Abstract
The purpose of this chapter is to discuss the role of the fragile X mental retardation protein (FMRP) in the spinal sensory system and the potential for use of the mouse model of fragile X syndrome to better understand some aspects of the human syndrome as well as advance knowledge in other areas of investigation, such as pain amplification, an important aspect of clinical pain disorders. We describe how the Fmr1 knockout mouse can be used to better understand the role of Fmrp in axons using cultures of sensory neurons and using manipulations to these neurons in vivo. We also discuss the established evidence for a role of Fmrp in nociceptive sensitization and how this evidence relates to an emerging role of translation control as a key process in pain amplification. Finally, we explore opportunities centered on the Fmr1 KO mouse for gaining further insight into the role of translation control in pain amplification and how this model may be used to identify novel therapeutic targets. We conclude that the study of the spinal sensory system in the Fmr1 KO mouse presents several unique prospects for gaining better insight into the human disorder and other clinical issues, such as chronic pain disorders, that affect millions of people worldwide.
4.1 Why Study Fmrp in the Spinal Sensory System?
4.1.1 Links to Fragile X Syndrome in Humans
Silencing of the fragile X mental retardation gene (FMR1) causes fragile X syndrome. This gene encodes a protein, fragile X mental retardation protein (FMRP), which plays a multifunctional role in protein synthesis and neuronal development (Bagni and Greenough 2005). FMRP binds to mRNAs and is involved in transporting them to distal sites in cells while repressing their translation. In neurons, upon intense synaptic stimulation, Fmrp is thought to dissociate from its target mRNA, leading to a derepression of translation (Bassell and Warren 2008). Synaptic synthesis of new proteins plays a key role in synaptic plasticity initiation and maintenance and all evidence indicate that Fmrp plays a crucial role in this process (Bassell and Warren 2008). Two forms of synaptic plasticity are altered in several brain regions in a mouse model of fragile X syndrome (Fmr1 knockout mouse): long-term depression (LTD) is enhanced (Bear et al. 2004) and long-term potentiation (LTP) is absent in some, but not all, brain regions (Li et al. 2002; Larson et al. 2005; Wilson and Cox 2007; Hu et al. 2008). A mouse model of fragile X syndrome was created in 1994 (Consorthium 1994) and the long-standing existence of this mouse, coupled with interest in the role of translation regulation in synaptic plasticity (Kelleher et al. 2004) and the high prevalence of fragile X syndrome (Turner et al. 1996) has led to an extraordinarily in-depth understanding of the role Fmrp plays in synaptic plasticity that continues to develop into new areas of discovery and possible therapeutic intervention.
While the primary focus of research into the role of Fmrp in neuronal plasticity is aimed at understanding this from the perspective of developing therapeutics around the developmental intellectual disability (Bear et al. 2004), there is good evidence from humans that the disorder includes pathology of the sensory spinal system. This is implied by the prominence of self-injurious behavior (SIB), especially among males affected by fragile X syndrome (Symons et al. 2010). Despite the prevalence of SIB in many genetic developmental disorders associated with severe intellectual impairment, very little is known about the neurobiological underpinnings of this comorbidity. SIB occurs in different sectors of the normal population, but its frequency is much higher among individuals with developmental disorders, including fragile X syndrome (Symons et al. 2003, 2010), that negatively influence brain function. The reasons for this are unclear; however, several recent advances in preclinical models of such disorders (including fragile X syndrome and Rett syndrome) have led to a greater understanding of how mutations in genes that cause these diseases lead to changes in the structure and function of the central nervous system (CNS). At the same time, a greater appreciation of plasticity in the CNS as it pertains to chronic pain conditions has led to the recognition that molecular mechanisms of learning and memory and pain amplification are remarkably similar (Ji et al. 2003). We undertook a study using the preclinical model of fragile X syndrome in an effort to ascertain whether loss of Fmrp led to deficits in sensitization of pain pathways (Price et al. 2007). This study, which will be discussed at length below, concluded that Fmr1 knockout (KO) mice have profound and specific deficits in nociceptive sensitization. Based on this evidence, we speculated that the persistence of SIB in humans with fragile X syndrome may be linked to a failure of the nociceptive system to amplify incoming pain signals, leading to the absence of a neurobiological stop signal for SIB. This hypothesis requires further testing and is unlikely to explain the emergence of SIB but does provide a testable neurological basis for the persistence of SIB in fragile X syndrome and other developmental intellectual disorders.
