Abstract
Alcoholic liver disease (ALD) is a major cause of chronic liver disease worldwide and can lead to fibrosis and cirrhosis. The latest surveillance report published by the National Institute on Alcohol Abuse and Alcoholism showed that liver cirrhosis was the 12th leading cause of death in the United States, with a total of 29,925 deaths in 2007, 48% of which were alcohol related. The spectrum of ALD includes simple steatosis, alcoholic hepatitis, fibrosis, cirrhosis, and superimposed hepatocellular carcinoma. Early work on the pathogenesis of the disease focused on ethanol metabolism–associated oxidative stress and glutathione depletion, abnormal methionine metabolism, malnutrition, and production of endotoxins that activate Kupffer cells. We review findings from recent studies that have characterized specific intracellular signaling pathways, transcriptional factors, aspects of innate immunity, chemokines, epigenetic features, microRNAs, and stem cells that are associated with ALD, improving our understanding of its pathogenesis. Despite this progress, no targeted therapies are available. The cornerstone of treatment for alcoholic hepatitis remains as it was 40 years ago: abstinence, nutritional support, and corticosteroids. There is an urgent need to develop new pathophysiology-oriented therapies. Recent translational studies of human samples and animal models have identified promising therapeutic targets.
Keywords: Alcohol Liver Disease, Innate Immunity, Adaptive Immunity, Cytokines, Inflammation
Alcohol consumption is a major risk factor for chronic disease worldwide; it accounted for 3.8% of all deaths in 2004.1 It is also a major cause of chronic liver disease in Western countries. Alcohol-related liver deaths account for up to 48% of cirrhosis-associated deaths in the United States2 and are also major contributors to liver disease-related mortality in other countries.1 Research on alcoholic liver disease (ALD) has been rapidly growing since the early 1960s; at that time, Lieber et al used experimental models to show that alcohol is a true hepatotoxin, which causes hepatocellular damage, and that ALD is not simply caused by malnutrition.3 Early studies indicated that ethanol metabolism-associated oxidative stress, glutathione depletion, abnormal methionine metabolism, malnutrition, ethanol-mediated induction of leakage of gut endotoxins, and subsequent activation of Kupffer cells have important roles in the pathogenesis of ALD.4-9 We review advances made in the past 10 years in our understanding of the roles of lipopolysaccharide (LPS) signaling, innate immunity, and transcription factors in the pathogenesis of ALD. We also review recent studies showing that alcohol mediates changes in epigenetic features, microRNAs (miRNAs), and stem cells, which could also contribute to ALD.
Despite the profound economic and health impact of ALD, little progress has been made in the management of patients with this severe clinical condition. There are no modern diagnostic tools to assess individual susceptibility to the development of ALD, and the pathogenesis of ALD in humans is incompletely understood. As a consequence, no new drugs for ALD have been successfully developed since the early 1970s, at which time the use of corticosteroids was proposed for the treatment of severe alcoholic hepatitis.10 The poor therapeutic progress in the field of ALD has, in part, resulted from the lack of experimental models of advanced ALD and from difficulties in performing clinical trials in patients with an active addiction. We review several potential targets that could generate therapeutic interventions for ALD.
Spectrum, Risk Factors, and Comorbidities
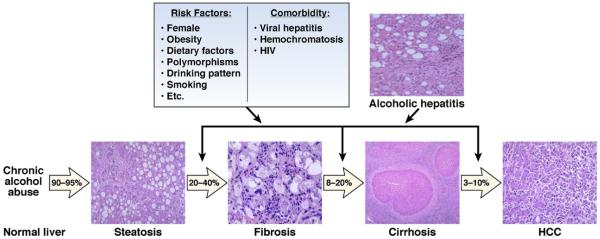
ALD presents as a broad spectrum of disorders, ranging from simple fatty liver to more severe forms of liver injury, including alcoholic hepatitis (AH), cirrhosis, and superimposed hepatocellular carcinoma (HCC) (Figure 1). Fatty liver, an early response to alcohol consumption, develops in most (more than 90%) heavy drinkers, with early-mild steatosis in zone 3 (perivenular) hepatocytes; it can also affect zone 2 and even zone 1 (periportal) hepatocytes when liver injury is more severe. Interestingly, only about 30% of heavy drinkers develop more severe forms of ALD, such as advanced fibrosis and cirrhosis. In patients with underlying ALD and heavy alcohol intake, episodes of superimposed AH may occur. In severe cases and in patients with liver cirrhosis, AH leads to severe complications related to liver failure and portal hypertension and has high short-term mortality.
Figure 1.
Spectrum of ALD, risk factors, and comorbidity. More than 95% of heavy drinkers develop fatty liver, but only up to 35% of this population develops more severe forms of ALD, including fibrosis, alcoholic hepatitis, cirrhosis, and HCC. Many risk factors have been proposed for the severe forms of ALD. Alcohol consumption and comorbid factors act in synergy to accelerate the progression of ALD.
