/CD45− fractions from inoculated cell suspensions were stained for the pan germ cell marker VASA (green) and counterstained with DAPI (blue). Scale bar = 50 µm. (D) Quantification of percentage of cells in each unsorted and sorted fraction that were VASA+ from each replicate experiment and the calculated VASA+ spermatogonial number is shown for each fraction. The percent recovery of VASA+ spermatogonia in the CD90+/CD45− fraction is presented relative to the starting number in the Unsorted fraction. Statistically significant differences (ANOVA on nested generalized linear mixed-effects models) in the VASA+ percentage between the Unsorted and either CD90+/CD45− (P = 2.2 × 10−16; *) or CD90−/CD45− (P = 6.17 × 10−8, **) are noted in superscript.
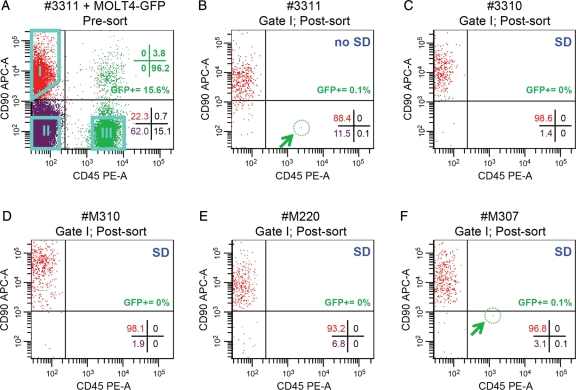
Next, we examined the malignant cell contamination in the sorted putative stem cell fraction (CD90+/CD45−; Gate I; Fig. 4A and B). In the initial sorting experiment (donor animal 3311), a purity check of the CD90+/CD45− fraction indicated that it contained 0.1% contamination with GFP+ CD90−/CD45+ MOLT4 cells (Fig. 4B and Table II). Furthermore, the tumorigenicity assay demonstrated that this fraction produced tumors in 3/3 mice (Table III). Thus, even a low level of MOLT4-GFP contamination (0.1%) was sufficient to produce GFP+ tumors in nude mice. While it is possible that some MOLT4-GFP cells were appropriately sorted into the CD90+/CD45− fraction based on phenotype (Table I), the contaminating MOLT4-GFP cell identified in post-sort reanalysis actually exhibited a CD90−/CD45+ phenotype (Fig. 4B). Thus, an alternative explanation could be that cell clumps containing MOLT4-GFP cells were inappropriately sorted into the CD90+/CD45− fraction.
Figure 4.
Post-sorting purity checks of the CD90+/CD45− fraction. (A) Scatter plot shows an example (animal 3311) of pre-sort prepubertal rhesus testis cell suspensions inoculated with MOLT4-GFP cells and stained for CD90 and CD45. Pre-sort analyses are not shown for replicates 2–5 (animals 3310, M310, M220 and M307). Quadrant statistics shown in the lower-right corner of this and the remaining scatter plots reflect the % of viable cells that fall within the indicated quadrant. GFP+ quadrant statistics are shown in green text in the upper right. Aqua polygons indicate the gates used to sort cells: CD90+/CD45− (Gate I; putative spermatogonial fraction), CD90−/CD45− (Gate II; negative testis cells) and CD90−/CD45+ (Gate III; putative MOLT4-GFP fraction). Note that the quadrant statistic values do not relate to the gates. Percentages of cells within each gate for all replicates as well as the overall percentage of GFP+ cells in the ungated, unsorted suspensions are in Supplementary data, Table SI. After sorting, scatter plots show reanalysis of sorted CD90+/CD45− fractions (Gate I) from five replicate inoculation experiments performed using testis cells from five different prepubertal rhesus macaques, one without SD (B) 3311, four with SD (C) 3310, (D) M310, (E) M220 and (F) M307. Blue text in the upper-right corner of each plot indicates whether SD was employed. Post-sort purity values (based on cells that fall back into Gate I shown in panel A) are described in Table II.
Table II.