Further evidence of a pathology in the spinal sensory system in fragile X syndrome comes from emerging evidence of deficits in pain sensation in humans with the disorder (Symons et al. 2010). This study suggests, based on parental reports, that children with fragile X syndrome have higher pain thresholds than other children unaffected by the disorder. Unfortunately, to date, no studies have assessed this directly with quantitative sensory testing [as has been done in Rett syndrome (Downs et al. 2010)]. Based on studies in the preclinical model (discussed below), we would not anticipate changes in acute pain thresholds in fragile X syndrome; however, pain amplification would be expected to be impaired. This is a fundamentally more difficult problem to address in human studies because it requires some intervention to induce sensitization, a manipulation that may be viewed as unethical in humans with fragile X syndrome. However, the fragile X premutation tremor/ataxia syndrome does provide some interesting potential insight into the hypothesis that pain amplification should be decreased in humans with fragile X syndrome. The fragile X premutation tremor/ataxia syndrome, unlike the full mutation that leads to fragile X syndrome (Hagerman and Hagerman 2002), does not repress FMRP expression but, rather, leads to an increase in FMRP mRNA expression (Hessl et al. 2005). Based on the hypothesis stated above, an increase in pain amplification might be expected in the premutation based on the increase, as opposed to loss, in FMRP expression. Interestingly, humans with the fragile X premutation tremor/ataxia syndrome frequently develop peripheral neuropathies, which have a high frequency of associated pain (Berry-Kravis et al. 2007; Brega et al. 2009). Moreover, the incidence of the functional pain disorder, fibromyalgia, is significantly increased in patients with fragile X premutation tremor/ataxia syndrome (Coffey et al. 2008). Hence, taken together, the preclinical and clinical evidence strongly suggest a major role for the Fmr1 gene in pain amplification. We believe that this clinical evidence provides a strong rationale to further understand the role of Fmrp in the spinal sensory system using the Fmr1 KO mouse.
4.1.2 Role of Fmrp in Axons
While the majority of work in CNS structures has focused on the role of Fmrp in dendrites (Bassell and Warren 2008), there is emerging evidence that Fmrp is found in axons as well and may play a functional role during development or even in synaptic plasticity (Akins et al. 2009; Antar et al. 2006; Centonze et al. 2008). Sensory neurons of the dorsal root ganglion (DRG) and trigeminal ganglion (TG) are the initial gateway of the pain, proprioceptive, and tactile sensation pathways. These neurons, unlike CNS neurons, are pseudo-unipolar and are made up of a single axon that emerges from the soma and bifurcates at close distance from the cell body, sending an axonal extension both to the periphery and into the spinal cord. These axons are longer than most CNS axons, with the possible exception of corticospinal and spinal motor neurons. Moreover, removing these neurons from adult rodents and developing primary cultures of these cells are a relatively straightforward process (see Malin et al. 2007 for detailed methods). Extensions from these neurons in vitro maintain axonal properties even after many days or weeks in vitro and these neurons maintain a phenotype consistent with their in vivo properties as well (Price et al. 2005; Malin et al. 2007). For manipulations in vivo, axons of these neurons are relatively accessible. Manipulations can be made to the sciatic nerve with a straightforward surgery that is selective for DRG and motor neurons (Decosterd and Woolf 2000) and the spinal process of the DRG (which contains no motor component) is also accessible for manipulation (Kim and Chung 1992). Lesions to these nerves are often used to assess neuropathic pain, which is caused by injury to peripheral sensory neurons, but such manipulations can also be made to assess a response to axonal injury (with caveats to include difference in regrowth capacity of CNS vs. peripheral neurons). We have utilized a model of injury to the sciatic nerve, to assess the role of Fmrp in neuropathic pain using the mouse model of fragile X syndrome.
As mentioned above, DRG and TG neurons can be used to generate primary cultures for in vitro studies. These neurons, if cultured from adult animals, can be grown in the presence or absence of growth factors (such as nerve growth factor) and they can survive for days to weeks in vitro. These neurons do not extend neurites with dendritic properties but, rather, extend neurites with axonal properties, affording the ability to study axons in isolation. The proliferation of new techniques to study selectively different compartments of these neurons at distance from the soma [e.g., microfluidic devices (Taylor et al. 2005; Park et al. 2006)] also provides a unique opportunity to study the role of Fmrp in axons when using these neurons from Fmr1 KO mice. Hence, these neurons may provide a readily accessible model for studying the role of Fmrp in the axonal compartment that may be relevant to gaining better insight into pathologies related to the human disorder.
4.1.3 Translation Control of Nociceptive Plasticity
A growing body of evidence indicates that translation control plays an important role in sensitization of the pain pathway (Price and Geranton 2009), both in sensory neurons of the DRG and in second-order neurons of the spinal dorsal horn. In fact, some of the first evidence for a direct role of translation control in pain amplification came from studies done in Fmr1 KO mice (Price et al. 2007). Because Fmrp is involved in transporting mRNAs to distal sites in neurons and in releasing these mRNAs for translation upon neuronal stimulation (Bassell and Warren 2008), as this area of research continues to blossom, the Fmr1 KO mouse may play an important role in identifying novel therapeutic targets. These opportunities and the existing evidence for a role of Fmrp in the spinal sensory pathway will be discussed at length below.
4.2 Evidence for a Role of Fmrp in Pain Pathology
4.2.1 Sensory Neurons and Their Axons
In the CNS, Fmrp localizes primarily to the soma and the dendritic compartment, leading to the view that Fmrp was segregated from the axon, at least in the adult. While several studies have now indicated that Fmrp localizes to the axonal compartment in the adult CNS (Akins et al. 2009; Christie et al. 2009), DRG and TG neurons lack dendritic arbors but robustly express Fmrp in the soma, and Fmrp immunoreactivity is also observed in the axons of these neurons (Price et al. 2006). Most, if not all, DRG and TG neurons express Fmrp and, in the peripheral branch of these pseudo-unipolar neurons, Fmrp immunoreactivity localizes to most axons. Interestingly, we noted that in the centrally projecting branch of DRG neurons, there appears to be less Fmrp expression and many axons do not contain Fmrp immunoreactivity (Price et al. 2006). Finally, at the central terminal of these neurons, located throughout the dorsal horn of the spinal cord, we did not observe any Fmrp immunoreactivity. Somewhat remarkably, Fmrp is not the only protein involved in translation control that shows this distribution as mammalian target of rapamycin (mTOR) immunoreactivity also is excluded from the central terminals of DRG neurons in the spinal dorsal horn (Geranton et al. 2009). This is in stark contrast to sensory neuropeptides and channels (e.g., TRPV1) that are robustly expressed at central terminals of sensory neurons. Hence, it is possible that translation machinery is excluded from the projection of DRG neurons as they traverse the dorsal root entry zone. The reasons and mechanisms of this apparent exclusion are not currently known.