The fact that only about 35% of heavy drinkers develop advanced ALD indicates that other factors are involved. Several risk factors for ALD have been identified (Figure 1). These include sex, obesity, drinking patterns, dietary factors, non-sex-linked genetic factors, and cigarette smoking (Figure 1).11-13 Female sex is a well-documented risk factor for susceptibility to ALD; the increased risk among women likely results from lower levels of gastric alcohol dehydrogenase, a higher proportion of body fat, and the presence of estrogens. Obesity represents another important risk factor that accelerates fibrosis progression and the development of cirrhosis in ALD.14,15 Experimental studies indicate that the synergistic effects of obesity and alcohol abuse involve the endoplasmic reticulum response to cell stress, type I macrophage activation, and adiponectin resistance.16 Daily or near-daily heavy drinking, begun at an early age, increases the risk of the development of severe forms of ALD compared with episodic or binge drinking.17 Genetic factors might also influence susceptibility to advanced ALD, but little data are available. Variations in genes that encode antioxidant enzymes, cytokines and other inflammatory mediators, and alcohol-metabolizing enzymes could have a role.13 Also, recent studies indicate that variations in patatin-like phospholipase domain-containing protein 3 (PNPLA3) affect development of alcoholic cirrhosis in white alcoholic subjects.18-20 Despite the strong link between the PNPLA3 polymorphisms and fatty liver diseases, deletion of this gene did not affect obesity-associated fatty liver or levels of liver enzymes in mice fed a high-fat diet.21 Further studies are required to clarify the role of PNPLA3 variants in the pathogenesis of ALD.
Finally, long-term alcohol drinking has synergistic effects with hepatitis virus B or C and/or human immunodeficiency virus infection, nonalcoholic fatty liver disease, and disorders such as hemochromatosis to accelerate progression of liver diseases. For example, many patients with viral hepatitis consume alcohol, which accelerates progression of liver fibrosis, cirrhosis, and HCC, likely via multiple mechanisms.22,23 A greater understanding of the interaction between alcohol and these comorbid factors could help us design better therapies for the treatment of chronic liver disease.
Pathogenesis
Alcoholic Fatty Liver (Steatosis)
Steatosis, the earliest response of the liver to alcohol abuse, is characterized by the accumulation of fat (mainly triglycerides, phospholipids, and cholesterol esters) in hepatocytes. Early studies indicated that alcohol consumption increases the ratio of reduced nicotinamide adenine dinucleotide/oxidized nicotinamide adenine di-nucleotide in hepatocytes, which disrupts mitochondrial β-oxidation of fatty acids and results in steatosis.24 Alcohol intake has also been shown to augment the supply of lipids to the liver from the small intestine, increasing mobilization of fatty acids from adipose tissue and uptake of fatty acids by the liver.24 However, the contribution of these mechanisms to the development of steatosis after long-term alcohol consumption is not clear and requires further investigation.
Recent studies indicate that alcohol exposure, directly or indirectly, regulates lipid metabolism-associated transcription factors; this stimulates lipogenesis and inhibits fatty acid oxidation (Figure 2). Ethanol increases fatty acid synthesis in hepatocytes via up-regulation of sterol regulatory element-binding protein 1c (SREBP-1c), a transcription factor that promotes fatty acid synthesis via up-regulation of lipogenic genes. Alcohol consumption could directly increase transcription of SREBP-1c gene via its metabolite acetaldehyde25 or indirectly up-regulate SREBP-1c expression by activating processes and factors that stimulate SREBP-1c expression, such as the endoplas mic reticulum response to cell stress,26,27 adenosine,28 endocannabinoids,29 LPS signaling via Toll-like receptor (TLR) 4, and its downstream proteins, including IRF-3, Egr-1, or tumor necrosis factor (TNF)-α.27,30-34 Alcohol also down-regulates factors that reduce SREBP-1c expression, such as AMP-activated protein kinase (AMPK),35 Sirtuin1,36 adiponectin,37 and signal transducer and activator of transcription 3 (STAT3).38 Disruption of SREBP-1c in mice reduced ethanol-induced fatty liver, indicating its role in ALD.39
Figure 2.
Mechanisms of alcoholic fatty liver. (1) Alcohol consumption can directly (via acetaldehyde) or indirectly (via regulation of multiple factors) up-regulate the expression of SREBP-1c and down-regulate the expression of PPAR-α, leading to the induction of fatty acid synthesis and inhibition of fatty liver β-oxidation, which results in the development of alcoholic fatty liver. Alcohol exposure also inhibits AMPK and subsequently increases ACC activity but decreases carnitine palmitoyltransferase 1 (CPT-1) activity, leading to an increase in fatty acid synthesis and a decrease in fatty acid β-oxidation. (2) Alcohol consumption can also modify many factors, including HIF-1, C3, C1qa, PKC[H9255], and iNOS, that subsequently contribute to the development of fatty liver. The mechanisms underlying the effects of these factors remain unclear.
