Abstract
Previously, we identified human papillomavirus type 16 (HPV-16) E5 as a tumor rejection antigen that can induce cytotoxic T lymphocytes (CTLs) to protect against tumor growth (D. W. Liu et al., J. Virol. 74:9083-9089, 2000). In the present study, we further mapped the CTL epitope of E5 protein by analyzing E5-specific CD8+ gamma interferon-positive (IFN-γ+) double-positive cells in C57BL/6 mice with flow cytometry. The results showed the region spanning amino acids 25 to 33 (VCLLIRPLL) contained the potential Db-restricted CTL epitope. Subsequently, to determine whether peptide E5 25-33-based vaccination could induce E5-specific CTL activity, syngeneic animals received E5 25-33 emulsified with either CpG oligodeoxynucleotide (CpG ODN 1826) or Freund's adjuvant, and the growth of the tumors was monitored. The results showed that although both adjuvants induced E5-specific CD8+ IFN-γ+ T cells and eradicated E5-containing tumor growth, CpG ODN was found to stimulate stronger CTL response than Freund's adjuvant. We also compared the immune response of the effector/memory/recall phase induced by E5 25-33 peptide or by E5 protein that was synthesized in vivo by adenovirus-based E5 gene delivery. E5 25-33 peptide plus CpG ODN was shown to be a superior vaccine compared to the adenovirus-based E5 gene. Interestingly, their chronological patterns of immune response were similar, suggesting that E5 25-33 is a major CTL peptide of E5 protein.
Carcinoma of the cervix uteri is the second most common cancer in women worldwide, and the vast majority of cases occur in developing countries (26, 66). Human papillomaviruses (HPVs) are naturally occurring DNA tumor viruses that cause epithelial cell proliferation during the course of infection. HPV type 16 (HPV-16) is the predominant type of virus identified in cervical cancers. It encodes three transforming oncogenes: E5, E6, and E7 (26, 66). E5, E6, and E7 are unique tumor antigens and may serve as ideal materials to be used as a tumor vaccine. Most evidence for HPV antigen-directed immunotherapy against cervical cancer comes from the experimental and natural papillomavirus-associated tumors that can be controlled by immunization with E6/E7 antigen (5-6, 8-9, 15, 17-18, 21, 32-33, 37-40, 49, 63). Our recent study of E5 protein explored its potential as a tumor antigen to induce E5-specific CD8+ T cells to eradicate tumor growth (37).
HPV-16 E5 consists of 83 amino acids of hydrophobic protein (4, 23) found in the endoplasmic reticulum (ER), Golgi apparatus, endosomal compartment, and cell membrane (12). HPV-16 E5 associates with the epidermal growth factor receptor (EGFR) (27), increasing the ligand-dependent activation of EGFR in HaCaT cells (13), and in primary human keratinocytes (55). The effect of E5 on EGFR activation in the presence of ligand may be due to its binding to the 16-kDa protein subunit of the vacuolar proton ATPase that is responsible for acidification of the endosomal milieu and consequently for ligand receptor degradation. HPV-16 E5 binds the 16-kDa subunit of vacuolar proton-ATPase (13) and perturbs its activity, leading to blockage of the acidification of endosomes in human keratinocytes (54, 56). The alkalization of endosomes may be responsible for increased recycling of EGFR to the cell surface, resulting in enhancement of EGFR-mediated biological activity in the presence of EGF (55, 56). EGFRs, along with other tyrosine kinase growth factor receptors, initiate diverse biochemical events and ultimately result in the transcription of a variety of genes, including c-jun, c-fos, and junB (10, 11). In vivo studies demonstrate that E5 is expressed soon after infection, with both mRNA and protein detectable in low-grade squamous intraepithelial lesions (3, 7, 29, 59); the prevalence of E5-containing mRNA increases with advancing severity of the disease (3). Therefore, when E5 is expressed in earlier stages of neoplastic transformation of the cervical epithelium during viral infection, the early lesions usually contain fewer tumor cells. The immune response cells in premalignant lesions may eradicate tumor more efficiently than in the invasive cervical cancers. Our previous study demonstrated that a single muscle injection of the recombinant adenovirus encoding HPV-16 E5 (i.e., rAd-E5) into syngeneic animals could reduce tumor growth in lesions that contain the E5 gene expression and that E5 vaccine-induced tumor inhibition occurs via CD8+ T cells (37).
Although viral oncoproteins such as HPV-16 E5, E6, and E7 are experimentally identified as target antigens in immune intervention protocols against cervical cancer, the use of vaccines encoding entire oncoproteins for the induction of T-cell immunity poses the risk of transformed healthy cells. To avoid this risk, vaccination with subunit vaccines limited to the T-cell epitopes of oncogenic proteins is preferable. Subunit vaccines that contain small synthetic peptides corresponding to minimal cytotoxic-T-lymphocyte (CTL) epitopes have been shown to be highly effective for the induction of strong, protective CTL-mediated immunity against infectious virus and tumor growth in murine models (17). Recently, several groups have mapped major histocompatibility complex class I (MHC-I) and MHC-II epitopes of HPV E6 and E7 proteins in murine and human systems (17, 28, 45, 48, 52, 58, 60, 67). Hence, in the present study, we mapped the CTL epitope of E5 protein by analyzing E5-specific CD8+ gamma interferon-positive (IFN-γ+) double-positive cells in H-2b C57BL/6 mice by using flow cytometry. Amino acids 25 to 33 (VCLLIRPLL) of the E5 protein were identified as a Db-restricted CTL epitope in C57BL/6 mice. The E5 25-33 peptide was then further tested for its ability to elicit anti-tumor immunity in C57BL/6 mice.
MATERIALS AND METHODS
Peptide synthesis.
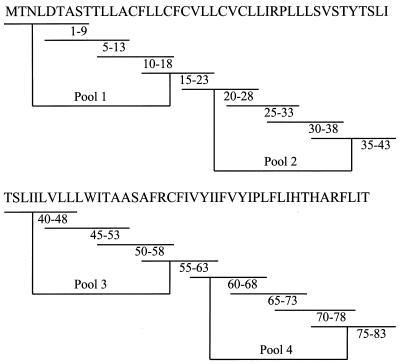
To determine potential vaccine candidates, we generated a complete set of 16 overlapping synthetic peptides of 9 amino acids in length with 5-amino-acid overlap covering the entire HPV-16 E5 protein that contains 83 amino acids (Fig. 1). Moreover, five potential Kb- and eight Db-binding peptides were synthesized (see Table 1). These peptides were synthesized by solid-phase strategies on an automated peptide synthesizer (Abimed AMS 422) by using Fmoc (9-fluorenylmethoxy carbonyl) chemistry. Peptides were analyzed by reverse-phase high-performance liquid chromatography; they were dissolved in dimethyl sulfoxide at 1 to 5 mg/ml and stored at −70°C.
FIG. 1.
Sixteen sets of 9-mer peptides covering the entire E5 protein. A complete set of 16 overlapping synthetic peptides, each nine amino acids long and overlapping each other sequences by five amino acids, covering the entire HPV-16 E5 protein was synthesized by using an automated peptide synthesizer (Abimed AMS 422) by using Fmoc chemistry. Peptides were analyzed by reverse-phase high-pressure liquid chromatography, dissolved in dimethyl sulfoxide at 1 to 5 mg/ml, and stored at −70°C. The peptides were divided into four groups: pool 1, including peptides 1-9, 5-13, 10-18, and 15-23; pool II, including peptides 20-28, 25-33, 30-38, and 35-43; pool III, including peptides 40-48, 45-53, 50-58, and 55-63; and pool IV, including peptides 60-68, 65-73, 70-78, and 75-83.
