Abstract
The spring-loaded model stipulates that influenza virus infection is coupled to the transition of the virus hemagglutinin (HA) from a metastable conformation to a highly stable conformation at low pH. The properties of retrovirus envelope glycoproteins indicate that infection is coupled to an analogous conformational change. As a test of this hypothesis, the requirements for avian leukosis virus A (ALV-A) infection were examined. These studies indicate that, like HA, the conformation of the mature ALV-A envelope glycoprotein is metastable and that infection is linked to refolding at low pH. However, unlike HA, low-pH activation is only observed after priming by receptor. Therefore, ALV-A infection is dependent on the synergistic effects of receptor binding and low pH, suggesting that receptor binding superimposes an additional constraint on activation of ALV-A fusion that proceeds by a mechanism comparable to that of influenza virus.
Distinct classes of enveloped viruses have adopted remarkably similar strategies to gain entry into cells. Influenza virus, Ebola virus, retroviruses, and the paramxyoviruses each encode a single membrane glycoprotein that forms a homotrimer and is cleaved during maturation, forming a complex that is incorporated into the virus (17). In this complex, the N-terminal subunit functions as a clamp to restrain the C-terminal transmembrane (TM) subunit. Infection occurs when cell factors release the clamp, and the TM protein refolds into a highly stable conformation (13, 37). The central feature of the stable conformation is a six-stranded bundle in which the membrane-proximal portion of TM folds back onto the grooves of an extended, three-stranded coiled coil formed by heptad repeats in the N-terminal portion of TM. There is mounting evidence that virus fusion and entry are coupled to formation of these helical hairpins (23, 24, 31).
For retroviruses, a central issue is how receptor binding to the surface subunit (SU) of the envelope glycoprotein (Env) triggers this conformational change in the TM subunit. Avian leukosis viruses (ALVs) offer several advantages for studying this problem. First, they have been engineered by Hughes et al. to create vectors that contain the marker gene for enhanced green fluorescent protein (EGFP) and replicate to high titer in avian cells (5, 16, 25). Second, these viruses are defective for assembly in mammalian cells, thereby providing a means for quantitative measurement of a single round of infection (15). Third, the TM domains of the cellular receptors for these viruses are not required for infection (9, 22). Protocols have been developed in which addition of the ectodomain of these receptor proteins to the culture medium is sufficient to confer ALV infection to nonpermissive cells. In contrast, soluble, recombinant forms of receptors for murine leukemia virus (MLV) and human immunodeficiency virus (HIV) have not been identified to date. Finally, the postactivation conformation of ALV TM is a highly stable oligomer that is readily detected on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (25). This oligomer is likely indicative of the formation of the six-stranded bundle analogous to the postfusion conformation of HIV TM and influenza virus hemagglutinin 2 (HA2) and therefore forms the basis for an assay to monitor the activation of the ALV fusion mechanism in vitro.
Previous studies indicate that direct binding of ALV-A to its receptor, Tva, is sufficient for virus association with lipid membranes (10, 12, 20) but not for infection, which is blocked by inhibition of endosomal acidification (25). Based on these studies, it has been proposed that ALV-A infection is the end result of a multistep process that requires the sequential effects of binding to receptor and exposure to acid pH. In this report, we have investigated this model by examining the activities of receptor and acid pH in inducing conformational changes in TM under defined conditions in vitro and by correlating these findings with infection. Our studies demonstrate that the threshold for refolding of TM is significantly reduced by receptor binding and acid pH, but not by either factor alone, indicating that a single large kinetic barrier stabilizes TM in the prefusion conformation.
MATERIALS AND METHODS
Cell lines.
Human 293 cells were obtained from the American Type Culture Collection. Avian DF-1 cells that produce a replication-competent ALV-A encoding EGFP [RCASBP(A)-EGFP, DF-1-ALV-A] and a 293-derived cell line that expresses Tva (293-Tva or 2.1) have been described previously (25). These cells were propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 4 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C in 5% CO2. The growth medium of 293-Tva cells was supplemented with 1 μg of puromycin per ml.
ALV-A production.
DF-1-ALV-A cells were grown in roller bottles with an extended surface area in 50 ml of medium. Supernatants were harvested twice a day, and individual collections were pooled until 1 liter of supernatant accumulated. Supernatants were precleared by low-speed centrifugation and filtered through 0.45-μm-pore filters to remove cells and cell debris. Aliquots were frozen for later use. Virus was purified and concentrated by pelleting through a sucrose cushion containing 15% sucrose in HN buffer (10 mM HEPES, 130 mM NaCl [pH 8.0]) for 1 h at 4°C at 25,000 rpm in a Beckman SW28 swinging bucket rotor. The virus pellet was resuspended in HN buffer for 1 h at 4°C before use.
sTva purification.
