Abstract
Cross-sectional studies in both humans and animals have demonstrated associations between obesity and altered reward functions at the behavioral and neural level, but it is unclear whether these alterations are cause or consequence of the obese state. Reward behaviors were quantified in male, outbred Sprague-Dawley (SD) and selected line obesity-prone (OP) and obesity-resistant (OR) rats after induction of obesity by high-fat diet feeding and after subsequent loss of excess body weight by chronic calorie restriction. As measured by the brief access lick and taste-reactivity paradigms, both obese SD and OP rats “liked” low concentrations of sucrose and corn oil less, but “liked” the highest concentrations more, compared with lean rats, and this effect was fully reversed by weight loss in SD rats. Acute food deprivation was unable to change decreased responsiveness to low concentrations but eliminated increased responsiveness to high concentrations in obese SD rats, and leptin administration in weight-reduced SD rats shifted concentration-response curves toward that seen in the obese state in the brief access lick test. “Wanting” and reinforcement learning as assessed in the incentive runway and progressive ratio lever-pressing paradigms was paradoxically decreased in both obese (compared with lean SD rats) and OP (compared with OR rats). Thus, reversible, obesity-associated, reduced “liking” and “wanting” of low-calorie foods in SD rats suggest a role for secondary effects of the obese state on reward functions, while similar differences between select lines of OP and OR rats before induction of obesity indicate a genetic component.
Keywords: motivation, sucrose, corn oil, incentive runway, taste-reactivity, calorie restriction, reward, taste, preference
overindulgence in palatable foods is considered an important factor for explaining the increased prevalence of obesity. A considerable body of literature exists demonstrating differences in reward behaviors and neural reward functions between lean and obese animals and humans (1, 21, 22, 33, 36, 56, 60, 66; see Refs. 6, 8, 24, 46, 61, and 67 for reviews). For example, obese humans have been reported to exhibit increased hedonic hunger—they are more often thinking about food and have a more powerful urge to have the foods they like, even when not metabolically hungry (56). For the same perceived sweetness, “liking” increases as body mass index increases (2). Obese rats lacking a functional CCK-1 receptor show a rightward shift in the concentration-responses to oral sucrose and exhibit increased operant performance for high, but not low, concentrations of sucrose solutions compared with their lean counterparts (36). A similar rightward shift in the concentration-response to sucrose and corn oil was observed in high-fat diet-induced obese rats subjected to sham surgery, compared with rats that lost all excess body weight after Roux-en-Y gastric bypass surgery and chow-fed lean rats (60).
However, it is not clear from these studies whether such differences exist before the development of obesity and could thus be causative, or whether they are a consequence of the obese state. Using fMRI, Stice et al. (65) demonstrated that individuals with hypo-functioning dopamine signaling in the dorsal striatum, due to point mutations in the dopamine D2 receptor gene, may overeat and gain weight, that women who gained weight over a 6-mo period showed reduced striatal responses to palatable food consumption relative to weight-stable women (68), and that normal weight youth at high risk for obesity showed greater activation of striatal somatosensory regions (69). In selectively bred lines of obesity-prone (OP) and obesity-resistant (OR) rats, several differences in reward behaviors and mesolimbic dopamine signaling have been reported (22, 32), but only one study found differences in mesolimbic dopamine signaling at an early age, before development of obesity (32).
All of these observations led to two competing hypotheses, making either hyper- or hypo-sensitivity to reward responsible for overeating palatable food and development of obesity (21). The hypersensitivity hypothesis suggests that the more reward a food generates, the more we eat, with apparently no upper limit of reward (3, 19). The hypo-sensitivity or reward-deficit hypothesis suggests that increased intake of palatable food is an attempt to reach a certain reward set point by compensating for hypo-functioning reward generation, specifically for reduced mesolimbic dopamine transmission (12, 32, 33, 48, 65, 77). Although the prospective studies in humans with genetically reduced dopamine signaling and the one observation in preobese obesity-prone rats suggest that genetic predisposition is an important factor in determining the response to palatable food, the contributions of these two competing hypotheses to the development of obesity, with no known genetic anomaly, is not well understood. Perhaps, such “common” obesity is characterized by a biphasic relationship with reward (17). In an early phase (gluttony phase) high-reward sensitivity is driving intake of palatable food and leads to alterations of some underlying neural mechanisms, such as mesolimbic dopamine signaling in vulnerable individuals (20, 69). In a later phase (reward deficiency phase), prone individuals overeat palatable foods as a form of self-medication to overcome a hypofunctioning reward system (10, 53, 75).
As a first step in dissecting some of the potential factors determining the relationship between reward and obesity, we focused on specific reward behaviors in lean and obese outbred rats and compared it with a model of genetically select lines of obesity-prone (OP) and obesity-resistant (OR) rats. The aim was to test the reversibility of obesity-induced alterations in behavioral reward functions by means of calorie restriction-induced weight loss and to test reward functions before and after induction of obesity in OP and OR rats. Three test paradigms were used, each sensitive to specific aspects of food reward: 1) hedonic evaluation in Grill and Norgren's taste reactivity test (“liking”), 2) reinforcement learning and motivation to obtain food reward in the incentive runway and progressive ratio lever press paradigms (“wanting”), and 3) a combination of taste-guided “liking” and low-effort “wanting” in the brief-access lick paradigm.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats weighing 200 g were purchased from Harlan Industries (Indianapolis, IN), and male OP and OR rats weighing ∼170 g from Charles River Laboratories (Wilmington, MA). Animals were housed individually in wire-mesh cages at a constant temperature of 21–23°C with a 12:12-h light-dark cycle (lights on 0700, off at 1900). When appropriate, subsets of animals were put on either a high-fat diet (in kcal%: 20 carb, 60 fat, and 20 prot; D12492, Research Diets, New Brunswick, NJ) or a sweet high-fat diet (in kcal%: 35 carb, 45 fat, 20 prot; D12451, Research Diets) to make them obese. Normal laboratory chow (in kcal%: 58 carb, 13.5 fat, and 28.5 prot; # 5001, Purina LabDiet, Richmond, IN) was always available in addition to the specific diets. All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center, in accordance with guidelines established by the National Institutes of Health.
Experiment 1: brief access licking and taste reactivity in fed and fasted, lean and obese outbred Sprague-Dawley rats.
An overview of the different rat strains and experimental conditions used for each experiment is provided in Table 1. Experiment 1 was designed to test the hypothesis that the obese state is associated with differences in “liking” of sucrose and corn oil. To this end, 3-mo-old male Sprague-Dawley (SD) rats were fed either chow only (n = 9) or chow plus high-fat diet (60% energy from fat, n = 14) for 16 wk to induce obesity (for details of the diet, see above). All rats were then subjected to training and testing in the brief access and taste reactivity paradigms lasting about 2 wk, as described in detail below.
Table 1.
Overview of different rat strains and experimental conditions
| Exp. 1 | Exp. 2a | Exp. 2b | Exp. 3 | Exp. 4a | Exp. 4b | |
|---|---|---|---|---|---|---|
| Rat strain | Outbred SD | Outbred SD | Outbred SD | OP and OR | Outbred SD | OP and OR |
| Groups (adiposity level) | Chow “lean” and high-fat “obese” | High-fat “obese”, restricted, and regain “obese” | Restricted, i.p. leptin, and i.p. saline | Chow and high-fat | Chow “lean” and high-fat “obese” | Chow “before” and high-fat “obese” |
| Test paradigms | Brief-access and taste reactivity | Brief-access | Brief-access | Brief-access and taste reactivity | Incentive runway | Incentive runway and progressive ratio |
| Reward types | Sucrose and corn oil | Sucrose and corn oil | Sucrose and corn oil | Sucrose and corn oil | Fruit Loop cereal | Sucrose pellet |
| Metabolic states | Ad libitum and food deprived | Ad libitum and restricted | Ad libitum and restricted | Ad libitum | Ad libitum | Ad libitum and deprived |
To assess the effect of acute food deprivation on brief-access lick performance, a separate cohort of 3-mo-old male SD rats was fed either chow only (n = 8) or chow plus sweet high-fat diet (45% energy from fat, n = 9) for 26 wk to induce obesity. After induction of obesity, all rats were subjected to training and testing in the brief-access lick paradigm, first under conditions of ad libitum food availability (except for 2 h before testing), and 2–5 days later after 16 h of food deprivation.
