Abstract
Despite considerable efforts, malaria is still one of the most devastating infectious diseases in the tropics. The rapid spread of antimalarial drug resistance currently compounds this grim picture. In this paper, we review the history of antimalarial drug resistance and the methods for monitoring it and assess the current magnitude and burden of parasite resistance to two commonly used drugs: chloroquine and sulfadoxine-pyrimethamine. Furthermore, we review the factors involved in the emergence and spread of drug resistance and highlight its public health importance. Finally, we discuss ways of dealing with such a problem by using combination therapy and suggest some of the research themes needing urgent answers.
INTRODUCTION
The true magnitude of the mortality and morbidity attributable to malaria worldwide is at best a scientific guess, although it is not disputable that the greatest burden is in sub-Saharan Africa. Those at highest risk are children younger than 5 years and pregnant women, particularly primigravidae.
In the late 1950s and early 1960s, the eradication of malaria seemed possible because the parasite does not have an animal reservoir and effective agents to interrupt transmission or to obtain a radical cure existed. On the basis of such observations, the World Health Organization (WHO) spearheaded projects for malaria eradication by using indoor residual spraying and large mass drug administration programs using chloroquine (CQ) and pyrimethamine (M. Pinotti, Abstr. Fifth Congr. Trop. Med. Malaria, p. 244, 1953). However, many of the national programs lacked adequate epidemiological skills and knowledge and administrative organization. These deficiencies were overlooked because of the humanitarian appeal of the program, the sense of urgency, and the feeling that peer pressure could eventually shake the chronic apathy of the health services (142). As time progressed, evidence started to accumulate indicating that although it was possible to reduce or even interrupt malaria transmission by insecticide spraying in large areas, it was very difficult to establish effective surveillance in the absence of a solid health infrastructure. Some of the factors responsible for the lower-than-expected impact of the eradication programme include the following (47): (i) DDT resistance in vector mosquitoes, (ii) objections by local inhabitants to the entry of spray men into their households (130), (iii) selection of exophilic mosquitoes which do not rest long enough indoors to pick up a lethal dose, and (iv) vertical organization of vector control programs, which required an efficient and stable organizational infrastructure. Furthermore, it was realised that in the great majority of countries, eradication was not a realistic goal (234) and that there was a need to change from highly prescriptive, centralized control programs to flexible, cost-effective, and sustainable programs adapted to local conditions and responding to local needs (233). Moreover, the emergence and spread of resistance to pyrimethamine and later CQ further compromised the mass drug administration programs and the eradication strategy. A global Malaria Control Strategy aimed at preventing mortality and reducing morbidity was adopted by the Ministerial Conference held in Amsterdam in 1992 (234). One of its components is the provision of early diagnosis and prompt treatment, the latter based on affordable antimalarial drugs such as CQ and sulfadoxine-pyrimethamine (SP). However, resistance of Plasmodium falciparum to CQ and SP and, more recently, resistance of P. vivax to CQ has compromised this strategy and has increased the need for new, affordable antimalarial drugs or combination of drugs.
Resistance to all known antimalarial drugs, with the exception of the artemisinin derivatives, has developed to various degrees in several countries (24). The geographic distribution of P. falciparum resistance to CQ corresponds almost exactly to that of the parasite, and the prevalence of resistance is high in many countries (223). The only current exceptions are Central America northwest of Panama, Haiti and the Dominican Republic, and the Middle East, where the magnitude of P. falciparum resistance is still the subject of investigation. Increasing drug resistance has prompted several countries to change from CQ to other regimens as first line treatment, usually to SP, which is affordable, relatively safe, and easy to administer (232). Parasite resistance to SP has developed very quickly in Southeast Asia (202). In Africa, SP resistance was low until 5 to 6 years ago (148). However, recently it has reached critical levels in some areas of East and Central Africa, prompting some experts to be concerned that a public health disaster may be imminent (18, 25, 104, 139, 200). In this paper, we review the history of, the methods for monitoring, and the dynamics for the emergence and spread of drug resistance. Furthermore, we describe the magnitude and current distribution of CQ and SP resistance and highlight its public health importance.
METHODOLOGY
We searched the published literature in the National Library of Medicine via the PubMed and MEDLINE search engines for research articles, reviews, books, and other reports. We identified the published reports by using key word searches such as malaria and drug resistance, in vivo efficacy and malaria, chemosensitivity and malaria, and in vitro drug resistance and malaria. Other key words were dihydrofolate reductase (dhfr), dihydropteroate synthase (dhps), Plasmodium falciparum multidrug resistance gene 1 (pfmdr1), Plasmodium falciparum chloroquine resistance related transporter (pfcrt) gene, candidate gene 1 and 2 (cg1 and cg2), and drug resistance and molecular markers and malaria. In addition, the relevant cited bibliographies in the reports identified were reviewed. The current magnitude of CQ and SP resistance was assessed by abstracting, in a semisystematic way, all in vivo test reports published in English for the period from 1996 to 2002. We used a “minimum data reported” criterion to identify the relevant in vivo test reports and abstracted them by using a standard data collection tool. The minimum data needed for the reports to be analyzed were age group, period of follow-up, entry parasite species, statement whether symptomatic or asymptomatic subjects were studied, definition of outcome measures, and whether drug administration was supervised. Moreover, at least 16 study participants should have completed the required period of follow-up and pregnant women, patients with severe disease, and those with concomitant infections should have been excluded. Data from all studies that recruited symptomatic patients and satisfied the minimum data requirements were entered into a computerized database. The data were summarized by continent; within each continent, further categorization by region was done: Africa (West, eastern/great lakes, central/southern, and North), Asia (Southeast Asia, India and Pakistan, Pacific Islands, and Middle East), and South America (Brazil and Columbia).
In view of the heterogeneous nature of the epidemiology of drug resistance, summary estimates derived by pooling results from different studies and applied to a whole country could be misleading. We therefore used the median as the measure of the central tendency and the range as the measure of the variability or dispersion.
HISTORY OF ANTIMALARIAL DRUG RESISTANCE
Development of the First Antimalarial Agents and Anecdotal Reports of Drug Resistance
Quinine, extracted from the bark of the cinchona tree, was used as an antimalarial agent as early as 1632 (15), and by the 19th century it was still the only known antimalarial agent. Primaquine and quinacrine were produced after the First World War. CQ followed shortly thereafter in 1934 (203), around 1946 it was designated the drug of choice for treatment of malaria (43). The earliest anecdotal reports of resistance to an antimalarial agent are those for quinine in 1844 and 1910 (67, 143, 144).
Emergence of Chloroquine-Resistant P. falciparum
Probably, CQ-resistant P. falciparum (CRPF) emerged from four independent foci. The first focus was in Southeast Asia around the Thai-Cambodian border, where CRPF infections were identified in 1957 and spread quickly to Thailand (88). Two other foci were identified in 1960 in South America (in Venezuela and in the Magdalena Valley, Colombia [136]). In 1976, two confirmed cases of CRPF infection were reported from Port Moresby in Papua New Guinea (PNG) (86) and probably represent the emergence of the fourth independent focus of CRPF infection. A recent population genetic survey has shown that despite the close proximity, strains from PNG are remarkably different from those from Southeast Asia (133). In Africa, CRPF was first found in 1978 in nonimmune travelers from Kenya and Tanzania (34, 72). This was followed 2 to 3 years later by reports from Madagascar (11) and by reports in semi-immune patients in Tanzania (106, 155) and Kenya (128, 193). Resistance spread from the African coastal areas inland and by 1983 had been observed in Sudan, Uganda (154), Zambia (65), and Malawi (73, 157, 189), leading to the view that CRPF may have spread from Southeast Asia to Africa as a result of population movements. This hypothesis is supported by a population genetic survey showing the similarity of parasites of African and Asian origin and their difference from those from South America and PNG (133; reviewed in reference 222).
In 1973 Thailand was the first country to replace CQ as a first-line drug; several other countries in Asia and South America followed soon after. In Africa CQ had a longer useful therapeutic life (UTL), and it was not until 1988 that the South African province of KwaZulu-Natal replaced CQ with SP (30). However, the first African country to change the national treatment policy from CQ to SP was Malawi in 1993, followed 4 years later by Kenya, South Africa (other than KwaZulu-Natal), and Botswana (26, 27).
Emergence of Chloroquine-Resistant P. vivax
CQ resistance in P. vivax (CRPV), the second most common malaria parasite (17), has been very limited despite the widespread CQ use. Resistance to primaquine, quinine, proguanil, and pyrimethamine was reported in 1987 (160), and resistance to the 4-aminoquinolines was reported in 1989 among repatriated Australians from two different areas in the northern part of PNG (174, 182). Other cases were reported from Nias Island in Indonesia in 1991 (183) and from Myanmar in 1992 to 1993 (140). CRPV malaria remained rare in Africa and South America, although 11 cases of suspected low responses to CQ and amodiaquine were observed in Colombia in 1989 (9). In 1996 a confirmed case of CRPV was reported from Guyana, South America (163). In Asia, CRPV has been limited to Sumatra in Indonesia (17), particularly to Irian Jaya (225), and to PNG, with a few cases in Myanmar and Mumbai, India (108). Recently, RII parasitologic failure to CQ has been reported from the Brazilian Amazon region (3), while parasite reappearance by day 16 was observed in 7% of 113 patients treated with CQ in Vietnam (162), suggesting the emergence of CRPV in this subregion. The other Plasmodium species have maintained their sensitivity to CQ, although there is a recent report of P. malariae resistant to CQ (123). However, Collins and Jeffery (44) have suggested that an extended parasite clearance time after treatment of infections with Plasmodium malariae may not be indicative of CQ resistance by this species. Indeed, in some patients parasitemia has persisted for up to 16 days following treatment with a full course of CQ (44). Such observations suggest that an extended parasite clearance time is not an adequate criterion for documenting resistant P. malariae and that other criteria are needed.
Emergence of Parasite Resistance to Other Drugs
Antifolate resistance, unlike that to CQ, emerged almost instantaneously and independently from several areas where the drugs had been introduced. For example, resistance to the type 2 antifolate proguanil was observed just 1 year after its introduction in 1947 in peninsular Malaya (160). It is thought that P. falciparum strains resistant to pyrimethamine and cross-resistant to proguanil, chlorproguanil, and cycloguanil originated under drug pressure in late 1953 in Muheza, Tanzania, and became consolidated locally during the following 2 years. The resistant strain is reported to have subsequently spread slowly, in the absence of drug pressure, through the areas of northern Tanzania with hyperendemic malaria. Consequently after about 8 years, the resistant strain formed two-fifths of the P. falciparum population in a 15-mile radius around Muheza (42). Similarly, SP was introduced in 1967 in Thailand and resistance was reported the same year (160, 224). The shorter time before the appearance of antifolate resistance is probably linked to the smaller number of genetic mutations involved (compared to those involved in CQ resistance) and to the long half-life of the drugs (71, 74, 222). Resistance to mefloquine was first reported in 1982, 5 years after it had been introduced (146), while resistance to atovaquone developed in 1996, the same year the drug was introduced (118). Confirmed resistance to the artemisinin derivatives has not been reported yet, although recrudescence among patients receiving a short course (less than 5 days) of therapy has been observed (132). Additionally, patients who have undergone splenectomy may experience a longer mean parasite clearance time (209).
MONITORING OF ANTIMALARIAL DRUG RESISTANCE
Definition of Parasite Resistance
The term “parasite resistance” has been used with different meanings. It has been defined as “the ability of a parasite strain to survive and/or multiply despite the administration and absorption of a drug in doses equal to or higher than those usually recommended but within the limits of tolerance of the subject” (232). This definition was subsequently modified to specify that “the drug must gain access to the parasite or the infected red blood cell for the duration of the time necessary for the normal action of the drug” (32). Despite this modification, the term “parasite resistance” has been used interchangeably in the published literature when referring to the parasite phenotype characterized in vitro by the confirmed ability to survive a threshold (nanomolar) concentration of the drug under standard conditions of continuous culture. Resistance has also been used when referring to therapeutic failure after administration of a standard dose of a drug; this definition is used in the WHO standard in vivo test protocol (229). However, in this test, serum drug levels are not normally measured and the observed therapeutic failure might be due to malabsorption, rapid or abnormal metabolism, or the presence of latent infections other than malaria. Therefore, it is important to define the terms being used in order to avoid confusion.
Although the definition of resistance in vitro more accurately reflects biological resistance to the drug, true parasite resistance requires a demonstration of the ability of parasites to survive in vivo in the presence of an adequate therapeutic concentration of the drug in serum. When serum drug concentrations are not measured, the in vivo therapeutic failure data should be interpreted with caution since these might overestimate the true parasite resistance; this is particularly true for slowly acting or long-acting drugs such as SP and mefloquine.
Methods for Monitoring Parasite Resistance
Several methods for monitoring antimalarial drug resistance exist; they include in vivo and in vitro tests and, more recently, molecular markers.
In vivo tests.
In vivo tests are traditionally the “gold standard” method for detecting drug resistance (231). The advantage of the in vivo tests over the in vitro assays is that they can be conducted in the field with little equipment and personnel and the results are easy to interpret. They reflect the true biological nature of treatment response, which involves a complex interaction between the parasites, the drugs, and the host response, while in vitro tests measure only the interaction between the parasites and the drugs. The classical 28-day extended test and the 7-day test based on parasitologic response were the first to be used and were interpreted using the standard S-RI-RII-RIII classification system (Table 1). The major limitation of these earlier tests is that the study subjects are typically asymptomatic (often schoolchildren) and the results cannot be easily applied to malaria patients. This has led to the development of the revised and simplified 14-day test of therapeutic efficacy. The main difference between this protocol and the previous one is that the study subjects are clinical patients and the outcome takes into account their clinical response (229) (Table 1). One of the advantages of the 14-day test is that reinfection is less likely than in the 28-day test. However, there are also some limitations. First, a poor concordance between early treatment failure (ETF) and RIII (167) and a tendency to overestimate ETF (175) has been observed. Indeed, it is common for some patients classified as ETF who are not given rescue treatment but are closely followed up to have adequate clinical response (ACR). Second, the adequate clinical response (ACR) category as defined in the 1996 WHO protocol includes patients on day 14 with parasitemia but without fever, thereby underestimating the true treatment failure rate, particularly the parasitological failure. However, in the 2002 modification to this test, the ACR category in the 1996 protocol (Table 1) is stratified into two classes: adequate clinical and parasitological response (ACPR) and late parasitological failure (LPF). Therefore, in future tests patients with parasitaemia on day 14 will be classified as LPF (Table 2). Third, in areas with intense transmission the test is restricted to children younger than 5 years because they are the group at greatest risk of malaria mortality and often have a less favorable response to antimalarial drugs than do older children and adults. Moreover, more children than adults with malaria tend to visit the outpatient clinics. However, adults contribute to the burden of malaria, and they could be included in the in vivo test for completeness and more comprehensive information, even in areas of intense transmission. This would increase costs and logistical problems and would be hardly feasible in countries with limited resources such as those in sub-Saharan Africa, where tests of children will continue to provide the relevant information for the reevaluation of malaria treatment policy.
TABLE 1.
Clinical and parasitologial classifications according to the WHO test protocol
| Parasitological classifications | Treatment failure classifications |
|---|---|
| Sensitive | Adequate clinical response (ACR) |
| Clearance of parasites after treatment without subsequent recrudescence within a defined period | (i) Absence of parasitemia on day 14, irrespective of fever status, without previously meeting any of the criteria for ETF or LTF |
| (ii) Absence of fever irrespective of the parasitemia status without previously meeting any of the criteria for ETF or LTF | |
| RI parasitologic failure | Early treatment failure (ETF) |
| Initial clearance followed by recrudescence after day 7 | (i) Danger signs or severe malaria on day 1, 2, or 3 in the presence of parasitemia |
| (ii) Fever (axillary temperature, ≥37.5°C) persists on day 2 and the parasite density is greater than that at enrollment (D0 parasite density) | |
| (iii) fever and parasitaemia on day 3 | |
| (iv) parasite density on day 3 is ≥25% of the day 0 parasite density | |
| RII parasitologic failure | Late treatment failure (LTF) |
| Reduction of parasitemia on day 2 to less than 25% of day 0 parasitemia, but no complete clearance | (i) Danger signs or severe malaria develop in the presence of parasitemia on any day from day 4 to day 14 |
| (ii) Fever and parasitemia on any day from day 4 to day 14, and yet the patient could not be classified as ETF | |
| RIII parasitologic failures | |
| On day 2, either no reduction of parasitemia or reduction to a level equal to or greater than 25% of the day 0 parasitemia |
TABLE 2.