Alcohol consumption inhibits fatty acid oxidation in hepatocytes mainly via inactivation of the peroxisome proliferator-activated receptor (PPAR)-α, a nuclear hormone receptor that controls transcription of a range of genes involved in free fatty acid transport and oxidation.40,41 The ethanol metabolite acetaldehyde, but not ethanol itself, directly inhibits the transcriptional activation activity and DNA-binding ability of PPAR-α in hepatocytes.42 Ethanol consumption can also indirectly inhibit PPAR-α via up-regulation of cytochrome P450 2E1-derived oxidative stress43 and adenosine,28 both of which inhibit PPAR-α, or via down-regulation of adiponectin44 and zinc,45 which each activate PPAR-α.
In addition to regulating fat metabolism-associated transcription factors, ethanol can also affect the activities of enzymes involved in fat metabolism by inhibiting AMPK, which reduces fat metabolism and fatty liver. AMPK is a serine-threonine kinase that can phosphorylate and subsequently inactivate acetyl-CoA carboxylase (ACC), a rate-limiting enzyme for fatty acid synthesis. Inactivation of ACC also reduces levels of malonyl-CoA, a precursor in fatty acid synthesis and an inhibitor of carnitine palmitoyltransferase 1, a rate-limiting enzyme for fatty acid oxidation.46 In addition, AMPK directly phosphorylates and inhibits SREBP activity in hepatocytes, thereby attenuating steatosis.47 In this man ner, AMPK inhibits fatty acid synthesis but promotes fatty acid oxidation via the inactivation of ACC enzyme activity. Alcohol consumption inhibits AMPK activity in the liver, leading to decreased phosphorylation and increased activity of ACC and decreased activity of carnitine palmitoyltransferase 1; each has an important role in the development of alcoholic fatty liver.35
Ethanol-induced steatosis is markedly reduced in many strains of mice, including HIF-1−/−,48 C3−/−,49 C1qa−/−,50 PKCε−/−,51 and iNOS,−/−52 indicating that regulation of these genes also contributes to the pathogenesis of alcoholic fatty liver. However, the underlying mechanisms remain to be determined.
Finally, autophagy has an important role in removing lipid droplets in hepatocytes.53 Long-term alcohol consumption inhibits autophagy.54,55 However, a recent study showed that short-term ethanol exposure activates autophagy by generating reactive oxygen species and inhibiting the mammalian target of rapamycin, indicating that acute ethanol activation of autophagy could have a compensatory role that prevents development of steatosis during the early stages of alcoholic liver injury.56 The inhibitory and stimulatory effects of ethanol on autophagy require further studies to clarify.
AH
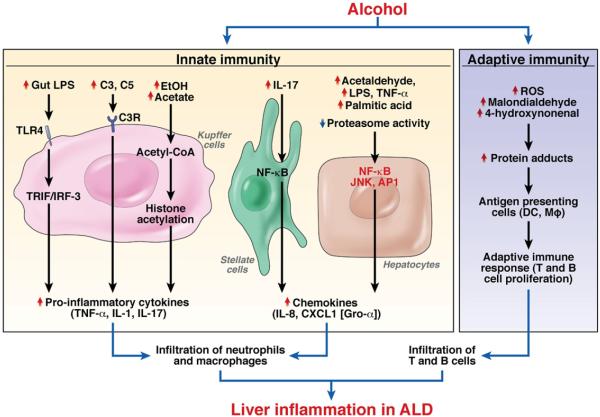
AH is a syndrome characterized by infiltration of the liver by inflammatory cells and hepatocellular injury. AH develops in patients with steatosis and is usually associated with progressive fibrosis. The prevalence of AH has not been accurately determined; it is believed to occur in 10% to 35% of heavy drinkers. AH includes a spectrum of diseases that range from mild injury to severe, life-threatening injury.11,57 The histologic characteristics of AH include centrilobular ballooning of hepatocytes, neutrophilic infiltration, Mallory–Denk hyaline inclusions, steatosis, and a “chicken wire”–like pattern of fibrosis. In many cases, there is underlying cirrhosis.11,57 A large body of evidence indicates that many factors contribute to alcohol-induced inflammation (Figure 3).
Figure 3.
Mechanisms underlying inflammation in ALD. (1) Activation of innate immunity. Parenchymal infiltration of neutrophils and macrophages is a prominent feature of ALD and is likely due to ethanol-mediated activation of innate immunity and subsequent induction of proinflammatory cytokines and chemokines. Alcohol consumption up-regulates a variety of factors that activate Kupffer cells, stellate cells, and hepatocytes, resulting in the production of cytokines and chemokines. Alcohol exposure also decreases proteasome activity and elevates IL-8 expression in hepatocytes. (2) Activation of adaptive immunity. ALD is associated with infiltration of CD4+ and CD8+ T cells in the liver. Alcohol consumption induces reactive oxygen species (ROS) and causes the formation of many protein adducts that might serve as antigens in the adaptive immune response, resulting in the accumulation of T and B cells in the liver.