Body weight was monitored daily for the first month, and weekly body weight was recorded afterward. Body composition (% fat mass, fluid, and lean mass) was measured before and after induction of obesity by using a Minispec LF 90 NMR Analyzer (Bruker BioSpin, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (44).
The brief-access lick test (Davis MS-160, DiLog Instruments, Tallahassee, FL) was used to measure taste-guided reward behavior (64). A short time window (10 s) for the animals to lick from a spout minimizes postingestive feedback modulation of food reward behavior. Rats were first adapted to the special cage and trained to lick from the spout filled with highly palatable chocolate Ensure under conditions of overnight (∼14 h) food and water deprivation in two successive daily sessions lasting 10–30 min, depending on when a particular rat started ingesting. Next, rats were trained to lick from serially presented (10 s) spouts containing Ensure in 2–5 successive daily sessions without food and water deprivation, until each rat licked repeatedly with a short latency. Testing started 1 day after the last training session with test sessions separated from each other by at least 1 day. On test days, animals were presented with increasing concentrations of either sucrose solutions (0, 0.005–1.5 M, in distilled water) or corn oil emulsions (0, 0.03–64%, in 1% Emplex emulsifier and distilled water). Each concentration was available for 10 s with 5-s intervals in two consecutive ascending series, and the number of licks/10 s was averaged for each concentration.
A modification of the taste reactivity test of Grill and Norgren (35) was used to quantify “liking” (5). Instead of delivering the tastants through intraoral cannulas, a 200-μl volume of sucrose solution was placed on the transparent floor of a cylindrical test cage, and the rat's orofacial expressions were videotaped from below after bouts of voluntary intake. Voluntary intake has been applied previously to the taste reactivity test to measure “liking” in rats and mice (27, 51, 52). We initially used intraoral fistulas for the delivery of tastants, but we switched to voluntary intake because of problems with patency and because we did not find significant differences in the monotonic increase of positive hedonic reactions in a subset of lean rats. The number of characteristic tongue protrusions was assessed by inspection of slow-motion videos (5), averaged over three consecutive bouts of ingestion, and for 3 concentrations of sucrose (0.01, 0.1, and 1.0 M) administered on separate days in random order. Positive hedonic orofacial responses included midline tongue protrusions, lateral tongue protrusions, and paw licking. None of the animals displayed neophobia toward the sucrose solutions during the test.
Experiment 2a: brief-access licking after weight loss and weight regain.
To test the hypothesis that some obesity-associated differences in taste-guided “liking” are reversible by weight loss and reinstated by subsequent weight regain, outbred Sprague-Dawley rats made obese as in experiment 1 (n = 6) were subjected to a weight loss regimen by restricting access to the high-fat plus chow diet. Food intake was restricted by placing 50–70% of each rat's daily baseline intake of both high-fat and chow diet (measured during 3 days before restriction) into the home cage 2 h before dark onset, so as to achieve a steady decrease in body weight of about 20% in 3 wk. This weight loss regimen was based on earlier studies with chronic food restriction in genetically obese rats (31, 70) and weight loss achieved with gastric bypass surgery in diet-induced obese rats (60, 83). Body weight was then maintained at the reduced level by providing rats ∼60–80% of their baseline intake during the subsequent test period. To avoid acute food restriction before testing, half the portion of the reduced food ration was given to the rats in the morning, ∼6 h before testing.
The same training and testing procedure as for the other two groups was then carried out, except that 60 min before brief-access tests, either sterile saline or leptin (1 mg/kg ip) was administered in a counterbalanced fashion (for details, see Experiment 2b). Finally, these weight-reduced (formerly obese) rats were allowed to regain body weight by allowing them to feed freely on chow plus high-fat diet, and brief-access testing was repeated after regaining 55% and 85% of their lost body weight.
Experiment 2b: brief-access licking after leptin administration in formerly obese rats.
To test the hypothesis that leptin can modulate taste-responsiveness, particularly, in the weight-reduced state when leptin sensitivity is restored (54), leptin (1 mg/kg ip) was injected 1 h before the start of brief-access testing. In addition, to assess plasma leptin levels, blood samples (100 μl) were taken from the cut tip of the tail at the end of testing. Blood was transferred into centrifuge tubes containing 0.5 M EDTA. Tubes were spun at 2,000 rpm for 10 min, and plasma was collected and stored at −80°C.
Leptin was measured using a rat leptin enzyme immunoassay kit (Assay Designs, Ann Arbor, MI). The assay was performed according to the manufacturer's specifications, and the samples were run in duplicate after diluting 1:4 with assay buffer as recommended. The color generated by stop solution was detected at 450 nm using a luminescence spectrometer (LS 50B, Perkin Elmer, Waltham, MA). The sensitivity of the kit was 67.2 pg/ml, and the intra-assay coefficient of variation was 4.4%.
Finally, the effectiveness of this dose of leptin to suppress food intake was verified by measuring 2 h of chow intake starting 4 h after injection.
Experiment 3: brief-access licking in genetically selected OP and OR rats.
To identify differences in genetic lines of OP and OR rats before and after development of overt obesity, we used 2-mo-old OP (n = 12) and OR (n = 12) rats that were initially maintained on regular chow. They were tested sequentially in the brief-access lick test with sucrose and corn oil (2 wk) and the taste reactivity test with sucrose (1 wk), as described in detail above. After completion of testing in the preobese state, all rats were exposed to chow plus sweet high-fat diet for 8 wk (45% energy from fat), and the same sequence of behavioral tests was repeated. Body weight and composition were monitored as above.
Experiment 4a: incentive runway performance in lean and obese SD rats.
To test the hypothesis that obesity affects motivation to obtain food, we compared incentive runway performance in chow-fed, lean, and high fat-fed, obese outbred SD rats.
The incentive runway test was used to assess goal-directed behavior, the motivation to obtain a food reward (25), also termed “wanting” (51). The runway consisted of start and goal boxes connected with a 158-cm-long running alley and a video camera mounted above. Rats were habituated to the runway during two daily 10-min sessions. During two additional sessions, overnight food-deprived rats were enticed to eat a small food reward [Fruit Loops cereal, ∼2 g, 88% carb (half of it as sugar), 8% fat, 4% protein] in the goal box. Runway behavior was then assessed in the non-food-deprived state in daily sessions of two consecutive trials over a period of 11 days. After placing the rats in the start box, the door was opened and the time to reach the goal box and consume the reward (completion time) was measured and expressed as completion speed. In addition, the time spent walking/running forward, standing still, moving backward, and any other distractions, as well as the net running speed were assessed by replaying the video recordings in slow motion. Analyses for both tests were conducted by two independent observers that were blind to the experimental conditions.
Experiment 4b: incentive runway and progressive ratio performance in OP and OR rats.
To test the hypothesis that genetic predisposition affects food motivation, the same OP and OR rats used in experiment 3 and maintained on chow, were subjected to the incentive runway test described in detail above. They were then exposed to a high-fat diet for 10 wk to induce overt obesity and retested in the incentive runway paradigm. Finally, they were trained and tested in the progressive ratio lever press paradigm described in detail below. Body weight and body composition were measured at the beginning and end of each test period.
