Abstract
Bone-marrow-derived mesenchymal stem cells (MSCs) have attracted considerable attention as tools for the systemic delivery of therapeutic proteins in vivo, and the ability to efficiently transfer genes of interest into such cells would create a number of therapeutic opportunities. We have designed and tested a series of human immunodeficiency virus type 1 (HIV-1)-based vectors and vectors based on the oncogenic murine stem cell virus to deliver and express transgenes in human MSCs. These vectors were pseudotyped with either the vesicular stomatitis virus G (VSV-G) glycoprotein (GP) or the feline endogenous virus RD114 envelope GP. Transduction efficiencies and transgene expression levels in MSCs were analyzed by quantitative flow cytometry and quantitative real-time PCR. While transduction efficiencies with virus particles pseudotyped with the VSV-G GP were found to be high, RD114 pseudotypes revealed transduction efficiencies that were 1 to 2 orders of magnitude below those observed with VSV-G pseudotypes. However, chimeric RD114 GPs, with the transmembrane and extracellular domains fused to the cytoplasmic domain derived from the amphotropic Moloney murine leukemia virus 4070A GP, revealed about 15-fold higher titers relative to the unmodified RD114 GP. The transduction efficiencies in human MSCs of HIV-1-based vectors pseudotyped with the chimeric RD114 GP were similar to those obtained with HIV-1 vectors pseudotyped with the VSV-G GP. Our results also indicate that RD114 pseudotypes were less toxic than VSV-G pseudotypes in human MSC progenitor assays. Taken together, these results suggest that lentivirus pseudotypes bearing alternative Env GPs provide efficient tools for ex vivo modification of human MSCs.
Adult bone marrow contains hematopoietic stem cells, as well as nonhematopoietic stem cells, which are also termed mesenchymal stem cells (MSCs). MSCs have the potential of differentiating into cells of the mesenchymal lineage (6, 20, 50) and were previously referred to as marrow stromal cells or CFU fibroblasts (CFU-Fs), reflecting their origin and morphology in culture (9, 24). MSCs derived from bone marrow can be readily isolated and expanded in vitro. Due to their ability for self-renewal and their potential to differentiate into terminal osteocytes, chondrocytes, myocytes, tenocytes, adipocytes, and neural cells in vitro (27, 56, 57, 62), bone-marrow-derived MSCs have attracted considerable attention as potential tools for therapeutic gene transfer.
A variety of studies using different viral vector systems have attempted to transduce MSCs (18, 21, 25, 42, 72). Due to their capacity to integrate into the host genome, oncogenic retrovirus vectors have been used extensively for transgene delivery into MSCs. This has led to the successful expression of a number of proteins, such as Escherichia coli β-galactosidase (2, 21, 49) and green fluorescent protein GFP (34, 46), as well as many therapeutic proteins, including coagulation factors VIII (13, 16, 17) and IX (12, 28, 32, 38), interleukins 3 (2, 42, 54) and 7 (7), human growth hormone (32), human erythropoietin (3), arylsulfatase A (47, 48), tyrosine hydroxylase GTP cyclohydrolase I (64, 65), α-l-iduronidase (4), β-hexosaminidase A (45), and bone morphogenetic protein (29). However, gene transfer into MSCs using oncogenic retroviruses is limited overall due to a low efficiency of transduction and a general lack of long-term transgene expression, possibly caused by promoter inactivation.
Previously, we optimized human immunodeficiency virus type 1 (HIV-1)-based lentivirus vectors to deliver and express transgenes in human MSCs (73). The results obtained indicated that a single round of transduction using unconcentrated HIV-1-based vectors pseudotyped with vesicular stomatitis virus G (VSV-G) can lead to the efficient transduction of human MSCs. Stable expression of reporter genes up to at least 10 months was observed. Clonogenic stromal progenitor cells (MPCs) could also be transduced by HIV-1-based lentivirus vectors, with transgene expression being maintained by their mesenchymal progeny cells over several cell divisions and during differentiation into adipocytes, providing evidence that the capacity of differentiation of the cells was unaffected by lentivirus-mediated reporter gene transfer (73). However, lentivirus pseudotypes were found to be toxic to such progenitors at multiplicities of infection (MOIs) above 0.5, possibly due to the VSV-G envelope (Env) glycoprotein (GP).
Here we report improved transduction conditions for MSCs and MPCs involving HIV-1-based lentivirus vectors pseudotyped with native and chimeric RD114 Env GPs.
MATERIALS AND METHODS
Cell lines and human MSCs.
Human embryonic kidney 293T cells (22) and human osteosarcoma (HOS) cells (American Type Culture Collection; CRL-1543) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) with 10% heat-inactivated fetal bovine serum (FBS) (HyClone), 2.0 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Cryopreserved human marrow low-density (<1.078) cells were thawed and plated at a density of 107 cells per T150 flask for bulk cultures. For identification of individual MSC colonies derived from individual MPCs or CFU-Fs, cells were plated at a density of 2.5 × 105 cells in 60-mm-diameter dishes. MSC cultures were carried out in long-term culture medium (LTCM) as described previously (73, 74). Immunophenotyping and fluorescence-activated cell sorter (FACS) analyses were performed with a representative pooled batch of MSCs (73).
Plasmid constructs.
The NL-EGFP/CMV, NL-EGFP/CEF, and NL-EGFP/CAG lentivirus vectors were described previously (59, 61, 73). The newly designed NL-EGFP(MSCV) vector lacks an internal promoter and contains a hybrid murine stem cell virus (MSCV)/HIV 3′ long terminal repeat (LTR) in which a portion of the U3 region of the 3′ LTR of HIV-1 was replaced with the U3 region of the MSCV LTR. This hybrid LTR was generated by PCR cloning (55), with pNL4-3 (1) (obtained from Malcom Martin through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and pMSCV-neo (31) (obtained from Robert Hawley, American Red Cross, Rockville, Md.) as templates. The primers used were as follows: HIV-U3-S, 5′ AGTTACTCTTCATTTTGCCTGTACTGGGTC; HIV-U3-AS, 5′ AGTTACTCTTCACCACAGATCAAGGATATC; MSCV-U3-S, 5′ AGTTACTCTTCATGGGTAACGCCATTTTGCAAG; and MSCV-U3-AS, 5′ AGTTACTCTTCAAAATGTGGGCTCTTTTATTGA. The pMGIN plasmid (11) was provided by Robert Hawley (American Red Cross). The pMG plasmid was constructed as follows. Plasmid pMGIN was cut with BamHI, and the resulting ends were blunted with mung bean nuclease (New England Biolabs) and subsequently cut with EcoRI. To the resulting 5.1-kb vector fragment, a 700-bp DNA fragment encoding enhanced GFP (EGFP) (Clontech) and bearing a blunted NotI site at the 5′ end and an EcoRI site at the 3′ end was ligated to yield pMG. The pLTR-G plasmid, encoding the VSV-G GP, was previously described (60). Plasmid pLTR-RD114, encoding the feline endogenous retrovirus Env GP, was constructed by inserting a 2.4-kb fragment derived from plasmid pFBRDSALF (58) (provided by Yasuhiro Takeuchi, University College, London, United Kingdom) into pLTR (60). Plasmid pLTR-RD114A was constructed as follows. A 505-bp DNA fragment encoding amino acids 372 to 530 of the RD114 Env GP was PCR amplified, with pLTR-RD114 as the template. The following primers were used: forward primer, 5′ TACATAGACCTAAACGAGCTGT; and reverse primer, 5′ CCATCGATTGAAAACGCATGGCCCAATGGT. The PCR product was digested with BsrGI and ClaI, and the fragment was cloned between the BsrGI and ClaI sites in pLTR-4070A (51) to generate pLTR-ARD114A, in which the sequences encoding amino acids 63 through 611 of the amphotropic Moloney murine leukemia virus (MLV) 4070A Env GP were replaced by RD114 sequences. An ∼2.3-kb XhoI-BsrGI fragment from pLTR-RD114, encoding amino acids 1 to 371 of the RD114 Env GP, was cloned between the XhoI and BsrGI sites of plasmid pLTR-ARD114A to generate pLTR-RD114A. Plasmid pLTR-RD114A encodes a hybrid RD114 GP with its intact ectodomain and transmembrane domain fused to a 33-amino-acid cytoplasmic tail derived from the amphotropic MLV 4070A Env GP. During construction of pLTR-RD114A, a mutated version of the plasmid, bearing an A-to-G mutation affecting amino acid 473 of the ectodomain, was fortuitously identified. This plasmid is referred to as pLTR-RD114Am. The helper plasmids used included pCD/NL-BH* (73), pCD/NL-BH*ΔΔΔ, which is similar to pC-HelpΔvifΔvprΔvpu (51) but carries a more extended deletion of the putative packaging signal, from nucleotides (nt) 747 to 787, between the 5′ major splice donor site and the beginning of the Gag coding region of the HIV-1 NL4-3 proviral DNA (1). The pCD/NL-BH*ΔRT− helper plasmid, with a D110E mutation in the reverse transcriptase (RT) coding region, was derived from pCD/NL-BH*ΔΔΔ. It contains sequences (nt 2005 to 5742) derived from pNLNgoMIVR−E−.HSA (35) (provided by Stephen H. Hughes, NCI—Frederick, Frederick, Md.) and carries a deletion in the Vpu coding region (51).
