Abstract
Borna disease virus (BDV) induces a nonpurulent CD4- and CD8-T-cell-dependent meningoencephalitis in susceptible animals. Upon intracerebral infection, BDV replicates in the mouse central nervous system (CNS), but only a few mouse strains develop neurological disorder. The antiviral T cells appear to suppress BDV replication by a noncytolytic mechanism. Since BDV does not replicate in standard mouse cell cultures, the putative role of gamma interferon (IFN-γ) in virus control could not be tested experimentally. Here, we report that mouse organotypic slice cultures can be used to elucidate the complex interactions of BDV, the CNS, and the immune system. We show that BDV replicated in various cell types of mouse cerebellar slice cultures in vitro. In infected slice cultures, a moderate upregulation of the chemokine genes CCL5 and CXCL10 was observed, while expression of various neural genes as well as other chemokine and cytokine genes was not altered. IFN-γ inhibited the multiplication of BDV in cerebellar and hippocampal slice cultures in a dose-dependent manner. However, while complete suppression of BDV was observed in cerebellar slice cultures, inhibition was incomplete in hippocampal slice cultures. Kinetic studies indicated that IFN-γ protects noninfected cells from infection rather than clearing the virus from infected cells. These results demonstrate that BDV can replicate in cultured neural cells of the mouse if organ integrity is well preserved. They further show that IFN-γ is a powerful inhibitor of BDV in the absence of blood-borne leukocytes in mouse cerebellar slice cultures.
Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus that can persistently infect the central nervous system (CNS) of a variety of animal species (for a review, see reference 21), which may then develop a severe nonpurulent meningoencephalitis (9, 13, 17). Natural infection with BDV occurs in horses, sheep, and other species (21). Although natural BDV infection of mice has not been reported, BDV can replicate in the mouse CNS after intracerebral infection (10, 11). However, most mouse strains are resistant to BDV-induced disease, and only mice of few strains, such as MRL, develop neurological disorder following infection with BDV (10, 11). In MRL mice, the antiviral CD8 T cells recognize a single immunodominant epitope located in the BDV nucleoprotein (20). These cells mediate both antiviral protection and neurological disease depending on whether they arrive in the brain before or after BDV has disseminated widely through the organ. Because the antiviral response toward BDV is unaltered in mutant MRL mice lacking perforin or lacking a functional fas system, it appears that the antiviral T cells employ as-yet-undefined, noncytolytic mechanisms to target BDV in infected cells (12). Using knockout and transgenic mice, we showed that alpha interferon (IFN-α) has the potential to control BDV in the CNS (22). However, the IFN-α and IFN-β genes are barely expressed in response to BDV infection, indicating that these cytokines might only play a minor role (8, 22). By contrast, antiviral T cells that migrate into BDV-infected mouse brains strongly express IFN-γ, raising the possibility that this cytokine plays an important role. We further showed BDV inhibition in transgenic mice (termed GF-IL-12) expressing interleukin 12 (IL-12) in astrocytes of the cerebellum (8). Although IFN-γ is an obvious candidate, the IL-12-induced factor which accounts for BDV inhibition in these animals remained elusive. Because BDV does not replicate in standard mouse cell culture systems, the putative inhibitory effect of IFN-γ could not be examined directly.
The aim of the present work was to establish an in vitro system that allows one to study the interactions of BDV and the CNS. Furthermore, we wanted to elucidate in more detail the effects of IL-12 and IFN-γ on BDV-infected neural cells of the mouse. We introduce organotypic cerebellar slice cultures as a novel tool to investigate the interactions of BDV and neural cells as well as the effects of antiviral cytokines in a system devoid of immune cells. We show that IFN-γ but not IL-12 can block BDV multiplication in this system.
MATERIALS AND METHODS
Cerebellar and hippocampal slice cultures.