Progressive ratio lever press performance was tested in operant conditioning chambers (Med Associates, St. Albans, VT). For the first 4 days of training, high-fat diet maintained, obesity-prone, and obesity-resistant animals were subjected to an autoshaping paradigm (one 45-mg sucrose pellet delivered whenever 10 min passed without a lever press) and an FR1 schedule of reinforcement in daily 4-h sessions. The rats were food-deprived for the first two training sessions only. On days 5–8, non-food-deprived rats were successively subjected to FR2 and FR3 schedules of reinforcement in daily 20-min sessions. Final testing was then performed with a progressive ratio schedule of reinforcement (1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268), first in the fed state and 3 days later after overnight food deprivation. The total number of presses during the 1-h test was averaged across the two days in fed and fasted state. Progressive ratio performance was defined as the highest number of lever presses achieved to obtain one sucrose pellet, with sessions terminated if no further lever presses were emitted within 30 min. Break point was defined as the last completed ratio requirement.
Statistical analysis.
Adiposity (fat %) and 2-h food intake were analyzed by Student's t-test. Plasma leptin levels were analyzed by one-way ANOVA. Number of licks in the brief-access test and number of positive hedonic orofacial responses in the taste reactivity test were analyzed by two-way repeated-measures ANOVA, with treatment as a between-subject factor and concentrations as a within-subject factor. Brief-access “liking” index of lean and obese rats was analyzed by two-way repeated-measures ANOVA, with phenotype as a between-subject factor and nutritional status (fed vs. fasted) as a within-subject factor. Brief-access index of formerly obese animals was analyzed by one-way repeated-measures ANOVA. Brief-access index and taste reactivity “liking” index of OP and OR rats were analyzed by two-way repeated-measures ANOVA, with genotype as a between-subject factor and dietary treatment as a within-subject factor. Taste reactivity “liking” index of lean and obese SD rats was analyzed by Student's t-test. Completion speed in the incentive runway was analyzed by three-way repeated-measures ANOVA, with treatment as a between-subject factor, and session and order of run in each session (first and second) as within-subject factors. Net running speed and distractions (latency, reversals, and pauses) were analyzed separately by Student's t-test. Runway “wanting” index of OP and OR rats was analyzed by two-way repeated-measures ANOVA, with genotype as a between-subject factor and dietary treatment as a within-subject factor. The same index of lean and obese SD rats was analyzed by Student's t-test. Progressive ratio lever press tests (break point) were analyzed by two-way repeated-measures ANOVA, with genotype as a between-subject factor and nutritional status as a within-subject factor. All ANOVAs were followed by Bonferroni-adjusted multiple-comparison tests. Simple linear regression analyses were performed to determine how well the adiposity can predict “liking” of sucrose and corn oil. Statistical significance was set at P < 0.05. Data were expressed as means ± SE.
RESULTS
High-fat diet-induced obesity in outbred SD rats shifts “liking” of sucrose and corn oil to higher concentrations (experiment 1).
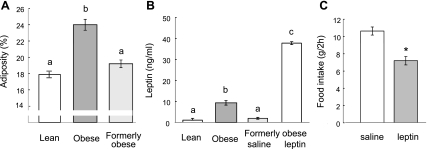
At the start of the behavioral tests, diet-induced obese rats weighed significantly more (578 ± 21 g vs. 446 ± 9 g, P < 0.01) and had significantly more fat mass (136 ± 7 g vs. 82 ± 3 g, P < 0.01). The adiposity index and plasma leptin levels were also significantly higher in the obese, compared with the lean chow-fed rats (Fig. 1, A and B).
Fig. 1.
Adiposity index, plasma leptin levels, and food intake in chronically calorie-restricted outbred Sprague-Dawley (SD) rats. A: adiposity of chow-fed lean and high-fat fed obese rats before and after calorie restriction. B: plasma leptin levels in chow-fed lean and high-fat fed obese rats before and after calorie restriction-induced 20% weight loss with saline or leptin (1 mg/kg ip) administration. C: dose of leptin (1 mg/kg ip) administered in the brief-access lick tests was able to significantly suppress 2-h food intake in a separate test. a,b,cBars that do not share the same letter are significantly (P < 0.05) different from each other (based on one-way ANOVA followed by Bonferroni-adjusted multiple comparison test). *P < 0.05, based on Student's t-test.
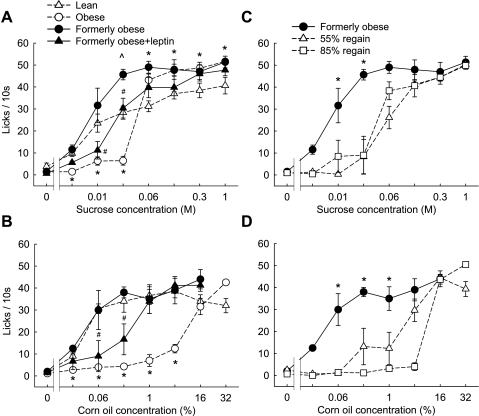
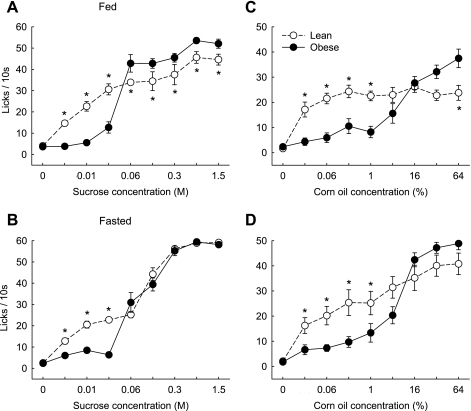
Lean, chow-fed SD rats showed the typical sigmoid concentration-response curve for the number of licks per 10 s of ascending concentrations of sucrose and corn oil in the brief-access lick test (Fig. 2, A and B). The lowest sucrose concentration eliciting significantly more licks compared with water was 0.006 M; the lowest corn oil concentration was 0.03%. Strikingly, diet-induced obese SD rats almost completely abstained from lower concentrations but responded strongly to the higher concentrations of sucrose and to the highest concentration of corn oil. For sucrose, the transition from abstaining to strongly responding was between 0.03 and 0.06 M; for corn oil, it was between 4% and 16%.
Fig. 2.
Brief-access licking responses for ascending concentrations of sucrose and corn oil in non food-deprived outbred SD rats. A and B: in Experiment 1, separate groups of chow-fed lean (△; n = 9) and high-fat diet obese (○; n = 11–14) rats were initially compared. In Experiments 2a and 2b, some of the obese rats were then calorie restricted to induce ∼20% weight loss and were tested again 1 h after intraperitoneal saline (formerly obese; ●; n = 6) or leptin injection (1 mg/kg; formerly obese + leptin, ▲; n = 6). C and D: formerly obese rats were then allowed to gain back body weight by ad libitum access to a two-choice high-fat or chow diet. Brief access licking was tested again after the rats had gained back 55% (△) and 85% (□) of the lost body weight. *P < 0.05, lean vs. obese and formerly obese vs. both regain groups; ^P < 0.05, formerly obese vs. lean; #P < 0.05, formerly obese vs. formerly obese + leptin; based on two-way repeated-measures ANOVA followed by Bonferroni-adjusted multiple comparison test.
