Abstract
The Minute syndrome in Drosophila melanogaster is characterized by delayed development, poor fertility, and short slender bristles. Many Minute loci correspond to disruptions of genes for cytoplasmic ribosomal proteins, and therefore the phenotype has been attributed to alterations in translational processes. Although protein translation is crucial for all cells in an organism, it is unclear why Minute mutations cause effects in specific tissues. To determine whether the heart is sensitive to haplo-insufficiency of genes encoding ribosomal proteins, we measured heart function of Minute mutants using optical coherence tomography. We found that cardiomyopathy is associated with the Minute syndrome caused by haplo-insufficiency of genes encoding cytoplasmic ribosomal proteins. While mutations of genes encoding non-Minute cytoplasmic ribosomal proteins are homozygous lethal, heterozygous deficiencies spanning these non-Minute genes did not cause a change in cardiac function. Deficiencies of genes for non-Minute mitochondrial ribosomal proteins also did not show abnormal cardiac function, with the exception of a heterozygous disruption of mRpS33. We demonstrate that cardiomyopathy is a common trait of the Minute syndrome caused by haplo-insufficiency of genes encoding cytoplasmic ribosomal proteins. In contrast, most cases of heterozygous deficiencies of genes encoding non-Minute ribosomal proteins have normal heart function in adult Drosophila.
THE Minute phenotype in Drosophila melanogaster is characterized by delayed development, poor fertility, short slender bristles, and smaller body size (Lambertsson 1998; Marygold et al. 2007). The distribution of Minute loci throughout the genome and the uniformity of the phenotype have been of great interest to geneticists for nearly a century. Many Minute loci correspond to cytoplasmic ribosomal genes, suggesting that the basis of the Minute phenotypes involves disruption of ribosomal function (Hart et al. 1993; Andersson et al. 1994; Mckim et al. 1996; Saeboe-Larssen and Lambertsson 1996; Schmidt et al. 1996; Saeboe-Larssen et al. 1997; Van Beest et al. 1998; Torok et al. 1999; Fauvarque et al. 2001; Marygold et al. 2005; Alexander et al. 2006; Tyler et al. 2007).
Eukaryotic cells contain cytoplasmic ribosomes and mitochondrial ribosomes, each containing many subunits. While all of these subunits are translated from nuclear genes, cytoplasmic ribosomes translate proteins in the cytosol and on the endoplasmic reticulum, whereas mitochondrial ribosomes synthesize proteins from mitochondrial genes in the mitochondrial matrix. Recent genetic and bioinformatic efforts have shown that Drosophila melanogaster have 88 genes encoding 79 different cytoplasmic ribosomal proteins (CRPs) and 75 nuclear-encoded mitochondrial ribosomal proteins (MRPs) (Marygold et al. 2007). While mutations in many CRP genes result in a Minute phenotype, mutations in MRP genes are not associated with the Minute syndrome (Marygold et al. 2007).
Drosophila CRPs correspond with all 79 human CRPs, while MRPs are more divergent between species (Marygold et al. 2007). Mutations in human CRP genes as well as genes for other proteins involved in ribosomal biogenesis have been shown to cause a variety of syndromes (reviewed in Freed et al. 2010), most notably CRP gene mutations in Diamond Blackfan anemia, characterized by congenital anemia and a variety of developmental abnormalities. Several MRP genes also map to loci associated with human disorders as well (reviewed in O’brien et al. 2005).
Since ribosomal proteins are important in every cell of an organism, we tested whether abnormal heart function is part of the Minute syndrome. We addressed this question using optical coherence tomography (OCT) (Wolf et al. 2006; Kim and Wolf 2009; Kim et al. 2010; Yu et al. 2010) to examine in vivo cardiac size and function in adult Drosophila with ribosomal deficiency. We show that haplo-insufficiency of RpS15Aa, a predicted Minute gene, results in characteristic short, slender bristles as well as significant dilation and dysfunction of the Drosophila heart. By examining the heart phenotype of many other deficiencies across CRP and MRP genes we also show that cardiac abnormalities are common in Minute flies, indicating that the heart is sensitive to deletion of a single copy of some ribosomal genes.
Materials and Methods
Drosophila stocks
All ribosomal deficiency stocks were obtained from the Bloomington Drosophila Stock Center. All UAS-RNAi stocks were obtained from the Vienna Drosophila RNAi Center (Dietzl et al. 2007): 19198 (RpS15Aa), 106321 (RpS3), 36060 (RpS5), 105182 (mRpS33).
wy1;+/+;p{tinC-Gal4}/p{tinC-Gal4} stock was provided by Manfred Frasch (Yin and Frasch 1998). wy1;p{tubP-GAL80ts}/p{tubP-GAL80ts};p{tinC-Gal4}/p{tinC-Gal4} stock was created as described (Kim and Wolf 2009; Yu et al. 2010). All stocks were maintained on standard media at room temperature.
Custom genomic deficiencies
PBac{WH}f08066, PBac{WH}f07791, PBac{RB}e00904, and P{XP}d02459 insertions stocks were obtained from the Exelixis Collection at Harvard Medical School. The genomic deletion Df(1)f08066-f07791 was generated using PBac{WH}f08066 and PBac{WH}f07791 stocks, and Df(1)e00904-d02459 was generated using PBac{RB}e00904 and P{XP}d02459 stocks according to previously established protocols (Parks et al. 2004). The phenotype of these custom deletions are described in the Results for Df(1)f08066-f07791 and shown in Table 1 for both Df(1)f08066-f07791 and Df(1)e00904-d02459.