Virus production.
Vector particles were produced in 293T cells by transient cotransfection involving a three-plasmid expression system (51). Briefly, 293T cells were plated in 6-well plates (6 × 105 cells per well), and 24 h later vector plasmid DNA (5 μg), helper plasmid DNA (3.5 μg), and pLTR-G DNA (1.75 μg) were added. Alternatively, pLTR-RD114 (5 μg) or pLTR-RD114A (5 μg) DNA was used. Transfection by calcium phosphate in the presence of 25 μM chloroquine was carried out for 12 to 15 h. The medium was replaced, and virus particles released into the medium were harvested 60 to 65 h after transfection. In some cases, virus particles were concentrated by centrifugation at 25,000 rpm (15°C) for 2 h in a Beckman SW28 rotor (44). The generation of replication-competent lentivirus was tested as described before (51). p24 assays were performed with a commercial kit (RETRO-TEK HIV-1 p24 antigen ELISA; ZeptoMetrix Corporation). MG vector stocks were prepared by quadruple transfection of 293T cells with pMG, pHIT60 (66), pLTR-G or pLTR-RD114, and pTAT-REV (67) (provided by Michael R. Green, Howard Hughes Medical Institute, University of Massachusetts Medical School, Worcester, Mass.). Vector titers were derived from quantitative FACS analysis of HOS cells or MSCs. For calculation of titers, the number of target cells was multiplied by the percentage of EGFP-positive cells (derived from the linear range of the titration curves) divided by the volume of the input virus.
Transduction of cells.
MSCs grown in bulk (in T150 flasks) for 2 weeks were detached by trypsin-EDTA treatment and replated in 6-well plates at a density of 5 × 104 cells in 2 ml of LTCM per well. Transductions were carried out at various MOIs in the presence of 8 μg of Polybrene (Sigma) per ml. After incubation at 37°C for 20 h, the transduction medium was replaced with fresh LTCM. MPCs were transduced by plating 2.5 × 105 marrow low-density cells in 4 ml of medium per 60-mm-diameter culture dish. Twenty-four hours later, all nonadherent cells were removed and replaced with 1.5 ml of fresh medium containing 8 μg of Polybrene/ml and viral vectors. Mock transduction was performed under the same conditions but without added virus. After incubation at 37°C for 20 h, the medium was replaced. The cells were kept for 2 more weeks with medium changes at weekly intervals before FACS analyses were done. MSC colonies were stained (with crystal violet) and counted under an inverted microscope. Transduction of HOS cells maintained in DMEM-10% FBS was performed in the same manner as that described previously (61).
Determination of transgene copy numbers by real-time PCR.
Real-time PCR was performed with an ABI PRISM 7700 sequence detector (Applied Biosystems) in a final volume of 50 μl. The PCR mix contained 25 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 5 μl of DNA sample, and forward and reverse primers and probe at a final concentration of 200 nM (each). EGFP-specific primers and probe were designed with Primer Express software 1.5A (Applied Biosystems). The sequences were as follows: forward primer, 5′ AGTCCGCCCTGAGCAAAGA; reverse primer, 5′ TCACGAACTCCAGCAGGACC; and probe, 5′ FAM-CCCAACGAGAAGCGCGATCACA-TAMRA. The cycling conditions were 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The virus was treated with DNase I (Sigma) at a concentration of 1 μg/ml at 37°C for 15 min prior to infection. High-molecular-weight genomic DNA from transduced cells was purified as described previously (73). Copy number determination of viral sequences in transduced cells was done by using serial dilutions of pNL-EGFP/CMV vector DNA as a standard. Genomic vector copies in each sample were normalized to human RNase P gene copies with specific primers and probes (TaqMan DNA template reagent kit; Applied Biosystems).
RESULTS
Development of retrovirus vector systems for transgene delivery and expression in human MSCs.
We previously used HIV-1-based lentivirus vectors and the oncogenic MSCV-based MGIN retrovirus vector (11) pseudotyped with the VSV-G Env GP to deliver and express transgenes in human bone marrow-derived MSCs (73). These results revealed that a single round of transduction using unconcentrated lentivirus vectors resulted in efficient transduction and sustained transgene expression in human MSCs. It was also evident from these studies that transduction efficiencies and expression levels in MSCs with HIV-1-derived lentivirus vectors were higher than those observed with the MGIN oncoretrovirus vector (73).
For quantitative comparisons of transduction efficiencies and transgene expression levels in MSCs, HIV-1-based vectors carrying the EGFP reporter gene under the control of different internal promoters were tested side-by-side by FACS analysis and quantitative real-time PCR. The internal promoters used in the experiments included the human cytomegalovirus (CMV) immediate-early (IE) promoter (8) and the hybrid CEF (70) and CAG (53) promoters (Fig. 1A). For direct comparison of transgene expression levels from lentivirus vectors and MSCV-based oncoretrovirus vectors, a lentivirus vector, referred to as NL-EGFP(MSCV), which lacks an internal promoter but carries a hybrid MSCV/HIV-1 LTR, was constructed along with a derivative (MG) of the original MGIN oncoretrovirus vector that expresses EGFP from the MSCV LTR (Fig. 1A).
FIG. 1.
Representation of HIV-1-based lentivirus vectors. (A) Vector constructs. pNL-EGFP/CMV, pNL-EGFP/CEF, and pNL-EGFP/CAG harbor CMV-IE, CEF, and CAG promoters, respectively (61). pNL-EGFP(MSCV), lentivirus vector lacking an internal promoter but harboring a hybrid LTR in which the U3 region of the HIV-1 LTR was replaced by the MSCV U3 region; pMG, vector derived from the MSCV oncoretrovirus vector (11); PCMV, human CMV-IE promoter; PCEF, Hybrid promoter consisting of the enhancer region of the CMV-IE promoter fused to EF-1α promoter elements; PCAG, hybrid promoter consisting of the enhancer region of the CMV-IE promoter fused to the chicken β-actin promoter; PPT, central polypurine tract. (B) Helper (packaging) constructs. pCD/NL-BH* (73) and pCD/NL-BH*ΔΔΔ, lentiviral helper constructs. pCD/NL-BH*ΔΔΔ carries deletions of all accessory protein-encoding regions. Plasmids pHIT60 (66) and pTAT-REV (67) were used to package MG vector genomes. (C) Envelope constructs. The pLTR-G plasmid (60) encodes the VSV-G GP, and pLTR-RD114 encodes the RD114 GP. Plasmids pLTR-RD114A and pLTR-RD114Am encode hybrid RD114 Env GPs. pLTR-RD114Am carries a point mutation in the ectodomain. ACT, cytoplasmic tail domain derived from the MLV 4070A Env.