TABLE 1.
E5 peptides
| MHC-I molecule | Peptide | Sequence | Scorea |
|---|---|---|---|
| Db | E5 9-17 | TTLLACFLL | 12.773 |
| E5 20-28 | CVLLCVCLL | 95.880 | |
| E5 24-32 | CVCLLIRPL | 26.633 | |
| E5 25-33 | VCLLIRPLL | 9.275 | |
| E5 34-42 | LSVSTYTSL | 33.748 | |
| E5 40-48 | TSLIILVLL | 123.742 | |
| E5 41-49 | SLIILVLLL | 15.807 | |
| E5 53-61 | AASAFRCFI | 12.524 | |
| Kb | E5 14-22 | CFLLCFCVL | 10.000 |
| E5 34-42 | LSVSTYTSL | 22.000 | |
| E5 37-45 | STYTSLIIL | 6.600 | |
| E5 61-69 | IVYIIFVYI | 16.500 | |
| E5 63-71 | YIIFVYIPL | 24.000 |
That is, the estimate of the half-time of dissociation of a molecule containing this sequence with MHC-I molecules.
Cells.
TC-1 is an E6/E7-expressing tumorigenic cell line from primary lung epithelial cells of C57BL/6 mice immortalized by HPV-16 E6 and E7 and then transformed with an activated ras oncogene (62). To establish a C57BL/6 syngeneic mice tumor model containing the E5 gene, TC-1 cells were transfected by using the liposome method with HPV-16E5/pHOOK plasmid containing the HPV-16 E5 gene with a 5′ tagged HA1 epitope in the plasmid vector pHOOKs (Invitrogen, Carlsbad, Calif.) (37). E5 gene expression was driven by a minimal hCMV early promoter. At 3 or 4 weeks after transfection at least 80 to 100 zeocin-resistant colonies were selected, pooled, and named TC-1/E5. TC-1 and TC-1/E5 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin-streptomycin (50 U/ml), l-glutamine (2 mM), sodium pyruvate (1 mM), nonessential amino acids (2 mM), G418 (0.4 mg/ml), and hygromycin (0.2 mg/ml). They were grown at 37°C in 5% CO2.
Intracytoplasmic cytokine staining and flow cytometry analysis.
Splenocytes from rAd-E5- or peptide-vaccinated mice or controls were incubated for 12 h with stimulator (E5 synthetic peptides) for the detection of E5 peptide-specific CD8+-T-cell precursors. Golgistop (Pharmingen, San Diego, Calif.) was added 6 h before the cells were harvested. Cells were then washed twice in FACScan buffer and stained with fluorescein isothiocyanate-conjugated rat anti-mouse CD8b.2 (Ly-3.2) monoclonal antibody (Pharmingen). Cells were subjected to intracellular cytokine staining by using the Cytofix/Cytoperm kit according to the manufacturer's instructions (Pharmingen). Phycoerythrin-conjugated rat anti-mouse IFN-γ monoclonal antibody was purchased from Pharmingen. Fluorescence-activated cell sorting analysis was performed on a Becton Dickinson FACScan with CellQuest software (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.).
Animals.
C57BL/6 (H-2b) mice were obtained from the National Laboratory Animal Breed and Research Center (Taipei, Taiwan) and maintained in our institute under specific-pathogen-free conditions. The mice were used at 4 to 6 weeks of age. Knockout I (KO I) mice are β2-microglobulin (β2m)−/− mutants that fail to express MHC-I molecules on the cell surface and thus are virtually devoid of functional CD8+ T cells (30, 64). KO II mice are H-2-IAβ−/− mutants that lack surface expressed MHC-II molecules (22) and thus are virtually devoid of functional CD4+ T lymphocytes.
In vivo tumor prevention and elimination experiments.
To assay tumor prevention experiments, 100 μg of E5 peptide 25-33 was extensively mixed with 0.2 μmol of CpG ODN 1826 (bioactive) or complete Freund's adjuvant (CFA) (1, 14, 46). The mixtures were intramuscularly or intraperitoneally (i.p.) injected in C57BL/6 mice and, 1 week later, these vaccinated mice were boosted with the same regimens as previously. Mice from control groups received either peptide with CpG ODN 1982 (nonbioactive) or CFA only or 100 μl of phosphate-buffered saline (PBS). At 1 week after the first immunization, 5 × 104 tumor cells were injected subcutaneously (s.c.) into the left flank of C57BL/6 mice, and secondary vaccines were boosted simultaneously; mice were then monitored once a week for tumor growth. To assay tumor elimination experiments, 5 × 104 tumor cells were injected s.c. into the left flank of C57BL/6 mice. After 1 week, mice were immunized and boosted with vaccines as described above at a 1-week interval. Mice were then monitored once a week for tumor growth.
RESULTS
The E5 CTL epitope was mapped by analyzing double-staining CD8+ IFN-γ+ cells with flow cytometry.
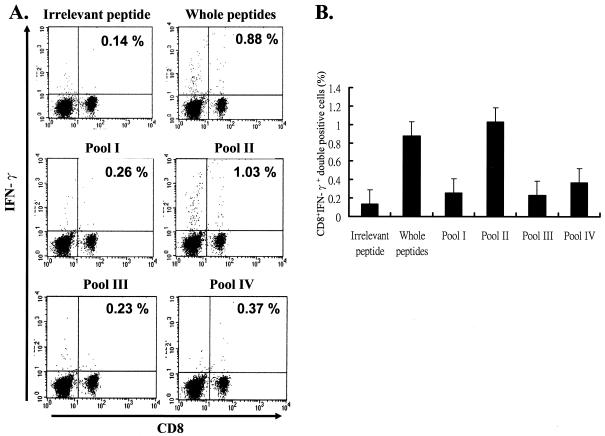
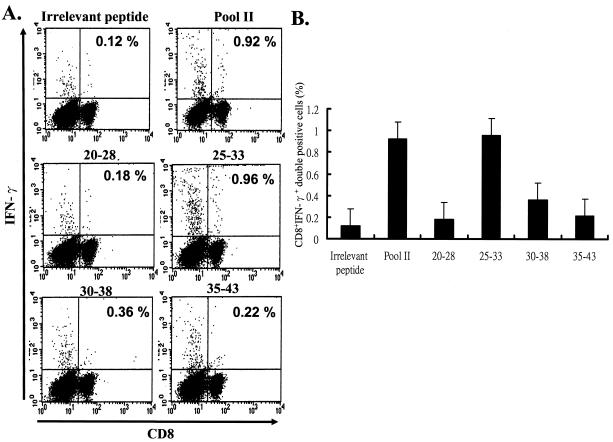
We have previously demonstrated that vaccination with rAd-E5 eradicates tumor growth in C57BL/6 mice through CD8-dependent CTL (37). In order to identify the CTL epitope here, we performed intracellular cytokine staining, a sensitive functional assay for examining IFN-γ production at the single-cell level (32). First, we synthesized a series of peptides, each of which contains 9 amino acids overlapping by 5 amino acids with its sequential neighbor; since E5 contains 83 amino acids in all, amino acids 1 to 9, 5 to 13, 10 to 18, 15 to 23, 20 to 28, 25 to 33, 30 to 38, 35 to 43, 40 to 48, 45 to 53, 50 to 58, 55 to 63, 60 to 68, 65 to 73, 70 to 78, and 75 to 83 were therefore synthesized according to the method of Gill et al. (20). These 16 sets of 9-mer peptides were divided into four groups, with each group containing four peptides, designated pools I (containing amino acids 1 to 9, 5 to 13, 10 to 18, and 15 to 23), II (containing amino acids 20 to 28, 25-33, 30 to 38, and 35 to 43), III (containing amino acids 40 to 48, 45 to 43, 50 to 58, and 55 to 63), and IV (containing amino acids 60 to 68, 65 to 73, 70 to 78, and 75 to 83) (Fig. 1). Mice were vaccinated with the rAd-E5 gene twice at a 1-week interval. Two weeks after vaccination, splenocytes were harvested and stimulated with each group peptide (pools I, II, III and IV separately), CTL was assayed by CD8+ IFN-γ+ double staining with flow cytometry. It is well recognized that the increase in CD8+ IFN-γ+ T cells is a phenotype consistent with CTL activity (43). As shown in Fig. 2, the peptides in pool II (including peptides 20-28, 25-33, 30-38, and 35-43) stimulated an approximately 7.4-fold increase (0.14 to 1.03%) in E5-specific CD8+ IFN-γ+ T cells compared to the control group (irrelevant peptide), implying that pool II contained CTL peptide epitope that could induce an E5-specific CTL response. Consequently, the CTL activity of these four peptides was measured further. As shown in Fig. 3, the epitope data clearly showed that peptide 25-33 (VCLLIRPLL) could stimulate an ∼8-fold increase (0.12 to 0.96%) in E5-specific CD8+ IFN-γ+ T cells compared to the control group (irrelevant peptide), implying that peptide E5 25-33 contained the E5 CTL epitope.