The cDNA encoding Tva residues 10 to 56 was amplified by PCR and subcloned into the plasmid pMMHb (26) using HindIII and BamHI restriction sites. Tva expressed from this construct is fused to a modified form of a His9-trpLE sequence in which the methionine and cysteine residues have been replaced by leucine and alanine (4). The fusion protein was expressed in Escherichia coli strain BL21(DE3) Codon Plus (Stratagene), isolated from inclusion bodies, and purified by the principles outlined by Blacklow and Kim (4). Inclusion bodies were dissolved in a mixture containing 50 mM Tris (pH 8.5), 6 M guanidine hydrochloride, and 8 mM 2-hydroxyethyl disulfide and oxidized overnight at room temperature. The fusion protein was precipitated from solution by 10-fold dilution in double-distilled H2O (ddH2O). The insoluble portion containing the fusion protein was dissolved in 70% formic acid, and the hydrophobic His9-trpLE leader was removed by cleavage with cyanogen bromide. The cleavage reaction was quenched with glycine and dialyzed extensively against 10% glycine and HCl overnight. Insoluble peptides including uncleaved fusion protein and His9-trpLE leader peptide were removed by centrifugation. Precipitation of additional insoluble protein was induced by adjusting the pH to 8 in 3 mM Tris and 5 mM dithiothreitol (DTT). The soluble fraction containing soluble Tva (sTva) was dialyzed against a refolding buffer consisting of 10 mM Tris, 2 mM CaCl2, 2 mM reduced glutathione, and 1 mM oxidized glutathione (pH 8.0) over 48 h with buffer changes every 24 h. After a final overnight dialysis without glutathione, the protein was acidified with 5% acetic acid and lyophilized to dryness. The lyophilized protein was dissolved in 5% acetic acid, and sTva was purified as a single peak by reverse-phase high-performance liquid chromatography (HPLC) with a C18 column. sTva identity was confirmed by matrix-assisted laser desorption ionization-time of flight(MALDI-TOF) mass spectometry. The sTva concentration was determined by spectrophotometry (1 A280 unit = 0.38 mg/ml, as calculated by the Protean program [DNAStar, Madison, Wis.]). Purified sTva was stored lyophilized at −80°C or at a high concentration (>40 mg/ml) in ddH20 at 4°C and was stable for months.
bTva purification.
The Tva sequence encoding residues 1 to 82 was cloned into the baculovirus expression vector pFastBac 1 (Invitrogen) as a fusion to three copies of an HA epitope tag and a His6 motif. Recombinant baculovirus was generated using the Bac-to-Bac system as described by the manufacturer (Invitrogen). Recombinant baculovirus was expanded by three passages on SF21 cells propagated in JC100 medium supplemented with 10% FBS, 4 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 27°C. Protein was produced in infected Hi5 cells grown in suspension in baffled flasks in a mixture of Excell 405 medium (250 ml) and SF900 II medium (150 ml) supplemented with 50 μg of gentamicin per ml at 27°C. Tva form baculovirus (bTva) was purified by nickel chelation chromatography from cell supernatant as described previously (1). The protein concentration was determined by bicinchoninic acid assay. Aliquots were stored at −80°C and thawed immediately before use.
Spinoculation and inactivation assays.
For spinoculation, ALV-A-containing supernatant was thawed and incubated for 20 min on ice with the indicated concentrations of sTva or bTva to allow binding. The virus-and-TVA mixture was serially diluted on ice in growth medium supplemented with 40 mM HEPES and added on ice to 12-well plates containing 293 or 293-Tva cells that had been prechilled to 4°C. The plates were centrifuged for 2 h at 1,640 × g in a Beckman Allegra 6R centrifuge at 4°C and then centrifuged for an additional 30 min while the temperature was gradually raised to 35°C as previously described (9). The plates were then transferred to a 37°C incubator and cultured for 36 to 48 h before being fixed in paraformaldehyde and analyzed by flow cytometry for GFP expression. Viral titers were determined by end point dilution.
For inactivation, the virus-containing supernatant was thawed on ice and incubated for 20 min at 4°C with sTva or bTva to allow binding. The pH was adjusted with predetermined volumes of buffer 1 (0.66 M MES [morpholineethanesulfonic acid], 1.69 M acetic acid), and the sample was warmed to 37°C for 30 min. The sample was neutralized to pH 7.4 with predetermined volumes of buffer 2 (0.63 M HEPES, 0.74 M NaOH) and chilled on ice before being serially diluted, added to cells, centrifuged, and analyzed as described above.
TMCA.
The TM SU conformation assay (TMCA) was performed as follows. Pelleted virus was resuspended in a small volume of HN buffer supplemented with 1 mM CaCl2 (HNC) and incubated for 20 min on ice with sTva to allow binding. The pH was adjusted with predetermined volumes of 100 mM Tris-acetate or Tris-HCl, and the sample was incubated at 37°C for 30 min. The samples were then neutralized with 1 M HEPES (pH 8.0), lysed with 1% SDS, and incubated for 10 min at 37°C. Samples were reduced with 100 mM DTT and analyzed by immunoblotting after SDS-PAGE on 13% polyacrylamide gels with an antibody specific for the carboxyl terminus of TM as described previously (25).
To study the effect of calcium, all HN buffers including the sucrose cushion used to purify the virus were treated with Chelex-100 resin (Sigma) to remove divalent metals. EDTA was added where indicated to 100 μM. CaCl2 was added where indicated to 1.1 mM. To determine the temperature threshold to trigger conformational change, purified virions in HN were incubated at the indicated temperature for 30 min or boiled for 5 min prior to lysis in SDS. To study the effects of urea, freshly prepared urea was added at the indicated concentration, and the samples were incubated at 37°C for 30 min. To determine the effects of receptor and pH alone or in combination on the temperature threshold for triggering conformational change, 20 nM sTva was added to purified virions in HNC where indicated, and the mixture was incubated on ice for 20 min to allow binding. The pH of the samples was adjusted with 100 mM Tris-HCl or Tris-acetate to the indicated pH. Samples were divided into aliquots and incubated at the indicated temperatures based on a calculated gradient across the heating block of a PTC-200 thermal cycler (MJ Research) for 30 min before being neutralized and analyzed as described above.
Liposomes.