Post FACS-sort purity checks of the CD90+/CD45− fraction (Gate I).
| Donor animal | In Gate I (%)a | Out of Gate I (%)b | GFP+c (%) |
|---|---|---|---|
| 3311 | 95.3 | 4.7 | 0.1 |
| 3310 | 97.9 | 2.1 | 0 |
| M310 | 97.2 | 2.8 | 0 |
| M220 | 95.5 | 4.5 | 0 |
| M307 | 96 | 4 | 0.1 |
| Avg ± SEM | 96.4 ± 0.6 | 3.6 ± 0.6 | 0.04 ± 0.03 |
aPercentage of viable cells in the CD90+/CD45− fraction that fall into Gate I on purity check (see sort gates on Fig. 4A).
bPercentage of viable cells in the CD90+/CD45− fraction that fall outside of Gate I on purity check (see Fig. 4A).
cPercentage of total viable cells in the CD90+/CD45− fraction with a GFP+ phenotype.
Table III.
Tumor analysis of unsorted-inoculated testis cell suspensions and sorted spermatogonia (CD90+/CD45−) and MOLT4 (CD90−/CD45−) fractions.
| Donor animal | Sort method | Tumor analysisa |
||
|---|---|---|---|---|
| Unsorted | CD90+/CD45− sorted | CD90−/CD45+ sorted | ||
| 3311 | CD90 × CD45 | 2/3 | 3/3 | 3/3 |
| 3310 | CD90 × CD45 + SD | 1/2 | 0/3 | 1/2 |
| M310 | CD90 × CD45 + SD | 2/3 | 0/2 | 2/3 |
| M220 | CD90 × CD45 + SD | 2/2 | 0/3 | 3/3 |
| M307 | CD90 × CD45 + SD | 1/3 | 0/3 | 2/3 |
SD, singlet discrimination.
aNumber mice with tumors/number analyzed (not all animals survived to analysis).
SD was subsequently employed to reduce the likelihood that cell clumps would be inappropriately sorted. Four additional sorting experiments were conducted using SD (Fig. 4C–F; animals 3310, M310, M220 and M307). In these experiments, single cells (singlets) were identified as those falling into a gate comprising cells with forward scatter height: area ratios (i.e. FSC-A × FSC-H) that were ∼1:1 (data not shown). Post-sort purity analysis of the putative SSC fraction (CD90+/CD45−, Gate I) demonstrated an absence of MOLT4-GFP contamination in three out of four replicates (animals 3310, M310, M220, Fig. 4C–E, Table II). The CD90+/CD45− fractions from these replicates were transplanted into nude mice and no tumors were formed (Table III). It is not clear how cells from M307 were inappropriately sorted, but it was encouraging that low level contamination (0.1%) could be detected upon purity analysis (Fig. 4F). Therefore, post-sort purity checks provide an essential safety screen prior to transplantation. The inoculated unsorted cell suspensions and sorted CD90−/CD45+ fractions always produced tumors when transplanted into nude mice (Table III). Although the GFP label will not be available when this paradigm is translated to the clinic, strategies are available to screen for tumor cells in human cell suspensions and are discussed later.
Discussion
SSC transplantation is an experimental technology that may have clinical utility for regenerating spermatogenesis in male survivors of childhood cancers. However, before this approach can be translated to the clinic, feasibility studies are required to demonstrate that testicular cell suspensions isolated from cancer patients are safe and free of malignant contamination. Such studies will be most compelling in a primate model that is relevant to human testis physiology.
Current data for eliminating cancer cells from contaminated human testicular cell suspensions is contradictory (Fujita et al., 2007; Geens et al., 2007a). Fujita et al. (2006) demonstrated that several leukemia and lymphoma cancer cell lines exhibited different major histocompatibility complex (MHC) Class I and CD45 phenotypes from germ cells in an adult human testis cell suspension, which could theoretically be used to separate malignant cells from germ cells. However, the testis cell suspension was never inoculated with cancer cells in that study, nor was the efficiency of isolating germ cells assessed (Fujita et al., 2006). In contrast, Geens and coworkers contaminated adult human testis cell suspensions with SB cells (B-cell acute lymphoblastic leukemia) and were not able to remove the malignant contamination using a FACS-based negative selection against MHC Class I (Geens et al., 2007b). The feasibility of using FACS-based strategies to decontaminate prepubertal human testis cell suspensions is not known because access to normal testis tissue from boys is limited. This deficit can be addressed using prepubertal non-human primate models that are relevant to human testis physiology.
In the current study, we employed a combination positive (CD90+) and negative (CD45−) selection strategy to isolate spermatogonia from a contaminated suspension of prepubertal non-human primate testis cells. The resulting fractions were evaluated for the efficiency of germ cell isolation as well as malignant cell elimination. To enhance our sensitivity for detecting contaminating cancer cells, we used a highly tumorigenic cancer cell line (MOLT4) that was marked with a GFP transgene. This allowed us to efficiently track cancer cells through in vitro manipulations and in vivo following transplantation.