FIG. 2.
Mapping HPV-16 E5-specific CTL peptide epitope in C57BL/6 mice. Four- to six-week-old C57BL/6 mice were vaccinated and boosted 7 days later with rAd-E5. Two weeks after the first vaccination, splenocytes were harvested and stimulated with each group of peptides. Iintracellular cytokine staining with flow cytometry was then performed to determine the number of CD8+ IFN-γ+ double-positive cells. (A) Splenocytes from vaccinated mice were stimulated in vitro with each pool of peptides and stained for both CD8+ and IFN-γ+ antibodies. “Whole peptides” refers to all of the 16 synthesized peptides. The result of one representative assay from five similar independent experiments is shown. (B) Summary of the five independent experiments. The data represent the means and standard errors of five experiments. The y axis shows the percent positive cells, i.e., (the number of E5-specific CD8+ IFN-γ+ T cells/the total number of tested lymphocytes) × 100%.
FIG. 3.
The E5 peptide 25-33 (VCLLIRPLL) is the CTL epitope in C57BL/6 mice. CTL activity among these four peptides of pool II (peptides 20-28, 25-33, 30-38, and 35-43) was measured by analyzing CD8+ IFN-γ+ double-positive cells from rAd-E5-vaccinated mice as described in Materials and Methods. (A) Splenocytes from vaccinated mice were stimulated in vitro with each peptide and stained for both CD8+ and IFN-γ+ antibodies. The result of one representative assay from five similar independent experiments is shown. (B) Summary of the five independent experiments. The data represent the means and standard errors of five experiments. The y axis shows (the number of E5-specific CD8+ IFN-γ+ T cells/the total number of tested lymphocytes) × 100%.
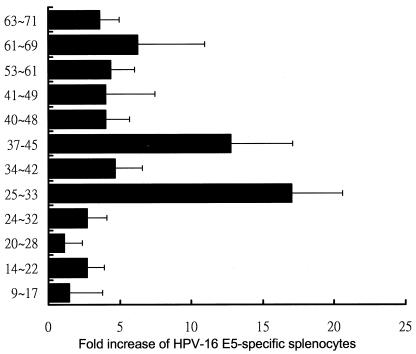
Moreover, we screened the primary structure of E5 for sequences fitting the allele-specific motifs of the C57BL/6 MHC-I molecules Kb and Db. Using the HLA peptide binding prediction offered by the National Institutes of Health (http://www.bimas.dcrt.nih.gov/cgi-bin/molbio/hla-bind/), the potential CTL epitopes were scored by estimating their dissociation half-lives from MHC-I molecules. Five potential Kb- and eight Db-binding peptides were synthesized (Table 1) and were then used as a stimulator to monitor CTL activity in rAd-E5 vaccinated mice as described in Fig. 2 and 3. As shown in Fig. 4, Db-restricted peptide 25-33 was the highest E5-specific CTL activity, corroborating the result shown in Fig. 3. The second highest CTL activity was associated with the Kb-restricted peptide 37-45.
FIG. 4.
Screening the CTL activity of the potential Kb- and Db-binding peptides of HPV-16 E5. The splenocytes from rAd-E5 vaccinated C57BL/6 mice were harvested and stimulated with each E5 peptide, and then intracellular cytokine staining with flow cytometry was performed to determine the number of CD8+ IFN-γ+ double-positive cells as described for Fig. 2. The data represent the means and standard error of five experiments. The x axis denotes the fold increase of E5-specific splenocytes, calculated as follows: (the number of vaccinated splenocytes stimulated with peptide 25-33/the number of vaccinated splenocytes stimulated with irrelevant peptide) × 100%.
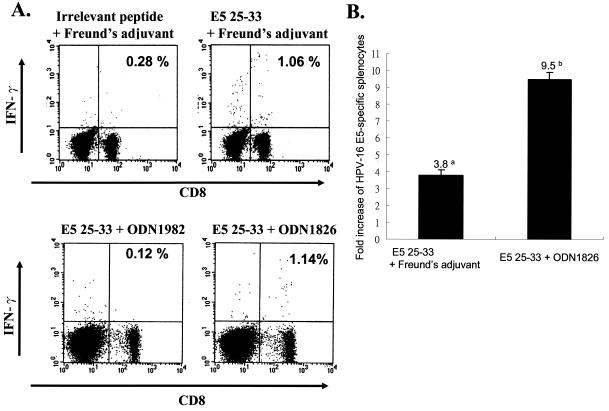
In the subsequent immunization experiments, we chose the highest CTL epitope (Db-restricted peptide 25-33) to determine whether peptide E5-based vaccination could induce E5-specific CTL activity. We chose two adjuvants for vaccination; one was Freund's adjuvant, and the other CpG phosphorethioate oligodeoxynucleotide (CpG ODN). CpG ODN, found in bacterial DNA but not vertebrate DNA, contains unmethylated CpG dinucleotides within specific sequence contexts (CpG motifs) (1, 19, 24-25, 41, 53, 57). DNA containing these CpG motifs can cause dendritic cells (DC) to mature and stimulate macrophages and B cells. It has been reported that CpG ODN acts as a powerful stimulator of leukocyte function in vitro and in vivo (19, 24-25, 41, 53, 57). Moreover, ODN 1826 containing two optimal murine CpG motifs (5′-GACGTT-3′) has strong immunostimulatory activities, such as natural killer lytic activity, B-cell proliferation, induction of tumor necrosis factor alpha, interleukin-6 (IL-6), IFN-γ, and IL-12 in murine spleen cells (25); ODN 1982 is negative or weak compared to CpG ODN 1826. Hence, in the present study, we used CpG ODN 1826 as an adjuvant rather than the conventional Freund's adjuvant to test the immunostimulating capability. One group of C57BL/6 mice (n = 5) were i.p. injected with peptide E5 25-33 emulsified with CFA. One week later, these vaccinated mice were boosted i.p. with peptide 25-33 with incomplete Freund's adjuvant. The other groups of mice were vaccinated (two immunizations, given 1 week apart) with peptide E5 25-33 with CpG ODN 1826 (bioactive) (19) or with a control nucleotide (ODN 1982, nonbioactive) as described in Materials and Methods. One week after the booster immunization, the splenocytes of all tested mice were explanted, and E5-specific CD8+ IFN-γ+ double-positive cells were measured by using flow cytometry. Figure 5A shows that mice vaccinated with peptide E5 25-33, together with either Freund's adjuvant or CpG ODN 1826, demonstrated E5-specific CTL activity. The increases in CTL activity induced by E5 25-33 with CpG ODN 1826 and E5 25-33 with Freund's adjuvant were 9.5- and 3.8-fold, respectively, compared to the control group (Fig. 5B), indicating that CpG ODN is superior to Freund's adjuvant in terms of immunostimulatory effects in conjunction with E5 25-33.