Table 1. Cardiomyopathy correlates with the Minute phenotype in Drosophila melanogaster.
| Gene (synonym) | Bristle phenotypea | Heart phenotype | Allele | Genes affected | EDDb (µm) | ESDb (µm) | FSb (%) | n = |
|---|---|---|---|---|---|---|---|---|
| Control | Normal | Normal | w1118 | 81 ± 4 | 2 ± 1 | 98 ± 1 | 16 | |
| Chromosome X | ||||||||
| RpL36 | Minute | Abnormal | Df(1)y74k24.1 | >30 | 123 ± 6 | 29 ± 9 | 78 ± 6 | 8 |
| RpL7A | Minute | Abnormal | Df(1)dx81 | >100 | 96 ± 5 | 23 ± 5 | 78 ± 5 | 14 |
| RpS15Aa | Minute | Abnormal | Df(1)f08066-f07791 | 3 | 131 ± 12 | 32 ± 10 | 78 ± 5 | 9 |
| eIF-2α | Minute | Abnormal | Df(1)ED7364 | 45 | 107 ± 4 | 22 ± 4 | 79 ± 4 | 4 |
| RpS5a | Minute | Abnormal | RpS5a1 | 1 | 104 ± 6 | 24 ± 5 | 78 ± 4 | 14 |
| mRpS14 | Normal | Normal | Df(1)e00904-d02459 | 9 | 70 ± 3 | 5 ± 1 | 93 ± 4 | 22 |
| Chromosome 2 | ||||||||
| RpLP1 | Minute | Abnormal | RpLP1beo | 1 | 84 ± 4 | 17 ± 4 | 81 ± 4 | 16 |
| RpS21 (oho23B) | Minute | Abnormal | Df(2L)JS31 | ∼70 | 91 ± 10 | 29 ± 10 | 71 ± 11 | 4 |
| RpL40 | Minute | Abnormal | Df(2L)BSC217 | 20 | 82 ± 5 | 28 ± 4 | 67 ± 4 | 11 |
| RpS13 | Minute | Abnormal | RpS131 | 1 | 109 ± 5 | 35 ± 6 | 69 ± 5 | 12 |
| RpL27A | Minute | Abnormal | RpL27A1 | 1 | 90 ± 5 | 28 ± 5 | 69 ± 4 | 11 |
| RpS26 | Minute | Abnormal | Df(2L)Exel8038 | 26 | 89 ± 4 | 22 ± 6 | 75 ± 7 | 7 |
| RpL5 | Minute | Abnormal | RpL52d2 | 1 | 98 ± 7 | 22 ± 5 | 80 ± 4 | 7 |
| mRpL10 | Normal | Normal | Df(2L)Exel7002 | 18 | 82 ± 7 | 7 ± 3 | 93 ± 3 | 14 |
| mRpL48 | Normal | Normal | Df(2L)Exel6005 | 16 | 76 ± 6 | 5 ± 2 | 95 ± 2 | 16 |
| mRpS2 | Normal | Normal | mRpS2EY10086 | 1 | 63 ± 3 | 1 ± 1 | 99 ± 1 | 14 |
| mRpL24 | Normal | Normal | Df(2L)Exel7021 | 22 | 92 ± 7 | 12 ± 5 | 87 ± 5 | 10 |
| mRpL28 | Normal | Normal | Df(2L)Exel7021 | 22 | 92 ± 7 | 12 ± 5 | 87 ± 5 | 10 |
| RpS28-like | Normal | Normal | Df(2L)ED678 | 65 | 94 ± 3 | 1 ± 1 | 99 ± 0 | 8 |
| RpS2 (sop) | Normal | Normal | Df(2L)ED695 | 40 | 76 ± 4 | 1 ± 0 | 99 ± 0 | 11 |
| mRpL52 | Normal | Normal | Df(2R)Exel7092 | 20 | 54 ± 5 | 11 ± 5 | 85 ± 6 | 12 |
| RpS15Ab | Normal | Normal | Df(2R)ED2098 | 56 | 85 ± 4 | 4 ± 1 | 96 ± 2 | 12 |
| Chromosome 3 | ||||||||
| RpS17 | Minute | Abnormal | RpS176 | 1 | 92 ± 6 | 15 ± 4 | 83 ± 4 | 11 |
| RpS29 | Minute | Abnormal | Df(3R)ED5454 | 68 | 97 ± 2 | 19 ± 5 | 81 ± 5 | 9 |
| RpS25 | Minute | Abnormal | Df(3R)ED5518 | 80 | 86 ± 4 | 23 ± 5 | 73 ± 6 | 9 |
| RpS3 | Minute | Abnormal | RpS3Plac92 | 1 | 84 ± 3 | 28 ± 4 | 64 ± 6 | 7 |
| mRpL17 | Normal | Normal | Df(3L)Exel6084 | 43 | 66 ± 6 | 18 ± 6 | 86 ± 7 | 7 |
| mRpL23 | Normal | Normal | Df(3L)ED4287 | 85 | 76 ± 9 | 9 ± 3 | 86 ± 5 | 7 |
| mRpS35 | Normal | Normal | Df(3L)Exel6091 | 13 | 69 ± 6 | 7 ± 2 | 93 ± 3 | 12 |
| mRpS6 | Normal | Normal | Df(3L)ED4342 | 48 | 83 ± 5 | 5 ± 3 | 94 ± 3 | 14 |
| mRpL12 | Normal | Normal | mRpL1210534 | 1 | 81 ± 3 | 6 ± 3 | 94 ± 3 | 11 |
| mRpL2 | Normal | Normal | Df(3L)ED4470 | 121 | 85 ± 5 | 7 ± 3 | 93 ± 3 | 11 |
| RpL10Ab | Normal | Normal | Df(3L)ED4470 | 121 | 85 ± 5 | 7 ± 3 | 93 ± 3 | 11 |
| mRpL20 | Normal | Normal | Df(3L)ED4486 | 64 | 78 ± 6 | 3 ± 3 | 97 ± 3 | 15 |
| RpS12 | Normal | Normal | Df(3L)ED4486 | 64 | 78 ± 6 | 3 ± 3 | 97 ± 3 | 15 |
| mRpL39 | Normal | Normal | Df(3L)ED217 | 98 | 84 ± 4 | 4 ± 2 | 96 ± 3 | 16 |
| mRpS34 | Normal | Normal | Df(3L)ED4606 | 148 | 113 ± 5 | 1 ± 1 | 99 ± 1 | 14 |
| mRpS26 | Normal | Normal | Df(3L)ED4710 | 81 | 79 ± 4 | 2 ± 2 | 96 ± 3 | 14 |