Revised WHO guidelines for assessing the response to treatment (2002)
| Early treatment failure (ETF): days 0, 1, 2, and 3 |
| Development of danger signs or severe malaria on days 0-3 in the presence of parasitemia |
| Parasitemia on day 2 higher than on day 0, irrespective of temperature |
| Parasitemia on day 3 with temperature of ≥37.5°C (axillary) |
| Parasitemia on day 3 is >25% of count on day 0 |
| Late clinical failure, (LCF): days 4 to 14 or 28 |
| Development of danger signs or severe malaria after day 3 in the presence of parasitaemia, without previously meeting any of the criteria of early treatment failure |
| Temperature of ≥37.5°C (axillary), or history of fever in past 24 h, on days 4 to 14 or 28 in the presence of parasitemia, without previously meeting any of the criteria of early treatment failure |
| Late parasitological failure (LPF): days 7 to 14 or 28 |
| Presence of parasitemia on any day from day 7 to day 14 or 28, and temperature of < 37.5°C (axillary), without previously meeting any of the criteria of early or late treatment failure |
| Adequate clinical and parasitological response (ACPR) |
| Absence of parasitemia on day 14 or 28, irrespective of temperature, without previously meeting any of the criteria of early or late treatment failure |
Fourth, it has been argued that a follow-up of 14 days underestimates overall treatment failure rates, particularly for long-acting drugs such as SP and mefloquine (see, for example, reference 58). A 28-day or even longer follow-up would be preferable, but the reappearance of parasitemia after day 14 would require the differentiation between a new infection and a recrudescence. This requires relatively expensive PCR techniques that use polymorphic markers such as the merozoite surface proteins (MSP1 and MSP2), glutamate-rich protein (GLURP), or microsatellite markers (112). Such techniques, based on the assumption that genetically different parasites at recruitment and at follow-up indicate a new infection (172), have some limitations (70). Some genotypes may not be detected at recruitment because they represent a minority of the parasite population; these subpopulations could be detected during the follow-up if they are resistant and if all the other sensitive parasites have been eliminated by the drug action. Such an infection would be wrongly classified as a new infection, overestimating the efficacy of the study drug. Furthermore, daily differences in the diversity of an infection have been observed (A. Farnert, M. Lebbad, and I. Rooth, Abstr., 3rd Pan Africa Multilateral Initiative for Malaria Conf., abstr., 145, 2002). Conversely, in places where there are few circulating parasite genotypes, new infections might have a similar genotype to the one eliminated by the drug (85). Furthermore, for isolates where both new and recrudescent genotypes are present, there is no agreed standard on whether such mixed infections should be classified as new, recrudescent, or undetermined genotypes. Some studies have excluded them from analysis (31, 211), while others have classified them as either reinfections or recrudescence (103, 172, 215). A recent study in Uganda has suggested that classifying such infections as new is the most accurate option (38). If validated, this could improve the accuracy of these molecular techniques in classifying the outcome in in vivo studies with an extended follow-up.
Fifth, according to the WHO 1996 in vivo protocol (231), the parasite density at recruitment should be 2,000 to 100,000/μl of blood. The lower limit of 2,000 asexual parasites/μl of blood increases the likelihood that the clinical illness under study is due to the parasites seen. However, the upper limit of 100,000 asexual parasites/μl could result in the exclusion of a substantial number of patients who would have been otherwise eligible, increasing the time for recruiting the required number of patients and consequently the costs and logistics of the test. Furthermore, some studies have observed a significant association between higher parasite density and increased risk of treatment failure (55). For example in Kampala, Uganda, approximately 25% of the children younger than 5 years have parasite densities above 100,000/μl but do not meet other criteria for severe malaria (G. Dorsey, personal communication). Exclusion of such patients could result in the underestimation of the true prevalence of treatment failure. Finally, the 1996 in vivo protocol has been designed more as a screening tool for treatment failure above a certain critical threshold. This is why the sample size estimation has been based on lot quality assurance sampling (113). However, this has often resulted in a small number of patients and point estimations of resistance with large confidence intervals, making meaningful temporal comparisons difficult because of random variability. Furthermore, results from these tests have often been presented as if a clinical trial had been carried out, resulting in misleading inferences.
In view of these limitations, a recent WHO consultative meeting (234a) revised the study protocol and proposed several modifications. The revisions relate mainly to the following areas; inclusion and exclusion criteria; classification of response to treatment; analytic and statistical issues (including sample size calculations), and recommended duration of follow-up.
With respect to the inclusion and exclusion criteria, the following modifications have been proposed. Children (younger than 5 years) with clinical malaria are the study subjects in all areas. However, where transmission is low or young children are at substantially lower risk of infection than adults (occupational exposure), all ages can be enrolled in sufficient numbers to allow stratification by age (younger than 5 years, 5 years and older). Furthermore, specific drug age limitations have to be taken into account. For example, atovaquone-proguanil (Malarone), artemether-lumefantrine (Co-artem, Riamet), and halofantrine are associated with a minimum age or weight threshold below which treatment is not recommended.
With respect to fever versus measured temperature elevation, it has been recommended that fever, as defined in the protocol, is a useful working definition and excludes normal diurnal variation as a cause of an elevated temperature reading. Fever has been defined as (i) axillary temperature of ≥37.5°C or (ii) rectal or tympanic temperature of ≥38°C. Consequently, each test protocol should specify the method used for measuring temperature. Furthermore, in areas of intense transmission, enrolment of patients should be based on measured fever (an elevated body temperature as per the definitions above) and not on history of fever alone. Similarly, the determination of outcome should also be based on fever only and not on history of fever alone. However, in areas of low transmission, where history of fever is deemed reliable, either fever or a history of fever (within the previous 24 h) can be used as an adequate enrolment criterion. Moreover, patients will no longer be excluded on the basis of a measured temperature of ≥39.5°C, so as to maintain consistency with the updated definitions of severe malaria (234b). Finally, the upper limit parasite density has also been revised from 100,000/μl to 200,000/μl in areas with intense transmission and from 30,000/μl to 100,000/μl in areas of low transmission.
Regarding the classification of the response to treatment, notable changes are the new classification system that has been recommended of early treatment failure (ETF), late clinical failure (LCF), late parasitologic failure (LPF) and adequate clinical and parasitological response (ACPR) (Table 2). In addition, the use of the classical method for sample size calculation based on the expected treatment failure and the desired confidence level (preferably 95%) and precision (either 5 or 10%) has been recommended. Data analysis should be by intention-to-treat rather than as per protocol (234a). With regard to the duration of follow-up, the recommended minimum length is 14 days. In areas of intense transmission, studies with a longer follow-up period should also include molecular genotyping of blood samples to help distinguishing between recrudescence and new infection.
In vitro tests.
In vitro assays are based on the inhibition of the growth and development of malaria parasites by different concentrations of a given drug relative to drug-free controls. The WHO in vitro microtest is based on counting the parasites developing into schizonts, while the isotopic microtest is based on measurement of the quantity of radiolabeled hypoxanthine, a DNA precursor, incorporated into the parasites (40). There are newer colorimetric tests that can test low parasitaemias taken directly from patients in the field: the parasite lactate dehydrogenase enzymatic assay (126), the parasite lactate dehydrogenase double-site enzyme-linked immunodetection assay (60) and the histidine-rich protein II assay (145). However, because of the limited amount of data published, it is still unclear whether these new tests will replace the traditional (micro and isotopic) tests. In the latter, drug resistance is identified when parasite growth occurs above a threshold concentration. This threshold is usually defined as the concentration (in nanomoles per liter) at which 50% of parasite growth (geometric mean IC50) (WHO microtest) or the incorporation of 50% hypoxanthine is inhibited (IC50) compared to the drug-free control wells. A major advantage of the in vitro assay is that it yields quantitative results and identifies the phenotype of the parasite independently of the immune and physiopathological conditions of the host. However, in vitro assays have several problems and drawbacks. (i) They require highly skilled personnel and laboratory equipment. (ii) Parasites isolated from patients who have taken medication on their own initiative a few days before consultation usually do not grow in vitro, making the tests akin to a measure of the amount of drug use. (iii) There is no consensus about the determination of the threshold IC50 that distinguish susceptible from resistant parasites, and there are currently no fully validated cutoff points for assessing in vitro resistance (175). (iv) The in vitro activity of drugs that are administered in combination, like the type 1 and 2 antifolates, may not accurately reflect the in vivo conditions. (v) The relevance of the in vitro tests for prodrugs that are metabolized into active drugs in vivo, such as proguanil and chlorproguanil metabolized to cycloguanil and chlorcycloguanil, is a pertinent issue because metabolism varies considerably among individuals (poor and extensive metabolisers). Consequently, the in vitro test provides little information on the efficacy of the prodrug. Furthermore, there is poor correlation between in vivo and in vitro test results, especially in areas of intense transmission, presumably due to the influence of host immunity. The accuracy of the inhibitory concentrations for a given sample is influenced by several factors such as the in vitro test conditions, the presence of mixed resistant and sensitive parasite populations in the same sample, and humoral factors from the donor that can interfere with parasite maturation (222). Despite these shortcomings, in vitro tests are of value, particularly for testing parasite resistance to new drugs and agents that have not been used previously. Furthermore, they can provide important longitudinal data on changes in parasite response to drugs, which is important collateral information about the emergence and spread of drug resistance. Finally, although in vitro techniques are not frequently used in malaria control activities in Africa, they play an important role in Southeast Asia.
Molecular markers.
Before discussing the role of molecular markers for the detection of drug resistance, it is important to present a brief overview of the known molecular mechanisms underlying resistance to the most commonly used antimalarial drugs, CQ and SP.
Molecular basis for resistance to antifolates.
The molecular basis for resistance to the antifolates has been extensively studied. Antifolate antimalarial drugs interrupt DNA replication in the parasite by competitively inhibiting folate synthesis, which is essential for the synthesis of pyrimidines (reviewed in reference 74). Pyrimethamine and the biguanides bind to and inhibit the bifunctional enzyme dihydrofolate reductase-thymidylate synthase (DHFR-TS) (33, 186), and the sulfonamides and sulfones inhibit the enzyme dihydropteroate synthase (DHPS) (reviewed in references 72 and 186). Point mutations at codons 108, 51, 59, and 164 in the dhfr-ts gene (which encodes the DHFR-TS enzyme) alter the binding active-site cavity where the drugs bind to the enzyme (45). A point mutation at codon 108 in the dhfr-ts gene that results in a change of amino acid from serine to asparagine seems to be the key mutation that confers resistance to pyrimethamine in vitro (45, 50). An asparagine-to-isoleucine amino acid change at codon 51 and a cysteine-to-arginine change at codon 59 appear to confer higher levels of in vitro pyrimethamine resistance when they occur with the asparagine-108 mutation (188). A mutation that results in an isoleucine-to-leucine substitution at codon position 164 in combination with the other three mutations (at codons 108, 51, and 59) has been found in P. falciparum strains that are highly resistant to both pyrimethamine and cycloguanil in Asia (20). Recently, the dhfr mutation at codon 164 has been reported in one study in Tanzania that used a yeast-based system (96). A serine-to-threonine change at codon 108 of the dhfr-ts gene plus a mutation at codon 16 confers parasite resistance to cycloguanil, the active form of proguanil (161).
The gene encoding DHPS, the target enzyme in P. falciparum for sulfadoxine has also been well studied, and point mutations associated with in vitro resistance to these drugs have been identified (210). These mutations include the following substitutions: serine to alanine at position 436, alanine to glycine at position 437, alanine to glycine at position 581, serine to phenylalanine at position 436 coupled with alanine to threonine or serine at position 613, and lysine to glutamate at position 540. It has been suggested that the sequence of mutations occurs in a stepwise fashion, with selection for mutations in dhfr-ts gene probably occurring first and the dhps gene mutations following later (186). Furthermore, very few field studies have observed mutations at codons 51 or 59 of the dhfr gene when the codon 108 mutation is absent. Such observation could be exploited to identify reliable molecular indices to detect antimalarial drug resistance.
Molecular basis for resistance to chloroquine.
CQ acts by binding to and interrupting hematin detoxification, resulting in the accumulation of large quantities of the toxic hematin that eventually kills the parasite (197). Parasite resistance to CQ is caused by the ability of the resistant parasites to limit the accumulation of CQ in the parasite digestive food vacuole (212). The exact mechanism leading to low intracellular CQ levels is not clear yet. However, several mechanisms have been proposed, such as altered pH (60), decreased drug uptake into the parasite food vacuole (180), or increased drug efflux out of the vacuole (107). CQ resistance has been linked to a number of mutations in the P. falciparum genes. Earlier studies of the mechanism of CQ resistance identified and investigated the role of the P. falciparum multidrug resistance (pfmdr1) gene, located on chromosome 5 and encoding the P-glycoprotein 1 (Pgh-1). Resistance to CQ was thought to be linked to point mutations, such as the asparagine-to-tyrosine substitution at position 86, and to other probably compensatory mutations at positions 184, 1034, 1042, and 1246 (75, 173). However, the results of field studies of the association of these mutations with the in vivo and in vitro resistance were not consistent. Subsequent studies identified other mutations in different genes of chromosome 7 (221). A single candidate gene (cg2), a polymorphic gene, was proposed as the one responsible for CQ resistance (187). However, the observed association was probably due to its close proximity on chromosome 7 to the pfcrt gene encoding the P. falciparum CQ resistance-related transporter protein (71, 187). Current evidence from transfection studies (71, 187) strongly suggests that the mechanism of P. falciparum resistance to CQ is linked to mutations in the pfcrt gene, especially the substitution of threonine for lysine at position 76. However, other mutations in the pfcrt gene at positions 72 to 78, 97, 220, 271, 326, 356, and 371, as well as mutations in other genes such as pfmdr1, might be involved in the modulation of resistance (173, 223). CQ resistance seems to involve a progressive accumulation of mutations in the pfcrt gene, and the mutation at position 76 seems to be the last in the long process leading to CQ clinical failure (53, 92).
Molecular markers as tools for detecting drug resistance.
Our increased understanding of the molecular mechanisms for parasite resistance to the antifolates and, to some extent, to CQ has led to a proliferation of field studies investigating the role of molecular markers in detecting drug resistance. Some studies have demonstrated a causal relationship between discrete polymorphism in the candidate genes and parasite resistance to either CQ or SP in vitro and in vivo, but others have not. The precise role of molecular markers in detecting and estimating P. falciparum resistance still needs to be established because the genetic mutations that predict parasite resistance remain unclear. A genotype resistance index (GRI) and genotype failure index (GFI), derived by computing the ratio between the age-adjusted frequency of the pfcrt mutation at position 76 and the prevalence of parasitologic and clinical CQ resistance, respectively (53), have been proposed for the surveillance of CQ resistance. Such indices consistently gave a stable value of 2 to 3 for several sites or different years (54), indicating that the prevalence of clinical or parasitologic failure was two to three times lower than the prevalence of the gene mutation. The association of the pfcrt codon 76 mutations with in vivo CQ treatment failure has been observed in several settings (13, 21, 39, 61, 127, 164, 214) but not in others (57, 111), especially in areas where CQ resistance was very high. In these situations, the GRI and GFI might not predict CQ treatment failure because the prevalence of the gene mutation in these populations has reached a plateau (111). In such settings, other indices are needed. In Uganda, a strong positive correlation between the pfcrt codon 76 mutant/wild (M/W) ratio and the late stages of high-grade CQ treatment failure (i.e., ETF and RIII parasitologic failure) has been observed (199). Such a ratio could be useful in estimating high-grade CQ resistance in cases where the prevalence of the pfcrt mutation is already high.
For SP resistance, there is growing evidence that the dhfr triple mutations with or without the dhps mutations can predict SP treatment failure (SP-TF). Studies conducted in East Africa have observed that the triple (dhfr Asn-108, Ile-51, and Arg-59) mutant genotype was associated with SP treatment failure (139, 149). However, a study in Malawi observed that the quintuple mutant genotype (dhfr Asn-108, Ile-51, and Arg-59 plus dhps Gly-437 and Glu-540) was more strongly associated with SP-TF than was the dhfr triple mutant (109). Lack of consistency in the results is probably a consequence of differences in the laboratory methods used to identify the mutations and in the estimation of the frequency of the gene mutations. In most studies, the prevalence of the gene mutations in host infections, i.e., the number of infected individuals carrying the mutations divided by the total number of infected individuals sampled, has often been used. However, the prevalence of infections with the mutant genotype might differ from the frequency of the mutant allele in the parasite population, and the latter is likely to be a more appropriate measure for estimating the number of circulating mutant parasites. We have recently demonstrated, using a population-based survey, that the prevalence of infections with mutant genotypes indeed differs from the mutant allele frequency. However, the ratio of the prevalence of infections with mutants to those with the wild type and the M/W ratio do not differ. These observations indicate that the M/W ratio might be a more robust index because it is not affected by parasite clone multiplicity. Furthermore, the dhfr codon 59 M/W ratio, but not the prevalence of infections with the dhfr codon 59 mutation, was positively correlated with SP treatment failure in Ugandan sentinel sites (201).
An improvement of molecular techniques that is urgently required is the standardization of the methods for detection of mutations. A recent critical comparison of three different techniques for the identification of the dhfr codon 108 mutation, i.e., mutation-specific PCR, restriction fragment length polymorphism, and probe hybridization to dot blots, observed significant discrepancies (171). Furthermore, analysis and interpretation of the observed prevalence of gene mutations needs to take into consideration the multiplicity of infections, particularly in areas of intense transmission where these are extremely common. This is particularly relevant if multiple loci or multiple genes encode resistance. Molecular markers need to be taken a step forward toward the surveillance of resistance at population level rather than for the diagnosis of treatment failure in individual patients. Because the samples are easy to collect, validated molecular markers could become a fundamental tool for an early-warning system and could give policy makers some lead-time to prepare for policy change. As a complementary tool, and not as a replacement for in vivo tests, they could be used for large-scale mapping and estimation of parasite resistance. In vivo tests could then be conducted in sites chosen on the basis of the molecular data to estimate more precisely the actual prevalence of resistance. Validation of molecular markers is therefore urgently required and needs strong collaborative partnerships between subregional and regional networks.
