Abstract
Gradients of acetylcholine can stimulate growth cone turning when applied to neurons grown in culture, and it has been suggested that acetylcholine could act as a guidance cue. However, the role acetylcholine plays in directing axon migrations in vivo is not clear. Here, we show that acetylcholine positively regulates signaling pathways that mediate axon responses to guidance cues in Caenorhabditis elegans. Mutations that disrupt acetylcholine synthesis, transportation, and secretion affect circumferential axon guidance of the AVM neuron and in these mutants exogenously supplied acetylcholine improves AVM circumferential axon guidance. These effects are not observed for the circumferential guidance of the DD and VD motor neuron axons, which are neighbors of the AVM axon. Circumferential guidance is directed by the UNC-6 (netrin) and SLT-1 (slit) extracellular cues, and exogenously supplied acetylcholine can improve AVM axon guidance in mutants when either UNC-6– or SLT-1–induced signaling is disrupted, but not when both signaling pathways are perturbed. Not in any of the mutants does exogenously supplied acetylcholine improve DD and VD axon guidance. The ability of acetylcholine to enhance AVM axon guidance only in the presence of either UNC-6 or SLT-1 indicates that acetylcholine potentiates UNC-6 and SLT-1 guidance activity, rather than acting itself as a guidance cue. Together, our results show that for specific neurons acetylcholine plays an important role in vivo as a modulator of axon responses to guidance cues.
CELLS secrete molecules that help guide axon growth cone migrations. In Caenorhabditis elegans, UNC-6 (netrin) and SLT-1 (slit) are secreted guidance cues and are ligands for the UNC-40 (DCC) and SAX-3 (Robo) receptors, which are present at the surface of the migrating axons (Hedgecock et al. 1990; Serafini et al. 1994; Chan et al. 1996; Keino-Masu et al. 1996; Wadsworth et al. 1996; Zallen et al. 1998; Brose et al. 1999; Hao et al. 2001). During nervous system development, different cells can spatially and temporally express a guidance cue to create dynamic patterns (Wadsworth et al. 1996). This expression can provide pathway and long-range signals, allowing for complex axon trajectories during the formation of neural circuits (Wadsworth and Hedgecock 1996). Additional extracellular guidance cues and other factors can further modify growth cone responses and alter trajectories. How multiple extracellular molecules together direct growth cone migrations is still not well understood.
Acetylcholine is best known as a molecule secreted at synapses, where it acts as a neurotransmitter. However, there is evidence to suggest that during early development acetylcholine has other roles, including the role of an axon guidance cue (Ruediger and Bolz 2007). Studies using chick, Xenopus, and Drospophila embryonic neurons indicate that acetylcholine is also secreted before synapses form (Hume et al. 1983; Young and Poo 1983; Yao et al. 2000). During this time, acetylcholine might influence different aspects of nervous system development, including the process of axon guidance. Moreover, under cell culture conditions, defined extracellular gradients of acetylcholine elicit turning responses from neuronal growth cones (Zheng et al. 1994; Kuffler 1996). A developmental role for acetylcholine in axon pathfinding in vivo was revealed when it was shown that Drosophila photoreceptor axons do not properly project to their targets when acetylcholine synthesis or metabolism is altered or eliminated (Yang and Kunes 2004).
In this article, we present evidence that extracellular acetylcholine is required for a migrating axon to properly respond to guidance cues in vivo. In C. elegans, the ventral axon migration of the AVM neuron is directed by the UNC-6 and SLT-1 guidance cues through signaling mediated by the UNC-40 and SAX-3 receptors (Hedgecock et al. 1990; Wadsworth et al. 1996; Hao et al. 2001; Yu et al. 2002; Gitai et al. 2003). At the beginning of the L2 larval stage, the AVM axon migrates ventrally. When the growth cone reaches the ventral nerve cord it turns anteriorly, eventually reaching the nerve ring in the head where it makes the majority of its synapses (Figure 1). We show that extracellular acetylcholine potentiates the response of the AVM axon to the UNC-6 and SLT-1 guidance cues, indicating that acetylcholine can play an important role in patterning neural connections during nervous system development.
Figure 1—
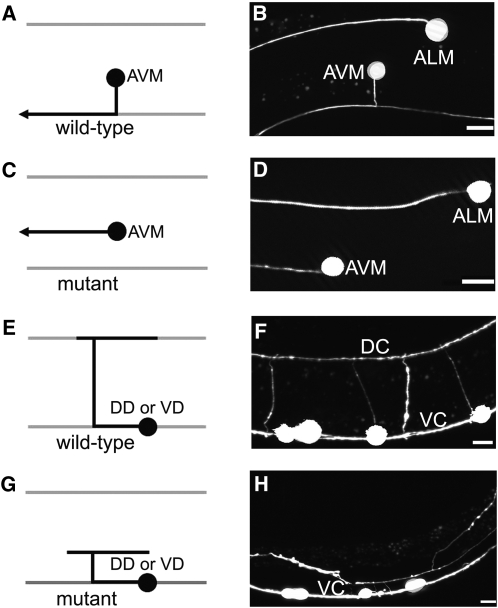
Mutations cause axon migration phenotypes. Left column, schematic diagram of the AVM neuron (A and C) and the DD and VD neurons (E and G); right column, fluorescence photomicrographs of larvae bearing a mec-4::gfp transgene that expressed GFP in the AVM neuron (B and D) or a unc-47::gfp transgene that expressed GFP in the DD and VD motor neurons (F and H). In each panel, left is anterior and up is dorsal. Bars, 10 µm. (A and B) In wild-type larvae the AVM neuron is located on the lateral right side of the C. elegans body wall. During the L2 stage, the AVM axon is guided ventrally to the ventral nerve cord where it turns and migrates anteriorly to the nerve ring. (C and D) In mutant larvae that have axon migration defects caused by loss of UNC-6 or SLT-1 guidance signaling, the AVM axon often fails to migrate ventrally and instead travels anteriorly. (E and F) In wild-type larvae the DD and VD motor neurons send processes along the ventral nerve cord and circumferentially along the body wall to the dorsal midline. DC, dorsal nerve cord; VC, ventral nerve cord. (G and H) In mutant larvae that have axon migration defects caused by loss of UNC-6 guidance signaling the DD and VD axons wander across the body wall and rarely reach the dorsal midline.