Hepatotoxicity of ethanol
In hepatocytes, ethanol is primarily metabolized into acetaldehyde by alcohol de hydrogenase in the cytosol, cytochrome P450 in micro-somes, and catalase in peroxisomes. Ethanol metabolism generates reactive oxygen species and causes lipid peroxidation, mitochondrial glutathione depletion, and S-adenosylmethionine depletion; all of these products subsequently prime and sensitize hepatocytes to injury. Acetaldehyde is rapidly metabolized into acetate by alde-hyde dehydrogenase in mitochondria. Acetaldehyde is a reactive compound; it is highly toxic to hepatocytes because it forms a variety of protein and DNA adducts that promote glutathione depletion, lipid peroxidation, and mitochondrial damage.58,59 The acetate that results from acetaldehyde breakdown is rapidly released from the liver into the circulation and is then metabolized into CO2 via the TCA cycle in heart, skeletal muscle, and brain. Although acetate has no direct hepatotoxicity, it is believed to regulate the inflammatory response in patients with AH via the up-regulation of proinflammatory cytokines in macrophages.60,61
Hepatocyte apoptosis
Hepatocyte apoptosis is an important pathologic feature of human ALD. Apoptosis results from multiple mechanisms, including ethanol-mediated hepatotoxicity, induction of oxidative stress, inhibition of survival genes (c-Met), and induction of proapoptotic signaling molecules (TNF-α and Fas ligand).62,63
Activation of innate immunity
Alcohol consumption not only causes enteric dysbiosis and bacterial over-growth64 but also increases gut permeability and the translocation of bacteria-derived LPS from the gut to the liver.65 These changes affect each other and ultimately contribute to the increased levels of LPS observed in patients with ALD. In Kupffer cells, LPS interacts with TLR4 to activate the MyD88-independent (TRIF/IRF-3) signaling pathway, leading to production of oxidative stress and proinflammatory cytokines, including TNF-α, that cause hepatocellular damage.30,31,33,34 Alcohol consumption also activates complement C3 and C5, which subsequently activate Kupffer cells via binding to their receptors on these cells; complement activation is followed by production of TNF-α and induction of hepatocyte injury.49,50,66 Interestingly, activation of TLR4 and complement factors also cause Kupffer cells to produce hepatoprotective cytokines such as interleukin (IL)-6 and anti-inflammatory cytokines such as IL-10. These cytokines activate STAT3 in hepatocytes and macrophages/ Kupffer cells, respectively, to prevent alcohol-induced liver injury and inflammation.38,67,68 Collectively, the activation of innate immunity components not only initiates alcoholic liver injury but also triggers hepatoprotective, regenerative, and anti-inflammatory responses that reduce alcohol-induced hepatocellular damage.
Infiltration by neutrophils
Infiltration of liver by parenchymal neutrophils is a prominent feature of AH. Up-regulation of IL-8, CXCL1 (Gro-α), and IL-17 in liver contributes to this infiltration and the severity of AH.69-71 Multiple factors stimulate parenchymal and nonparenchymal cells to produce these chemokines and cytokines in patients with AH. These include acetaldehyde, LPS, TNF-α, palmitic acid, and down-regulation of proteasome functions.72-74 IL-17, which is increased in patients with AH, not only directly induces neutrophil recruitment but also stimulates hepatic stellate cells (HSCs) to produce IL-8 and CXCL1; this is followed by induction of neutro-phil infiltration.71 In addition, IL-8 and CXCL1 are secreted by activated HSCs75 and Kupffer cells,76 which might promote recruitment of neutrophils. Furthermore, many other chemokines (eg, CXCL5, CXCL6, CXCL4) and cytokines (eg, TNF-α, IL-1, osteopontin) are markedly up-regulated and could promote infiltration of neutrophils during progression of AH.69-71 Although neutrophil infiltration correlates with the severity of AH, because there is no rodent model of this disease, no direct experimental evidence supports the role of neutrophils in pathogenesis. Studies from other liver injury models in rodents indicate that, after systemic activation, neutrophils migrate into the liver parenchyma and subsequently kill sensitized hepatocytes by releasing reactive oxygen species and proteases, likely contributing to alcoholic liver injury.77
Activation of adaptive immunity
Patients with AH have increased levels of circulating antibodies against lipid peroxidation adducts and increased numbers of T cells in the liver, indicating that activation of adaptive immunity is involved in the pathogenesis of ALD, including AH.78-81 Long-term alcohol consumption increases oxidative stress, which generates lipid peroxidation products such as malondialdehyde and 4-hydroxynonenal; these compounds can modify many proteins, inducing the formation of protein adducts that can serve as antigens to activate the adaptive immune response.78-81 However, the precise mechanisms by which adaptive immune responses induce hepatocellular damage and inflammation in patients with AH are not known.