CURRENT DISTRIBUTION OF IN VIVO PARASITE RESISTANCE TO CHLOROQUINE AND SULFADOXINE-PYRIMETHAMINE
Accurate comparison of the current prevalence of in vivo parasite resistance is complicated by the enormous variability in study methods, period of follow-up, type of subjects recruited, and definition of outcomes. Furthermore, the completeness of the study reports is variable. The lack of an agreed drug resistance threshold at which the antimalarial drug policy should be changed also complicates comparisons. Several thresholds have been proposed, such as an RIII prevalence at 5 to 10% (27) or at 14 to 31% (195) or the prevalence of treatment failure (TTF) at 25% (180). The latter is used by WHO as the threshold for a four-stage framework of treatment failure (ETF plus LTF) to guide the actions to be taken by malaria control programs: grace (0 to 5%), alert (6 to 14%), action (15 to 24%), and change (≥25%).
In this section, we summarize the in vivo studies that we identified in an attempt to show general geographic differences in the prevalence of resistance to CQ and SP. A more accurate comparison would require inclusion of only studies that used a standardized method with uniform follow-up, similar outcome measures, and similar study populations. Such strict criteria commonly used for the meta-analysis of randomized trials would have resulted in the exclusion of many in vivo studies. For CQ, a total of 108 studies carried out in Africa were identified, 21 from the central/southern region, 55 from the eastern/great lakes region, 29 from West Africa, and 3 from North Africa. Twenty-two studies from Asia and seven from South America were identified. There are fewer studies from Asia and South America because many countries stopped using CQ in the 1970s and 1980s and consequently no recent in vivo studies have been carried out. For SP, 92 studies were identified in Africa, 14 from the central/southern region, 65 from the eastern/great lakes region, 11 from West Africa, and 2 from North Africa. In Africa, the age of the patients varied considerably among the different studies (younger than 5 years, younger than 10 or 12 years, or all age groups) and the outcome reported was either the parasitological or clinical failure or both, making comparisons rather difficult. Some studies conducted in the eastern/great-lakes region were comparable, a consequence of the standardization of the method by the East Africa network for monitoring treatment efficacy. In Asia and South America, most studies recruited all age groups and reported mostly parasitological outcomes.
The recent (1996 to 2002) prevalence and distribution of CQ and SP resistance in Africa, Asia, and South America is presented in Tables 3 to 8. In Africa, median CQ-TTF among children younger than 5 years was above the critical value (TTF = 25%) in all the countries in the eastern/great lakes (seven of seven) and central/southern (four of four) regions. However, in West Africa only one of five countries had a median CQ-TTF above the critical value (Table 3). In Asia the median CQ parasitologic failure (CQ-PF) varied from 38 to 91% in Southeast Asia, from 23 to 38% in the Pacific region, from 46 to 77% in the Indian subcontinent, and from 12 to 69% in the Middle East (Table 4). In South America the lowest median CQ-PF was 33% and the highest was 50%, while the corresponding lowest and highest estimates for CQ-TTF were 44 and 100%, respectively (Table 5).
TABLE 3.
P. falciparum CQ treatment failure in vivo for selected countries in Africa, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median %CQ-PF (range)a | Median %CQ-TTF (range)a | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| Central | Central African Republic | 1 | 1998 | 0.5-5 | WHO 1996 | 14 | 64 | WHO/AFRO 1998 | |
| Gabon | 1 | 1996-1998 | 1-55 | WHO 1973 | 14 | 19 | 29 | ||
| Mozambique | 1 | 1999 | <5 | WHO 1973, 1996 | 28 | 58 | 26 | 129 | |
| Zambia | 7 | 1998-2000 | 0.5-5 | WHO, 1973 | 14, one study 7 | 55 (33-70) | 42 (22-54) | 18, 23, WHO/AFRO 1998 | |
| Zimbabwe | 8 | 1996-1999 | NSb | WHO 1973 | 7 | 27 (8-33) | 19, 125, 127 | ||
| Southern | Botswana | 3 | 1998 | 0.5-5 | WHO 1996 | 14 | 25 (21-44) | WHO/AFRO 1998 | |
| Eastern and great lakes | Burundi | 1 | 1998 | 0-14 | WHO, 1973 | 7 | 41 | 52 | |
| Ethiopia | 5 | 1998 | 0.5-5 | WHO 1996 | 14 | 79 (51-93) | WHO/AFRO 1998 | ||
| Ethiopia combined with Eritrea | 5 | 1989-1991 | 1-80 | WHO 1973 | 7 | 88 (82-94) | 4 | ||
| Kenya | 3 for clinical, 1 for parasitologic outcomes | 1996-2001 | 0.5-5 for clinical, 2-12 for parasitologic outcomes | WHO 1996, WHO 1973 | 14, 28 | 71 | 64 (32-87) | 5; www.eanmat.org WHO/AFRO 1998 | |
| Kenya | 1 | 1996-2001 | All ages | Parasite clearance at day 14 | 14 | 18 | 41 | ||
| Rwanda | 6 clinical, 4 parasitologic | 1999-2000 | <5 | WHO 1996 | 14 | 81 (73-88) | 52 (19-60) | www.eanmat.org, WHO/AFRO 1998 | |
| Tanzania | 9 | 1998-2002 | 0.5-5 | WHO 1996, day 14 parasite failure | 14 | 55 (11-98) | 42 (17-71) | 66; www.eanmat.org, WHO/AFRO 1998 | |
| Tanzania | 2 | 1998-2002 | 1-14 | WHO 1973, day 14 parasite failure | 14, 28 | 55 (11-98) | 42 (17-71) | 97, 218 | |
| Uganda | 17 clinical, 15 parasite outcome | 1996-2000 | 0.5-5 | WHO 1973, 1996 | 14 | 41 (10-96) | 28 (9-89) | 56, 104, 105, 113, 137, 199; Kilian 1998 (Uganda MoH report) | |
| Zanzibar | 2 | 2000-2001 | <5 | WHO 1996 | 14 | 60-61 | www.eanmat.org | ||
| West | Burkina Faso | 4 clinical, 2 parasite outcome | 1998-2000 | 0.5-5 | WHO 1973, 1996 | 14 | 42 (18-65) | 18 (12-23) | 29, 204, WHO/AFRO 1998 |
| Cameroon | 4 | 1994-2001 | 1-55 | WHO, 1973 | 7, 14 | 31 (8-53) | 29, 114, 205 | ||
| Cote d'lvoire | 1 | 1996 | >10 | WHO 1973 | 28 | 33 | 99 | ||
| The Gambia | 1 | 1994-1996 | 0.5-10 | Day 7 and 28 parasite failures | 28 | 23, 50 | 138 | ||
| Ghana | 2 | 1999-2000 | 0.5-5 | WHO 1973, 1996 | 14 | 58 (56-61) | 41 (29-53) | 59, 63 | |
| Guinea-Bissau | 1 | 1998 | 0.5-5 | WHO 1996 | 14 | 11 | WHO/AFRO 1998 | ||
| Mali | 5 | 1997 | <5 | WHO 1973, 1996 | 14 | 25 (20-30) | 21 (18-24) | 53, 167 | |
| Nigeria | 1 | 1997 | 0.5-5 | WHO 1973 | 14, 28 | 64 | 8 | ||
| Nigeria | 2 | 2001 | 1-12 | WHO 1973 | 14, 28 | 31 (21-42) | 191 | ||
| Nigeria | 2 | 1996 | All | WHO 1973 | 14, 28 | 27 (13-54) | 68 | ||
| Senegal | 4 | 1996-1998 | 1-55 | WHO 1973 | 14 | 42 (24-59) | 13 (10-16) | 29; WHO/AFRO 1998 | |
| Northern | Somalia | 2 | 2002 | All | WHO, 1996 | 14 | 56 (35-77) | 42 (33-51) | 217 |
| Sudan | 1 | 1994-1995 | >5 | WHO 1973 | 14 | 10 | 87 |
CQ-PF, CQ parasitologic failure (either R1-R3 or cumulative parasite failure); CQ-TTF, clinical failure (either ETF + LTF or cumulative clinical failure).
NS, not stated.
TABLE 8.
P. falciparum SP treatment failure in vivo for selected countries in South America, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median % SP-PFa | Median % SP-TTFa | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| Latin America/Caribbean | Colombia | 1 | 1998 | 1-60 | WHO 1973, 1996 | 14 | 6 | 6 | 156 |
| Colombia | 1 | 1999 | 14-66 | WHO 1973, 1996 | 28 | 20 | 37 | ||
| Venezuela | 1 | 1999 | All | WHO 1996 | 14 | 20 | 32 | 1 | |
| Venezuela | 1 | 1999 | 14-60 | WHO 1973 | 28 | 20 | 35 | ||
| Venezuela | 1 | 1999-2000 | All | WHO 1996 | 21 | 3 | 133 |
SP-PF, SP parasitologic failure (either R1-R3 or cumulative parasite failure); SP-TTF, SP clinical failure (either ETF + LTF or cumulative clinical failure).
TABLE 4.
P. falciparum CQ treatment failure in vivo for selected countries in Asia, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median %CQ-PF (range)a | Median %CQ-TTF (range)a | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| East Asia | China | 1 | 1995-1996 | 6-60 | WHO 1973 | 28 | 91 | 102 | |
| Laos | 1 | 2001 | >1 | WHO 1973 | 28 | 46 | 164 | ||
| Myanmar | 2 | 1995 | All | WHO 1973 | 28 | 82 (77-86) | 190 | ||
| Indonesia | 5 | 1995-1999 | ≥5 | WHO 1973 | 28 | 38 (25-82) | 15, 77, 78, 79, 123, 141 | ||
| Malaysia | 1 | 1996 | 5-51 | WHO 1973 | 28 | 64 | 117 | ||
| Pacific | Philippines | 1 | 1995 | 5-67 | WHO 1973 | 28 | 38 | 14 | |
| Solomon Islands | 1 | 1994-1996 | 5-14 | WHO 1973 | 7 | 23 | 100 | ||
| Indian subcontinent | Bangladesh | 1 | 1996-1997 | 12-60 | WHO 1973 | 28 | 77 | 66 | 170 |
| India | 1 | 2000 | 16-72 | WHO 1973 | 7 | 82 | 81 | ||
| Pakistan | 1 | 1994 | All | WHO 1973 | 28 | 46 | 179 | ||
| Middle East | Afghanistan | 3 | 1999 | >1 | WHO 1973 | 28 | 69 (60-83) | 169 | |
| Saudi Arabia | 1 | 1997-1998 | 1-75 | WHO 1973 | 14 | 12 | 80 | ||
| Saudi Arabia | 1 | 1997-1998 | 1-12 | WHO 1973 | 28 | 18 | 2 |
CQ-PF, CQ parasitologic failure (either R1-R3 or cumulative parasite failure); CQ-TF, CQ clinical failure (either ETF + LTF or cumulative clinical failure).
TABLE 5.
P. falciparum CQ treatment failure in vivo for selected countries in South America, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median %CQ-PF (range)a | Median %CQ-TTF (range)a | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| South America/Caribbean | Colombia | 3 | 1998 | 1-65 | WHO | 14, 28 | 50 (44-56) | 44 (25-61) | 37, 156 |
| Guyana | 1 | 1998 | >5 | Day 7, 14, and 28 cumulative parasite failure | 28 | 33, 48, 56 | 16 | ||
| Venezuela | 1 | 1999 | All | WHO, 14, 28 | 28 | 100 | 1 | ||
| Venezuela | 2 | 1999 | 14-60 | WHO, 14, 28 | 28 | 34 (20-47) | 35 |
CQ-PF, CQ parasitologic failure (either R1-R3 or cumulative parasite failure); CQ-TTF, CQ clinical failure (either ETF + LTF or cumulative clinical failure).
In Africa, the median SP-TF varied from 0 to 35% and the median SP parasitologic failure (SP-PF) ranged from 0 to 76% (Table 6). In Asia the SP-TF varied from 9 to 35%, and in South America it varied from 3 to 32% (Table 7). The SP-PF was higher and varied between 5 and 67% in Asia and between 6 and 20% in South America (Table 8).
TABLE 6.
P. falciparum SP treatment failure in vivo for selected countries in Africa, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median % SP-PF (range)a | Median % SP-TTF (range)a | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| Central | Gabon | 1 | 1999 | <10 | WHO 1973 | 28 | 30 | 2 | 49 |
| Malawi | 5 | 1994-1998 | 0.5-5 | WHO 1973 | 28 | 10 (0-35) | 120, 148, 198, 213 | ||
| Malawi | 2 | 1994-1998 | 0.5-5 | WHO 1996 | 14 | 9 (0-19) | 119, 213 | ||
| Zambia | 3 | 1995-2000 | 0.5-5 | WHO 1973, 1996 | 7, 14 | 55 (30-70) | 3 | 18, 23 | |
| Eastern and great lakes | Kenya | 22 | 1998-2002 | 0.5-5 | WHO 1996 | 14 | 8 (0-52) | www.eanmat.org | |
| Kenya | 6 | 1998-2002 | <12 | WHO 1973 | 7 | 23 (13-38) | 56, 82, 150, 153 | ||
| Rwanda | 3 | 1999-2000 | 0.5-5 | WHO 1996 | 14 | 35 (12-36) | www.eanmat.org | ||
| Tanzania | 11 | 1999-2000 | 0.5-5 | WHO 1996 | 14 | 10 (1-34) | www.eanmat.org | ||
| Tanzania | 1 | 1994-1994 | 1-10 | WHO 1973 | 7 | 74 | 178 | ||
| Tanzania | 1 | 1997-1997 | 1-10 | WHO 1973 | 14 | 26 | 82 | ||
| Tanzania | 3 | 1996-1999 | 1-10 | WHO 1973 | 28 | 45 (31-92) | 122, 139, 218 | ||
| Uganda | 16 | 1996-2002 | 0.5-5 | WHO 1973, 1996 | 14 | 17 (0-73) | 10 (0-25) | (57, 104, 111, 188, 194), Mpeka and Ndezil, (1996), personal communication; Kilian 1998, (Uganda MoH report); Priotto, personal communication, www.eanmat.org. | |
| Zanzibar | 2 | 2001 | 0.5-5 | WHO 1996 | 14 | 9 (5-13) | www.eanmat.org | ||
| South | South Africa | 3 | 1999-2001 | All | WHO 1973 | 42 | 37 (6-92) | 30, 83, 119 | |
| West | Burkina Faso | 1 | 1998-2000 | 0.5-5 | WHO 1973, 1996 | 14 | 0.6 | 0.3 | 204 |
| Cameroon | 1 | 2002 | <10 | WHO 1996 | 14 | 0 | 0 | 22 | |
| Cameroon | 1 | 2002 | <10 | WHO 1996 | 28 | 10.3 | 15.3 | 22 | |
| The Gambia | 2 | 1994-1996 | 0.5-10 | Parasite failure at day 7 | 7 | 5 (3-6) | 28, 138 | ||
| The Gambia | 2 | 1998-2001 | 0.4-18 | WHO 1973, 1996 | 28 | 3 | 3 | 55, 215 | |
| Ghana | 1 | 1999-2000 | 0.5-5 | WHO 1973, 1996 | 28 | 29 | 19 | 59 | |
| Nigeria | 2 | 1997-2001 | 1-10 | WHO 1973 | 14 | 32 (24-40) | 30 | 69, 192 | |
| Nigeria | 1 | 1997-2001 | All | WHO 1973 | 14 | 29 | 68 | ||
| Senegal | 1 | 1996-1997 | 14-45 | WHO 1973 | 28 | 0 | 176 | ||
| Northern | Somalia | 1 | 1997 | All | WHO 1996 | 14 | 76 | 2 | 217 |
SP-PF, SP parasitologic failure (either R1-R3 or cumulative parasite failure); SP-TTF, SP clinical failure (either ETF + LTF or cumulative clinical failure).
TABLE 7.
P. falciparum SP treatment failure in vivo for selected countries in Asia, 1996 to 2002
| Region | Country | No. of data points identified | Period | Age range (yr) | Outcome classification | Period of follow-up (days) | Median %SP-PF (range)a | Median %SP-TTF (range)a | Source or reference |
|---|---|---|---|---|---|---|---|---|---|
| East Asia | Indonesia | 2 | 1992-1999 | All | WHO 1973 | 28 | 43 (15-71) | 9 | 76, 124 |
| Malaysia | 1 | 1996 | 5-51 | WHO 1973 | 28 | 47 | 117 | ||
| Myanmar | 2 | 1995 | All | Day 28 clinical failure, WHO 1973 | 28 | 67 (64-70) | 19 (18-20) | 190 | |
| Myanmar | 1 | 1995 | All | WHO 1996 | 14 | 35 | 62 | ||
| Pakistan | 1 | 1994 | All | WHO 1973 | 28 | 5 | 179 |
SP-PF, SP parasitologic failure (either R1-R3 or cumulative parasite failure); SP-TTF, SP clinical failure (either ETF + LTF or cumulative clinical failure).