A series of different packaging (helper) plasmids were used, including pCD/NL-BH* (73), pCD/NL-BH*ΔΔΔ (which is similar to pCD/NL-BH* but lacks all accessory protein-encoding regions), and pCD/NL-BH*ΔRT− (which encodes a defective RT bearing a D110E mutation [35] as well as a deletion in the Vpu HIV-1 accessory protein coding region [Fig. 1B]). Plasmids pHIT60 (66) and pTAT-REV (67) were used to produce MG vector particles.
The Env-encoding plasmids used included pLTR-G (60). Novel Env GP plasmids encoding native and engineered feline endogenous virus RD114 Env GPs (58, 71) were also designed (Fig. 1C).
Quantitative analysis of transgene expression in MSCs transduced with lentivirus vectors bearing internal promoters.
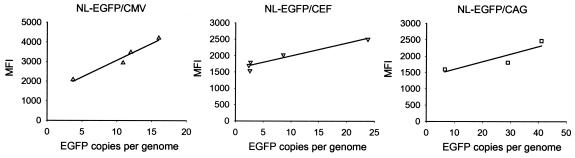
In previous studies, we found that transgene expression in human MSCs was highest with vectors containing the CMV-IE promoter, followed by vectors bearing the hybrid CEF and CAG promoters (73). It could not be ruled out, however, that the apparently higher EGFP expression levels with the NL-EGFP/CMV vector in MSCs were primarily caused by transgene copy number effects rather than differences in promoter strength. To quantitatively correlate EGFP expression levels and transgene copy numbers, HOS cells were transduced side-by-side with the NL-EGFP/CMV, NL-EGFP/CEF, and NL-EGFP/CAG lentivirus vectors at various MOIs (Fig. 2). The percentages of EGFP-positive cells were determined by FACS. In Fig. 2A, the percentages of EGFP-positive cells as a function of the amount of virus added (displayed as nanograms of p24) are shown. These results revealed a linear relationship between the percentages of EGFP-positive cells and the amount of virus added up to a level at which 40% of the cells in the analyzed population were EGFP positive. In parallel experiments, the numbers of EGFP transgene copies per genome were determined by quantitative real-time PCR. The percentages of EGFP-positive cells relative to the numbers of EGFP transgene copies per cell are shown in Fig. 2, panels B. A linear relationship was apparent up to a level at which 40% of the cells in the analyzed population were EGFP positive. The mean fluorescence intensity (MFI) values of EGFP-positive cells were also determined by FACS (Fig. 2C). For the NL-EGFP/CMV vector, there was a linear relationship between the MFI values and the numbers of EGFP transgene copies per genome up to at least 25 copies. For the NL-EGFP/CEF and NL-EGFP/CAG vectors, the linear relationship extended up to at least 13 and 11 copies, respectively. In transduced HOS cells, a single NL-EGFP/CEF proviral copy per genome revealed an MFI value of 1,300, while single proviral copies of NL-EGFP/CMV and NL-EGFP/CAG yielded MFIs of 190 and 720, respectively. Since the MFI values reflect EGFP expression levels, we conclude that EGFP transgene expression from the CEF promoter was more efficient than that from the CAG or CMV-IE promoter. To rule out the presence of PCR artifacts due to carryover of plasmid DNA present in the virus preparation, virus stocks were prepared in parallel with a packaging construct (pCD/NL-BH*Δvpu/RT−) lacking a functional RT (35). The results obtained indicated that plasmid carryover did not contribute significantly to the signals observed (Table 1). The relative strengths of the CMV-IE, CEF, and CAG promoters in MSCs were also determined. The results shown in Fig. 3 demonstrate that the CMV-IE promoter yielded the highest MFI values. At 10 NL-EGFP/CMV proviral copies per genome, the MFI value was 3,000, while 10 proviral copies of the NL-EGFP/CEF or NL-EGFP/CAG vector per genome yielded MFI values of 2,000 and 1,600, respectively. This shows that the CMV-IE promoter was superior to the CEF and CAG promoters in MSCs. Previous studies have revealed a good agreement between the percentages and MFI values of EGFP-positive cells analyzed 3 days after transduction and of cells analyzed 5.5 months later (73). This indicates that FACS analyses performed after 3 days provide reliable measures for determination of gene transfer efficiencies and transgene expression levels in MSCs. Table 2 shows a comparison of the titers obtained by quantitative real-time PCR and those obtained by FACS. The ratios of DNA-based titers and FACS-based titers varied depending on the promoter used and the type of cell transduced. While DNA-based titers in HOS cells and MSCs were similar for a given vector, FACS-based titers were lower in MSCs, possibly indicating that transgene expression in MSCs was less efficient compared to that in HOS cells. Taken together, these results confirm our earlier results obtained with virus stocks whose titers had been adjusted by using a FACS-based approach (73). They also show that transgene copy number effects are not responsible for the apparently higher efficiency of the CMV-IE promoter in MSCs.
FIG. 2.
Influence of promoters and vector copy numbers on EGFP transgene expression in HOS cells. Cells were transduced with NL-EGFP/CMV, NL-EGFP/CEF, and NL-EGFP/CAG vector stocks at various MOIs in DMEM-10% FBS containing 8 μg of Polybrene/ml at 37°C for 20 h. (A) Percentages of EGFP-positive cells as a function of the amount (nanograms) of p24 added. (B) Percentages of EGFP-positive cells as a function of the number of EGFP transgene copies. (C) MFI values of the EGFP-positive cell populations as a function of the number of EGFP transgene copies. The cells were analyzed by FACS 3 days after transduction. Aliquots were processed for quantitative real-time PCR with EGFP-specific primers. The MFI values and the numbers of EGFP copies per genome are displayed. The data shown were obtained from two independent experiments. Virus titers were 3.6 × 104 infectious units (IU)/ng of p24 for NL-EGFP/CMV, 1.42 × 104 IU/ng of p24 for NL-EGFP/CEF, and 7.6 × 103 IU/ng of p24 for NL-EGFP/CAG.
TABLE 1.
Absence of transgene DNA and lack of transgene expression in cells transduced with vector stocks prepared with helper constructs encoding a defective RT
| Vector | Helper plasmid | Cell type | % EGFP positive cellsa | MFI valuea | No. of EGFP copies per genomeb |
|---|---|---|---|---|---|
| None | None | HOSc | 0.13 | 35 | <0.01 |
| pNL-EGFP/CMV | pCD/NL-BH*d | HOS | 35.5 | 162 | 0.75 |
| pNL-EGFP/CMV | pCD/NL-BH*Δvpu/RT−e | HOS | 0.23 | 47 | 0.02 |
| pNL-EGFP/CEF | pCD/NL-BH* | HOS | 39.0 | 1,239 | 0.65 |
| pNL-EGFP/CEF | pCD/NL-BH*Δvpu/RT− | HOS | 0.45 | 45 | <0.01 |
| pNL-EGFP/CAG | pCD/NL-BH* | HOS | 38.6 | 633 | 0.57 |
| pNL-EGFP/CAG | pCD/NL-BH*Δvpu/RT− | HOS | 0.10 | 117 | <0.01 |
| None | None | MSCc | 0.23 | 68 | 0.02 |
| pNL-EGFP/CMV | pCD/NL-BH* | MSC | 36.6 | 2,377 | 3.72 |
| pNL-EGFP/CMV | pCD/NL-BH*Δvpu/RT− | MSC | 0.09 | 84 | 0.01 |
| pNL-EGFP/CEF | pCD/NL-BH* | MSC | 29.9 | 1,794 | 2.75 |
| pNL-EGFP/CEF | pCD/NL-BH*Δvpu/RT− | MSC | 0.12 | 69 | 0.01 |
| pNL-EGFP/CAG | pCD/NL-BH* | MSC | 24.6 | 1,805 | 29.2 |
| pNL-EGFP/CAG | pCD/NL-BH*Δvpu/RT− | MSC | 0.11 | 54 | 0.02 |
The percentages of EGFP-positive cells and the MFI values were determined by FACS 3 days after transduction.
Copy numbers were determined by real-time PCR.
Mock-transduced cells.
Helper plasmid encoding wild-type RT.
Helper plasmid encoding mutant RT.
FIG. 3.