We previously demonstrated that VASA+ germ cells (including Adark and Apale spermatogonia) are in the CD90+ fraction of prepubertal rhesus macaque testis cells. Furthermore, the FACS-sorted CD90+ fraction produced colonies of spermatogonia upon xenotransplantation into nude mouse testes (Hermann et al., 2009). FACS sorting performed in the present study similarly demonstrated that nearly all VASA+ spermatogonia from prepubertal rhesus macaques were recovered in the CD90+/CD45− fraction. This fraction was significantly enriched for VASA+ germ cells and did not produce tumors in nude mice when SD was employed and the post-sort purity check showed no contamination. Thus, a combination positive/negative selection strategy can be used to enrich undifferentiated spermatogonia and remove malignant contamination from a prepubertal testis cell suspension. However, post-sort purity checks are required to confirm removal of cells with a cancer phenotype.
Looking forward to clinical application, it is important to acknowledge that the cancer phenotype in patient samples is likely to be more heterogeneous than the cell line used in the current study. Phenotypic discrimination between cancerous cells and SSCs in the primary malignancies observed in clinical situation can be appropriately tailored by taking advantage of the known phenotype of the particular malignancy. For instance, extensive data on the cell surface phenotype of hematological malignancies is often used for diagnostic purposes and could be exploited to design multi-parameter cell sorting strategies. This approach would be facilitated by ongoing studies to identify additional positive and negative markers of primate SSCs.
While FACS-based strategies can effectively decontaminate testicular cell suspensions, each additional processing step results in further loss of spermatogonia. This is an important consideration in the clinical setting, where the amount of testicular tissue obtained from pre-pubertal boys is likely to be limited (Ginsberg et al., 2010). Thus, as the technology develops, it will be essential retain the maximum number of SSCs and also confirm the absence of malignant contamination.
In the clinical setting, an important first step prior to any manipulation would be to determine whether the testis cells banked prior to cancer treatment are already safe for potential transplant. Quantitative PCR-based assays are available for some malignancies to identify MRD at high sensitivity (10−3 to 10−6) and could be employed to detect miniscule levels of malignant cell contamination in small aliquots of patient testicular cell samples (Willemse et al., 2002; Jolkowska et al., 2007). For other malignancies where sensitive PCR-based MRD assays are not available, cancer cell contamination could be assessed by flow cytometry using cell surface markers that distinguish the specific cancer from spermatogonia. In cases where malignant cell contamination is indicated, purification steps such as those performed in this study would be required. The GFP marker employed in this study will not be accessible in the clinical setting, but served as a surrogate for MRD PCRs to assess malignant cell contamination in the current study. Our results using this approach demonstrate that follow-up MRD assessment after sorting is essential to ensure safety prior to transplant. In clinical cases where contamination is still evident after sorting to remove suspected residual malignant cells, re-sorting of sorted fractions could be used to increase purity (Davies, 2007), but likely with an associated loss of some SSCs. Culture may enable amplification of SSCs from clones or small enriched fractions of testis cells and also alleviate malignant cell contamination. Some progress culturing human SSCs has been reported in recent years (Sadri-Ardekani et al., 2009; Wu et al., 2009; He et al., 2010; Lim et al., 2010). In all cases, safety of the testis cell suspension must be clearly established prior to transplantation using the best methods available for the particular malignancy in question.
Testicular tissue xenografting is an alternative technique that may provide a therapeutic option for prepubertal cancer patients and avoids the risk of malignant cell contamination. Using this approach, intact testicular tissue grafts from immature mice, rats, hamsters, pigs, goats and nonhuman primates, which contain undifferentiated spermatogonia, were competent to produce complete spermatogenesis following ectopic transplantation under the skin of mouse hosts [reviewed by (Rodriguez-Sosa and Dobrinski, 2009)]. Sperm retrieved from rodent grafts (freshly transplanted or cryopreserved) could be used for ICSI to produce offspring (Schlatt et al., 2002; Schlatt et al., 2003). However, to date, there has only been one report of sperm production in grafts of cryopreserved prepubertal rhesus macaque testicular tissue from among numerous studies using primate tissue (Honaramooz et al., 2004; Ehmcke and Schlatt, 2008; Jahnukainen et al., 2007; Jahnukainen et al., 2011). For a prepubertal cancer patient, tissue could be retrieved surgically and processed into small fragments to be xenografted immediately or cryopreserved for future grafting. Thus, testicular tissue xenografting could theoretically serve as a sperm bioreactor for prepubertal cancer patients to be used in conjunction with additional assisted reproductive techniques (i.e. ICSI), but more work is needed to perfect the conditions for generating sperm. Alternatively, grafts could be implanted back into the patients in the homotopic site (within the testis) or at a heterotopic site such as beneath the skin (Jahnukainen et al., 2011), but this approach bears the same risk of malignant cell contamination as SSC transplantation.