FIG. 5.
E5 25-33 peptide-based vaccination can induce E5-specific CTL activity. Four- to six-week-old C57BL/6 mice were intramuscularly vaccinated with E5 peptide 25-33 (VCLLIRPLL) plus CpG ODN 1826 (bioactive) or peptide 25-33 plus control nucleotide (ODN 1928; nonbioactive), or peptide 25-33 plus Freund's adjuvant, or irrelevant peptide with Freund's adjuvant twice at 1-week intervals. One week after boosting, splenocytes were isolated and stimulated with peptide 25-33 (VCLLIRPLL), and then CD8+ IFN-γ+ double-positive cells were analyzed by flow cytometry as described in Materials and Methods. (A) Splenocytes from vaccinated mice were stimulated in vitro with E5 peptide 25-33 and stained for both CD8+ and IFN-γ+ antibodies. The result of one representative assay from five mice is shown. (B) Summary of the data for five mice in each group. The data represents the means and the standard errors of five experiments. The y axis shows the fold increase of HPV-16 E5 specific splenocytes. Superscripts: a, (the number of E5-specific CD8+ IFN-γ+ T cells from vaccinated mice with E5 25-33 plus Freund's adjuvant/the number of E5-specific CD8+ IFN-γ+ T cells of mice vaccinated with irrelevant peptide plus Freund's adjuvant) × 100; b, (the number of E5-specific CD8+ IFN-γ+ T cells from vaccinated mice with E5 25-33 plus CpG ODN 1826/the number of E5-specific CD8+ IFN-γ+ T cells of mice vaccinated with E5 25-33 plus ODN 1982) × 100%.
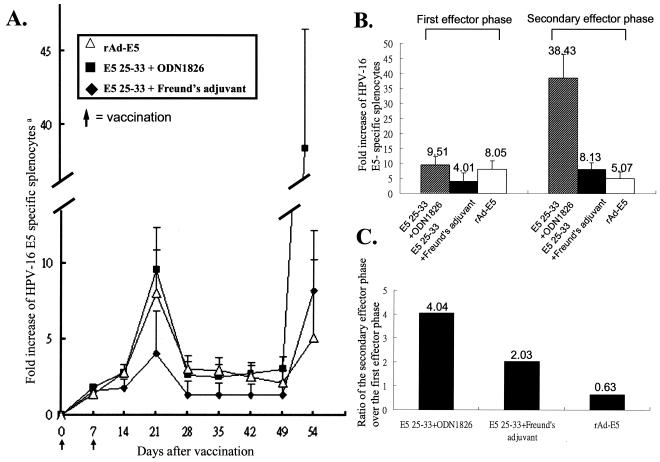
To analyze and compare the kinetics of E5-specific CD8+ IFN-γ+ T cells by intramuscularly vaccinating them with rAd-E5, peptide 25-33 with CpG ODN 1826 or i.p. immunizing peptide E5 25-33 with Freund's adjuvant as shown in Fig. 6A, the peak of immune response in all of these three vaccinations occurred at 21 days postvaccination. At 28 days postvaccination, the splenic response had declined to memory levels. At 49 days postinjection, mice were again injected with the same antigen via the same route in each group. At 7 days after secondary immunization of primed mice, E5-specific CD8+ IFN-γ+ T cells in mice immunized with E5 25-33 plus CpG ODN 1826 greatly increased in number (Fig. 6B), the secondary effector cell activity being fourfold greater than the primary effector cell activity (Fig. 6C). In mice injected with E5 25-33 plus Freund's adjuvant, a twofold increase in the second effector cell activity compared to the primary effector activity was observed (Fig. 6C). However, in the rAd-E5-vaccinated mice, there was decrease of secondary effector cell activity (0.63-fold) compared to its primary effector activity, implying that the inducing neutralization antibody to adenovirus from the primary rAd-E5 vaccination reduced the delivery efficiency of antigen by the secondary adenovirus infection. Thus, E5 peptide 25-33, along with either CpG ODN 1826 or Freund's adjuvant, generated E5-specific memory T cells able to mount a more rapid and effective secondary immune response than the primary response.
FIG. 6.
Kinetics of E5-specific CD8+ IFN-γ+ T cells in C57BL/6 mice vaccinated with rAd-E5, peptide 25-33 plus CpG ODN 1826, or peptide E5 25-33 plus Freund's adjuvant. (A) In the primary immunization, mice were vaccinated intramuscularly with rAd-E5 or peptide 25-33 plus CpG ODN 1826 (bioactive) or immunized i.p. peptide 25-33 plus Freund's adjuvants twice at days 0 and 7; secondary vaccination was performed with the same regimens as the first immunization at day 49. The splenocytes were isolated at the indicated time in days postinfection and then stimulated with E5 peptide 25-33, stained with CD8+ and IFN-γ+ antibodies, and subsequently analyzed by flow cytometry. (B) Summary of five independent experiments. The data represent the means and standard errors of five experiments. Superscripts: a, the y axis shows the fold increase of E5-specific splenocytes calculated as (the number of vaccinated splenocytes stimulated with peptide 25-33/the number of vaccinated splenocytes stimulated with irrelevant peptide) × 100%. The number on the top of each bar represents the fold increase of E5-specific splenocytes from panel A. (C) Ratio, calculated as follows: (secondary effector cell activity/first effector cell activity) × 100%.
Peptide E5 25-33-based vaccination can induce tumor elimination.