The C57BL/6 mice used in this study were obtained from the specific-pathogen-free breeding facility of the University of Freiburg. The preparation of cerebellar and hippocampal slice cultures was performed as described before with minor modifications (23). Briefly, 6- to 7-day-old mice were decapitated and the brain of each mouse was removed. The cerebellum or the hippocampus was placed on a tissue chopper (McIllwain, The Mickle Laboratory Engeneering, Gonshall, Surrey, United Kingdom), and 400-μm-thick sections were prepared. Six to fourteen sections were placed on Milliwells (Millipore, Bedford, Mass.) in six-well plates and cultured over tissue culture medium containing 37.5% MEM, 37.5% HBSS, 25 mM HEPES, and 25% heat-inactivated horse serum (all from Gibco/Invitrogen, Karlsruhe, Germany). Cytokines (IFN-γ [PeproTech, London, United Kingdom] or IL-12 [R&D Systems, Wiesbaden, Germany]) were added to the culture medium at concentrations given in the results section and the figure legends. Tissue culture medium was changed every 2 days.
Infection of slice cultures.
A rat-adapted strain of BDV was adapted to the mouse by four consecutive passages through brains of newborn BALB/c mice and two further passages through brains of adult MRL mice. The seed virus which was originally assumed to be derived from strain He/80 (10) has recently been identified as the strain designated rat BDV, also designated RW98 (7). Brains were homogenized (1:10, wt/vol) in phosphate-buffered saline (PBS), aliquoted, and stored at −70°C. Infection of slice cultures was performed by applying 10-μl samples of 5% brain homogenate in culture medium (stock 76 or 97) onto cerebellar or hippocampal slices. Infection with either stock showed comparable kinetics of BDV mRNA expression in cerebellar slices. For mock infections, brain homogenate of noninfected mice was used.
RNA isolation and RPA.
The tissue slices were harvested and total RNA was extracted with Trizol reagent (Gibco/Invitrogen) according to the manufacturer's protocol. Each RNA sample was derived from 12 to 15 tissue slices. The RNA was dissolved in TE (10 mM Tris [pH 8], 1 mM EDTA) and stored at −80°C. RNase protection assays (RPA) for the detection of cytokine RNAs were performed as described previously (15). The RNA samples were hybridized with labeled probe set AP9 containing probes for CD3, BDV-N (19), nitric oxide synthase 1 (NOS-1), NOS-2 and NOS-3 as well as tumor necrosis factor alpha, IFN-γ, IL-1α, IL-10, IL-12, and glial fibrillary acidic protein (GFAP), a novel probe set containing probes for the neuronal genes encoding GAP43, the 68-kDa subunit of neurofilament, synaptophysin, β-tubulin, calbindin, the oligodendroglial genes proteolipoprotein and myelin basic protein, the astrocytic GFAP, and β2-microglobulin (Table 1) and the mCK1 probe set (Promega, Madison, Wis.) as described before (8). A fragment of the RPL32-4A gene (5) served as an internal loading control. The NIH Image software (version 1.62) was used to quantify the band density in the autoradiographs.
TABLE 1.
Gene probes included in the ontogenesis RPA probe set
| Gene product | Sequence | Length (nt) | Reference or accession no. |
|---|---|---|---|
| GAP-43 | 254-593 | 340 | NM_008083.1 |
| NF-68 | 987-1297 | 311 | M20480.1 |
| β2-Microglobulin | 58-317 | 260 | NM_009735.2 |
| β-Tubulin | 923-1155 | 233 | X04663.1 |
| Synaptophysin | 471-670 | 200 | x95818 |
| PLP | 53-225 | 173 | M14674.1 |
| MBPa | 657-811 | 155 | NM_010777 |
| Calbindin | 418-557 | 140 | NM_009788.1 |
| GFAP | 129 | 4 | |
| L32 | 61-139 | 79 | NM_172086.1 |
MBP, myelin basic protein.
Immunohistochemistry.
The slice cultures were fixed for 12 h in 4% buffered paraformaldehyde. Following washes with Tris-buffered saline (two washes, 10 min each) and PBS (two washes, 10 min each), slices were permeabilized in blocking buffer (1% bovine serum albumin, 0.3% Triton X-100, 0.1% NaN3 in PBS). The slices were incubated for 4 to 6 days at 4°C with antibodies against GFAP (DAKO, Hamburg, Germany), BDV-N (kind gift from I. Lipkin, University of California—Irvine), calbindin (Swant, Bellinzona, Switzerland), and NeuN and GABA A receptor (both from Chemicon, Temecula, Calif.). Sections were then thoroughly washed (three times for 2 h each, with 0.1% Triton X-100 in PBS) and incubated for 48 h at 4°C with Cy2- or Alexa 488-labeled secondary antibodies (Molecular Probes, Eugene, Oreg.). Sections were washed for 3 h in 0.1% Triton in PBS, incubated in tap water for 15 min, and mounted in Elvanol. Following polymerization overnight in the dark, slices were examined with a confocal laser scanning microscope.
