Abstract
Background
Marrow damage from chemo- and radiation therapies has been suggested to affect quality and quantity of Hematopoietic stem cell (HSC) products. We tested the hypothesis that CD34+ cells (HSC) from low mobilizers are qualitatively inferior to HSC from high mobilizers.
Materials and Methods
HSC quality was defined by proportion of primitive HSC subsets (CD34+CD38−, CD34+HLA-DR− and CD34+ in G0 stage of cell cycle), the proportion of HSCs that express CXCR4 and CD26 homing proteins and days, to neutrophil and platelet engraftments post transplant. HSC content and CD34 subsets analyses were performed using flow cytometry following ISHAGE protocol. We evaluated the HSC quantity and quality of 139 autologous filgrastim mobilized HSC products. Patients were categorized into low, moderate and high mobilizers if their total HSC collection was <3 ×106/kg, ≥3 ×106/kg and <5 ×106/kg, and ≥5 × 106/Kg respectively.
Results
The median number of primitive CD34 subsets increases with increasing HSC numbers and this association was statistically significant (p = 0.001). However, when the ratios of the primitive CD34 subsets to total HSC counts were compared among the mobilization groups, the ratios were not significantly different. Co-expression of neither CD26 nor CXCR4 with CD34 antigen correlated with HSC mobilization. Evaluation of days to neutrophil engraftment among the mobilization groups did not show a statistically significant difference (p = 0.1). However, days to platelet engraftment among the mobilization groups was statistically significantly different (p = 0.05).
Conclusion
The quality of HSCs from low mobilizers was comparable to HSCs from high mobilizers.
Keywords: Hematopoietic stem cells, mobilization, Poor Mobilizers, CD34+ Cells
Introduction
For patients with hematological malignancies, such as multiple myeloma (MM) and high-risk or relapsed non-Hodgkin’s lymphoma (NHL), autologous hematopoietic stem cell (HSC) transplant after high-dose chemotherapy has been widely accepted as a standard therapy due, to its ease of collection and the faster tempo of engraftment.1
HSCs exist at a very low amount (0.04%) in steady-state peripheral blood (PB).2,3 Most HSCs remain tethered to the bone marrow through adhesion molecule/receptor interactions with bone marrow stromal cells, osteoblasts, and osteoclasts, forming the stem niche.4 After chemotherapy administration, HSCs in PB increase as the patient's white blood cell count starts to recover.5 Current mobilization regimens increase HSCs in the PB above steady state levels by disrupting the normal bone marrow microenvironment through several mechanisms. Filgrastim (G-CSF) is thought to catalyze HSC mobilization by down-regulating mRNA and protein levels of the chemokine stromal cell-derived factor-1 (SDF-1). It is also believed to promote accumulation of matrix metalloprotienase-9 (MMP-9), neutrophil elastase, and cathepsin G, which can cleave kit ligand and SDF-1 in addition to VCAM-16–10.
Common strategies for PB stem cell mobilization include the use of growth factors alone or combined with conventional chemotherapy, which may cause a 100–160 folds increase of HSC in the peripheral blood.11 However, numerous factors, including age, bone marrow involvement with disease, previous exposure to chemotherapeutic agents such as lenalidomide and alkylating agents, and/or radiation therapies, have been identified as risk factors for poor mobilization.12–16 Moreover, many factors including the low quantity of HSCs, high dose chemotherapy, and prior radiation will have adverse effects on the engraftment of neutrophils and platelets.17,18
There is no general consensus about adequate number of CD34+ cell dose needed for successful engraftment following a transplant. In general, 5 million CD34+ cell /Kg of recipient body weight is considered an adequate cell dose and 2 million CD34+ cell /Kg is considered as the minimum acceptable cell dose for an autologous HSC transplant.19 This cell dose is usually obtained within a simple mobilization attempt (by means of 1–4 apheresis collection procedures) 20; otherwise, such patients will undergo other strategies including large-volume leukapheresis 21,22, different mobilizing regimens, or allogeneic HSC transplant.