Inhibition of liver regeneration
The liver can regenerate after injury or loss of tissue; recovery of liver mass occurs mainly via proliferation of remaining adult hepatocytes, biliary epithelial cells, and endothelial cells. Under pathogenic conditions in which proliferation of hepatocytes is inhibited, liver progenitor cells (oval cells or ductular hepatocytes) proliferate and differentiate into hepatocytes or biliary epithelial cells.82 Although long-term alcohol consumption inhibits hepatocyte proliferation after partial hepatectomy in a rodent model,83 the effect of alcohol on hepatocyte proliferation in patients has not been explored. It is probable that long-term alcohol consumption not only causes hepatocyte death but also inhibits hepatocyte proliferation in patients, thereby contributing to the pathogenesis of ALD.
Alcoholic Fibrosis
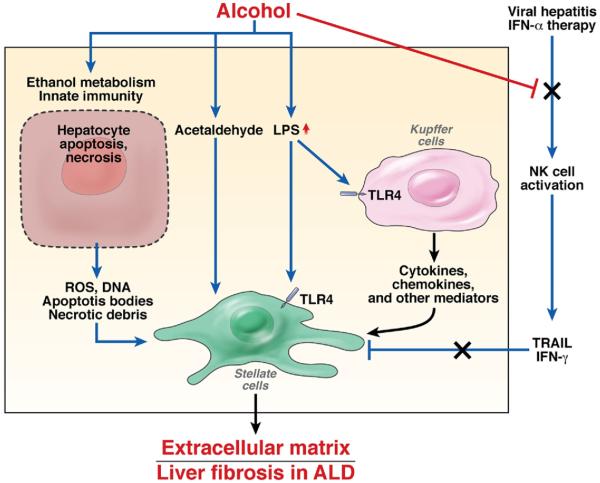
Liver fibrosis is a wound-healing response to virtually all forms of chronic liver injury; it is characterizedby excessive accumulation of collagen and other extracellular matrix proteins.84,85 Activated HSCs are the major source of the increased production of extracellular matrix proteins, along with portal fibroblasts and bone marrow–derived myofibroblasts. Hepatocellular damage increases levels of and activates many cytokines, chemokines, neuroendocrine factors, angiogenic factors, and components of the innate immune system that subsequently induce HSC activation and fibrogenesis.84,85 Most of these fibro genic mechanisms occur during alcoholic liver fibrogenesis, whereas some unique mechanisms also contribute to alcoholic liver fibrosis (Figure 4).86,87
Figure 4.
Mechanisms of liver fibrosis in patients with ALD. (1) Alcohol consumption causes hepatocyte damage, which leads to the release of a variety of mediators and the subsequent induction of stellate cell activation. (2) Acetaldehyde directly targets stellate cells and up-regulates the expression of collagens in these cells. (3) Alcohol consumption results in elevation of LPS levels in the liver. LPS can directly enhance stellate cell activation via up-regulation of TGF-β signaling and indirectly promote stellate cell activation via activation of Kupffer cells to release profibrotic cytokines and chemokines. (4) Natural killer (NK) cells are activated during viral hepatitis or IFN-α therapy. Activated natural killer cells can kill stellate cells by releasing TRAIL and inhibit stellate cell proliferation by releasing IFN-γ; they therefore have an important role in inhibiting liver fibrosis. Alcohol consumption suppresses the antifibrotic effects of NK cells and IFN-γ, thereby promoting liver fibrosis.
LPS activates TLR4
Increased serum levels of LPS are commonly found in patients with ALD. LPS not only stimulates Kupffer cells to produce reactive oxygen species and cytokines that subsequently promote activation of HSCs86,87 but also directly activates HSCs via TLR4.88 LPS can also activate TLR4 signaling in liver sinusoidal endothelial cells, resulting in the regulation of angiogenesis and subsequent promotion of fibrogenesis.89 Collectively, TLR4 signaling in immune cells (Kupffer cells) and liver nonparenchymal cells (HSCs and endothelial cells) therefore contribute to the pathogenesis of fibrosis, which is further supported by a recent report using chimeric mice in which wild-type mice underwent transplant with bone marrow cells from TLR4 knockout mice and vice versa.90
Activation of HSCs by acetaldehyde
Acetalde-hyde is produced mainly by hepatocytes and acts on HSCs in a paracrine manner; it directly increases expression of collagen I in HSCs via activation of multiple signaling pathways and transcription factors.91 Acetaldehyde reacts rapidly with cellular components, producing adducts such as malondialdehyde, 4-hydroxynonenal, and malondialdehyde-acetaldehyde, which help maintain HSC activation.91
Ethanol suppresses antifibrotic effects
Activated natural killer cells inhibit liver fibrosis by destroying activated HSCs92,93 or producing interferon (IFN)-γ, which directly induces HSC cell cycle arrest and apoptosis.94 These could play an important role in preventing progression of liver fibrosis in humans but are blocked by long-term alcohol consumption.95 Disrupted activity of natural killer cells and IFN-γ could be important factors in the pathogenesis of alcoholic liver fibrosis and alcohol-mediated acceleration of liver fibrosis in patients with viral hepatitis.