SSC transplantation has proven effective for regenerating spermatogenesis and fertility in small and large animal models (Brinster and Avarbock, 1994; Ogawa et al., 2000; Shinohara et al., 2001; Nagano et al., 2001; Brinster et al., 2003; Honaramooz et al., 2003; Orwig and Schlatt, 2005; Mikkola et al., 2006; Kim et al., 2008). Clinical translation of this technique is imminent and may provide hope for future fertility in cases where there are no other options to preserve and/or restore fertility, such as prepubertal cancer patients. Indeed clinics around the world (including our own) are already cryopreserving testicular tissue for prepubertal male cancer patients in anticipation that this tissue can be used to restore their future fertility (Keros et al., 2007; Wyns et al., 2008; Ginsberg et al., 2010; Sadri-Ardekani et al., 2011). In this paradigm it is essential to eliminate the risk of re-introducing malignant cells into a cancer survivor. We have established some parameters for decontaminating and screening prepubertal testis cell suspensions prior to SSC transplantation and speculated about how these methods could be extrapolated to the clinic. Responsible development and implementation of this stem cell-based therapy could permanently restore natural fertility and enhance quality of life for cancer survivors.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors’ roles
B.P.H. contributed to conception and design of the study, collection and assembly of data, data analysis and interpretation, financial support and manuscript writing. M.S. contributed to collection and assembly of data as well as data analysis and interpretation. J.S. contributed to collection and assembly of data. Y.S. contributed to conception and design of study as well as collection and assembly of data. T.C. contributed to data analysis and interpretation. K.E.O. contributed to conception and design of the study, collection and assembly of data, data analysis and interpretation, financial support, administrative support, manuscript writing, and final approval of manuscript.
Funding
This work was supported by NIH grants R01 HD055475 and R21 HD061289 to KEO; the Magee-Womens Research Institute Postdoctoral Fellowship program and NIH grant K99/R00 HD062687 to BPH; and the Magee-Womens Research Institute and Foundation. This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH) through cooperative agreement (U54 HD08160) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPIR).
Supplementary Material
Acknowledgements
The authors thank Lynda Guzik and Dr Eric Lagasse from the McGowan Institute for Regenerative Medicine, University of Pittsburgh for assistance with cell sorting. Lentivirus particles were produced by the Magee-Womens Research Institute Transgenic and Molecular Core (http://www.mwrif.org/125/transgenic-and-molecular-core).