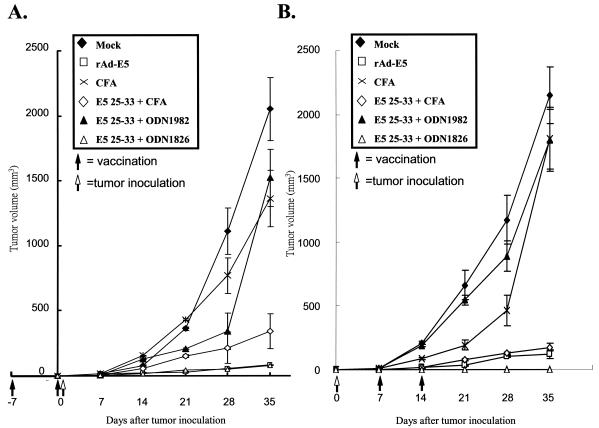
E5 is a transforming oncogene; therefore, use of the full-length E5 protein as a tumor vaccine has the potential risk of transforming healthy cells. To alleviate safety concerns, peptide vaccines are the best choice. In addition to the lack of E5 gene expression in invasive cancers, vaccination with E5 may target premalignant cells in which E5 genes are highly expressed (3, 7, 29, 59). In the present study, we chose TC-1/E5 cells as a tumor cell model (37) that contained E5, E6, and E7 proteins as described in Materials and Methods. Hence, we investigated the vaccine efficacy of E5 CTL peptide, along with CpG ODN 1826, in tumor prevention and elimination assays. The positive control was performed by vaccination with rAd-E5 that had been reported to induce CD8+ CTLs to eradicate tumor growth previously (37). To assess the degree of prevention of tumor cell growth, 10 C57BL/6 mice of each group were intramuscularly immunized and boosted with E5 25-33 plus CpG ODN 1826, i.p. immunized with E5 25-33 plus Freund's adjuvant, or intramuscularly immunized with rAd-E5. One week after the first vaccination, the mice were s.c. injected with 5 × 104 TC-1/E5 cells. The tumor volume was measured once a week. As shown in Fig. 7A, mice vaccinated with E5 25-33 plus CpG ODN 1826 showed significant retardation of TC-1/E5 cell-induced tumor development, as did rAd-E5-vaccinated mice. Mice vaccinated with E5 25-33 plus Freund's adjuvant also prevented E5-containing tumor growth, but this combination was less effective than E5 25-33 plus CpG ODN and rAd-E5; inoculations with E5 25-33 plus the nonbioactive nucleotide ODN 1982 or with Mock (i.e., CFA or PBS alone) had no effect.
FIG. 7.
In vivo tumor prevention and elimination assays. (A) Tumor prevention assay. Groups of 10 mice were immunized with 100 μg of E5 25-33 (VCLLIRPLL) plus 0.2 μmol of CpG ODN 1826 (bioactive), ODN 1982 (nonbioactive), CFA or PBS without any peptide (Mock), or rAdE5 (positive control). The mixtures were intramuscularly injected into C57BL/6 mice, and 1 week later, these vaccinated mice were boosted with the same regimens. One week after the first immunization, 5 × 104 tumor cells were injected s.c. into the left flank of C57BL/6 mice; booster vaccinations were given simultaneously, and then mice were monitored once a week for tumor growth. The data represent the means and standard errors of each group. (B) Tumor elimination assay. Groups of 10 mice were injected s.c. with 5 × 104 tumor cells into the left flank. After 1 week, the mice were immunized and boosted with 100 μg of E5 25-33 peptide alone plus CpG ODN 1826 (bioactive), ODN 1982 (nonbioactive), CFA or PBS (Mock), or rAdE5 (positive control) alone at 1-week intervals as described above. Mice were then monitored once a week for tumor growth. The data represent the means and standard errors of each group.
To assess the effect of the E5 25-33 on an established tumor, 10 C57BL/6 mice from each group were s.c. injected in their left flank with 5 × 104 TC-1/E5 cells. Based on previous studies (33, 37), the tumor size at 7 days after tumor cell inoculation should have been ∼9 mm3. Therefore, 1 week after tumor cell injection, we immunized and boosted these small tumor-bearing mice with peptide E5 25-33 with CpG ODN 1826 intramuscularly or immunized them i.p. with E5 25-33 with Freund's adjuvant or i.m. with rAd-E5. Tumor volume was measured once a week thereafter. As shown in Fig. 7B, vaccination of E5 25-33 with CpG ODN 1826 significantly regressed the tumor growth induced by E5 expression cells (TC-1/E5). Vaccinations with E5 25-33 plus Freund's adjuvant and with rAd-E5 had a similar effect on tumor regression but were inferior to the treatment with E5 25-33 plus CpG ODN 1826, although vaccinations with E5 25-33 plus nonbioactive ODN 1982 or Mock had no effect on tumor elimination. In summary, E5 25-33 peptide vaccination with either Freund's adjuvant or CpG ODN 1826 prevented and eradicated E5-containing tumor growth, but CpG ODN 1826 as an adjuvant was superior to Freund's adjuvant.
Vaccination-induced tumor protection is CD8 T cell dependent.
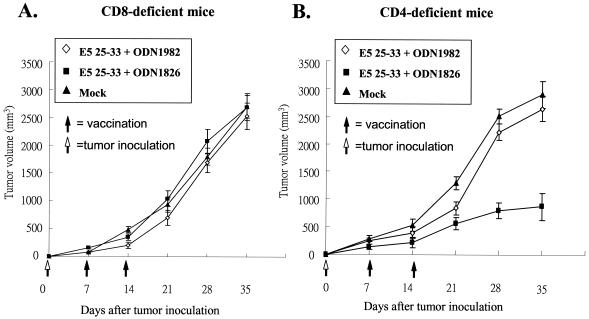
To determine the relative roles of CD4+ and CD8+ T cells in E5 peptide-based vaccine protection against tumors, mice deficient in CD4+ and CD8+ T cells as a result of targeted gene disruption at β2m and MHC-II, respectively, were studied. The sources of CD8+- and CD4+-T-cell-deficient mice were β2m−/− and MHC-II−/− C57BL/6 mice, respectively (22, 30, 64). Breeders for β2m−/− and MHC-II−/− mice were kindly provided by John Kung (Academia Sinica, Taipei, Taiwan) and were bred under specific-pathogen-free conditions. Groups (n = 5) of CD4+-T-cell-deficient mice (KO II) and CD8+-T-cell-deficient mice (KO I) were injected with 5 × 104 TC-1/E5 cells, followed 1 week later by vaccination with peptide E5 25-33 with CpG ODN 1826 or nonbioactive ODN 1982 or Mock twice at a 1-week interval. Figure 8 shows clearly evident tumor growth in CD8+-T-cell-deficient groups but not in CD4+-T-cell-deficient mice.
FIG. 8.
The CTL response is CD8+ dependent. KO I mice (CD8+ deficient) and KO II mice (CD4+ deficient) were injected s.c. with 5 × 104 TC-1/E5 tumor cells that contained the E5 gene. One week later, the animals were divided into three groups of five mice each for intramuscular vaccination with 100 μg of E5 25-33 peptide plus CpG ODN 1826 (bioactive), ODN 1982 (nonbioactive), or Mock. Booster peptide injections were administered 1 week after the first vaccination. The tumor volume was monitored once a week. (A) KO I mice (CD8+ deficient); (B) KO II (CD4+ deficient) mice.
