Abstract
We analyzed the kinetics of nonphotochemical quenching of chlorophyll fluorescence (qN) in spinach (Spinacia oleracea) leaves, chloroplasts, and purified light-harvesting complexes. The characteristic biphasic pattern of fluorescence quenching in dark-adapted leaves, which was removed by preillumination, was evidence of light activation of qN, a process correlated with the de-epoxidation state of the xanthophyll cycle carotenoids. Chloroplasts isolated from dark-adapted and light-activated leaves confirmed the nature of light activation: faster and greater quenching at a subsaturating transthylakoid pH gradient. The light-harvesting chlorophyll a/b-binding complexes of photosystem II were isolated from dark-adapted and light-activated leaves. When isolated from light-activated leaves, these complexes showed an increase in the rate of quenching in vitro compared with samples prepared from dark-adapted leaves. In all cases, the quenching kinetics were fitted to a single component hyperbolic function. For leaves, chloroplasts, and light-harvesting complexes, the presence of zeaxanthin was associated with an increased rate constant for the induction of quenching. We discuss the significance of these observations in terms of the mechanism and control of qN.
Under different physiological conditions the efficiency with which absorbed light energy is harvested by photosynthesis is altered by the operation of a regulatory mechanism that determines how much excitation energy is used and how much is dissipated as heat (Horton, 1987; Demmig-Adams and Adams, 1992; Horton and Ruban, 1992; Björkman and Demmig-Adams, 1995; Demmig-Adams et al., 1995). The function of this mechanism is to harmlessly dissipate excess energy when photosynthesis is light saturated, thereby protecting the pigments and proteins of the chloroplast membrane from photo damage. The extent of energy dissipation, measured by the quenching of chlorophyll fluorescence, has been found to correlate with the extent of de-epoxidation of the xanthophyll cycle carotenoids (Demmig-Adams, 1990).
The kinetics of the formation and relaxation of quenching and the effects of inhibitors established the role of the energization of thylakoid by the ΔpH, so this quenching was referred to as qE (Briantais et al., 1979). It is therefore widely accepted that these two factors control the induction of qE (Horton et al., 1996). Various reports suggest that qE occurs in LHCII (Horton and Ruban, 1992; Horton et al., 1996), where the xanthophyll cycle carotenoids are bound (Peter and Thornber, 1991; Bassi et al., 1993; Ruban et al., 1994b) and where proton-active sites involved in qE have been identified (Walters et al., 1996; Pesaresi et al., 1997). It has been suggested that CP26 and CP29, two of the minor components of LHCII, have a major role in qE (Horton and Ruban, 1992; Bassi et al., 1993; Crofts and Yerkes, 1994; Walters et al., 1994; Pesaresi et al., 1997; Gilmore et al., 1996b).
It is unclear how the xanthophyll cycle and qE are related. Some have suggested that the de-epoxidized pigments antheraxanthin and zeaxanthin directly quench excited chlorophyll singlet states (Demmig-Adams, 1990; Owens et al., 1992; Frank et al., 1994; Gilmore et al., 1995, 1996a, 1996b). However, quenching does not depend on the presence of these pigments, as has been shown in isolated chloroplasts (Rees et al., 1989; Noctor et al., 1991) and in Chlamydomonas reinhardtii mutants defective in zeaxanthin formation (Niyogi et al., 1997a). Horton et al. (1991) have therefore proposed that the xanthophyll cycle is a modulator of qE. Experimental support for this model came from a titration of the ΔpH dependency of qE for chloroplasts with and without zeaxanthin; a shift to a lower ΔpH requirement was found in the presence of zeaxanthin (Rees et al., 1989; Noctor et al., 1991).
The mechanism by which zeaxanthin exerts this effect has been explored by observing the effects of exogenous carotenoids on the quenching displayed by purified light-harvesting complexes in vitro. These experiments demonstrated marked differences in the behavior of violaxanthin and zeaxanthin; violaxanthin was an inhibitor of quenching, whereas zeaxanthin was a stimulator (Ruban et al., 1994a, 1996; Phillip et al., 1996). In this model for qE, quenching is caused by a conformational change in one or more of the proteins of the LHCII system, a change that is induced by protonation and controlled allosterically by the xanthophyll cycle carotenoids (Horton et al., 1991, 1994, 1996; Ruban and Horton, 1995b). Recently, this quenching has been directly related to the oligomerization state of LHCII, with violaxanthin and zeaxanthin inhibiting and promoting, respectively, the formation of protein aggregates (Ruban et al., 1997b).
A mechanism for quenching can therefore be proposed, because the changes in chlorophyll orientation and conformation in the oligomeric state are associated with increased energy dissipation (Ruban et al., 1997a). An alternative explanation of the activating role of zeaxanthin is that quenching in the absence of zeaxanthin arises from lutein (Gilmore and Yamamoto, 1991). Support for this idea comes from the complete absence of quenching in double mutants of C. reinhardtii, which lack both lutein and zeaxanthin (Niyogi et al., 1997b); because lutein is a weaker quencher, more protonation is needed. The in vitro effects of violaxanthin and zeaxanthin on quenching were explained by their displacement of endogenous lutein in the light-harvesting complexes. Such a direct role of these carotenoids in quenching cannot, however, explain why they affect LHCII oligomerization.
Although the results of these experiments lend support to a coherent mechanism for qE that can explain all experimental observations to date, there is clearly a need to devise new approaches that can distinguish between the direct and indirect roles of the xanthophyll cycle. It is particularly important to provide a firmer basis for relating observations made on isolated LHCII to events occurring with qE in isolated chloroplasts and in leaves. For this paper we established conditions in spinach (Spinacia oleracea) leaves so that the effect of zeaxanthin on qE could be kinetically analyzed. We also isolated intact chloroplasts from these leaves for similar but more detailed investigation. Finally, we purified LHCII components to discover if endogenous zeaxanthin and violaxanthin exert the predicted effects in vitro. In this way it was possible to show that the basic features of light activation of qE by the xanthophyll cycle can be observed at all three levels of organization. The analyses of the rate of induction of quenching in all systems suggest a qE mechanism that controls this rate by the pH and the de-epoxidation state of the xanthophyll-cycle pool.
MATERIALS AND METHODS
Spinach plants were grown for 6 weeks in a greenhouse under supplemented light with an 8-h photoperiod. Light treatment of dark-adapted leaves to induce zeaxanthin formation was performed as described by Rees et al. (1992). Chloroplasts were isolated using Percoll (Noctor et al., 1991). The reaction medium contained 0.33 m sorbitol, 5 mm MgCl2, 10 mm KCl, 1 mm EDTA, 10 mm Hepes, 0.1 mm methyl viologen, and 35 μm chlorophyll, pH 8.0. We avoided breaking the chloroplasts (by osmotic shock) to preserve the intactness of the grana stacking, which affects the extent and kinetics of the qN. The medium pH was increased to 8.0, the optimum pH for qE (Walters et al., 1994). The medium also contained 1 μm 9-aminoacridine to monitor the extent of ΔpH. Chlorophyll and 9-aminoacridine fluorescence were assayed simultaneously, as described by Noctor et al. (1991).
We used pulses of saturating light at 2-min intervals to obtain data on the kinetic properties of qN formation. However, because close spacing of pulses changes the character of qN, we needed to change the start time for the first pulse from sample to sample to accumulate a sufficient number of Fm′ values at different time intervals. This was possible because of the high reproducibility of qN kinetics over a significant number of replicates.
Simultaneous recordings of chlorophyll fluorescence and absorption changes at 505 nm for leaves were carried out using a fluorimeter (Walz, Effeltrich, Germany) and a spectrophotometer (model DW2000, Aminco, Silver Spring, MD), as described previously (Ruban et al., 1993).