References
- Avarbock MR, Brinster CJ, Brinster RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med. 1996;2:693–696. doi: 10.1038/nm0696-693. doi:10.1038/nm0696-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. Package Version 0.999375-39. Vienna, Austria: R Foundation for Statistical Computing; 2011. lme4: Linear mixed-effects models using S4 classes. http://CRAN.R-project.org/package=lme4 . [Google Scholar]
- Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. doi:10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. doi:10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. doi:10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. doi:10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26. doi: 10.1002/bdrc.20142. doi:10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- Davies D. Cell sorting by flow cytometry. In: Macey MG, editor. Flow Cytometry: Principles and Applications. Totowa, NJ: Humana Press; 2007. pp. 257–276. [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. doi:10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. doi:10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. Animal models for fertility preservation in the male. Reproduction. 2008;136:717–723. doi: 10.1530/REP-08-0093. doi:10.1530/REP-08-0093. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. doi:10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Gassei K, Westernstroer B, Schlatt S. Immature rhesus monkey (Macaca mulatta) testis xenografts show increased growth, but not enhanced seminiferous differentiation, under human chorionic gonadotropin treatment of nude mouse recipients. Int J Androl. 2011;34(5 pt2):e459–e467. doi: 10.1111/j.1365-2605.2011.01179.x. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, Matsumiya K, Wakayama T, Okuyama A. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115:1855–1861. doi: 10.1172/JCI24189. doi:10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Tsujimura A, Miyagawa Y, Kiuchi H, Matsuoka Y, Takao T, Takada S, Nonomura N, Okuyama A. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006;66:11166–11171. doi: 10.1158/0008-5472.CAN-06-2326. doi:10.1158/0008-5472.CAN-06-2326. [DOI] [PubMed] [Google Scholar]
- Fujita K, Tsujimura A, Okuyama A. Isolation of germ cells from leukemic cells. Hum Reprod. 2007;22:2796–2797. doi: 10.1093/humrep/dem212. doi:10.1093/humrep/dem212. [DOI] [PubMed] [Google Scholar]
- Geens M, Van de Velde H, De Block G, Goossens E, Tournaye H. Reply: isolation of germ cells from leukemic cells. Hum Reprod. 2007a;22:2797–2798. doi:10.1093/humrep/dem213. [Google Scholar]
- Geens M, Van de Velde H, De BG, Goossens E, Van SA, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod. 2007b;22:733–742. doi: 10.1093/humrep/del418. doi:10.1093/humrep/del418. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod. 2010;25:37–41. doi: 10.1093/humrep/dep371. doi:10.1093/humrep/dep371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. doi:10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, et al. Characterization, cryopreservation and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. doi:10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. doi:10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. doi: 10.1095/biolreprod.103.018788. doi:10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. doi:10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Immunomagnetic separation of normal rat testicular cells from Roser's T-cell leukaemia cells is ineffective. Int J Androl. 2009;32:66–73. doi: 10.1111/j.1365-2605.2007.00819.x. doi:10.1111/j.1365-2605.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22:1060–1067. doi: 10.1093/humrep/del471. doi:10.1093/humrep/del471. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25:287–302. doi: 10.1016/j.beem.2010.09.007. doi:10.1016/j.beem.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Jolkowska J, Derwich K, Dawidowska M. Methods of minimal residual disease (MRD) detection in childhood haematological malignancies. J Appl Genet. 2007;48:77–83. doi: 10.1007/BF03194661. doi:10.1007/BF03194661. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Shinohara T. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum Reprod. 2003;18:2660–2667. doi: 10.1093/humrep/deg483. doi:10.1093/humrep/deg483. [DOI] [PubMed] [Google Scholar]
- Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. doi:10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis A. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–831. doi: 10.1530/REP-08-0226. doi:10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. doi:10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43:405–417. doi: 10.1111/j.1365-2184.2010.00691.x. doi:10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. doi:10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, Katila T, Andersson M. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41:124–128. doi: 10.1111/j.1439-0531.2006.00651.x. doi:10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Saunders PT, Sharpe RM, Kelnar CJ, Wallace WH. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr Dev. 2009;15:101–134. doi: 10.1159/000207612. doi:10.1159/000207612. [DOI] [PubMed] [Google Scholar]
- Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. doi:10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. doi:10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. doi:10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta) Biol Reprod. 2000;63:82–88. doi: 10.1095/biolreprod63.1.82. doi:10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- Rodriguez-Sosa JR, Dobrinski I. Recent developments in testis tissue xenografting. Reproduction. 2009;138:187–194. doi: 10.1530/REP-09-0012. doi:10.1530/REP-09-0012. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Avarbock MR, Brinster RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–263. doi: 10.1016/j.ydbio.2003.07.010. doi:10.1016/j.ydbio.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biology. 2004;274:158–170. doi: 10.1016/j.ydbio.2004.07.004. doi:10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van d Veen F, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. doi:10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. doi:10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. doi:10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. doi:10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–284. doi: 10.1002/pbc.22001. doi:10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci USA. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. doi:10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. doi:10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. doi:10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- Willemse MJ, Seriu T, Hettinger K, d'Aniello E, Hop WC, Panzer-Grumayer ER, Biondi A, Schrappe M, Kamps WA, Masera G, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99:4386–4393. doi: 10.1182/blood.v99.12.4386. doi:10.1182/blood.V99.12.4386. [DOI] [PubMed] [Google Scholar]
- Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, Ginsberg JP, Brinster RL. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci USA. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. doi:10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, Van LA, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23:2402–2414. doi: 10.1093/humrep/den272. doi:10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.