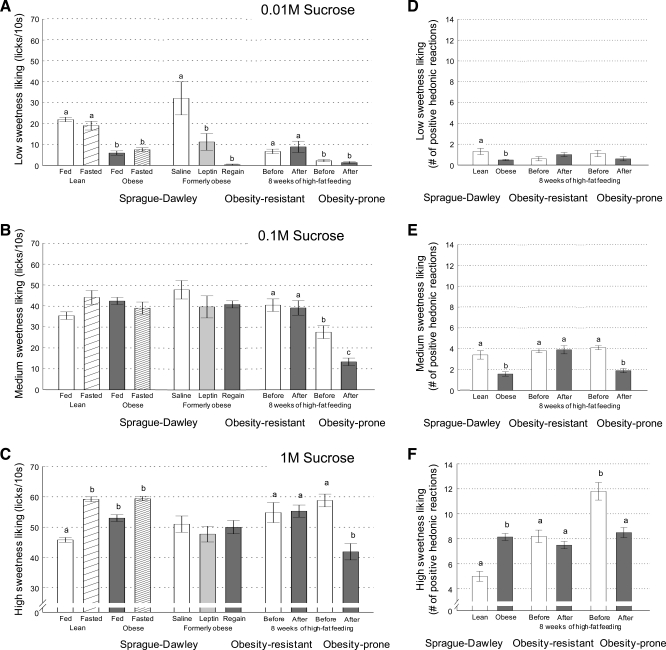
In distinction to the brief-access test, the taste reactivity test measures the number of positive hedonic orofacial reactions and does not rely on motivation to obtain a rewarding stimulus; it merely reflects the hedonic value or pleasure associated with tasting the stimulus (5, 35). As expected, lean, chow-fed SD rats showed a significant dose-response relationship in this test (Fig. 3, D–F; note that brief-access lick counts for low, medium, and high sucrose concentrations from Fig. 2 are replotted as bar graphs in Fig. 3, A–C for direct comparison with taste reactivity performance). High-fat diet-induced obese SD rats significantly decreased “liking” of the lowest (0.01 M, Fig. 3D) and the intermediate (0.1 M, Fig. 3E) sucrose concentration, but significantly increased “liking” of the high (1.0 M, Fig. 3F) sucrose concentration. Thus, in both tests, obese rats shift their “liking” of sucrose to higher nutrient concentrations.
Fig. 3.
Cross comparisons of “Liking” index for sucrose extracted from brief-access lick performance assessed in experiments 1, 2a, 2b, and 3 and shown in Figs. 2, 4, and 5 (A–C) and separately assessed by measuring the number of positive hedonic orofacial responses in Grill and Norgren's taste reactivity test (D–F). “Liking” of low ( 0.01 M, A and D), medium (0.1 M, B and E), and high (1 M, C and F) sucrose concentrations is shown for lean, obese, and formerly obese outbred SD rats in the fed and fasted state and after leptin administration and weight regain, as well as for genetically select lines of obesity resistant and obesity-prone rats before and after high-fat feeding, as indicated. For each method of “liking” assessment, groups were analyzed separately. a,b,cBars that do not share the same letter are significantly different from each other (P < 0.05, based on ANOVA followed by Bonferroni-adjusted multiple comparison test).
To test whether the right shift in the concentration-response relationship was affected by the metabolic state, we compared the fed to the fasted state in a separate group of rats (experiment 2, Fig. 4). Similar to experiment 1, non-food-deprived obese SD rats showed a significant right shift in the concentration-response curves for both sucrose and corn oil, with significantly less responding to low concentrations but significantly more responding to high concentrations (Fig. 4, A and C). When fasted, the obese rats maintained the significantly reduced responding to the low concentrations of both sucrose and corn oil, but the significant increase in responding to the higher concentrations was no longer observed (Fig. 4, B and D). While responding to the three lowest sucrose concentrations and the five lowest corn oil concentrations was very similar under fed and fasted conditions in both lean and obese rats, fasting specifically increased responding to the higher concentrations of sucrose in lean rats, and corn oil in both lean and obese rats.
Fig. 4.
Brief access responding for sucrose (A and B) and corn oil (C and D) in fed (A and C) and fasted (B and D) outbred SD rats of experiment 1. Groups of chow-fed, lean (○; n = 8) and high-fat, diet-induced obese rats (●; n = 9) were first tested in the fed state and 3–5 days later in 16-h food-deprived rats. *P <0.05, obese vs. lean rats, based on two-way repeated-measures ANOVA followed by Bonferroni-adjusted multiple comparison test.
Weight loss induced by calorie restriction reverses the effects of obesity on “liking” (experiment 2a).
Some of the diet-induced obese rats were subjected to the brief-access training and testing procedure after weight loss induced by chronic calorie restriction. The restriction regimen resulted in significant body weight (−93 ± 9.5 g, or −17 ± 1.4%, P < 0.01) and fat mass loss (−40 ± 4.7 g, or −33 ± 2.5%, P < 0.001), and it significantly decreased adiposity index (from 24 ± 0.7% to 19.0 ± 0.5%, or −21 ± 1.9%, P < 0.001), and plasma leptin levels (Fig. 1, A and B). Body weight, fat mass, and adiposity index of weight-reduced, formerly obese rats, was not significantly different from age-matched chow-fed lean control rats.
These weight-reduced, formerly obese rats regained their strong response to low concentrations of both sucrose and corn oil (Fig. 2, A and B). For sucrose, there was even a tendency to respond more strongly to the medium concentration of 0.03 and 0.06 M sucrose compared with lean, chow-fed rats (for 0.03 M sucrose, P < 0.01; for 0.6 M sucrose, P = 0.063).
Weight regain after the calorie restriction regimen shifted the concentration-response relationships for both sucrose and corn oil back to the right, characteristic for the obese state (Fig. 2, C and D). Regain of about half of the lost body weight was sufficient to produce a significant right shift.
Leptin administration and weight regain in weight-reduced rats shift taste responsiveness toward a profile seen in obese rats (experiment 2b).
We argued that calorie restriction-induced weight loss led to reinstatement of full leptin sensitivity and that raising leptin by either exogenous leptin administration or weight regain would shift taste responsivity in the brief-access test back to reduced “liking” of low nutrient concentrations, as seen in the obese state. Leptin injection in weight-reduced SD rats significantly suppressed responding to low sucrose and corn oil concentrations compared with saline injection, shifting the concentration-response curve partially toward the obese pattern (Fig. 2, A and B). Leptin administration led to plasma leptin levels (37.8 ± 0.7 ng/ml) that were significantly above levels of saline-injected, weight-reduced rats, as well as obese SD rats (P < 0.01) (Fig. 1B). In a separate test, we found that this dose of leptin significantly suppresses food intake in food-deprived rats compared with saline injection (7.0 ± 0.5 g vs. 10.6 ± 0.5 g/2 h, P < 0.01, Fig. 1C).
Genetically selected obesity-prone rats show a right shift of sucrose and corn oil concentration response curve at a young age before high-fat, diet-induced obesity (experiment 3).
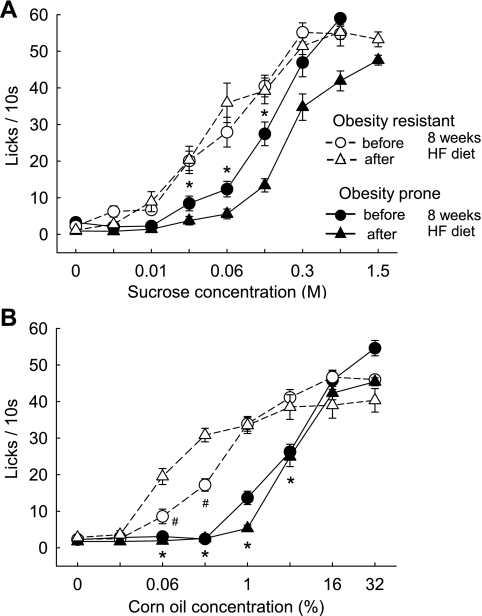
At 7–8 wk of age, the earliest feasible age for behavioral testing, OP rats already weighed significantly more (333 ± 6.1 g vs. 271 ± 5.6 g; P < 0.001) and had significantly more fat mass (68 ± 1.4 g vs. 54 ± 1.0 g; P < 0.001) than OR rats. At this stage, OP rats showed a right shift of the concentration response curve for both sucrose and corn oil compared with OR rats in the brief-access test (Fig. 5, A and B), similar to obese vs. lean outbred SD rats. Specifically, these young OP rats responded very little up to 0.06 M sucrose and up to 1% corn oil, but readily licked the higher concentrations.