Influence of promoters and vector copy numbers on EGFP transgene expression in MSCs. Cells were transduced with NL-EGFP/CMV, NL-EGFP/CEF, and NL-EGFP/CAG vector stocks at various MOIs in LTCM containing 8 μg of Polybrene/ml at 37°C for 20 h. The cells were analyzed by FACS 3 days after transduction. Aliquots were processed for quantitative real-time PCR with EGFP-specific primers. The MFI values and the numbers of EGFP copies per genome are displayed. The data shown were obtained from two independent experiments.
TABLE 2.
Comparison of vector titers in HOS cells and MSCs as determined by real-time PCR and FACS analysis
| Vector | Cell type | FACS titera (IU/ml) | DNA titerb (IU/ml) | Ratio of DNA titer/FACS titer |
|---|---|---|---|---|
| NL-EGFP/CMV | HOS | 3.15 × 107 ± 0.29 × 107 | 7.10 × 107 ± 2.61 × 107 | 2.3 |
| NL-EGFP/CEF | HOS | 1.16 × 107 ± 0.14 × 107 | 2.13 × 107 ± 0.44 × 107 | 1.2 |
| NL-EGFP/CAG | HOS | 6.50 × 106 ± 0.74 × 106 | 8.30 × 106 ± 2.19 × 106 | 1.3 |
| NL-EGFP/CMV | MSC | 3.85 × 106 ± 0.77 × 106 | 9.50 × 107 ± 0.41 × 107 | 31 |
| NL-EGFP/CEF | MSC | 7.80 × 105 ± 1.81 × 105 | 2.04 × 107 ± 0.95 × 107 | 26 |
| NL-EGFP/CAG | MSC | 4.30 × 106 ± 0.35 × 105 | 3.50 × 107 ± 1.30 × 107 | 81 |
Titers were determined by quantitative FACS analysis. For calculations of titers, the number of target cells was multiplied by the percentage of EGFP- positive cells (derived from the linear range of the titration curves) divided by the volume of the input virus. Mean values ± standard deviations are presented. IU, infectious units.
Titers were determined by quantitative real-time PCR with EGFP-specific primers. Mean values ± standard deviations are presented.
Transgene expression from MSCV-based oncoretrovirus vectors and lentivirus vectors bearing MSCV promoter sequences.
Our earlier findings suggested that transduction efficiencies and transgene expression levels in MSCs were higher with lentivirus vectors than with the MGIN oncoretrovirus vector (73). However, since the promoters used in the two vector systems were different, a direct comparison was not possible. For direct comparison of EGFP transgene expression levels in MSCs from HIV-1-based lentivirus vectors and MSCV-based oncoretrovirus vectors, HIV-1-based vectors and MSCV-based vectors bearing identical transgene and promoter sequences were constructed. The U3 region of the 3′ LTR of the NL-EGFP/CMV vector was deleted from positions −13 to −401 with respect to the transcription start site and replaced by a 318-bp fragment derived from the U3 region of the MSCV virus (positions −13 to −331 with respect to the transcription start site). The internal CMV-IE promoter was also deleted from this plasmid to yield NL-EGFP(MSCV) (Fig. 1A), thus putting EGFP transgene expression under the control of the hybrid MSCV/HIV promoter. The MGIN vector was also modified to make it more similar to the NL-EGFP(MSCV) vector. The resulting MG vector harbors an EGFP reporter gene under the control of the MSCV LTR but lacks the internal ribosome entry site (IRES) and neo gene sequences present in MGIN (Fig. 1A).
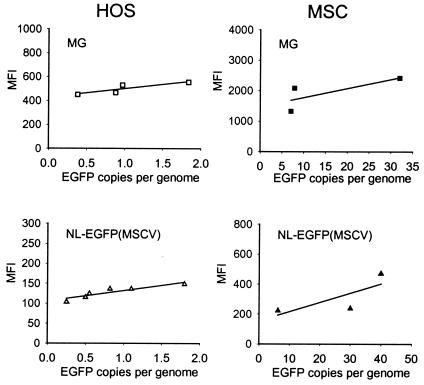
HOS cells and MSCs were transduced side-by-side with NL-EGFP(MSCV) and MG vector stocks at various MOIs, and EGFP expression levels were determined by quantitative FACS analysis. Transgene copies were analyzed by quantitative real-time PCR using EGFP-specific primers. A comparison of the MFI values and the numbers of transgene copies is shown in Fig. 4. The results obtained indicate that at identical transgene copy numbers, EGFP expression levels in HOS cells with the MG vector were higher than those with the NL-EGFP(MSCV) vector (Fig. 4, left panels). MSCs transduced side-by-side with the MG and NL-EGFP(MSCV) vectors revealed the same pattern (Fig. 4, right panels). From these results, we conclude that EGFP transgene expression levels in HOS cells and in MSCs from MSCV-based oncoretrovirus vectors are higher than those from HIV-1-based lentivirus vectors bearing identical transgene and promoter sequences. It was also interesting that the newly designed MG vector performed more efficiently than the MGIN vector, indicating that the IRES and neo sequences present in MGIN may have negatively impacted EGFP expression levels in HOS cells and in MSCs (X.-Y. Zhang, unpublished data).
FIG. 4.
Transgene expression in HOS cells and MSCs from oncoretrovirus and lentivirus vectors bearing MSCV promoter sequences. Cells were transduced with oncogenic MG vector stocks and NL-EGFP(MSCV) lentivirus vector stocks at various MOIs. The cells were analyzed by FACS 3 days after transduction. Aliquots were processed for quantitative real-time PCR with EGFP-specific primers. The MFI values and the numbers of EGFP copies per genome are displayed. The data shown were obtained from two independent experiments.
Transduction of MSCs with HIV-1 pseudotypes bearing RD114 Env.
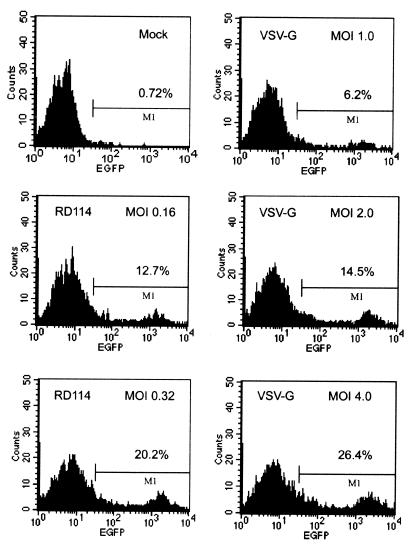
The feline endogenous virus RD114 Env GP has previously been shown to form pseudotypes with MLV-based vectors (19, 71), HIV-1-based vectors (30, 33), and simian immunodeficiency virus-based vectors (63). Such pseudotypes led to the efficient transduction of human bone marrow-derived hematopoietic progenitor cells, including CD34+ cells (30, 58, 63). We wanted to test the capacity of HIV-1-based vectors pseudotyped with the RD114 Env GP to transduce MSCs. MSCs were analyzed by FACS following transduction with the NL-EGFP/CEF vector pseudotyped with the RD114 Env GP (Fig. 5, left panels) or VSV-G (Fig. 5, right panels). With RD114 pseudotypes used at an MOI of 0.32 (HOS units), 20% EGFP-positive cells were observed, while MOIs above 2 were required in order to yield a similar percentage of EGFP-positive cells with VSV-G pseudotypes (Fig. 5, right panels). This shows that the relative transduction efficiency with RD114 pseudotypes in MSC was at least 10-fold higher than with VSV-G pseudotypes.
FIG. 5.
Transduction of MSCs with lentivirus vectors pseudotyped with the RD114 Env GP. MSCs were transduced with NL-EGFP/CEF/RD114 vector stocks at MOIs of 0.16 and 0.32. NL-EGFP/CEF/VSV-G vector stocks were tested in parallel at the MOIs indicated. The cells were analyzed by FACS 3 days after transduction. MOIs were adjusted based on titers determined on HOS cells. Panels: top left, mock (control); middle and bottom left, RD114 pseudotypes; right, VSV-G pseudotypes.