What is the purpose of Fmrp in the axonal extensions of DRG and TG neurons? The traditional view of the axon is that translation does not occur in this compartment, but studies over the past decade have made it clear that the axons of these neurons contain mRNAs (Mohr and Richter 2000; Aronov et al. 2001; Tohda et al. 2001; Willis et al. 2005), a variety of RNA transport proteins (e.g., Fmrp and staufen) (Bassell et al. 1998; Hirokawa and Takemura 2005; Antar et al. 2006; Price et al. 2006; Li et al. 2009), ribosomal proteins (Koenig 1979; Twiss et al. 2000), golgi components (Merianda et al. 2009), and functional RNA interference (Murashov et al. 2007) and that protein synthesis does, indeed, occur in this compartment (Brittis et al. 2002; Martin 2004; Willis and Twiss 2006; Lin and Holt 2008; Melemedjian et al. 2010). Most of the work in this area has focused on axonal regeneration and growth cone guidance and collapse (Brittis et al. 2002; Martin 2004; Willis and Twiss 2006; Lin and Holt 2008); however, we have recently demonstrated that growth factors (such as NGF) and cytokines (such as interleukin-6, IL-6) are capable of stimulating protein synthesis in the axon of DRG and TG neurons and this process is directly linked to the development of nociceptive sensitization by these endogenous pain mediators (Melemedjian et al. 2010). We presume that Fmrp may play an important role in NGF- and IL-6-mediated translation and, in support of this, we have recently shown that IL-6-induced sensitization is strongly blunted in FMRP KO mice (Asiedu et al. 2011).
4.2.2 Fmrp in the Spinal Dorsal Horn
In addition to Fmrp expression in DRG and TG neurons, FMRP is richly expressed in the spinal cord of mice (Price et al. 2006). Like many other CNS regions, FMRP expression in the spinal cord is isolated to neurons and our previous findings suggest that all spinal cord neurons express Fmrp. Fmrp localization to the dendrites of spinal cord neurons has not been assessed and, in the Fmr1 KO, it is not known if dendritic spines of spinal neurons show abnormal morphology as they do in many other CNS regions (Comery et al. 1997).
4.2.3 Behavioral Pain Phenotype of Fmr1 Knockout Mice
4.2.3.1 Deficits in Nociceptive Sensitization Linked to Peripheral Stimulation
Sensitization of peripheral nociceptors is a primary mechanism of pain amplification. This process involves local signaling within the peripheral terminal of the nociceptor. One of the best-studied forms of peripheral sensitization is thermal hyperalgesia. Research on thermal hyperalgesia was greatly enhanced by the discovery of the noxious heat and capsaicin receptor TRPV1 in the late 1990s (Caterina et al. 1997). TRPV1 is expressed on peripheral terminals of nociceptors and its activity is enhanced by a number of kinase signaling cascades, such as protein kinases A and C, through direct phosphorylation of the receptor (Caterina et al. 1997, 2000; Tominaga et al. 1998; Caterina and Julius 2001). TRPV1 phosphorylation leads to a leftward shift in the temperature response curve of the channel such that it becomes more sensitive to temperature, leading to a drop in threshold for activation of the receptor. Interestingly, recent evidence suggests that this process is mediated by an endogenous agonist and not temperature itself (Patwardhan et al. 2009, 2010). Hence, in many cases, thermal hyperalgesia can be explained simply as a signaling cascade that occurs locally to change the activation threshold of nociceptive sensory neurons (Tominaga et al. 1998).