Materials and Methods
Strains
Animals were cultivated and maintained according to standard techniques at 20° or 25° (Brenner 1974). Bristol strain N2 was used as wild-type genetic background. All genetic lesions used for this study are strong loss-of-function or null alleles unless otherwise indicated. Strains used in study as follows: IM1147 unc-40(e1430)I; oxIs12X, IM936 unc-104(e1265)II; zdIs5I, IM941 unc-40(e1430)I, zdIs5I; unc-104(e1265)II, IM939 rpm-1(ur299)V; unc-104(e1265)II; zdIs5I, IM945 unc-104(e1265)II; clec-38(ur280)V; zdIs5I, IM940 unc-104(e1265)II; sax-3(ky123)X; zdIs5I, IM942 unc-104(e1265)II; unc-6(ev400)X; zdIs5I, IM938 unc-104(e1265)IV; slt-1(eh15)X; zdIs5I, IM1148 unc-104(e1265)II; oxIs12X, IM946 rpm-1(ur299)V; unc-104(e1265)II; sax-3(ky123)X; zdIs5I, IM948 unc-104(e1265)II; clec-38(ur280)V; unc-40(e1430)I; zdIs5I, IM1053 unc-17(e245)IV; zdIs5I, IM944 unc-40(e1430)I; unc-17(e245)IV; zdIs5I, IM945 unc-17(e245)IV; sax-3(ky123)X; zdIs5I, IM1054 unc-17(e245); unc-6(ev400)X; zdIs5I, IM1043 unc-17(e245)IV; slt-1(eh15)X; zdIs5I, IM1149 unc-17(e245)IV; oxIs12 X, IM937 cha-1(p1152)IV; zdIs5I, IM943 cha-1(p1152)IV; slt-1(eh15)X; zdIs5I, IM1150 cha-1(p1152)IV; oxIs12 X, IM1151 unc-5(e53)IV; oxIs12 X, IM947 deg-3(u701)V; slt-1(eh15)X; zdIs5I, IM648 unc-40(e1430)I; zdIs5I, IM712 sax-3(ky123)X; zdIs5I, IM650 unc-6(ev400)X; zdIs5I, IM1152 unc-6(ev400), oxIs12 X, IM647 slt-1(eh15)X; zdIs5I, IM950 deg-3(u701)V; zdIs5I, IM649 unc-6(ev400), slt-1(eh15)X; zdIs5I, IM1112 urEx386 [mec-4::unc-104, odr-1::dsred], IM1113 unc-104(e1265); zdIs5I; urEx386 [mec-4::unc-104, odr-1::dsred], IM1110 urEx385 [unc-3::unc-104, odr-1::dsred], IM1111 unc-104(e1265); zdIs5I; urEx385 [unc-3::unc-104, odr-1::dsred], IM1098 urEx380 [mec-4::unc-17, odr-1::dsred], IM1105 unc-17(e245)I; slt-1(eh15)X; zdIs5I; urEx380 [mec-4::unc-17, odr-1::dsred], IM1097 urEx379 [unc-3::unc-17, odr-1::dsred], IM1106 unc-17(e245)I; slt-1(eh15)X; zdIs5I; urEx379 [unc-3::unc-17, odr-1::dsred], and AGC11 deg-3(u701)V; slt-1(eh15)X; zdIs5; cueEx6 [mec-4::deg-3, odr-1::dsred].
DNA constructs
pIM 218 encodes mec-4::unc-104 and was made by PCR amplifying unc-104 cDNA sequence from a C. elegans cDNA library (Invitrogen, Carlsbad, CA) using primers: forward, GATCGCATCCTAGGATGTCATCGGTTAAAGTAGCTGT and reverse, GATCGCATGGTACCTTATGAAGCAATTGAAGATGATGTT. The PCR product was digested with AvrII and KpnI restriction enzymes and was cloned into the NheI and KpnI sites behind the mec-4 promoter sequence of plasmid pIM 207 (Quinn et al. 2006). pIM 219 is a plasmid with an unc-3 promoter. The unc-3 promoter is amplified from plasmid pBP6-1 using primers: forward, CCTGCAGGAAGCTTGATCAAACCGTGA and reverse, CTGTCAACCCCGGGCCACAGTTTT. The PCR product was digested with HindIII and SmaI and was cloned into pPD52-102 to replace the mec-7 promoter. pIM 220 encodes unc-3::unc-104. It was made by cloning the AvrII and KpnI digested unc-104 cDNA into the NheI and KpnI sites behind the unc-3 promoter sequence of pIM 219. pIM 221 encodes mec-4::unc-17 and pIM222 encodes unc-3::unc-17. pAGC1 encodes mec-4::deg-3 and was made by PCR amplifying deg-3 cDNA sequence from a C. elegans cDNA library.
