Abstract
Regulation of transcription can be a complex process in which many cis- and trans-interactions determine the final pattern of expression. Among these interactions are trans-interactions mediated by the pairing of homologous chromosomes. These trans-effects are wide ranging, affecting gene regulation in many species and creating complex possibilities in gene regulation. Here we describe a novel case of trans-interaction between alleles of the Malic enzyme (Men) locus in Drosophila melanogaster that results in allele-specific, non-additive gene expression. Using both empirical biochemical and predictive bioinformatic approaches, we show that the regulatory elements of one allele are capable of interacting in trans with, and modifying the expression of, the second allele. Furthermore, we show that nonlocal factors—different genetic backgrounds—are capable of significant interactions with individual Men alleles, suggesting that these trans-effects can be modified by both locally and distantly acting elements. In sum, these results emphasize the complexity of gene regulation and the need to understand both small- and large-scale interactions as more complete models of the role of trans-interactions in gene regulation are developed.
THE regulation of gene expression is a complex process often involving many levels of organization. In a simple model, gene expression is determined by intragenic interactions (e.g., enhancer–promoter interactions occurring in cis on the same chromosome). In more complex models of regulation, expression is also influenced by the three-dimensional genomic structure and organization of chromosomes. In the latter, more realistic models, nuclear organization governs interactions between neighboring genetic elements, such as transcription factories, heterochromatin, homologous chromosomes, and genes that are capable of acting in trans to modulate gene expression (reviewed in Henikoff and Comai 1998; Wu and Morris 1999; Lanctot et al. 2007; Xu and Cook 2008). These trans-interactions create levels of complexity in gene regulation involving interphase chromatin structuring, its impact on pairing of homologs, and the potential exchange of transcriptional or regulatory proteins between homologs. One such trans-interaction, transvection, is the modification of gene activity through interactions between the regulatory elements of one allele and its homolog on the homologous chromosome (reviewed by Pirrotta 1999; Wu and Morris 1999; Duncan 2002). Here we describe a case of trans-interaction at the Malic enzyme locus (Men) (Merritt et al. 2005), potentially transvection, and examine interactions between putative regulatory elements on homologous chromosomes.
The term “transvection” was first coined by E. B. Lewis (1954) to describe complementation and trans-interactions between two Ultrabithorax (Ubx) alleles in Drosophila. Lewis found that certain Ubx alleles were able to complement each other and that this complementation could be interrupted by chromosomal rearrangements that disrupted local homolog pairing (Lewis 1954). Since this initial description, the term transvection has generally been used to describe interallelic interactions at a single locus in which two mutant alleles on paired homologous chromosomes interact, leading to either higher or lower gene expression than would be predicted from each allele independently [e.g., enhancer elements acting in trans to promote gene expression or zeste-mediated silencing, reducing gene expression in paired alleles (reviewed in Duncan 2002; Southworth and Kennison 2002)]. Transvection effects have been described for more than a dozen Drosophila melanogaster genes (e.g., Gelbart and Wu 1982; Davison et al. 1985; Babu et al. 1987; Geyer et al. 1990; Leiserson et al. 1994; Gindhart and Kaufman 1995; Hopmann et al. 1995; Morris et al. 1999; Southworth and Kennison 2002; Coulthard et al. 2005; Merritt et al. 2005; Gohl et al. 2008; Ou et al. 2009) and in other species, including humans (e.g., Koeman et al. 2008). Several models for the molecular mechanisms of transvection have been described, including trans enhancer–promoter interactions, insulator bypassing, and pairing-dependent silencing (reviewed in Duncan 1987; Henikoff 1997; Henikoff and Comai 1998; Southworth and Kennison 2002). Of particular interest for the study presented here are enhancer–promoter interactions occurring in trans—the type of interactions that our work suggests are responsible for the trans-interactions that we observe at the Men locus.
In Drosophila and many other dipteran species, homologous chromosomes are extensively paired throughout the nucleus of interphase somatic cells (Stevens 1908; Metz 1916; Fung et al. 1998; reviewed in McKee 2004). This pairing facilitates a wide array of homology effects, including transvection, that have remarkable roles in gene regulation (Wu and Morris 1999). Similar pairing and transvection-like effects have also been found in plants (reviewed in Chandler and Stam 2004; Grant-Downton and Dickinson 2004; Stam 2009), mammals (Thatcher et al. 2005; Bacher et al. 2006; Xu et al. 2006), and fungi (reviewed in Shiu et al. 2006; Vyas et al. 2006), emphasizing the importance of trans-interactions in gene regulation across a wide variety of species. With the availability of a wide array of genetic tools, and because extensive pairing occurs between homologous chromosomes, D. melanogaster is an excellent system in which to study these interallelic pairing effects on gene expression.
Cytosolic malic enzyme oxidizes malate to pyruvate and is one of four enzymes primarily responsible for the reduction of the cofactor NADP+ to NADPH (Wise and Ball 1964). In earlier work using D. melanogaster Men knockout alleles to study the interaction between the NADPH enzymes, unexpectedly high, non-additive amounts of MEN activity were observed when small deletion knockout alleles were heterozygous with wild-type alleles (Merritt et al. 2005, 2009). The high level of MEN activity was shown to be dependent on the specific deletion creating the knockout allele and not simply on physiological up-regulation, suggesting transvection, or a similar type of trans-interaction, between the functional and nonfunctional Men alleles (Merritt et al. 2005).
Here we characterize these trans-interactions between functional and nonfunctional alleles at the Men locus. Using a suite of 19 Men null activity (knockout) alleles (MenExi−) that differ only in their genomic lesions, we tested MEN enzyme activity and Men gene expression in MenExi−/MenExi+ heterozygotes using two different wild-type alleles (MenExi+). We found significant differences in MEN activity across these heterozygotes that are dependent upon specific characteristics of each deletion. The presence or absence of putative regulatory elements, based upon computational prediction, was correlated with differences in trans-interaction across the five sets of knockout alleles with significantly different levels of trans-interaction. In addition, we examined the impact of five different genetic backgrounds on the trans-interaction effects, finding significant interactions between specific deletions and specific backgrounds. These significant interactions illustrate the importance of large- and small-scale interactions between the functional and nonfunctional alleles in trans-interactions.
Materials and Methods
Stock lines
Isothird chromosome lines (VT26, HFL53, JFL12, CT21, MD76, and MD5) were a subset of nonlethal third chromosomes extracted from isofemale lines (Duvernell and Eanes 2000; Merritt et al. 2005). Inbred lines used as common genetic backgrounds were obtained either from Bloomington Drosophila Stock Center at Indiana University (BDSC, Bloomington, IN; line no. 6326) or from the Eanes lab (VT83) (Merritt et al. 2005). The P-element line used for the P-element-mediated deletion, EP(3)0517, was obtained from the Szeged Drosophila Stock Center (Szeged, Hungary). The line used as the source of transposase (stock #2030) and a line containing a deficiency that covers the entire Men gene, Df(3R)kar31 (stock #6160), were obtained from the BDSC. Three P-element-derived excision alleles for the Men gene initially described in an earlier article—MenEx3+(wild type), MenEx9− (knockout), and MenEx15− (knockout) (Merritt et al. 2005)—were also included in both the enzyme activity and quantitative PCR (qPCR) analysis.
Culture conditions
Flies were maintained on a standard cornmeal media at 25° with a 12-hr/12-hr photocycle. All enzyme activity and qPCR assays were conducted on male flies aged for 4–6 days after emergence.