HCC
Like cirrhosis of any other etiology, alcoholic cirrhosis is a major risk factor for HCC. The mechanisms that contribute to development of HCC in patients with cirrhosis, including alcoholic cirrhosis, are complex and include telomere shortening (which induces chromosomal instability), alterations of the microenvironment and macroenvironment that promote tumor cell survival and proliferation, impairment of hepatocyte proliferation, loss of cell cycle checkpoints, and activation of oncogenic pathways.96
There are some unique mechanisms that contribute to the development of HCC specifically in patients with ALD.97,98 These include the formation of acetaldehyde, which is a carcinogen with mutagenic properties, ethanol-stimulated induction of CYP2E1, which metabolizes many of the procarcinogenic compounds in alcoholic beverages, and the immunosuppressive effect of alcohol. Increased levels of LPS in patients with ALD might synergize with HCV infection to promote liver tumorigenesis via up-regulation of cancer stem cells, indicated by analysis of stem cell markers.99
Epigenetics, miRNA, and Stem Cells
Epigenetics is the study of heritable changes in phenotype (appearance) or gene expression that are caused by mechanisms other than alterations in DNA coding sequences. Epigenetic modifications include DNA methylation, histone modifications, and RNA-based mechanisms. Ethanol consumption causes epigenetic changes that contribute to alcohol-induced organ damage, including ALD.100 In an extensive study, ethanol affected metabolism of methionine and thereby DNA methylation. Methionine metabolism occurs primarily in the liver, where homocysteine is methylated to methio-nine and then S-adenosylmethionine (SAMe) in a transmethylation reaction catalyzed by methionine adenosyltransferase.101 SAMe is a principal methyl donor in methylation and has an important role in inducing DNA and histone methylation. Long-term ethanol consumption reduces hepatic levels of SAMe and DNA and histone methylation, increasing expression of genes that regulate the endoplasmic reticulum stress response and alcoholic liver injury.26 Alcohol feeding has been shown to modulate histone methylation and acetylation in livers of mice, although the mechanism by which ethanol induces these modifications and their roles in the pathogenesis of ALD are not known. Exposure to ethanol or its metabolite acetate up-regulates histone acetylation in macrophages, contributing to the up-regulation of several proinflamma-tory cytokines that could promote AH.60,61
miRNAs are short, noncoding RNAs that are an average of only 22 nucleotides long; they control expression of genes involved in cell growth, differentiation, and apoptosis and are believed to be involved in the pathogenesis of liver disease, especially cancer.102,103 Several studies have examined the role of miRNAs in liver disorders such as ALD.103,104 Ethanol exposure up-regulates miRNA-155 in macrophages, which increases TNF-α production (via increased mRNA stability)105; short-term ethanol exposure up-regulates miRNA-212 in intestinal epithelial cells, which down-regulates zonula occludens-1 protein,106 a factor that maintains intestinal permeability. Expression of liver miRNAs has also been shown to be significantly altered in ethanol-fed mice,107 but the functions of these miRNAs in the pathogenesis of ALD are not clear.
The number of oval cells (liver progenitor cells) is significantly increased in patients with ALD; it correlates with disease severity and might increase the risk of alcoholic liver cancer.108,109 There is also evidence that bone marrow-derived stem cells contribute to the pathogenesis of ALD.110 However, in early-stage clinical studies, infusion of autologous, expanded, mobilized, adult bone marrow-derived CD34+ stem cells111 (or in vivo mobilization of these cells by injections of granulocyte colony-stimulating factor)112 did benefit patients with ALD. It is therefore important to clarify the pathogenic and therapeutic roles of CD34+ bone-derived stem cells in patients with ALD.
Current Therapies and Future Approaches
Patients with ALD are most commonly treated with approaches to eliminate alcohol intake; continued alcohol ingestion is the single most important risk factor for progression of the disease. Abstinence is also critical for patients with advanced disease who could eventually require liver transplantation, because patients who actively engage in alcohol consumption are not eligible for most transplantation programs. Referral to rehabilitation programs, in combination with family support, is usually necessary. Some patients also require specific pharmacologic treatment. Disulfiram, an irreversible inhibitor of alcohol dehydrogenase, is frequently prescribed to treat alcoholism113 but is not recommended for patients with advanced ALD because of potential severe hepatotoxicity.114 Anticraving drugs (eg, acamprosate) are effective at preventing relapses, but special care must be taken because of the potential hepatotoxicity of some of these drugs.113 An agonist of the γ-aminobutyric acid B receptor, baclofen, has been found to be effective in maintaining abstinence and is safe even for cirrhotic patients.115 The opioid antagonist naltrexone has been shown to reduce relapse, although its efficacy is modest.113
There are no approved antifibrotic drugs to prevent disease progression in patients with moderate ALD. The extent of liver fibrosis can be estimated by liver biopsy analysis or with noninvasive tools such as elastography or measurements of serum markers.116,117 Clinical trials are hampered by the poor compliance of patients with alcohol abuse and by the fact that patients enrolled in therapeutic studies often modify their drinking behavior, which can have a positive influence on disease severity.