Transgenes
Transgenic strains were created by injecting DNA into N2 hermaphrodites using described methods (Mello and Fire 1995). A total of 5 ng/µl of pIM220 was injected into N2 animals along with 50 ng/µl of odr-1::dsred co-injection marker. The transgenic lines were maintained as extrachromosomal arrays by following the red fluorescent protein (RFP). Three independent lines were established. The array of one line, IM1110, was crossed into unc-104 (e1265); zdIs5I to generate strain IM1111. The strain IM1112 and IM1113 were similarly made. The strain IM1097 was generated by injecting 5 ng/μl of pIM222 along with 50 ng/μl of odr-1::dsred into the N2 strain. The IM1097 was then crossed into unc-17(e245)IV; slt-1(eh15)X; zdIsI animals to generate strain IM1106. The strains IM1098 and IM1105 were made similarly. The cueEx6 transgene was generated by injecting 5 ng/μl of pAGC1 along with 50 ng/μl of odr-1::dsred into the N2 strain. The cueEx6 transgene was then crossed into deg-3(u701)V; slt-1(eh15)X; zdIs5 animals to generate strain AGC11.
Fluorescence microscopy
Animals were mounted on 5% agarose pads in M9 buffer containing 10 mm levamisole and observed with ×40 as well as ×63 objectives on a Carl Zeiss Axio-Imager Z1 microscope. Touch receptor neuron AVM was visualized using a mec-4::gfp marker in wild-type or mutant backgrounds. The axon guidance defect was scored as failure of the axon to reach the ventral nerve cord. The DD and VD motor neurons were visualized using an unc-47::gfp marker in wild-type or mutant backgrounds. The axon guidance defect was scored as failure of the axons to reach the dorsal nerve cord.
Exogenous acetylcholine treatment
Embryos were placed on the plates containing 1 mg/ml acetylcholine. After the animals hatched and grew to the L3 stage, the axon guidance defects of the AVM or the DD/VD neurons were scored under fluorescence microscopy.
Results
Exogenously supplied acetylcholine can modulate UNC-6– and SLT-1–mediated AVM axon guidance
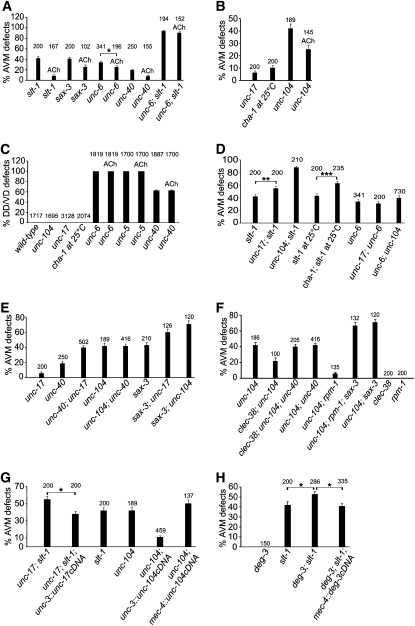
Acetylcholine can influence growth cone turning in cell cultures, so we wondered whether acetylcholine could influence AVM axon guidance if it was simply added to the growth medium. Although the exogenous acetylcholine is noxious under these experimental conditions, it does not cause AVM axon guidance defects. We find that exogenous acetylcholine rescues AVM ventral axon guidance defects in unc-6(ev400), slt-1(eh15), unc-40(e1430) and sax-3(ky123) mutants. However, it could not rescue the AVM axon guidance defects of the double unc-6(ev400), slt-1(eh15) mutants, in which both of the ventral axon guidance cues are disrupted (Figure 2A). These results indicate that acetylcholine can modulate the responsiveness of AVM to the UNC-6 and SLT-1 guidance cues.