For all bone marrow transplants (BMT), it is important to collect HSCs that will allow durable engraftment and rapid hematopoietic recovery. These goals are affected not only by the quantity of HSCs but also by the cell types present in the graft and by the intrinsic qualities of these cells.23 The quality of collected HPC is defined by proportion of primitive HSC subsets (CD34+CD38−, CD34+HLA-DR−, and CD34+ in G0 stage of cell cycle) 24, the proportion of HSCs that express CXCR4 and CD26 homing proteins 8,25, and days to neutrophil and platelet engraftments post transplant.26 However, the correlation of the quantity and the quality of the HSCs in the autologous BMT setting is unclear. In this study, we tested the hypothesis that HSCs collected from low mobilizers are qualitatively inferior to HSCs from high mobilizers.
Materials and Methods
Patient and graft characteristics
We evaluated the HSC quantity and quality of 139 autologous filgrastim (G-CSF) mobilized HSC products. Patients were prospectively followed from the time of initial evaluation to transplant. Patients’ consents were obtained with Mayo Clinic Institutional Review Board approval (IRB No.242-02) before the study was conducted. The median patient age was 58 years (range 18 – 76 years), and male to female ratio was 3 to 2. Patients were diagnosed with plasma cells dyscrasia, mainly multiple myeloma, or non-Hodgkin's lymphoma (Table 1).
Table 1.
Patient characteristics
| Mobilization Groups | |||
|---|---|---|---|
| Variables | Low | Moderate | High |
| Number of cases | 33 | 70 | 36 |
| Age years median (range) | 56 (19 – 73) | 59 (27 – 76) | 56 (23 – 74) |
| Sex | |||
| Female | 15 | 28 | 11 |
| Male | 18 | 42 | 25 |
| Type of diseases | |||
| MM | 13 | 51 | 26 |
| NHL | 20 | 19 | 10 |
Peripheral blood stem cell collection procedure
Patients were first mobilized with 10 µg/kg filgrastim for approximately 4 to 5 days. On the day their peripheral HSC count reached 10 per µL or higher, collection was initiated.
Leukapheresis procedures were performed with an apheresis system (COBE spectra, Gambro BCT, Lakewood, CO). The machine was run with operating software version 5.1. The venous access for all autologous donors was via a trilumen central venous catheter. All donors were subjected to 4-blood-volume collection, which took approximately 4 to 5 hours to complete. The same operator and machine were used for all collections for each donor. Each donor, however, was randomly assigned to an operator and a machine. All operators were competent and passed their annual competency evaluations. All apheresis machines were validated and received routine biannual preventive maintenance.
HSCs and subsets count
Samples for HSC counts were collected on each day of collection. However, CD34 subset analysis for all patients was consistently performed on samples from first day of collection. The HSC counts and HSC subsets (CD38−, HLA-DR−, CD26+, and CXCR4+) were performed following the ISHAGE protocol.27 Total HSC yield is defined as cumulative total number of CD34+ cells from all collection. The HSC product was assayed for the total number of mononuclear cells (MNCs) and CD34+ cells. MNCs were stained with anti-CD45-FITC (Pharmingen, San Diego, CA) and anti-CD34-PE (Becton-Dickinson Immunocytometry Systems, San Jose, CA) and analyzed with a flow cytometer (FACSCalibur, BD Biosciences, Waltham, MA). The number of CD34+ cells was determined before cryopreservation. The data analysis was performed with computer software (Cellquest, BD Biosciences) after acquisition layouts (Procount, BD Biosciences). The percentage of HSCs in each sample was determined and the total number of CD34+ cell infused was calculated.
For calculating the subsets of CD34+ cells, cells are stained with anti-45, anti-CD34, anti-CD38, anti-HLA-DR, anti-CD26, and anti-CXCR4 (Pharmingen, San Diego, CA) and were analyzed. All antibodies were conjugated with either FITC or PE or PerCP. HSC subset doses were determined by calculating the percentage of total HSCs that expressed or did not express CD38, HLA-DR, CD26, or CXCR4 and multiplying the percentages with total HSC dose/kg that had been previously determined.