| mRpS9 | Normal | Normal | Df(3R)ED7665 | 131 | 75 ± 5 | 7 ± 3 | 91 ± 3 | 12 |
| mRpL1 | Normal | Normal | Df(3R)ED5230 | 103 | 73 ± 3 | 4 ± 2 | 96 ± 3 | 21 |
| mRpL19 | Normal | Normal | Df(3R)ED5330 | 71 | 75 ± 6 | 3 ± 3 | 96 ± 4 | 5 |
| mRpS21 | Normal | Normal | Df(3R)ED5612 | 104 | 71 ± 5 | 1 ± 1 | 90 ± 1 | 14 |
| mRpS33 | Normal | Abnormal | mRpS33f01766 | 1 | 97 ± 6 | 24 ± 6 | 78 ± 5 | 16 |
| mRpS11 | Normal | Normal | Df(3R)ED5780 | 100 | 87 ± 3 | 4 ± 2 | 96 ± 2 | 13 |
| mRpL55 | Normal | Normal | Df(3R)ED5938 | 73 | 69 ± 5 | 7 ± 3 | 91 ± 4 | 12 |
| Chromosome 4 | ||||||||
| RpS3A | Minute | Abnormal | RpS3A57g | 1 | 117 ± 5 | 42 ± 7 | 65 ± 5 | 10 |
Bristle phenotypes were confirmed as published (Duffy et al. 1996; Marygold et al. 2007) or visually confirmed in the case of custom deletions or obtained P-element stocks.
Cardiac chamber measurements: end-diastolic dimension (EDD), end-systolic dimension (ESD), and fractional shortening (FS: (EDD − ESD)/EDD × 100). Values are expressed as the mean ±SEM. Abnormal heart function is noted by a significant increase in ESD and a significant decrease in fractional shortening compared to w1118 control. One-way Anova with Dunnett Multiple Comparisons test.
OCT measurement of adult cardiac function
In vivo adult Drosophila cardiac function was measured in 7-day-old awake female flies as described (Wolf et al. 2006; Kim and Wolf 2009; Kim et al. 2010; Yu et al. 2010), using an OCT microscopy system (Bioptigen, Inc., Durham, NC).
The cardiac chamber in the first abdominal segment was first visualized using two-dimensional B-mode OCT, then recorded as one-dimensional line scans (M-mode images) that represent systolic and diastolic changes in cardiac chamber size as a function of time. OCT M-modes were analyzed using ImageJ software and a 125-μm standard. End-diastolic dimension (EDD) and end-systolic dimension (ESD) were calculated from three consecutive beats per M-mode trace. Fractional shortening (FS) was calculated as (EDD − ESD)/EDD × 100.
Images of bristles
Bristles were directly visualized using a Leica M165FC stereomicroscope with a DFC310x camera.
Statistical analysis
Data are expressed as mean ±SEM. Comparison of difference in heart dimensions was tested by either a student’s t-test for two samples or an analysis of variances (ANOVA) for multiple comparisons. GraphPad statistical software (GraphPad Software Inc.) was used for analyses. P < 0.05 was considered significant.
Results
Df(1)Exel6245 causes dilated cardiomyopathy
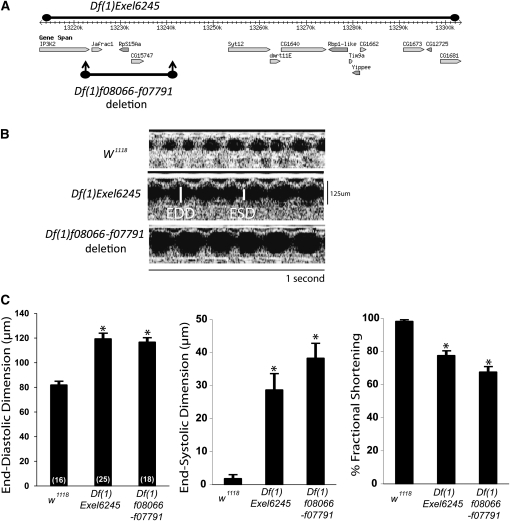
We used OCT to measure cardiac function in a screen of genomic deficiency mutants from the Exelixis and DrosDel collections (Parks et al. 2004; Thibault et al. 2004; Ryder et al. 2007). OCT allows assessment of in vivo heart function in awake adult Drosophila in a manner similar to that of echocardiography in mammals (Wolf et al. 2006; Kim and Wolf 2009; Kim et al. 2010; Yu et al. 2010). EDD measures the heart chamber in the fully relaxed state, and ESD measures the fully contracted state during each beat. Fractional shortening reflects the level of cardiac function of the heart and is calculated as the difference between EDD and ESD divided by EDD. When compared to w1118 controls, Df(1)Exel6245/FM7c adult females had increased EDD and ESD and a decrease in fractional shortening (Figure 1). These changes in cardiac parameters were consistent with an enlarged heart and reduced cardiac function.
Figure 1 .