Figure 2—
Acetylcholine enhances UNC-6 and SLT-1 guidance signaling for AVM, but not for DD and VD neurons. Quantification of axon guidance defects is shown in each panel. Number above bar indicates number of animals scored (A, B, D–H) or total number of axons scored (C). Error bars represent standard error of proportions (A–H). (A) Exogenously supplied acetylcholine rescues AVM axon guidance defects in unc-6(ev400), slt-1(eh15), unc-40(e1430), or sax-3(ky123) mutant background but not in unc-6(ev400), slt-1(eh15) mutants. Asterisks indicate statistically significant difference (*P < 0.05, z-test for two proportions). (B) Strong loss-of-function mutation in unc-17, unc-104, or cha-1 causes AVM ventral axon guidance defect, while exogenously supplied acetylcholine rescues the AVM axonal guidance defect caused by unc-104(e1265). (C) Strong loss-of-function mutation in unc-17, unc-104, or cha-1 does not cause DD or VD axon guidance defect. Exogenously supplied acetylcholine has no effect on DD/VD axon guidance in the unc-6(ev400), unc-40(e1430), and unc-5(e53) mutant backgrounds. (D) Strong loss of function of unc-17, unc-104, or cha-1 enhances AVM axon guidance defects caused by slt-1(eh15). Asterisks indicate statistically significant difference (**P < 0.005, ***P < 0.0000, z-test for two proportions). (E) Strong loss of function in unc-17 and unc-104 enhance AVM axon guidance defects caused by unc-40(e1430) as well as sax-3(ky123), respectively. (F) Either rpm-1(ur299) or clec-38(ur280) suppresses the AVM axon guidance defects in the unc-104(e1265) mutants. However, the axon guidance defects are not suppressed in either rpm-1(ur299); unc-104(e1265); sax-3(ky123) or clec-38(ur280); unc-104(e1265); unc-40(e1430) mutants. (G) unc-17 cDNA expression, which is driven by unc-3 promoter but not by mec-4 promoter, rescues the axon guidance defects in unc-17(e245); slt-1(eh15) mutants. Similarly, unc-104 cDNA expression, which is driven by unc-3 promoter but not by mec-4 promoter, rescues the axon guidance defects in unc-104(e1265) mutants. Asterisks indicate statistically significant difference (**P < 0.005, z-test for two proportions). (H) The deg-3(u701) mutation enhances the AVM ventral axon guidance defect caused by slt-1(eh15). When deg-3 cDNA is expressed in AVM by using the mec-4 promoter to drive expression in touch receptor neurons, the enhancement is suppressed. Asterisks indicate statistically significant difference (*P < 0.05, z-test for two proportions).
Mutations that disrupt acetylcholine synthesis, transportation, and secretion affect AVM ventral guidance
To study the acetylcholine effect, we used mutants that should have a deficiency of extracellular acetylcholine. We found that mutations of cha-1, unc-17, and unc-104 that cause a reduction of function have AVM ventral axon guidance defects (Figure 2B). CHA-1 is a choline acetyltransferase and is expressed in ventral nerve cord cholinergic neurons (Alfonso et al. 1994). UNC-17 is a vesicular acetylcholine transporter that loads acetylcholine into synaptic vesicles (Alfonso et al. 1993) and UNC-104 is a kinesin that transports vesicles (Otsuka et al. 1991). In unc-104 mutants, vesicles accumulate in neuronal cell bodies and consequently VAChT and ChAT immunoreactivity are abnormally concentrated in the cell bodies; thus unc-104 mutations likely also disrupt CHA-1 and UNC-17 activities (Hall and Hedgecock 1991; Duerr et al. 2008). We note that the alleles used are not null and that the null alleles of these genes are lethal. Therefore it is difficult to make inferences about the relative contribution of each gene for guidance on the basis of the severity of the guidance defect caused by these partial loss-of-function alleles.
To experimentally test whether the mutations are affecting the ability of acetylcholine to influence AVM axon guidance, we grew unc-104(e1265) mutants on the plates containing acetylcholine. We find when exogenous acetylcholine is supplied, the AVM ventral axon guidance defect is suppressed in the mutant (Figure 2B). This further supports that the mutations disrupt AVM axon guidance by limiting the availability of extracellular acetylcholine.
The acetylcholine effect is specific to certain neurons
It is possible that exogenous acetylcholine improves the AVM guidance in the unc-104(e1265) mutants by stimulating UNC-6 processing and increasing the availability of extracellular UNC-6. In fact, a recent study provides evidence that UNC-104 might be required for UNC-6 secretion (Asakura et al. 2010). In this study, a Venus-tagged UNC-6 showed a punctate distribution pattern throughout the cytoplasm and axons of wild-type neurons, but in unc-104(e1265) mutants the UNC-6 was observed evenly distributed in the cell body with little detected in the axon. If this mislocalization prohibited UNC-6 secretion, other axons that require UNC-6 for guidance should likewise be affected by the unc-104(e1265) mutation. We therefore examined the guidance of the neighboring DD and VD motor neuron axons, which also require UNC-6–mediated guidance. We find that unlike AVM axon guidance, the DD and VD axons migrate normally in the unc-17(e245), unc-104(e1265), and cha-1(p1152) mutants (Figure 2C). Because the DD and VD axons are guided normally, we conclude that the AVM guidance defects observed in unc-17, unc-104, and cha-1 mutants are not explained by a deficiency in the synthesis, transportation, or secretion of UNC-6. We also note that neither the addition of exogenous acetylcholine, which presumably causes higher levels of acetylcholine, nor the mutations, which presumably cause lower levels of acetylcholine, affect the DD and VD axon migrations (Figure 2C). Together, these observations suggest that acetylcholine does not indirectly affect the AVM axon migration, as an indirect mechanism would likely affect all nearby axon migrations.