Large unilamellar vesicles (LUVs) were created by mixing a 2:1 molar ratio of phosphatidylcholine (800 nmol) and cholesterol (400 nmol) in chloroform. The chloroform was evaporated under an argon stream, and the lipid film was lyophilized to dryness. The lipid mixture was rehydrated by vigorous shaking with 154 μl of HN buffer for 1 h at room temperature. The rehydrated lipids were subjected to 5 freeze-thaw cycles before being extruded 21 times through an Avanti mini-extruder with 100-nm pores. LUV formation was verified by transmission electron microscopy using uranyl acetate as a negative stain. For experiments to study the effect of LUVs on the TMCA, purified ALV-A and the indicated concentration of sTva were incubated with 8.47 μl of the LUV suspension in a final volume of 100 μl on ice for 50 min. Samples were divided into two aliquots, the pH was adjusted to 7.4 or 5.0 with Tris, and the samples were treated and analyzed as described above.
RESULTS
Comparison of receptor function of two soluble forms of Tva.
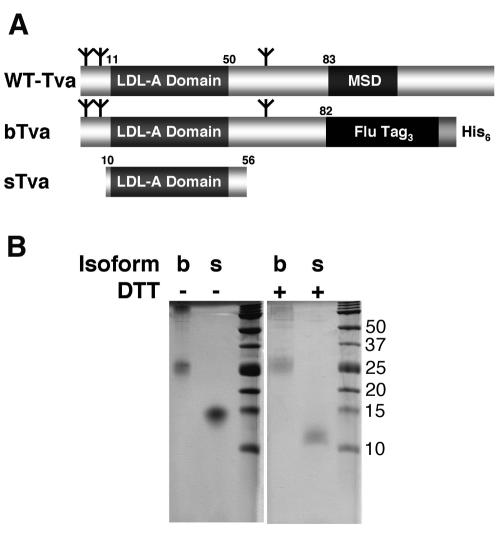
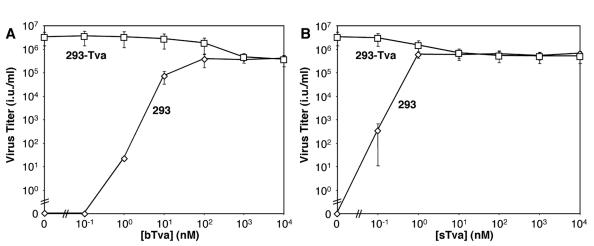
In our initial studies of ALV-A infection, we compared the properties of bTva and sTva, two recombinant forms of the ALV-A receptor (Fig. 1A). bTva consists of the entire ectodomain (residues 1 to 82) of the receptor linked at the carboxyl terminus to an influenza HA epitope tag and a His6 motif. bTva was expressed in insect cells using a recombinant baculovirus vector and purified from supernatant by nickel chelation chromatography (Fig. 1B). sTva is smaller (residues 10 to 56) than bTva and is composed primarily of the domain homologous to the type A ligand-binding repeats (LAs) of the low-density lipoprotein receptor. sTva was expressed in E. coli, purified and refolded in vitro, and recovered in a single peak by HPLC (Fig. 1B). The receptor activities of these proteins were compared by using a spinoculation protocol (9) to measure infection of human 293 cells by RCASBP(A)-EGFP as a function of bTva or sTva concentration in the medium. The capacities of the recombinant receptors to support RCASBP(A)-EGFP infection were similar. The maximum titer of infection mediated by bTva was (3.93 ± 1.22) × 105 infectious units (i.u.)/ml (≥100 nM bTva; Fig. 2A), and that mediated by sTva was (6.46 ± 1.69) × 105 i.u./ml (≥1 nM sTva; Fig. 2B). These findings indicate that both the bTva and sTva isoforms of the ALV-A receptor are functional and confirm that receptor activity resides in the LA module (3, 27-29, 39). The specific activity of sTva exceeded that of bTva by approximately 10-fold, possibly due to heterogeneity in the bTVA preparation caused by incomplete purification or variation in glycosylation and/or in disulfide bond formation. In addition, RCASBP(A)-EGFP infection on 293 cells expressing the Tva receptor (293-Tva) was inhibited by 8- to 10-fold (Fig. 2) when either bTva or sTva was added to the culture medium. This strongly suggests that these proteins compete with membrane-bound receptor for a common binding site on the ALV-A Env glycoprotein.
FIG. 1.
Tva isoforms. (A) Schematic diagram of Tva-derived proteins used in this study. Numbers refer to the positions of residues in mature wild-type (WT) Tva. MSD, membrane-spanning domain. Flu Tag3, three copies of an influenza virus HA epitope tag. Branched symbols denote positions of N-linked glycosylation sites. (B) Coomassie R-250-stained gels of purified bTva and sTva. Where noted, samples were boiled in 100 mM DTT prior to loading.
FIG. 2.
sTva and bTva are functional virus receptors. RCASBP(A)-EGFP virus was incubated with bTva (A) or sTva (B) at the indicated concentrations and spinoculated onto 293 (⋄) or 293-Tva (□) cells as described in Materials and Methods. Forty-eight hours later, cells were analyzed for acquired EGFP expression by flow cytometry, and virus titers (infectious units per milliliter) were determined by end point dilution. Error bars represent the standard deviation of three measurements.
Requirements for in vitro inactivation of ALV-A.