Mutagenesis
We performed P-element-directed mutagenesis (Rorth 1996) to create small deletion alleles of the Men locus using EP(3)0517. The P-element construct carries the eye color marker gene mini-white (w+mC) and is inserted 473 bases 5′ to the Men start codon (Merritt et al. 2005). The EP(3)0517 element was mobilized by crossing virgin females to males carrying the Δ2-3 source of transposase. Dysgenic males, EP(3)517/Δ2-3,TM3, were crossed to w−; 6326; Dr/TM8, Sb females and w−, Sb, Dr+, males; i.e., males containing a copy of the EP(3)517 chromosome with the P-element excised were collected. Candidate excision males were again crossed with TM8 for complete third chromosome isolation, and male and female progeny were backcrossed to establish each line. The excision chromosomes generated are essentially isogenic, differing only in a small region at the point of P-element excision. Recovered excision chromosomes were screened for MEN activity. Flies containing chromosomes showing no MEN activity, i.e., knockout allele candidates, were placed in a common X and second chromosome background using simple crosses (w1118;6326) (Merritt et al. 2005, 2009). Seventeen new alleles with no apparent MEN activity (knockout lines) were generated (Table 1).
Table 1. Detailed summary of MenExi− alleles.
| Excision | Deletion size (bp) | Insertion | 5' deletion from TSS | 3' deletion from TSS | MenExi−/MenEx3+ activity |
|---|---|---|---|---|---|
| MenEx8 | 1,651 | TCATCATCATAACATAAAG | −1347 | 304 | 0.7249 ± 0.0397 |
| MenEx9 | 4,080 | NA | −2203 | 1877 | 0.6876 ± 0.0313 |
| MenEx12 | 2,500 | TTAATA | −1635 | 865 | 0.7422 ± 0.0397 |
| MenEx15 | 2,414 | NA | −2306 | 108 | 0.7560 ± 0.0364 |
| MenEx30 | NA | Not sequenced | NA | NA | 0.7922 ± 0.0374 |
| MenEx43 | 2,682 | NA | −2044 | 638 | 0.6639 ± 0.0352 |
| MenEx48 | 3,581 | NA | −2014 | 1567 | 0.7553 ± 0.0345 |
| MenEx52 | 3,058 | TAAACAGACATT | −1623 | 1435 | 0.6619 ± 0.0361 |
| MenEx55 | 16,231 | NA | −10245 | 5986 | 0.5414 ± 0.0356 |
| MenEx57 | 1,379 | GATATATAG | −1304 | 75 | 0.7454 ± 0.0462 |
| MenEx58 | 535 | AACAATTCGCAGAGTCCT | −215 | 320 | 0.8400 ± 0.0401 |
| MenEx60 | 646 | CATGATGAAATAATAAATAATAATA | −213 | 433 | 1.0569 ± 0.0490 |
| MenEx76 | 669 | CATGATGAAATAACATAA | −215 | 454 | 0.9097 ± 0.0443 |
| MenEx77 | 2,070 | TAAATAA | −1404 | 666 | 0.7648 ± 0.0440 |
| MenEx81 | 2,765 | NA | −1759 | 1006 | 0.6670 ± 0.0300 |
| MenEx86 | 2,239 | NA | −2147 | 92 | 0.7665 ± 0.0391 |
| MenEx109 | 3,551 | NA | −2635 | 916 | 0.7523 ± 0.0521 |
| MenEx119 | 1,379 | GATATATAG | −1304 | 75 | 0.7663 ± 0.0451 |
| MenEx125 | 2,378 | GTT | −1513 | 865 | 0.7032 ± 0.0317 |
MenExi− lines are partitioned into overlapping groups by a Tukey’s HSD test of differences in transvection-influenced enzyme activity. MenExi− alleles that do not share a letter code are significantly different.
For each new allele, ∼15 kb of genomic sequence surrounding the transcription start site (TSS) was characterized using a series of overlapping PCR amplifications. Primer pairs were designed to amplify ∼1-kb regions that overlapped with the next primer pair (Supporting Information, Figure S1). Regions that did not amplify in comparison with a wild-type positive control indicated regions of the excision site. Primers that flanked the excision site were used to directly amplify and sequence the excision break points.
Fly homogenizations
Flies were homogenized for enzyme activity determination in 100 μl of grinding buffer (50 mM Tris–HCl, pH 7.4) per fly. In general, five flies per sample were homogenized; however, if there were insufficient flies, fewer were assayed and the homogenate volume was adjusted accordingly. Each sample was centrifuged at 20,000 × g for 5 min at 4° to pellet and remove all insoluble residues.
Enzyme kinetic assay
Malic enzyme activity was measured using 10 μl of whole-fly homogenate in 100 μl of assay solution in a SpectraMax 384Plus 96-well plate spectrophotometer (Molecular Devices). Absorbance at 320 nm was measured every 9 sec for 3 min at 25° and activity was quantified as the slope of this line. Each sample was assayed three times and the mean was used for statistical analysis. The assay solution consisted of 100 mM Tris–HCl, 0.34 mM NADP+, 50 mM MnCl2, and 50 mM malate (pH 7.4).
Quantitative RT-PCR
Total RNA was extracted and purified from four groups of five male flies for each genotype using the RNeasy kit (QIAGEN). One microgram of total RNA was reverse-transcribed using random hexamers and High Capacity cDNA Reverse Transcription Kits with RNase Inhibitor (Applied Biosystems). The PCR reaction consisted of 2 μl of undiluted cDNA template, 0.4 μM of each primer and 0.2-μM probe, and Quantitect Probe PCR Master Mix (QIAGEN) in a total volume of 25 μl. cDNA synthesis of samples lacking reverse transcriptase were used to ensure that there was no genomic DNA contamination, and “no-template” blanks were used to ensure that there was no contamination within our reagents. The primers and probe flank the intron between exon 2 and exon 3 [3R: 8540309–8540365; exon 3 and 4, respectively, as annotated by FlyBase; forward (GTATTGCCAACCTGTGCC), reverse (AGC TTGTGTTCGGTGAGT), and probe (56-FAM/ATGGTGGATAGCCGTGGTGTCA/3IABkFQ)]. FlyBase annotates four exons with two transcript variants that differ in the genomic location of the first exon for Men. However, on the basis of the examination of expressed sequence tags (ESTs) (Figure S2) and annotation by the Paired-End Analysis of TSS (PEAT) project (Ni et al. 2010), we suspect that the true TSS is downstream of this predicted first exon, and thus this first “exon” was ignored in our analysis. Two qPCR reactions per template were performed in parallel using a Mastercycler ep realplex Thermal Cycler (Eppendorf). All sample expression results were normalized to RpL32 [(forward: CCATTTGTGCGACAGCTT), (reverse: ATACAGGCCCAAGATCGT), and (probe: 56-FAM/ACCAAGCACTTCATCCGCCAC/3IABlk_FQ)] and quantified/reported relative to MenEx3+ using the ΔΔCT method (Livak and Schmittgen 2001).
Data analysis
Flies of specific genotypes were generated by mating five males and five virgin females in single vials. Each cross was done in six separate vials (replicates) to allow for statistical analysis. All samples were weighed prior to homogenizations, and this wet weight was used as a covariance to standardize the MEN activity for differences in fly size between individuals. Multivariate analysis of variance tests were conducted to ascertain possible significant differences in MEN activity across heterozygote individuals for Men knockouts. Tukey’s honestly significant difference (HSD) multiple comparison tests were conducted to group excision lines for similarity in MEN activity.