Identification of a deletion on the X chromosome causing dilated cardiomyopathy. (A) Schematic of the Df(1)Exel6245 deletion containing 14 genes on the X chromosome. Df(1)f08066-f07791 represents the flp-mediated recombination deletion made with transposons f08066 and f07791, covering 3 genes including RpS15Aa. (B) Representative M-mode recordings of Drosophila cardiac function reflecting the change in cardiac chamber dimension over time. Control w1118 and representative dilated images of Df(1)Exel6245/FM7c and Df(1)f08066-f07791/FM7c are shown. End-diastolic and end-systolic positions are delineated with vertical, white lines. (C) Summary of cardiac function for w1118, Df(1)Exel6245/FM7c, and Df(1)f08066-f07791/FM7c. Mean ±SEM for each group, n = 16–25 per group shown in parentheses. *P < 0.01 compared to w1118, one-way ANOVA.
The deficiency corresponding to Df(1)Exel6245 spans 95 kb from cytology region 11E11 to 11F4 and encodes 14 genes on the X chromosome (Figure 1A). Due to homozygous lethality, the cardiac phenotype of Df(1)Exel6245 was observed in heterozygous females maintained with an X chromosome balancer. The abnormal cardiac phenotype persisted even after removal of the balancer chromosome by crossing into a w1118 genetic background (data not shown). To narrow the candidate interval within Df(1)Exel6245, we engineered custom deletions using transposons containing FRT sites from the Exelixis collection (Parks et al. 2004). One heterozygous 19-kb deletion, denoted as Df(1)f08066-f07791/FM7c, corresponded to a genomic region that encoded three genes (Figure 1A). Cardiac function in Df(1)f08066-f07791/FM7c Drosophila was abnormal and phenocopied the cardiac dysfunction seen in Df(1)Exel6245, indicating cardiomyopathy (Figure 1, B and C).
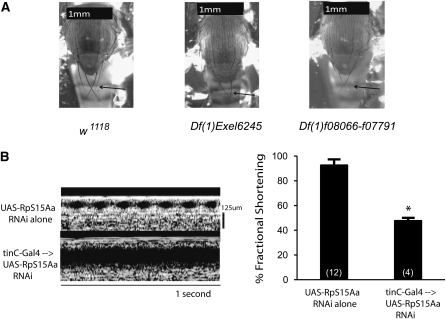
One of the genes within the narrowed region of Df(1)f08066-f07791, designated RpS15Aa, encoded a ribosomal protein subunit. Since RpS15Aa is predicted to be a causative gene for a Minute locus mapped to this region (Marygold et al. 2007), we examined these stocks for other Minute phenotypes. Both Df(1)Exel6245/FM7c and Df(1)f08066-f07791/FM7c had short, thin bristles compared to w1118 control, consistent with the expected phenotype of a haplo-insufficiency for this gene encoding a CRP (Figure 2A).
Figure 2 .
Heart-specific knockdown of RpS15Aa severely impairs cardiac function in adult Drosophila. (A) Representative images of bristles in w1118 control, Df(1)Exel6245/FM7c, and Df(1)f08066-f07791/FM7c. Both deletions have characteristically Minute bristles that are shorter and thinner than wild-type bristles. (B) Heart-specific tinC-Gal4 driving expression of RNAi against RpS15Aa causes a severe cardiac phenotype and severely reduced eclosion. Representative OCT M-mode images of the few eclosed adults (n = 4) showed severely impaired heart function compared to control of UAS-RNAi alone without driver (n = 12). *P < 0.001 vs. no driver control, unpaired t-test.
RpS15Aa disruption causes severe cardiomyopathy
Next we examined the effects of ubiquitous and cardiac-specific knockdown of RpS15Aa. Cardiac expression of UAS-RpS15Aa RNAi using a heart-specific driver tinC-Gal4 (Yin and Frasch 1998; Qian and Bodmer 2009; Yu et al. 2010) resulted in very few progeny; however, flies that escaped lethality had severely impaired heart function (Figure 2B). Ubiquitous expression of UAS-RpS15Aa RNAi by using an actin5C-Gal4 driver resulted in lethality during development. Flies with UAS-RpS15Aa RNAi in the absence of any Gal4 driver had normal cardiac function (Figure 2B). These data suggest that RpS15Aa expression in the heart is important for both heart function and viability of the fly.
Abnormal cardiac function is a common trait in Minute mutants
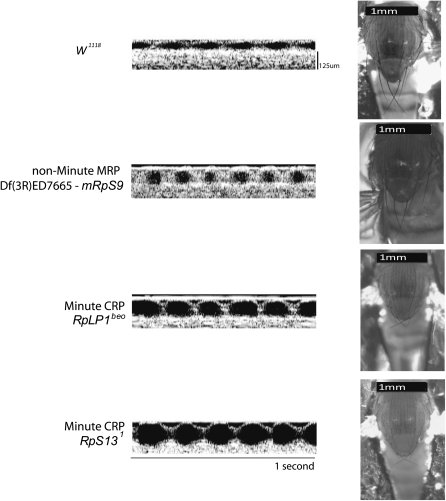
Since the Minute phenotypes are highly consistent across the numerous loci in the Drosophila genome (Lambertsson 1998), we examined other Minute mutants to determine whether cardiomyopathy is also associated with these stocks. We obtained publicly available Drosophila stocks that had genetic deficiencies spanning ribosomal protein genes and measured cardiac chamber size and function. All of the Minute mutants screened demonstrated severely impaired heart function with significantly decreased fractional shortening as compared to w1118 controls (Figure 3 and Table 1). While most Minutes correspond to mutations in CRP genes, a single Minute locus is caused by mutation of eIF-2α, a translation initiation factor gene (Marygold et al. 2007). Cardiac function of a mutant deficient for eIF-2α (Df(1)ED7364/FM7h) showed enlarged EDD and ESD with reduced fractional shortening (Table 1). Although eIF-2α is not a CRP gene, disruption of eIF-2α likely leads to impaired protein translation resulting in a Minute phenotype and cardiomyopathy.