Acetylcholine modulates the AVM signaling response to UNC-6 and SLT-1
We found that exogenous acetylcholine can rescue AVM ventral axon guidance defects in unc-6(ev400), slt-1(eh15), unc-40(e1430) and sax-3(ky123) mutants, but could not rescue the AVM axon guidance defects of the double unc-6(ev400), slt-1(eh15) mutants (Figure 2A). Since these results suggest that acetylcholine can modulate the responsiveness of AVM to UNC-6 and SLT-1, we examined the requirements of acetylcholine for the guidance signaling pathways by using the unc-17(e245), unc-104(e1265), and cha-1(p1152) mutations. The AVM axon migrates toward the UNC-6 sources, which are ventral nerve cord neurons, and it migrates away from the SLT-1 sources, which are the dorsal muscles (Wadsworth et al. 1996; Hao et al. 2001). In the slt-1(eh15), unc-6(ev400) mutants that lose both SLT-1 and UNC-6, 94% of the AVM axons fail to migrate ventrally. Either slt-1(eh15) or unc-6(ev400) causes ∼40% of AVM ventral guidance defects (Figure 2A). We found that unc-17(e245), unc-104(e1265), or cha-1(p1152) enhances the AVM ventral axon guidance defect caused by slt-1(eh15), which suggests that acetylcholine potentiates UNC-6–mediated axon guidance (Figure 2D). Our results are similar to those previously reported for unc-104(e1265) (Asakura et al. 2010).
We found that unc-17(e245) or unc-104(e1265) does not enhance the AVM ventral axon guidance defect caused by unc-6(ev400) (Figure 2B). However, these results do not indicate that acetylcholine has no effect on SLT-1 signaling in AVM. Because the alleles used in these experiments are not null, they might not be strong enough to enhance the axon guidance defects in unc-6(ev400) mutants. Furthermore, our results indicate that exogenously supplied acetylcholine improves guidance in response to the SLT-1 guidance cue, albeit not as strongly as for the response to the UNC-6 guidance cue (Figure 2A). Consistent with the idea that acetylcholine affects both UNC-6 and SLT-1 signaling, there is evidence that the UNC-6 and SLT-1 signaling pathways in AVM act synergistically, rather than independently (Quinn et al. 2006). Thus, acetylcholine might affect both UNC-6 and SLT-1 signaling and it has stronger effect on UNC-6 signaling.
Since UNC-6 and SLT-1 guidance is mediated by the UNC-40 and SAX-3 receptors (Hedgecock et al. 1990; Wadsworth et al. 1996; Hao et al. 2001; Yu et al. 2002; Gitai et al. 2003), we also examined unc-17(e245) and unc-104(e1265) mutants with unc-40(e1430) or sax-3(ky123) loss-of-function mutations. We observe that the unc-40(e1430) and sax-3(ky123) loss-of-function AVM axon guidance phenotypes are enhanced by unc-17(e245) and unc-104(e1265), respectively (Figure 2E). This supports the hypothesis that acetylcholine affects both UNC-6 and SLT-1 signaling mediated by the receptors.
If disrupting acetylcholine secretion inhibits UNC-40– and SAX-3–mediated signaling, then increasing the activity of these receptors might suppress the AVM axon guidance defects observed in the unc-104(e1265) mutants. To test this idea, we used strong loss-of-function mutations in rpm-1 and clec-38. RPM-1 is an E3-ubiquitin ligase that influences axon outgrowth by negatively regulating SAX-3 and UNC-5 (Li et al. 2008). CLEC-38 is a transmembrane protein with C-type lectin-like domains that regulates axon outgrowth by negatively regulating UNC-40 activity (Kulkarni et al. 2008). We find that rpm-1 or clec-38 loss-of-function mutations can suppress AVM ventral axon guidance defects caused by unc-104(e1265) (Figure 2F). Furthermore, there is no suppression in either unc-104(e1265); rpm-1(ur299); sax-3(ky123) or in unc-104(e1265); clec-38(ur280); unc-40(e1430) mutants. These results are consistent with the idea that acetylcholine modulates the UNC-40– and SAX-3–mediated signaling responses to UNC-6 and SLT-1 in AVM.
Acetylcholine does not modulate the DD and VD signaling response to UNC-6
Our results indicate that acetylcholine can modulate UNC-40–mediated signaling to improve the ability of UNC-6 to guide the AVM axon ventrally. We also presented evidence that loss of extracellular acetylcholine affects AVM axon guidance but not the guidance of the neighboring DD or VD axons. On the basis of these results, we hypothesized that in unc-40(e1430) and unc-5(e53) mutants, exogenous acetylcholine would not suppress the guidance defects of the DD or VD axons, which require UNC-6 and the UNC-6 receptors, UNC-40 and UNC-5. We found that this is the case (Figure 2C), further indicating that the DD and VD neurons respond differently than AVM to acetylcholine. The different responses also suggest that the enhancement of AVM axon guidance by exogenous acetylcholine is not the result of improving the extracellular environment for the migrating AVM axon, since this would likely also improve the ability of the DD and VD axons to reach their targets.