Phylogenetic footprinting
Approximately 15 kb of genomic sequence around the Men locus, 9 kb upstream of the TSS and 6 kb downstream, was aligned, and the degree of sequence divergence was quantified across 10 Drosophila species (D. melanogaster, D. simulans, D. sechellia, D. yakuba, D. erecta, D. ananassae, D. pseudoobscura, D. persimilis, D. mojavensis, and D. virilis). D. melanogaster genomic sequence was obtained from FlyBase (http:www.flybase.org) while the other Drosophila sequences were obtained from the assembly/alignment/annotation of 12 related Drosophila species project (rana.lbl.gov/drosophila/; the corresponding genomic sequence could not be reliably aligned from D. grimshawi and D. willistoni so these two species were not included in our phylogenetic analysis). BigFoot, a Bayesian alignment and phylogenetic footprinting software, was used to align genomic sequences and score regions for the degree of conservation with settings of 1 million burn-in cycles and 5 million samples (Satija et al. 2009). MatInspector was used to examine aligned sequences for potential transcription factor-binding sites (TFBS) across the same 10 species with an optimized core matrix similarity of 0.75 (Cartharius et al. 2005). The combination of BigFoot and MatInspector analysis was used to determine regions and potential regulatory elements at the Men loci that affect trans-interactions.
Results
P-element excision-derived Men alleles
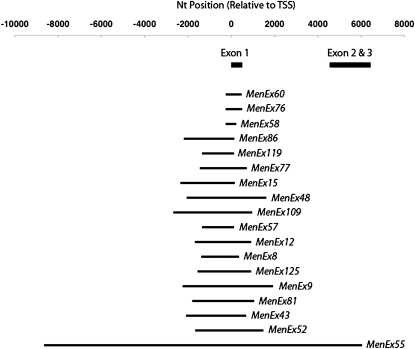
We generated a series of excision-based Men knockout alleles that varied in the size and location of the genomic excisions. Using P-element-mediated dysgenesis (Tsubota and Schedl 1986; Salz et al. 1987; Rorth 1996) with the EP(3)0517 fly stock line, we generated 17 novel knockout alleles (denoted as MenExi−) (Table1). Two other knockout alleles were previously reported (MenEx9− and MenEx15−) (Merritt et al. 2005). Regions flanking the deletion sites were amplified and sequenced (summarized in Table 1). We were unable to amplify the excision site of MenEx30−, suggesting that this allele has a more complex excision site; therefore, this allele was not considered further in this study. All 18 (i.e., not including MenEx30−) deletions remove regions of the TSS, annotated by the PEAT project (Ni et al. 2010) and examination of Men ESTs (Figure S2), and as such are considered to be promoter deficient. All deletions were found to excise some portion of the protein-coding region and upstream nonprotein-coding genomic regions (Figure 1). MenEx55− was the largest deletion generated and has a 16,321-bp excision that removes a majority of the Men coding region, as well as a substantial portion upstream of the TSS. MenEx60− and MenEx58− have the two smallest deletions, 646 and 535 bp, respectively, which remove a portion of the first exon, including the TSS. The remainder of the deletion alleles have 1.3- to 4-kb deletions that are roughly centered around the EP(3)0517 insertion site. All the deletions are homozygous viable, but as homozygotes show no MEN enzyme activity. P-element mutagenesis largely modifies only the site of insertion/excision (Cooley et al. 1988; Spradling et al. 1995). Since all alleles are placed into a common genetic background (w;6326;MenExi−), we expect that the only differences in genetic architecture between the lines are at the Men locus excision site, although it is possible that other changes could exist elsewhere on the third chromosome.
Figure 1—
Genomic map of MenExi− excisions. Predicted Men TSS is at “0.” The bars indicate excised regions.
Trans-interaction and MEN activity in heterozygotes is dependent on the deletion of the knockout allele
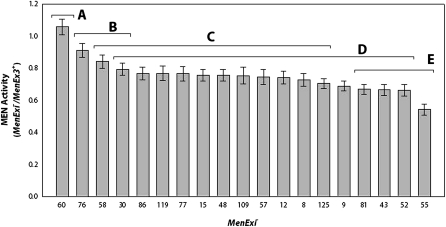
Transvection is the modulation of gene expression due to interactions between paired homologous chromosomes (reviewed in Duncan 2002 and Southworth and Kennison 2002), and our results are consistent with the high levels of expression that we observe being driven by transvection. At paired loci, enhancer elements have been shown to act on a trans-promoter when its cis-promoter is deficient, increasing overall gene expression (Geyer et al. 1990; Morris et al. 1998, 1999, 2004; Lee and Wu 2006). In the specific case of the Men locus, and MEN enzyme activity, we expect that excision/wild-type heterozygotes would have 50% wild-type activity if the MenExi− allele contributed no activity. Instead, MenExi− heterozygotes have shown greater-than-expected levels of MEN activity that are dependent on the deletion of the MenExi− allele, a phenomena that has been previously attributed to transvection (Merritt et al. 2005, 2009). Previous examination of MenEx9− and MenEx15− suggested that the level of trans-interaction might be dependent on the specific deletion allele (data not shown). To test for variation in up-regulation between deletion alleles, MEN enzyme activity in MenExi− allele heterozygotes was quantified using two different wild-type third chromosome lines (w;6326/VT83i;MenExi−/HFL53 and w;6326/VT83i;MenExi−/VT26). The average MEN enzyme activity between both sets of crosses was used in the analysis (Figure 2; the activities of the excision alleles in either background are shown individually in Figure S3 and Figure S4). While all homozygous MenExi− alleles have no MEN activity, we found that the amount of MEN enzyme activity in MenExi− heterozygotes varied significantly depending on the specific MenExi− deletion in each cross (F18,222 = 18.8916, P < 0.0001). Tukey’s HSD test placed the lines into five overlapping bins on the basis of their activity (Figure 2).
Figure 2—
Heterozygote MEN enzyme activity graphed as a ratio of wild-type activity across two isochromosomal backgrounds, HFL53 and VT26; i.e., heterozygote activity was calculated as the average activity of MenExi−/HFL53 and MenExi−/VT26. All MenExi− lines are grouped into overlapping bins on the basis of Tukey’s HSD classification. For each excision, n = 6, and samples were measured in triplicate. Error bars represent standard error.
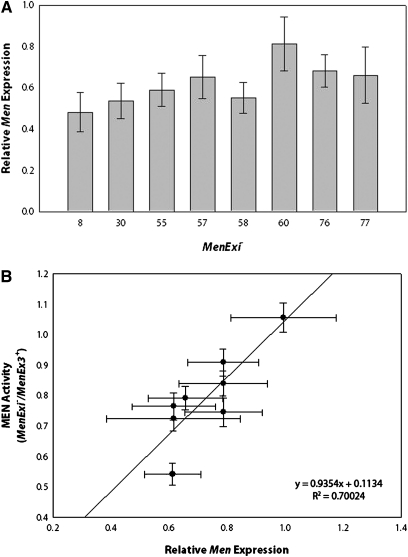
Trans-interactions are transcriptional phenomena, and we expected that the up-regulated levels of MEN enzyme activity observed resulted from elevated transcription from the functional Men copy in the heterozygotes due to trans-interactions with the promoter-deficient MenExi− alleles. To test if the up-regulation of MEN activity that we observed truly was a transcriptional phenomenon, Men gene expression was measured using quantitative RT-PCR (qRT-PCR) from heterozygotes from each Tukey’s HSD test bin of MenExi−/VT26 (Figure 3A). As predicted, we found a significant effect on gene expression of the specific deletion alleles, similar to the allele-specific differences in enzyme activity (F7, 53 = 3.0665, P = 0.0098). MEN enzyme activity and Men gene activity were significantly correlated with R2 = 0.700 (Figure 3B), strongly suggesting that the observed differences in enzyme activity are transcriptionally driven. MenEx55−, the largest deletion, shows no up-regulation with heterozygotes expressing essentially 50% of wild-type gene and enzyme activity. Similarly, MenEx60− heterozygotes have essentially wild-type levels of gene expression and enzyme activity, even though one allele is completely nonfunctional, suggesting very strong up-regulation. The lack of exact fit in the comparison of MEN enzyme activity and Men gene expression may simply reflect the greater error associated with measuring gene expression rather than protein activity, a technical limitation of qRT-PCR.