Figure 3 .
Minute deficiencies display cardiomyopathy while non-Minute mutants do not. Representative OCT images and the corresponding bristle images of Drosophila with mutations or deficiencies in genes for ribosomal proteins. Representative heterozygous Minute mutants display small thin bristles as well as significantly enlarged heart phenotype. Heterozygous deletion of a non-Minute MRP has normal bristle and heart phenotypes. Summary data are shown in Table 1.
Deficiencies of non-Minute ribosomal genes are not generally associated with cardiomyopathy
Approximately 25% of CRP genes are not associated with a Minute phenotype (Marygold et al. 2007). Additionally, heterozygous deletions of MRP genes have not been associated with Minute phenotypes (Marygold et al. 2007). To test whether non-Minute CRP or MRP genes are associated with cardiomyopathy, we measured heart function in heterozygous deletions that span genes encoding MRPs and non-Minute CRPs. Of the 29 non-Minute deficiencies or insertions screened, 28 had normal heart function (Figure 3 and Table 1), while only mRpS33f01766 showed an abnormal heart phenotype (Figure 4). These findings suggest that, similar to the Minute syndrome, not all deficiencies of CRP genes cause a cardiac phenotype. Likewise, heterozygous deletions of MRP genes do not generally cause an abnormal heart phenotype. These data support our hypothesis that deficiencies that cause a Minute phenotype also cause cardiomyopathy, whereas deficiencies of non-Minute ribosomal protein genes do not generally have an altered heart phenotype.
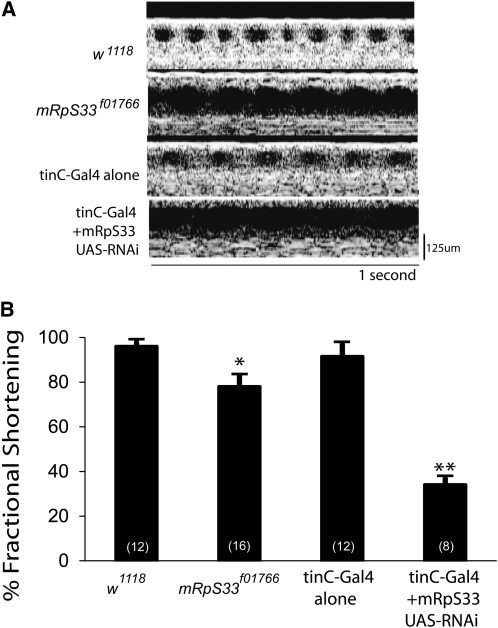
Figure 4 .
Disruption of mRpS33 causes cardiomyopathy. (A) Representative OCT images of control w1118, transposon insertion PBac{WH}mRpS33f01766, tinC-Gal4 control, and UAS-RNAi against mRpS33 driven by tinC-Gal4. (B) PBac{WH}mRpS33f01766, and heart-specific mRpS33 knockdown have significant decrease in heart function compared to controls. n = 8–16 per group shown in parentheses. *P < 0.05, **P < 0.01 vs. w1118, one-way Anova with Dunnett Multiple Comparisons test.
Interestingly, a stock heterozygous for PBac{WH}mRpS33f01766, an insertion in mRpS33, has significantly decreased heart function (Figure 4). Knockdown of mRpS33 in the heart with RNAi driven by tinC-Gal4 also results in severely abnormal heart function without causing significant lethality (Figure 4). To date this is the only MRP gene we have found to cause a heart phenotype as a heterozygous mutant, and knockdown in the heart confirms that disruption of mRpS33 causes severely impaired heart function.
Knockdown of Minute-causing genes postdevelopmentally in the heart results in cardiomyopathy
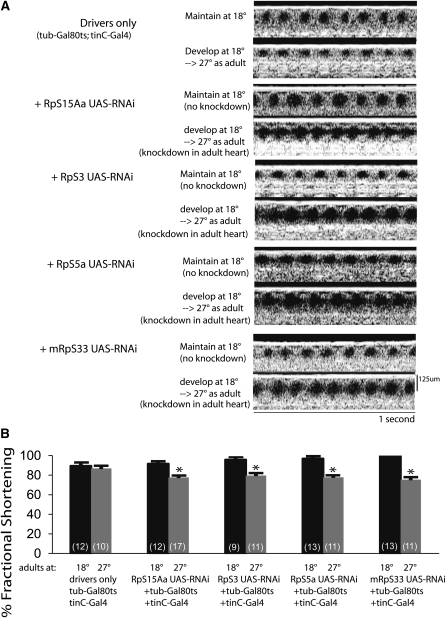
We next tested the effect of postdevelopmental knockdown of RpS15Aa and two other known Minute genes, RpS5a and RpS3, specifically in the adult fly heart. We used the tubulin-Gal80ts; tinC-Gal4 driver system to knock down these genes in a temporally and spatially restricted manner (Kim and Wolf 2009; Kim et al. 2010; Yu et al. 2010). Drosophila with tub-Gal80ts, tinC-Gal4, and UAS-RNAi against RpS15Aa, RpS5a, or RpS3 were kept at 18° throughout development, keeping knockdown off, and then either maintained at 18° or moved to 27° after eclosion to allow RNAi expression in the adult heart. The postdevelopmental expression of RNAi directed against RpS15Aa, RpS5a, and RpS3 all resulted in abnormal heart size and reduced cardiac function compared to control flies maintained at 18° (Figure 5). These data show that CRP function remains important for adult cardiac function. Additionally, adult cardiac knockdown of the MRP gene mRpS33 resulted in impaired heart function in adult Drosophila indicating its importance for maintaining normal adult heart function (Figure 5).
Figure 5 .