RESULTS
BDV proliferates in mouse cerebellar slice cultures.
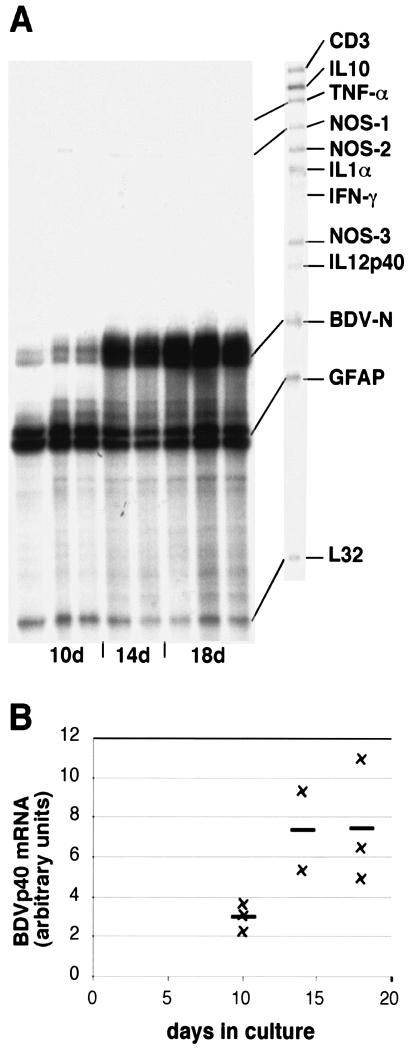
In order to determine whether BDV can infect and replicate in slice cultures, slices were harvested at different time points between 10 and 18 days postinfection. RPA analysis demonstrated that the BDV-N RNA amounts increased with longer incubation periods, indicating replication of the virus in cerebellar slice cultures (Fig. 1). After day 14, the amount of BDV-N RNA did not increase much further (Fig. 1), and virtually no difference in BDV-N RNA levels was observed between 14- and 18-day-old cultures (Fig. 1B).
FIG. 1.
Expression of various cytokine and NOS genes as well as BDV-N RNA in BDV-infected cerebellar slice cultures. (A) RPA with probe set AP9. The time course of BDV-N RNA expression shown here is a representative example of two experiments. Each lane consists of 2 μg of RNA from 12 to 15 pooled cerebellar slice culture specimens. The RPA was performed as stated in Materials and Methods. (B) Quantitative densitometric analysis of BDV-N RNA expression shown in panel A.
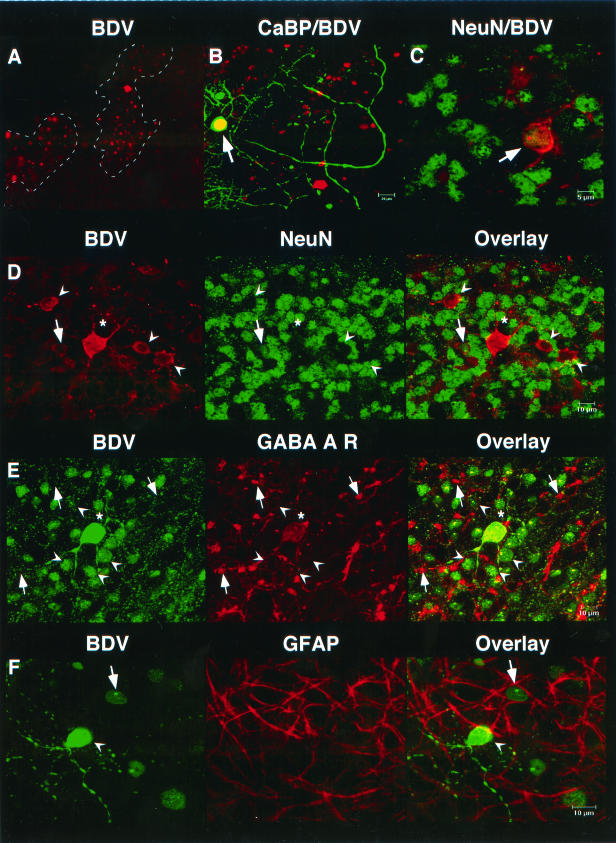
Immunohistochemistry of BDV-infected slice cultures with an antibody against BDV-N revealed that viral antigen was not evenly distributed throughout the cerebellar slices (Fig. 2A). Patches of immunoreactive cells were surrounded by areas that showed no staining for BDV-N. A dotted nuclear immunoreactivity pattern that is a hallmark of BDV infection was observed in many cells. Double labeling was performed to further characterize BDV-infected cells. Confocal laser scanning microscopy revealed that most BDV-infected cells were neuronal cells, many of which were NeuN immunoreactive (Fig. 2C and D). There was another significant portion of infected cells, however, that resembled granule cells or interneurons (Fig. 2D) that showed strong immunoreactivity for BDV-N while no immunoreactivity for NeuN was observed. The majority of BDV-infected neurons were granule cells that were immunoreactive for the GABA-A receptor α6 subunit (Fig. 2E). These cells showed a faint staining on the perinuclear cell surface (Fig. 2E); however, stronger immunoreactivity was observed on structures resembling cerebellar glomeruli (Fig. 2E). A small number of calbindin-immunoreactive Purkinje cells (Fig. 2B) and a few GFAP-immunoreactive astrocytes (Fig. 2F) were immunoreactive for BDV-N.