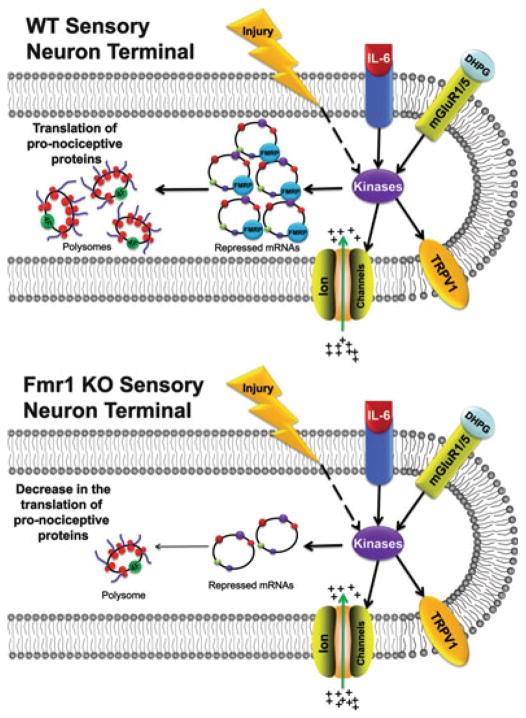
As mentioned above, we did not observe any deficits in normal mechanical or thermal thresholds in Fmr1 KO mice. On the contrary, several deficits in nociceptive sensitization were found, some of which were linked to peripheral sensitization, while others gave clear indications of CNS deficits (Price et al. 2007). We were able to separate the contributions of peripheral and central sensitization based largely on the administration of a group I metabotropic glutamate receptor (mGluR1/5) agonist. These experiments were facilitated by two lines of evidence: experiments leading to the development of the mGluR theory of fragile X syndrome (Bear et al. 2004) and experiments demonstrating a clear role of mGluR1/5 in nociceptive sensitization (Karim et al. 2001; Adwanikar et al. 2004; Hu et al. 2007). In terms of peripheral sensitization, previous experiments had demonstrated that mGluR1/5 are expressed by DRG neurons and that these receptors localize to the peripheral terminals of these neurons (Bhave et al. 2001). Stimulation of mGluR1/5 with the specific agonist DHPG leads to the development of thermal hyperalgesia in normal animals (Bhave et al. 2001), an event which has subsequently been linked, on the molecular level, to sensitization of the noxious heat and capsaicin receptor TRPV1 (Kim et al. 2009). We found that while thermal hyperalgesia was present in wild-type mice in response to intradermal DHPG administration, it failed to develop in Fmr1 KO mice (Price et al. 2007). Because thermal hyperalgesia in response to local injection of DHPG likely occurs through local sensitization of TRPV1, effectively dropping the thermal threshold of this subset of nociceptors, this finding provides strong evidence of a lack of peripheral sensitization in response to mGluR1/5 stimulation in the periphery in Fmr1 KO mice (Fig. 4.1).
Fig. 4.1.
In the peripheral termini of WT sensory neurons, Fmrp facilitates the transport and translational repression of mRNA destined for the axon. Injury, cytokines such as IL-6, and the mGluR1/5 agonist DHPG activate various kinases that increase the excitability of sensory neurons by modulating the activity of TRPV1 and other ion channels. Moreover, activated kinases can induce the initiation of translation [via increased eIF4F complex formation (4 F)], leading to the local synthesis of pronociceptive proteins that enhance and maintain nociceptive sensitization of the primary afferents. In contrast, absence of Fmrp results in the dysregulation of mRNA trafficking and translational repression. Hence, nociceptive inputs that induce prolonged sensitization of the primary afferents may not efficiently induce the local translation of pronociceptive proteins. This results in abrogated responses to injury, IL-6, and DHPG in Fmr1 KO mice
4.2.3.2 Deficits in Nociceptive Sensitization Linked to Spinal Processing
Windup
When a noxious stimulus, such as biting one’s hand, is applied, it causes an initial stinging or sharp pain with a short latency (called “first pain”) and is followed by a more persistent burning-type pain, which commonly possesses a burning quality. This so-called second pain has a longer latency and is thought to be associated with windup of dorsal horn neurons (Price et al. 1977; Price 1972). Windup involves a progressive increase in action potential generation in spinal dorsal horn neurons in response to repetitive firing of peripheral afferents synapsing in the dorsal horn. This windup takes less than 1 sec to begin and can be observed in most dorsal horn neurons that receive a nociceptive input. While the pharmacology of windup is complex, its basic mechanisms involve glutamatergic neurotransmission and postsynaptic glutamate receptors of the NMDA type. Existing evidence points to progressive depolarization through NMDA channels as a primary means through which frequency-dependent amplification of dorsal horn neuron firing is augmented (Dickenson and Sullivan 1987). In addition to the increase in the output firing of dorsal horn neurons relative to the afferent input, windup can also lead to after-discharge in these neurons (continued firing despite the absence of continued input). Because windup takes place over such a short time course, it is commonly viewed as a primary mechanism for short-term plasticity in the nociceptive system (Herrero et al. 2000).
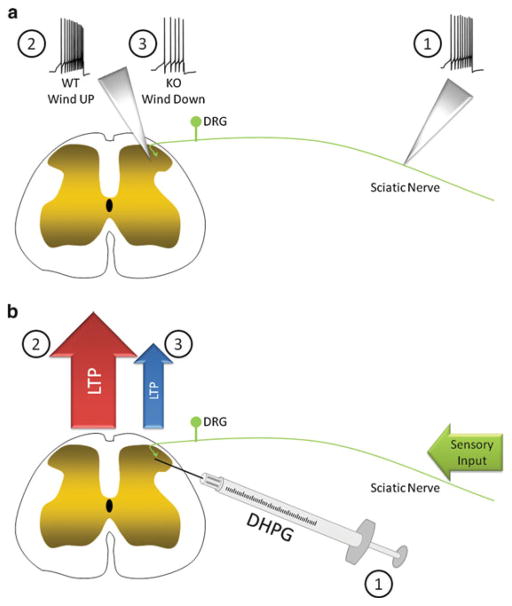
We recorded responses in ascending fibers of second-order dorsal horn neurons from Fmr1 KO mice after afferent volleys that are sufficient to induce windup in most wild-type neurons. Strikingly, windup was absent in the vast majority of fibers in FMRP KO mice and some of these fibers even demonstrated a decrease in their input–output function, which we termed winddown (Fig. 4.2a) (Price et al. 2007). The molecular mechanisms of this effect are not known, but this provides compelling evidence for a specific deficit in this form of short-term sensitization in the spinal dorsal horn of Fmr1 KO mice. This effect may be explained by abnormal synaptic connections and/or changes in NMDA receptor expression in the spinal dorsal horn, but these possibilities have not been tested. However, these hypotheses are supported by alterations in dendritic morphology in the absence of Fmrp and Fmrp-mediated control, via microRNA association, of NMDA receptor expression (Edbauer et al. 2010).