The recent data show the high prevalence of CQ resistance (TTF > 25%) in the eastern/great lakes and central/southern regions of Africa, where SP resistance is emerging. Such a situation could quickly lead to multidrug resistance, particularly if SP is adopted widely as first-line treatment. The CQ and SP data summarized here have important implications for the choice of the first-line antimalaral drug after CQ. For example, Malawi was the first African country to change from CQ to SP in 1993; Table 3 clearly shows that there are no recent CQ in vivo studies in Malawi because these would be unethical. However, there are interesting in vitro data from Malawi suggesting a significant increase in CQ chemosensitivity, probably a consequence of the removal of CQ from public and private drug outlets (197).
Furthermore, a recent study of the trends of the prevalence of pfcrt codon 76 and pfmdr1 mutant alleles linked to CQ resistance suggests their significant decline over the period 1992 to 2000 (110). The molecular data suggest that a return to CQ use would probably quickly reselect resistant genotypes. However, such observations have important public health implications because they offer hope that concerted efforts to withdraw a failing drug could be associated with a retrieved sensitivity. This could allow the reintroduction of a modified CQ in combination with efficacious drugs like the artemisinin derivatives. Trend analyses like those reported by Kublin et al. (110) are needed in other areas to support this assertion.
DYNAMICS FOR EMERGENCE AND SPREAD OF ANTIMALARIAL DRUG RESISTANCE
The factors responsible for the emergence and rate of spread of parasite resistance are not fully known. What is clear, though, is that parasite resistance can occur for any antimalarial drug and that drug selection pressure is a critical and essential prerequisite for the development of resistance. However, what determines the rate at which resistance spreads is still a matter for scientific investigation. Several theoretical models have been proposed (46, 48, 89, 93, 95, 121, 135, 227). However, except for the role of the drug selective pressure, these models are not consistent and the predictions obtained are contradictory. The various factors that have been considered in the models include the degree of drug use, the drug elimination half-life, host heterogeneity (95), parasite biomass (93, 227), malaria transmission intensity (93, 135) and its proxy factors such as the number of parasite clones in a single infection (clone multiplicity), the immunity of hosts, and intrahost dynamics (89). We have tried to identify most of the factors cited in the published literature.
Drug Use
Circumstantial evidence for the role of mass drug administration in the emergence of antimalarial drug resistance was highlighted by Payne (159), who observed that resistance to CQ appears to have developed from different sites whose common denominator was the long-term use of CQ for either prophylaxis or for treatment. Several years later, studies at the Kenyan coast, Malawi, Mali, and Bolivia have observed a positive correlation between the pattern of drug use and in vitro parasite resistance or the prevalence of mutations linked to resistance (51, 150; Pinotti, Abstr. Fifth Congr. Trop. Med. Malaria). A recent study in Uganda (200) observed that the prevalence of CQ resistance was higher in sites with a high frequency of CQ use (measured as the percentage of people with detectable CQ metabolites in the urine). However, SP resistance was highest in sites with relatively low SP use but with high transmission, suggesting the role of other factors in addition to drug pressure in the spread of drug resistance.
Drug Elimination Half-Life
The role of drug elimination half-life in the evolution of parasite resistance has recently been reviewed and modeled (95). Readily absorbed drugs with a long elimination half-life, such as mefloquine and SP, have multiple therapeutic advantages. Patient compliance is improved because these treatments are given either as a single dose, or as a short regimen, which can be directly observed by the clinic staff. Moreover, residual drug levels during the post-therapeutic period offer a certain protection against the reemergence of parasitemia for several weeks (up to 8 weeks for SP) and may help patients to recover from anemia, a major cause of ill health and death in areas of intense malaria transmission. However, these drugs are likely to exert undesirable drug selection pressure for the period when their concentrations drop below a critical threshold that can still prevent reinfection by sensitive parasites but not by resistant ones. This has been shown in Kenya, where a potent selective pressure for resistance operates even under conditions of supervised drug administration and optimal dosage (220). P. falciparum infections appearing between days 15 and 52 after SP treatment were more likely to be resistant to pyrimethamine in vitro. Although drugs with a long elimination half-life benefit the individual, they create a potent selective pressure that can accelerate the evolution of resistance.
One of the recent developments in the management of uncomplicated malaria in African countries with endemic infection that needs a cautious approach is the strategy of home-based management of fevers spearheaded by WHO. This strategy has been recently launched in Uganda and will probably become a key strategy for many sub-Saharan countries in Africa. It aims at increasing access to prepackaged first-line drugs and promptly treating febrile children. In settings where physical access to formal health care is difficult, such a strategy can decrease childhood mortality by decreasing the risk of uncomplicated malaria cases evolving into the severe form of the disease. However, home-based management of fevers will inevitably be associated with the supply and distribution of large quantities of CQ+SP, the current first-line drug in Uganda, to the most peripheral level. Although CQ has a long terminal half-life (1 to 2 months), the period of therapeutically useful concentrations in the blood is much shorter than that for SP (95, 227). Such a large-scale presumptive treatment project will increase drug use and the percentage of individuals with subtherapeutic levels of SP. This will probably result in increased selective pressure on the parasite populations that could contribute to the rapid emergence of resistance. It is crucial that such a project incorporate a component for monitoring drug resistance.
Malaria Transmission Intensity and Its Proxy Factors
There are contrasting views on whether malaria transmission plays an independent role in the spread of resistance. Babiker and Walliker (12) have suggested that intensity of transmission is an important determinant of drug resistance as a result of its relationship to clone multiplicity. As transmission intensity increases, the number of parasite clones per infected individual increases (10). However, such an increase is probably nonlinear, and a plateau is reached at approximately 50 infectious bites/person/year. There are presently three contrasting theories on the role of malaria transmission. The first suggests that low transmission intensity increases the spread of resistance (89, 228) because monoclonal infections are common and sexual recombination, responsible for the breakdown of spontaneously arising resistant genotypes, is less likely (101). Consequently, self-fertilization (selfing) by gametocytes from the same parasite clone (151) occurs more often, increasing the probability that a combination of resistant mutations will be transmitted to the next generation of parasites (93). Furthermore, most infections will progress to clinical disease as a consequence of low immunity and the proportion of treated infections will be higher (177), increasing the contact between the parasite population and the drugs and thus the drug pressure (89, 93, 228). In addition, the frequency of parasite infections with a large biomass (see below) is probably higher in low-transmission areas because of low population immunity. The second theory suggests that resistant parasites spread faster when transmission is high if intrahost dynamics (crowding effects) exist (89, 90, 93). It assumes that the density-dependent constraints on parasite survival or establishment, a remarkable feature of the population biology of helminth species (6), apply also to P. falciparum. If this were true, the high transmission that is associated with higher clone multiplicity could facilitate efficient recolonization by resistant parasites after the elimination of susceptible ones following chemotherapy (89). The third theory states that transmission intensity plays no role in the early stages of the evolution of parasite resistance (89, 95). Theoretical predictions based on mathematical models of this important issue are therefore not consistent and depend on underlying assumptions of P. falciparum population biology. The effect of transmission intensity on the spread of drug resistance seems to be complex, and the net result is probably a balance between the effects of sexual recombination, intrahost dynamics, and the number of genes involved. Resistance might spread faster at the extremes of the transmission spectrum if two or more genes are involved, while spread of resistance could be faster in areas of high transmission if only one gene encodes resistance (91). However, malaria transmission indirectly affects drug use, and a high frequency of drug use could also result in a faster spread in low-transmission areas when only one gene is involved. A field study carried out in seven Ugandan sentinel sites observed the highest CQ resistance at the extremes of transmission intensity. However, SP resistance was higher in the sites with the most intense transmission (200), an observation supporting the direct and indirect roles of malaria transmission in the spread of resistance. The study in Uganda also provides indirect evidence for the role of intrahost dynamics in the population biology of P. falciparum. Most inferences about the role of transmission intensity are based on mathematical models. We are aware of only one study (200) that has attempted to determine the role of transmission intensity under field conditions. However, transmission intensity is an ecological exposure and the identification of an appropriate study design to prove its role is problematic.
Parasite Biomass
Mutant parasites are statistically more likely to occur in infections with a large number of parasites. It has been proposed that large parasite biomass infections are more common in nonimmune individuals, who can clear mutant parasites less easily than those with a partial immunity (228). The failure to clear parasites by the nonimmune individuals is supported by the higher prevalence of infections with mutations linked to CQ resistance or higher CQ treatment failure observed in African children compared to adults (53, 56, 199). Large-parasite-biomass infections are thought to be very important for the de novo generation of gene mutation (226). Although, the parasite biomass theory is statistically attractive, proving it will probably depend on indirect epidemiological observations because total parasite biomass is difficult to measure accurately. The peripheral parasite density is a poor proxy for total parasite biomass because the former is confounded by several factors such as the age group studied and the sequestration of parasites in organs such as the spleen, the brain, and the microvessels. Indeed the effects of sequestration are best observed in pregnant women who may have large-parasite-biomass infections with parasites sequestered in the placenta and a negative blood slide by microscopy examination of the peripheral blood.
Migration of Humans or Vectors
Population or vector movements could introduce new and resistant parasite genotypes that could be rapidly selected according to the amount of drug use. The spread of multidrug-resistant malaria around the Thai-Burmese border might have been the result of population movements as well as the occurrence of CQ resistance on the eastern coast of Africa from a focus in Southeast Asia. Indeed, it has been proposed that the genetic events that confer resistance to CQ are so rare (226) that they might have occurred in few foci around the world and then spread as a result of human or vector movements.
Genetic Basis of Resistance
The emergence and spread of drug resistance will be faster if the number of mutations required to encode resistance is low and their effects on parasite fitness are minimal (227). For example, the emergence and development of resistance to atovaquone occurs rapidly because it needs only a single mutation in the gene encoding cytochrome P450 2C19. Similarly, in vitro resistance to pyrimethamine requires only a single mutation at codon 108 in the dhfr gene. Conversely, resistance is slower to emerge if the genetic basis involves more than one gene. Furthermore, the rate of evolution depends on whether the effects of each subsequent mutation are additive (independent) or epistatic (all the relevant mutations are required) (90). It has been suggested that resistance to SP developed very fast because the dhfr mutations that confer a selective advantage are additive. The rapid development of SP resistance is consistent with both monogenic and additive mechanisms. The mutations in the dhps gene might modulate parasite tolerance to the drug. Conversely, the observation that CQ resistance took longer to develop suggests that the genetic basis for CQ resistance involves two or more genes. However, it is also consistent with multiple mutations on the same gene, all of them required (epistatic basis).
PUBLIC HEALTH IMPORTANCE OF ANTIMALARIAL DRUG RESISTANCE
Morbidity, Mortality, and Drug Resistance
In Senegal, a 2- to 11-fold increase in malaria-specific mortality in children aged 0 to 4 years has been associated with CQ resistance (207, 208). Initially, in vivo resistance is observed as an increased number of RI recrudescences among nonimmune individuals and young children with low acquired immunity. As resistance increases, the time between treatment and recrudescence shortens and RII/RIII parasitologic failure appears. Eventually, the lack of resolution of clinical symptoms occurs in high-grade resistance. In children with clinical malaria treated with a failing drug, the period of clinical improvement is shortened and there is no hematological recovery (26, 27). The latter increases the risk of severe anemia and consequently the risk for human immunodeficiency virus transmission through blood transfusions. In some African countries in the early 1990s, blood transfusions accounted for approximately one-quarter of the human immunodeficiency virus infections in children (184). Drug resistance also increases the number of clinically ill patients who come in contact with the health services. For example, in the former Zaire, an increase in the number of hospitalizations for malaria and of the specific case fatality rate (CFR) was observed when CQ resistance increased (36, 84). In Malawi, the hospital-based CFR for malaria increased when CQ started failing (Malawi Ministry of Health, unpublished data). Similarly, in Ugandan hospitals the average CFR for malaria increased from 3% in 1990 to about 8% in 1998 (Uganda Health Bulletin, 2001), while in Kenya the CFR decreased by 60 to 70% when quinine or SP instead of CQ was used for treatment (235). Hospital-based observations such as the proportion of admissions or deaths are prone to spurious errors and biases because of variation in accessibility, reporting, and diagnostic criteria. Nevertheless, measures such as the CFR are directly related to the quality of the case management, including the efficacy and quality of the drugs used.
Malaria Epidemics and Drug Resistance
An epidemic is a dramatic increase of the disease incidence in a defined population at a specific time. Epidemic thresholds have been established for several infectious diseases. However, for endemic diseases that have seasonal variations, such as malaria, the determination of an epidemic threshold is not obvious. Although the recognition of a large malaria epidemic does not pose any problem, small epidemics could easily be confused for seasonal fluctuations and vice versa. Theoretically, a failing drug that does not clear parasitemia increases the infective reservoir and, in areas with previously low transmission, could lead to increased malaria transmission and malaria epidemics. Nonetheless, it is difficult to attribute the occurrence of an epidemic to the decreasing efficacy of a drug. There are few reports that identify drug resistance as the major reason for the occurrence of a malaria epidemic. In Balcad, Somalia, a town with previously low malaria transmission, the incidence of malaria rose more than 20-fold between 1986 and 1988 and the emergence of CQ resistance, coupled with favorable meteorological conditions, was identified as the cause (219). A similar phenomenon occurred in 1994 in Rajasthan, India, and resulted in many deaths (185). The recent epidemics in the highlands of Eastern Africa have been attributed to several factors such as drug resistance, long-term climate changes, and rapid meteorological change (98).
Increased Costs of Malaria Control
Drug resistance is of enormous importance to malaria control, especially in resource-constrained Africa, where alternatives to CQ and SP are limited due to cost. Each full adult treatment course of CQ or SP costs less than 20c. However, the cost is about 10-fold for quinine ($2.28) and mefloquine ($2.4), approximately 30 to 40 times higher for halofantrine ($7), and about 300 to 400 times higher for other efficacious drugs like Malarone (atovaquone plus proguanil) (40). The most heavily affected countries, which are also some of the poorest, now have to increase their health expenditure to cope with the higher cost.
PERSPECTIVES FOR THE FUTURE
The use of antimalarial drugs in combination might increase their efficacy and might also delay the emergence of resistance because the drugs may have different modes of action and different mechanisms for resistance (228).
Combination Therapy with an Artemisinin Derivative
Artemisinin-based combination therapy (ACT) is probably the best option available nowadays for the treatment of malaria (228). The artemisinin derivatives have several advantages such as rapid reduction in the parasite biomass, gametocytocidal properties that reduce gametocyte carriage and transmissibility, rapid elimination and consequently little selective pressure, and rapid clinical relief. Some Asian countries have adopted the combination artesunate-mefloquine (ART + MQ) as first-line treatment against P. falciparum malaria, a near-desperate therapeutic choice to confront multidrug resistance (230). Its use has halted the progression of MQ resistance and reduced the incidence of P. falciparum malaria, possibly because of its antigametocyte properties (147, 168). However, it is not clear whether the same results can be obtained in sub-Saharan Africa, where the intensity of transmission is much higher.
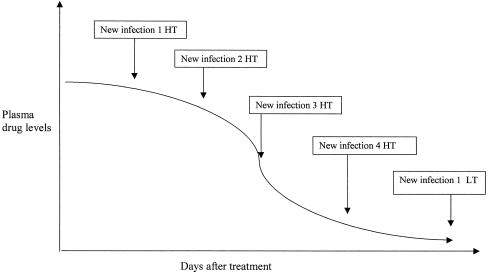
Most of the drugs that can be associated with the artemisinin derivatives have a much longer terminal half-life (t1/2). Probably such a mismatch does not have important consequences in areas of low to medium transmission; the artemisinin derivative rapidly reduces the parasite biomass, while the long-acting drug eliminates the residual parasitemia (228). However, in areas of intense transmission, new infections with mixed susceptible and partially or fully resistant parasites emerging after the artemisinin component has been eliminated could be exposed to subcritical levels of the partner drug (Fig. 1), and resistant parasites may be selected (95). If this were the case, the artemisinin component would not “protect” the partner drug.
FIG. 1.
Scheme showing plasma drug levels and new infections in high-transmission (HT) and low-transmission (LT) areas.
Several randomized trials supported by WHO/TDR (152) have reported a good efficacy of ACT when the partner drug had also a good efficacy. When this was not the case, as with ACT containing SP tested in areas of Africa with high SP resistance, the efficacy was lower than expected (179a, 194). Furthermore, a recent innovative longitudinal drug efficacy study with a 1-year follow-up has shown that the efficacy of SP combined with artesunate (SP + ART) is lower than that of SP plus amodiaquine (SP + AQ) if the follow-up is extended beyond 2 weeks (58). Such an observation can be explained by the lower efficacy of SP (parasite failure, 26 to 30%) as than of AQ (parasite failure, 16%) (104, 194). In areas of high transmission, the ideal ACT is likely to include a partner drug with a short elimination half-life. Presently, the only known drug that comes close to that description is chlorproguanil plus dapsone (Lapdap). However, a coformulated ACT with Lapdap is at least 2 to 3 years in the future.