FIG. 2.
BDV infection of cerebellar slice cultures. (A) Immunoreactivity for BDV-N (BDV) showed a patchy distribution. (B) Colabeling of BDV and calbindin (CaBP) occasionally revealed infected Purkinje cells (arrow; yellow color indicates colabeling). (C and D) Most infected cells were neurons. Many infected granule cells showed NeuN immunoreactivity (arrow) and coexpression of BDV (red) and NeuN (green), while other granule cells (arrowheads) and Golgi cells (asterisk) were negative for this antigen. (E) Infected granule cells (arrowheads) show coexpression of BDV-N and the GABA-A receptor α6 subunit (GABA A R). The GABA A R immunoreactivity appears as a faint red band of different width on the surface of the granule cells. The globular structures that present with strong immunoreactivity represent cerebellar glomeruli ([arrows]). (F) Few GFAP-immunoreactive astrocytes were infected with BDV (arrow and arrowhead, BDV-infected neuron).
Neural, chemokine, and cytokine genes are almost unaltered in infected slice cultures.
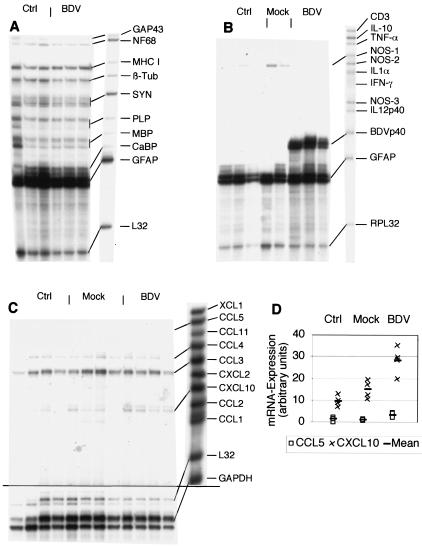
Cerebral BDV infection of mice and rats can induce chemokine expression in astrocytes in the absence of infiltrating leukocytes (19). Therefore, a number of different RPA probe sets were utilized to determine whether BDV infection leads to altered expression of neural, chemokine, and cytokine genes in 18-day-old cerebellar slice cultures (Fig. 1 and 3). All neural genes covered by the probe set were constitutively expressed and were not altered by BDV infection (Fig. 3A). Gene expression of GFAP was very strong in controls as well as in BDV-infected slices reflecting the astrogliosis that develops in organotypic slice cultures. There was no detectable alteration in the expression of cytokine and NOS genes in BDV-infected cerebellar slice cultures (Fig. 3B). The chemokine genes CCL3 and CCL4 (and in some samples CXCL10) were also constitutively expressed in cerebellar slice cultures. A two- to threefold up-regulation of CCL5 and CXCL10 gene expression was observed in BDV-infected slice cultures (Fig. 3C and D).