Fig. 4.2.
Spinal plasticity in Fmr1 KO mice. (a) Repetitive stimulation of peripheral nociceptors (1) induces windup (an increase in the number of spikes relative to the peripheral input) in the ascending second-order neurons of the spinal cord in WT mice (2). However, in Fmr1 KO mice, a lack of windup, and even winddown (3), was observed in response to the stimulation of peripheral nociceptors (1). (b) Intrathecal injection of mGluR1/5 agonist DHPG (1) may induce LTP in second-order neurons of the spinal cord in WT mice (2), resulting in robust nociceptive behavior. However, intrathecal injection of DHPG fails to induce nociceptive behavior in Fmr1 KO mice (3) compared to that in WT. This lack of nociceptive behavior may reflect a reduction in, or absence of, spinal LTP in Fmr1 KO mice
Long-term Potentiation
Unlike windup, LTP involves an increase in synaptic efficacy that has a longer latency to onset and can persist for days to weeks and may even be permanent. As such, most work on LTP has focused on establishing its mechanistic role in learning and memory. In fact, several lines of evidence suggest that LTP occurs during learning and memory (Whitlock et al. 2006) and inhibition of molecular maintenance mechanisms of LTP reverses established memories (Pastalkova et al. 2006; Shema et al. 2007). Moreover, LTP is impaired in preclinical models of Rett syndrome (Moretti et al. 2006) and fragile X syndrome (Zhao et al. 2005; Wilson and Cox 2007). In terms of pain signaling, LTP has recently been recognized as an important synaptic amplifier mechanism in the dorsal horn (Ikeda et al. 2006; Sandkuhler 2007). While LTP can be induced in dorsal horn neurons by artificial high-frequency stimulation of nociceptors, it can also be observed after natural stimulation that mimics persistent inflammation and/or injury to the peripheral nervous system (Ikeda et al. 2006), pointing to the physiological importance of this type of plasticity in chronic pain states. While the ability of LTP to explain the full sequelae of chronic pain symptoms is still controversial (Sandkuhler 2010; Latremoliere and Woolf 2010), it is, nevertheless, a critical amplification mechanism for pain pathways that leads to enhanced pain perception in human subjects (Lang et al. 2007; Klein et al. 2004).
We tested the effect of intrathecal (direct spinal injection) DHPG in Fmr1 KO mice. Previous studies indicated that DHPG elicits a nociceptive response when injected intrathecally through postsynaptic stimulation of mGluR1/5 receptors, leading to extracellular signaling-regulated kinase (ERK) activation (Karim et al. 2001; Adwanikar et al. 2004; Hu et al. 2007). While DHPG stimulated a robust nociceptive response in wild-type animals, this response was virtually absent in Fmr1 KO mice (Price et al. 2007). Our findings on links between mGluR1/5 and Fmrp and deficits in nociceptive sensitization in the mouse model of fragile X syndrome may seem to contradict findings in the hippocampus where mGluR1/5-dependent LTD is enhanced in the absence of Fmrp, suggesting hyperactive mGluR1/5 signaling in neurons lacking FMRP (Bear et al. 2004). However, mGluR1/5-dependent LTP is absent in the visual neocortex of Fmr1 KO mice, indicating that differences in mGluR1/5-mediated plasticity in the absence of Fmrp can differentially influence LTP and LTD (Wilson and Cox 2007). In the hippocampus, DHPG can induce LTD, however, in the spinal cord, and in the visual cortex, mGluR1/5 recruitment is required for the establishment of LTP, similarly to the visual neocortex (Wilson and Cox 2007). Although mGluR1/5 activation does induce LTD in some spinal cord neurons (Heinke and Sandkühler 2005), the clear role of mGluR1/5 in spinal LTP (Azkue et al. 2003) leads us to speculate that there may be deficits in spinal LTP in Fmr1 KO mice (Fig. 4.2b). While we speculate, based on this evidence, that spinal LTP may be absent in Fmr1 KO mice, this hypothesis has not been tested to date.
4.2.3.3 Neuropathic Pain
Neuropathic pain presents one of the greatest clinical challenges facing pain neuroscientists and clinicians today (Campbell and Meyer 2006; Woolf 2010). Neuropathic pain is largely intractable to common analgesics and the prolonged duration of the disease state makes the use of such medicines challenging due to adverse side effects (Baron 2006; Campbell and Meyer 2006). Hence, gaining a better understanding of neuropathic pain mechanisms and identifying new targets for neuropathic treatment are of great importance. Neuropathic pain is generally caused by injury to the peripheral nervous system, although it is not always the case that an injury can be directly identified. In such cases, neuropathic pain is often assigned as a diagnosis based on symptoms and effective analgesics (e.g., serotonin and norepinephrine reuptake inhibitors). Injury to the peripheral nervous system causes the generation of ectopic activity in sensory neurons, leading to consistent afferent input into the spinal dorsal horn causing continuous activation of pain pathways (Baron 2006; Campbell and Meyer 2006; Devor 2006). It is also thought that this afferent discharge induces changes in spinal circuitry (Latremoliere and Woolf 2009; Woolf 2010) and even abnormal neuroimmune interactions (Romero-Sandoval et al. 2008) in the spinal dorsal horn that drive amplification of pain pathways. These pathologies manifest as continuous ongoing pain, mechanical allodynia (in most cases), and the presence of thermal (generally cold) hypersensitivities (Baron 2006).