Cell cycle analysis
Cell-cycle analysis involved simultaneous staining of DNA and RNA, which allowed us to distinguish HSCs that were in G0, G1, and S/M states of the cell cycle. Simultaneous staining of DNA and RNA with Hoechst 33342 (Hst) and Pyronin Y allowed further fractionation of the G0/G1 phase. Cell suspensions of 1–2 million cells/mL were made and Hst dye (Molecular Probes, Eugene, OR, USA), to a final concentration of 10 µg/mL, was added and incubated at 37°C for 45 minutes for staining extracellular markers of HSC, anti-CD34-FITC was added and incubated in the dark for 30 minutes after staining, cells were suspended in 2 ml 5% paraformaldehyde and kept overnight in a refrigerator. The next day, Pyronin Y (Sigma, St Louis, MO, USA) was added at a 1:100 dilution and incubated for 30 minutes at 4°C. Finally cells were washed once and resuspended in 500 µL 5% paraformaldehyde. After staining, cells were analyzed using a FACS-Vantage (Becton Dickinson) that was equipped with an argon laser, providing the 488-nm excitation needed for Pyronin Y, and a krypton laser, providing the 350-nm excitation needed for Hst. The Pyronin Y signal was selected with a 575 ± 13-nm band-pass filter and Hst was detected with a 424 ± 22-nm bandpass filter.
Neutrophil and platelet count
Neutrophil engraftment was defined as the first of three consecutive days on which the neutrophil count reached 500/µL. Platelet engraftment was defined as the first of three consecutive days on which the platelet count reached 20,000/µL without platelet transfusion. The neutrophil and platelet counts were part of complete blood count (CBC). CBC was performed with a cell and particle counter (Coulter Counter, Beckman Coulter, Fullerton, CA).
Statistical analysis
The Kruskal-Wallis test was used for testing differences among the three mobilization groups. When only two categorical groups were compared, the Wilcoxon rank-sum test was used. Linear correlation between two continuous factors was assessed by Spearman’s rank correlation. No statistical adjustment was made for performing multiple tests. All probability values are 2-sided and a p-value less than or equal to 0.05 was considered statistically significant.
Results
The patients were categorized into low (n=33), moderate (n=70) and high (n=36) mobilizers if their total CD34+ cell yield in one collection series was <3 × 106/kg, ≥3 × 106/kg and <5 106/kg, and ≥5 × 106/kg respectively. The median count of HSCs was 3 × 106/kg for the low mobilizers; 4.6 × 106/kg for the moderate mobilizers; and 6.6 × 106/kg in high mobilizers (Table 2). The overall median count of HSCs in all 139 patients was 4.9 × 106/kg.
Table 2.
CD34+ Subsets and Mobilization
| Mobilization (median × 106/kg) | ||||
|---|---|---|---|---|
| CD34+ Subsets | Low | Moderate | High | P value |
| Number of cases | 33 | 70 | 36 | |
| CD34+ | 3 | 4.6 | 6.6 | <0.001 |
| CD34+CD38− | 1.2 | 2.6 | 3.8 | <0.001 |
| CD34+HLA-DR− | 1.1 | 1.6 | 2 | 0.001 |
| CD34+CD26+ | 0 | 0 | 0 | 0.88 |
| CD34+CXCR4+ | 0 | 0 | 0 | 0.55 |
| CD34+G0 stage | 1.5 | 3.3 | 4.6 | <0.001 |
Early CD34+ subsets included CD34+CD38− and CD34+HLA-DR− cells. The median number of CD34+CD38− cells (× 106/kg) was 1.2 in low; 2.6 in moderate; and 3.8 in high mobilizers. The proportion of CD34+CD38− cells in the HSC products (CD34+CD38−/HSC count ratio) was 52% in low, 55% in moderate, and 53% in high mobilizers with an overall median of 54% in all patients (range 0.78 – 99.6%). The median number of CD34+HLA-DR− cells (× 106/kg) was 1.1 in low, 1.6 in moderate, and 2 in high mobilizers; and the ratio of CD34+HLA-DR/HSCs was 30% in low, 35% in moderate, and 27% in high mobilizers with an overall median 31.5% (range 0 – 97%). The median number of quiescent CD34+ subset of G0 stage (× 106/kg) was 1.5 in low and 3.3 in moderate and 4.6 in high mobilizers. The median percent of HSCs in G0 stage cells 72.1% (range 0 – 100%), and was 71% in low and 75% in moderate and 64% in high mobilizers. Only a small amount of HSCs co-expressed CD26 (median 0% range 0 – 31.6%) and CXCR4 antigens (median 0%, range 0 – 16.7%). All the data were summarized in tables 2 and 3.
Table 3.