DISCUSSION
In this study, we identified amino acids 25 to 33 (VCLLIRPLL) of E5 as a potential Db-restricted CTL epitope in H-2b C57BL/6 mice. The E5 peptide was determined as a CTL epitope from rAd-E5 vaccinated splenocytes by measuring E5-specific CD8+ IFN-γ+ double-positive cells via flow cytometry. Previously, mapping MHC-I and -II epitopes of HPV E6 and E7 protein in murine and human systems has been well studied by various assay systems. For example, Tindle et al. (58) used a T-cell proliferation assay to determine HPV-16 E7 amino acids 44 to 60 as a T-cell proliferation epitope that can stimulate lymphocytes isolated from the lymph nodes of immunized C57BL/6 mice with mixtures of different peptides that cover the whole E7 protein (58). Another method used RMA-S cells to identify an H-2b-specific peptide that binds to MHC-I molecules (52). RMA-S cells, from the H-2b-derived mutant thyoma cell line, express small amounts of “empty” Kb and Db molecules at the cell surface that are devoid of peptide. Hence, MHC-I molecule expression on RMA-S cells is low but increased when Kb binding peptides were added to the culture medium. Based on this method, HPV-16 E6 peptide 41-50 was identified as a CTL epitope. When vaccinated with peptide E6, 41-50 can elicit CTL activity (52). Likewise, the HPV-16 E7 CTL peptide epitope in H-2b C57BL/6 mice was identified as E7 49-57 (17). Immunization with peptide HPV-16 E7 amino acids 49 to 57 renders mice immune from subsequent challenge with HPV-16-transformed tumor cells in vivo and induces a CTL response that lyses the tumor cells in vitro. In addition, a peptide competitive binding assay has been used to determine the peptide epitope of five human HLA-A alleles: HLA-A1 (A*0101), A2.1 (A*0201), A3 (A*0301), A11 (A*1101), and A24 (A*2401) (28). Moreover, the CTL peptide has also been analyzed in vivo in HLA-A*0201Kb transgenic mice (45). Mice transgenic for a chimeric HLA-A*0201/Kb molecule have been used successfully by many researchers to identify HLA-A*0201-restricted CTL epitopes derived from tumor antigens (60). It has been reported that if a peptide is immunogenic in HLA-A*0201/Kb transgenic mice, it is also highly immunogenic in CTL induction with peripheral blood mononuclear cells of HLA-A*0201+ healthy donors (60). Based on this method, HPV-16 E7-derived peptides (11-20, YMLDLQPETT ;82-90, LLMGTLGIV; 86-93, TLGIVCPI), were identified as human CTL epitopes of HPV-16. In the present study, we provide another method for determining the CTL peptide epitope of HPV-16 E5 gene in H-2b C57BL/6 mice.
When vaccinated with peptide E5 25-33 emulsified with Freund's adjuvant via i.p. injection or with peptide E5 25-33 plus CpG ODN via intramuscular injection into H-2b C57BL/6 mice, both preparations induced E5-specific CD8+ IFN-γ+ T cells capable of reducing the growth of tumors expressing E5. This indicates that the segment of E5 from amino acid 25-33 is indeed a CTL peptide epitope of the HPV-16 E5 gene in H-2b C57BL/6 mice. In addition, the immune response of the effector/memory/recall phase in the vaccination with rAd-E5 or peptide E5 25-33 with Freund's adjuvant or peptide E5 25-33 with CpG presented similar patterns but reactions of different strengths. The peak of effector phase among all of these three vaccines occurred 3 weeks after the first immunization and then simultaneously dropped after 4 weeks. Therefore, we infer that E5 protein antigen might be digested by the proteosome to generate various peptides. Antigenic peptides are then transported across the membrane of the ER by transporters associated with antigen presentation. In the ER, newly synthesized MHC-I heavy chain, β2-microglobulin, and peptide form a trimolecular complex that is then transported to the cell surface (44). Based on the similar patterns of immune response to E5 peptide 25-33 and E5 protein, E5 peptide 25-33 is at least a naturally processed Db-restricted CTL peptide that can bind to MHC-I molecule present on the surface of antigen-presenting cells. Recently, the molecular details of the peptide-MHC-I interaction have been established by the solution of the three-dimensional structure of the peptide-binding groove occupied either by heterogeneous mixtures of naturally processed peptides or by a single peptide epitope. Therefore, the crystal structure of E5 protein is interesting to investigate.
In the present study, we compared two different adjuvants, along with E5 25-33, for use in vaccination. Both the primary and the secondary effector cell activities induced by peptide with CpG ODN were higher than that induced by peptide with Freund's adjuvant, suggesting that CpG ODN as an adjuvant is superior to CFA in inducing peptide-specific effector and memory cells (Fig. 6B and C). In addition, although E5 25-33 plus either Freund's adjuvant or CpG ODN 1826 can protect against E5 containing tumor growth, the adjuvant CpG ODN 1826 was more efficient in helping to eradicate tumor growth than was the Freund's adjuvant. In summary, CpG ODN 1826 as an adjuvant is more efficient than Freund's adjuvant in terms of CTL activity and tumor protection. We cannot rule out the possibility that the difference in the route of vaccination may account for the differences in the intensity of the response. However, similar reports have demonstrated that the adjuvant activity of CpG ODN exceeds that achieved by Freund's adjuvant (1-2, 35-36, 41-42, 47, 50, 57). For example, immunization peptide H11.1 of T-cell lymphoma with CpG ODN induced a 2.5-fold-higher frequency of peptide-specific memory cells than it did with Freund's adjuvant (53). The strong immunogenic activity of the CpG ODN-peptide combination is due to the fact that CpG ODN stimulates macrophages and DC to synthesize various cytokines, including IL-12, IL-18, tumor necrosis factor alpha, IFN-α, IFN-β, and IFN-γ, and upregulates costimulatory molecules such as CD40 and MHC-II (14, 16, 24, 31, 34, 51, 61). The range and level of cytokine production vary according to each ODN sequence and its particular modifications. Therefore, the production of IL-12, IL-18, and other soluble mediators from activated DC induced by CpG ODN causes a more physiologic cognate interaction between the DC and T cells in lymphoid tissues, resulting in both a qualitatively and quantitatively different type of CD4+- and CD8+-T-cell response (14, 19, 53, 65, 67). Because Freund's adjuvant utilizes mycobacterial extracts as a major constituent in the adjuvant formula and because components involving paraffin oil and Mycobacterium cell wall product cause a severe inflammatory response at the injection site, the Freund's adjuvant is not suited for human vaccination. CpG ODN has minimal toxicity and is a powerful adjuvant (47). Thus, CpG ODN might be a promising adjuvant for the future human subunit vaccination approach.
We show here that E5 25-33 is a mouse Db-specific peptide of HPV-16 E5. Like the HPV-16 E7 peptide 49-57, identified as a Db CTL epitope (17), this E5 25-33 peptide can induce CTL activity that efficiently protects against E5-containing tumor growth in a mouse tumor model. Based on the E7 49-57 peptide, numerous strategies of E7 vaccination were established in C57BL/6 mice. These studies paved the way for the later development of candidate peptides of human vaccine. The E7 peptides 11-20, 82-90, and 86-93 have been determined to be human HLA-A2-specific CTL epitopes and are currently being investigated for their efficacy in human vaccinations. Although we have only identified the peptide of HPV-16 E5, which is immunogenic for mouse CTLs in the present study, this established animal model can provide us with the tool to comprehensively investigate the mechanisms of immunoregulation of peptide stimulation, to develop different E5 vaccination strategies, and to evaluate the efficacy of immunotherapy by a single or combined peptide(s) of E5 and/or E7 immunization at the various stages of cancer for the future human vaccination. It is indeed our goal to identify candidate E5 peptide(s) for human vaccine. Experiments are with HLA-A2 transgenic mice are under way to identify human HLA-A2-specific peptide(s) of HPV-16 E5.
Acknowledgments
We are grateful to T. C. Wu providing TC-1 cells, John Kung for providing β2m−/− and MHC-II−/− mice, and Kernick James Deen and Michelle Monis for editing the manuscript.