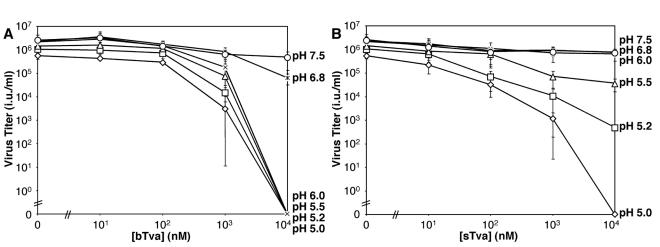
The conditions for recombinant Tva to inactivate virus in vitro were assessed. We observed that incubation of RCASBP(A)-EGFP for 30 min at 37°C and pH 7.5 in the presence of bTva or sTva did not inhibit subsequent infection of 293-Tva cells by spinoculation (Fig. 3). Virus inactivation was not observed even when the concentration of bTva (1,000-fold) or sTva (10,000-fold) markedly exceeded that required for maximal infection. However, RCASBP(A)-EGFP infection was progressively reduced by exposure to these proteins at acid pH. The threshold for inhibition of infection by bTva was pH 6.0 to 6.8 (Fig. 3A), and that for sTva was pH 5.5 to 6.0 (Fig. 3B). Complete inactivation of infection required a high concentration of receptor (>10 μM) and low pH (pH 6.0 for bTva and 5.0 for sTva). These studies demonstrate that low pH is required for receptor-induced inactivation in vitro.
FIG. 3.
In vitro inactivation of ALV-A infection by sTva and bTva is dependent upon exposure to acid pH. RCASBP(A)-EGFP virus was exposed to bTva (A) or sTva (B) at the indicated concentrations. After a 20-min incubation on ice, the pH was adjusted to 5.0 (⋄), 5.2 (□), 5.5 (▵), 6.0 (×), 6.8 (*), or 7.5 (○), and samples were incubated for an additional 30 min at 37°C. After neutralization, virus-receptor complexes were spinoculated onto 293-Tva cells, and these cells were analyzed for acquired EGFP expression 48 h later. Viral titers (infectious units per milliliter) were determined by end point dilution. Error bars represent the standard deviation of three measurements.
Inactivation of ALV-A infection correlates with a conformational change in ALV-A TM.
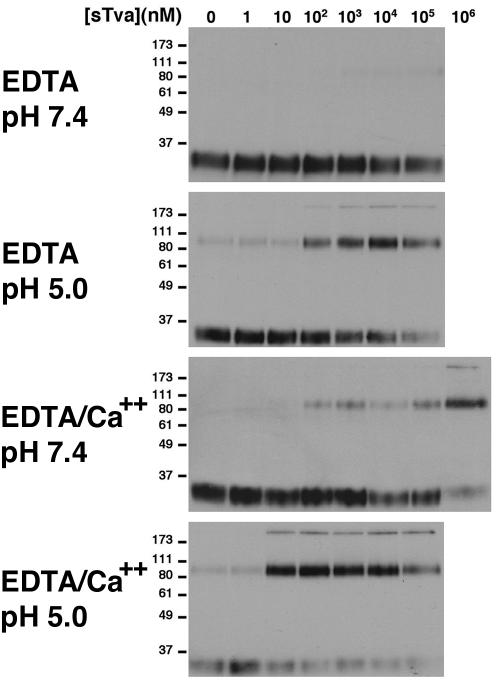
Previously, we observed that inactivation of ALV-A by a recombinant Tva-epidermal growth factor (EGF) fusion protein correlated with a conformational change in TM (25). This conformational change was detected by the reduced mobility of TM when assayed by immunoblotting after SDS-PAGE (TMCA). The mobility of the 90-kDa receptor-induced conformation of TM is consistent with the formation of an SDS-resistant homotrimer analogous to the highly stable postfusion conformation of the influenza virus HA2 (6). The reported stability of this conformation in SDS to 80°C supports this conclusion. We determined the conformation of TM of RCASBP(A)-EGFP after exposure to defined concentrations of sTva at neutral (pH 7.4) and acid (pH 5.0) pH. At neutral pH, the presence of sTva did not induce formation of the 90-kDa conformation (Fig. 4, top panel). However at pH 5.0 (Fig. 4, upper middle panel), a significant fraction of TM was converted to this stable isoform in sTva (≥100 nM). We also observed a >170-kDa species that likely represents a higher-order oligomer of TM, perhaps formed by the packing of the carboxyl-terminal α helix from the TM subunit of one trimer against the amino-terminal coiled coil from a neighboring trimer. Precise quantitative assessment of the percentage of conversion following receptor or low-pH-induced conformational change in TM was confounded by the enhanced recognition of oligomers by the bivalent anti-TM antibody.
FIG. 4.
Acid pH and calcium increase the specific activity of sTva for inducing a conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with increasing concentrations of sTva. After incubation, samples were exposed to DTT and loaded onto SDS-PAGE gels without boiling. All experiments were performed in 100 μM EDTA, and 1.1 mM CaCl2 was added where indicated. Formation of the 90- and >170-kDa species indicative of the postfusion conformation of TM was determined by immunoblotting (TMCA). Note that the 90-kDa and >170-kDa species observed in this experiment are equivalent to the 70- and 150-kDa species described in our previous report (25). The discrepancy in assigned molecular masses is dependent upon the concentration of polyacrylamide in SDS-PAGE. In lower-percentage gels (4 to 15% gradient gels and 10% gels), the oligomeric TM species migrate at approximately 70 and 150 kDa (25; data not shown). However, in the 13% gels shown here, they migrate at approximately 90 and >170 kDa.