In Table 3, the transduction efficiencies in HOS cells and MSCs of MSCV-based MG oncoretrovirus vectors and of lentivirus vectors pseudotyped with the RD114 or VSV-G GPs are compared. With HOS cells, the titers observed with NL-EGFP(MSCV)/RD114 pseudotypes and NL-EGFP(MSCV)/VSV-G pseudotypes differed 304-fold, while MG/RD114 and MG/VSV-G pseudotypes differed 3-fold. However, the titers observed with NL-EGFP(MSCV)/RD114 and NL-EGFP(MSCV)/VSV-G pseudotypes on MSCs differed just 15-fold, while the corresponding MG pseudotypes had a ratio of 1.3 (Table 3). This indicates that RD114 lentivirus pseudotypes were relatively more efficient at transducing MSCs than HOS cells. The results in Table 3 also show that the absolute titers of MG/RD114 pseudotypes on HOS cells and on MSCs were higher than those of NL-EGFP(MSCV)/RD114 pseudotypes. Assuming that the activity of the MG vector LTR and that of the NL-EGFP(MSCV) vector are similar, this may indicate that RD114 GP incorporation into HIV-1 vector particles was inefficient compared to that into MG vector particles.
TABLE 3.
Efficiency of gene transfer into HOS cells and MSCs by use of oncoretroviral vectors and HIV-1 vectors pseudotyped with the RD114 Env protein
| Vector | Cell type | Env GP | Titer (IU/ml)a | Ratiob |
|---|---|---|---|---|
| MG | HOS | VSV-G | 2.26 × 106 ± 1.20 × 106 | 3.0 |
| MG | HOS | RD114 | 7.57 × 105 ± 1.32 × 105 | |
| NL-EGFP(MSCV) | HOS | VSV-G | 1.47 × 107 ± 0.47 × 107 | 304 |
| NL-EGFP(MSCV) | HOS | RD114 | 4.83 × 104 ± 0.35 × 104 | |
| MG | MSC | VSV-G | 1.30 × 105 ± 0.10 × 105 | 1.3 |
| MG | MSC | RD114 | 1.00 × 105 ± 0.10 × 105 | |
| NL-EGFP(MSCV) | MSC | VSV-G | 3.00 × 105 ± 1.8 × 105 | 15 |
| NL-EGFP(MSCV) | MSC | RD114 | 2.00 × 104 ± 0.8 × 104 |
Titers were obtained as described in Table 2. Results are means ± standard deviations.
Ratios of the titers obtained with vector particles pseudotyped with VSV-G and the titers obtained with vector particles pseudotyped with the RD114 Env GP.
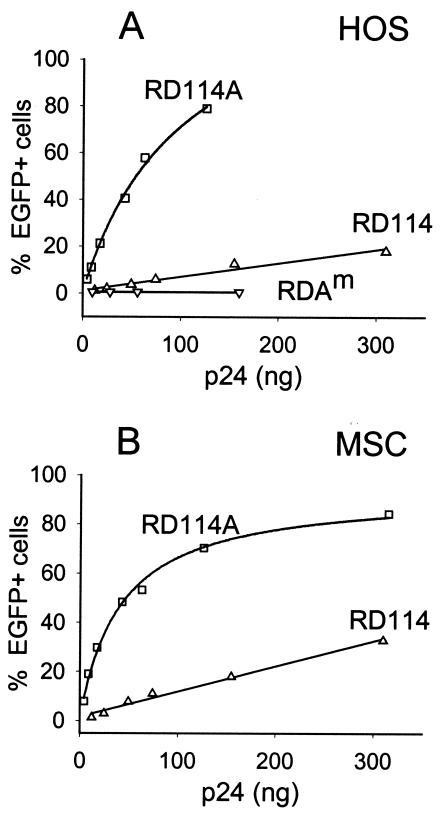
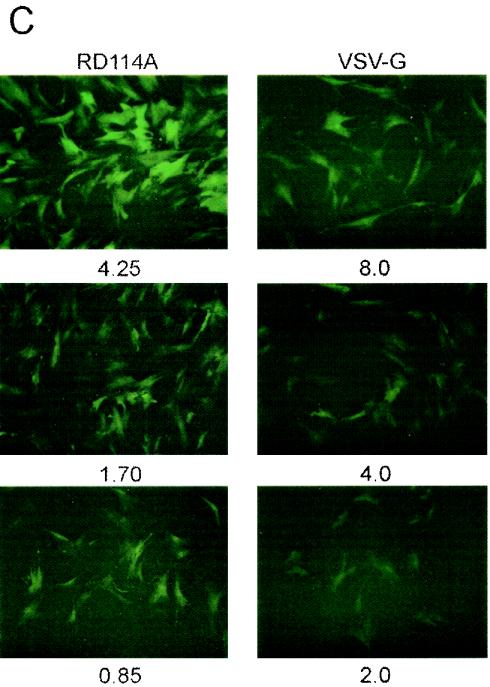
In an attempt to increase the titers of lentivirus/RD114 pseudotypes, the RD114 GP was modified. Because the amphotropic MLV 4070A Env GP was previously found to form efficient pseudotypes with HIV-1 vectors (51, 59), we replaced the cytoplasmic tail of RD114 with that of the amphotropic MLV 4070A GP to yield plasmid pLTR-RD114A (Fig. 1C). In HOS cells, the hybrid RD114A Env GP yielded >20-fold higher titers than the unmodified RD114 Env (Fig. 6A), while in MSCs the titers differed by a factor of 15 (Fig. 6B), resulting in titers similar to those observed with VSV-G pseudotypes. It was interesting that a point mutation that was fortuitously introduced into one of the clones during construction of the hybrid GP, resulting in a Gly-to-Glu change at amino acid 473, completely abolished virion infectivity (Fig. 6A). The corresponding Env GP is referred to as RD114Am (Fig. 1C). MSCs transduced with the NL-EGFP/CMV vector pseudotyped with RD114A or VSV-G at MOIs ranging from 0.8 to 8.0 were analyzed by fluorescence microscopy. Consistent with the view that RD114A pseudotypes were relatively more efficient than VSV-G pseudotypes for transducing MSCs, MSCs transduced with RD114A pseudotypes were found to be brighter than cells transduced with VSV-G pseudotypes at similar MOIs (Fig. 6C).
FIG. 6.
Transduction of MSCs with lentivirus vectors bearing chimeric RD114 Env GPs. (A and B) HOS cells and MSCs were trans-duced with NL-EGFP/CMV vector stocks bearing RD114 or chimeric RD114A GP. Both vector stocks had been prepared side-by-side to minimize differences in titers due to variations in vector production. The amount of virus added is indicated (expressed as nanograms of p24). The cells were analyzed by FACS 3 days after transduction. (C) Fluorescence microscopy of MSCs transduced with RD114A (left panels) and VSV-G (right panels) pseudotypes 3 days after transduction. The MOIs used (HOS units) are indicated.
Transduction of MPCs.
To assess the ability of lentivirus vectors pseudotyped with the RD114 and RD114A Env GPs to deliver transgenes into MPCs, we transduced strongly adherent marrow cells 1 day after plating. The percentage of fluorescent colonies (CFU-Fs) was scored 2 weeks later. The results shown in Table 4 indicate that cells which had been infected with NL-EGFP/CMV vector stocks 24 h after plating gave rise to bright fluorescent progeny cells in an MOI-dependent fashion. Up to 51% of the cells arising from 1-day cells transduced with RD114 pseudotypes at an MOI of 3 were EGFP positive, while up to 74% of the cells transduced with RD114A pseudotypes at an MOI of 2.5 were EGFP positive. The sizes and numbers of CFU-Fs were unchanged following transduction with RD114 and RD114A pseudotypes at MOIs up to 60, while VSV-G pseudotypes yielded reduced numbers of CFU-Fs (Table 4), possibly caused by toxicity problems related to VSV-G. Toxicity problems with VSV-G pseudotypes in MPCs at MOIs above 0.5 have been noticed before (73).
TABLE 4.