Figure 3—
(A) The average Men gene expression measured from MenExi−/HFL53 and MenExi−/VT26 heterozygotes relative to MenEx3+/HFL53 and MenEx3+/VT26, respectively. Expression was normalized to RpL32. Relative expression was calculated using the ∆∆CT method (Livak and Schmittgen 2001). (B) MEN enzyme activity plotted against Men gene expression.
Given the nature of the knockout alleles (removing the TSS) and the general model for trans-interactions, we expected that all observed gene expression (and protein activity) was from the wild-type functional allele. To test this expectation, we crossed a subset of the deletion alleles to a wild-type allele that could be distinguished by a single SNP site in the coding region. The Men gene has a G/C polymorphism at position 338 (Sezgin et al. 2004); all of the MenExi− alleles have a G at this position. Three MenGExi− lines (MenEx55−, MenEx43−, and MenEx60−) were crossed to a MenC line, MD5, to create MenG/C heterozygotes (w;6326/VT83i;MenExi−,MenG/MD5,MenC). Total RNA was extracted from male MenExi−/MD5 heterozygotes, cDNA was synthesized, and the polymorphism-containing region of the Men gene was amplified and sequenced. MenEx55− acts as a negative control since the deletion removes this polymorphic site. Both MenEx43− and MenEx60− have this site intact. If both alleles were actively being transcribed, the sequence of the polymorphic site would have appeared as a double G/C peak. Only MenC transcript was found upon analysis of the sequence, indicating that transcription occurs only on the functional allele and not on the excision allele (Figure S5).
Phylogenetic footprinting and the prediction of regulatory elements
Our results indicated that the amount of trans-interaction is dependent on the excision allele present, suggesting that the size, location, or sequence—or a combination of all three—of the deletion modulates the interactions. In general, we found a broad correlation between deletion size and trans-interactions: the largest deletion (MenEx55−) is associated with no up-regulation, midsize deletions have moderate levels of up-regulation, and small deletions have high levels of up-regulation. A regression analysis of activity and deletion size, however, found no significant relationship between activity and deletion size (Figure S6). In general, there is no correlation between deletion size and amount of trans-interaction (up-regulation) across the midsize deletions in the data set. This lack of correlation could be a function of the low amount of variation in trans-interaction between midsize deletions and the relatively low accuracy of our gene expression assay. However, it seems more likely, given the lack of pattern across all 19 alleles, that this lack of simple correlation with deletion size indicates that deletion size alone does not fully explain the impact of each allele on the trans-interactions. Instead, interactions appear to be a function of the presence or absence of specific genomic sequences in each allele, suggesting that specific regulatory elements are present or absent in the different excision alleles.
To identify such potential elements, we used both phylogenetic footprinting and regulatory element prediction analysis. In general, regulatory elements are likely associated with regions of high phylogenetic conservation (Tagle et al. 1988; Blanchette et al. 2002), while consensus sequences can be used to predict specific elements. Regulatory element prediction analysis within these regions allowed us to identify consensus regulatory sites. We attempted to identify potential modifying elements by comparing this in silico regulatory element discovery with genomic regions that differ between alleles that cause different amounts of trans-interactions (heterozygous MEN activity).
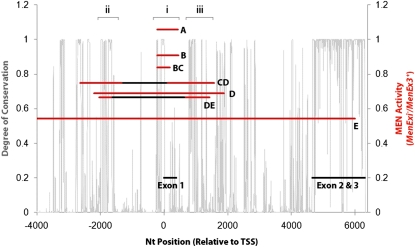
We examined ∼15 kb of genomic sequence across 10 Drosophila species for conservation using BigFoot, a combined statistical alignment and phylogenetic footprinting software that uses a Markov chain Monte Carlo sampling method to detect conserved functional elements without assuming a fixed alignment (Satija et al. 2009). The 15-kb region encompasses the entire Men coding region as well as ∼8.6 kb upstream of the TSS, covering all of the deletion sites in the alleles used. We identified three potentially informative regions of high conservation that may impact trans-interactions and Men expression: regions i–iii (Figure 4). The first region of interest is an ∼650-bp region that centers on the Men TSS and spans both the MenEx60− and MenEx58− deletions (Figure 5). Regions ii and iii are conserved regions that are absent in Tukey’s HSD test groups CD, D, and DE (Figure S7). All three of these regions are rich with predicted regulatory binding elements and provide a list (Table S1) of potential regions that impact overall Men gene regulation and transvection. Although we limited our search to regions of high conservation, we recognize that it is possible for D. melanogaster to have functional elements that are not conserved across all the species examined. In some cases, however, predicted TFBS do coincide very well with regions of high conservation. For example, knirps, found ∼920 bp downstream of ATG within region iii (Table S1; Figure S7), was predicted well within a peak of high conservation. This approach of associating quantified trans-interactions between MenExi− and wild-type alleles and an in silico discovery of regulatory elements provides a step in identifying potential regions in the genomic architecture that impact trans-interactions.
Figure 4—
The degree of conservation within the Men genomic region measured across 10 Drosophila genomes (gray lines, left y-axis) and average relative activity of the excision heterozygotes (height of bars representing excision groups). Tukey’s HSD groups (overlapping groups A–E) were determined by relative MEN enzyme activity (right y-axis). Red lines show total deletion size within that bin, and the black lines indicate common deletion size per bin. Deletion groups without a black line are made up of only one MenExi− allele. Regions of interest have a bracket above and are denoted i, ii, and iii.
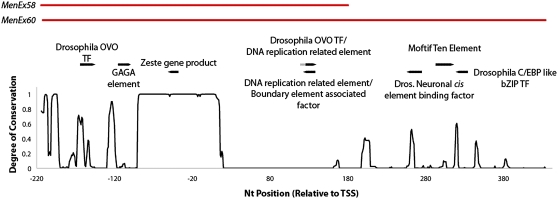
Figure 5—
MenEx58 and MenEx60 mapped above the degree of conservation (Figure 4) and the predicted regulatory elements. The degree of conservation is measured from 0 to 1 in which 0 represents no conservation and 1 represents high conservation. Overlapping predicted regulatory elements are shown in different shades. Regulatory elements in the top row are in the forward direction whereas regulatory elements in the bottom row are in the reverse direction.