The LHCII components LHCIIb and CP26 were isolated by nondenaturing IEF (Ruban et al., 1994b). Fluorescence quenching in isolated LHCII was measured using a fluorimeter (Walz) as described by Ruban et al. (1994a). For pH titration experiments the required amount of HCl was added 20 s after the dilution of the LHCII sample in buffer. The quenched fluorescence level was taken 30 s after the addition of acid. To analyze fluorescence quenching kinetics in LHCII, leaves, and chloroplasts, a curve-fitting procedure was applied using SigmaPlot software (SPSS, Chicago, IL).
Analysis of the LHCIIb aggregation state was carried out using the Suc-gradient centrifugation method, as described by Ruban et al. (1997b). A seven-step exponential Suc gradient (0.1–0.9 m) was used to resolve aggregates of LHCIIb generated by dilution of the sample in low-detergent medium and by acidification. The sample concentration was 25 μm chlorophyll and the detergent (n-dodecyl β-maltoside) concentration was varied from 6 to 200 μm. Incubation time was 5 min. Immediately after the sample was applied to the gradient, it was centrifuged at 200,000g for 17 h. We gently collected different LHCIIb fractions by syringe and the spectrophotometer recorded their absorption spectra. The chlorophyll content in the samples was estimated from integration of the absorption spectra from 600 to 750 nm. This method was more sensitive than chlorophyll estimation using acetone extraction and was valid for the calculation of the relative amount of chlorophyll in each LHCIIb fraction.
Analysis of the carotenoid composition of leaves, chloroplasts, and LHCII fractions was carried out by HPLC, as described previously (Ruban et al., 1994b). Carotenoid contents were determined as the percentage of total carotenoids and DEPS was expressed as 100 × (zeaxanthin + ½[antheraxanthin])/(violaxanthin + antheraxanthin + zeaxanthin).
RESULTS
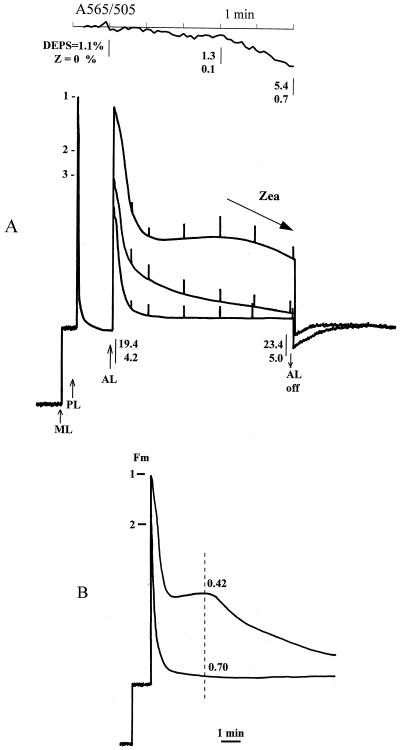
Figure 1A shows the quenching of chlorophyll fluorescence in spinach leaves. Trace 1 is for a dark-adapted leaf with no detectable zeaxanthin and a DEPS of 1.1%. Quenching was induced during the 1st min of illumination, followed by a period when the fluorescence was stable before the beginning of further quenching after 3 to 4 min. Most of the quenching was nonphotochemical. Simultaneous recording of the 505-nm absorption change indicated very little accumulation of zeaxanthin during the first 3 min of illumination; this was confirmed by HPLC analysis. The onset of further quenching after 4 min was correlated with increased zeaxanthin formation. Upon darkening for 10 min a substantial amount of qN reversed, and the Fm′ remained quenched by approximately 25% compared with the initial Fm. Re-illumination of the leaf resulted in a deeper quenching being reached in the 1st min, followed by a period of substantial induction of qN (trace 2). Dark adaptation of this leaf resulted in reversal of almost all of the extra quenching obtained in the second illumination. Re-illumination for a third time (trace 3) caused the fluorescence level to drop rapidly (within 1 min) to the minimum level. This was the maximum attainable level of qN for this actinic light intensity and was associated with a DEPS of approximately 20%.
Figure 1.
A, Chlorophyll fluorescence traces and A505 measured on a spinach leaf. Plants were dark adapted for 24 h and given 5 min of actinic illumination (800 μm PAR m−2 s−1), followed by 10 min of dark adaptation to allow recovery of the fast qN (qE) component. 1, 2, and 3 indicate Fm′ before each illumination cycle. Fv /Fm (or Fv ′/ Fm′) before each run was 0.8, 0.75, and 0.7, respectively. For clarity, only the initial fluorescence and the effect of the initial saturation pulse for the first trace and only the initial fluorescence recovery after the first and third illumination are shown. ML, Measuring beam light; PL, light-saturation pulse; AL, actinic light. Also shown are the DEPS and zeaxanthin (Z) percentages of the total carotenoid content taken at the time intervals indicated by the open arrows. This was done repeatedly for a number of different leaves and averaged. The experimental error was not greater than 15%. A505 was measured simultaneously with the first fluorescence-quenching cycle in differential mode against A565 and is presented as the reversed value (A565 − A505). B, Induction of chlorophyll fluorescence quenching in dark-adapted spinach leaf induced by actinic light of 5000 μm PAR m−2 s−1. Horizontal bars and 1 and 2 indicate the Fm before each illumination cycle. Dark-adaptation period between cycles was 15 min. Fv/Fm (or Fv′/Fm′) was 0.79 and 0.70 for the first and second run, respectively. The vertical dashed line indicates a point where qN was calculated as (Fm − Fm′)/Fm, and the value is displayed above each curve.
Figure 1B shows the results of an experiment that was similar to the one described above, except that the illumination was stronger and longer. This experiment was carried out to light-saturate the photosynthetic electron transport (photochemical quenching < 0.1 throughout) and the ΔpH. The same fluorescence pattern (of a dark-adapted leaf) was observed as that for the lower-intensity illumination, with rapid initial quenching, a period of almost constant fluorescence, and then a further phase of quenching. The transition to a state of maximum quenching occurred after 10 min of illumination. A second illumination resulted in a more rapid quenching to the minimum fluorescence level; the leaf had been “light-activated.” After 3 min of illumination the dark leaf had a qN of 0.42 and the fully light-activated leaf had a qN of 0.70.
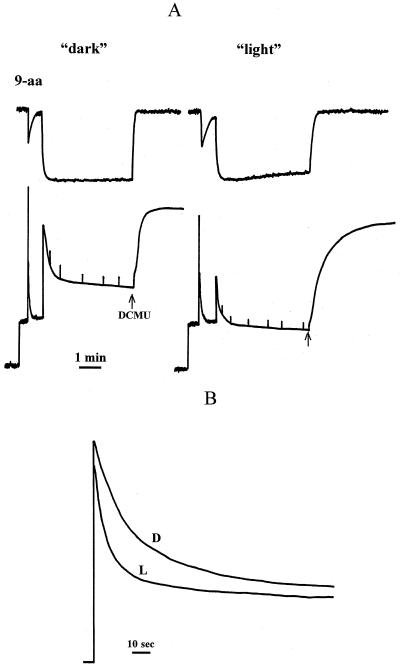
Light-activated leaves showing the behavior seen in Figure 1B typically have a DEPS of 40% to 50%. Chloroplasts isolated from these leaves retained the difference between the light-activated and the dark-adapted states. Figure 2A illustrates that the fluorescence quenching of the dark chloroplasts was significantly less and slower to develop than that of the light chloroplasts. Final values of qN, calculated as (Fm − Fm′)/Fm, where Fm′ was measured for the pulse just prior to the addition of DCMU, were approximately 0.50 for dark and 0.70 for light. In both cases the ΔpH, as estimated from the quenching of 9-aminoacridine fluorescence, formed with the same kinetics and reached the same steady-state level. Most of the qN was ΔpH-dependent, because it relaxed when the ΔpH collapsed after the addition of DCMU. The characteristics of these chloroplasts are summarized in Table I.