Fig. 5.
Brief-access responding for ascending concentrations of sucrose (A) and corn oil (B) in selectively bred obesity-resistant (OR, ○ and △; n = 12) and obesity-prone (OP, ● and ▲, n = 12) rats, before (○ and ●) and after (△ and ▲) exposure to high-fat diet of experiment 3. Young, chow-fed rats of both lines were first tested at 7–8 wk of age, when their body weight and adiposity were still only moderately different, and after 8 wk on a high-fat diet to induce overt obesity. *P < 0.05, OP vs. OR rats. #P < 0.05, before vs. after obesity induction in OR rats, based on two-way repeated-measures ANOVA followed by Bonferroni-adjusted multiple comparison test.
The right shift in responding for sucrose, found with the brief-access test, was reflected by higher “liking” of the 1 M sucrose concentration in the taste reactivity test in these chow-fed young OP rats (Fig. 3F). The two lower sucrose concentrations were similarly “liked” by OP and OR rats (Fig. 3, D and E).
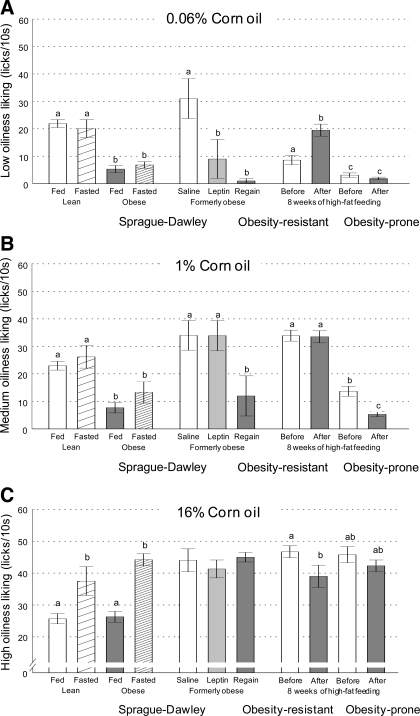
When the behavioral tests were repeated after the 8-wk high-fat feeding period, OP rats weighed significantly more (598 ± 19.2 g vs. 478 ± 10.6 g; P < 0.001) and had significantly more fat mass (148 ± 7.6 g vs. 114 ± 3.3 g; P < 0.001) compared with OR rats. For both genotypes, the pattern of brief-access sucrose and corn oil licking across the concentrations changed little compared with before the high-fat diet. Specifically, the OP rats showed a further right shift of the sucrose concentration response curve, with significantly less licks for 0.1 M and 1.0 M sucrose (Fig. 5A), while the OR rats did not appreciably change their responsiveness to sucrose. In response to corn oil, OP rats showed little change, while OR rats unexpectedly showed a slight left shift in the concentration-response curve (Fig. 5B). To facilitate comparisons in brief-access lick performance among the various rat strains and conditions, the lick responses for low, medium, and high corn oil concentrations are replotted as bars in Fig. 6.
Fig. 6.
Cross comparisons of “Liking” index for corn oil extracted from brief-access lick performance in experiments 1, 2a, 2b, and 3, shown in Figs. 2, 4, and 5, for low (0.06%, A), medium (1%, B), and high (16%, C) corn oil concentrations. Data are shown for lean, obese, and formerly obese outbred SD rats in the fed and fasted state and after leptin administration and weight regain, as well as for genetically select lines of obesity-resistant and obesity-prone rats before and after high-fat feeding, as indicated. a,b,cBars that do not share the same letter are significantly different from each other (P < 0.05, based on ANOVA followed by Bonferroni-adjusted multiple comparison test).
Findings with the taste reactivity test were similar to the brief-access test in that high-fat fed OP rats significantly decreased “liking” of the two higher sucrose concentrations compared with the prediet measurement (Fig. 3, E and F), and OR rats did not change “liking” of any sucrose concentration (Fig. 3, D–F).
Adiposity is negatively correlated with “liking” of sucrose and corn oil.
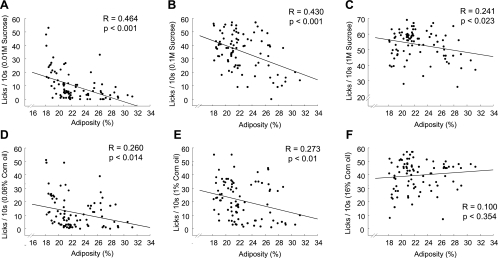
Simple regression analyses between the level of adiposity and the number of licks for specific sucrose and corn oil concentrations in the brief-access test across all three rat strains (outbred Sprague-Dawley, obesity-prone, and obesity-resistant) revealed significant negative relationships for 0.01, 0.1, and 1.0 M sucrose and for 0.06 and 1.0% corn oil, but not for 16% corn oil (Fig. 7).
Fig. 7.
Linear regression analysis of relationships between adiposity and the brief-access responses to low, medium, and high sucrose (A–C) or corn oil concentrations (D–F) from the combined data of both outbred SD and genetically select obesity-prone and obesity-resistant rats.
High-fat diet-induced obesity paradoxically decreases motivation for food reward as measured by incentive runway and progressive ratio lever press performance (experiments 4a and b).
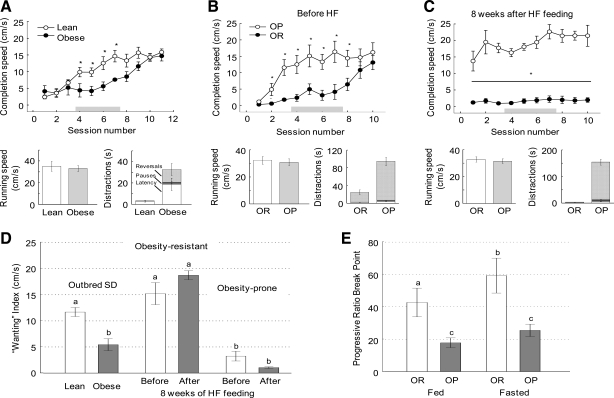
Completion speed in the incentive runway is a measure of reinforcement learning and “wanting” to obtain a food or drug reward (25, 51). In experiment 4a, compared with lean chow-fed rats, high-fat diet-induced obese SD rats showed significantly reduced completion speed during sessions 4–7, although they eventually reached the goal box just as fast as the lean rats during sessions 10 and 11 (Fig. 8A). This was not due to nonspecific motor impairment, as the net running speed was not different. However, obese rats spent significantly more time being distracted, as indicated by significantly increased latency to leave the start box and duration of pauses and reversals.
Fig. 8.
Incentive runway (A–D) and progressive ratio lever press (E) performance as a measure of “wanting” in outbred Sprague-Dawley rats (experiment 4a) and genetically select lines of obesity-resistant (OR) and obesity-prone (OP) rats (experiment 4b). The incentive runway test was conducted with lean (○/open bars; n = 6) and obese (●/filled bars; n = 7) outbred SD rats (A and D) and with obesity-resistant (○; n = 11) and obesity-prone (●; n = 11) rats before (B) and after (C) 8 wk of high-fat feeding to induce obesity. All tests were conducted in the fed state. Completion speed (based on time required to move from start to goal box), net running speed (after subtraction of distraction time), and duration of distractions (latency to leave the start box, pauses, and reversals) are also shown in bar graphs. The gray bars in A-C indicate the time window used for calculation of a “wanting” index (D). The index was calculated on the average completion speed in sessions 4–7. The progressive ratio break point was measured only in obesity-resistant and prone rats after 10 wk of high-fat feeding, in the fed and fasted state. a,b,cBars in D and E that do not share the same letter are significantly different from each other. *P < 0.05, lean vs. obese SD rats, and OR vs. OP rats, based on ANOVA followed by Bonferroni-adjusted multiple comparisons.