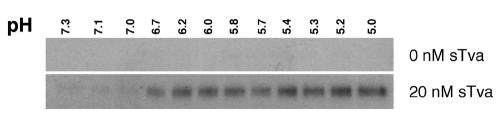
These studies were performed under conditions in which calcium was depleted by EDTA. However, structural studies reveal a calcium ion coordinated in the LA module of Tva that may be required for receptor function (34-36). Therefore, the influence of calcium ion repletion on sTva activity was also measured. Low but consistently detectable formation of the 90- and >170-kDa conformations of TM was mediated by sTva at neutral pH in the presence of 1 mM Ca2+ (Fig. 4, lower middle panel). However, at acid pH, 10 nM sTva was sufficient to convert >90% of TM to the 90- and >170-kDa isoforms (Fig. 4, bottom panel). Equivalent results were observed with virus that had not been exposed to EDTA (data not shown). In the presence of Ca2+ and sTva, the threshold for formation of the TM oligomer is pH 6.7 (Fig. 5). Consistent with the previous studies of in vitro inactivation, these findings indicate that the specific activity of sTva is markedly increased at acid pH (>10,000-fold). However, the sTva-dependent formation of the stable TM conformations was never complete.
FIG. 5.
Determination of the pH threshold for sTva-induced conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with or without 20 nM sTva in the presence of 100 μM EDTA and 1.1 mM CaCl2 at the indicated pHs and analyzed by TMCA as described in the legend to Fig. 4. Only the 90-kDa species of TM is shown.
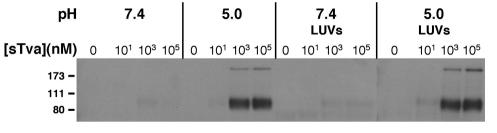
Presence of lipid membranes does not alleviate the acid pH requirement for Tva-induced conformational change in TM.
We considered the possibility that the presence of a target membrane might alter the requirements for in vitro inactivation of ALV-A infection, possibly by providing a hydrophobic environment to accommodate exposure of the fusion peptide in TM. To test this hypothesis, the TMCA was repeated in the presence of liposomes. LUVs were prepared according to an established protocol (10, 12, 20), and their capacity to associate with receptor-activated ALV-A was verified (data not shown). The presence of LUVs did not alter the threshold for sTva- or pH-induced conformational change at neutral or acid pH (Fig. 6). Moreover, varying the LUV concentration over a 300-fold range did not significantly increase the fraction (<10%) of TM that was converted to oligomer by sTva at pH 7.4 (data not shown). We conclude that the presence of lipid bilayers did not alter the receptor or pH requirement for formation of the TM oligomer that is likely indicative of the postfusion conformation. Similar findings were obtained with crude membrane preparations from either 293 or 293-Tva cells (data not shown), thereby suggesting that an additional component specific to cell membranes does not trigger the change in TM.
FIG. 6.
The presence of lipid targets does not alter the receptor and pH requirements for conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with increasing concentrations of sTva in the presence or absence of liposomes at pH 5.0 or 7.4 and analyzed for the presence of the 90- and >170-kDa isoforms by TMCA as described in the legend to Fig. 4.
The native conformation of ALV-A Env is metastable.
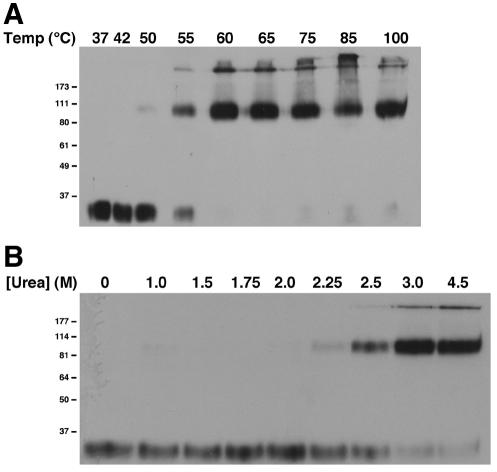
The ability to monitor the conversion of ALV-A TM to an SDS-resistant oligomer provided the basis for an experiment to directly test whether the native ALV-A Env is kinetically trapped in a metastable conformation. Previously, Carr and colleagues showed that mild chemical or thermal denaturation was equivalent to acid pH in surmounting the kinetic barrier to formation of the stable, postfusion conformation of influenza virus HA (7). We adapted this protocol for the study of ALV-A. Aliquots of purified virions were incubated at temperatures from 37 to 85°C for 30 min or were boiled for 5 min at pH 7.4 and then cooled to room temperature. We observed that formation of the 90-kDa conformation of TM occurred at 55°C (Fig. 7A) under these assay conditions. Further increases in temperature resulted in complete loss of the 34-kDa isoform and partial conversion to the high-molecular-mass (>170 kDa) isoform. Similarly, exposure of virions to >2.25 M urea also induced formation of the 90- and >170-kDa TM isoforms (Fig. 7B). Therefore, both thermal denaturation and chemical denaturation of ALV-A induced a conformational change in TM equivalent to that induced by receptor and acid pH, strongly suggesting that the prefusion conformation of ALV-A Env, like that of HA, is metastable.
FIG. 7.
Mild denaturation induces the formation of oligomeric ALV-A TM. RCASBP(A)-EGFP virus was incubated at the indicated temperatures for 30 min or boiled for 5 min (A) or incubated at 37°C in the presence of increasing concentrations of urea (B) and analyzed by TMCA as described in the legend to Fig. 4.
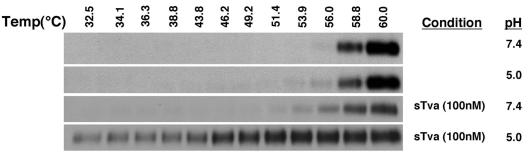
Tva and acid pH are synergistic in reducing the kinetic barrier to ALV-A TM conformational change.