Transduction of MSC progenitor cellsa
| Vector (MOI) | Env GP | Average no. of CFU-Fs per plateb | Result of FACS analysis
|
|
|---|---|---|---|---|
| % Positive cells | MFI value | |||
| Nonec | 21.2 | 0.35 | 50 | |
| Mockd | 17.0 | 0.82 | 113 | |
| NL-EGFP/CMV (0.25) | RD114 | 16.2 | 2.1 | 1,312 |
| NL-EGFP/CMV (0.5) | RD114 | 16.5 | 11.3 | 2,886 |
| NL-EGFP/CMV (1.0) | RD114 | 15.8 | 17.5 | 4,017 |
| NL-EGFP/CMV (3.0) | RD114 | 18.0 | 50.9 | 5,789 |
| NL-EGFP/CMV (2.5) | RD114A | 17.5 | 74.0 | 4,831 |
| NL-EGFP/CMV (5.0) | RD114A | 15.0 | 61.0 | 4,428 |
| NL-EGFP/CMV (10) | RD114A | 15.0 | 50.5 | 5,045 |
| NL-EGFP/CMV (60) | RD114A | 14.0 | 93.4 | 6,785 |
| NL-EGFP/CMV (0.25) | VSV-G | 17.2 | 28.3 | 4,749 |
| NL-EGFP/CMV (0.5) | VSV-G | 14.2 | 36.9 | 3,377 |
| NL-EGFP/CMV (3.0) | VSV-G | 8.5 | 21.7 | 2,801 |
For each experiment, 5 × 104 1-day-old cells were transduced in 60-mm-diameter plates at the indicated MOI. MOIs were adjusted based on titers determined on MSCs. The medium was changed 20 h after virus addition, and the cells were analyzed 14 days later. The total number of colonies was determined following staining with crystal violet. The cells from parallel plates were collected and analyzed by FACS.
Four plates were used.
Cells were grown with medium changes at weekly intervals.
Cells were grown in transduction medium in the absence of virus.
DISCUSSION
This work deals with the transduction of human bone marrow-derived stromal cells, MSCs, with HIV-1-based lentivirus vectors and MSCV-based oncoretrovirus vectors pseudotyped with the VSV-G and RD114 Env GPs. In an attempt to optimize HIV-1 vectors for efficient transgene delivery and expression in MSCs, we have tested a number of different promoters and investigated their abilities to direct high-level EGFP transgene expression in MSC cultures by using FACS analysis and quantitative real-time PCR. Our results show that HIV-1 vectors containing an internal CMV-IE promoter induce high levels of reporter gene expression in MSCs, as judged by the MFI values of the cell population (Fig. 3). Expression from the hybrid CEF (70) and CAG (53) promoters at identical proviral copy numbers was found to be less efficient, consistent with our earlier findings (73). EGFP transgene expression from the hybrid MSCV/HIV-1 LTR, present in the NL-EGFP(MSCV) vector, was also less efficient than that from the CMV-IE promoter. This contrasts with the results obtained with the MG oncoretrovirus vector, which harbored identical MSCV promoter sequences and which resulted in high levels of EGFP expression in MSCs, revealing MFI values some eightfold above those seen with the NL-EGFP(MSCV) vector (Fig. 4). It should be kept in mind, however, that cis-acting sequences located downstream of the HIV-1 promoter and affecting its chromatin structure and transcriptional activity (23) may have negatively impacted transgene expression from the hybrid MSCV/HIV LTR. Similar lentivirus vectors bearing MSCV/HIV-1 LTR promoter sequences were previously described by Choi et al. (14) and Gao et al. (26). Tests performed with these vectors in CD34+ human hematopoietic stem cells indicated that EGFP transgene expression from lentivirus vectors bearing hybrid MSCV/HIV-1 LTRs was significantly higher than that from vectors bearing internal promoters, including the MSCV, CMV-IE, and Rous sarcoma virus promoters. It was also clear from those studies that transgene expression levels from lentivirus vectors approached those seen with the MSCV-based oncoretrovirus vectors, although a quantitative assessment of transgene copy numbers in CD34+ cells was not reported.
We and others have previously documented the versatility of HIV-1-based lentivirus vectors to form pseudotypes with a number of Env GPs besides that of VSV-G, including the MLV-derived ecotropic and amphotropic Env GPs (40, 52, 60) and the human T-cell leukemia virus type 1 Env (41, 69). A growing list of GPs derived from a variety of different enveloped viruses have since been reported to form stable pseudotypes with HIV-1 vector cores in vitro. These include the gibbon ape leukemia virus Env (15, 68), the Env proteins of the MLV 10A1 polytropic and xenotropic subtypes (15, 68), the avian leukosis virus subtype A Env (43), and the RD114 Env of the cat endogenous retrovirus (30). Nonretrovirus-derived GPs were also tested, including the rabies G GP (51), the G GP of a related lyssavirus, Mokola virus (51), the lymphocytic choriomeningitis virus GP (5, 15), the hemagglutinin protein of influenza virus (15, 36), the respiratory syncytial virus F and G surface GPs (36), the Ebola virus and Marburg virus GPs (10, 36), the Sendai virus fusion protein F (37), and the baculovirus GP64 Env (39). These alternative pseudotypes may ultimately help us to bypass toxicity problems that are inherent to VSV-G (5), although the titers obtained with these alternative GPs are at present lower than those observed with VSV-G (51, 59).
In this study, we have tested the RD114 Env GP, its potential to form pseudotypes with HIV-1-based vectors, and the capacity of such pseudotypes to infect MSCs. The transduction efficiencies of lentivirus/RD114 pseudotypes in MSCs were 10- to 15-fold below those seen with VSV-G pseudotypes, and in HOS cells the difference was much more pronounced (300-fold), indicating that the transduction efficiency with RD114 pseudotypes in MSCs is relatively higher than that with VSV-G pseudotypes (Table 3). Lentivirus vectors pseudotyped with the RD114A hybrid GP reached significantly higher titers in HOS cells and MSCs, approaching those obtained with VSV-G pseudotypes (Fig. 6). Thus, RD114A pseudotypes appear to be viable alternatives to VSV-G and may help alleviate some of the toxicity problems that are inherent to the VSV-G GP. This was clearly evident from experiments carried out with stromal progenitor cells in which MOIs up to 60 had no adverse effect as far as the numbers and sizes of CFU-Fs were concerned (Table 4). A chimeric RD114 GP similar to that described in this report was designed by Sandrin et al. (63). Simian immunodeficiency virus-derived vectors pseudotyped with this chimeric GP had significantly higher titers than vectors pseudotyped with unmodified RD114. Ikeda et al. (33) constructed an RD114 variant with an HIV-1 protease site introduced at the R-peptide cleavage site. HIV-1 vectors pseudotyped with this variant displayed titers up to 8.5 × 106 infectious units/ml (33). It was interesting in our work that a point mutation affecting amino acid 473 within the RD114 ectodomain completely abolished virion infectivity. The steps in virus assembly and/or uptake affected by this mutation will need to be determined.
In conclusion, our results show that HIV-1-based vectors appear to be very efficient for delivering and expressing transgenes in MSCs. The clinical utility of genetically modified MSCs and their progenitors requires stable and long-term expression of the desired gene product as well as regulation of gene expression according to disease status. HIV-1-based vectors in the context of MSCs may have clinical applications once the safety issues that are inherent to this vector system have been resolved.
Acknowledgments
We are grateful to Sherry Price for providing stromal cells and to Connie Porretta for help with the FACS analysis. We thank Robert Kutner for help with virus production.
This work was supported in part by NIH grant R01 NS044832-01 (J.R.) and the Louisiana Board of Regents Health Excellence Fund (J.R.).