Different genetic backgrounds and their impact on different excisions
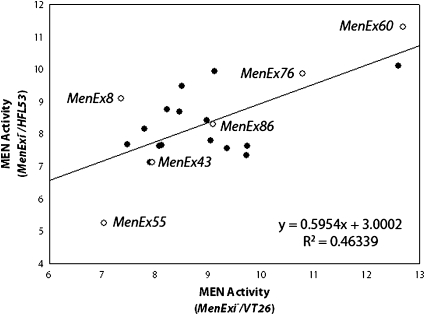
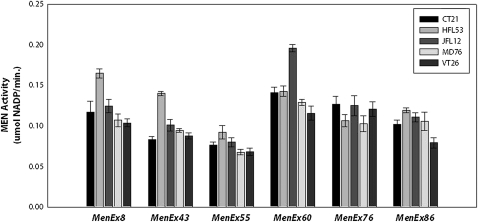
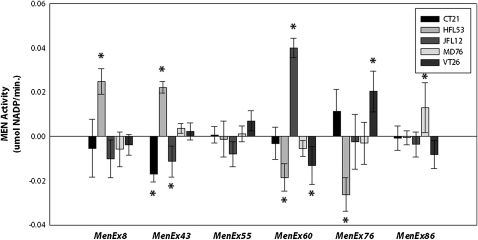
Overall, examination of the 19 excision alleles heterozygous with the VT26 and HFL53 third chromosomes indicated that different excision alleles differentially impact trans-interactions and suggested a general grouping of the alleles into sets with similar impact on trans-interactions. However, a linear regression between the VT26 (Figure S3) and the HFL53 (Figure S4) heterozygote activities suggested that some excisions have a different impact in different backgrounds—a background by MenExi− allele interaction effect (Figure 6). To further examine this possibility, we selected a subset of the excision alleles and crossed these to five different third chromosome genetic backgrounds (VT83, HFL53, JFL12, CT21, and MD76) (Figure 7). We selected an allele that deviated from the correlation (MenEx8−), alleles that followed the correlation (MenEx43−, MenEx86−, and MenEx76−), and low- and high-activity alleles (MenEx55− and MenEx60−). Importantly, we found similar levels of MEN enzyme activity across the five background lines to that observed in the first two backgrounds studied, confirming the impact of individual excision alleles, and genomic regions, on the observed trans-interactions. For example, MenEx60− heterozygotes consistently had high levels of activity across all five isogenic backgrounds, while MenEx55− consistently had low levels of activity. We standardized all crosses by both excision allele and genetic background and looked for outliers to determine if any excision allele by genetic background effects deviated from that average (Figure 8). Genetic backgrounds (third chromosomes) differ from each other in MEN activity, consistent with previous studies of this locus (Merritt et al. 2005, 2009). We therefore standardized all crosses to a given third chromosome to the average activity of that chromosome (Figure S8). Similarly, all crosses to a specific MenExi− allele were standardized to the average activity of that excision allele. By standardizing for both background and excision alleles, if no interactions are present, then the individual crosses will not significantly differ from zero. With the exception of MenEx55−, we found very specific interactions between genetic backgrounds and specific genetic lesions, indicating that genetic backgrounds play a substantial role in transvection with their ability to complement each lesion independently. The lack of interaction with Me0nEx55− is not surprising; this is the largest deletion in our set and the only deletion that does not show evidence for trans-interactions (up-regulation). Specific cases in which interactions may differ between MenExi− alleles and genetic backgrounds appear to be highly dependent on variables that affect trans-regulation, such as transcription factor expression in a particular genetic background and the presence or absence of a binding site for that transcription factor on the MenExi− allele.
Figure 6—
The correlation between MenExi−/HFL53 and MenExi−/VT26 heterozygote MEN enzyme activity. Certain alleles deviate away from the regression, suggesting that there may be a genetic background by the MenExi− allele interaction effect. Alleles tested across multiple backgrounds (Figure 7; Figure 8; Figure S8) are indicated by an open circle.
Figure 7—
Heterozygote MEN enzyme activity measured across five third isochromosomal backgrounds. Third chromosomes were CT21, HFL53, JFL12, MD76, and VT26. For each data point, n = 4, and samples were measured in triplicate. Error bars represent standard error.
Figure 8—
Heterozygote MEN enzyme activity measured across five isochromosomal backgrounds (Figure 7). Within each excision group, activities are standardized by both average excision allele and third chromosome activities. Stars indicate lines that are significantly different from the standardized average at a 0.95 threshold, i.e., significant excision alleles by genetic background interactions.
Discussion
Here we characterize interallelic trans-interactions in gene regulation at the Men locus in D. melanogaster. Our results are consistent with a specific type of trans-interaction—transvection—driving high levels of gene expression at the Men locus. Using small deletions in the 5′ region of the gene, we identified genomic regions, putative local regulatory elements, which may be involved in interallelic trans-interactions. Our approach identifies regulatory elements that are capable of acting in trans, elements that are still poorly understood, and begins to annotate the regulatory region of Men, a region largely uninvestigated to date. In addition, we determined the impact of larger-scale variation (the entire third chromosome) on these trans-interactions to determine the impact of nonlocal genetic factors on local trans-interactions at the Men locus. Although other transvection systems—e.g., the yellow gene (Morris et al. 1998, 1999, 2004; Lee and Wu 2006), Ubx (Lewis 1954; Qian et al. 1991), and the white gene (Benson and Pirrotta 1988; Pirrotta 1999)—have better-characterized regulatory regions, the strength of the Men system is the ability to accurately quantify the trans-interactions using simple assays for both gene expression and enzyme activity. Enzyme activity can be quantified with much more sensitivity and accuracy than most morphological changes. These assays, therefore, allow us to very accurately determine the role of different genomic regions on the observed trans-interactions.
While transvection is often studied through the complementation between two mutant alleles (e.g., Lewis 1954; Geyer et al. 1990; Morris et al. 1998, 2004; Lee and Wu 2006), we used interaction between mutant and wild-type alleles to identify elements that modify trans-interactions. We identified these putative regulatory regions by creating a matched suite of synthetic Men null activity alleles (denoted MenExi−) and by quantifying differences in trans-regulation (up-regulation of Men expression and MEN activity) across these lines (Table 1). By placing the knockout alleles in a heterozygous condition with wild-type third chromosomes, we found that different MenExi− alleles resulted in significantly different amounts of MEN activity (Figure 2). Because genetic background is controlled for, these differences can be directly attributed to differences at the excision sites. Heterozygotes of all but one excision allele have significantly higher activity than the expected 50% wild-type activity; this up-regulation is attributed to trans-interactions (Merritt et al. 2005). The significant differences in the amount of up-regulation between alleles appears to be a function of the different genomic elements present or absent in different deletions—i.e., the size, location of the lesions, or a combination of both. Our results could also be explained by changes in the gene topology of the functional allele driven by the excision allele (Morris et al. 1999). In this model, potential negative regulators are “looped-out” of the functional allele, causing the observed up-regulation. Although this type of interaction has been observed in other transvection systems (Morris et al. 1998, 1999), given that no potential negative regulators were identified in the region of this gene and the variation seen between interactions of the different excision alleles, regulatory elements acting in trans appears to be a simpler and more likely explanation of our observations.