Figure 2.
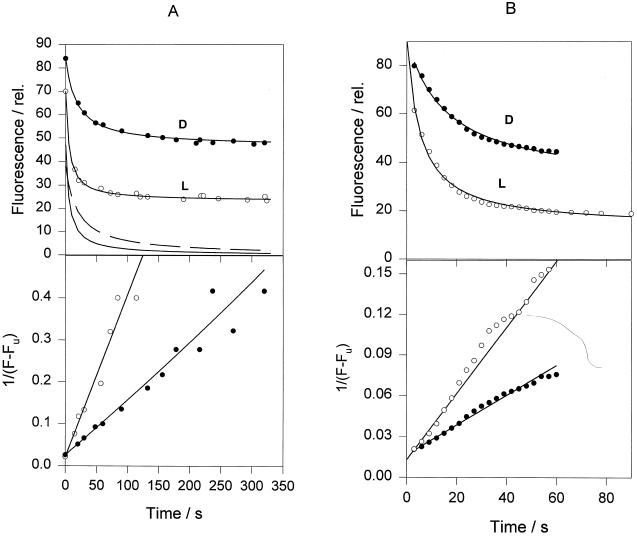
A, Simultaneous measurements of chlorophyll fluorescence quenching and 9-aminoacridine (9-aa) fluorescence quenching for intact spinach chloroplasts isolated from dark-adapted (“dark”) and light-treated (“light”) leaves. Fluorescence quenching was induced by actinic light of 800 μm PAR m−2 s−1. Quenching was reversed by the addition of 5 μm of DCMU (arrow). B, Kinetics of the chlorophyll fluorescence quenching in LHCIIb diluted in buffer at pH 5.5. D and L, Samples from dark-adapted and light-treated leaves, respectively. The chlorophyll concentration of the samples was 3 μm, and the detergent concentration was 6 μm.
Table I.
Chlorophyll fluorescence quenching and ΔpH for intact chloroplasts
| Sample | Actinic Light | q9-aa | qE | Fv/Fm | DEPS |
|---|---|---|---|---|---|
| μmol PAR m−2 s−1 | % | ||||
| Dark | 50 | 0.62 ± 0.02 | 0.08 ± 0.02 | 0.76 ± 0.01 | 4.0 ± 2.0 |
| 600 | 0.84 ± 0.02 | 0.48 ± 0.03 | |||
| Light | 50 | 0.65 ± 0.01 | 0.54 ± 0.02 | 0.69 ± 0.02 | 58.0 ± 2.0 |
| 600 | 0.82 ± 0.03 | 0.61 ± 0.02 | |||
Data are shown for chloroplasts isolated from dark-adapted and light-activated leaves. The coefficient of quenching of 9-aminoacridine (q-9aa) was calculated as the amplitude of quenching divided by total fluorescence. qE = (Fmr − Fm′)/Fm, where Fmr is the recovered (5 min after addition of 5 μm DCMU) fluorescence levels. For details, see legend to Figure 2A. Data are the means (±se) of six to seven assays obtained from two different preparations of chloroplasts.
At a higher light intensity, one sufficient to give a 9-aminoacridine quenching coefficient of 0.82 to 0.84, qE was 0.48 for dark chloroplasts and 0.61 for light chloroplasts. At a lower light intensity and a much lower ΔpH, much larger differences in qE were observed, with a 9-aminoacridine quenching coefficient of 0.62 to 0.65 and qE values of 0.08 and 0.54 for dark and light chloroplasts, respectively. Such data are consistent with the previously published ΔpH titration curves for qE, which show that light activation is associated with a shift in pK to a smaller ΔpH, so that the ΔpH-saturated levels of qE are equal in light and dark chloroplasts (Rees et al., 1989; Noctor et al., 1991).
There is strong evidence that qE occurs in LHCII (Horton and Ruban, 1992; Horton et al., 1996). Light activation of qE has been interpreted according to a model for qE in which a ΔpH-dependent conformational change in the LHCII proteins is responsible for quenching via a change in pigment interactions, and zeaxanthin formation modulates the conformation of the proteins and thereby explains light activation (Horton et al., 1991). Therefore, LHCII complexes were isolated from dark and light chloroplasts. As reported previously, approximately 6% to 10% of the xanthophylls bound to spinach LHCIIb are xanthophyll cycle carotenoids (Ruban et al., 1994b). When isolated from light-activated chloroplasts, a DEPS of 62% was found (Table II).
Table II.
Chlorophyll fluorescence quenching and xanthophyll-cycle parameters for LHCIIb
| Sample | Quenching | DEPS | Zeaxanthin | V + A + Z |
|---|---|---|---|---|
| % | ||||
| Dark | 0.67 ± 0.02 | 10.0 ± 2.0 | 0.1 ± 0.05 | 10.2 ± 2.9 |
| Light | 0.71 ± 0.02 | 62.0 ± 4.0 | 3.7 ± 0.9 | 6.3 ± 1.3 |
Data are shown for LHCIIb isolated from dark-adapted and light-treated leaves. Zeaxanthin content and the xanthophyll-cycle pool of violaxanthin plus antheroxanthin plus zeaxanthin (V + A + Z) are expressed as a percentage of total carotenoid in the sample. “Quenching” is the maximum extent of Chl fluorescence quenching induced by a decrease in pH to 4.2, expressed as a percentage of the Fm. Data are means (±se) of four to five different preparations of LHCIIb.
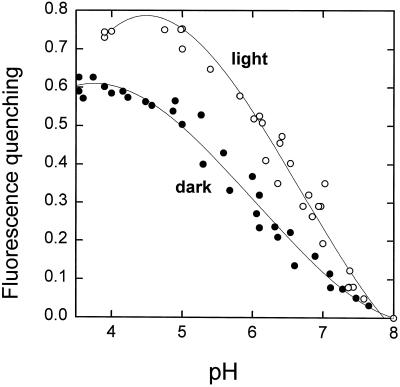
Isolated LHCII can be induced to show fluorescence quenching in vitro upon acidification (Ruban et al., 1994a, 1996). The extent of this quenching was almost the same for “light” and “dark” LHCIIb (Fig. 2B). However, the rate of induction of quenching was markedly different. For dark LHCIIb the half-time was approximately 20 s, whereas it was less than 5 s for light LHCIIb. By determining the fluorescence level after 30 s (light LHCIIb was nearly completely quenched by this time), it was possible to detect a difference between light and dark LHCIIb that resembled the behavior of chloroplasts and leaves. This assay allowed the extent of quenching to be determined as a function of pH (Fig. 3). For dark LHCIIb the quenching titrated with a midpoint around pH 6.0, whereas a value of approximately 6.5 was found for light LHCIIb. This shift in pH dependency was similar to that observed for ΔpH (Noctor et al., 1991) and pH (Rees et al., 1992) titration of fluorescence quenching in chloroplasts. The difference in the rate of induction of quenching provides the first evidence, to our knowledge, of an effect of endogenous xanthophyll cycle carotenoids on the behavior of an isolated LHCII component.
Figure 3.
pH titration of chlorophyll fluorescence quenching in LHCIIb from dark-adapted (•) and light-treated (○) leaves. Fluorescence quenching was calculated as (F − F′)/F, where F is the fluorescence level at the moment of addition of HCl and F′ is the fluorescence level 30 s after the addition of HCl.
The consistent difference in quenching kinetics between light and dark samples for leaves, chloroplasts, and LHCII prompted a more detailed analysis of these kinetics to obtain essential parameters such as the rate constant and the reaction half-time. The process of describing the kinetics of the quenching process involved testing a limited number of mathematical models with increasing complexity (Table III). The objective was to identify the best fit among a small range of simple models, not to rigorously establish a reaction mechanism based on the fit. First, the data were fitted to a one-component first-order exponential, a*exp (− k*t), where a is the maximum fluorescence level, k is the rate constant, and t is time, or a second-order hyperbolic, 1/(k*t + 1/a), and one constant. Fitting with two components was then tested. This program allowed the calculation of the parameter dependencies and the coefficients of variation of parameters.