FIG. 3.
Expression of various neural, chemokine and cytokine genes as well as BDV-N RNA in BDV-infected cerebellar slice cultures. RPA with the probe set AP10 containing various neural genes and β2-microglobulin (abbreviations: MHC I, major histocompatibility complex type I; β-Tub, β-tubulin) (A); the probe set AP9, which contains probes for cytokine and NOS genes as well as BDV-N RNA (B); and the probe set mCK1 for chemokine genes (C). Each lane consists of 2 μg of RNA from 12 to 15 pooled cerebellar slice culture specimens. (D) Quantitative densitometric analysis of the bands in panel C.
IFN-γ but not IL-12 inhibits proliferation of BDV in cerebellar slice cultures.
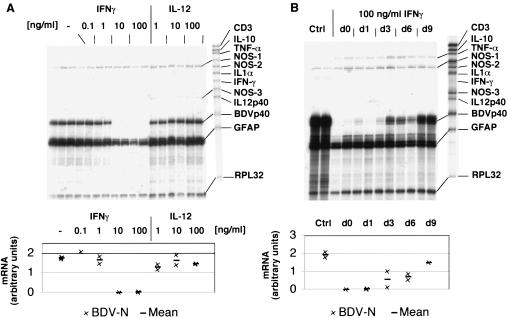
In order to study the influence of IFN-γ and IL-12 on the multiplication of BDV in cerebellar slice cultures, various amounts of these cytokines were added to the culture medium. RPA analysis revealed that IFN-γ inhibited BDV proliferation in a dose-dependent manner if given from the time of infection until the end of the experiment (Fig. 4A). While IFN-γ at a concentration of 1 ng/ml of showed no effect on BDV-N RNA levels, at 100 ng/ml it abolished BDV multiplication virtually completely. With IFN-γ at a concentration of 10 ng/ml, inhibition of BDV ranged from severalfold to below the detection limit in different experiments. In contrast, IL-12 showed no effect on BDV-N RNA expression at all doses tested (Fig. 4A).
FIG. 4.
Effect of IFN-γ and IL-12 on BDV in infected cerebellar slice cultures. RPA with the probe set AP9. (A) The dose response of BDV-N RNA expression shown here is a representative example of three (IFN-γ) or two (IL-12) experiments. Each lane consists of 2 μg of RNA from 12 to 15 pooled cerebellar slices. (B) Effect of the time period between BDV infection and addition of IFN-γ to the culture medium. Abbreviations: d0, immediate addition of IFN-γ; d9, IFN-γ added at day 9 of the incubation period; Ctrl, no IFN-γ added. The lower panels show the quantitative densitometric analysis.
In order to discriminate between the possibility that IFN-γ protects noninfected cells from infection or that it acts by clearing virus from infected cells, IFN-γ was added to the slice cultures at different time points after infection. BDV-N RNA levels in the infected slice cultures were clearly higher if IFN-γ treatment was initiated late (Fig. 4B). Treatment with high doses of IFN-γ concomitantly reduced the levels of GFAP RNA in the slice cultures by about threefold (Fig. 4A).
IFN-γ treatment is less effective in BDV-infected hippocampal slice cultures.