Combination Therapy without an Artemisinin Derivative
Combination therapy without an artemisinin derivative (CT) is not new in malaria treatment (153). Current definitions of CT do not include SP, which is considered a single agent, because its activity is due to synergy between pyrimethamine and sulfadoxine rather than the independent action of the two drugs (24). In the early 1980s, when the efficacy of SP started to decline, countries in Southeast Asia such as Thailand adopted a combination of SP and mefloquine (MSP). No delay in the development of SP resistance was observed. In South America SP + CQ was adopted, but again resistance to such CT developed very fast (156). In Africa the efficacy of CQ + SP has been assessed in some studies but the combination has not been adopted for wide-scale use. The threat of multidrug-resistant malaria in the eastern and central African regions has obliged Rwanda and Uganda to adopt SP + AQ and SP + CQ, respectively, as interim policies. Such decisions were based mainly on the high cost of the artemisinin derivatives and the lack of data on the efficacy of possible ACTs. AQ and SP have matched elimination half-lives and could “protect” each other against resistance. Whether SP + AQ will have a long UTL remains to be seen, but the study by Dorsey et al. (58) offers some hope in terms of CT options that could be adopted as interim policies in resource-constrained countries in sub-Saharan Africa.
Although the epidemiology of malaria in South America and Southeast Asia is different from that in sub-Saharan Africa, the experiences and lessons learned from these areas need to be considered when changing treatment policies in Africa. The approach of combining a drug with very low therapeutic efficacy with one that is still efficacious did not delay the development of resistance to the partner drug. The level of therapeutic failure at which a drug ceases to protect the partner drug is not clearly known. However, most of the available data do not demonstrate a significant benefit of CQ + SP over SP monotherapy, although there seems to be a tendency toward faster resolution of clinical symptoms (131). There is only one study from Africa that reported a greater efficacy of CQ + SP than of SP mono-therapy (G. Dorsey, S. Staedke, N. Bakyaita, and P. J. Rosenthal, Abstr. 50th Annu. Meet. Am. Soc. Trop. Med. abstr. 591, 2001). Although CQ + SP may have relatively good efficacy, it is predicted that its UTL will be short-lived. Interim policies such as that adopted by Uganda could buy time for mobilization of the necessary resources before a CT with a good short- and long-term efficacy is adopted. However, the danger is that an interim policy might be replaced by another interim policy with disastrous consequences. Combining a low-efficacy drug such as CQ with another drug, such as SP, to which there is existing and rapidly developing resistance, is not the best policy to implement. These regimens will probably fail rapidly, but in the process SP and possibly other drugs with a similar basis for resistance like Lapdap could also be lost as partner drugs in CT. The notion of interim policies, especially when there is cross-resistance between drugs, might therefore be dangerous.
CONCLUSIONS
The challenge that antimalarial drug resistance poses to the health services, especially in Africa, is enormous. To deal with drug resistance, the most severely affected countries need to select the best affordable treatment option as quickly and cautiously as possible so as to avert deaths and also “protect” the drugs from the development of resistance. At the moment, CT offers the best potential option for providing efficacious treatment that should last for long time. However, the appropriate CT for sub-Saharan Africa is still unclear. Furthermore, CT raises several questions, such as its delivery at the most peripheral level, patient compliance, and the involvement of private practitioners. The implementation of CT will require innovative ways of delivering it to the peripheral health units, and operational research is required in this area. CT will also need innovative ways of confirming the diagnosis of malaria so as to reduce the number of patients treated unnecessarily. The rapid diagnostic tests that are currently available could be exploited for this purpose. However, CT and such tests will increase costs and the logistical constraints for the management of malaria cases. Furthermore, innovative approaches to validly documenting adverse drug reactions are required. Monitoring the potency and bioavailability of antimalarial drugs is an extremely important but difficult task that will need efficient drug regulatory bodies. Longitudinal studies to measure the long-term efficacy of CT and its impact on malaria transmission intensity in sub-Saharan Africa are also needed. These research themes will require cross-disciplinary collaborative partnerships between research institutions in the north and the south that are closely linked to government departments responsible for malaria control, public health surveillance, and public health policy. In Africa, three projects investigating some of these research themes are under way: the IMPACT project in Tanzania, the Uganda Malaria Surveillance Project, and the Southeast African Combination Antimalarial Therapy project. The faster implementation of research evidence into policy will be possible only when such partnership is established more often.
Acknowledgments
We thank the East Africa Network for Monitoring Antimalarial Treatment (EANMAT) for allowing us to use some of their in vivo efficacy data. We also thank colleagues who have made helpful comments on the manuscript, in particular Stella Alamo, Annette Enhart, Sarah Staedke, and Grant Dorsey.
Ambrose O. Talisuna is supported by Belgian Development Co-Operation in collaboration with the Institute of Tropical Medicine Antwerp (BEADC-ITM) and the Fonds voor Wetenschappeljk Onderzoek-Vlanderen (FWO).
REFERENCES
- 1.Ache, A., M. Escorihuela, E. Vivas, E. Paez, L. Miranda, A. Matos, W. Perez, O. Diaz, and E. Izarra. 2002. In vivo drug resistance of falciparum malaria in mining areas of Venezuela. Trop. Med. Int. Health 7:737-743. [DOI] [PubMed] [Google Scholar]
- 2.al Arishi, H. M., A. F. el Awad, and L. A. al Bishi. 2001. Chloroquine-resistant Plasmodium falciparum malaria among children seen in a regional hospital, Tabuk, Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 95:439-440. [DOI] [PubMed] [Google Scholar]
- 3.Alecrim, M. D., W. Alecrim, and V. Macedo. 1999. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 32:67-68. [DOI] [PubMed] [Google Scholar]
- 4.Alene, G. D., and S. Bennett. 1996. Chloroquine resistance of Plasmodium falciparum malaria in Ethiopia and Eritrea. Trop. Med. Int. Health 1:810-815. [DOI] [PubMed] [Google Scholar]
- 5.Anabwani, G. M., F. O. Esamai, and D. A. Menya. 1996. A randomised controlled trial to assess the relative efficacy of chloroquine, amodiaquine, halofantrine and Fansidar in the treatment of uncomplicated malaria in children. East Afr. Med. J. 73:155-158. [PubMed] [Google Scholar]
- 6.Anderson, R. M., and R. M. May. 1991. Infectious diseases of humans. Oxford University Press, Oxford, United Kingdom.
- 7.Reference deleted.
- 8.Antia-Obong, O. E., A. A. Alaribe, M. U. Young, A. Bassy, and B. V. Etim. 1997. Chloroquine-resistant Plasmodium falciparum among children in Calabar, south eastern Nigeria. Trop. Doct. 27:146-149. [DOI] [PubMed] [Google Scholar]
- 9.Arias, A. E., and A. Corredor. 1989. Low response of Colombian strains of Plasmodium vivax to classical antimalarial therapy. Trop. Med. Parasitol. 40:21-23. [PubMed] [Google Scholar]
- 10.Arnot, D. 1998. Unstable malaria in Sudan: the influence of the dry season. Clone multiplicity of Plasmodium falciparum infections in individuals exposed to variable levels of disease transmission. Trans. R. Soc. Trop. Med. Hyg. 92:580-585. [DOI] [PubMed] [Google Scholar]
- 11.Aronsson, B., E. Bengtsson, A. Bjorkman, P. O. Pehrson, L. Rombo, and M. Wahlgren. 1981. Chloroquine-resistant falciparum malaria in Madagascar and Kenya. Ann. Trop. Med. Parasitol. 75:367-373. [DOI] [PubMed] [Google Scholar]
- 12.Babiker, A., and D. Walliker. 1997. Current views on the population structure of Plasmodium falciparum: implications for control. Parasitol. Today 13:262-267. [DOI] [PubMed] [Google Scholar]
- 13.Babiker, H. A., S. J. Pringle, A. Abdel-Muhsin, M. Mackinnon, P. Hunt, and D. Walliker. 2001. High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J. Infect. Dis. 183:1535-1538. [DOI] [PubMed] [Google Scholar]
- 14.Baird, J. K., E. Caneta-Miguel, S. Masbar, D. G. Bustos, J. A. Abrenica, A. V. Layawen, J. M. Calulut, B. Leksana, and F. S. Wignall. 1996. Survey of resistance to chloroquine of falciparum and vivax malaria in Palawan, The Philippines. Trans. R. Soc. Trop. Med. Hyg. 90:413-414. [DOI] [PubMed] [Google Scholar]
- 15.Baird, J. K., M. F. Sustriayu Nalim, H. Basri, S. Masbar, B. Leksana, E. Tjitra, R. M. Dewi, M. Khairani, and F. S. Wignall. 1996. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:409-411. [DOI] [PubMed] [Google Scholar]
- 16.Baird, J. K., T. Tiwari, G. J. Martin, C. L. Tamminga, T. M. Prout, J. Tjaden, P. P. Bravet, S. Rawlins, M. Ferrel, D. Carucci, and S. L. Hoffman. 2002. Chloroquine for the treatment of uncomplicated malaria in Guyana. Ann. Trop. Med. Parasitol. 96:339-348. [DOI] [PubMed] [Google Scholar]
- 17.Baird, J. K., I. Wiady, D. J. Fryauff, M. A. Sutanihardja, B. Leksana, H. Widjaya, Kysdarmanto, and B. Subianto. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627-631. [DOI] [PubMed] [Google Scholar]
- 18.Barat, L. M., B. Himonga, S. Nkunika, M. Ettling, T. K. Ruebush, W. Kapelwa, and P. B. Bloland. 1998. A systematic approach to the development of a rational malaria treatment policy in Zambia. Trop. Med. Int. Health 3:535-542. [DOI] [PubMed] [Google Scholar]
- 19.Barduagni, P., U. Schwartz, W. Nyamayaro, and T. L. Chauke. 1998. In vivo testing of the therapeutic efficacy of chloroquine on falciparum malaria infections in Chirundu, Mashonaland West, Zimbabwe. Cent. Afr. J. Med. 44:251-254. [PubMed] [Google Scholar]
- 20.Basco, L. K., D. P. Eldin, C. M. Wilson, J. Le Bras, and A. Mazabraud. 1995. Point mutations in the dihydrofolate reductase-thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol. Biochem. Parasitol. 69:135-138. [DOI] [PubMed] [Google Scholar]
- 21.Basco, L. K., and P. Ringwald. 2001. Analysis of the key pfcrt point mutation and in vitro and in vivo response to chloroquine in Yaounde, Cameroon. J. Infect. Dis. 183:1828-1831. [DOI] [PubMed] [Google Scholar]
- 22.Basco, L. K., A. Same-Ekobo, V. F. Ngane, M. Ndounga, T. Metoh, P. Ringwald, and G. Soula. 2002. Therapeutic efficacy of sulfadoxine-pyrimethamine, amodiaquine and the sulfadoxine-pyrimethamine-amodiaquine combination against uncomplicated Plasmodium falciparum malaria in young children in Cameroon. Bull. W. H. O. 80:538-545. [PMC free article] [PubMed] [Google Scholar]
- 23.Bijl, H. M., J. Kager, D. W. Koetsier, and T. S. van der Werf. 2000. Chloroquine- and sulfadoxine-pyrimethamine-resistant Falciparum malaria in vivo—a pilot study in rural Zambia. Trop. Med. Int. Health 5:692-695. [DOI] [PubMed] [Google Scholar]
- 24.Bloland, P. 2001. Drug resistance in malaria. WHO monograph WHO/CDS/CSR/DRS/2001.4. World Health Organization, Geneva, Switzerland.
- 25.Bloland, P. B., and M. Ettling. 1999. Making malaria-treatment policy in the face of drug resistance. Ann. Trop. Med. Parasitol. 93:5-23. [DOI] [PubMed] [Google Scholar]
- 26.Bloland, P. B., P. N. Kazembe, A. J. Oloo, B. Himonga, L. M. Barat, and T. K. Ruebush. 1998. Chloroquine in Africa: critical assessment and recommendations for monitoring and evaluating chloroquine therapy efficacy in sub-Saharan Africa. Trop. Med. Int. Health 3:543-552. [DOI] [PubMed] [Google Scholar]
- 27.Bloland, P. B., E. M. Lackritz, P. N. Kazembe, J. B. Were, R. Steketee, and C. C. Campbell. 1993. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J. Infect. Dis. 167:932-937. [DOI] [PubMed] [Google Scholar]
- 28.Bojang, K. A., G. Schneider, S. Forck, S. K. Obaro, S. Jaffar, M. Pinder, J. Rowley, and B. M. Greenwood. 1998. A trial of Fansidar plus chloroquine or Fansidar alone for the treatment of uncomplicated malaria in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 92:73-76. [DOI] [PubMed] [Google Scholar]
- 29.Brasseur, P., R. Guiguemde, S. Diallo, V. Guiyedi, M. Kombila, P. Ringwald, and P. Olliaro. 1999. Amodiaquine remains effective for treating uncomplicated malaria in west and central Africa. Trans. R. Soc. Trop. Med. Hyg. 93:645-650. [DOI] [PubMed] [Google Scholar]
- 30.Bredenkamp, B. L., B. L. Sharp, S. D. Mthembu, D. N. Durrheim, and K. I. Barnes. 2001. Failure of sulphadoxine-pyrimethamine in treating Plasmodium falciparum malaria in KwaZulu-Natal. S. Afr. Med. J. 91:970-972. [PubMed] [Google Scholar]
- 31.Brockman, A., R. E. Paul, T. J. Anderson, I. Hackford, L. Phaiphun, S. Looareesuwan, F. Nosten, and K. P. Day. 1999. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am. J. Trop. Med. Hyg. 60:14-21. [DOI] [PubMed] [Google Scholar]
- 32.Bruce-Chwatt, L. J., R. H. Black, C. J. Canfield, D. F. Clyde, W. Peters, and W. Wernsdorfer, 1986. Chemotherapy of malaria. WHO Monogr. Ser. 2:27. [Google Scholar]
- 33.Bzik, D. J., W. B. Li, T. Horii, and J. Inselburg. 1987. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc. Natl. Acad. Sci. USA 84:8360-8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell, C. C., W. Chin, W. E. Collins, S. M. Teutsch, and D. M. Moss. 1979. Chloroquine-resistant Plasmodium falciparum from East Africa: cultivation and drug sensitivity of the Tanzanian I/CDC strain from an American tourist. Lancet ii:1151-1154. [DOI] [PubMed] [Google Scholar]
- 35.Caraballo, A., and A. Rodriguez-Acosta. 1999. Chemotherapy of malaria and resistance to antimalarial drugs in Guayana area, Venezuela. Am. J. Trop. Med. Hyg. 61:120-124. [DOI] [PubMed] [Google Scholar]
- 36.Carme, B., H. Guillo du Bodan, and M. Lallemant. 1992. Infant and child mortality and malaria in the Congo. The trend in the suburbs of Brazzaville between 1981 and 1988. Trop. Med. Parasitol. 43:177-180. [PubMed] [Google Scholar]
- 37.Castillo, C. M., L. E. Osorio, and G. I. Palma. 2002. Assessment of therapeutic response of Plasmodium vivax and Plasmodium falciparum to chloroquine in a Malaria transmission free area in Colombia. Mem. Inst. Oswaldo Cruz 97:559-562. [DOI] [PubMed] [Google Scholar]
- 38.Cattamanchi, A., D. Kyabayinze, A. Hubbard, P. J. Rosenthal, and G. Dorsey. 2003. Distinguishing recrudescence from re-infection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of MSP1, MSP2 and GLURP. Am. J. Trop. Med. Hyg. 68:133-139. [PubMed] [Google Scholar]
- 39.Chen, N., B. Russell, J. Staley, B. Kotecka, P. Nasveld, and Q. Cheng. 2001. Sequence polymorphisms in pfcrt are strongly associated with chloroquine resistance in Plasmodium falciparum. J. Infect. Dis. 183:1543-1545. [DOI] [PubMed] [Google Scholar]
- 40.Childs, G. E., T. Wimonwattrawatee, and N. Pooyindee. 1988. Evaluation of an in vitro assay system for drug susceptibility of field isolates of Plasmodium falciparum from southern Thailand. Am. J. Trop. Med. Hyg. 38:19-23. [DOI] [PubMed] [Google Scholar]
- 41.Clarke, D., H. Odialla, J. Ouma, V. Kenny, R. MacCabe, B. Rapuoda, and W. M. Watkins. 1996. A malariometric survey in Turkana District, Kenya: chemosensitivity in vivo of Plasmodium falciparum infections and identity of the vector. Trans. R. Soc. Trop. Med. Hyg. 90:302-304. [DOI] [PubMed] [Google Scholar]
- 42.Clyde, D. F. 1966. Drug resistance of malari parasites in Tanzania. East Afr. Med J. 43:405-408. [PubMed] [Google Scholar]
- 43.Coatney, G. R. 1963. Pitfalls in a discovery: the chronicle of chloroquine. Am. J. Trop. Med. Hyg. 12:121-128. [DOI] [PubMed] [Google Scholar]
- 44.Collins, W. E., and G. M. Jeffery. 2002. Extended clearance time after treatment of infections with Plasmodium malariae may not be indicative of resistance to chloroquine. Am. J. Trop. Med. Hyg. 67:406-410. [DOI] [PubMed] [Google Scholar]
- 45.Cowman, A. F., M. J. Morry, B. A. Biggs, G. A. Cross, and S. J. Foote. 1988. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:9109-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cross, A. P., and B. Singer. 1991. Modelling the development of resistance of Plasmodium falciparum to anti-malarial drugs. Trans. R. Soc. Trop. Med. Hyg. 85:349-355. [DOI] [PubMed] [Google Scholar]