CD34+ subsets/CD34 ratio and mobilization
| CD34 subsets/CD34+ ratio | |||||
|---|---|---|---|---|---|
| CD34 subsets | Low | Moderate | High | Average | P value |
| Number of cases | 33 | 70 | 36 | ||
| CD34+CD38− | 0.52 | 0.55 | 0.53 | 0.54 | 0.41 |
| CD34+HLA-DR− | 0.30 | 0.35 | 0.27 | 0.31 | 0.84 |
| CD34+G0 stage | 0.71 | 0.75 | 0.64 | 0.72 | 0.58 |
Therefore, the median number of primitive stem cells increases with increasing HSC numbers (from low to high mobilization), and this association was statistically significant (p=0.001, Kruskal Walis test) (table 2). However, when the ratios of the primitive CD34 subsets to total HSC counts were compared among the mobilization groups, the ratios were not significantly different (table 3). This suggests the proportions of primitive HSCs in products from low mobilizers were comparable to those from high mobilizers. Co-expression of neither CD26 nor CXCR4 homing proteins with CD34 antigen correlated with HSC mobilization.
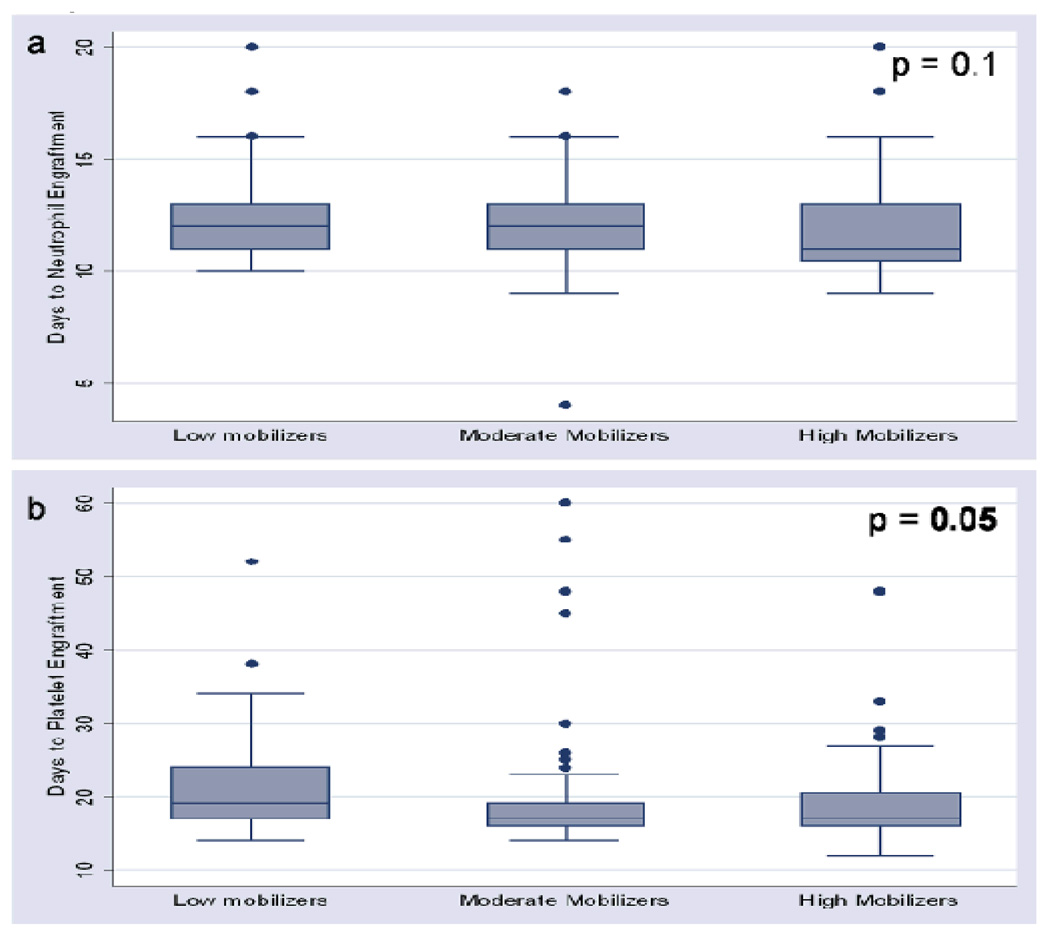
The median time to neutrophil and platelet engraftment was 12 and 18 days respectively. Evaluation of days to neutrophil engraftment among the mobilization groups did not show a statistically significant difference (p = 0.1, figure 1a). This finding agrees with observed comparable primitive HSC subsets among the mobilization groups and further supports the qualitative equivalence of HSCs from low with moderate and high mobilizers. Days to platelet engraftment among the mobilization groups was statistically significantly different (p = 0.05, figure 1b).