This study was supported by National Science Council grants NSC 91-2320-B-016-019 and NSC 91-3112-B-016-003-M51 and by National Health Research Institute grant NHRI-EX91-9013BL.
REFERENCES
- 1.Ballas, Z. K., A. M. Krieg, T. Warren, W. Rasmussen, H. L. Davis, M. Waldschmidt, and G. J. Weiner. 2001. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J. Immunol. 167:4878-4886. [DOI] [PubMed] [Google Scholar]
- 2.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 3.Biswas, C., B. Kell, C. Mant, R. J. Jewers, J. Cason, P. Muir, K. S. Raju, and J. M. Best. 1997. Detection of human papillomavirus type 16 early gene transcription by reverse transcription-PCR is associated with abnormal cervical cytology. J. Clin. Microbiol. 35:1560-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobb, V., D. J. McCance, and R. Schlegel. 1988. DNA sequence of the HPV-16 E5 ORF and the structural conservation of its encoded protein. Virology 163:243-246. [DOI] [PubMed] [Google Scholar]
- 5.Borysiewicz, L. K., A. Fiander, M. Nimako, S. Man, G. W. Wilkinson, D. Westmoreland, A. S. Evans, M. Adams, S. N. Stacey, M. E. Boursnell, E. Rutherford, J. K. Hickling, and S. C. Inglis. 1996. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347:1523-1527. [DOI] [PubMed] [Google Scholar]
- 6.Boursnell, M. E., E. Rutherford, J. K. Hickling, E. A. Rollinson, A. J. Munro, N. Rolley, C. S. McLean, L. K. Borysiewicz, K. Vousden, and S. C. Inglis. 1996. Construction and characterization of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine 14:1485-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, J. L., Y. P. Tsao, D. W. Liu, S. J. Huang, W. H. Lee, and S. L. Chen. 2001. The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J. Biomed. Sci. 8:206-213. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L. P., E. K. Thomas, S. L. Hu, I. Hellstrom, and K. E. Hellstrom. 1991. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc. Natl. Acad. Sci. USA 88:110-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., M. T. Mizuno, M. C. Singhal, S. L. Hu, D. A. Galloway, I. Hellstrom, and K. E. Hellstrom. 1992. Induction of cytotoxic T lymphocytes specific for a syngeneic tumor expressing the E6 oncoprotein of human papillomavirus type 16. J. Immunol. 148:2617-2621. [PubMed] [Google Scholar]
- 10.Chen, S. L., C. H. Huang, T. C. Tsai, K. Y. Lu, and Y. P. Tsao. 1996. The regulation mechanism of c-jun and junB by human papillomavirus type 16 E5 oncoprotein. Arch. Virol. 141:791-800. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. L., Y. K. Lin, L. Y. Li, Y. P. Tsao, H. Y. Lo, W. B. Wang, and T. C. Tsai. 1996. E5 proteins of human papillomavirus types 11 and 16 transactivate the c-fos promoter through the NF1 binding element. J. Virol. 70:8558-8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad, M., V. J. Bubb, and R. Schlegel. 1993. The human papillomavirus type 6 and 16 E5 are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 67:6170-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crusius, K., E. Auvinen, B. Steuer, H. Gaissert, and A. Alonso. 1998. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp. Cell Res. 241:76-83. [DOI] [PubMed] [Google Scholar]
- 14.Davila, E., and E. Celis. 2000. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J. Immunol. 165:539-547. [DOI] [PubMed] [Google Scholar]
- 15.De Bruijn, M. L., D. H. Schuurhuis, M. P. Vierboom, H. Vermeulen, K. A. de Cock, M. E. Ooms, M. E. Ressing, M. Toebes, K. L. Franken, J. W. Drijfhout, T. H. Ottenhoff, R. Offringa, and C. J. Melief. 1998. Immunization with human papillomavirus type 16 (HPV16) oncoprotein-loaded dendritic cells as well as protein in adjuvant induces MHC class I-restricted protection to HPV16-induced tumor cells. Cancer Res. 58:724-731. [PubMed] [Google Scholar]
- 16.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 17.Feltkamp, M. C. W., H. L. Smits, M. P. M. Vierboom, R. P. Minnaaar, B. M. de Jongh, J. W. Drijfhout, J. ter Schegget, C. J. M. Melief, and W. M. Kast. 1993. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 23:2242-2249. [DOI] [PubMed] [Google Scholar]
- 18.Gao, L., B. Chain, C. Sinclair, L. Crawford, J. Zhou, J. Morris, X. Zhu, and H. Stauss. 1994. Immune response to human papillomavirus type 16 E6 gene in a live vaccinia vector. J. Gen. Virol. 75:157-164. [DOI] [PubMed] [Google Scholar]
- 19.Gierynska, M., U. Kumaraguru, S. K. Eo, S. Lee, A. Krieg, and B. T. Rouse. 2002. Induction of CD8 T-cell-specific systemic and mucosal immunity against herpes simplex virus with CpG-peptide complexes. J. Virol. 76:6568-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill, D. K., J. M. Bible, C. Biswas, B. Kell, J. M. Best, N. A. Punchard, and J. Cason. 1998. Proliferative T-cell responses to human papillomavirus type 16 E5 are decreased amongst women with high grade neoplasia. J. Gen. Virol. 79:1971-1976. [DOI] [PubMed] [Google Scholar]
- 21.Greenstone, H. L., J. D. Nieland, K. E. de Visser, M. L. De Bruijn, R. Kirnbauer, R. B. Roden, D. R. Lowy, W. M. Kast, and J. T. Schiller. 1998. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA 95:1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 23.Halbert, C. L., and D. A. Galloway. 1988. Identification of the E5 open reading frame of human papillomavirus type 16. J. Virol. 62:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann, G., and A. M. Krieg. 2000. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164:944-952. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 26.Howley, P. M. 1991. Role of the human papillomaviruses in human cancer. Cancer Res. 51:5019s-5022s. [PubMed] [Google Scholar]
- 27.Hwang, E. S., T. Nottoli, and D. DiMaio. 1995. The HPV-16E5 protein: expression, detection and stable complex formation with transmenbrane proteins in COS cells. Virology 211:227-233. [DOI] [PubMed] [Google Scholar]
- 28.Kast, W. M., R. M. P. Brangt, J. Sidney, J. W. Drijfhout, R. T. Kubo, H. M. Grey, C. J. M. Melief, and A. Sette. 1994. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J. Immunol. 152:3904-3912. [PubMed] [Google Scholar]
- 29.Kell, B., R. J. Jewers, J. Cason, F. Parkarian, J. N. Kaye, and J. M. Best. 1994. Detection of E5 oncoprotein in human papillomavirus type 16-positive cervical scrapes using antibodies raised to synthetic peptides. J. Gen. Virol. 75:2451-2456. [DOI] [PubMed] [Google Scholar]
- 30.Koller, B. H., O. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248:1227-1230. [DOI] [PubMed] [Google Scholar]
- 31.Krieg, A. M., L. Love-Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 32.Lin, C. W., J. Y. Lee, Y. P. Tsao, C. P. Shen, H. C. Lai, and S. L. Chen. 2002. Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can regress tumor growth in mice. Int. J. Cancer 102:629-637. [DOI] [PubMed] [Google Scholar]
- 33.Lin, K. Y., F. G. Guarnieri, O. C. K. F. Staveley, H. I. Levitsky, J. T. August, D. M. Pardoll, and T. C. Wu. 1996. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 56:21-26. [PubMed] [Google Scholar]