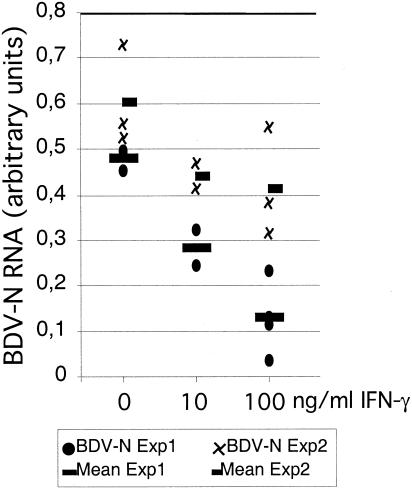
It has been reported that IFN-γ may have different antiviral effects in various brain regions. Binder and Griffin showed that IFN-γ-expressing recombinant Sindbis virus is cleared from the spinal cord but not from the brain stem, cortex, and hippocampus in SCID mice (2). In order to examine whether IFN-γ exerts site-specific effects on BDV multiplication in slice cultures from various brain regions too, hippocampal slice cultures were infected with BDV and treated with different amounts of IFN-γ. After 18 days in culture, control hippocampal slices revealed BDV-N RNA levels that were comparable to those observed in cerebellar slice cultures (Fig. 5). In contrast to cerebellar slice cultures, however, IFN-γ treatment of BDV-infected hippocampal slice cultures even at the highest dose of 100 ng/ml induced only a 30 to 70% reduction of BDV-N RNA levels (Fig. 5). A complete suppression of BDV-N RNA below the detection limit of the RPA technique was never observed in hippocampal slice cultures.
FIG. 5.
Effect of IFN-γ on BDV in infected hippocampal slice cultures. Quantitative densitometric analysis of RPA with the probe set AP9. Treatment with IFN-γ at a concentration of 10 or 100 ng/ml was started concomitantly with BDV infection. Results of two separate experiments are shown.
DISCUSSION
In the present study, we have demonstrated that the BDV-infected murine organotypic slice culture is a novel in vitro model to study the interactions of BDV, the CNS, and soluble immune effector molecules.
BDV inoculation leads to lifelong persistent infection of the CNS of various species (for a review, see reference 21). In susceptible hosts BDV infection may lead to a nonpurulent meningoencephalitis. The demonstration that upon intracerebral inoculation of BDV certain mouse strains are susceptible hosts for Borna disease (10) opened the possibility of investigating the interaction of BDV and the host animal in a wide range of mouse strains with targeted mutations. Using mice with a disruption of the β2-microglobulin gene, we demonstrated that CD4 as well as CD8 T cells are involved in the immune response that causes neurological disorder (11).
In order to further dissect the interactions of BDV, the CNS, and the immune system we established BDV-infected organotypic cerebellar or hippocampal slice cultures as a novel model system. Since infiltrating leukocytes are absent from slice cultures, this system allows the study of the role of defined factors such as cytokines in the immune response against BDV. Moreover, and in contrast to primary cultures of dissociated cells, in organotypic slice cultures the brain-resident cells (neurons, astrocytes, oligodendrocytes, microglia, and endothelial cells) are present at their physiological ratio, and the different cell types can maintain their physiological interactions.
In BDV-infected cerebellar slice cultures we observed an increase of BDV-N RNA amounts with longer incubation periods, indicating that BDV proliferated in the cerebellar slice cultures. Additionally, the demonstration of BDV-N immunoreactivity in the nuclei of various neural cell types in the slice cultures supported the notion that a proliferative BDV infection was established. Therefore, to our knowledge, the organotypic cerebellar slice culture is the first in vitro model for BDV infection of mouse neural cells. Interestingly, not only neuronal cells such as granule cells, Purkinje cells, and interneurons but also astrocytes were infected with BDV. This correlates to observations in the cerebellum of BDV-infected mice in which mainly neuronal cells and only few GFAP-immunoreactive astrocytes show immunoreactivity for BDV-N (8; S. Freude and A. Pagenstecher, unpublished observations). The patchy distribution of BDV-N immunoreactivity in cerebellar slices contrasts with the more even distribution of BDV-N in BDV-infected mouse brains (8, 10). A possible explanation for this difference might be the interruption of neuronal connections in the slice cultures. Application of BDV to the surface of the cerebellar slices may infect superficially located cells, and from these seeds the virus spreads to connecting neurons. The interruption of the cerebellar three-dimensional connectivity at the sections of the 400-μm-thick slices may inhibit further virus spread throughout the cerebellar slice.