Table III.
Analysis of the curve-fitting procedures for kinetics of fluorescence quenching in LHCII and chloroplasts
| Sample | Eq | Norm | a1
|
a2
|
k1
|
k2
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| cv | dep | cv | dep | cv | dep | cv | dep | |||
| relative units | relative units | relative units | relative units | relative units | ||||||
| LHCIIb | 1 | 13.1 ± 2.3 | 3.5 ± 1.0 | 0.7 ± 0.2 | 2.1 ± 0.2 | 0.7 ± 0.1 | 6.1 ± 0.8 | 0.8 ± 0.1 | ||
| 2 | 8.3 ± 2.1 | 2.5 ± 0.5 | 0.9 ± 0.06 | 3.1 ± 0.2 | 0.9 ± 0.1 | 11.0 ± 1.6 | 0.87 ± 0.1 | 6.0 ± 0.7 | 0.87 ± 0.12 | |
| 3 | 5.1 ± 1.4 | 2.2 ± 0.3 | 0.6 ± 0.1 | 1.2 ± 0.2 | 0.7 ± 0.07 | 3.6 ± 0.6 | 0.8 ± 0.1 | |||
| 4 | 3.9 ± 1.6 | 1.9 ± 0.4 | 0.8 ± 0.2 | 2.1 ± 0.2 | 0.8 ± 0.12 | 7.1 ± 0.8 | 0.85 ± 0.1 | 2.5 ± 0.7 | 0.95 ± 0.05 | |
| Chloroplast | 1 | 6.3 ± 1.0 | 9.0 ± 2.2 | 0.4 ± 0.1 | 5.1 ± 1.3 | 0.3 ± 0.1 | 7.1 ± 1.1 | 0.4 ± 0.2 | ||
| 2 | 4.1 ± 0.6 | 8.1 ± 1.5 | 0.3 ± 0.1 | 7.2 ± 2.4 | 0.9 ± 0.3 | 6.1 ± 1.2 | 0.4 ± 0.2 | 14.0 ± 2 | 0.86 ± 0.2 | |
| 3 | 3.0 ± 0.5 | 4.0 ± 1.3 | 0.06 ± 0.02 | 0.8 ± 0.2 | 0.3 ± 0.1 | 5.0 ± 0.6 | 0.4 ± 0.2 | |||
| 4 | 2.9 ± 0.5 | 5.3 ± 1.4 | 0.4 ± 0.01 | 2.0 ± 1.2 | 0.9 ± 0.2 | 9.0 ± 1.1 | 0.7 ± 0.2 | 6.3 ± 2.1 | 0.87 ± 0.3 | |
| CP26 | 1 | 5.0 ± 0.3 | 7.3 ± 0.3 | 0.8 ± 0.1 | 10.3 ± 0.8 | 0.4 ± 0.2 | 7.0 ± 0.6 | 0.6 ± 0.2 | ||
| 2 | 3.0 ± 0.1 | 6.3 ± 0.6 | 0.8 ± 0.1 | 5.6 ± 0.3 | 0.9 ± 0.1 | 13.2 ± 1.4 | 0.9 ± 0.1 | 5.3 ± 0.9 | 0.9 ± 0.1 | |
| 3 | 3.0 ± 0.1 | 6.3 ± 0.5 | 0 | 2.1 ± 0.3 | 0.2 ± 0.06 | 1.7 ± 0.5 | 0.4 ± 0.06 | |||
| 4 | 2.2 ± 0.06 | 4.0 ± 0.3 | 0.4 ± 0.03 | 2.0 ± 0.2 | 0.7 ± 0.1 | 2.1 ± 0.6 | 0.8 ± 0.1 | 2.0 ± 0.8 | 0.7 ± 0.1 | |
Fluorescence quenching curves were fitted to four different kinetic models: Eq 1 − Ft = a1.exp(−k1.t) + a2; Eq 2 − Ft = a1.exp(−k1.t) + a2.exp(−k2.t); Eq 3 − Ft = 1/(k1.t + 1/a2) + a2; Eq 4 − Ft = 1/(k1.t + 1/a2) + 1/(k2.t + 1/a2). cv, Coefficient of variation; dep, parameter dependency. Data were obtained from the analysis of three replicate quenching curves.
The other criterion used to evaluate the quality of the curve fit was the norm, calculated from the sum of the squares of the deviations of the calculated values. The overall criterion for best fit was to achieve minimum values for parameter dependency, coefficient of variation, and the norm. The best fit of those tested was obtained using a hyperbolic decay describing the second-order reaction F(t) = 1/(k*t + 1/Fq) + Fu. For this model the coefficients of variation were 1% to 6%, the parameter dependencies 0 to 0.8, and the norm 3% to 5%. The norm divided by the square root of the number of points in the curve represents an average accuracy of the curve fit. If this parameter is normalized to the scale and multiplied by 100%, this yields an average error of the procedure of 0.6% to 1%. Using exponential decay instead of the hyperbolic function caused an approximately 2-fold increase in the value of the norm. Increasing the number of components slightly reduced the norm but increased the parameter dependencies and coefficients of variation.
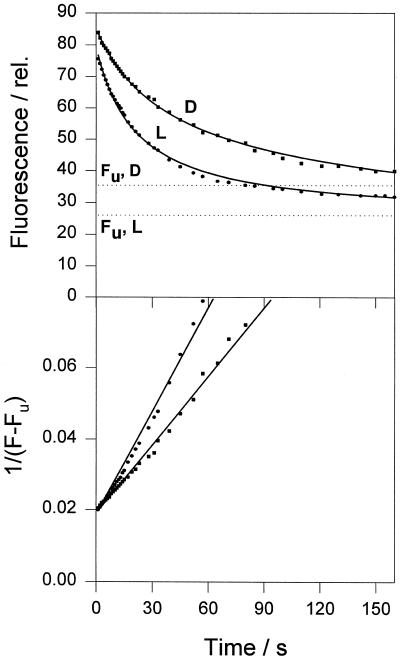
This analysis for pH-induced quenching of light and dark LHCIIb gave a good fit to both LHCII samples (Fig. 4). Curve-fitting parameters are given in Table IV. At both pH 4.2 and 5.5, the quenching rate constant was 2 to 3 times higher in the light LHCIIb. Both samples showed a response to differing pH, with a 3-fold increase in the rate constant at pH 4.2 compared with 5.5. At the lower pH the total amount of quenching was the same in light and dark LHCIIb, but at higher pH the light sample showed a larger quenching. The greater differences in the extent of quenching at subsaturating pH between light and dark LHCIIb are consistent with the data shown in Figure 3 and Table I, and with previous observations on chloroplasts (Noctor et al., 1991).
Figure 4.
Analysis of the kinetics of chlorophyll fluorescence quenching in LHCIIb induced by pH 5.5. Squares and circles are experimental data for LHCII from dark-adapted (D) and light-treated (L) leaves, respectively. Solid lines are theoretical curves derived from curve-fitting analysis using two components; the first expresses a second-order reaction, and the second is a constant component, Fu (shown as dotted lines; for more details, see text). The bottom plot represents a linearization of the experimental and theoretical data from the upper plot using a calculated value of Fu (see Table III). Parameter dependencies were within 0.4 to 0.8. The norm was equal to 4.0. The average curve-fit error was 1.3%. The coefficient of variation of parameters was within 0.7% to 3.6%. See text for definitions.
Table IV.