- 34.Lipford, G. B., T. Sparwasser, S. Zimmermann, K. Heeg, and H. Wagner. 2000. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven response. J. Immunol. 165:1228-1235. [DOI] [PubMed] [Google Scholar]
- 35.Lipford, G. B., M. Bauer, C. Black, R. Reiter, H. Wagner, and K. Heeg. 1997. CpG-containing synthetic oligonucleotides promote B and cytotoxic T-cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J. Immunol. 27:2340-2344. [DOI] [PubMed] [Google Scholar]
- 36.Lipford, G. B., T. Sparwasser, M. Bauer, S. Zimmermann, E. S. Koch, K. Heeg, and H. Wagner. 1997. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur. J. Immunol. 27:3420-3426. [DOI] [PubMed] [Google Scholar]
- 37.Liu, D. W., Y. P. Tsao, C. H. Hsieh, J. T. Hsieh, J. T. Kung, C. L. Chiang, S. J. Huang, and S. L. Chen. 2000. Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J. Virol. 74:9083-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, X. S., I. Abdul-Jabbar, Y. M. Qi, L. H. Frazer, and J. Zhou. 1998. Mucosal immunization with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology 252:39-45. [DOI] [PubMed] [Google Scholar]
- 39.Londono, L. P., S. Chatfield, R. W. Tindle, K. Herd, X. M. Gao, I. Frazer, and G. Dougan. 1996. Immunization of mice using Salmonella typhimurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine 14:545-552. [DOI] [PubMed] [Google Scholar]
- 40.Meneguzzi, G., C. Cerni, M. P. Kieny, and R. Lathe. 1991. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology 181:62-69. [DOI] [PubMed] [Google Scholar]
- 41.Messina, J. P., G. S. Gilkeson, and D. S. Pisetsky. 1993. The influence of DNA structure on the in vitro stimulation of murine lymphocytes by natural and synthetic polynucleotide antigens. Cell. Immunol. 147:148-157. [DOI] [PubMed] [Google Scholar]
- 42.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 43.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 44.Pamer, E., and P. Cresswell. 1998. Mechanisms of MHC class 1-restricted antigen processing. Annu. Rev. Immunol. 16:323-358. [DOI] [PubMed] [Google Scholar]
- 45.Ressing, M. E., A. Sette, R. M. P. Brandt, J. Ruppert, P. A. Wentworth, M. Hartman, C. Oseroff, H. M. Grey, C. J. M. Melief, and W. M. Kast. 1995. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J. Immunol. 154:5934-5943. [PubMed] [Google Scholar]
- 46.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 48.Sadovnikova, E., X. J. Zhu, S. M. Collins, J. Zhou, K. Bousden, L. Crawford, P. Beverley, and H. J. Stauss. 1994. Limitations of predictive motifs revealed by cytotoxic lymphocyte-T epitope mapping of the human papilloma virus E7 protein. Int. Immunol. 6:289-296. [DOI] [PubMed] [Google Scholar]
- 49.Schafer, K., M. Muller, S. Faath, A. Henn, W. Osen, H. Zentgraf, A. Benner, L. Gissmann, and I. Jochmus. 1999. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles: induction of cytotoxic T cells and specific tumor protection. Int. J. Cancer 81:881-888. [DOI] [PubMed] [Google Scholar]
- 50.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 51.Sparwasser, T., R. M. Vabulas, B. Villmow, G. B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T-cell responses to soluble proteins. Eur. J. Immunol. 30:3591-3597. [DOI] [PubMed] [Google Scholar]
- 52.Stauss, H. J., H. Davies, E. Sadovnikova, B. Chain, N. Horowitz, and C. Sinclair. 1992. Induction of cytotoxic T lymphocytes with peptides in vitro: identification of candidate T-cell epitopes in human papilloma virus. Proc. Natl. Acad. Sci. USA 89:7871-7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern, B. V., B. O. Boehm, and M. Tary-Lehmann. 2002. Vaccination with tumor peptide in CpG adjuvant protects via IFN-γ-dependent CD4 cell immunity. J. Immunol. 168:6099-6105. [DOI] [PubMed] [Google Scholar]
- 54.Straight, S. W., B. Herman, and D. J. McCance. 1995. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 69:3185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straight, S. W., P. M. Hinkle, R. J. Jewers, and D. J. McCance. 1993. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the down-regulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 67:4521-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen, P., B. van Deurs, B. Norrild, and L. Kayser. 2000. The HPV16 E5 oncogene inhibits endocytic trafficking. Oncogene 19:6023-6032. [DOI] [PubMed] [Google Scholar]
- 57.Tighe, H., K. Takabayashi, D. Schwartz, R. Marsden, L. Beck, J. Corbeil, D. D. Richman, J. J. Eiden, Jr., H. L. Spiegelberg, and E. Raz. 2000. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur. J. Immunol. 30:1939-1947. [DOI] [PubMed] [Google Scholar]
- 58.Tindle, R. W., G. J. P. Fernando, J. C. Sterling, and I. H. Frazer. 1991. A“public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides papillomavirus genotypes. Proc. Natl. Acad. Sci. USA 88:5887-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai, T. C., and S. L. Chen. 2003. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch. Virol. 148:1445-1453. [DOI] [PubMed]
- 60.van der Burg, S. H., M. E. Ressing, K. M. C. Kwappenberg, A. de Jong, K. Straathof, J. de Jong, A. Geluk, K. E. van Meijgaarden, K. L. M. C. Franken, T. H. M. Ottenhoff, G. J. Fleuren, G. Kenter, C. J. M. Melief, and R. Offringa. 2001. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int. J. Cancer 91:612-618. [DOI] [PubMed] [Google Scholar]
- 61.Vicari, A. P., C. Chiodoni, C. Vaure, S. Aït-Yahia, C. Dercamp, F. Matsos, O. Reynard, C. Taverne, P. e Merle, M. P. Colombo, A. O'Garra, G. Trinchieri, and C. Caux. 2002. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196:541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, T. C., F. G. Guarnieri, O. C. K. F. Staveley, R. P. Viscidi, H. I. Levitsky, L. Hedrick, K. R. Cho, J. T. August, and D. M. Pardoll. 1995. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc. Natl. Acad. Sci. USA 92:11671-11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu, X., X. Zhao, W. F. Burkholder, A. Gragerov, C. M. Ogata, M. E. Gottesman, and W. A. Hendrickson. 1996. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272:1606-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zijlstra, M., E. Li, F. Sajjadi, S. Subramani, and R. Jaenisch. 1989. Germ-line transmission of a disrupted β2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342:435-438. [DOI] [PubMed] [Google Scholar]
- 65.Zimmerrmann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Röcken, H. Wagner, and K. Heeg. 1998. Cutting edge: CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]
- 66.zur Hausen, H., and E. M. de Villiers. 1994. Human papillomaviruses. Annu. Rev. Microbiol. 48:427-447. [DOI] [PubMed] [Google Scholar]
- 67.Zwaveling, S., S. C. F. Mota, J. Nouta, M. Johnson, G. B. Lipford, R. Offringa, S. H. van der Burg, and C. J. M. Melief. 2002. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J. Immunol. 169:350-358. [DOI] [PubMed] [Google Scholar]