Acetylcholine from cholinergic neurons modulates AVM ventral axon migration
To determine a source of the acetylcholine that influences AVM ventral axon guidance, we expressed unc-17 cDNA and unc-104 cDNA using the unc-3 promoter to drive expression in ventral midline cholinergic motor neurons (Prasad et al. 1998). We also expressed unc-17 cDNA and unc-104 cDNA in touch receptor neurons, including AVM, by using the mec-4 promoter (Lai et al. 1996). We found that expression of unc-17 cDNA in cholinergic motor neurons could rescue AVM ventral axon guidance defects caused by the unc-17(e245) mutation in the slt-1(eh15) background (Figure 2G). We did not observe rescue in the mec-4 promoter experiments. We also found that expression of unc-104 cDNA in the ventral midline cholinergic motor neurons could rescue the AVM ventral axon guidance defects caused by the unc-104(e1265) mutation (Figure 2G). Again, we did not observe rescue using the mec-4 promoter. These results indicate that release of acetylcholine from cholinergic neurons is sufficient to influence AVM axon guidance. We further note that since these motor neurons send processes to the dorsal midline, the source of acetylcholine may not be well localized in the animals, and, therefore, similar to the interpretation of the exogenous acetylcholine experiments, we conclude the acetylcholine effect does not require gradients to form.
Acetylcholine nicotinic receptor functions cell autonomously to influence the AVM axon guidance response to UNC-6
The turning responses of growth cones in culture are dependent on the activation of neuronal nicotinic acetylcholine receptors (Zheng et al. 1994). We therefore examined whether the effects of acetylcholine on AVM axon guidance might involve such receptors in C. elegans. The nicotinic acetylcholine receptor DEG-3/DES-2 is a heteromeric receptor formed by DEG-3 and DES-2; it is expressed in the touch receptor neurons and is localized in the cell body and neuronal processes but not at the synapse (Treinin and Chalfie 1995; Treinin et al. 1998; Yassin et al. 2001). We found that the null mutation deg-3(u701) enhances the AVM ventral axon guidance defect caused by slt-1(eh15), which suggests that the response to the UNC-6 guidance cue is inhibited by disrupting the acetylcholine receptor. Furthermore, expression of DEG-3 in AVM by using the mec-4 promoter to drive expression in touch receptor neurons rescues the axon guidance defects caused by deg-3(u701) in the slt-1(eh15) background (Figure 2H). Together these observations indicate that the activity of DEG-3/DES-2 receptors expressed by AVM can regulate guidance responses to UNC-6.
Discussion
Although it acts as a neurotransmitter, acetylcholine may also have other conserved roles that help regulate the development of nervous systems. One of these may be the ability to influence axon guidance. We found that mutations that should reduce extracellular acetylcholine levels cause AVM axon guidance defects and that these defects can be rescued by exogenous acetylcholine. The AVM axon is guided by the UNC-6 and SLT-1 cues and when either UNC-6– or SLT-1–mediated signaling is disrupted, exogenous acetylcholine can improve AVM axon guidance. However, if both UNC-6 and SLT-1 signaling pathways are deficient, exogenous acetylcholine has no effect. Together these results suggest that acetylcholine has the ability to potentiate AVM axon guidance through UNC-6– and SLT-1–induced signaling.
We considered several models that could explain our observations. We favor a model that predicts acetylcholine directly affects guidance signaling pathways within AVM (Figure 3). When strong loss-of-function mutations in unc-17, unc-104, or cha-1 cause lower extracellular levels of acetylcholine, AVM axon guidance is defective because the responsiveness of AVM to the UNC-6 and SLT-1 guidance cues is reduced. We also considered a model where acetylcholine affects the secretion of the guidance cues. In this case, lower extracellular levels of acetylcholine caused by the unc-17, unc-104, or cha-1 mutations result in lower levels of extracellular UNC-6 and SLT-1, which in turn causes AVM axon guidance defects because of a loss of guidance information. Exogenous acetylcholine might be able to rescue the guidance defects in the unc-6 or slt-1 loss-of-function mutants because it somehow stimulates the secretion of the guidance cue. We also considered models whereby the many developmental and morphological defects caused by unc-17, unc-104, or cha-1 mutations physically alter the extracellular distribution of guidance cues or other pathway components used by the axon. These conditions would alter the ability of the axon to interpret guidance cues or even physically block the AVM axon from reaching its target. Again, exogenous acetylcholine would somehow reverse these conditions. We do not favor these latter models because in these situations all axon migrations that depend on the guidance cues would likely be affected. This is not the case, however, since we observe that neighboring DD and VD axons are correctly guided in the mutants, indicating that the UNC-6 guidance cue, which is required for DD and VD axon guidance, is present and can guide these axons to their targets despite any changes to the axon’s environment. Furthermore, we have found that DEG-3 functions cell autonomously in the AVM neuron to mediate the influence of acetylcholine on the AVM’s response to UNC-6. This observation supports the idea that acetylcholine functions directly on the AVM neuron and is inconsistent with the idea that it might function indirectly by regulating other cells.
Figure 3—
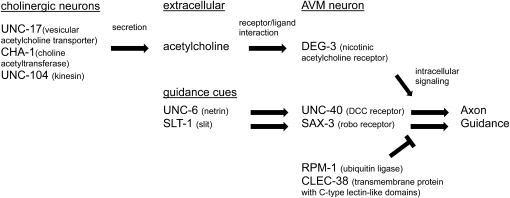
Acetylcholine potentiates AVM axon guidance through UNC-6– and SLT-1–induced signaling. Diagram showing the sites of action of the molecules described. Acetylcholine secreted by ventral nerve cord cholinergic neurons is sufficient to enhance the AVM response to UNC-6 and SLT-1 guidance cues through its interaction with AVM acetylcholine receptors. RPM-1 and CLEC-38 act within AVM to negatively regulate the guidance signaling and have the opposite effect of acetylcholine.