Results of curve fitting for the kinetics of quenching in LHCIIb, chloroplasts, and leaves
| Sample | D/L | pH | Fq | Fu | k | τ1/2 | qN0 |
|---|---|---|---|---|---|---|---|
| relative units | 1/s | s | |||||
| LHCII | D | 4.2 | 63 | 29 | 0.001 | 15.7 | 0.68 |
| D | 5.5 | 47 | 35 | 0.0004 | 43.6 | 0.57 | |
| L | 4.2 | 70 | 28 | 0.003 | 4.5 | 0.71 | |
| L | 5.5 | 54 | 25 | 0.0009 | 20.6 | 0.68 | |
| Chloroplasts | D | 38 | 46 | 0.0013 | 20.0 | 0.45 | |
| L | 46 | 24 | 0.004 | 5.4 | 0.66 | ||
| Leaves | D | 60 | 31 | 0.001 | 15.2 | 0.65 | |
| L | 78 | 14 | 0.0024 | 5.3 | 0.85 | ||
D and L, Samples prepared from dark-adapted and light-treated leaves, respectively; k, second-order rate constant of quenching; τ1/2, quenching half-time calculated as 1/(Fq*k); qN0, maximum potential quenching calculated as Fq/(Fq + Fu). See text for details of calculation.
This method of kinetic analysis of fluorescence quenching was applied to chloroplasts and leaves (Fig. 5) and, again, the quenching could be fitted to a hyperbolic function (Table III). The curve-fitting parameters were again different between light and dark samples (Table IV). For chloroplasts the rate constant for the induction of quenching was 3 times higher for the light-activated sample. Also, the total amount of quenching was higher and the amount of residual unquenchable fluorescence was lower. We found a similar pattern for leaves: a higher rate constant and smaller residual fluorescence for the light-adapted leaf.
Figure 5.
Analysis of qN kinetics in intact chloroplasts (A) and leaves (B). Actinic light intensity for chloroplasts was 600 μm PAR m−2 s−1, and for leaves it was 5000 μm PAR m−2 s−1. Data were obtained as for Figures 1 and 2. A, Top solid and dashed line at the bottom of the graph are kinetic components derived from the curve-fit procedure for light (L) and dark (D) chloroplasts, respectively. Other details are as described in the Figure 4 legend. Parameter dependencies for chloroplasts and leaves were within 0.11 to 0.5 and 0.5 to 0.9, respectively. The norm was 2.9 for chloroplasts and 5.3 for leaves. The coefficient of variation of parameters was within 1.4% to 4.9% for chloroplasts and 1.9% to 7.4% for leaves. See text for definitions.
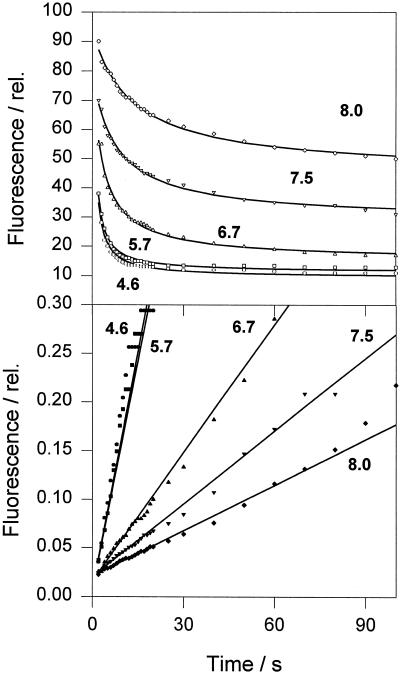
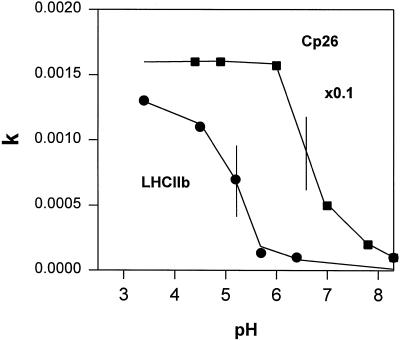
We also applied the kinetic analysis to CP26, one of the minor components of LHCII, and found no detectable difference between the quenching kinetics of samples prepared from light-treated and dark-adapted leaves. Figure 6 illustrates data for a dark CP26 sample. We obtained good data fits at a range of pH values. We found rapid quenching even at pH 8.0 for this complex and, in contrast to LHCIIb (see Table IV), we found no difference in kinetics between 4.6 and 5.7. A titration of the rate constant determined for CP26 and LHCIIb confirmed this difference (Fig. 7): for CP26 a half-maximum effect at pH 6.8, with the rate saturated by pH 6.0, whereas for LHCIIb these values were pH 5.2 and 4.0, respectively.
Figure 6.
Analysis of chlorophyll fluorescence quenching kinetics in CP26 induced by acidification. The lines are theoretical curves derived from curve-fitting analysis using two components; the first expresses a second-order reaction and the second is a constant component, Fu. The bottom plot represents a linearization of the experimental and theoretical data from the upper plot using a calculated value of Fu (see Table III). Parameter dependencies were within 0.2 to 0.4, and the norm was 3.0. The average curve-fit error was 1.3%. The coefficient of variation of parameters was within 1.7% to 6.3%. Numbers on the plot correspond to pH values in the incubation medium. See text for definitions.
Figure 7.
pH dependency of the fluorescence quenching rate constant for CP26 and LHCIIb from dark-adapted leaves. See text for explanation.
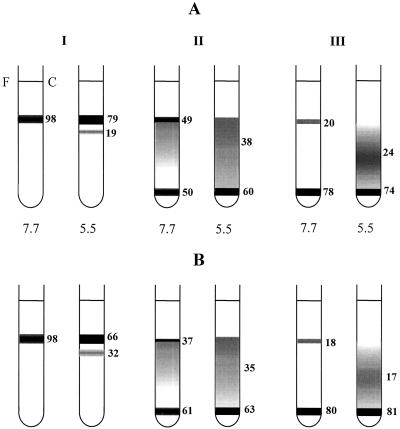
The above data show that LHCIIb from light-adapted plants had an increased tendency for fluorescence quenching in vitro. Previous work had established that in vitro quenching in these complexes was related to the formation of protein aggregates (Ruban et al., 1997b). We therefore examined the effect of light adaptation on the LHCII-aggregation state by determining the distribution of LHCII on Suc gradients. LHCII trimers appeared at the top of the gradient and macromolecular aggregates at the bottom, and between these were oligomers of varying size. The observed pattern on the gradient was a function of the detergent concentration and the pH; low detergent concentrations and pH favored oligomerization more than high detergent concentrations and pH. These tendencies were clearly shown in both light and dark LHCIIb (Fig. 8): At pH 5.5 there was a consistent trend towards increased oligomerization compared with pH 7.7 at all detergent concentrations, and a similar trend for increased oligomerization was found by comparing high (200 μm), medium (100 μm), and low (15 μm) n-dodecyl β-maltoside concentrations.
Figure 8.
Suc-gradient centrifugation of LHCII at different pH values and detergent concentrations (I, II, and III correspond to 200, 100, and 15 μm of n-dodecyl β-maltoside, respectively). Numbers beside each tube are the chlorophyll concentration as a percentage of the total. Numbers below each tube are the pH values. A and B correspond to LHCIIb from dark-adapted and light-treated leaves, respectively. Data represent the average from four experiments. The experimental error was within 5%. See text for details.
To expose differences between light and dark samples we needed to select the appropriate detergent concentration and pH; if the disaggregated state was too stable (at a high detergent concentration and pH), no effect would be observed. Conversely, if the aggregated state was too strongly induced (at low detergent concentration and pH), again, no effect would be predicted. At 200 μm n-dodecyl β-maltoside there was no difference between light and dark LHCII at pH 7.7, but we found an increased proportion of oligomers at pH 5.5 for light LHCII. At 100 μm n-dodecyl β-maltoside we found increased oligomerization at pH 7.7 in the light LHCIIb, but there was no difference at pH 5.5. At 15 μm n-dodecyl β-maltoside there was no significant difference between light and dark at either pH value.