Cardiac-specific knockdown of ribosomal proteins in the adult results in postdevelopmental dilated cardiac phenotype. (A) Representative OCT images of Drosophila containing the following transgenes: tub-Gal80ts, tinC-Gal4 drivers control; tub-Gal80ts, tinC-Gal4, and UAS-RNAi for RpS15Aa; tinC-Gal4 and UAS-RNAi for RpS3; tub-Gal80ts, tinC-Gal4, and UAS-RNAi for RpS5a; tub-Gal80ts; tinC-Gal4 and UAS-RNAi for mRpS33. Flies developed at 18° keeping the RNAi transgene from being expressed. Adult flies were either kept at 18° (no knockdown) or moved to 27° to turn on heart-specific knockdown in the adult. (B) Summary data showing that with drivers only, heart function remains normal when adults are moved to 27°; however, heart function is significantly impaired when RNAi expression is induced in the adult heart. n = 9–17 flies per group shown in parentheses. *P < 0.05 vs. 18° control, unpaired t-test. No error bars are shown on mRpS33 at 18° since all measurements of FS were 100%.
Discussion
In this study we demonstrate that RpS15Aa is a candidate gene for cardiomyopathy and that cardiomyopathy is associated with the Minute phenotype observed in many CRP gene deficiencies. Drosophila heterozygous for CRP and MRP genes that did not cause a Minute phenotype in general did not demonstrate abnormal cardiac function. However, deficiency of mRpS33 appears to be an exception since mRpS33f01766 had abnormal heart function. We also demonstrate that postdevelopmental, cardiac-specific knockdown of ribosomal protein genes that are associated with the Minute phenotype results in an impairment of Drosophila heart function.
We narrowed the candidate region of a Minute locus on the X chromosome to three coding regions using the custom deletion Df(1)f08066-f07791 and showed that heart-specific RNAi knockdown of RpS15Aa, a gene within the deletion, caused severe cardiac dysfunction in adult Drosophila. On the basis of these observations, we show that many Minute stocks exhibit cardiomyopathy in addition to the previously described characteristics that define the Minute phenotype. The Minute stocks screened included deletions of CRP genes as well as the one non-CRP gene eIF-2α (Marygold et al. 2007). Many Minute stocks have poor viability, and deletions across these Minute loci are often not available in many deletion collections. Our results suggest that cardiac abnormalities may contribute to the poor viability of Minute flies. While we have not screened all the Minute loci (17 of 61 loci were screened), we postulate that cardiomyopathy is likely a common phenotype of the Minute syndrome.
The mechanism for decreased cardiac function in Minute stocks is not known. Previous work has suggested that a specific balance of all subunits is needed in the ribosome such that ribosome assembly and translation capacity is very sensitive to the least abundant component available (Marygold et al. 2007). Recent work indicates that mutations in genes that specifically affect ribosomal assembly result in marked decrease of functional ribosomes (Freed et al. 2010). Prior work has also investigated possible extra-ribosomal functions of some ribosomal proteins (Warner and Mcintosh 2009), as well as tissue specific ribosomal activity (Kondrashov et al. 2011; Noben-Trauth and Latoche 2011). Alternatively, it has been proposed that differences in basal expression of various ribosomal protein genes could correspond with the differential sensitivity of the genes to haplo-insufficiency (Marygold et al. 2007). Therefore, heart size and function may be sensitive to decreases in CRP-mediated translation, or subsets of CRPs could have heart-specific function as well. It is interesting that knockdown of CRPs in the adult fly can cause a postdevelopmental phenotype, indicating that decreasing ribosomal subunit expression in the adult fly can adversely affect heart function. The mechanism for the heart phenotype may be due to a decrease in the translational capacity of cardiac cells, or as previously mentioned, CRPs may have extra-ribosomal functions important for the integrity of the heart.
MRPs are essential for cell growth and proliferation, and mutant alleles of several MRP genes have been characterized in the fly, with defects in cell growth and development (Frei et al. 2005; Tselykh et al. 2005; Zhan et al. 2010). In our screen, we observed that 28 of 29 disruptions of non-Minute CRP or MRP genes retained a normal adult cardiac function, representing 5 of 22 non-Minute CRPs and 24 of 75 MRPs. While all of these genes are required to produce proteins for proper ribosome formation and protein translation, only a subset of ribosomal genes cause a Minute phenotype, including cardiomyopathy. mRpS33 is the only exception we identified in which a heterozygous deficiency of a MRP gene causes significantly dilated cardiomyopathy.
Human CRP and MRP genes have been established as candidates for causing human syndromes and diseases (Draptchinskaia et al. 1999; Ruggero and Pandolfi 2003; O’brien et al. 2005; Da Costa et al. 2010; Freed et al. 2010; Ito et al. 2010; Narla and Ebert 2010). Diamond Blackfan anemia is associated with mutations in several haplo-insufficient CRP genes (Draptchinskaia et al. 1999; Da Costa et al. 2010; Ito et al. 2010) and is characterized by congenital defects, including cardiac abnormalities (Ito et al. 2010; Doherty et al. 2010). Mutations in the gene encoding ribosomal protein RPS19 have been identified in approximately 25% of Diamond Blackfan anemia families and haplo-insufficiency of several other CRP genes have subsequently been found in affected patients (Da Costa et al. 2010). Studies in transgenic mice expressing a mutated RPS19 gene suggest that one mechanism by which mutations in RPS19 can cause Diamond Blackfan anemia is by its effect as a dominant negative protein (Devlin et al. 2010).