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allay, J. A., J. E. Dennis, S. E. Haynesworth, M. K. Majumdar, D. W. Clapp, L. D. Shultz, A. I. Caplan, and S. L. Gerson. 1997. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum. Gene Ther. 8:1417-1427. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew, A., S. Patil, A. Mackay, M. Nelson, D. Buyaner, W. Hardy, J. Mosca, C. Sturgeon, M. Siatskas, N. Mahmud, K. Ferrer, R. Deans, A. Moseley, R. Hoffman, and S. M. Devine. 2001. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum. Gene Ther. 12:1527-1541. [DOI] [PubMed] [Google Scholar]
- 4.Baxter, M. A., R. F. Wynn, J. A. Deakin, I. Bellantuono, K. G. Edington, A. Cooper, G. T. Besley, H. J. Church, J. E. Wraith, T. F. Carr, and L. J. Fairbairn. 2002. Retrovirally mediated correction of bone marrow-derived mesenchymal stem cells from patients with mucopolysaccharidosis type I. Blood 99:1857-1859. [DOI] [PubMed] [Google Scholar]
- 5.Beyer, W. R., M. Westphal, W. Ostertag, and D. von Laer. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco, P., M. Riminucci, S. Gronthos, and P. G. Robey. 2001. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180-192. [DOI] [PubMed] [Google Scholar]
- 7.Bolotin, E., M. Smogorzewska, S. Smith, M. Widmer, and K. Weinberg. 1996. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88:1887-1894. [PubMed] [Google Scholar]
- 8.Boshart, M., F. Weber, G. Jahn, K. Dorsch-Hasler, B. Fleckenstein, and W. Schaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41:521-530. [DOI] [PubMed] [Google Scholar]
- 9.Castro-Malaspina, H., R. E. Gay, G. Resnick, N. Kapoor, P. Meyers, D. Chiarieri, S. McKenzie, H. E. Broxmeyer, and M. A. Moore. 1980. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 56:289-301. [PubMed] [Google Scholar]
- 10.Chan, S. Y., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, L., C. Du, D. Murray, X. Tong, Y. A. Zhang, B. P. Chen, and R. G. Hawley. 1997. A GFP reporter system to assess gene transfer and expression in human hematopoietic progenitor cells. Gene Ther. 4:1013-1022. [DOI] [PubMed] [Google Scholar]
- 12.Cherington, V., G. G. Chiang, C. A. McGrath, A. Gaffney, T. Galanopoulos, W. Merrill, C. B. Bizinkauskas, M. Hansen, J. Sobolewski, P. H. Levine, J. S. Greenberger, and D. R. Hurwitz. 1998. Retroviral vector-modified bone marrow stromal cells secrete biologically active factor IX in vitro and transiently deliver therapeutic levels of human factor IX to the plasma of dogs after reinfusion. Hum. Gene Ther. 9:1397-1407. [DOI] [PubMed] [Google Scholar]
- 13.Chiang, G. G., H. L. Rubin, V. Cherington, T. Wang, J. Sobolewski, C. A. McGrath, A. Gaffney, S. Emami, N. Sarver, P. H. Levine, J. S. Greenberger, and D. R. Hurwitz. 1999. Bone marrow stromal cell-mediated gene therapy for hemophilia A: in vitro expression of human factor VIII with high biological activity requires the inclusion of the proteolytic site at amino acid 1648. Hum. Gene Ther. 10:61-76. [DOI] [PubMed] [Google Scholar]
- 14.Choi, J. K., N. Hoang, A. M. Vilardi, P. Conrad, S. G. Emerson, and A. M. Gewirtz. 2001. Hybrid HIV/MSCV LTR enhances transgene expression of lentiviral vectors in human CD34(+) hematopoietic cells. Stem Cells 19:236-246. [DOI] [PubMed] [Google Scholar]
- 15.Christodoulopoulos, I., and P. M. Cannon. 2001. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J. Virol. 75:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuah, M. K., H. Brems, V. Vanslembrouck, D. Collen, and T. Vandendriessche. 1998. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum. Gene Ther. 9:353-365. [DOI] [PubMed] [Google Scholar]
- 17.Chuah, M. K., A. Van Damme, H. Zwinnen, I. Goovaerts, V. Vanslembrouck, D. Collen, and T. Vandendriessche. 2000. Long-term persistence of human bone marrow stromal cells transduced with factor VIII-retroviral vectors and transient production of therapeutic levels of human factor VIII in nonmyeloablated immunodeficient mice. Hum. Gene Ther. 11:729-738. [DOI] [PubMed] [Google Scholar]
- 18.Conget, P. A., and J. J. Minguell. 2000. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp. Hematol. 28:382-390. [DOI] [PubMed] [Google Scholar]
- 19.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deans, R. J., and A. B. Moseley. 2000. Mesenchymal stem cells: biology and potential clinical uses. Exp. Hematol. 28:875-884. [DOI] [PubMed] [Google Scholar]
- 21.Ding, L., S. Lu, R. Batchu, R. S. Iii, and N. Munshi. 1999. Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther. 6:1611-1616. [DOI] [PubMed] [Google Scholar]
- 22.DuBridge, R. B., P. Tang, H. C. Hsia, P. M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.el Kharroubi, A., and M. A. Martin. 1996. cis-acting sequences located downstream of the human immunodeficiency virus type 1 promoter affect its chromatin structure and transcriptional activity. Mol. Cell. Biol. 16:2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenstein, A. J., J. F. Gorskaja, and N. N. Kulagina. 1976. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 4:267-274. [PubMed] [Google Scholar]
- 25.Frolova-Jones, E. A., A. Ensser, A. J. Stevenson, S. E. Kinsey, and D. M. Meredith. 2000. Stable marker gene transfer into human bone marrow stromal cells and their progenitors using novel herpesvirus saimiri-based vectors. J. Hematother. Stem Cell Res. 9:573-581. [DOI] [PubMed] [Google Scholar]
- 26.Gao, Z., J. Golob, V. M. Tanavde, C. I. Civin, R. G. Hawley, and L. Cheng. 2001. High levels of transgene expression following transduction of long-term NOD/SCID-repopulating human cells with a modified lentiviral vector. Stem Cells 19:247-259. [DOI] [PubMed] [Google Scholar]
- 27.Gerson, S. L. 1999. Mesenchymal stem cells: no longer second class marrow citizens. Nat. Med. 5:262-264. [DOI] [PubMed] [Google Scholar]
- 28.Gordon, E. M., M. Skotzko, R. K. Kundu, B. Han, J. Andrades, M. Nimni, W. F. Anderson, and F. L. Hall. 1997. Capture and expansion of bone marrow-derived mesenchymal progenitor cells with a transforming growth factor-beta1-von Willebrand's factor fusion protein for retrovirus-mediated delivery of coagulation factor IX. Hum. Gene Ther. 8:1385-1394. [DOI] [PubMed] [Google Scholar]
- 29.Gysin, R., J. E. Wergedal, M. H. Sheng, Y. Kasukawa, N. Miyakoshi, S. T. Chen, H. Peng, K. H. Lau, S. Mohan, and D. J. Baylink. 2002. Ex vivo gene therapy with stromal cells transduced with a retroviral vector containing the BMP4 gene completely heals critical size calvarial defect in rats. Gene Ther. 9:991-999. [DOI] [PubMed] [Google Scholar]
- 30.Hanawa, H., P. F. Kelly, A. C. Nathwani, D. A. Persons, J. A. Vandergriff, P. Hargrove, E. F. Vanin, and A. W. Nienhuis. 2002. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 5:242-251. [DOI] [PubMed] [Google Scholar]
- 31.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 32.Hurwitz, D. R., M. Kirchgesser, W. Merrill, T. Galanopoulos, C. A. McGrath, S. Emami, M. Hansen, V. Cherington, J. M. Appel, C. B. Bizinkauskas, H. H. Brackmann, P. H. Levine, and J. S. Greenberger. 1997. Systemic delivery of human growth hormone or human factor IX in dogs by reintroduced genetically modified autologous bone marrow stromal cells. Hum. Gene Ther. 8:137-156. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda, Y., Y. Takeuchi, F. Martin, F. L. Cosset, K. Mitrophanous, and M. Collins. 2003. Continuous high-titer HIV-1 vector production. Nat. Biotechnol. 21:569-572. [DOI] [PubMed] [Google Scholar]
- 34.Jaalouk, D. E., N. Eliopoulos, C. Couture, S. Mader, and J. Galipeau. 2000. Glucocorticoid-inducible retrovector for regulated transgene expression in genetically engineered bone marrow stromal cells. Hum. Gene Ther. 11:1837-1849. [DOI] [PubMed] [Google Scholar]