DISCUSSION
It had been shown previously that light treatment of isolated thylakoids caused an increase in the sensitivity of qE to pH, so that the pK shifted to a higher lumen pH and, thus, a smaller ΔpH (Rees et al., 1989; Noctor et al., 1991). This activation was attributed to the light-dependent de-epoxidation of violaxanthin to zeaxanthin. It was proposed, therefore, that the xanthophyll-cycle carotenoids controlled qE by modulating protonation-dependent quenching in the LHCII system according to a simple process of allosteric control (Horton et al., 1991, 1994, 1996; Ruban and Horton, 1995b).
In the current study we describe some important new features of the putative activating role of the xanthophyll cycle. First, the activating effect appeared in leaves and isolated LHCIIb in addition to chloroplasts. qN developed slowly and weakly upon illumination of a dark-adapted leaf. Preillumination for several minutes induced a large increase in both the rate of formation and the extent of qE, such that maximum quenching was attained after approximately 2 min. In the dark-adapted leaf photosynthetic electron transport was restricted by the low activity of the Calvin cycle during the induction period of photosynthetic carbon assimilation (Walker, 1976). During the induction period there was a buildup of ATP (Giersch et al., 1980; Quick and Horton, 1986) and ΔpH (Horton, 1983). Preillumination removed this type of photosynthetic induction period (Walker, 1976), suggesting that the ΔpH was equal to or smaller than that found in the dark-adapted leaf. Therefore, the observation of more rapid qE formation in the light-treated leaf clearly showed the presence of light activation in vivo.
The development of the activation of qE was correlated with the accumulation of zeaxanthin. We should note that a de-epoxidation state of only about 20% was necessary for the maximum extent of qE, which explains the fact that the extent of activation needed to reach maximum qE depended on the ΔpH. Examination of the titration of ΔpH and qE in thylakoids showed that the extent of the shift was related to the DEPS (Noctor et al., 1991), and what was required to reach the ceiling level of qE depended on the value of the ΔpH attained in the leaf. There is additional complexity because the activity of the violaxanthin de-epoxidase, which determined the DEPS, was dependent on the lumen pH (Pfündel and Bilger, 1994; Eskling et al., 1997), and this dependency was also subject to light activation (Delrieu, 1998). It is also important to point out that the development of the light-activated state, for both chloroplasts and leaves, was associated with a sustained qN and an associated decrease in Fv /Fm, which is in agreement with previous work (Ruban and Horton, 1995a).
To our knowledge, the effect of light activation on the rate of qE formation has not yet been analyzed, although there are numerous reports of faster quenching after preillumination (Demmig-Adams et al., 1989; Ruban et al., 1993; Johnson et al., 1994). A 2- to 3-fold increase in the rate constant was found in both leaves and chloroplasts in the light-activated state. For the latter, any differences in ΔpH were totally excluded by direct measurement of 9-aminoacridine fluorescence. It is particularly important to point out that the differences in the rate of qE formation between light and dark chloroplasts were not due to differences in the rate of ΔpH formation. In fact, the difference in the rate of formation of quenching compared with ΔpH is a fundamental characteristic of qE, which was the first line of evidence that quenching arose from a (slow) conformational change (Horton, 1996).
From this and other evidence it is widely concluded that the effect of low pH is due to protonation-induced conformational changes in one or more of the components of the PSII antenna (Horton et al., 1991; Gilmore and Yamamoto 1992; Noctor et al., 1993; Ruban et al., 1993; Bilger and Björkman, 1994). Support for the view that both protonation and zeaxanthin induce conformational changes in the PSII antenna came later from an analysis of fluorescence lifetimes (Gilmore et al., 1995, 1996a, 1996b) and steady-state fluorescence (Walters and Horton, 1993), which indicated that the qE was associated with a switch from an unquenched to a quenched conformation. This conformational change was thought to give rise to the absorption change at 535 nm (Noctor et al., 1993; Ruban et al., 1993; Bilger and Björkman, 1994). More rapid qE formation induced in leaves (by preillumination similar to that used here, see Ruban et al. [1993]) or in isolated chloroplasts (Noctor et al., 1993) was indeed associated with a similarly accelerated ΔA535. Therefore, we concluded that in the presence of zeaxanthin this conformational change occurs more rapidly. Such a conclusion is easily accommodated within (and indeed predicted by) the allosteric conformation-change model for qE, but is not so easily explained by a model in which zeaxanthin is a direct quencher of fluorescence.
The accelerating effect of zeaxanthin on fluorescence quenching was clearly found in isolated LHCIIb. Even though xanthophyll cycle carotenoids account for only 6% to 10% of the bound xanthophylls, the rate of quenching was significantly faster in light compared with dark LHCIIb. Previous work has demonstrated the accelerating effect of zeaxanthin and the inhibitory effect of violaxanthin with exogenously added carotenoids (Ruban et al., 1994a, 1996; Phillip et al., 1996). In the current study it was significant that we showed the same effect, this time caused by different DEPS of endogenously bound carotenoids. Similarly, just as exogenous carotenoids have been found to control the state of oligomerization of LHCIIb, light-treated complexes have shown an increased tendency for oligomerization.
The increase in the rate of induction of quenching in samples of LHCIIb with an increased DEPS was similar to that found for qE in leaves and thylakoids, providing new evidence that the quenching observed in vivo and in vitro shared some common features. Using the in vitro system the accelerating effects of both the decrease in pH and the increase in DEPS can be quantified. The result predicts that both the extent of qE and the rate of formation are dependent on ΔpH. The measurements made on isolated chloroplasts have confirmed this finding. To our knowledge there are no published studies of the analysis of the rate of qE formation as a function of ΔpH in leaves determined, for instance, by alterations in light intensity, although in the current study we found that in spinach leaves both the rate and extent of quenching showed a similar dependency on light intensity (A.V. Ruban, unpublished data).
The kinetics of quenching in LHCII, chloroplasts, and leaves was found to fit a single-component hyperbolic function, providing further support for the view that the quenching mechanism is the same in all three systems. The kinetic model describes the existence of a proportion of fluorescence that is not quenchable; moreover, its amplitude is not fixed, but instead depends on the DEPS and the pH. Although we obtained the best fit using a second-order hyperbolic function, the known heterogeneities of the systems indicate that it is premature to attach a mechanistic interpretation to this finding.
The participation of LHCIIb in qE has been questioned (e.g. Demmig-Adams and Adams, 1996; Gilmore et al., 1996b), and some have suggested that the minor LHCII components play a major role in qE in vivo (Horton and Ruban, 1992; Bassi et al., 1993; Crofts and Yerkes, 1994; Walters et al., 1994; Gilmore et al., 1996b; Pesaresi et al., 1997). The doubt about the involvement of LHCIIb is based partly on the study of mutants and/or developing chloroplasts, which lack LHCIIb but still show qE (Härtel and Lokstein, 1995; Jahns and Schweig, 1995; Gilmore et al., 1996b). However, it is important to point out that in these plants quenching is less efficient (Briantais, 1994) and is expressed in terms of quenching efficiency with respect either to the de-epoxidation state of the xanthophyll pool (Gilmore et al., 1996b) or to ΔpH (Schonknecht et al., 1996). Efficient quenching and its physiological regulation may therefore require a complete system containing all of the LHCII proteins, implying that the xanthophyll cycle and ΔpH exert control over the organization of the whole PSII antenna, as depicted in Horton et al. (1994). It should also be emphasized that the xanthophyll cycle carotenoids bound by the minor LHCII account for only approximately 30% of the PSII-associated pool, with the remainder associated with LHCIIb (Ruban et al., 1994b). All of the xanthophyll cycle carotenoids except those bound to CP29 appear to be approximately equally available for de-epoxidation (Ruban et al., 1994b; Färber et al., 1997).