The up-regulation that we observe at the Men locus appears to be a function of trans-interactions between the functional and nonfunctional alleles and is consistent with a specific type of trans-interaction—transvection (Figure 9). Our results, and those of other labs and systems, support this model. Often the definitive case for transvection is the disruption of trans-interaction by chromosomal rearrangements. In our case, we have not created rearrangements, but we do show that a large deletion (MenEx55−) (Figure 2) abolishes the observed up-regulation in a similar way as we speculate rearrangements would, supporting our claim that the observed phenomena is transvection. This effect of the large deletion is not, however, definitive proof of transvection as the deletion removes regulatory elements, as well as potentially disrupting pairing, and our results are therefore consistent with forms of trans-interaction other than transvection. Alteration of the observed effects by chromosomal rearrangements would be strong evidence for transvection and is the subject of ongoing research in the Merritt laboratory. Specific genomic regions have been shown to modulate transvection at other loci. At the yellow locus, for example, specific wing and body enhancer elements are capable of acting in trans to drive gene expression (Morris et al. 1998, 1999, 2004). These examples are dependent on the alleles being cis-promoter deficient, and our genomic analysis suggests that the MenExi− alleles are similarly promoter deficient (Figure 4). We hypothesize that the regulatory elements of the nonfunctional alleles act in trans to up-regulate the activity of the functional allele. The differences in MenExi− heterozygote activity, then, result from the differences between the deletions: different size and location of deletions remove different regulatory elements that are capable of interacting in trans to modulate transcription on the promoter-competent allele. The difference in MEN activity and deletion size between MenEx55− and MenEx60− heterozygotes strongly supports this model. As homozygotes, both alleles have no MEN activity (the case for all the knockout alleles used). As heterozygotes, however, the two alleles have very different activities. The MenEx60− allele heterozygotes have essentially wild-type levels of MEN activity, while the MenEx55− alleles have essentially 50% wild-type activity (no up-regulation). The MenEx55− allele has an ∼16-kb deletion, the largest in this study, while the MenEx60− allele has one of the smallest deletions in this study. The lack of trans-interaction effects in MenEx55− heterozygotes is consistent with removal of all the genomic elements required for trans-interactions. Presumably, the MenEx55− deletion has removed all, or nearly all, regulatory elements capable of acting in trans. The pronounced up-regulation of the MenEx60− allele is consistent with this allele retaining a large number of elements capable of acting in trans to modify the activity of the intact allele.
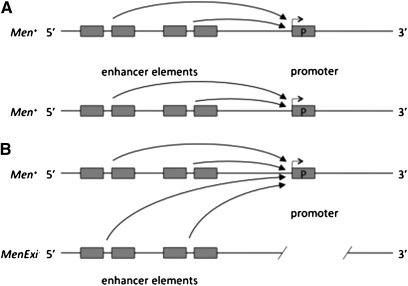
Figure 9—
(A) Proper gene expression occurs in wild-type flies in which regulatory elements drive the expression on the cis-promoter for each individual allele. (B) In our current model of transvection, MenExi− alleles lack a promoter. When MenExi− alleles are paired with a wild-type allele, enhancer elements of the null activity allele are capable of acting in trans and driving gene expression on the functional promoter of the wild-type allele.
Given the difference in deletion size and activity between MenEx60− and MenEx55− heterozygotes, a reasonable and simple model to explain the differences in trans-interaction between the deletion alleles is that the size of the deletion influences the amount of activity. In general, we found an overall trend in which the largest deletion shows no up-regulation, midsize deletions have moderate levels of up-regulation, and small deletions have high levels of up-regulation. The correlation between size and activity, however, is low (Figure S6), although this may be due to the relatively large number of the midsize deletions with little variation in transvection levels between them. The lack of an exact fit in the midsize deletions does suggest that the presence or absence of specific genomic regions—putative regulatory elements—is driving the trans-interactions observed. Phylogenetic footprinting, in combination with the Tukey’s HSD-grouped MenExi− allele sets and their specific deletions (Figure 4), identified three potential genomic regions of interest that are conserved across 10 Drosophila species (identified as i–iii in Figure 4). These three regions differentiate between the deletions of the MenExi− bins (discussed further below), and the high degree of evolutionary conservation in these regions suggests that they may be of functional importance. Furthermore, motif prediction (Cartharius et al. 2005) identified potential elements within these regions, namely TFBS and their associated transcription factors that may interact in trans (Table S1).
In at least one case, deletion of a putative element appears to increase up-regulation at this locus. We predicted that larger deletions would decrease the possibilities of trans-interactions between the nonfunctional and the wild-type allele by decreasing available binding sites for regulatory proteins capable of trans-interactions, leading to lower activity in heterozygotes (i.e., larger deletions would result in less transvection and lower activity as heterozygotes). Counter to this expectation, MenEx60−, with a 252-bp larger deletion region than MenEx58−, has more Men expression and enzyme activity as heterozygotes. Motif prediction (Cartharius et al. 2005) identifies potential motif ten element (MTE) ∼290 bases downstream of the predicted Men TSS. MTEs are core promoter elements generally found within 20–30 bases of the TSS (Lim et al. 2004). MenEx60− and MenEx58− differ by only this region (Figure 5). MenEx60−, which lacks the MTE sequence, shows significantly greater MEN enzyme activity than MenEx58− (Figure 2); i.e., trans-interactions between the knockout and functional allele are increased when this region is removed. Lee and Wu (2006) demonstrated that regulatory elements have a greater preference for cis- than for trans-promoter interactions. Although we predict that all of the MenExi− alleles are promoter deficient, core cis-promoter sequences that still exist may have an affinity to some regulatory elements; such affinity could restrict their ability to interact in trans. The deletion of such an element would then lead to an increase in trans-interaction, as seen in the comparison of MenEx60− and MenEx58−. MTEs have previously been shown to function across tens, not hundreds, of base pairs, and this predicted element is almost 300 bp from the TSS. This distance could suggest that the sequence is not truly a MTE or could suggest a novel, longer distance function for MTEs. Only experimental testing will resolve this uncertainty. This is in fact true of all of our in silico results; they present interesting possibilities that all require experimental testing for verification.
Transvection at many loci, including w, decapentaplegic, and Ubx, is dependent on zeste expression (reviewed by Duncan 2002). At the Men locus, we found a predicted zeste-binding site with high matrix identity within a region of high conservation near the Men TSS (Figure 5 and Table S1). Zeste proteins may facilitate trans-interactions by binding specific DNA sites and forming multimers, bringing trans and long-distance cis sequences into close proximity (Benson and Pirrotta 1988; Bickel and Pirrotta 1990). Therefore, loci located near zeste-binding sites may be susceptible to zeste-dependent transvection. At the Men locus, only a single zeste-binding site was predicted within the region of phylogenetic footprinting. However, all MenExi− deletions remove the predicted zeste-binding site, making it unlikely that transvection at the Men locus is zeste dependent (given that all but one of the alleles show the up-regulation that we are attributing to transvection). We cannot rule out, however, a model of hemizygous zeste-dependent transvection in which a single binding site on the functional allele allows transvection.
The transcription factor-binding sites that we identified in regulatory regions that differentiate between low and high trans-interaction MenExi− alleles are also required for proper gene expression at other loci that show transvection effects. We found putative binding sites for knirps, hunchback, and tailless, three transcription factors that are also required for proper expression of the Drosophila homeotic gene Ultrabithorax (White and Lehmann 1986; Irish et al. 1989; Qian et al. 1991). As mentioned, transvection has been previously documented at the Ubx locus. Given that these TFBS are required for gene expression at another loci that also exhibits transvection effects, aside from proper gene regulation, these transcription factors may have a role in transvection. Interestingly, and indicative of how widespread transvection effects may be, transvection has also been documented at the knirps locus (Lunde et al. 1998), showing that regulation of an element that appears to play a role in modulating transvection may itself be a function of transvection.