How could acetylcholine enhance SLT-1– and UNC-6–induced signaling? We suggest that during AVM axon outgrowth, AVM acetylcholine-activated receptors regulate calcium influx in response to extracellular acetylcholine levels. Cytosolic Ca2+ is one of the key regulators of growth cone motility and it helps mediate both attractive and repulsive responses to many extracellular guidance cues (see review in Zheng and Poo 2007). In support of this idea, the turning response of growth cones in culture to netrin-1 gradients depends on Ca2+ influx through plasma membrane Ca2+ channels (Hong et al. 2000) and there is an increase in cytosolic Ca2+ on the side of the growth cone facing the source, which is a micropipette delivering the netrin (Henley and Poo 2004).
Significantly, we present evidence that extracellular levels of acetylcholine can influence guidance cue signaling in vivo. If this is due to the ability of acetylcholine to alter cytosolic Ca2+ levels within a migrating growth cone, then it raises the possibility that localized sources of acetylcholine in vivo could alter the type of response that a growth cone has to guidance cues at specific sites. This idea is based on the observations that in culture, different patterns of Ca2+ elevation can trigger attractive and repulsive turning responses to netrin-1 (Hong et al. 2000). Thus, depending on how cytosolic Ca2+ levels are altered, acetylcholine sources could promote, inhibit, or even alter the direction of an axon’s outgrowth. Furthermore, we have also shown genetic interactions between genes that could regulate extracellular acetylcholine levels and clec-38 or rpm-1. CLEC-38 and RPM-1 not only influence axon guidance receptor activity but they also affect the ability of neurons to form presynaptic structures (Li et al. 2008). An intriguing possibility is that acetylcholine and netrin secretion by intermediate target neurons could be important signals that coordinate axon outgrowth responses to guidance cues and synaptogenesis. In the case of the AVM axon, acetylcholine and UNC-6 are secreted by the target neurons of the ventral nerve cord. Apparently, the responsiveness of AVM to UNC-6 changes at the ventral nerve cord as the axon turns and migrates anteriorly. AVM also makes a choice of producing only a few synapses within the ventral nerve cord, instead making the majority of its synapses in the nerve ring within the head (White et al. 1986).
Although the same guidance cue is used by several neurons, the effects can be regulated to produce unique responses. Acetylcholine has an effect on UNC-6 guidance for the AVM axon, but not for the DD and VD axons. However, acetylcholine levels do have an effect on an UNC-6–mediated activity that regulates DD and VD axon branching (Wang and Wadsworth 2002). In unc-6 null mutants, DD and VD axons often fail to branch and extend processes dorsally (Wang and Wadsworth 2002). Experiments using the expression of an UNC-6 protein that lacks the C domain suggest that a branching activity is controlled by the activity of the C domain (Lim et al. 1999). It is proposed that UNC-6 becomes associated with receptor complexes on the surface of the motor neurons and domain C silences a branching outgrowth activity induced by the N-terminal domains. These motor neurons might normally branch at specific locations where the domain C-mediated inhibition becomes repressed. It was shown that the N-terminal–mediated branching outgrowth activity is sensitive to acetylcholine release (Wang and Wadsworth 2002). Like the guidance response, this branching activity also requires the UNC-6 receptors. Furthermore, loss-of-function and gain-of-function alleles of unc-43 enhance or suppress, respectively, the DD and VD branching induced by the N-terminal domains of UNC-6. UNC-43 is a calcium/calmodulin-dependent protein kinase (CaMKII). These observations again support an idea that acetylcholine can influence UNC-6–induced signaling pathways that control axon outgrowth by influencing cytosolic Ca2+ levels.
Acknowledgments
We thank Millet Treinin for strain TU1851 deg-3(u701) and Scott Clark for strain SK4005. We thank Randall R. Reed for plasmid pBP6-1; Chris Li for the odr-1::rfp construct; and members of the Wadsworth, Soto, and Kramer laboratories for helpful discussions. We thank the Caenorhabditis Genetics Center for strains. This work was funded by National Institutes of Health grants NS033156 (to W.G.W.) and 5R03HD060787 (to C.C.Q.), by a New Jersey Commission on Spinal Cord Research grant (to W.G.W.), by start up funding from the University of Wisconsin-Milwaukee (UWM) (to C.C.Q.), and by a UWM research foundation fellowship (to Y.X.).