Once cirrhosis is established, abstinent patients usually have slower disease progression than those consuming alcohol. Moreover, patients with persisting alcohol intakecan develop some degree of alcoholic steatohepatitis, which increases the risk of decompensation (conditions such as ascites, hepatic encephalopathy, variceal bleeding, or renal dysfunction) and HCC. There is no evidence that different management strategies prevent clinical decompensation in patients with alcoholic cirrhosis compared with other causes of cirrhosis, other than encouragement of alcohol intake cessation.
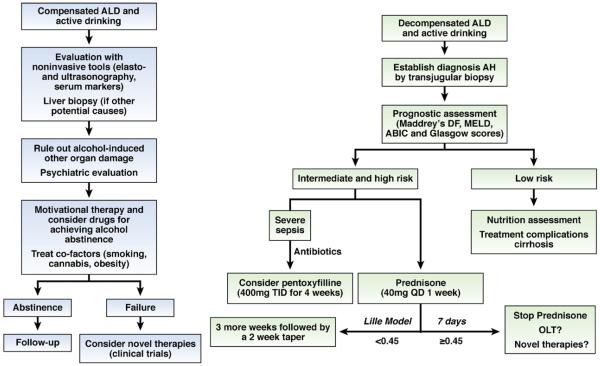
AH is a severe form of ALD; its treatment has been summarized in many excellent reviews.11,57,118-120 We briefly summarize the treatment of AH, focusing mainly on recently identified potential therapeutic targets (Figure 5).
Figure 5.
Algorithms for treatment of compensated ALD and decompensated ALD with superimposed AH. DF, discriminant function; MELD, Model for End-Stage Liver Disease; ABIC, age/bilirubin/international normalized ratio/creatinine scoring system; OLT, orthotopic liver transplantation.
Treatment of AH
Supportive treatment
Patients with severe AH could require admission to an intensive care unit. The airway should be protected in patients with acute alcoholic intoxication or an advanced degree of hepatic encephalopathy. The use of benzodiazepines is contraindicated in these patients. Given the potential risk of Wernicke encephalopathy among alcoholic and malnourished patients, the administration of B-complex vitamins is often required. A daily protein intake of 1.5 g/kg body wt has been proposed. Patients with AH are predisposed to develop severe infections, so early diagnosis and empiric antibiotic treatment are advised.
Corticosteroids
The efficacy of corticosteroids in treating patients with AH is controversial, with different findings from individual studies and meta-analyses. Several meta-analyses reported that corticosteroids increased patient survival times,121,122 and one did not support use of corticosteroids because of the heterogeneity of the clinical trials and the high risk of bias.123 Nevertheless, the current American Association for the Study of Liver Diseases practice guidelines recommend the use of corticosteroids for patients with severe AH, defined by Maddrey’s discriminant function ≥32 or hepatic encephalopathy.11 Patients must also be intensively monitored for evidence of infections, which occur in almost 25% of patients during corticosteroid treatment and are associated with a poor prognosis.124 The decision to stop corticosteroids can be based on calculation of the Lille Model after 7 days of treatment; a Lille Model score greater than 0.45 indicates failure to respond to corticosteroid treatment and predicts a 6-month survival rate of less than 25%.125 Severe acute AH is associated with significant lymphocyte corticosteroid insensitivity, which can be reduced ex vivo by theophylline administration126 or reagents that block the IL-2 receptor.127 These drugs might therefore improve the efficacy of corticosteroids in treating AH.
Pentoxifylline
Pentoxifylline is a phosphodiesterase inhibitor that blocks transcription of TNF-α to decrease serum levels of the gene product. Pentoxifylline can be used to treat patients with severe AH who cannot be given corticosteroids. It was shown to reduce the mortality of patients with severe AH, which was related to decreased development of hepatorenal syndrome.128,129 However, this effect was not appreciable after adjustment for multiple testing.130 Importantly, pentoxifylline was not effective as rescue therapy for patients who did not respond to corticosteroids.131
Anti-TNF-α agents
TNF-α has an important role in the pathogenesis of ALD in animal models, so there have been several clinical studies of the effects of infliximab or etanercept (reagents that block this cytokine) in patients with AH. Early-stage studies showed positive results in terms of survival and safety,132 but later-stage clinical trials showed that these drugs actually increased mortality and risk of infection among patients with AH.133,134 These reagents are therefore not recommended for the treatment of AH.