BDV infection of mice and rats can induce cerebral expression of CXCL10 and CCL5 in the absence of infiltrating B and T cells (19). The induction of these genes was also observed in BDV-infected cerebellar slice cultures, however, at levels lower than the expression levels observed in brains of mice and rats. An explanation for this difference might be the short incubation period of the cerebellar slices (18 days) compared to the in vivo experiments in which neonatally infected mice and rats showed high levels of CXCL10 and CCL5 gene expression at later time points (19). It is noteworthy that both CXCL10 and CCL5 not only act as potent attractants for leukocytes but also exert antiviral effects (1, 14). The effects of chronic expression of CXCL10 in the CNS have been studied in a transgenic mouse model (3). These mice showed infiltration of the CNS and the meninges by mainly neutrophils and to a lesser extent T cells. No tissue destruction was observed, indicating that the infiltrating leukocytes need further signals to become fully activated effector cells (3). Importantly, we observed no alteration in the expression of neural, cytokine, and NOS isoform genes in cerebellar slice cultures. The GFAP gene was expressed at high levels in control slice cultures that were not further increased by BDV infection. Most likely this reflects the reactive astrogliosis in this cell culture model. However, we did not find upregulation of GFAP mRNA expression and GFAP immunoreactivity in the CNS of BDV-infected mice, indicating that BDV does not increase GFAP expression in this species (8, 10). Interestingly, there was a significant portion of BDV-infected granule cells that did not show immunoreactivity for NeuN. Since NeuN is differentially expressed during the maturation of granule cells, this finding raises the question whether BDV infection interferes with the maturation of granule cells in cerebellar slice cultures. Together, these findings are in line with the inconspicuous appearance and behavior of BDV-infected mice on BDV-resistant genetic backgrounds such as C57BL/6, adding further evidence that Borna disease is mainly immunologically triggered.
We have recently shown that transgenic expression of IL-12 in the murine CNS results in reduced proliferation of BDV in areas of transgene expression (8). Although a number of factors including tumor necrosis factor alpha, IL-1α, and NOS-2 that may exert an antiviral effect were upregulated in the CNS of BDV-infected GF-IL-12 mice, the factors that accounted for the observed inhibition of BDV remained elusive. Here, we demonstrate that IFN-γ inhibited the proliferation of BDV in cerebellar slice cultures, suggesting that IFN-γ decreases virus multiplication also in the cerebellum of BDV-infected GF-IL-12 mice (8). It has been shown that IFN-γ can both induce an antiviral state in the cell that protects the cell from infection and inhibit the replication of infectious agents via different mechanisms (for a review, see reference 18). Our finding that the amount of BDV-N RNA in cerebellar slice cultures increased with the time period between infection of the slices and the initiation of IFN-γ treatment suggests that IFN-γ did not eliminate established BDV from infected cells but inhibited the infection of noninfected neural cells. Thus, IFN-γ seems to block an early step of the BDV multiplication cycle. Results of recent experiments with cultured human cells are in good agreement with this hypothesis (C. Sauder, unpublished results). A similar phenomenon was observed in measles virus-infected murine hippocampal neurons in which a time period between infection and IFN-γ treatment resulted in higher virus RNA amounts compared to cultures that were immediately treated with IFN-γ (16). Importantly, IFN-γ did not eliminate measles virus from hippocampal neuron cultures completely. In the present study, we observed a complete suppression of BDV-N RNA in IFN-γ-treated cerebellar slice cultures, while an incomplete suppression was seen in hippocampal slice cultures. This corroborates observations in Sindbis virus-infected mice, in which IFN-γ mediates the clearance of established virus from neurons in certain regions of the CNS (2). This difference suggests a different IFN-γ-sensitivity of different brain regions. Similarly, IFN-γ mediates partial resistance of passively immunized mice that have no functional receptors for IFN-α/β against herpes simplex virus (24). In these experiments, IFN-γ together with neutralizing antibodies was sufficient to eliminate herpes simplex virus from a portion of brains. Although we can exclude effects of antibodies and infiltrating T cells and NK cells on BDV in organotypic slice cultures, it remains unclear whether IFN-γ is the only factor that inhibits BDV proliferation in GF-IL-12 mice. Preliminary results with BDV-infected GF-IL-12 mice deficient for IFN-γ suggest that IFN-γ is crucial for the inhibition of BDV in GF-IL-12 mice since these mice showed no inhibition of BDV multiplication in areas of transgene expression (M. Hofer and A. Pagenstecher, unpublished data).