We hypothesized that translation control may play a key role in neuropathic pain (Price and Géranton 2009). Previous studies had indicated that injury to the peripheral nervous system in the form of a preconditioning nerve crush lesion induces alterations in mRNA localization to DRG neuron axons (Zheng et al. 2001; Willis et al. 2005). As an initial test of this hypothesis, we assessed whether Fmr1 KO mice develop neuropathic pain after injury to branches of the sciatic nerve. Strikingly, Fmr1 KO mice failed to develop neuropathic allodynia (the major measure of neuropathic pain in preclinical models) for several weeks after injury to the sciatic nerve. Moreover, even when these mice did develop a drop in mechanical thresholds, these mice failed to develop full neuropathic allodynia compared to wild-type mice (Price et al. 2007). Subsequent studies from our group have indicated that injury to the peripheral nervous system in mice and rats induces a robust increase in Fmrp localization in peripheral sensory neuron axons (Melemedjian and Price, unpublished observations). This finding, taken together with data from Fmr1 KO mice, strongly suggests a role of Fmrp in neuropathic pain.
More recent investigations have substantiated the case for translation control as a key aspect of neuropathic pain. Local inhibition of mTOR acutely reduces hyperalgesia to mechanical stimulation after injury of the peripheral nervous system (Jiménez-Díaz et al. 2008; Geranton et al. 2009). Another study has indicated that the mRNA for the voltage-gated sodium channel, NaV1.8, increases in DRG axons after injury to the peripheral nervous system (Thakor et al. 2009). This finding parallels other studies that have demonstrated that NaV1.8 protein increases within the sciatic nerve in the setting of neuropathic pain (Gold et al. 2003). Pharmacological blockade of NaV1.8 reduces neuropathic pain in preclinical models, as does knockout or knockdown of NaV1.8 with antisense technology (Lai et al. 2002; Roza et al. 2003; Jarvis et al. 2007). Hence, local synthesis of this voltage-gated sodium channel may contribute to sensory neuron hyperexcitability and ectopic activity (Devor 2006), providing a direct link between translation control and sensory neuron pathology in neuropathic pain. How Fmr1-based mouse models and Fmrp association with mRNAs may be utilized to advance our understanding of neuropathic pain will be discussed below.
4.3 Open Questions and How to Address Them
4.3.1 Dissecting the Role of Fmrp in DRG Versus Spinal Neurons (Conditional Knockouts)
Although certain pain phenotypes in the Fmr1 KO mouse model are indicative of altered peripheral (mGluR1/5 thermal hyperalgesia) or central (windup) sensitization, others are harder to categorize and will require further experimentation. One example is decreases in the nociceptive responses of the Fmr1 KO mouse in the formalin test (Price et al. 2007), a common test for assessing analgesic efficacy of novel therapeutics (Mogil 2009). The formalin test consists of two phases: the first, which lasts for 10 min, is associated with the initial nociceptor discharge in response to formalin and the second, which lasts from 20 to 45 min postformalin injection, is classically considered a test of central sensitization (Mogil 2009). Despite this commonly held view, there is clear evidence of a peripheral component to the second phase of the formalin test (Taylor et al. 1995; Puig and Sorkin 1996). It is currently not clear if decreased sensitization in the mouse model of fragile X syndrome is due to peripheral or central effects, or both, but there are several tools available for the potential solution to this and other problems.
The generation of mice harboring LoxP sites to excise the Fmr1 gene in a conditional fashion has the potential to advance fragile X syndrome research greatly (Mientjes et al. 2006). Likewise, the generation of CRE-expressing mice for conditional knockout of floxed alleles in certain populations of sensory neurons has led to major advances in our basic understanding of pain mechanisms. One such Cre recombinase-harboring mouse is the NaV1.8-Cre mouse (Nassar et al. 2004; Stirling et al. 2005). Because this voltage-gated sodium channel is only expressed in a population of nociceptors, this mouse can be used to generate mice with conditional knockout of genes only in this subset of cells. A decrease in formalin-induced pain in NaV1.8-Cre mice crossed with Fmr1-floxed mice would strongly suggest a predominate role of peripheral Fmrp in formalin-induced sensitization. Likewise, peripherin-Cre mice have been created and these mice can be used for conditional knockout of floxed alleles selectively in unmeylinated sensory neurons (Zhou et al. 2002). Similar technologies can be used to delete genes in the spinal cord conditionally but, thus far, no Cre-harboring mice have been created that generate a dorsal horn-specific knockout of floxed alleles.
While we have used the formalin pain phenotype of the Fmr1 KO mouse as an example above, these types of experiments have broader implications than simply parceling out peripheral vs. central components of the formalin test. These model systems have been particularly important for neuropathic pain research and a sensory neuron-specific deletion of Fmr1 would be useful for advancing our understanding of the contribution of sensory neuron Fmrp expression for neuropathic pain.