Separate analysis of performance in the first and subsequent runs was performed to differentiate between motivation driven more by reward expectancy (first run in session) and motivation driven by having recently consumed a reinforcing reward (second run in session). Although, as expected, rats were moving significantly faster to the goal box in the second run, obesity equally affected performance in the first and second run (data not shown).
In experiment 4b, young, chow-fed OP rats similarly exhibited significantly reduced completion speed compared with their OR counterparts (Fig. 8B). Net running speed was similar but again, the reduced completion speed was due to increased duration of distractions. After 8 wk of a high-fat diet, OP rats further reduced completion speed compared with their leaner state before the high-fat diet, while obesity-resistant rats reached the goal box fast, even in the first session (Fig. 8C). Again, even though the “obese” obesity-prone rats were extremely sluggish in reaching the goal box, this was not due to their net running speed (Fig. 8C) but to drastically more time spent with distractions along the alley.
A “wanting” index was derived from the average completion speed during sessions 4–7, showing that high-fat diet-induced obesity significantly reduced “wanting” in outbred SD rats with a trend for such a suppression (P = 0.07) in obesity-prone rats, but not in obesity-resistant rats (Fig. 8D). It also shows significantly lower “wanting” in young, mildly obese OP rats compared with OR rats. However, the outcome in obesity-resistant rats is confounded with retention of memory from the first series of runway tests before obesity induction. Administration of this test in naïve obesity-resistant rats will be necessary to assess the true effect of obesity in this group.
Progressive ratio lever press performance as determined by the break point was also lower in obesity-prone rats that had become overtly obese during 16 wk on a high-fat diet compared with obesity-resistant rats, both in the fed and fasted state (Fig. 8E). There were significant effects of genotype [F(1,11) = 7.55; P = 0.019] and nutritional status [F(1,11) = 12.65; P < 0.005] but no significant interaction [F(1,11) = 1.71; P = 0.22].
DISCUSSION
Indulgence in highly palatable and energy-dense foods is widely seen to contribute to the current obesity crisis, but the mechanisms involved are far from clear. One emerging explanation is altered brain responsiveness to food and food cues in obese subjects, with brain areas involved in reward and cognition receiving the most attention. Because the neural mechanisms of reward are most likely influenced by both preexisting (genetic and/or nongenetic) differences and secondary effects of the obese state, it has not been clear what the relative contribution is of these two factors. In an initial attempt to separate effects of the two factors on reward behaviors, here we measure food reward behaviors in rats before and after high-fat diet-induced obesity, after reversal of obesity by chronic food restriction, and in a genetic model of obesity-prone and -resistant rats. We demonstrate that in outbred SD rats, obesity-induced changes in reward behaviors are more or less reversed by subsequent weight loss, suggesting that they were largely secondary to the obese state. Specifically, we found an obesity-induced shift in taste-guided responsiveness (“liking”) for sucrose and corn oil—away from low concentrations toward higher concentrations—and a full reversal after weight loss. Partial prevention of this reversal by exogenous leptin administration in weight-reduced rats further suggests that leptin may play a role in obesity-induced decreases of sucrose and corn oil sensitivity. Finally, we confirm earlier observations of decreased “wanting” in high-fat diet-induced obese SD and in genetically obesity-prone rats, suggesting that while obese rats readily indulge in easily available strong sweet and fatty food stimuli, they are not ready to make an extra effort to obtain them.
For the remainder of this discussion, we make the assumption that the brief access and taste reactivity tests are primarily measures of “liking” and that the incentive runway and progressive ratio paradigms are measures of “wanting”. However, it is clear that none of these tests is able to completely separate “liking” and “wanting”. In both the brief access and modified taste reactivity tests, “liking” is inherently accompanied by the appetitive phase and consummation and, therefore, some degree of “wanting” of the reward. In the taste reactivity test, this “wanting” component may have been augmented by presenting the tastants on the floor instead of being delivered via intraoral cannulas. Therefore, it is possible that decreased lick performance in the brief access and taste reactivity tests could at least partially be explained by decreased motivation (or “wanting”) rather than decreased “liking”. However, because hyperdopaminergic mice exhibit a very selective increase in “wanting” but not “liking” (assessed with voluntary intake of the sucrose solutions from the floor) (51), the “wanting” component of this test is likely very small and insignificant.
In the brief access lick test, the hesitance (or refusal) to lick low concentrations of sucrose and corn oil of obese rats could also be interpreted as lack of motivation or “wanting”, rather than decreased “liking”, just as the increased distractibility in the incentive runway and operant responding are interpreted as lack of “wanting”. However, the fact that in the same session, highly concentrated taste stimuli elicited immediate responding in the same rat, clearly distinguishes the brief access lick test from the incentive runway and progressive ratio tests, where “wanting” in obese rats was lower, even for highly palatable rewards. Furthermore, the similar response patterns in the brief-access and taste reactivity tests for sucrose (see direct comparison in Fig. 3) suggest that the two tests measure “something” very similar, and, on the basis of the strong evidence that the oro-facial hedonic responses measured by the taste reactivity test reflect hedonic value, it appears to be “liking”. Clearly, future experiments using additional measurements of taste reactivity, such as taste acuity and preference, as well as measurements of neurochemical correlates will be necessary for a better distinction.
Obesity differentially affects “liking” of diluted and concentrated sweet and oily taste stimuli, and the effects are reversible by weight loss.
Clearly, sucrose “liking” was changed by both high-fat, diet-induced obesity and genetic predisposition to obesity. However, the effect was concentration-dependent: strong suppression at low, and mild enhancement at high sucrose concentrations. The increased “liking” of high sucrose concentrations in obese rats is consistent with findings in obese humans, in which “liking” increases as a function of sweetness more in obese subjects and more as body mass index (BMI) increases, and for the same perceived sweetness, “liking” increases as BMI increases (2). Using the Power of Food Scale (45), Schultes et al. (56) reported increased hedonic hunger in severely obese patients. Obese patients were more often thinking about food and had a more powerful urge to have the foods they liked, even when not metabolically hungry. Such increased hedonic hunger could be due to increased “liking”, “wanting”, or both.
Others have applied the Physical Anhedonia Scale (16), a 61-item questionnaire designed to quantify the degree to which individuals take pleasure from rewarding behaviors, to overweight and obese subjects (20). While sensitivity to reward (note that high sensitivity to reward is reflected by a low anhedonia score) was increased in overweight, it was decreased in obese (BMI >30) women, suggesting a nonlinear relationship between obesity and sensitivity to reward (17, 20).
Given that in our tests “liking” is strictly dependent on taste (and perhaps smell), it seems reasonable to assume that taste processing is different in the obese, but it is not clear what level of taste processing might be affected: peripheral, central, or both. In another rat model of obesity, the CCK1-receptor-deficient rat, taste-responsive neurons in the parabrachial nucleus had a reduced overall sensitivity to sucrose, with decreased responsiveness to lower concentrations and augmented responses to higher concentrations (38), similar to the present findings in diet-induced obese rats. However, it is not clear whether these changes in the pontine taste relay are causally related to the absence of CCK1R signaling in that brain area or are secondary to the obese state.