The decreased levels of GFAP gene expression in IFN-γ-treated cerebellar slice cultures are in contrast to earlier reports demonstrating that IFN-γ enhances immunoreactivity for GFAP in dissociated human fetal spinal cord cells (6). This contrast might be explained by the different culture systems that were used. While Erkman and coworkers used human fetal spinal cord cells, in our case, neonatal cerebellar tissue was investigated that is considerably more mature than fetal tissue.
Together, our results demonstrate for the first time that mouse organotypic slice cultures represent a suitable in vitro system to study the role of defined soluble or cellular factors such as cytokines or lymphocytes in the interaction of the CNS and viruses. Our data further reveal that the noncytolytic antiviral activities of IFN-γ render this cytokine a crucial factor for CNS immunity.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Beate Hobmaier and Rolf Knoth for help at the CLSM.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to A.P.
REFERENCES
- 1.Appay, V., and S. L. Rowland-Jones. 2001. RANTES: a versatile and controversial chemokine. Trends Immunol. 22:83-87. [DOI] [PubMed] [Google Scholar]
- 2.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 3.Boztug, K., M. J. Carson, N. Pham-Mitchell, V. C. Asensio, J. DeMartino, and I. L. Campbell. 2002. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J Immunol. 169:1505-1515. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, C. S., A. Stalder, A. Samimi, and I. L. Campbell. 1994. Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev. Neurosci. 16:212-221. [DOI] [PubMed] [Google Scholar]
- 5.Dudov, K. P., and R. P. Perry. 1984. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37:457-464. [DOI] [PubMed] [Google Scholar]
- 6.Erkman, L., L. Wuarin, D. Cadelli, and A. C. Kato. 1989. Interferon induces astrocyte maturation causing an increase in cholinergic properties of cultured human spinal cord cells. Dev. Biol. 132:375-388. [DOI] [PubMed] [Google Scholar]
- 7.Formella, S., C. Jehle, C. Sauder, P. Staeheli, and M. Schwemmle. 2000. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J. Virol. 74:7878-7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freude, S., J. Hausmann, M. Hofer, N. Pham-Mitchell, I. L. Campbell, P. Staeheli, and A. Pagenstecher. 2002. Borna disease virus accelerates inflammation and disease associated with transgenic expression of interleukin-12 in the central nervous system. J. Virol. 76:12223-12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 10.Hallensleben, W., M. Schwemmle, J. Hausmann, L. Stitz, B. Volk, A. Pagenstecher, and P. Staeheli. 1998. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J. Virol. 72:4379-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausmann, J., W. Hallensleben, J. C. de la Torre, A. Pagenstecher, C. Zimmermann, H. Pircher, and P. Staeheli. 1999. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 96:9769-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausmann, J., K. Schamel, and P. Staeheli. 2001. CD8+ T lymphocytes mediate Borna disease virus-induced immunopathology independently of perforin. J. Virol. 75:10460-10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 14.Mahalingam, S., J. M. Farber, and G. Karupiah. 1999. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J. Virol. 73:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagenstecher, A., A. K. Stalder, and I. L. Campbell. 1997. RNase protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J. Immunol. Methods 206:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 18.Rottenberg, M., and K. Kristensson. 2002. Effects of interferon-gamma on neuronal infections. Viral Immunol. 15:247-260. [DOI] [PubMed] [Google Scholar]
- 19.Sauder, C., W. Hallensleben, A. Pagenstecher, S. Schneckenburger, L. Biro, D. Pertlik, J. Hausmann, M. Suter, and P. Staeheli. 2000. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J. Virol. 74:9267-9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schamel, K., P. Staeheli, and J. Hausmann. 2001. Identification of the immunodominant H-2Kk-restricted cytotoxic T-cell epitope in the Borna disease virus nucleoprotein. J. Virol. 75:8579-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 22.Staeheli, P., M. Sentandreu, A. Pagenstecher, and J. Hausmann. 2001. Alpha/beta interferon promotes transcription and inhibits replication of Borna disease virus in persistently infected cells. J. Virol. 75:8216-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauer, U., B. Volk, and B. Heimrich. 1996. Differentiation of Purkinje cells in cerebellar slice cultures: an immunocytochemical and Golgi EM study. Neuropathol. Appl. Neurobiol. 22:361-369. [DOI] [PubMed] [Google Scholar]
- 24.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]