- 47.Curtis, C. F., and J. D. Lines. 1985. Impregnated fabrics against malaria mosquitoes. Parasitol. Today 1:147. [DOI] [PubMed] [Google Scholar]
- 48.Curtis, C. F., and L. N. Otoo. 1986. A simple model of the build-up of resistance to mixtures of anti- malarial drugs. Trans. R. Soc. Trop. Med. Hyg. 80:889-892. [DOI] [PubMed] [Google Scholar]
- 49.Deloron, P., J. Mayombo, A. Le Cardinal, J. Mezui-Me-Ndong, C. Bruzi-Baert, F. Lekoulou, and N. Elissa. 2000. Sulfadoxine-pyrimethamine for the treatment of Plasmodium falciparum malaria in Gabonese children. Trans. R. Soc. Trop. Med. Hyg. 94:188-190. [DOI] [PubMed] [Google Scholar]
- 50.de Pecoulas, P. E., L. K. Basco, J. Le Bras, and A. Mazabraud. 1996. Association between antifol resistance in vitro and DHFR gene point mutation in Plasmodium falciparum isolates. Trans. R. Soc. Trop. Med. Hyg. 90:181-182. [DOI] [PubMed] [Google Scholar]
- 51.Diourte, Y., A. Djimde, O. K. Doumbo, I. Sagara, Y. Coulibaly, A. Dicko, M. Diallo, M. Diakite, J. F. Cortese, and C. V. Plowe. 1999. Pyrimethamine-sulfadoxine efficacy and selection for mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase in Mali. Am. J. Trop. Med. Hyg. 60:475-478. [DOI] [PubMed] [Google Scholar]
- 52.Di Perri, G., P. Olliaro, S. Nardi, R. Deganello, B. Allegranzi, S. Bonora, S. Vento, and E. Concia. 1998. Response of uncomplicated falciparum malaria to oral chloroquine and quinine in Burundi highlands. Acta Trop. 70:25-33. [DOI] [PubMed] [Google Scholar]
- 53.Djimde, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourte, A. Dicko, X. Z. Su, T. Nomura, D. A. Fidock, T. E. Wellems, C. V. Plowe, and D. Coulibaly. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 54.Djimde, A., O. K. Doumbo, R. W. Steketee, and C. V. Plowe. 2001. Application of a molecular marker for surveillance of chloroquine- resistant falciparum malaria. Lancet 358:890-891. [DOI] [PubMed] [Google Scholar]
- 55.Doherty, J. F., A. D. Sadiq, L. Bayo, A. Alloueche, P. Olliaro, P. Milligan, L. von Seidlein, and M. Pinder. 1999. A randomized safety and tolerability trial of artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone for the treatment of uncomplicated malaria in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 93:543-546. [DOI] [PubMed] [Google Scholar]
- 56.Dorsey, G., M. R. Kamya, G. Ndeezi, J. N. Babirye, C. R. Phares, J. E. Olson, E. T. Katabira, and P. J. Rosenthal. 2000. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am. J. Trop. Med. Hyg. 62:686-692. [DOI] [PubMed] [Google Scholar]
- 57.Dorsey, G., M. R. Kamya, A. Singh, and P. J. Rosenthal. 2001. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J. Infect. Dis. 183:1417-1420. [DOI] [PubMed] [Google Scholar]
- 58.Dorsey, G., D. Njama, M. R. Kamya, A. Cattamanchi, D. Kyabayinze, S. G. Staedke, A. Gasasira, and P. J. Rosenthal. 2002. Sulfadoxine/pyrimethamine alone or with amodiaquine or artesunate for treatment of uncomplicated malaria: a longitudinal randomised trial. Lancet 360:2031-2038. [DOI] [PubMed] [Google Scholar]
- 59.Driessen, G. J., S. van Kerkhoven, B. J. Schouwenberg, G. Bonsu, and J. P. Verhave. 2002. Sulphadoxine/pyrimethamine: an appropriate first-line alternative for the treatment of uncomplicated falciparum malaria in Ghanaian children under 5 years of age. Trop. Med. Int. Health 7:577-583. [DOI] [PubMed] [Google Scholar]
- 60.Druilhe, P., A. Moreno, C. Blanc, P. H. Brasseur, and P. Jacquier. 2001. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 64:233-241 [DOI] [PubMed] [Google Scholar]
- 61.Durand, R., S. Jafari, O. Bouchaud, P. Ralaimazava, A. Keundjian, and J. Le Bras. 2001. Plasmodium falciparum: pfcrt and DHFR mutations are associated with failure of chloroquine plus proguanil prophylaxis in travelers. J. Infect. Dis. 184:1633-1634. [DOI] [PubMed] [Google Scholar]
- 62.Dzekunov, S. M., L. M. Ursos, and P. D. Roepe. 2000. Digestive vacuolar pH of intact intraerythrocytic P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 110:107-124. [DOI] [PubMed] [Google Scholar]
- 63.Ehrhardt, S., F. P. Mockenhaupt, P. Agana-Nsiire, A. Mathieu, S. D. Anemana, K. Stark, R. N. Otchwemah, and U. Bienzle. 2002. Efficacy of chloroquine in the treatment of uncomplicated, Plasmodium falciparum malaria in northern Ghana. Ann. Trop. Med. Parasitol. 96:239-247. [DOI] [PubMed] [Google Scholar]
- 64.Ejov, M. N., T. Tun, S. Aung, and K. Sein. 1999. Response of falciparum malaria to different antimalarials in Myanmar. Bull. W. H. O. 77:244-249. [PMC free article] [PubMed] [Google Scholar]
- 65.Ekue, J. M., A. M. Ulrich, and E. K. Njelesani. 1983. Plasmodium malaria resistant to chloroquine in a Zambian living in Zambia. Br. Med. J. Clin. Res. Ed. 286:1315-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekvall, H., Z. Premji, and A. Bjorkman. 1998. Chloroquine treatment for uncomplicated childhood malaria in an area with drug resistance: early treatment failure aggravates anaemia. Trans. R. Soc. Trop. Med. Hyg. 92:556-560. [DOI] [PubMed] [Google Scholar]
- 67.Elliotson, J. 1844. The principles and practice of medicine. Carey and Hart, Philadelphia, Pa.
- 68.Ezedinachi, E. 1996. In vivo efficacy of chloroquine, halofantrine, pyrimethamine-sulfadoxine and qinghaosu (artesunate) in the treatment of malaria in Calabar, Nigeria. Cent. Afr. J. Med. 42:109-111. [PubMed] [Google Scholar]
- 69.Falade, C. O., L. A. Salako, A. Sowunmi, A. M. Oduola, and P. Larcier. 1997. Comparative efficacy of halofantrine, chloroquine and sulfadoxine-pyrimethamine for treatment of acute uncomplicated falciparum malaria in Nigerian children. Trans. R. Soc. Trop. Med. Hyg. 91:58-62. [DOI] [PubMed] [Google Scholar]
- 70.Farnert, A., A. P. Arez, H. A. Babiker, H. P. Beck, A. Benito, A. Bjorkman, M. C. Bruce, D. J. Conway, K. P. Day, L. Henning, O. Mercereau-Puijalon, L. C. Ranford-Cartwright, J. M. Rubio, G. Snounou, D. Walliker, J. Zwetyenga, and V. E. do Rosario. 2001. Genotyping of Plasmodium falciparum infections by PCR: a comparative multicentre study. Trans. R. Soc. Trop. Med. Hyg. 95:225-232. [DOI] [PubMed] [Google Scholar]
- 71.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. Ursos, A. B. Sidhu, B. Naude, K. W. Deitsch, X. Z. Su, J. C. Wootton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fogh, S., S. Jepsen, and P. Effersoe. 1979. Chloroquine-resistant Plasmodium falciparum malaria in Kenya. Trans. R. Soc. Trop. Med. Hyg. 73:228-229. [DOI] [PubMed] [Google Scholar]
- 73.Fogh, S., S. Jepsen, and R. H. Mataya. 1984. R-III chloroquine-resistant Plasmodium falciparum malaria from northern Malawi. Trans. R. Soc. Trop. Med. Hyg. 78:282. [DOI] [PubMed] [Google Scholar]
- 74.Foote, S. J., and A. F. Cowman. 1994. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Trop. 56:157-171. [DOI] [PubMed] [Google Scholar]
- 75.Foote, S. J., D. E. Kyle, R. K. Martin, A. M. Oduola, K. Forsyth, D. J. Kemp, and A. F. Cowman. 1990. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature 345:255-258. [DOI] [PubMed] [Google Scholar]
- 76.Fryauff, D. J., B. Leksana, S. Masbar, I. Wiady, P. Sismadi, A. I. Susanti, H. S. Nagesha, Syafruddin, S. Atmosoedjono, M. J. Bangs, and J. K. Baird. 2002. The drug sensitivity and transmission dynamics of human malaria on Nias Island, North Sumatra, Indonesia. Ann. Trop. Med. Parasitol. 96:447-462. [DOI] [PubMed] [Google Scholar]
- 77.Fryauff, D. J., Soekartono, S. Tuti, B. Leksana, Suradi, S. Tandayu, and J. K. Baird. 1998. Survey of resistance in vivo to chloroquine of Plasmodium falciparum and P. vivax in North Sulawesi, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 92:82-83. [DOI] [PubMed] [Google Scholar]
- 78.Fryauff, D. J., I. Sumawinata, Purnomo, T. L. Richie, E. Tjitra, M. J. Bangs, A. Kadir, and G. Ingkokusumo. 1999. In vivo responses to antimalarials by Plasmodium falciparum and Plasmodium vivax from isolated Gag Island off northwest Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 60:542-546. [DOI] [PubMed] [Google Scholar]
- 79.Fryauff, D. J., S. Tuti, A. Mardi, S. Masbar, R. Patipelohi, B. Leksana, K. C. Kain, M. J. Bangs, T. L. Richie, and J. K. Baird. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513-518. [DOI] [PubMed] [Google Scholar]
- 80.Ghalib, H. W., S. Al Ghamdi, M. Akood, A. E. Haridi, A. A. Ageel, and R. E. Abdalla. 2001. Therapeutic efficacy of chloroquine against uncomplicated, Plasmodium falciparum malaria in south-western Saudi Arabia. Ann. Trop. Med. Parasitol. 95:773-779. [DOI] [PubMed] [Google Scholar]
- 81.Gogtay, N. J., S. Desai, V. S. Kadam, K. D. Kamtekar, S. S. Dalvi, and N. A. Kshirsagar. 2000. A randomized, parallel-group study in Mumbai (Bombay), comparing chloroquine with chloroquine plus sulfadoxine-pyrimethamine in the treatment of adults with acute, uncomplicated, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 94:309-312. [DOI] [PubMed] [Google Scholar]
- 82.Gorissen, E., G. Ashruf, M. Lamboo, J. Bennebroek, S. Gikunda, G. Mbaruku, and P. A. Kager. 2000. In vivo efficacy study of amodiaquine and sulfadoxine/ pyrimethamine in Kibwezi, Kenya and Kigoma, Tanzania. Trop. Med. Int. Health 5:459-463. [DOI] [PubMed] [Google Scholar]
- 83.Govere, J. M., J. J. la Grange, D. N. Durrheim, J. A. Freese, B. L. Sharp, A. Mabuza, N. Mugomezulu, and B. L. Bredenkamp. 1999. Sulfadoxine-pyrimethamine effectiveness against Plasmodium falciparum malaria in Mpumalanga Province, South Africa. Trans. R. Soc. Trop. Med. Hyg. 93:644. [DOI] [PubMed] [Google Scholar]
- 84.Greenberg, A. E., M. Ntumbanzondo, N. Ntula, L. Mawa, J. Howell, and F. Davachi. 1989. Hospital-based surveillance of malaria-related paediatric morbidity and mortality in Kinshasa, Zaire. Bull. W. H. O. 67:189-196. [PMC free article] [PubMed] [Google Scholar]
- 85.Greenwood, B. 2002. The molecular epidemiology of malaria. Trop. Med. Int. Health 7:1012-1021. [DOI] [PubMed] [Google Scholar]
- 86.Grimmond, T. R., K. O. Donovan, and I. D. Riley. 1976. Chloroquine resistant malaria in Papua New Guinea. Papua New Guinea Med. J. 19:184-185. [PubMed] [Google Scholar]
- 87.Guthmann, J. P., C. Cetre, F. Suzan, S. Darovare, and F. Morin. 1996. Field research, relief work and war: does chloroquine-resistance occur in displaced populations of southern Sudan? Trop. Doct. 26:89-90. [DOI] [PubMed] [Google Scholar]
- 88.Harinasuta, T., P. Suntharasamai, and C. Viravan. 1965. Chloroquine-resistant falciparum malaria in Thailand. Lancet ii:657-660. [DOI] [PubMed] [Google Scholar]
- 89.Hastings, I. M. 1997. A model for the origins and spread of drug-resistant malaria. Parasitology 115:133-141. [DOI] [PubMed] [Google Scholar]
- 90.Hastings, I. M. 2001. Modelling parasite drug resistance: lessons for management and control strategies. Trop. Med. Int. Health 6:883-890. [DOI] [PubMed] [Google Scholar]
- 91.Hastings, I. M. 2003. Malaria control and the evolution of drug resistance: an intriguing link. Trends Parasitol. 19:70-73. [DOI] [PubMed] [Google Scholar]
- 92.Hastings, I. M., P. G. Bray, and S. A. Ward. 2002. Parasitology. A requiem for chloroquine. Science 298:74-75. [DOI] [PubMed] [Google Scholar]
- 93.Hastings, I. M., and U. D'Alessandro. 2000. Modelling a predictable disaster: the rise and spread of drug- resistantmalaria. Parasitol. Today 16:340-347. [DOI] [PubMed] [Google Scholar]
- 94.Hastings, I. M., and M. J. Mackinnon. 1998. The emergence of drug-resistant malaria. Parasitology 117:411-417. [DOI] [PubMed] [Google Scholar]
- 95.Hastings, I. M., W. M. Watkins, and N. J. White. 2002. The evolution of drug-resistant malaria: the role of drug elimination half-life. Philos. Trans. R. Soc. London Ser. B 357:505-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hastings, M. D., S. J. Bates, E. A. Blackstone, S. M. Monks, T. K. Mutabingwa, and C. S. Sibley. 2002. Highly pyrimethamine-resistant alleles of dihydrofolate reductase in isolates of Plasmodium falciparum from Tanzania. Trans. R. Soc. Trop. Med. Hyg. 96:674-676. [DOI] [PubMed] [Google Scholar]
- 97.Hatz, C., S. Abdulla, R. Mull, D. Schellenberg, I. Gathmann, P. Kibatala, H. P. Beck, M. Tanner, and C. Royce. 1998. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1-5 years. Trop. Med. Int. Health 3:498-504. [DOI] [PubMed] [Google Scholar]
- 98.Hay, S. I., J. Cox, D. J. Rogers, S. E. Randolph, D. I. Stern, G. D. Shanks, M. F. Myers, and R. W. Snow. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henry, M. C., M. Kone, P. Guillet, and J. Mouchet. 1998. Chloroquine resistance and malaria control in Ivory Coast. Sante 8:287-291. (In French.) [PubMed] [Google Scholar]
- 100.Hess, F. I., A. Iannuzzi, J. Leafasia, D. Cowdrey, H. D. Nothdurft, F. von Sonnenburg, T. Loscher, and K. H. Rieckmann. 1996. Risk factors of chloroquine resistance in Plasmodium falciparum malaria. Acta Trop. 61:293-306. [DOI] [PubMed] [Google Scholar]
- 101.Hill, W. G., H. A. Babiker, L. C. Ranford-Cartwright, and D. Walliker. 1995. Estimation of inbreeding coefficients from genotypic data on multiple alleles, and application to estimation of clonality in malaria parasites. Genet. Res. 65:53-61. [DOI] [PubMed] [Google Scholar]
- 102.Hong-Ping, G., Z. Yao, D. Qian, Y. Jin-Hua, J. Kai-Xun, and Z. Min. 1998. The in vivo sensitivity of Plasmodium falciparum to chloroquine in the Red River basin, Yunnan, China. Southeast Asian J. Trop. Med. Public Health 29:692-695. [PubMed] [Google Scholar]
- 103.Irion, A., I. Felger, S. Abdulla, T. Smith, R. Mull, M. Tanner, C. Hatz, and H. P. Beck. 1998. Distinction of recrudescences from new infections by PCR-RFLP analysis in a comparative trial of CGP 56 697 and chloroquine in Tanzanian children. Trop. Med. Int. Health 3:490-497. [DOI] [PubMed] [Google Scholar]
- 104.Kamya, M. R., N. N. Bakyaita, A. O. Talisuna, W. M. Were, and S. G. Staedke. 2002. Increasing antimalarial drug resistance in Uganda and revision of the national drug policy. Trop. Med. Int. Health 7:1031-1041. [DOI] [PubMed] [Google Scholar]
- 105.Kamya, M. R., G. Dorsey, A. Gasasira, G. Ndeezi, J. N. Babirye, S. G. Staedke, and P. J. Rosenthal. 2001. The comparative efficacy of chloroquine and sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Trans. R. Soc. Trop. Med. Hyg. 95:50-55. [DOI] [PubMed] [Google Scholar]
- 106.Kihamia, C. M., and H. S. Gill. 1982. Chloroquine-resistant falciparum malaria in semi-immune African Tanzania. Lancet ii:43. [DOI] [PubMed] [Google Scholar]
- 107.Krogstad, D. J., I. Y. Gluzman, D. E. Kyle, A. M. Oduola, S. K. Martin, W. K. Milhous, and P. H. Schlesinger. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283-1285. [DOI] [PubMed] [Google Scholar]
- 108.Kshirsagar, N. A., N. J. Gogtay, D. Rajgor, S. S. Dalvi, and M. Wakde. 2000. An unusual case of multidrug-resistant Plasmodium vivax malaria in Mumbai (Bombay), India. Ann. Trop. Med. Parasitol. 94:189-190. [DOI] [PubMed] [Google Scholar]
- 109.Kublin, J. G., F. K. Dzinjalamala, D. D. Kamwendo, E. M. Malkin, J. F. Cortese, L. M. Martino, R. A. Mukadam, S. J. Rogerson, A. G. Lescano, M. E. Molyneux, P. A. Winstanley, P. Chimpeni, T. E. Taylor, and C. V. Plowe. 2002. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380-388. [DOI] [PubMed] [Google Scholar]
- 110.Kublin, J. G., J. F. Cortese, E. M. Njuju, R. A. G. Mukadam, J. J. Wirima, P. N. Kazembe, A. A. Djimde, B. Kouriba, T. E. Taylor, and C. V. Plowe. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187:1870-1875. [DOI] [PubMed] [Google Scholar]
- 111.Kyosiimire-Lugemwa, J., A. J. Nalunkuma-Kazibwe, G. Mujuzi, H. Mulindwa, A. Talisuna, and T. G. Egwang. 2002. The Lys-76-Thr mutation in PfCRT and chloroquine resistance in Plasmodium falciparum isolates from Uganda. Trans. R. Soc. Trop. Med. Hyg. 96:91-95. [DOI] [PubMed] [Google Scholar]
- 112.Leclerc, M. C., P. Durand, T. De Meeus, V. Rovert, and F. Renaud. 2002. Genetic diversity and population structure of Plasmodium falciparum isolates from Dakar, Senegal, investigated from microsatellite and antigen determinanat loci. Microbes Infect. 4:685-692. [DOI] [PubMed] [Google Scholar]
- 113.Legros, D., K. Johnson, P. Houpikian, M. Makanga, J. K. Kabakyenga, A. O. Talisuna, and W. R. Taylor. 2002. Clinical efficacy of chloroquine or sulfadoxine-pyrimethamine in children under five from south-western Uganda with uncomplicated falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 96:199-201. [DOI] [PubMed] [Google Scholar]
- 114.le Hesran, J. Y., C. Boudin, M. Cot, P. Personne, R. Chambon, V. Foumane, J. P. Verhave, and C. de Vries. 1997. In-vivo resistance of Plasmodium falciparum to chloroquine and amodiaquine in south Cameroon and age-related efficacy of the drugs. Ann. Trop. Med. Parasitol. 91:661-664. [DOI] [PubMed] [Google Scholar]
- 115.Reference deleted.