Human studies suggest that taste acuity, as measured by detection and recognition thresholds, is not different in lean and morbidly obese subjects (57). Obesity-induced differences at higher levels of taste processing are indicated by observations in obese adolescent girls showing greater activation of the gustatory cortex to anticipation or consumption of a palatable milkshake (66), and in obese children (14) and adults (55) showing greater activation to visual food stimuli in the anterior insula and orbitofrontal cortex, compared with their normal weight counterparts.
“Liking” is closely related to opioid signaling, as demonstrated by a number of pharmacological studies using selective mu-opioid receptor agonists and antagonists directed to the nucleus accumbens, ventral pallidum, and related brain areas (6, 21, 42, 50, 59, 62, 63, 82). The nucleus accumbens, which is a particular hotspot for opioid-induced “liking”, can be considered part of the system processing taste information, as it receives direct inputs from the nucleus of the solitary tract, the first taste relay in the brain (13), and from the amygdala and insular cortex (80). The idea that obesity affects opioid signaling in the nucleus accumbens is supported by the observations that consumption of a highly palatable diet reduces striatal enkephalin gene expression (43) and that 18 wk on a high-fat diet decreases sucrose preference and mu-opioid receptor expression via changes in DNA-methylation and chromatin remodeling (76).
To determine whether obesity-induced alterations of reward behaviors are permanent or reversible, we looked at the behaviors after food restriction-induced weight loss. Surprisingly, most obesity-induced changes in reward sensitivity to both sucrose and corn oil were fully reversed by weight loss. Weight-reduced rats “liked” the low concentrations of sucrose and corn oil as much as age-matched, chow-fed lean controls. Only 1 M sucrose continued to be significantly more “liked” in the taste reactivity test by the formerly obese rats after weight loss compared with lean controls. “Liking” of all other sucrose concentrations and all corn oil concentrations, as assessed by either test, was fully reversed and not significantly different compared with chow-fed lean controls.
This is the first demonstration of reversible changes in food reward behaviors induced by obesity and subsequent calorie restriction-induced weight loss in diet-induced obese rats. In the only other study using calorie restriction, sensitivity to lateral hypothalamic self-stimulation, a measure of “wanting”, rather than “liking” (7) was increased in obese Zucker rats after a 3- to 4-wk weight loss regimen, resulting in an ∼25% body weight loss (31). Together with the recent observation that sensitivity to lateral hypothalamic self-stimulation is reduced after a period of obesity-inducing, high-fat feeding in SD rats (40), the finding suggests that this measure of food reward sensitivity can also be reversed by weight loss.
Bariatric surgery is increasingly used to produce sustained weight loss in obese patients. Some of these surgeries, particularly Roux-en-Y gastric bypass surgery, has been shown to change hedonic reactions to food in both humans and rodent models (15, 37, 56, 60). We have recently demonstrated full reversal of obesity-induced alterations in brief access licking and taste reactivity, 5 mo after Roux-en-Y gastric bypass surgery in Sprague-Dawley rats, a time when body weight has long stabilized at a lower level. In fact, on the basis of the present findings, we can speculate that reversal after gastric bypass surgery is mostly secondary to weight loss, not some other consequences of surgery, such as increased levels of gut hormones.
Dieting-induced weight loss in obese subjects is typically followed by weight regain, and it has been shown that this relapse may be caused by a similar natural biological reaction to weight loss that is part of the homeostatic regulation in normal-weight individuals. Neural activity in a number of brain areas evoked by pictures of food is changed after weight loss, and some of these changes are restored after leptin treatment (54). This prompted us to examine a possible role for restored leptin sensitivity in the weight loss-induced upregulation of sucrose and corn oil sensitivity in the brief-access lick test. Although we have not treated obese and lean control rats with leptin, our preliminary results strongly suggest that formerly obese, weight-reduced rats are highly sensitive to exogenous leptin in reducing “liking” of low sucrose and corn oil concentrations. This preliminary finding is consistent with demonstrations in genetically leptin-deficient mice and humans of increased olfactory and gustatory perception capabilities, as well as “liking” of palatable foods; all reversed by leptin replacement therapy (26, 34, 58).
Obesity reduces “wanting” when effort is high but may increase “wanting” when effort is low.
In contrast to the brief-access and taste reactivity tests that measure primarily “liking”, the incentive runway and progressive ratio lever press paradigm primarily reflect the motivation to obtain a food reward, or briefly “wanting”. However, the food reward that the animals work for is typically also “liked”, and it is, therefore, impossible to rule out that changes in “wanting” are partially mediated through changes in “liking” or vice versa.
As seen by others before with the progressive ratio lever press paradigm (22), “wanting” of a palatable food reward, as assessed in the incentive runway here, was clearly reduced in high-fat diet-induced obese, compared with chow-fed lean SD rats, as well as in young, chow-fed, obesity-prone compared with obesity-resistant rats. Similarly, “wanting” as measured by the break point in the progressive ratio lever press paradigm was also significantly lower in obesity-prone than obesity-resistant rats that had been fed a high-fat diet for an extended period of time. This outcome seems paradoxical, given the popular belief that obese individuals are more, not less, motivated to eat palatable foods and the higher “liking” of concentrated sucrose and corn oil by our obese rats and in humans discussed above.
Possible explanations for this disconnect include a concentration-dependent effect. It could be that in parallel to changes in “liking”, only the highest concentrations of sucrose and corn oil are “wanted” more by the obese, but lower concentrations are “wanted” less. Although the “treats” that we used in the incentive runway (Fruit Loops) and lever press paradigm (sucrose pellets) appear to reflect high-concentration stimuli, it could be that 1 M liquid sucrose and 32% corn oil are more salient acute stimuli. To test the validity of this explanation, the same concentration range of liquid sucrose and corn oil stimuli needs to be offered in the “wanting” tests in future experiments.
Another possible explanation for the paradoxically reduced “wanting” associated with obesity is effort dependency of the effect. It could be that obesity is associated with increased “wanting” of a food reward only when the procurement effort is low, but with decreased “wanting” when the effort is high, such as in the incentive runway and progressive lever press paradigms. Support for such an interpretation comes from studies in mice demonstrating that when the unit cost is low, melanocortin-4 receptor-deficient obese mice obtaining all their food through operant responding increase daily food intake compared with wild-type controls, but when the unit cost is high, daily food consumption is decreased (1). Similar trends were observed in leptin-deficient ob/ob mice subjected to increased unit costs on fixed-ratio schedules (28). It should also be considered that the reduced spontaneous physical activity in preobese OP rats (70) and other models of obesity, such as ventromedial hypothalamic obesity (74), may at least partly be responsible for decreased performance in these high-effort tests. However, because the obese SD rats and the preobese OP rats eventually reach the same completion speed as their lean counterparts (Fig. 8), this explanation may only apply for the grossly obese OP rats after the high-fat diet, as they never increased completion speed.
Finally, the behavioral phenomenon known as delayed discounting—a measure of immediate over delayed gratification—could play a role in the reduced “wanting” observed in our study, in that obese rats overconsume readily available high concentrations of sucrose and corn oil from the spout, but underperform if the reward is delayed in the runway or lever press paradigms. Obviously, delay and effort are confounded in our experiments, and will have to be varied separately to study their specific effects.
However, this possibility is supported by observations in obese women showing significantly greater delayed discounting (78) and children with difficulties in delaying gratification being more likely to become obese (24).