Previously, we have shown that isolated CP29 and CP26 exhibited similar in vitro quenching to LHCIIb (Ruban et al., 1996); this is not unexpected given the high degree of structural homology among these proteins (Jansson, 1994; Green and Durnford, 1996). Therefore, irrespective of whether the principal site of qE is in LHCIIb or in the minor complexes, or whether qE is a property of the LHCII system as a whole, these experiments with LHCIIb serve as models for studying the potential for dynamic changes in the structural and photophysical properties of all of these complexes. CP26 more readily forms the quenched state than LHCIIb, the effect of pH saturating around 6.0. The strong potential for quenching in CP26 is consistent with the key role ascribed to qE. However, there was no effect of an increased DEPS on the rate of quenching for CP26, despite the fact that xanthophyll cycle carotenoids account for 30% of its bound carotenoids (Bassi et al., 1993; Ruban et al., 1994b). It may indicate that endogenous carotenoids do not regulate quenching in CP26. Another explanation is that this complex so readily adopts the quenched state in vitro that xanthophylls are not limiting. This idea may relate to the greater hydrophobicity of the complex arising from the higher lipid-to-protein ratio (Tremolieres et al., 1994).
It also has to be emphasized that in vitro experiments with purified complexes can only reveal the dynamic potential of each complex. There is much more freedom for protein-to-protein interactions and conformational changes in vitro than there are in vivo, where the LHCII components are organized in a strict stoichiometry in the PSII complex (Hankamer et al., 1997; Rhee et al., 1997). Thus, in vivo, interaction between CP26 complexes probably could not occur, but interaction of CP26 with different complexes could. Further work is needed to explore how the macrostructure of the PSII antenna and the associated xanthophyll cycle carotenoids control the structural and photophysical properties of the LHCII components.
In summary, we have shown here how the xanthophyll cycle controls not only the extent of qE but also its rate of formation. The protonation state and the de-epoxidation state of the associated xanthophylls determine the rate constant for the induction of quenching. This is evident from experiments on both leaves and chloroplasts. These observations are consistent with the allosteric conformational change model for qE in which four states of LHCII are described (Horton et al., 1991, 1996; Horton and Ruban, 1992). The unprotonated state with bound violaxanthin is quenched slowly and only partially at a subsaturating ΔpH. In contrast, the unprotonated state binding zeaxanthin can be quenched rapidly and deeply at physiological ΔpH. The primed state of LHCII is already weakly quenched. The fact that similar effects of light activation and similar quenching kinetics are found in isolated LHCII is consistent with this model and further validates the use of in vitro systems for exploring the molecular mechanism of qE.
Abbreviations:
- ΔpH
transthylakoid pH gradient
- DEPS
de-epoxidation state of the xanthophyll cycle carotenoids
- Fm
maximum level of fluorescence in the dark-adapted state
- Fm′
maximum level of fluorescence after illumination
- Fq
amplitude of quenchable fluorescence
- Fu
amplitude of unquenchable fluorescence
- Fv
variable fluorescence
- LHCII
light-harvesting complex of PSII consisting of CP26, CP24, CP29, and LHCIIb
- qE
component of nonphotochemical quenching dependent on the ΔpH
- qN
nonphotochemical quenching
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council of the United Kingdom (grant no. 50/C05874).
LITERATURE CITED
- Bassi R, Pineau B, Dainese P, Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993;212:297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Planta. 1994;193:238–246. [Google Scholar]
- Björkman O, Demmig-Adams B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze ED, Caldwell MM, editors. Ecophysiology of Photosynthesis. Berlin: Springer-Verlag; 1995. pp. 17–47. [Google Scholar]
- Briantais J-M. Light harvesting chlorophyll a/b complex requirement for regulation of photosystem II photochemistry by nonphotochemical quenching. Photosynth Res. 1994;40:287–294. doi: 10.1007/BF00034778. [DOI] [PubMed] [Google Scholar]
- Briantais J-M, Vernotte C, Picaud M, Krause GH. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979;548:128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Crofts AR, Yerkes CT. A molecular mechanism for qE quenching. FEBS Lett. 1994;352:265–270. doi: 10.1016/0014-5793(94)00976-7. [DOI] [PubMed] [Google Scholar]
- Delrieu MJ. Regulation of thermal dissipation of absorbed excitation energy and violaxanthin deepoxidation in the thylakoids of Lactuca sativa: photoprotective mechanism of a population of photosystem II reaction centres. Biochim Biophys Acta. 1998;1363:157–173. doi: 10.1016/s0005-2728(97)00097-2. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. Carotenoids and photoprotection: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta. 1990;1020:1–24. [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Demmig-Adams B, Adams WW, III, Logan BA, Verhoevan AS. Xanthophyll cycle-dependent energy dissipation and flexible photosystem II efficiency in plants acclimated to light stress. Aust J Plant Physiol. 1995;22:249–260. [Google Scholar]
- Demmig-Adams B, Winter K, Kruger A, Czygan F-C. Zeaxanthin and the induction and relaxation kinetics of the dissipation of excess energy in leaves in 2% O2, 0% CO2. Plant Physiol. 1989;90:887–893. doi: 10.1104/pp.90.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskling M, Arvidsson P-O, Åckerlund H-E. The xanthophyll cycle, its regulation and components. Physiol Plant. 1997;100:806–816. [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P. Dynamics of the xanthophyll cycle in different antenna sub-complexes in the photosynthetic membranes of higher plants. Plant Physiol. 1997;115:1609–1618. doi: 10.1104/pp.115.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank HA, Cua A, Chynwat V, Young AJ, Goztola D, Wasielewski MR. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- Giersch C, Heber U, Kobayashi Y, Inoue Y, Shibata K, Heldt HW. Energy charge, phosphorylation potential and proton motive force in chloroplasts. Biochim Biophys Acta. 1980;589:59–73. doi: 10.1016/0005-2728(80)90146-2. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Debruner PG, Govindjee Comparative time-resolved photosystem II chlorophyll a fluorescence analysis reveals distinctive differences between photoinhibitory reaction centre damage and xanthophyll cycle dependent energy dissipation. Photochem Photobiol. 1996a;64:552–563. doi: 10.1111/j.1751-1097.1996.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Debrunner PG, Govindjee Photosystem II chlorophyll a fluorescence lifetimes and intensity are independent of the antenna size differences between barley wild-type and chlorina mutants: photochemical quenching and xanthophyll cycle nonphotochemical quenching of fluorescence. Photosynth Res. 1996b;48:171–187. doi: 10.1007/BF00041007. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Govindjee Xanthophyll cycle dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short lifetime. Proc Natl Acad Sci USA. 1995;92:2273–2277. doi: 10.1073/pnas.92.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 1991;96:635–643. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA. 1992;89:1899–1903. doi: 10.1073/pnas.89.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BR, Durnford DG. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:685–714. doi: 10.1146/annurev.arplant.47.1.685. [DOI] [PubMed] [Google Scholar]
- Hankamer B, Barber J, Boekema EJ. Structure and membrane organisation of photosystem II in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:641–671. doi: 10.1146/annurev.arplant.48.1.641. [DOI] [PubMed] [Google Scholar]
- Härtel H, Lokstein H. Relationship between quenching of maximum and dark level of chlorophyll fluorescence in vivo: dependence on photosystem II antenna size. Biochim Biophys Acta. 1995;1228:91–94. [Google Scholar]