Previous results (T. Merritt, unpublished), as well as the variation in results between VT26/MenExi− and HFL53/MenExi− heterozygotes (Figure 6), suggested that genetic background can modify the effects of specific individual MenExi− alleles; i.e., genomic background and specific deletions may interact to determine the magnitude of trans-interactions. We examined this possibility by creating heterozygotes using a subset of the MenExi− alleles and five different third chromosome genetic backgrounds. Strikingly, certain MenExi− and genetic background combinations exhibit significantly different interactions (Figure 8), supporting the model of trans-interaction (transvection) being a product of the interaction of local and nonlocal factors. These interactions might be a result of nonlocal genetic factors (such as the expression of individual transcription factors required for the proper expression of Men), the capability of certain MenExi− alleles to pair with specific genetic backgrounds, or a combination of both. For example, the smaller deletion alleles, MenEx60− (646bp) and MenEx76− (669bp), show significantly less MEN activity with HFL53 than the group averages. However, two larger deletion alleles, MenEx8− and MenEx43− (1.65 and 2.68 kb, respectively), show significantly greater MEN activity with HFL53 than the group averages. The smaller deletions may have a factor that interacts significantly with HFL53 to repress trans-interaction at the Men locus. Consistent with the above result (Figure 5), the removal of this region could allow for greater transvection. We expected that MenEx86−, having a deletion size between MenEx8− and MenEx43−, would also follow this pattern and show significantly greater interaction with HFL53. However, MenEx86− does not show any significant interaction with HFL53, suggesting that the interactions are more complex than our simple model. MenEx55−, one the largest deletions, did not show any significant differences in activity across the five tested genetic backgrounds, supporting our previous hypothesis that the deletion has removed nearly all regulatory elements capable of trans-interaction. Possible future research then would be to examine these interactions with more genetic backgrounds and MenExi− alleles.
Given the widespread occurrence of transvection and other forms of trans-interaction effects across many species, an understanding of these phenomena and their mechanisms is fundamental to a complete understanding of gene regulation. Extensive homologous pairing that likely mediates or facilitates many of these trans-interactions is found across Diptera, and in Drosophila specifically, making this a rich system for the study of these trans-effects. Although other species do not necessarily undergo such extensive pairing, similar pairing-related phenomena have been well documented in non-dipterans (reviewed in Wu and Morris 1999).
Examining the Men genomic region using bioinformatic tools is a “first-pass” analysis; very little is known about regulation of this gene in this species. While this is only an initial study of regulatory elements, we are able to make interesting predictions. A strength in this gene system is our ability to quantify both gene expression and enzyme activity with high accuracy, a feature unique among systems used to study transvection. The ultimate strength of this system will be in comparing genomic regions of other genes that do, and do not, show the trans-effects that we document here. Here we have shown large trans-interaction effects at the Men locus. In some cases, the interactions between functional and nonfunctional alleles produce gene expression and enzyme activity as high as the expression of two functional alleles. Similar deletion heterozygotes in other NADPH enzymes (e.g., Idh and G6pd) have been previously examined, and limited or no evidence of transvection has been found in these other cases (Merritt et al. 2009), suggesting that these interactions are not a function of deletion/wild-type heterozygosity and likely involve more complex interactions or genomic requirements. The Men locus, for example, has a relatively large 5′ untranscribed region with many potential regions for trans-regulation. It is possible that this region contains a large number of regulatory elements or is packaged, or not packaged, in such a way to facilitate these trans-interactions. Genomic comparisons of loci that do, and do not, show such pronounced trans-interactions are ongoing and are expected to shed more light on this type of gene regulation. Further differentiating between regions and regulatory factors that are capable of interacting in trans will also shed light on specificities of trans-interactions as well as why some loci are capable of, or susceptible to, trans-interactions while others are not. In addition, we have shown that local trans-interactions can be influenced by nonlocal genetic background factors, although it is still unclear what specific factors are involved. Future research elucidating specific factors capable or involved in trans-interactions between two alleles will be important in understanding and further exploring the mechanisms of this pairing phenomenon.
Acknowledgments
The authors thank Joe Lachance, Luciano Matzkin, Eric Gauthier, Amadeo Parissenti, and two anonymous reviewers for constructive comments on earlier versions of this manuscript and Rahul Satija for assistance with the BigFoot software. We thank the Bloomington and Szeged stock centers for stocks. This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery (3414-07) and Research Tools and Instruments (346023-07) grants, a Canadian Foundation for Innovation Leaders Opportunity Fund grant (16729), a Canada Research Chair (950-215763) to T.J.S.M., and a NSERC Undergraduate Student Research Award to T.E.L.
Literature Cited
- Babu P., Selvakumar K. S., Bhosekar S., 1987. Studies on transvection at the bithorax complex in Drosophila melanogaster. Mol. Gen. Genet. 210: 557–563 [DOI] [PubMed] [Google Scholar]
- Bacher C. P., Guggiari M., Brors B., Augui S., Clerc P., et al. , 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell Biol. 8: 293–299 [DOI] [PubMed] [Google Scholar]
- Benson M., Pirrotta V., 1988. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J. 7: 3907–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S., Pirrotta V., 1990. Self-association of the Drosophila zeste protein is responsible for transvection effects. EMBO J. 9: 2959–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette M., Schwikowski B., Tompa M., 2002. Algorithms for phylogenetic footprinting. J. Comput. Biol. 9: 211–223 [DOI] [PubMed] [Google Scholar]
- Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., et al. , 2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21: 2933–2942 [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Stam M., 2004. Chromatin conversations: mechanisms and implications of paramutation. Nat. Rev. Genet. 5: 532–544 [DOI] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A., 1988. Insertional mutagenesis of the Drosophila genome with single P elements. Science 239: 1121–1128 [DOI] [PubMed] [Google Scholar]
- Coulthard A. B., Nolan N., Bell J. B., Hilliker A. J., 2005. Transvection at the vestigial locus of Drosophila melanogaster. Genetics 170: 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison D., Chapman C. H., Wedeen C., Bingham P. M., 1985. Genetic and physical studies of a portion of the white locus participating in transcriptional regulation and in synapsis-dependent interactions in Drosophila adult tissues. Genetics 110: 479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I., 1987. The bithorax complex. Annu. Rev. Genet. 21: 285–319 [DOI] [PubMed] [Google Scholar]
- Duncan I. W., 2002. Transvection effects in Drosophila. Annu. Rev. Genet. 36: 521–556 [DOI] [PubMed] [Google Scholar]