In studies of influenza virus HA, progressive exposure to acid pH reduced the temperature threshold for conformational change, suggesting that protonation reduced the kinetic barrier to refolding that is linked to fusion (7, 21). We assessed the capacity of sTva and of acid pH, alone or in combination, to reduce the threshold for heat-induced formation of the stable conformation of ALV-A TM. Virus was incubated in sTva (100 nM, 1 mM Ca2+) and/or acid pH, and the temperature threshold for the conformational change in TM was assessed by TMCA. We observed a small but detectable reduction in the temperature threshold for formation of the 90-kDa TM isoform at pH 5.0 or in the presence of sTva at neutral pH (Fig. 8). In each case and in the untreated control, significant conversion of TM to the 90-kDa isoform occurred at 58.8°C. However, a dramatic reduction in the thermal threshold was induced by exposure to sTva and acid pH together. Significant formation of the 90-kDa isoform was observed even at 32.5°C, the lowest temperature that was assayed. These observations strongly indicate that acid pH-induced refolding of ALV-A TM to the postfusion conformation is strictly dependent on receptor binding, possibly indicating that Tva exposes critical titratable residues in Env. Therefore, the behavior of ALV-A Env is distinct from that of influenza virus HA for which receptor binding is not required.
FIG. 8.
Determination of the effects of sTva and acid pH on the thermal threshold for triggering conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated at the indicated temperatures in the presence or absence of 100 nM sTva at pH 5.0 or 7.4 and analyzed by TMCA as described in the legend to Fig. 4. Only the 90-kDa species of TM is shown.
DISCUSSION
Previous work suggested that ALV infection is a two-step process in which receptor binding primes the envelope glycoprotein for triggering at acid pH (25). We have tested this conclusion by performing quantitative experiments under defined conditions. We have obtained evidence that the native ALV-A envelope glycoprotein resides in a metastable conformation and that both receptor binding and acid pH are required to induce the conformational change in TM that is coupled to fusion. These results are consistent with previous studies (11, 25) indicating that ALV-A infection occurs in acidified endosomes.
Requirements for Tva function.
Several studies have mapped the receptor function of Tva to the LA module (3, 27-29, 39). In this report, we demonstrate that this domain is sufficient to activate the virus fusion mechanism and to mediate infection. The possibility that residues in the ectodomain of Tva outside of the LA module might enhance receptor activity was also examined. Indeed, ALV-A treated with the entire ectodomain (bTva) was more sensitive to acid pH-induced inactivation of infection than virus treated with the LA module (sTva) alone (Fig. 3). However, the activities of bTva and sTva were equivalent in triggering formation of the stable TM conformation (data not shown), and the activity of sTva was greater than that of bTva in mediating ALV-A infection by spinoculation (Fig. 2). The observed differences between bTva and sTva may be due to the complex influence of residues outside of the LA module or to heterogeneity in the bTva preparation secondary to processing in insect cells. However, a well-defined role for the portion of the receptor ectodomain outside of the LA module was not revealed by these studies. To date, all LA modules that have been studied, including that of Tva, contain a structural calcium ion (14, 34-36). Our studies show that once sTva folding is completed, the extraction of calcium ion from the domain by reverse-phase chromatography and by EDTA chelation reduced (>100-fold) but did not abolish receptor activity. This indicates that calcium ion is not required for binding of receptor to ALV-A SU, likely because the conformation of the SU binding site is restricted by the three native intramolecular disulfide bonds in the LA module and does not require the locked conformation imposed by calcium ion coordination. This conclusion is consistent with the recent report by Yu et al. and with the reported roles of W48 and H38 in receptor activity (20, 28, 29, 38-40). The side chains of these two residues face the solvent and are not directly involved in calcium ion coordination (34, 36). Therefore, the SU binding site may be a small motif superimposed on a scaffold provided by the LA module. Similarly, a peptide derived from the receptor for the closely related ALV-B subgroup of viruses retains receptor activity (22).
In vitro inactivation of ALV-A requires acid pH.
A previous study identified the low-pH requirement for ALV-A infection and for receptor-dependent conformational change in TM (25). A key experiment in this study demonstrated in vitro inactivation of virus by Tva. Inactivation was measured as a reduction of infection after incubation of virus at low pH with a chimeric protein composed of the ectodomain of Tva fused to the ligand EGF (Tva-EGF) and subsequent exposure to cells expressing the EGF receptor (EGFR). Infection was dependent upon the binding of the chimeric protein to EGFR on the cell surface and to the ALV-A envelope glycoprotein (33). A potential shortcoming of this strategy was an inability to discriminate between a reduction in infection caused by low-pH-induced dissociation of the bridge protein from virus and inactivation caused by premature triggering of the virus fusion mechanism. Moreover, the sensitivity of this experiment was limited because virus titers were low, possibly due to the use of suboptimal (and undefined) concentrations of bridge protein. Our studies have addressed these shortcomings by substituting purified sTva as the source of receptor and by measuring its activity through spinoculation onto 293-Tva cells. This approach promotes high infection efficiency under defined sTva concentrations and eliminates the requirement for a bridge protein to mediate virus attachment in order to assess receptor activity.