- Horton P. Relations between electron transport and carbon assimilation simultaneous measurement of chlorophyll fluorescence, transthylakoid pH gradient and O2 evolution in isolated chloroplasts. Proc R Soc Lond Ser B. 1983;217:405–416. [Google Scholar]
- Horton P. Interplay between environmental and metabolic factors in the regulation of photosynthesis in higher plants. In: Biggins J, editor. Progress in Photosynthesis Research, Vol II. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 681–688. [Google Scholar]
- Horton P (1996) Nonphotochemical quenching of chlorophyll fluorescence. In RC Jennings, G Zucchelli, F Ghetti, G Colombetti, eds, Light as an Energy Source and Information Carrier in Plant Physiology. Plenum Press, New York, NY, pp 99–111
- Horton P, Ruban AV. Regulation of photosystem II. Photosynth Res. 1992;34:375–385. doi: 10.1007/BF00029812. [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett. 1991;292:1–4. doi: 10.1016/0014-5793(91)80819-o. [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Indication by non-photochemical quenching of chlorophyll fluorescence. Plant Physiol. 1994;106:415–420. doi: 10.1104/pp.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jahns P, Schweig S. Energy-dependent quenching of chlorophyll fluorescence in the thylakoids from intermittent-light grown pea plants: evidence for an interaction of zeaxanthin and the chlorophyll a/b binding proteins. Plant Physiol Biochem. 1995;33:683–687. [Google Scholar]
- Jansson S. The light harvesting chlorophyll a/b-binding proteins. Biochim Biophys Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Johnson GN, Young AJ, Horton P. Activation of nonphotochemical quenching in thylakoids and leaves. Planta. 1994;194:550–556. [Google Scholar]
- Noctor G, Rees D, Young A, Horton P. The relationship between zeaxanthin, energy-dependent quenching of chlorophyll fluorescence and the transthylakoid pH-gradient in isolated chloroplasts. Biochim Biophys Acta. 1991;1057:320–330. [Google Scholar]
- Noctor G, Ruban AV, Horton P. Modulation of ΔpH-dependent nonphotochemical quenching of chlorophyll fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1993;1183:339–344. [Google Scholar]
- Nyogi KK, Björkman O, Grossman AR. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell. 1997a;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyogi KK, Björkman O, Grossman AR. The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA. 1997b;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens TG, Shreve AP, Albrecht AC. Dynamics and mechanism of singlet energy transfer between carotenoids and chlorophylls: light harvesting and nonphotochemical fluorescence quenching. In: Murata N, editor. Research in Photosynthesis, Vol 4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 179–186. [Google Scholar]
- Pesaresi P, Sandonà D, Giuffra E, Bassi R. A single point mutation (E166Q) prevents dicyclohexylcarbodiimide binding to the photosystem II subunit CP29. FEBS Lett. 1997;402:151–156. doi: 10.1016/s0014-5793(96)01518-9. [DOI] [PubMed] [Google Scholar]
- Peter GF, Thornber P. Biochemical composition and organisation of higher plant photosystem II light harvesting proteins. J Biol Chem. 1991;266:16745–16754. [PubMed] [Google Scholar]
- Pfündel EE, Bilger W. Regulation and possible function of the xanthophyll cycle. Photosynth Res. 1994;42:89–109. doi: 10.1007/BF02187121. [DOI] [PubMed] [Google Scholar]
- Phillip D, Ruban AV, Horton P, Asato A, Young AJ. Quenching of chlorophyll fluorescence in the major light harvesting complex of photosystem II. Proc Natl Acad Sci USA. 1996;93:1492–1497. doi: 10.1073/pnas.93.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick WP, Horton P. Studies on the induction of chlorophyll fluorescence in barley protoplasts. III. Correlation between changes in the level of glycerate 3-phosphate and the pattern of fluorescence quenching. Biochim Biophys Acta. 1986;849:1–6. [Google Scholar]
- Rees D, Noctor G, Ruban AV, Crofts J, Young A, Horton P. pH dependent chlorophyll fluorescence quenching in spinach thylakoids from light treated or dark adapted leaves. Photosynth Res. 1992;31:11–19. doi: 10.1007/BF00049532. [DOI] [PubMed] [Google Scholar]
- Rees D, Young AJ, Noctor G, Britton G, Horton P. Enhancement of the ΔpH-dependent dissipation of excitation energy in spinach chloroplasts by light activation: correlation with the synthesis of zeaxanthin. FEBS Lett. 1989;256:85–90. [Google Scholar]
- Rhee K-H, Morris EP, Zhaleva D, Hankamer B, Kuhlbrandt W, Barber J. Two-dimensional structure of plant photosystem II at 8 Å resolution. Nature. 1997;389:522–526. doi: 10.1038/24421. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Calkoen F, Kwa SLS, van Grondelle R, Horton P, Dekker JP. Characterisation of LHCII in the aggregated state by linear and circular dichroism spectroscopy. Biochim Biophys Acta. 1997a;1321:61–70. [Google Scholar]
- Ruban AV, Horton P. An investigation of the sustained component of nonphotochemical quenching of chlorophyll fluorescence in isolated chloroplasts and leaves of spinach. Plant Physiol. 1995a;108:721–726. doi: 10.1104/pp.108.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Horton P. Regulation of non-photochemical quenching of chlorophyll fluorescence in plants. Aust J Plant Physiol. 1995b;22:221–230. [Google Scholar]
- Ruban AV, Philip D, Young AJ, Horton P. Carotenoid-dependent oligomerization of the major light harvesting complex of photosystem II in plants. Biochemistry. 1997b;36:7855–7859. doi: 10.1021/bi9630725. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Young AJ, Horton P. Induction on nonphotochemical energy dissipation and absorbance changes in leaves. Evidence for changes in the state of the light harvesting system of photosystem II in vivo. Plant Physiol. 1993;102:741–750. doi: 10.1104/pp.102.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Young AJ, Horton P. Modulation of chlorophyll fluorescence quenching in isolated light harvesting complex of photosystem II. Biochim Biophys Acta. 1994a;1186:123–127. [Google Scholar]
- Ruban AV, Young AJ, Horton P. Quenching of chlorophyll fluorescence in the minor chlorophyll a/b binding proteins of photosystem II. Biochemistry. 1996;35:674–678. doi: 10.1021/bi9524878. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Young AJ, Pascal AA, Horton P. The effects of illumination on the xanthophyll composition of the photosystem II light harvesting complexes of spinach thylakoid membranes. Plant Physiol. 1994b;104:227–234. doi: 10.1104/pp.104.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonknecht G, Neimanis S, Gerst U, Heber U. The pH dependent regulation of photosynthetic electron transport in leaves. In: Mathis P, editor. Photosynthesis: from Light to the Bio-sphere. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 843–846. [Google Scholar]
- Tremolieres A, Dainese P, Bassi R. Heterogeneous lipid distribution chlorophyll-binding proteins of photosystem II in maize mesophyll chloroplasts. Eur J Biochem. 1994;221:721–730. doi: 10.1111/j.1432-1033.1994.tb18785.x. [DOI] [PubMed] [Google Scholar]
- Walker DA. CO2 fixation by intact chloroplasts: photosynthetic induction and its relation to transport phenomena and control mechanisms. In: Barber J, editor. The Intact Chloroplast. Amsterdam: Elsevier; 1976. pp. 235–278. [Google Scholar]
- Walters RG, Horton P. Theoretical assessment of alternative mechanisms for non-photochemical quenching in barley leaves. Photosynth Res. 1993;27:121–133. doi: 10.1007/BF00016277. [DOI] [PubMed] [Google Scholar]
- Walters RG, Ruban AV, Horton P. Light-harvesting complexes bound by dicyclohexylcarbodiimide during inhibition of protective energy dissipation. Eur J Biochem. 1994;226:1063–1069. doi: 10.1111/j.1432-1033.1994.01063.x. [DOI] [PubMed] [Google Scholar]
- Walters RG, Ruban AV, Horton P. Identification of proton-active residues in a higher plant light-harvesting complex. Proc Natl Acad Sci USA. 1996;93:14204–14209. doi: 10.1073/pnas.93.24.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]