Figure 1.
Distribution of days to neutrophil and platelet engraftment among mobilization groups.
Discussion
Poor mobilization remains a major challenge for autologous transplantation.12–16 There are some suggestions that it is not only the quantity of the HSC that is limited in products from poor mobilizers but also the quality of the cells. We consider HSC quality to be the capacity of the HSC product to home and engraft in its natural microenvironment, following cell infusion, and the capacity of the cells to provide long term hematopoietic reconstitution.
To assess HSC homing capacity, we investigated the co-expression of CXCR4 or CD26 with CD34 antigens. These markers have been previously reported to play a significant role in stem cell mobilization and homing. However, our evaluation showed poor surface expression of these molecules on HSCs in vitro. This observation is consistent with previous reports that suggest surface expression of CXCR4 molecule is only induced in the presence of its ligand, SDF-1α, and that surface expressed CXCR4 molecules are quickly internalized and recycled.8 CD26 is a dipeptidase enzyme whose expression had been well studied in non-hematopoietic and metastatic tumor cells.25 It was shown in aminal models that lack of CD26 allows HSC to engraft more effieciently 28 and CD26 expression is essential for GCSF-induced HSC mobilization29. Prabhash et al reported significant correlation between overall expression of CD26 in HSC graft and early engraftment. However, they did not find significant CD26 co-expression by CD34+ cells25. Contrary to these reports, we did not observed significant association between overall CD26 expression or lack of with engraftment (data not shown) nor did we observe significant CD26 co-expression by CD34+ cells. Therefore, the significance of CD26 in HSC homing in humans is yet to be fully establsihed.
To assess long term hematopoietic reconstitution as a surrogate measure of HSC product quality, we evaluated the proportion of primitive HSC in each product. It was previously reported that CD34+CD38− and CD34+HLA-DR− cells are more primitive and have a longer term hematopoietic reconstituting capacity than CD34+CD38+ and CD34+HLA-DR+ cells30. The proportions of HSCs that lack CD38 and HLA-DR antigens expression observed in this study were consistent with previous reports by us24. We did not observe a significant difference in proportion of these primitive HSCs among the mobilization groups. This suggests poor mobilization has no influence on the proportions of primitive HSC.
In vitro studies and in vivo animal studies have shown that the most primitive HSC are in the G0/G1 phase of the cell cycle31–34. Similarly it was shown that quiescent, G0, HSC are superior over proliferating HSCs in rescuing lethally irradiated recipients.12 In humans, experimental studies examining the impact of cell cycle status on engraftment capacity were unattainable except in xenotransplant models, which are of limited value. Our assessment showed 72% of HSCs in all the HSC products analyzed were quiescent and mobilization capacity did not appear to significantly influence the cell cycle stage of the HSCs. Our findings agree with previous reports that suggest majority of HSC are quiescent. They proliferate upon receiving appropriate signal that involves engagement of soluble c-kit ligand to its receptor on the surface of the HSCs. This triggers binary division with one daughter cell renewing the parent cell and becoming quiescent while the second daughter cell continues to rapidly proliferate and turning into ‘rapid amplifying cells.’ As the cells proliferate they become more differentiated. To our knowledge, this is the first study that assesses the relationship of HSC cell cycle status and mobilization capacity.
The correlation of HSC dose and time to neutrophil and platelet engraftments was well studied and published by us35,36. We also previously reported that the time to neutrophil engraftment is the most sensitive predictor of long term engraftment35. Therefore, our finding that products from good and low mobilizers resulted in statistically similar time to neutrophil engraftment attests not only the equivalency of the product in terms of short term engraftment but also long term engraftment. We also observed in this study that mobilization capacity affected time to platelet engraftment but not time to neutrophil engraftment. Our previous report shows time to platelet engraftment is more responsive to HSC dose than time to neutrophil engraftment26. This finding explains the observation that time to platelet engraftment was statistically significantly shorter in high mobilizers. However, the 1 day median difference in time to platelet engraftment may not be clinically significant.