- 116.Lemeshow, S., and S. Taber. 1991. Lot quality assurance sampling: single- and double-sampling plans. World Health Stat. Q. 44:115-132. [PubMed] [Google Scholar]
- 117.Lokman, H. S., S. W. Sharifah Roohi, Y. Zurkurnai, R. A. Noor, S. M. Mansor, K. Palmer, V. Navaratnam, and J. W. Mak. 1996. Plasmodium falciparum: increased proportion of severe resistance (RII and RIII) to chloroquine and high rate of resistance to sulfadoxine-pyrimethamine in Peninsular Malaysia after two decades. Trans. R. Soc. Trop. Med. Hyg. 90:294-297. [DOI] [PubMed] [Google Scholar]
- 118.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 119.Mabuza, A., J. Govere, D. Durrheim, N. Mngomezulu, B. Bredenkamp, K. Barnes, and B. Sharp. 2001. Therapeutic efficacy of sulfadoxine-pyrimethamine in uncomplicated Plasmodium falciparum malaria 3 years after introduction in Mpumalanga. S. Afr. Med. J. 91:975-978. [PubMed] [Google Scholar]
- 120.MacArthur, J., G. M. Stennies, A. Macheso, M. S. Kolczak, M. D. Green, D. Ali, L. M. Barat, P. N. Kazembe, and T. K. Ruebush. 2001. Efficacy of mefloquine and sulfadoxine-pyrimethamine for the treatment of uncomplicated Plasmodium falciparum infection in Machinga District, Malawi, 1998. Am. J. Trop. Med. Hyg. 65:679-684. [DOI] [PubMed] [Google Scholar]
- 121.Mackinnon, M. J., and I. M. Hastings. 1998. The evolution of multiple drug resistance in malaria parasites. Trans. R. Soc. Trop. Med. Hyg. 92:188-195. [DOI] [PubMed] [Google Scholar]
- 122.Magesa, S. M., K. Y. Mdira, A. Farnert, P. E. Simonsen, I. C. Bygbjerg, and P. H. Jakobsen. 2001. Distinguishing Plasmodium falciparum treatment failures from re-infections by using polymerase chain reaction genotyping in a holoendemic area in northeastern Tanzania. Am. J. Trop. Med. Hyg. 65:477-483. [DOI] [PubMed] [Google Scholar]
- 123.Maguire, J. D., I. W. Sumawinata, S. Masbar, B. Laksana, P. Prodjodipuro, I. Susanti, P. Sismadi, N. Mahmud, M. J. Bangs, and J. K. Baird. 2002. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 360:58-60. [DOI] [PubMed] [Google Scholar]
- 124.Maguire, J. D., A. I. Susanti, Krisin, P. Sismadi, D. J. Fryauff, and J. K. Baird. 2001. The T76 mutation in the pfcrt gene of Plasmodium falciparum and clinical chloroquine resistance phenotypes in Papua, Indonesia. Ann. Trop. Med. Parasitol. 95:559-572. [DOI] [PubMed] [Google Scholar]
- 125.Mahomva, A. I., D. E. Peterson, and L. Rakata. 1996. Chloroquine resistant falciparum malaria in Mutare District, eastern Zimbabwe. Cent. Afr. J. Med. 42:112-113. [PubMed] [Google Scholar]
- 126.Makler, M. T., J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins, and D. J. Hinrichs. 1993. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 48:739-741. [DOI] [PubMed] [Google Scholar]
- 127.Makono, R., and S. Sibanda. 1999. Review of the prevalence of malaria in Zimbabwe with specific reference to parasite drug resistance (1984-96). Trans. R. Soc. Trop. Med. Hyg. 93:449-452. [DOI] [PubMed] [Google Scholar]
- 128.Markwalder, K. A., and H. E. Meyer. 1982. Possible sulfadoxine-pyrimethamine resistance in Plasmodium falciparum malaria from Kenya. Trans. R. Soc. Trop. Med. Hyg. 76:281. [DOI] [PubMed] [Google Scholar]
- 129.Mayor, A. G., X. Gomez-Olive, J. J. Aponte, S. Casimiro, S. Mabunda, M. Dgedge, A. Barreto, and P. L. Alonso. 2001. Prevalence of the K76T mutation in the putative Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene and its relation to chloroquine resistance in Mozambique. J. Infect. Dis. 183:1413-1416. [DOI] [PubMed] [Google Scholar]
- 130.McCormack, C. P. 1984. Human ecology and behaviour in malaria control in tropical Afica. Bull. W. H. O. 62:81-87. [PMC free article] [PubMed] [Google Scholar]
- 131.McIntosh, H. M., and B. M. Greenwood. 1998. Chloroquine or amodiaquine combined with sulfadoxine-pyrimethamine as a treatment for uncomplicated malaria—a systematic review. Ann. Trop. Med. Parasitol. 92:265-270. [DOI] [PubMed] [Google Scholar]
- 132.McIntosh, H. M., and P. Olliaro. 2000. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database Syst. Rev. CD000256. [DOI] [PMC free article] [PubMed]
- 133.Mehlotra, R. K., H. Fujioka, P. D. Roepe, O. Janneh, L. M. Ursos, V. Jacobs-Lorena, D. T. McNamara, M. J. Bockarie, J. W. Kazura, D. E. Kyle, D. A. Fidock, and P. A. Zimmerman. 2001. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. USA 98:12689-12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mendez, F., A. Munoz, G. Carrasquilla, D. Jurado, M. Arevalo-Herrera, J. F. Cortese, and C. V. Plowe. 2002. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am. J. Epidemiol. 156:230-238. [DOI] [PubMed] [Google Scholar]
- 135.Molyneux, D. H., K. Floyd, G. Barnish, and E. M. Fevre. 1999. Transmission control and drug resistance in malaria: a crucial interaction. Parasitol. Today 15:238-240. [DOI] [PubMed] [Google Scholar]
- 136.Moore, D. V., and J. E. Lanier. 1961. Observations on two Plasmodium falciparum infections with abnormal response to chloroquine. Am. J. Trop. Med. Hyg. 10:5-9. [DOI] [PubMed] [Google Scholar]
- 137.Mulindwa, H. C., H. Mayanja-Kizza, and J. Freers. 2002. Resistance patterns of Plasmodium falciparum malaria to chloroquine in Kampala, Uganda. East Afr. Med. J. 79:115-119. [DOI] [PubMed] [Google Scholar]
- 138.Muller, O., M. B. van Hensbroek, S. Jaffar, C. Drakeley, C. Okorie, D. Joof, M. Pinder, and B. Greenwood. 1996. A randomized trial of chloroquine, amodiaquine and pyrimethamine-sulphadoxine in Gambian children with uncomplicated malaria. Trop. Med. Int. Health 1:124-132. [DOI] [PubMed] [Google Scholar]
- 139.Mutabingwa, T., A. Nzila, E. Mberu, E. Nduati, P. Winstanley, E. Hills, and W. Watkins. 2001. Chlorproguanil-dapsone for treatment of drug-resistant falciparum malaria in Tanzania. Lancet 358:1218-1223. [DOI] [PubMed] [Google Scholar]
- 140.Myat, P. K., O. Myint, L. Myint, Z. Thaw, H. A. Kyin, and N. Y. Nwe. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687. [DOI] [PubMed] [Google Scholar]
- 141.Nagesha, H. S., S. Din, G. J. Casey, A. I. Susanti, D. J. Fryauff, J. C. Reeder, and A. F. Cowman. 2001. Mutations in the pfmdr1, dhfr and dhps genes of Plasmodium falciparum are associated with in-vivo drug resistance in West Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 95:43-49. [DOI] [PubMed] [Google Scholar]
- 142.Najera, J. A. 1990. Malaria control: present situation and need for historical research. Parassitologia 32:215-229. [PubMed] [Google Scholar]
- 143.Neiva, A. 1910. Ueber die Bildung einur chininresistenten Rasse des Malariaparasiten. Mem. Inst. Oswaldo Cruz 2:131-140. [Google Scholar]
- 144.Nocht, B., and H. Werner. 1910. Beobachtungen uber eine relative Chininresistenz bei malaria aus brasilien. Dtsch. Med. Wochenschr. 36:1557-1560. [Google Scholar]
- 145.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 46:1658-1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nosten, F., F. ter Kuile, T. Chongsuphajaisiddhi, C. Luxemburger, H. K. Webster, M. Edstein, L. Phaipun, K. L. Thew, and N. J. White. 1991. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet 337:1140-1143. [DOI] [PubMed] [Google Scholar]
- 147.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 148.Nwanyanwu, O. C., C. Ziba, P. Kazembe, L. Chitsulo, J. J. Wirima, N. Kumwenda, and S. C. Redd. 1996. Efficacy of sulphadoxine/pyrimethamine for Plasmodium falciparum malaria in Malawian children under five years of age. Trop. Med. Int. Health 1:231-235. [DOI] [PubMed] [Google Scholar]
- 149.Nzila, A. M., E. K. Mberu, J. Sulo, H. Dayo, P. A. Winstanley, C. H. Sibley, and W. M. Watkins. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nzila, A. M., E. Nduati, E. K. Mberu, S. C. Hopkins, S. A. Monks, P. A. Winstanley, and W. M. Watkins. 2000. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J. Infect. Dis. 181:2023-2028. [DOI] [PubMed] [Google Scholar]
- 151.Ogutu, B. R., B. L. Smoak, R. W. Nduati, D. A. Mbori-Ngacha, F. Mwathe, and G. D. Shanks. 2000. The efficacy of pyrimethamine-sulfadoxine (Fansidar) in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Trans. R. Soc. Trop. Med. Hyg. 94:83-84. [DOI] [PubMed] [Google Scholar]
- 152.Olliaro, P., W. R. Taylor, and J. Rigal. 2001. Controlling malaria: challenges and solutions. Trop. Med. Int. Health 6:922-927. [DOI] [PubMed] [Google Scholar]
- 153.Omar, S. A., A. Bakari, A. Owiti, I. S. Adagu, and D. C. Warhurst. 2001. Co-trimoxazole compared with sulfadoxine-pyrimethamine in the treatment of uncomplicated malaria in Kenyan children. Trans. R. Soc. Trop. Med. Hyg. 95:657-660. [DOI] [PubMed] [Google Scholar]
- 154.Onori, E. 1984. The problem of Plasmodium falciparum drug resistance in Africa south of the Sahara. Bull. W. H. O. 62(Suppl.):55-62. [PMC free article] [PubMed] [Google Scholar]
- 155.Onori, E., B. Grab, P. Ambroise-Thomas, and J. Thelu. 1982. Incipient resistance of Plasmodium falciparum to chloroquine among a semi-immune population of the United Republic of Tanzania. 2. The impact of chloroquine used as a chemosuppressant on the immune status of the population. Bull. W. H. O. 60:899-906. [PMC free article] [PubMed] [Google Scholar]
- 156.Osorio, L. E., L. E. Giraldo, L. F. Grajales, A. L. Arriaga, A. L. Andrade, T. K. Ruebush, and L. M. Barat. 1999. Assessment of therapeutic response of Plasmodium falciparum to chloroquine and sulfadoxine-pyrimethamine in an area of low malaria transmission in Colombia. Am. J. Trop. Med. Hyg. 61:968-972. [DOI] [PubMed] [Google Scholar]
- 157.Overbosch, D., van den Wall Bake AW, P. C. Stuiver, and H. J. van der Kaay. 1984. Chloroquine-resistant falciparum malaria from Malawi. Trop. Geogr. Med. 36:71-72. [PubMed] [Google Scholar]
- 158.Paul, R. E., M. J. Packer, M. Walmsley, M. Lagog, L. C. Ranford-Cartwright, R. Paru, and K. P. Day. 1995. Mating patterns in malaria parasite populations of Papua New Guinea. Science 269:1709-1711. [DOI] [PubMed] [Google Scholar]
- 159.Payne, D. 1988. Did medicated salt hasten the spread of chloroquine resistance in Plasmodium falciparum. Parasitol. Today 4:112-115. [DOI] [PubMed] [Google Scholar]
- 160.Peters, W. 1987. Chemotherapy and drug resistance in malaria, 2nd ed. Academic Press, Inc., New York, N.Y.
- 161.Peterson, D. S., W. K. Milhous, and T. E. Wellems. 1990. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 87:3018-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Phan, G. T., P. J. de Vries, B. Q. Tran, H. Q. Le, N. V. Nguyen, T. V. Nguyen, S. H. Heisterkamp, and P. A. Kager. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop. Med. Int. Health 7:858-864. [DOI] [PubMed] [Google Scholar]
- 163.Phillips, E. J., J. S. Keystone, and K. C. Kain. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 23:1171-1173. [DOI] [PubMed] [Google Scholar]
- 164.Pillai, D. R., A. C. Labbe, V. Vanisaveth, B. Hongvangthong, S. Pomphida, S. Inkathone, K. Zhong, and K. C. Kain. 2001. Plasmodium falciparum malaria in Laos: chloroquine treatment outcome and predictive value of molecular markers. J. Infect. Dis. 183:789-795. [DOI] [PubMed] [Google Scholar]
- 165.Reference deleted.