- 35.Julias, J. G., A. L. Ferris, P. L. Boyer, and S. H. Hughes. 2001. Replication of phenotypically mixed human immunodeficiency virus type 1 virions containing catalytically active and catalytically inactive reverse transcriptase. J. Virol. 75:6537-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobinger, G. P., D. J. Weiner, Q. C. Yu, and J. M. Wilson. 2001. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 19:225-230. [DOI] [PubMed] [Google Scholar]
- 37.Kowolik, C. M., and J. K. Yee. 2002. Preferential transduction of human hepatocytes with lentiviral vectors pseudotyped by Sendai virus F protein. Mol. Ther. 5:762-769. [DOI] [PubMed] [Google Scholar]
- 38.Krebsbach, P. H., K. Zhang, A. K. Malik, and K. Kurachi. 2003. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: human factor IX model. J. Gene Med. 5:11-17. [DOI] [PubMed] [Google Scholar]
- 39.Kumar, M., B. P. Bradow, and J. Zimmerberg. 2003. Large-scale production of pseudotyped lentiviral vectors using baculovirus GP64. Hum. Gene Ther. 14:67-77. [DOI] [PubMed] [Google Scholar]
- 40.Landau, N. R., and D. R. Littman. 1992. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J. Virol. 66:5110-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landau, N. R., K. A. Page, and D. R. Littman. 1991. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J. Virol. 65:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, K., M. K. Majumdar, D. Buyaner, J. K. Hendricks, M. F. Pittenger, and J. D. Mosca. 2001. Human mesenchymal stem cells maintain transgene expression during expansion and differentiation. Mol. Ther. 3:857-866. [DOI] [PubMed] [Google Scholar]
- 43.Lewis, B. C., N. Chinnasamy, R. A. Morgan, and H. E. Varmus. 2001. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector. J. Virol. 75:9339-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marino, M. P., M. J. Luce, and J. Reiser. 2003. Small-to high-scale production of lentivirus vectors. Methods Mol. Biol. 229:43-55. [DOI] [PubMed]
- 45.Martino, S., C. Cavalieri, C. Emiliani, D. Dolcetta, M. G. Cusella De Angelis, V. Chigorno, G. M. Severini, K. Sandhoff, C. Bordignon, S. Sonnino, and A. Orlacchio. 2002. Restoration of the GM2 ganglioside metabolism in bone marrow-derived stromal cells from Tay-Sachs disease animal model. Neurochem. Res. 27:793-800. [DOI] [PubMed] [Google Scholar]
- 46.Marx, J. C., J. A. Allay, D. A. Persons, S. A. Nooner, P. W. Hargrove, P. F. Kelly, E. F. Vanin, and E. M. Horwitz. 1999. High-efficiency transduction and long-term gene expression with a murine stem cell retroviral vector encoding the green fluorescent protein in human marrow stromal cells. Hum. Gene Ther. 10:1163-1173. [DOI] [PubMed] [Google Scholar]
- 47.Matzner, U., K. Harzer, R. D. Learish, J. A. Barranger, and V. Gieselmann. 2000. Long-term expression and transfer of arylsulfatase A into brain of arylsulfatase A-deficient mice transplanted with bone marrow expressing the arylsulfatase A cDNA from a retroviral vector. Gene Ther. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 48.Matzner, U., F. Schestag, D. Hartmann, R. Lullmann-Rauch, R. D'Hooge, P. P. De Deyn, and V. Gieselmann. 2001. Bone marrow stem cell gene therapy of arylsulfatase A-deficient mice, using an arylsulfatase A mutant that is hypersecreted from retrovirally transduced donor-type cells. Hum. Gene Ther. 12:1021-1033. [DOI] [PubMed] [Google Scholar]
- 49.Min, Y. H., G. X. Li, J. H. Jang, H. C. Suh, J. S. Kim, J. W. Cheong, S. T. Lee, J. S. Hahn, and Y. W. Ko. 2002. Long-term bone marrow culture-derived stromal fibroblasts as a potential target for gene therapy in acute myelogenous leukemia. Leukoc. Res. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 50.Minguell, J. J., A. Erices, and P. Conget. 2001. Mesenchymal stem cells. Exp. Biol. Med. 226:507-520. [DOI] [PubMed] [Google Scholar]
- 51.Mochizuki, H., J. P. Schwartz, K. Tanaka, R. O. Brady, and J. Reiser. 1998. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol. 72:8873-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 53.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 54.Nolta, J. A., M. B. Hanley, and D. B. Kohn. 1994. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood 83:3041-3051. [PubMed] [Google Scholar]
- 55.Padgett, K. A., and J. A. Sorge. 1996. Creating seamless junctions independent of restriction sites in PCR cloning. Gene 168:31-35. [DOI] [PubMed] [Google Scholar]
- 56.Pereira, R. F., K. W. Halford, M. D. O'Hara, D. B. Leeper, B. P. Sokolov, M. D. Pollard, O. Bagasra, and D. J. Prockop. 1995. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA 92:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147. [DOI] [PubMed] [Google Scholar]
- 58.Porter, C. D., M. K. Collins, C. S. Tailor, M. H. Parkar, F. L. Cosset, R. A. Weiss, and Y. Takeuchi. 1996. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum. Gene Ther. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 59.Reiser, J. 2000. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 7:910-913. [DOI] [PubMed] [Google Scholar]
- 60.Reiser, J., G. Harmison, S. Kluepfel-Stahl, R. O. Brady, S. Karlsson, and M. Schubert. 1996. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc. Natl. Acad. Sci. USA 93:15266-15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reiser, J., Z. Lai, X. Y. Zhang, and R. O. Brady. 2000. Development of multigene and regulated lentivirus vectors. J. Virol. 74:10589-10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez-Ramos, J., S. Song, F. Cardozo-Pelaez, C. Hazzi, T. Stedeford, A. Willing, T. B. Freeman, S. Saporta, W. Janssen, N. Patel, D. R. Cooper, and P. R. Sanberg. 2000. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 164:247-256. [DOI] [PubMed] [Google Scholar]
- 63.Sandrin, V., B. Boson, P. Salmon, W. Gay, D. Negre, R. Le Grand, D. Trono, and F. L. Cosset. 2002. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100:823-832. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz, E. J., G. M. Alexander, D. J. Prockop, and S. A. Azizi. 1999. Multipotential marrow stromal cells transduced to produce l-DOPA: engraftment in a rat model of Parkinson disease. Hum. Gene Ther. 10:2539-2549. [DOI] [PubMed] [Google Scholar]
- 65.Schwarz, E. J., R. L. Reger, G. M. Alexander, R. Class, S. A. Azizi, and D. J. Prockop. 2001. Rat marrow stromal cells rapidly transduced with a self-inactivating retrovirus synthesize l-DOPA in vitro. Gene Ther. 8:1214-1223. [DOI] [PubMed] [Google Scholar]
- 66.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Southgate, C., M. L. Zapp, and M. R. Green. 1990. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature 345:640-642. [DOI] [PubMed] [Google Scholar]
- 68.Stitz, J., C. J. Buchholz, M. Engelstadter, W. Uckert, U. Bloemer, I. Schmitt, and K. Cichutek. 2000. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology 273:16-20. [DOI] [PubMed] [Google Scholar]
- 69.Sutton, R. E., and D. R. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takada, T., K. Iida, T. Awaji, K. Itoh, R. Takahashi, A. Shibui, K. Yoshida, S. Sugano, and G. Tsujimoto. 1997. Selective production of transgenic mice using green fluorescent protein as a marker. Nat. Biotechnol. 15:458-461. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi, Y., F. L. Cosset, P. J. Lachmann, H. Okada, R. A. Weiss, and M. K. Collins. 1994. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J. Virol. 68:8001-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuda, H., T. Wada, Y. Ito, H. Uchida, H. Dehari, K. Nakamura, K. Sasaki, M. Kobune, T. Yamashita, and H. Hamada. 2003. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol. Ther. 7:354-365. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, X. Y., V. F. La Russa, L. Bao, J. Kolls, P. Schwarzenberger, and J. Reiser. 2002. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol. Ther. 5:555-565. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, X. Y., V. F. La Russa, and J. Reiser. 2003. Mesenchymal stem cells. Methods Mol. Biol. 229:131-140. [DOI] [PubMed]