Other diseases that have decreased ribosomal biogenesis and function are commonly associated with increased susceptibilities to cancer (Ruggero and Pandolfi 2003; Bilanges and Stokoe 2007; Montanaro et al. 2008; Narla et al. 2011). From human studies, disease-causing mutations often appear to disrupt the biogenesis of the ribosome, and thus mutation in one gene is able to greatly influence the translational capacity of the cell. In humans, hematopoietic tissues seem to be especially vulnerable to these ribosomopathies, which could be explained by the high proliferation rate in these cells; however, the tissue specificity of these diseases of ubiquitous proteins is intriguing (Freed et al. 2010). In addition, several human MRP genes map to loci associated with disorders consistent with impaired oxidative phosphorylation (O’Brien et al. 2005). Mutations of human MRPS22 and MRP16 have been shown to cause severe disease in the homozygous state (Miller et al. 2004; Saada et al. 2007; Smits et al. 2010), showing that complete lack of a MRP is extremely detrimental. Future investigation of human ribosomal protein gene mutations, both in the heterozygous and homozygous states, may reveal insights into ribosomal biology and cardiovascular disease.
Drosophila is a valuable model system for investigation into human disease, including cardiomyopathy. Previous work has found that alterations in structural and contractile proteins can alter contractility in the Drosophila heart (Wolf et al. 2006; Allikian et al. 2007; Taghli-Lamallem et al. 2008). Additionally, signaling in such pathways as Notch, Rhomboid 3, SMAD, and insulin can change heart function in flies (Wessells et al. 2004; Kim et al. 2010; Yu et al. 2010; Goldstein et al. 2011). In addition to the CRP genes that we identify in this study, large-scale RNAi screening has identified protein complexes important for Drosophila heart function (Neely et al. 2010).
Our data support the concept that alterations in ribosomal function can cause cardiac dysfunction as shown by the marked cardiomyopathy in Drosophila with a Minute phenotype. Additional investigation is needed to address the mechanism underlying the sensitivity of cardiac tissues in Drosophila to mutation in haplo-insufficient ribosomal protein genes.
Acknowledgments
This work was supported by grants from the National Institutes of Health HL-083965 to H.A.R., HL085072 to M.J.W, American Heart Association predoctoral fellowships 0715314U and 09PRE2110019 to M.E.C., and American Heart Association postdoctoral fellowship 0825499E to I.M.K.
Literature Cited
- Alexander S., Woodling N., Yedvobnick B., 2006. Insertional inactivation of the L13a ribosomal protein gene of Drosophila melanogaster identifies a new Minute locus. Gene 368: 46–52 [DOI] [PubMed] [Google Scholar]
- Allikian M. J., Bhabha G., Dospoy P., Heydemann A., Ryder P., et al. , 2007. Reduced life span with heart and muscle dysfunction in Drosophila sarcoglycan mutants. Hum. Mol. Genet. 16: 2933–2943 [DOI] [PubMed] [Google Scholar]
- Andersson S., Saeboe-Larssen S., Lambertsson A., Merriam J., Jacobs-Lorena M., 1994. A Drosophila third chromosome Minute locus encodes a ribosomal protein. Genetics 137: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilanges B., Stokoe D., 2007. Mechanisms of translational deregulation in human tumors and therapeutic intervention strategies. Oncogene 26: 5973–5990 [DOI] [PubMed] [Google Scholar]
- Da Costa L., Moniz H., Simansour M., Tchernia G., Mohandas N., et al. , 2010. Diamond-Blackfan anemia, ribosome and erythropoiesis. Transfus. Clin. Biol. 17: 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin E. E., Dacosta L., Mohandas N., Elliott G., Bodine D. M., 2010. A transgenic mouse model demonstrates a dominant negative effect of a point mutation in the RPS19 gene associated with Diamond-Blackfan anemia. Blood 116: 2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Doherty L., Sheen M. R., Vlachos A., Choesmel V., O’Donohue M. F., et al. , 2010. Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am. J. Hum. Genet. 86: 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draptchinskaia N., Gustavsson P., Andersson B., Pettersson M., Willig T. N., et al. , 1999. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet. 21: 169–175 [DOI] [PubMed] [Google Scholar]
- Duffy J. B., Wells J., Gergen J. P., 1996. Dosage-sensitive maternal modifiers of the Drosophila segmentation gene runt. Genetics 142: 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvarque M., Laurenti P., Boivin A., Bloyer S., Griffin-Shea R., et al. , 2001. Dominant modifiers of the polyhomeotic extra-sex-combs phenotype induced by marked P element insertional mutagenesis in Drosophila. Genet. Res. 78: 137–148 [DOI] [PubMed] [Google Scholar]
- Freed E. F., Bleichert F., Dutca L. M., Baserga S. J., 2010. When ribosomes go bad: diseases of ribosome biogenesis. Mol. Biosyst. 6: 481–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei C., Galloni M., Hafen E., Edgar B. A., 2005. The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth. EMBO J. 24: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. A., Kelly S. M., LoPresti P. P., Heydemann A., Earley J. U., et al. , 2011. SMAD signaling drives heart and muscle dysfunction in a Drosophila model of muscular dystrophy. Hum. Mol. Genet. 20: 894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart K., Klein T., Wilcox M., 1993. A Minute encoding a ribosomal protein enhances wing morphogenesis mutants. Mech. Dev. 43: 101–110 [DOI] [PubMed] [Google Scholar]
- Ito E., Konno Y., Toki T., Terui K., 2010. Molecular pathogenesis in Diamond-Blackfan anemia. Int. J. Hematol. 92: 413–418 [DOI] [PubMed] [Google Scholar]
- Kim I. M., Wolf M. J., 2009. Serial examination of an inducible and reversible dilated cardiomyopathy in individual adult Drosophila. PLoS One 4: e7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. M., Wolf M. J., Rockman H. A., 2010. Gene deletion screen for cardiomyopathy in adult Drosophila identifies a new notch ligand. Circ. Res. 106: 1233–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov N., Pusic A., Stumpf C. R., Shimizu K., Hsieh A. C., et al. , 2011. Ribosome-mediated specificity in hox mRNA translation and vertebrate tissue patterning. Cell 145: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsson A., 1998. The minute genes in Drosophila and their molecular functions. Adv. Genet. 38: 69–134 [DOI] [PubMed] [Google Scholar]