Literature Cited
- Alfonso A., Grundahl K., Duerr J. S., Han H. P., Rand J. B., 1993. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261: 617–619 [DOI] [PubMed] [Google Scholar]
- Alfonso A., Grundahl K., McManus J. R., Rand J. B., 1994. Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J. Neurosci. 14: 2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Waga N., Ogura K., Goshima Y., 2010. Genes required for cellular UNC-6/netrin localization in Caenorhabditis elegans. Genetics 185: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K., Bland K. S., Wang K. H., Arnott D., Henzel W., et al. , 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96: 795–806 [DOI] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., et al. , 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195 [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Han H. P., Fields S. D., Rand J. B., 2008. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 506: 398–408 [DOI] [PubMed] [Google Scholar]
- Gitai Z., Yu T. W., Lundquist E. A., Tessier-Lavigne M., Bargmann C. I., 2003. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37: 53–65 [DOI] [PubMed] [Google Scholar]
- Hall D. H., Hedgecock E. M., 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847 [DOI] [PubMed] [Google Scholar]
- Hao J. C., Yu T. W., Fujisawa K., Culotti J. G., Gengyo-Ando K., et al. , 2001. C. elegans Slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 32: 25–38 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85 [DOI] [PubMed] [Google Scholar]
- Henley J., Poo M. M., 2004. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 14: 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Nishiyama M., Henley J., Tessier-Lavigne M., Poo M., 2000. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403: 93–98 [DOI] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D., 1983. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature 305: 632–634 [DOI] [PubMed] [Google Scholar]
- Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., et al. , 1996. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175–185 [DOI] [PubMed] [Google Scholar]
- Kuffler D. P., 1996. Chemoattraction of sensory neuron growth cones by diffusible concentration gradients of acetylcholine. Mol. Chem. Neuropathol. 28: 199–208 [DOI] [PubMed] [Google Scholar]
- Kulkarni G., Li H., Wadsworth W. G., 2008. CLEC-38, a transmembrane protein with C-type lectin-like domains, negatively regulates UNC-40-mediated axon outgrowth and promotes presynaptic development in Caenorhabditis elegans. J. Neurosci. 28: 4541–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. C., Hong K., Kinnell M., Chalfie M., Driscoll M., 1996. Sequence and transmembrane topology of MEC-4, an ion channel subunit required for mechanotransduction in Caenorhabditis elegans. J. Cell Biol. 133: 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Kulkarni G., Wadsworth W. G., 2008. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 28: 3595–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. S., Mallapur S., Kao G., Ren X. C., Wadsworth W. G., 1999. Netrin UNC-6 and the regulation of branching and extension of motoneuron axons from the ventral nerve cord of Caenorhabditis elegans. J. Neurosci. 19: 7048–7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation. Methods Cell Biol. 48: 451–482 [PubMed] [Google Scholar]
- Otsuka A. J., Jeyaprakash A., Garcia-Anoveros J., Tang L. Z., Fisk G., et al. , 1991. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6: 113–122 [DOI] [PubMed] [Google Scholar]
- Prasad B. C., Ye B., Zackhary R., Schrader K., Seydoux G., et al. , 1998. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development 125: 1561–1568 [DOI] [PubMed] [Google Scholar]
- Quinn C. C., Pfeil D. S., Chen E., Stovall E. L., Harden M. V., et al. , 2006. UNC-6/Netrin and SLT-1/Slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/Lamellipodin. Curr. Biol. 16: 845–853 [DOI] [PubMed] [Google Scholar]
- Ruediger T., Bolz J., 2007. Neurotransmitters and the development of neuronal circuits. Adv. Exp. Med. Biol. 621: 104–115 [DOI] [PubMed] [Google Scholar]
- Serafini T., Kennedy T. E., Galko M. J., Mirzayan C., Jessell T. M., et al. , 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78: 409–424 [DOI] [PubMed] [Google Scholar]
- Treinin M., Chalfie M., 1995. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron 14: 871–877 [DOI] [PubMed] [Google Scholar]
- Treinin M., Gillo B., Liebman L., Chalfie M., 1998. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc. Natl. Acad. Sci. USA 95: 15492–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth W. G., Hedgecock E. M., 1996. Hierarchical guidance cues in the developing nervous system of C. elegans. Bioessays 18: 355–362 [DOI] [PubMed] [Google Scholar]
- Wadsworth W. G., Bhatt H., Hedgecock E. M., 1996. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16: 35–46 [DOI] [PubMed] [Google Scholar]
- Wang Q., Wadsworth W. G., 2002. The C domain of netrin UNC-6 silences calcium/calmodulin-dependent protein kinase- and diacylglycerol-dependent axon branching in Caenorhabditis elegans. J. Neurosci. 22: 2274–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Southgate E., Thompson J., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 327–348 [DOI] [PubMed] [Google Scholar]
- Yang H., Kunes S., 2004. Nonvesicular release of acetylcholine is required for axon targeting in the Drosophila visual system. Proc. Natl. Acad. Sci. USA 101: 15213–15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W. D., Rusch J., Poo M., Wu C. F., 2000. Spontaneous acetylcholine secretion from developing growth cones of Drosophila central neurons in culture: effects of cAMP-pathway mutations. J. Neurosci. 20: 2626–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin L., Gillo B., Kahan T., Halevi S., Eshel M., et al. , 2001. Characterization of the deg-3/des-2 receptor: a nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol. Cell. Neurosci. 17: 589–599 [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M., 1983. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature 305: 634–637 [DOI] [PubMed] [Google Scholar]
- Yu T. W., Hao J. C., Lim W., Tessier-Lavigne M., Bargmann C. I., 2002. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat. Neurosci. 5: 1147–1154 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Yi B. A., Bargmann C. I., 1998. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92: 217–227 [DOI] [PubMed] [Google Scholar]
- Zheng J. Q., Poo M. M., 2007. Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 23: 375–404 [DOI] [PubMed] [Google Scholar]
- Zheng J. Q., Felder M., Connor J. A., Poo M. M., 1994. Turning of nerve growth cones induced by neurotransmitters. Nature 368: 140–144 [DOI] [PubMed] [Google Scholar]