Using this protocol, we observed a correlation between inactivation of ALV-A infection and the formation of the stable TM oligomer. The endpoints of both assays were strictly receptor dependent and more efficient at acid pH. However, careful quantification of receptor concentration and pH revealed that in all cases, the threshold for formation of the stable TM oligomer was lower than that for virus inactivation. For example, high concentrations of sTva at neutral pH induced some oligomer formation (Fig. 4), although no virus inactivation was observed (Fig. 3). In addition, the threshold for receptor-dependent oligomer formation at low sTva concentration (Fig. 5) was observed at a higher pH than the threshold for virus inactivation (Fig. 3). Moreover, a higher concentration of receptor was required to inactivate virus (Fig. 3) than to trigger oligomer formation (Fig. 4, bottom panel). These findings likely reflect an inherent difference between the TMCA, which is a gain-of-function assay, and virus inactivation, which measures loss of function. They also indicate the existence of pools of Env with distinct thresholds for pH activation caused by heterogeneity in the affinity for sTva or susceptibility to conformational change. Such differences may arise from lateral interactions among Env glycoproteins during activation and/or from variation in posttranslational modification but are unlikely to arise from mutations, since the viruses were produced from cells transfected with a cloned provirus. Taken together, our results strongly support the conclusion from previous studies with Tva-EGF that the threshold for receptor-induced ALV-A infection is markedly decreased by acid pH (25). However, the possibility that other cellular factors play a role in infection efficiency remains open. In particular, the possibility that acid pH is a surrogate for another factor provided at the target cell membrane that functions coordinately with Tva and is indirectly blocked by inhibitors of endosome acidification cannot be excluded. However, a simple requirement for a lipid membrane to stabilize hydrophobic domains in the ALV-A Env exposed by sTva binding was not supported by our studies, which showed that the presence of liposomes did not mitigate the requirement for acid pH.
ALV-A Env is metastable.
Carr and colleagues provided direct evidence that the conformation of the mature influenza virus HA is metastable (7). They showed that exposing virus to denaturing conditions, such as elevated temperature or urea concentration, was sufficient to induce formation of the six-helix bundle in HA2. In their experiments, refolding of HA2 was abruptly triggered at a transition temperature between 58 and 60°C or at a urea concentration between 3.5 and 3.75 M. The conformation of HA2 induced by these perturbations was indistinguishable from the conformation induced by acid pH and was stable after removing the inciting conditions. These findings indicate that after cleavage during maturation, the conformation of HA2 on the virus surface is unstable but is prevented from refolding to the stable conformation by a significant kinetic barrier. Infection is coupled to refolding of HA2 favored by an acid-dependent decrease in this barrier.
Current models of retrovirus entry are based on the assumption that Env resides in a metastable conformation on the mature virion surface (13). Evidence for the metastability of Env is indirect and is based largely on the properties of inhibitory peptides and by analogy to influenza virus HA. In this report, we provide the first evidence to show that like HA2, ALV-A TM refolding to a stable oligomer is also induced by elevated temperature and urea concentration. Indeed, the temperature (between 58 and 60°C) and urea concentration (between 2.25 and 2.5 M) required to induce stable oligomer formation are strikingly similar to the conditions required for HA2 refolding. These findings directly demonstrate that, like HA2, the mature ALV-A TM resides in a kinetically trapped metastable conformation and provide more evidence to suggest that the postactivation conformation is a homotrimer.
Roles of receptor binding and acid pH in the formation of the putative TM homotrimer.
The notion that the TM oligomer is indicative of the postfusion conformation provided the basis for a direct assessment of the roles of receptor binding and low pH in surmounting the kinetic barrier to fusion. In Fig. 8, we show that acid pH or sTva at pH 7.4 each caused a small, but reproducible, reduction in the thermal threshold for TM acquiring the postfusion conformation. These findings are in contrast to the marked reduction in the thermal threshold in virions exposed to sTva and low pH. Moreover, the behavior of ALV-A TM is distinct from that of influenza virus HA, for which receptor binding is not required to establish a direct relationship between acid pH and the threshold for heat-induced conformational change (21, 30, 32). These findings support the previous conclusion that receptor binding primes ALV-A Env for a pH-dependent refolding of TM to the stable conformation containing the six-helix bundle. Although refolding to a stable hairpin conformation is central to fusion of influenza virus and retrovirus, each virus has adopted a distinct mechanism for triggering this change.
Proposed role for acid pH in ALV-A infection.
At present, the stage of the fusion reaction that requires the acid pH-induced conformational change in ALV-A Env is unknown. Recent work has revealed new details about the Env protein and lipid rearrangements that occur during fusion by HIV and ALV-A (12, 23). Based on these studies and our findings, we propose the following model for the mechanism of ALV-A infection and the role of acid pH. After budding, native ALV-A Env is a metastable trimer of heterodimers on the virion surface. The SU of Env binds to Tva at the plasma membrane (1, 2, 8, 18). Tva binding induces a conformational change in Env indicated by increased sensitivity of SU to thermolysin cleavage and exposure of TM to fusion peptide-specific antibodies (19). The exposed fusion peptide then is inserted into the target lipid bilayer, as shown by enhanced virus association with liposomes (10, 12, 20). In one of these reports, liposome association was not inhibited by peptides designed to prevent the formation of the stable hairpin conformation, suggesting that TM is in an elongated, prehairpin conformation (12). After insertion of the fusion peptide into the target bilayer, additional refolding of TM “pulls” the viral and target bilayers into closer apposition. Hemifusion resulting from merger of the opposed outer leaflets of the lipid bilayer and subsequent merger of the inner leaflets leads to formation of a fusion pore (24). Membrane merger was demonstrated in a recent study measuring the dequenching of pyrene in labeled ALV-A upon fusion with target cells; however, these studies could not distinguish hemifusion and fusion pore formation (12). In this study, pyrene dequenching was inhibited by peptides designed to prevent hairpin formation, suggesting that at least some refolding of TM to the low-energy conformation is coupled to fusion. Moreover, inhibition of endosomal acidification by bafilomycin did not prevent receptor-induced lipid mixing, suggesting that at least some hairpins had formed at neutral pH. In support of this conclusion, our studies directly demonstrate a low (<10%), but reproducible, level of stable homotrimer formation at neutral pH. However, protonation of Env at acid pH leads to nearly complete conversion of TM to stable trimer in this assay. Recent studies of HIV fusion have shown that the bulk of hairpin formation occurs after initial fusion pore formation and functions to stabilize and enlarge the fusion pore (23). If also true for ALV-A, these results suggest that protonation of ALV-A Env, although not strictly required for lipid mixing, is required for formation of a fusion pore that can permit entry of the core, perhaps by promoting more extensive local hairpin formation and/or by promoting lateral interactions between trimers. This hypothesis provides an explanation for the findings that receptor binding is sufficient to initiate virus-target membrane merger, but not ALV-A cDNA synthesis, which is dependent on entry of the core into the cytoplasm (12, 25). Although we hypothesize that the acid pH-dependent step is near the end of the entry process, a strict temporal requirement for the shift from neutral to acid pH is not required, since liposome association and lipid mixing occur equally well at acid pH and at neutral pH (12). A key test of this model will be provided by studies of the recently described peptide inhibitor of hairpin formation.