- Marygold S., Coelho C., Leevers S., 2005. Genetic analysis of RpL38 and RpL5, two Minute genes located in the centric heterochromatin of chromosome 2 of Drosophila melanogaster. Genetics 169: 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold S. J., Roote J., Reuter G., Lambertsson A., Ashburner M., et al. , 2007. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8: R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim K., Dahmus J., Hawley R., 1996. Cloning of the Drosophila melanogaster meiotic recombination gene mei-218: a genetic and molecular analysis of interval 15E. Genetics 144: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Saada A., Shaul N., Shabtai N., Ben-Shalom E., et al. , 2004. Defective mitochondrial translation caused by a ribosomal protein (MRPS16) mutation. Ann. Neurol. 56: 734–738 [DOI] [PubMed] [Google Scholar]
- Montanaro L., Trere D., Derenzini M., 2008. Nucleolus, ribosomes, and cancer. Am. J. Pathol. 173: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla A., Ebert B. L., 2010. Ribosomopathies: human disorders of ribosome dysfunction. Blood 115: 3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla A., Hurst S. N., Ebert B. L., 2011. Ribosome defects in disorders of erythropoiesis. Int. J. Hematol. 93: 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely G. G., Kuba K., Cammarato A., Isobe K., Amann S., et al. , 2010. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell 141: 142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K., Latoche J. R., 2011. Ectopic mineralization in the middle ear and chronic otitis media with effusion caused by RPL38 deficiency in the Tail-short (Ts) mouse. J. Biol. Chem. 286: 3079–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien T. W., O’Brien B. J., Norman R. A., 2005. Nuclear MRP genes and mitochondrial disease. Gene 354: 147–151 [DOI] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., et al. , 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292 [DOI] [PubMed] [Google Scholar]
- Qian L., Bodmer R., 2009. Partial loss of GATA factor Pannier impairs adult heart function in Drosophila. Hum. Mol. Genet. 18: 3153–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D., Pandolfi P. P., 2003. Does the ribosome translate cancer? Nat. Rev. Cancer 3: 179–192 [DOI] [PubMed] [Google Scholar]
- Ryder E., Ashburner M., Bautista-Llacer R., Drummond J., Webster J., et al. , 2007. The DrosDel Deletion Collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177: 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada A., Shaag A., Arnon S., Dolfin T., Miller C., et al. , 2007. Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J. Med. Genet. 44: 784–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeboe-Larssen S., Lambertsson A., 1996. A novel Drosophila Minute locus encodes ribosomal protein S13. Genetics 143: 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeboe-Larssen S., Urbanczyk Mohebi B., Lambertsson A., 1997. The Drosophila ribosomal protein L14-encoding gene, identified by a novel Minute mutation in a dense cluster of previously undescribed genes in cytogenetic region 66D. Mol. Gen. Genet. 255: 141–151 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Hollmann M., Schafer U., 1996. A newly identified Minute locus, M(2)32D, encodes the ribosomal protein L9 in Drosophila melanogaster. Mol. Gen. Genet. 251: 381–387 [DOI] [PubMed] [Google Scholar]
- Smits P., Saada A., Wortmann S. B., Heister A. J., Brink M., et al. , 2010. Mutation in mitochondrial ribosomal protein MRPS22 leads to Cornelia de Lange-like phenotype, brain abnormalities and hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 19: 394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghli-Lamallem O., Akasaka T., Hogg G., Nudel U., Yaffe D., et al. , 2008. Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell 7: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., et al. , 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36: 283–287 [DOI] [PubMed] [Google Scholar]
- Torok I., Herrmann-Horle D., Kiss I., Tick G., Speer G., et al. , 1999. Down-regulation of RpS21, a putative translation initiation factor interacting with P40, produces viable Minute imagos and larval lethality with overgrown hematopoietic organs and imaginal discs. Mol. Cell. Biol. 19: 2308–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselykh T. V., Roos C., Heino T. I., 2005. The mitochondrial ribosome-specific MrpL55 protein is essential in Drosophila and dynamically required during development. Exp. Cell Res. 307: 354–366 [DOI] [PubMed] [Google Scholar]
- Tyler D., Li W., Zhuo N., Pellock B., Baker N., 2007. Genes affecting cell competition in Drosophila. Genetics 175: 643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest M., Mortin M., Clevers H., 1998. Drosophila RpS3a, a novel Minute gene situated between the segment polarity genes cubitus interruptus and dTCF. Nucleic Acids Res. 26: 4471–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., McIntosh K. B., 2009. How common are extraribosomal functions of ribosomal proteins? Mol. Cell 34: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells R. J., Fitzgerald E., Cypser J. R., Tatar M., Bodmer R., 2004. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 36: 1275–1281 [DOI] [PubMed] [Google Scholar]
- Wolf M. J., Amrein H., Izatt J. A., Choma M. A., Reedy M. C., et al. , 2006. Drosophila as a model for the identification of genes causing adult human heart disease. Proc. Natl. Acad. Sci. USA 103: 1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Frasch M., 1998. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev. Genet. 22: 187–200 [DOI] [PubMed] [Google Scholar]
- Yu L., Lee T., Lin N., Wolf M. J., 2010. Affecting Rhomboid-3 function causes a dilated heart in adult Drosophila. PLoS Genet. 6: e1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Melian N. Y., Pantoja M., Haines N., Ruohola-Baker H., et al. , 2010. Dystroglycan and mitochondrial ribosomal protein l34 regulate differentiation in the Drosophila eye. PLoS One 5: e10488. [DOI] [PMC free article] [PubMed] [Google Scholar]