4.3.2 Viral Vectors to Assess Changes in Adult Animals
A common criticism of knockout mouse studies, including conditional knockouts, is the potential for developmental compensatory changes and their contribution to the presence or absence of phenotypes in such mice. While this is likely less of a concern for Fmr1 KO mice due to their link to the human disorder, one way around this problem is to allow for development to proceed normally in the presence of floxed alleles and then delete these genes in a conditional fashion in adult animals through transduction of cells with a viral vector expressing Cre (van der Neut 1997). In this regard, the spinal cord is accessible through a relatively simple procedure [intrathecal injection (Hylden and Wilcox 1980)] and several studies have shown that this is an effective route for viral transduction in adult mice (Milligan et al. 2005; Chou et al. 2005). Because selective Cre mice for dorsal horn expression have not been created to date, this may be a more effective approach for the investigator interested in deleting Fmr1 in the adult spinal cord. Somewhat surprisingly, the DRG and TG systems are also relatively accessible for viral transduction in adult animals. Certain viruses are selectively taken up by DRG and TG neurons and transduction of these ganglia can be induced by simple intradermal injection of virus at the appropriate anatomical location in adult animals (Tzabazis et al. 2007; Gu et al. 2005; Jackson et al. 2005). Hence, generating conditional deletion of Fmr1 in adult animals may prove to be particularly simple in the spinal sensory system with the use of floxed Fmr1 mice. These experiments may be important for better understanding how Fmrp contributes to pain plasticity.
4.3.3 Opportunities to Better Understand the Role of Fmrp in Axons: New Mechanisms and Targets for Pain Control
Our view is that understanding the role of Fmrp in sensory neuron axonal plasticity may play an important role in unlocking new therapeutic targets for neuropathic pain. The question is how to harness Fmrp to discover these new targets. Because Fmrp is a well-known RNA-binding protein, much research has been dedicated to identify its mRNA-binding targets (Darnell et al. 2005). Extremely stringent experimental protocols have been elucidated to identify these targets in CNS neurons (Brown et al. 2001; Darnell et al. 2001, 2009) but, thus far, these techniques have not been applied to sensory neurons and their axons. They have also not been applied to in vivo conditions that represent important preclinical models of neuropathic pain.
As mentioned above, cultured sensory neurons maintain their in vivo phenotype [although this phenotype may best represent a “neuropathic” one (Dussor et al. 2003)] in culture for days and even weeks. Certain phenotypes can also be enriched by altering the growth factors present in the culture media (Price et al. 2005; Malin et al. 2007). Hence, this model system affords the opportunity to enrich axons from these neurons (using microfluidics or other techniques) in an effort to identify Fmrp-bound mRNAs that localize to the axonal compartment. Identifying these mRNAs could provide insight into proteins that might be translated locally within the axonal compartment in an Fmrp-dependent fashion (a hypothesis that can be assessed using the Fmr1 KO mouse). The repertoire of mRNAs bound to Fmrp in the axons of sensory neurons in culture could then be compared to in vivo conditions, with or without injury to the peripheral nervous system. Because we have observed a striking neuropathic phenotype in Fmr1 KO mice (Price et al. 2007), and an increase in Fmrp in the axons of DRG neurons after peripheral nerve injury (Melemedjian and Price, unpublished observations), these studies have the potential to identify targets linked to Fmrp that may lead to the development of novel therapeutics for the treatment of neuropathic pain.
4.4 Conclusions
The sensory spinal system has received less attention than other, higher CNS structures in Fmrp research. From a fragile X syndrome therapeutic standpoint, there are good reasons for this disparity; however, we have tried to argue that studying Fmrp in the sensory spinal system is highly relevant both to a better understanding of certain aspects of the disorder and to gaining insight into other human diseases such as neuropathic pain. While work into translation control and nociceptive sensitization is just beginning, studying Fmrp is a natural gateway to understand how translation control contributes to nociceptive plasticity that may lead to the development of novel mechanism-based therapeutics for human pain disorders.
Acknowledgments
This work was supported by startup funds from The University of Arizona School of Medicine, The American Pain Society, The Rita Allen Foundation, and NIH Grant R01NS065926 to TJP. TJP is a Rita Allen Foundation Scholar in Pain.