Standardized mobilization regimen, prospective analysis of fresh samples before freezing and prospectively monitoring for neutrophil and platelet engraftments strengthen our study design. CD34 subset analysis was only performed on samples from first day of collection. Therefore, the total number of all CD34 subsets for each patient was based on the proportion of the subsets on first day of collect. This may introduce some element of bias since the CD34 subsets may vary with each collection even though we are unaware of any report claiming this assumption. Even if this assumption is true, we do not believe the interpretation of our results will be affected since all products for each mobilization subgroups were processed the same way and therefore the potential confounding factor will be cancelled out. The study will be further enhanced by corroborating the flow cytometric studies with molecular studies such as quantitative polymerase chain reaction (qPCR) assays particularly on purified HSCs.
In summary, we showed that based on the quality parameters been studied, HSC products from low mobilizers are qualitatively similar to HSC product from high mobilizers.
Acknowledgments
Source of funding: National Cancer Institute, NIH Grant no. CA102824
Footnotes
Financial Disclosure: None
Reference
- 1.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 2.Fruehauf S, Haas R, Zeller WJ, Hunstein W. CD34 selection for purging in multiple myeloma and analysis of CD34+ B cell precursors. Stem Cells. 1994;12:95–102. doi: 10.1002/stem.5530120116. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DR, Keating A, Nayar R, Anania S, Stewart AK. Sensitive detection and enumeration of CD34+ cells in peripheral and cord blood by flow cytometry. Exp Hematol. 1994;22:1003–1010. [PubMed] [Google Scholar]
- 4.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 5.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 6.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 8.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 9.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 10.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 12.Fruehauf S, Tricot G. Comparison of unmobilized and mobilized graft characteristics and the implications of cell subsets on autologous and allogeneic transplantation outcomes. Biol Blood Marrow Transplant. 2010;16:1629–1648. doi: 10.1016/j.bbmt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Mendrone A, Jr, Arrais CA, Saboya R, Chamone Dde A, Dulley FL. Factors affecting hematopoietic progenitor cell mobilization: an analysis of 307 patients. Transfus Apher Sci. 2008;39:187–192. doi: 10.1016/j.transci.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Drake M, Ranaghan L, Morris TC, Nolan L, Desai ZR, Irvine AE, Jordan A, Magill K, Price S. Analysis of the effect of prior therapy on progenitor cell yield: use of a chemotherapy scoring system. Br J Haematol. 1997;98:745–749. doi: 10.1046/j.1365-2141.1997.2743091.x. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi MK, Jestice K, Scott MA, Bloxham D, Bass G, Marcus RE. The minimum CD34 threshold depends on prior chemotherapy in autologous peripheral blood stem cell recipients. Bone Marrow Transplant. 1999;23:9–13. doi: 10.1038/sj.bmt.1701530. [DOI] [PubMed] [Google Scholar]
- 16.Perseghin P, Terruzzi E, Dassi M, Baldini V, Parma M, Coluccia P, Accorsi P, Confalonieri G, Tavecchia L, Verga L, Ravagnani F, Iacone A, Pogliani EM, Pioltelli P. Management of poor peripheral blood stem cell mobilization: incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41:33–37. doi: 10.1016/j.transci.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, Gooley T, Demirer T, Schiffman K, Weaver C, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz CH, Glassman JR, Wuest D, Maslak P, Reich L, Gucciardo A, Coady-Lyons N, Zelenetz AD, Nimer SD. Factors affecting mobilization of peripheral blood progenitor cells in patients with lymphoma. Clin Cancer Res. 1998;4:311–316. [PubMed] [Google Scholar]