As to the potential neural pathways and circuits responsible for these obesity-induced effects on food reward behaviors, there is considerable evidence for obesity-associated decreased signaling through the mesolimbic dopamine/D2-receptor system in both rodents (22, 30, 32, 33, 36, 39, 72) and humans (18, 21, 65, 67, 75, 77). This system is thought to play a pivotal role in both the motivational (“wanting”) and reinforcement learning component of food and drug reward (4, 79), and both hyperfunctioning and hypofunctioning of this system have been suggested to increase the risk of obesity. Although the more intuitive explanation is that a hyperactive dopamine system (or overstimulation of a normally functioning system) increases the risk of overeating and obesity (4, 21), the reward deficiency hypothesis suggests that a hypofunctioning dopamine system pushes a subject to overeat palatable foods in a “self-medication” manner to increase a preferred level of dopaminergic signaling (10, 11, 20, 75, 77). Under this latter scenario, dopamine signaling would have to be considered as providing negative feedback to a defended level of reward, just as nutrient-sensing mechanisms provide negative feedback to a defended level or set point of body weight. Dopamine signaling would then not constitute reward by itself; it would be more like a sensing mechanism leading to reward generation, just like nutrient-sensing mechanisms lead to satiation. A limitation of our study is that we have not investigated the status of dopamine signaling in rats displaying the different reward behaviors, and we can only assume that there was suppressed dopamine signaling in the obese state as reported by others (22, 30, 32, 33, 36, 39, 72).
Overall, the present findings provide support for both predetermined and acquired alterations in behavioral reward functions during development of high-fat diet-induced obesity, but they are only a first step. Many additional studies will be necessary to tease out the specific roles of dietary composition, oral vs. postoral detection, repeated exposure, effort, and reward salience in these behavioral alterations, and to identify the accompanying neuroplastic changes.
Perspectives and Significance
The present results and findings by others strongly indicate that high-fat diet-induced obesity affects reward behaviors, but the mechanisms involved remain elusive. It has become clear recently that a state of chronic, low-grade inflammation in various peripheral tissues and in the brain is associated with obesity and may even be a causative factor in high-fat diet-induced obesity (23, 71). Furthermore, obesity-associated inflammation can be reversed by calorie restriction-induced weight loss (29, 73). Therefore, at least some of the reversible obesity-induced changes in reward behaviors could be due to changes in inflammatory signaling in brain areas controlling reward functions. Such changes have been shown in the hypothalamus (47, 71), hippocampus (9, 81), and striatum (41, 49). It will be interesting to examine such changes in inflammatory signaling in other key structures controlling reward functions, such as the nucleus accumbens, ventral tegmental area, and orbitofrontal cortex.
GRANTS
This work was partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-071082 and DK-047348.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Paul Pistell for setting up the incentive runway and taste reactivity tests, Jennifer Dowden and Abby Duhé for running the operant conditioning boxes, and Katie Bailey for help with editing.
REFERENCES
- 1. Atalayer D, Robertson KL, Haskell-Luevano C, Andreasen A, Rowland NE. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am J Physiol Regul Integr Comp Physiol 298: R1667–R1674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci 361: 1137–1148, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci 26: 5160–5166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24: 173–198, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Berridge KC, Ho CY, Richard JM, Difeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res 1350: 43–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 300: R1266–R1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24: 2104–2115, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 32 Suppl: i-iv, 1–112, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long-term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model 5: 24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blum K, Chen TJ, Meshkin B, Downs BW, Gordon CA, Blum S, Mengucci JF, Braverman ER, Arcuri V, Varshavskiy M, Deutsch R, Martinez-Pons M. Reward deficiency syndrome in obesity: a preliminary cross-sectional trial with a Genotrim variant. Adv Ther 23: 1040–1051, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255–278, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 34: 1494–1500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 95: 666–670, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol 85: 374–382, 1976 [DOI] [PubMed] [Google Scholar]
- 17. Davis C, Fox J. Sensitivity to reward and body mass index (BMI): evidence for a non-linear relationship. Appetite 50: 43–49, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K, Hwang R, Kennedy JL. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry 32: 620–628, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite 48: 12–19, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite 42: 131–138, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 17: 1220–1225, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122: 1257–1263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav 100: 438–445, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav 91: 271–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science 317: 1355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feurte S, Nicolaidis S, Berridge KC. Conditioned taste aversion in rats for a threonine-deficient diet: demonstration by the taste reactivity test. Physiol Behav 68: 423–429, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Finger BC, Dinan TG, Cryan JF. Progressive ratio responding in an obese mouse model: Effects of fenfluramine. Neuropharmacology 59: 619–626, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol 44: 41–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Fulton S, Richard D, Woodside B, Shizgal P. Food restriction and leptin impact brain reward circuitry in lean and obese Zucker rats. Behav Brain Res 155: 319–329, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J 22: 2740–2746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159: 1193–1199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Getchell TV, Kwong K, Saunders CP, Stromberg AJ, Getchell ML. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol Behav 87: 848–856, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats Brain Res 143: 263–279, 1978 [DOI] [PubMed] [Google Scholar]
- 36. Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 293: R1846–R1854, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Hajnal A, Kovacs P, Ahmed TA, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967–G979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hajnal A, Norgren R, Kovacs P. Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci 1170: 347–364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang XF, Yu Y, Zavitsanou K, Han M, Storlien L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res 135: 150–161, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalonia H, Kumar P, Kumar A. Attenuation of proinflammatory cytokines and apoptotic process by verapamil and diltiazem against quinolinic acid induced Huntington like alterations in rats. Brain Res 1372: 115–126, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate EnsureR) alters striatal enkephalin gene expression. Eur J Neurosci 18: 2592–2598, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res 12: 1604–1615, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 91: 432–439, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Lowe MR, van Steenburgh J, Ochner C, Coletta M. Neural correlates of individual differences related to appetite. Physiol Behav 97: 561–571, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Macdonald L, Radler M, Paolini AG, Kent S. Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. Am J Physiol Regul Integr Comp Physiol 301: R172–R184, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, Zhang Y, Sparkes RS, Grandy DK. D2 dopamine receptor gene and obesity. Int J Eat Disord 15: 205–217, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia 53: 248–256, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23: 9395–9402, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol 97: 140–153, 1983 [PubMed] [Google Scholar]
- 53. Pothos EN, Sulzer D, Hoebel BG. Plasticity of quantal size in ventral midbrain dopamine neurons: possible implications for the neurochemistry of feeding and reward. Appetite 31: 405, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37: 410–421, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr 92: 277–283, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Scruggs DM, Buffington C, Cowan GS., Jr Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg 4: 24–28, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 145: 839–847, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Shin AC, Pistell PJ, Phifer CB, Berthoud HR. Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience 170: 580–588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (Lond) 33 Suppl 2:S44–S48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27: 1594–1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25: 8637–8649, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096–1109, 1996 [PubMed] [Google Scholar]
- 65. Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322: 449–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117: 924–935, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav 97: 551–560, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 30: 13105–13109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31: 4360–4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teske JA, Kotz CM. Effect of acute and chronic caloric restriction and metabolic glucoprivation on spontaneous physical activity in obesity-prone and obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 297: R176–R184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: Resolving the paradox. Front Neuroendocrinol 31: 79–84, 2010 [DOI] [PubMed] [Google Scholar]
- 72. Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse 62: 637–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vilberg TR, Keesey RE. Reduced energy expenditure after ventromedial hypothalamic lesions in female rats. Am J Physiol Regul Integr Comp Physiol 247: R183–R188, 1984 [DOI] [PubMed] [Google Scholar]
- 75. Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363: 3191–3200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vucetic Z, Kimmel J, Reyes TM. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology 36: 1199–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet 357: 354–357, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite 51: 563–569, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol 493: 115–121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci 16: 1877–1893, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience 160: 295–306, 2009 [DOI] [PubMed] [Google Scholar]
- 82. Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience 99: 267–277, 2000 [DOI] [PubMed] [Google Scholar]
- 83. Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]