Contributor Information
Theodore J. Price, Email: tjprice@email.arizona.edu, Department of Pharmacology, The University of Arizona School of Medicine, Tucson, AZ, USA. Bio5 Institute, The University of Arizona School of Medicine, Tucson, AZ, USA
Ohannes K. Melemedjian, Department of Pharmacology, The University of Arizona School of Medicine, Tucson, AZ, USA
References
- Adwanikar H, Karim F, Gereau RWt. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Akins MR, Berk-Rauch HE, Fallon JR. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits. 2009;3:17. doi: 10.3389/neuro.04.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32(1–2):37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Aronov S, Aranda G, Behar L, Ginzburg I. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci. 2001;21:6577–6587. doi: 10.1523/JNEUROSCI.21-17-06577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase m zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkue JJ, Liu X-G, Zimmermann M, Sandkühler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Baron R. Mechanisms of disease: neuropathic pain – a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Goetz CG, Leehey MA, et al. Neuropathic features in fragile X premutation carriers. Am J Med Genet A. 2007;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RWt. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Brega AG, Reynolds A, Bennett RE, Leehey MA, Bounds LS, Cogswell JB, Hagerman RJ, Hagerman PJ, Grigsby J. Functional status of men with the fragile X premutation, with and without the tremor/ataxia syndrome (FXTAS) Int J Geriatr Psychiatry. 2009;24:1101–1109. doi: 10.1002/gps.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, et al. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biolog Psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chou AK, Yang LC, Wu PC, Wong WT, Liu GS, Chen JT, Howng SL, Tai MH. Intrathecal gene delivery of glial cell line-derived neurotrophic factor ameliorated paraplegia in rats after spinal ischemia. Brain Res Mol Brain Res. 2005;133:198–207. doi: 10.1016/j.molbrainres.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29:1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consorthium TD-BFX. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB. FMRP RNA targets: identification and validation. Genes Brain Behav. 2005;4:341–349. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Darnell J, Fraser C, Mostovetsky O, Darnell R. Discrimination of common and unique RNA-binding activities among Fragile-X mental retardation protein paralogs. Hum Mol Genet. 2009;18(17):3164–3177. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Downs J, Geranton SM, Bebbington A, Jacoby P, Bahi-Buisson N, Ravine D, Leonard H. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am J Med Genet A. 2010;152A:1197–1205. doi: 10.1002/ajmg.a.33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor GO, Price TJ, Flores CM. Activating transcription factor 3 mRNA is upregulated in primary cultures of trigeminal ganglion neurons. Brain Res Mol Brain Res. 2003;118:156–159. doi: 10.1016/s0169-328x(03)00335-8. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, et al. Regulation of Synaptic Structure and Function by FMRP-Associated MicroRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci. 2009;29:15017–15027. doi: 10.1523/JNEUROSCI.3451-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na (V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Xu Y, Li GW, Huang LY. Remote nerve injection of mu opioid receptor adeno-associated viral vector increases antinociception of intrathecal morphine. J Pain. 2005;6:447–454. doi: 10.1016/j.jpain.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev. 2002;12:278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Heinke B, Sandkühler J. Signal transduction pathways of group I metabotropic glutamate receptor-induced long-term depression at sensory spinal synapses. Pain. 2005;118:145–154. doi: 10.1016/j.pain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- Hu H-J, Alter BJ, Carrasquillo Y, Qiu C-S, Gereau RW. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007;27:13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336:173–183. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jiménez-Díaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RWt. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park C-K, Back SK, et al. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci. 2009;29:10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hopf H-C, Sandkühler J, Treede R-D. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci. 2004;24:964–971. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig E. Ribosomal RNA in Mauthner axon: implications for a protein synthesizing machinery in the myelinated axon. Brain Res. 1979;174:95–107. doi: 10.1016/0006-8993(79)90806-0. [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Lang S, Klein T, Magerl W, Treede R-D. Modality-specific sensory changes in humans after the induction of long-term potentiation (LTP) in cutaneous nociceptive pathways. Pain. 2007;128:254–263. doi: 10.1016/j.pain.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Synaptic plasticity and central sensitization: author reply. J Pain. 2010;11:801–803. doi: 10.1016/j.jpain.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Li C, Bassell GJ, Sasaki Y. Fragile X mental retardation protein is involved in protein synthesis-dependent collapse of growth cones induced by semaphorin-3A. Front Neural Circuits. 2009;3:11. doi: 10.3389/neuro.04.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Martin KC. Local protein synthesis during axon guidance and synaptic plasticity. Curr Opin Neurobiol. 2004;14:305–310. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, et al. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Mohr E, Richter D. Axonal mRNAs: functional significance in vertebrates and invertebrates. J Neurocytol. 2000;29:783–791. doi: 10.1023/a:1010987206526. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, Katwa LC, Van Scott MR. RNAi pathway is functional in peripheral nerve axons. FASEB J. 2007;21:656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nat Protoc. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Price TJ, Geranton SM. Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci. 2009;29(12):2253–2263. doi: 10.1111/j.1460-9568.2009.06786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Price TJ, Louria MD, Candelario-Soto D, et al. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F, Hargreaves KM. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 2006;141:2107–2116. doi: 10.1016/j.neuroscience.2006.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550:921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Mol Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Central sensitization versus synaptic long-term potentiation (LTP): a critical comment. J Pain. 2010;11:798–800. doi: 10.1016/j.jpain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB., Jr Self-injurious behavior in young boys with fragile X syndrome. Am J Med Genet A. 2003;118:115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Byiers BJ, Raspa M, Bishop E, Bailey DB. Self-injurious behavior and fragile X syndrome: findings from the national fragile x survey. Am J Intellect Dev Disabil. 2010;115:473–481. doi: 10.1352/1944-7558-115.6.473. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci. 1995;15:7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, Spigelman I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- Tzabazis AZ, Pirc G, Votta-Velis E, Wilson SP, Laurito CE, Yeomans DC. Antihyperalgesic effect of a recombinant herpes virus encoding antisense for calcitonin gene-related peptide. Anesthesiology. 2007;106:1196–1203. doi: 10.1097/01.anes.0000267603.32634.03. [DOI] [PubMed] [Google Scholar]
- van der Neut R. Targeted gene disruption: applications in neurobiology. J Neurosci Methods. 1997;71:19–27. doi: 10.1016/s0165-0270(96)00123-9. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A. 2007;104:2454–2459. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2010;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/s0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]