Acknowledgments
We thank Adrienne Boerger for an initial experiment indicating that treatment of ALV-A with heat or urea leads to the conformational change in TM and Qing Yao for cloning Tva into the baculovirus vector.
This work was supported by the Howard Hughes Medical Institute (J.M.C.) and by grants from the National Institutes of Health (T32-HL07623 and T32-AI07498 to J.G.S.).
REFERENCES
- 1.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 3.Bélanger, C., K. Zingler, and J. A. T. Young. 1995. Importance of cysteines in the LDLR-related domain of the subgroup A avian leukosis and sarcoma virus receptor for viral entry. J. Virol. 69:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blacklow, S. C., and P. S. Kim. 1996. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat. Struct. Biol. 3:758-762. [DOI] [PubMed] [Google Scholar]
- 5.Boerkoel, C. F., M. J. Federspiel, D. W. Salter, W. Payne, L. B. Crittenden, H. J. Kung, and S. H. Hughes. 1993. A new defective retroviral vector system based on the Bryan strain of Rous sarcoma virus. Virology 195:669-679. [DOI] [PubMed] [Google Scholar]
- 6.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 7.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly, L., K. Zingler, and J. A. T. Young. 1994. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J. Virol. 68:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Griffero, F., S. A. Hoschander, and J. Brojatsch. 2002. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J. Virol. 76:12866-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earp, L. J., S. E. Delos, R. C. Netter, P. Bates, and J. M. White. 2003. The avian retrovirus avian sarcoma/leukosis virus subtype A reaches the lipid mixing stage of fusion at neutral pH. J. Virol. 77:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 14.Fass, D., S. Blacklow, P. S. Kim, and J. M. Berger. 1997. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691-693. [DOI] [PubMed] [Google Scholar]
- 15.Federspiel, M. J., P. Bates, J. A. Young, H. E. Varmus, and S. H. Hughes. 1994. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc. Natl. Acad. Sci. USA 91:11241-11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federspiel, M. J., and S. H. Hughes. 1997. Retroviral gene delivery. Methods Cell Biol. 52:179-214. [PubMed] [Google Scholar]
- 17.Fields, B. N., D. M. Knipe, P. M. Howley, and D. E. Griffin. 2001. Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 18.Gilbert, J. M., P. Bates, H. E. Varmus, and J. M. White. 1994. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope glycoprotein. J. Virol. 68:5623-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Q., R. Opitz, E. W. Knapp, and A. Herrmann. 2002. Protonation and stability of the globular domain of influenza virus hemagglutinin. Biophys. J. 82:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knauss, D. J., and J. A. T. Young. 2002. A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 76:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 26.North, C. L., and S. C. Blacklow. 1999. Structural independence of ligand-binding modules five and six of the LDL receptor. Biochemistry 38:3926-3935. [DOI] [PubMed] [Google Scholar]
- 27.Rong, L., and P. Bates. 1995. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J. Virol. 69:4847-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong, L., K. Gendron, and P. Bates. 1998. Conversion of a human low-density lipoprotein receptor ligand binding repeat to a virus receptor: identification of residues important for ligand specificity. Proc. Natl. Acad. Sci. USA 95:8467-8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong, L., K. Gendron, B. Strohl, R. Shenoy, R. J. Wool-Lewis, and P. Bates. 1998. Characterization of determinants for envelope binding and infection in Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 72:4552-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruigrok, R. W., S. R. Martin, S. A. Wharton, J. J. Skehel, P. M. Bayley, and D. C. Wiley. 1986. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology 155:484-497. [DOI] [PubMed] [Google Scholar]
- 31.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 33.Snitkovsky, S., and J. A. Young. 1998. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. USA 95:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonelli, M., R. J. Peters, T. L. James, and D. A. Agard. 2001. The solution structure of the viral binding domain of Tva, the cellular receptor for subgroup A avian leukosis and sarcoma virus. FEBS Lett. 509:161-168. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Q.-Y., K. Dolmer, W. Huang, P. G. W. Gettins, and L. Rong. 2001. Role of calcium in protein folding and function of Tva, the receptor of subgroup A avian sarcoma and leukosis virus. J. Virol. 75:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Q. Y., W. Huang, K. Dolmer, P. G. Gettins, and L. Rong. 2002. Solution structure of the viral receptor domain of Tva and its implications in viral entry. J. Virol. 76:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X., Q. Y. Wang, Y. Guo, K. Dolmer, J. A. T. Young, P. G. W. Gettins, and L. Rong. 2003. Kinetic analysis of binding interaction between the subgroup A Rous sarcoma virus glycoprotein SU and its cognate receptor Tva: calcium is not required for ligand binding. J. Virol. 77:7517-7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zingler, K., C. Bélanger, R. Peters, D. Agard, and J. A. T. Young. 1995. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J. Virol. 69:4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zingler, K., and J. A. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]