- 19.Gianni AM. Where do we stand with respect to the use of peripheral blood progenitor cells? Ann Oncol. 1994;5:781–784. doi: 10.1093/oxfordjournals.annonc.a059003. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Leisenring W, Bensinger WI, Holmberg LA, Rowley SD. The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 1999;39:442–450. doi: 10.1046/j.1537-2995.1999.39050442.x. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamsen JF, Stamnesfet S, Liseth K, Hervig T, Bruserud O. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion. 2005;45:248–253. doi: 10.1111/j.1537-2995.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- 22.Zubair AC, Rymer R, Young J, Keeton U, Befort R, Nolot B, Evans C, Bleach T, Torloni A. Multiple myeloma patients receiving large volume leukapheresis efficiently yield enough CD34+ cells to allow double transplants. J Clin Apher. 2009;24:6–11. doi: 10.1002/jca.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruehauf S, Ehninger G, Hubel K, Topaly J, Goldschmidt H, Ho AD, Muller S, Moos M, Badel K, Calandra G. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin's lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2010;45:269–275. doi: 10.1038/bmt.2009.142. [DOI] [PubMed] [Google Scholar]
- 24.Zubair AC, Kao G, Daley H, Schott D, Freedman A, Ritz J. CD34(+) CD38(−) and CD34(+) HLA-DR(−) cells in BM stem cell grafts correlate with short-term engraftment but have no influence on long-term hematopoietic reconstitution after autologous transplantation. Cytotherapy. 2006;8:399–407. doi: 10.1080/14653240600847241. [DOI] [PubMed] [Google Scholar]
- 25.Prabhash K, Khattry N, Bakshi A, Karandikar R, Joshi A, Kannan S, Sastry PS, Parikh P, Kode JA. CD26 expression in donor stem cell harvest and its correlation with engraftment in human haematopoietic stem cell transplantation: potential predictor of early engraftment. Ann Oncol. 2010;21:582–588. doi: 10.1093/annonc/mdp342. [DOI] [PubMed] [Google Scholar]
- 26.Zubair AC, Grant R, Wu W, Tun H, Rivera C, Moreno-Aspitia A, Joyce M, Roy V, Colon-Otero G, Solberg LA. Platelet count is a sensitive predictor of autologous peripheral blood progenitor cell collection yield in previously treated plasma cell disease patients. Transfusion. 2008;48:1106–1114. doi: 10.1111/j.1537-2995.2008.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 28.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 29.Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26−/− mice. Exp Hematol. 2003;31:1126–1134. doi: 10.1016/j.exphem.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Prosper F, Vanoverbeke K, Stroncek D, Verfaillie CM. Primitive long-term culture initiating cells (LTC-ICs) in granulocyte colony-stimulating factor mobilized peripheral blood progenitor cells have similar potential for ex vivo expansion as primitive LTC-ICs in steady state bone marrow. Blood. 1997;89:3991–3997. [PubMed] [Google Scholar]
- 31.Paz H, Wong CA, Li W, Santat L, Wong KK, Chatterjee S. Quiescent subpopulations of human CD34-positive hematopoietic stem cells are preferred targets for stable recombinant adeno-associated virus type 2 transduction. Hum Gene Ther. 2007;18:614–626. doi: 10.1089/hum.2006.188. [DOI] [PubMed] [Google Scholar]
- 32.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 33.Danova M, Porta C, Ferrari S, Brugnatelli S, Comolli G, Riccardi A, Ascari E. Cell cycle status and apoptosis of hematopoietic progenitor cells released into the peripheral blood after taxanes and granulocyte colony-stimulating factor in breast cancer patients. Oncology Reports. 2000;7:585–589. doi: 10.3892/or.7.3.585. [DOI] [PubMed] [Google Scholar]
- 34.Jetmore A, Plett PA, Tong X, Wolber FM, Breese R, Abonour R, Orschell-Traycoff CM, Srour EF. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99:1585–1593. doi: 10.1182/blood.v99.5.1585. [DOI] [PubMed] [Google Scholar]
- 35.Zubair A, Zahrieh D, Daley H, Schott D, Gribben JG, Freedman A, Ritz J. Early neutrophil engraftment following autologous BMT provides a functional predictor of long-term hematopoietic reconstitution. Transfusion. 2003;43:614–621. doi: 10.1046/j.1537-2995.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 36.Zubair AC, Zahrieh D, Daley H, Schott D, Gribben JG, Alyea EP, Schlossman R, Freedman A, Antin JH, Soiffer RJ, Neuberg D, Ritz J. Engraftment of autologous and allogeneic marrow HPCs after myeloablative therapy. Transfusion. 2004;44:253–261. doi: 10.1111/j.1537-2995.2004.00666.x. [DOI] [PubMed] [Google Scholar]