- Duvernell D. D., Eanes W. F., 2000. Contrasting molecular population genetics of four hexokinases in Drosophila melanogaster, D. simulans and D. yakuba. Genetics 156: 1191–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. C., Marshall W. F., Dernburg A., Agard D. A., Sedat J. W., 1998. Homologous chromosome pairing in Drosophila melanogaster proceeds through multiple independent initiations. J. Cell Biol. 141: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart W. M., Wu C. T., 1982. Interactions of zeste mutations with loci exhibiting transvection effects in Drosophila melanogaster. Genetics 102: 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G., 1990. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 9: 2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart J. G., Jr, Kaufman T. C., 1995. Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics 139: 797–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohl D., Muller M., Pirrotta V., Affolter M., Schedl P., 2008. Enhancer blocking and transvection at the Drosophila apterous locus. Genetics 178: 127–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant-Downton R. T., Dickinson H. G., 2004. Plants, pairing and phenotypes: Two’s company? Trends Genet. 20: 188–195 [DOI] [PubMed] [Google Scholar]
- Henikoff S., 1997. Nuclear organization and gene expression: homologous pairing and long-range interactions. Curr. Opin. Cell Biol. 9: 388–395 [DOI] [PubMed] [Google Scholar]
- Henikoff S., Comai L., 1998. Trans-sensing effects: the ups and downs of being together. Cell 93: 329–332 [DOI] [PubMed] [Google Scholar]
- Hopmann R., Duncan D., Duncan I., 1995. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics 139: 815–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish V. F., Martinez-Arias A., Akam M., 1989. Spatial regulation of the Antennapedia and Ultrabithorax homeotic genes during Drosophila early development. EMBO J. 8: 1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeman J. M., Russell R. C., Tan M. H., Petillo D., Westphal M., et al. , 2008. Somatic pairing of chromosome 19 in renal oncocytoma is associated with deregulated EGLN2-mediated [corrected] oxygen-sensing response. PLoS Genet. 4: e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot C., Cheutin T., Cremer M., Cavalli G., Cremer T., 2007. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat. Rev. Genet. 8: 104–115 [DOI] [PubMed] [Google Scholar]
- Lee A. M., Wu C. T., 2006. Enhancer-promoter communication at the yellow gene of Drosophila melanogaster: diverse promoters participate in and regulate trans interactions. Genetics 174: 1867–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson W. M., Bonini N. M., Benzer S., 1994. Transvection at the eyes absent gene of Drosophila. Genetics 138: 1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B., 1954. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 89: 73–89 [Google Scholar]
- Lim C. Y., Santoso B., Boulay T., Dong E., Ohler U., et al. , 2004. The MTE, a new core promoter element for transcription by RNA polymerase II. Genes Dev. 18: 1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lunde K., Biehs B., Nauber U., Bier E., 1998. The knirps and knirps-related genes organize development of the second wing vein in Drosophila. Development 125: 4145–4154 [DOI] [PubMed] [Google Scholar]
- McKee B. D., 2004. Homologous pairing and chromosome dynamics in meiosis and mitosis. Biochim. Biophys. Acta 1677: 165–180 [DOI] [PubMed] [Google Scholar]
- Merritt T. J., Duvernell D., Eanes W. F., 2005. Natural and synthetic alleles provide complementary insights into the nature of selection acting on the Men polymorphism of Drosophila melanogaster. Genetics 171: 1707–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt T. J., Kuczynski C., Sezgin E., Zhu C. T., Kumagai S., et al. , 2009. Quantifying interactions within the NADP(H) enzyme network in Drosophila melanogaster. Genetics 182: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C. W., 1916. Chromosome studies on the Diptera. II. The paired association of chromosomes in the Diptera, and its significance. J. Exp. Zool. 21: 213–279 [Google Scholar]
- Morris J. R., Chen J. L., Geyer P. K., Wu C. T., 1998. Two modes of transvection: enhancer action in trans and bypass of a chromatin insulator in cis. Proc. Natl. Acad. Sci. USA 95: 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Chen J., Filandrinos S. T., Dunn R. C., Fisk R., et al. , 1999. An analysis of transvection at the yellow locus of Drosophila melanogaster. Genetics 151: 633–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Petrov D. A., Lee A. M., Wu C. T., 2004. Enhancer choice in cis and in trans in Drosophila melanogaster: role of the promoter. Genetics 167: 1739–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni T., Corcoran D. L., Rach E. A., Song S., Spana E. P., et al. , 2010. A paired-end sequencing strategy to map the complex landscape of transcription initiation. Nat. Methods 7: 521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S. A., Chang E., Lee S., So K., Wu C. T., et al. , 2009. Effects of chromosomal rearrangements on transvection at the yellow gene of Drosophila melanogaster. Genetics 183: 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., 1999. Transvection and chromosomal trans-interaction effects. Biochim. Biophys. Acta 1424: M1–M8 [DOI] [PubMed] [Google Scholar]
- Qian S., Capovilla M., Pirrotta V., 1991. The bx region enhancer, a distant cis-control element of the Drosophila Ubx gene and its regulation by hunchback and other segmentation genes. EMBO J. 10: 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P., 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA 93: 12418–12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Cline T. W., Schedl P., 1987. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics 117: 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R., Novak A., Miklos I., Lyngso R., Hein J., 2009. BigFoot: Bayesian alignment and phylogenetic footprinting with MCMC. BMC Evol. Biol. 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E., Duvernell D. D., Matzkin L. M., Duan Y., Zhu C. T., et al. , 2004. Single-locus latitudinal clines and their relationship to temperate adaptation in metabolic genes and derived alleles in Drosophila melanogaster. Genetics 168: 923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu P. K., Zickler D., Raju N. B., Ruprich-Robert G., Metzenberg R. L., 2006. SAD-2 is required for meiotic silencing by unpaired DNA and perinuclear localization of SAD-1 RNA-directed RNA polymerase. Proc. Natl. Acad. Sci. USA 103: 2243–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southworth J. W., Kennison J. A., 2002. Transvection and silencing of the Scr homeotic gene of Drosophila melanogaster. Genetics 161: 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Stern D. M., Kiss I., Roote J., Laverty T., et al. , 1995. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc. Natl. Acad. Sci. USA 92: 10824–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam M., 2009. Paramutation: a heritable change in gene expression by allelic interactions in trans. Mol. Plant 2: 578–588 [DOI] [PubMed] [Google Scholar]
- Stevens N. M., 1908. A study of the germ cells of certain diptera, with reference to the heterochromosomes and the phenomena of synapsis. J. Exp. Zool. 5: 359–374 [Google Scholar]
- Tagle D. A., Koop B. F., Goodman M., Slightom J. L., Hess D. L., et al. , 1988. Embryonic epsilon and gamma globin genes of a prosimian primate (Galago crassicaudatus): nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J. Mol. Biol. 203: 439–455 [DOI] [PubMed] [Google Scholar]
- Thatcher K. N., Peddada S., Yasui D. H., Lasalle J. M., 2005. Homologous pairing of 15q11-13 imprinted domains in brain is developmentally regulated but deficient in Rett and autism samples. Hum. Mol. Genet. 14: 785–797 [DOI] [PubMed] [Google Scholar]
- Tsubota S., Schedl P., 1986. Hybrid dysgenesis-induced revertants of insertions at the 5' end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics 114: 165–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas M., Ravindran C., Kasbekar D. P., 2006. Chromosome segment duplications in Neurospora crassa and their effects on repeat-induced point mutation and meiotic silencing by unpaired DNA. Genetics 172: 1511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Lehmann R., 1986. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell 47: 311–321 [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Ball E. G., 1964. Malic enzyme and lipogenesis. Proc. Natl. Acad. Sci. USA 52: 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. T., Morris J. R., 1999. Transvection and other homology effects. Curr. Opin. Genet. Dev. 9: 237–246 [DOI] [PubMed] [Google Scholar]
- Xu M., Cook P. R., 2008. The role of specialized transcription factories in chromosome pairing. Biochim. Biophys. Acta 1783: 2155–2160 [DOI] [PubMed] [Google Scholar]
- Xu N., Tsai C. L., Lee J. T., 2006. Transient homologous chromosome pairing marks the onset of X inactivation. Science 311: 1149–1152 [DOI] [PubMed] [Google Scholar]