- 166.Plowe, C. V., A. Djimde, T. E. Wellems, S. Diop, B. Kouriba, and O. K. Doumbo. 1996. Community pyrimethamine-sulfadoxine use and prevalence of resistant Plasmodium falciparum genotypes in Mali: a model for deterring resistance. Am. J. Trop. Med. Hyg. 55:467-471. [DOI] [PubMed] [Google Scholar]
- 167.Plowe, C. V., O. K. Doumbo, A. Djimde, K. Kayentao, Y. Diourte, S. N. Doumbo, D. Coulibaly, M. Thera, T. E. Wellems, and D. A. Diallo. 2001. Chloroquine treatment of uncomplicated Plasmodium falciparum malaria in Mali: parasitologic resistance versus therapeutic efficacy. Am. J. Trop. Med. Hyg. 64:242-246. [DOI] [PubMed] [Google Scholar]
- 168.Price, R. N., F. Nosten, C. Luxemburger, F. O. ter Kuile, L. Paiphun, T. Chongsuphajaisiddhi, and N. J. White. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654-1658. [DOI] [PubMed] [Google Scholar]
- 169.Rab, M. A., T. W. Freeman, N. Durrani, D. de Poerck, and M. W. Rowland. 2001. Resistance of Plasmodium falciparum malaria to chloroquine is widespread in eastern Afghanistan. Ann. Trop. Med. Parasitol. 95:41-46. [DOI] [PubMed] [Google Scholar]
- 170.Rahman, M. R., D. C. Paul, M. Rashid, A. Ghosh, A. M. Bangali, M. A. Jalil, and M. A. Faiz. 2001. A randomized controlled trial on the efficacy of alternative treatment regimens for uncomplicated falciparum malaria in a multidrug-resistant falciparum area of Bangladesh—narrowing the options for the National Malaria Control Programme? Trans. R. Soc. Trop. Med. Hyg. 95:661-667. [DOI] [PubMed] [Google Scholar]
- 171.Ranford-Cartwright, L. C., K. L. Johnston, A. M. Abdel-Muhsin, B. K. Khan, and H. A. Babiker. 2002. Critical comparison of molecular genotyping methods for detection of drug-resistant Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 96:568-572. [DOI] [PubMed] [Google Scholar]
- 172.Ranford-Cartwright, L. C., J. Taylor, T. Umasunthar, L. H. Taylor, H. A. Babiker, B. Lell, J. R. Schmidt-Ott, L. G. Lehman, D. Walliker, and P. G. Kremsner. 1997. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans. R. Soc. Trop. Med. Hyg. 91:719-724. [DOI] [PubMed] [Google Scholar]
- 173.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 174.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 175.Ringwald, P., and L. K. Basco. 1999. Comparison of in vivo and in vitro tests of resistance in patients treated with chloroquine in Yaounde, Cameroon. Bull. W. H. O. 77:34-43. [PMC free article] [PubMed] [Google Scholar]
- 176.Robert, V., H. P. Awono-Ambene, J. Y. le Hesran, and J. F. Trape. 2000. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am. J. Trop. Med. Hyg. 62:210-216. [DOI] [PubMed] [Google Scholar]
- 177.Rogier, C., A. Tall, N. Diagne, D. Fontenille, A. Spiegel, and J. F. Trape. 1999. Plasmodium falciparum clinical malaria: lessons from longitudinal studies in Senegal. Parassitologia 41:255-259. [PubMed] [Google Scholar]
- 178.Ronn, A. M., H. A. Msangeni, J. Mhina, W. H. Wernsdorfer, and I. C. Bygbjerg. 1996. High level of resistance of Plasmodium falciparum to sulfadoxine-pyrimethamine in children in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 90:179-181. [DOI] [PubMed] [Google Scholar]
- 179.Rowland, M., N. Durrani, S. Hewitt, and E. Sondorp. 1997. Resistance of falciparum malaria to chloroquine and sulfadoxine-pyrimethamine in Afghan refugee settlements in western Pakistan: surveys by the general health services using a simplified in vivo test. Trop. Med. Int. Health 2:1049-1056. [DOI] [PubMed] [Google Scholar]
- 179a.Rwagacondo, C. E., F. Niyitegeka, J. Sarushi, C. Karema, V. Mugisha, J. C. Dujardin, C. Van Overmeir, J. van den Ende, and U. D’Alessandro. 2003. Efficacy of amodiaquine alone and combined with sulfadoxine-pyrimethamine and of sulfadoxine pyrimethamine combined with artesunate. Am. J. Trop. Med. Hyg. 68:743-747. [PubMed]
- 180.Sanchez, C. P., S. Wunsch, and M. Lanzer. 1997. Identification of a chloroquine importer in Plasmodium falciparum. Differences in import kinetics are genetically linked with the chloroquine-resistant phenotype. J. Biol. Chem. 272:2652-2658. [DOI] [PubMed] [Google Scholar]
- 181.Schapira, A., P. F. Beales, and M. E. Halloran. 1993. Malaria: living with drug resistance. Parasitol. Today 9:168-174. [DOI] [PubMed] [Google Scholar]
- 182.Schuurkamp, G. J., P. E. Spicer, R. K. Kereu, P. K. Bulungol, and K. H. Rieckmann. 1992. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 86:121-122. [DOI] [PubMed] [Google Scholar]
- 183.Schwartz, I. K., E. M. Lackritz, and L. C. Patchen. 1991. Chloroquine-resistant Plasmodium vivax from Indonesia. N. Engl. J. Med. 324:927. [DOI] [PubMed] [Google Scholar]
- 184.Shaffer, N., K. Hedberg, F. Davachi, B. Lyamba, J. G. Breman, O. S. Masisa, F. Behets, A. Hightower, and P. Nguyen-Dinh. 1990. Trends and risk factors for HIV-1 seropositivity among outpatient children, Kinshasa, Zaire. AIDS 4:1231-1236. [DOI] [PubMed] [Google Scholar]
- 185.Sharma, Y. D., S. Biswas, C. R. Pillai, M. A. Ansari, T. Adak, and C. U. Devi. 1996. High prevalence of chloroquine resistant Plasmodium falciparum infection in Rajasthan epidemic. Acta Trop. 62:135-141. [DOI] [PubMed] [Google Scholar]
- 186.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 187.Sidhu, A. B., D. Verdier-Pinard, and D. A. Fidock. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sims, P., P. Wang, and J. E. Hyde. 1999. Selection and synergy in Plasmodium falciparum. Parasitol. Today 15:132-134. [DOI] [PubMed] [Google Scholar]
- 189.Slatter, M. J., K. Pettengell, and R. K. Taylor. 1983. Chloroquine-resistant malaria. S. Afr. Med. J. 63:838. [PubMed] [Google Scholar]
- 190.Smithuis, F. M., F. Monti, M. Grundl, A. Z. Oo, T. T. Kyaw, O. Phe, and N. J. White. 1997. Plasmodium falciparum: sensitivity in vivo to chloroquine, pyrimethamine/sulfadoxine and mefloquine in western Myanmar. Trans. R. Soc. Trop. Med. Hyg. 91:468-472. [DOI] [PubMed] [Google Scholar]
- 191.Sowunmi, A., A. I. Ayede, A. G. Falade, V. N. Ndikum, C. O. Sowunmi, A. A. Adedeji, C. O. Falade, T. C. Happi, and A. M. Oduola. 2001. Randomized comparison of chloroquine and amodiaquine in the treatment of acute, uncomplicated, Plasmodium falciparum malaria in children. Ann. Trop. Med. Parasitol. 95:549-558. [DOI] [PubMed] [Google Scholar]
- 192.Sowunmi, A., A. M. Oduola, O. A. Ogundahunsi, and L. A. Salako. 1998. Comparative efficacy of chloroquine plus chlorpheniramine and pyrimethamine/sulfadoxine in acute uncomplicated falciparum malaria in Nigerian children. Trans. R. Soc. Trop. Med. Hyg. 92:77-81. [DOI] [PubMed] [Google Scholar]
- 193.Spencer, H. C., D. M. Kariuki, and D. K. Koech. 1983. Chloroquine resistance in Plasmodium falciparum from Kenyan infants. Am. J. Trop. Med. Hyg. 32:922-925. [DOI] [PubMed] [Google Scholar]
- 194.Staedke, S. G., M. R. Kamya, G. Dorsey, A. Gasasira, G. Ndeezi, E. D. Charlebois, and P. J. Rosenthal. 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet 358:368-374. [DOI] [PubMed] [Google Scholar]
- 195.Su, X., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 196.Sudre, P., J. G. Breman, and J. P. Koplan. 1990. Delphi survey of malaria mortality and drug resistance in Africa. Lancet 335:722. [DOI] [PubMed] [Google Scholar]
- 197.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Takechi, M., M. Matsuo, C. Ziba, A. Macheso, D. Butao, I. L. Zungu, I. Chakanika, and M. D. Bustos. 2001. Therapeutic efficacy of sulphadoxine/pyrimethamine and susceptibility in vitro of P. falciparum isolates to sulphadoxine-pyrimethamine and other antimalarial drugs in Malawian children. Trop. Med. Int. Health 6:429-434. [DOI] [PubMed] [Google Scholar]
- 199.Talisuna, A. O., J. Kyosiimire-Lugemwa, P. Langi, T. K. Mutabingwa, W. Watkins, E. Van Marck, T. Egwang, and U. D'Alessandro. 2002. Role of the pfcrt codon 76 mutation as a molecular marker for population-based surveillance of chloroquine (CQ)-resistant Plasmodium falciparum malaria in Ugandan sentinel sites with high CQ resistance. Trans. R. Soc. Trop. Med. Hyg. 96:551-556. [DOI] [PubMed] [Google Scholar]
- 200.Talisuna, A. O., P. Langi, N. Bakyaita, T. Egwang, T. K. Mutabingwa, W. Watkins, E. Van Marck, and U. D'Alessandro. 2002. Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: what is the relationship between these three factors? Trans. R. Soc. Trop. Med. Hyg. 96:310-317. [DOI] [PubMed] [Google Scholar]
- 201.Talisuna, A. O., P. Langi, T. K. Mutabingwa, E. Van Marck, W. Watkins, T. G. Egwang, and U. D'Alessandro. 2003. Population based validation of DHFR gene mutations for the prediction of sulphadoxine-pyrimethamine resistance in Uganda. Trans. R. Soc. Trop. Med. Hyg. in press. [DOI] [PubMed]
- 202.Thimasarn, K., S. Pinichpongse, S. Malikul, W. Rooney, and S. Tansophalaks. 1990. Phase III double-blind comparative study of Fansimef and Lariam for the curative treatment of Plasmodium falciparum infections in Thailand. Southeast Asian J. Trop. Med. Public Health 21:404-411. [PubMed] [Google Scholar]
- 203.Thompson, P. E., and L. M. Werbel. 1972. Antimalarial agents: chemistry and phamacology. In G. deStevens (ed.), Medicinal chemistry. Academic Press, Inc., New York, N.Y.
- 204.Tinto, H., E. B. Zoungrana, S. O. Coulibaly, J. B. Ouedraogo, M. Traore, T. R. Guiguemde, E. Van Marck, and U. D'Alessandro. 2002. Chloroquine and sulphadoxine-pyrimethamine efficacy for uncomplicated malaria treatment and haematological recovery in children in Bobo-Dioulasso, Burkina Faso during a 3-year period, 1998-2000. Trop. Med. Int. Health 7:925-930. [DOI] [PubMed] [Google Scholar]
- 205.Titanji, V. P., T. Nkuo-Akenji, W. Ntopi, and R. Djokam. 2001. Reduced levels of chloroquine resistant Plasmodium falciparum in selected foci of the South West Province, Cameroon. Cent. Afr. J. Med. 47:145-149. [PubMed] [Google Scholar]
- 206.Tjitra, E., S. Suprianto, B. J. Currie, P. S. Morris, J. R. Saunders, and N. M. Anstey. 2001. Therapy of uncomplicated falciparum malaria: a randomized trial comparing artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 65:309-317. [DOI] [PubMed] [Google Scholar]
- 207.Trape, J. F. 2001. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 64:12-17. [DOI] [PubMed] [Google Scholar]
- 208.Trape, J. F., G. Pison, M. P. Preziosi, C. Enel, A. Desgrees du Lou, V. Delaunay, B. Samb, E. Lagarde, J. F. Molez, and F. Simondon. 1998. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. III 321:689-697. [DOI] [PubMed] [Google Scholar]
- 209.Treeprasertsuk, S., P. Viriyavejakul, U. Silachamroon, S. Vannphan, P. Wilairatana, and S. Looareesuwan. 2000. Is there any artemisinin resistance in falciparum malaria? Southeast Asian J. Trop. Med. Public Health 31:825-828. [PubMed] [Google Scholar]
- 210.Triglia, T., J. G. Menting, C. Wilson, and A. F. Cowman. 1997. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 94:13944-13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.van Vugt, M., A. Brockman, B. Gemperli, C. Luxemburger, I. Gathmann, C. Royce, T. Slight, S. Looareesuwan, N. J. White, and F. Nosten. 1998. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 42:135-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Verdier, F., J. Le Bras, F. Clavier, I. Hatin, and M. C. Blayo. 1985. Chloroquine uptake by Plasmodium falciparum-infected human erythrocytes during in vitro culture and its relationship to chloroquine resistance. Antimicrob. Agents Chemother. 27:561-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Verhoeff, F. H., B. J. Brabin, P. Masache, B. Kachale, P. Kazembe, and H. J. van der Kaay. 1997. Parasitological and haematological responses to treatment of Plasmodium falciparum malaria with sulphadoxine-pyrimethamine in southern Malawi. Ann. Trop. Med. Parasitol. 91:133-140. [DOI] [PubMed] [Google Scholar]
- 214.Vieira, P. P., A. M. das Gracas, L. H. da Silva, I. Gonzalez-Jimenez, and M. G. Zalis. 2001. Analysis of the PfCRT K76T mutation in Plasmodium falciparum isolates from the Amazon region of Brazil. J. Infect. Dis. 183:1832-1833. [DOI] [PubMed] [Google Scholar]
- 215.von Seidlein, L., K. Bojang, P. Jones, S. Jaffar, M. Pinder, S. Obaro, T. Doherty, M. Haywood, G. Snounou, B. Gemperli, I. Gathmann, C. Royce, K. McAdam, and B. Greenwood. 1998. A randomized controlled trial of artemether/benflumetol, a new antimalarial and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. Am. J. Trop. Med. Hyg. 58:638-644. [DOI] [PubMed] [Google Scholar]
- 216.von Seidlein, L., M. Jawara, R. Coleman, T. Doherty, G. Walraven, and G. Targett. 2001. Parasitaemia and gametocytaemia after treatment with chloroquine, pyrimethamine/sulfadoxine, and pyrimethamine/sulfadoxine combined with artesunate in young Gambians with uncomplicated malaria. Trop. Med. Int. Health 6:92-98. [DOI] [PubMed] [Google Scholar]
- 217.Warsame, M., A. Abdillahi, O. N. Duale, A. N. Ismail, A. M. Hassan, A. Mohamed, and A. Warsame. 2002. Therapeutic efficacy of chloroquine and sulfadoxine/pyrimethamine against Plasmodium falciparum infection in Somalia. Bull. W. H. O. 80:704-708. [PMC free article] [PubMed] [Google Scholar]
- 218.Warsame, M., V. A. Kilimali, W. H. Wernsdorfer, M. Lebbad, A. S. Rutta, and O. Ericsson. 1999. Resistance to chloroquine and sulfadoxine-pyrimethamine in Plasmodium falciparum in Muheza district, Tanzania. Trans. R. Soc. Trop. Med. Hyg. 93:312-313. [DOI] [PubMed] [Google Scholar]
- 219.Warsame, M., W. H. Wernsdorfer, G. Huldt, and A. Bjorkman. 1995. An epidemic of Plasmodium falciparum malaria in Balcad, Somalia, and its causation. Trans. R. Soc. Trop. Med. Hyg. 89:142-145. [DOI] [PubMed] [Google Scholar]
- 220.Watkins, W. M., and M. Mosobo. 1993. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Trans. R. Soc. Trop. Med. Hyg. 87:75-78. [DOI] [PubMed] [Google Scholar]
- 221.Wellems, T. E., L. J. Panton, I. Y. Gluzman, R. Do, V, R. W. Gwadz, A. Walker-Jonah, and D. J. Krogstad. 1990. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature 345:253-255. [DOI] [PubMed] [Google Scholar]
- 222.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 223.Wernsdorfer, W. H. 1994. Epidemiology of drug resistance in malaria. Acta Trop. 56:143-156. [DOI] [PubMed] [Google Scholar]
- 224.Wernsdorfer, W. H., and D. Payne. 1991. The dynamics of drug resistance in Plasmodium falciparum. Pharmacol. Ther. 50:95-121. [DOI] [PubMed] [Google Scholar]
- 225.Whitby, M. 1997. Drug resistant Plasmodium vivax malaria. J. Antimicrob. Chemother. 40:749-752. [DOI] [PubMed] [Google Scholar]
- 226.White, N. J., and W. Pongtavornpinyo. 2003. The de novo selection of drug-resistant malaria parasites. Proc. R. Soc. Lond. Ser. B 270:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.White, N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. Lond. Ser. B 354:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.White, N. J. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41:301-308. [PubMed] [Google Scholar]
- 229.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]
- 230.Wongsrichanalai, C., K. Thimasarn, and J. Sirichaisinthop. 2000. Antimalarial drug combination policy: a caveat. Lancet 355:2245-2247. [DOI] [PubMed] [Google Scholar]
- 231.World Health Organization. 1996. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated malaria in areas with intense transmission. WHO/MAL/96.1077. World Health Organization, Geneva, Switzerland.
- 232.World Health Organization. 1965. Resistance of malaria parasites to drugs. W.H.O. Tech. Rep. Ser. 296:1-65. [PubMed]
- 233.World Health Organization. 1993. Implementation of the Global Strategy. Report of a WHO Study Group on the Implementataion of the Global Plan of Action for Malaria Control, 1993-2000. World Health Organization, Geneva. [PubMed]
- 234.World Health Organization. 1993. A global strategy for malaria control. World Health Organization, Geneva, Switzerland. 1993.
- 234a.World Health Organization. 2001. Monitoring antimalarial drug resistance. Report of WHO Consultation, Geneva, Switzerland, 3-5 December 2001. WHO monographs WHO/CDS/CSR/EPH/2002.7 and WHO/CDS/RBM/2002.29. World Health Organization, Geneva, Switzerland.
- 234b.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed]
- 235.Zucker, J. R., E. M. Lackritz, T. K. Ruebush, A. W. Hightower, J. E. Adungosi, J. B. Were, B. Metchock, E. Patrick, and C. C. Campbell. 1996. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. Am. J. Trop. Med. Hyg. 55:655-660. [DOI] [PubMed] [Google Scholar]