Nutrition therapy
Alcoholic patients often experience protein calorie malnutrition, which can promote bacterial infections. Nutritional support is recommended in patients with AH; it improves liver function and results from histologic analyses and might increase survival times based on results of short-term follow-up studies.135-137
SAMe
SAMe is a methyl donor that has been shown to protect against alcoholic liver injury via multiple mechanisms, including antioxidant functions, maintenance of mitochondrial function, and down-regulation of TNF-α.138 An early-stage trial showed that administration of SAMe as a supplemental agent significantly decreased mortality and need for liver transplantation among patients with ALD; it had a favorable safety profile.139 However, in a Cochrane report, there was no evidence to support the use of SAMe in treatment of patients with ALD. Long-term, high-quality, randomized trials are required to establish its therapeutic effects.140
Liver transplantation
Liver transplantation has been used to treat patients with decompensated ALD. Outcomes are equal to or better than those obtained when it is used to treat end-stage liver disease from other causes.141 Several liver transplantation centers have therefore proposed that this be a rescue option for patients with severe AH who do not respond to medical therapy and are unlikely to survive the mandatory, 6-month abstinence period but who fulfill all other standard criteria for transplantation, including a thorough psychosocial evaluation.118,142
Other therapies
Androgen corticosteroids have been used in attempts to improve the nutritional status of patients with AH. Although initial trials with oxandrolone had positive results, they were not confirmed in further studies; no benefit was shown in a meta-analysis.143 Pro pylthiouracil, an antithyroid drug, has also been evaluated for the treatment of acute AH. A meta-analysis of 6 clinical trials showed that propylthiouracil did not affect survival times and was associated with adverse effects.144 Because ALD is associated with increased levels of oxidative stress, a number of studies have investigated the benefits of antioxidants (eg, vitamin E and silymarin). Unfortunately, in early-stage studies, survival times of patients with AH did not increase.145,146 However, a study that evaluated the potential benefit of combining N-acetylcysteine with corticosteroids showed an increased number of patients survived a short follow-up period compared with controls.147
New Targets
The identification of therapeutic targets for ALD has been hampered by the fact that in most animal models, the extent of liver injury is mild; animals do not develop liver failure or severe portal hypertension. Animal models are urgently needed that have the features of liver injury in patients with severe forms of ALD so we can test the effects of targeting factors found to be involved in pathogenesis. Liver samples from patients with ALD are probably most suited to identify therapeutic targets because serum levels of cytokines such as TNF-α may correlate with disease severity148 but are likely to have less pathophysiological significance due to confounding factors such as impaired liver clearance or bacterial infections. A straightforward approach would be to investigate the expression and/or activation of different mediators in liver tissues from patients and correlate these with disease severity and patient outcomes and then test the biological significance of these factors in animal models. The following are examples of therapeutic targets that fulfill several of these criteria.
CXC chemokines
The CXC family of chemokines includes IL-8 and Gro-α; these usually attract neutrophils, which infiltrate livers of patients with ALD. In patients with AH, hepatic expression of CXC chemokines is increased and correlates with survival time and the degree of portal hypertension.70,149 Reagents that target CXC chemokines might be developed as therapeutics for AH.
IL-22
IL-22 is a member of the IL-10 family of cytokines, which control bacterial infection, homeostasis, and tissue repair. IL-22 might be used to treat patients with ALD because of its antioxidant, antiapoptotic, anti-steatotic, proliferative, and antimicrobial effects.150 In addition, the side effects of IL-22 could be minimal, because the IL-22 receptor is only expressed on epithelial cells such as hepatocytes. Corticosteroids, which are widely used to treat AH, have well-documented side effects that include increased risk of infection. Corticosteroids and IL-22 could therefore be a good combination treatment for AH, because IL-22 might overcome the corticosteroid-mediated promotion of infection.
Complement
Activation of complement is an important step in the development of ethanol-induced liver injury in mice.49,50,66 Therapeutic interventions to either block complement activation or increase the activity of negative regulators of complement might be used to treat patients with ALD. Several compounds that inhibit complement activation are in phase 1 or 2 trials for the treatment of age-related macular degeneration.151 These drugs may be developed to treat patients with ALD.
Gut microbiota and LPS pathway
Modulation of the gut microbiota and LPS pathways might also be used to treat patients with ALD. The gut microbiota and LPSsignaling can be modified by probiotics and TLR4 antagonists, respectively, with the latter being proposed as therapeutic agents for the treatment of chronic liver diseases, including ALD.152 Results from a placebo-controlled trial recently showed that the nonabsorbable antibiotic rifaximin, which modifies the gut microbiota, protected patients from hepatic encephalopathy.153 Reagents that alter the gut microbiota might also prevent ALD, so further studies are required.
Inhibition of apoptosis
Apoptosis is a prominent feature of chronic liver disease, so apoptosis inhibitors have been investigated in animal models of liver injury and patients with chronic liver diseases. Multiple clinical trials have shown that various caspase inhibitors reduced liver injury and fibrosis in patients with chronic HCV infection154,155 or nonalcoholic steatohepatitis.156 ALD is also associated with significant levels of hepatocyte apoptosis,62 so inhibitors might be a treatment option for ALD.
Osteopontin
Osteopontin is an extracellular matrix protein that is markedly up-regulated in patients with ALD.157 The basal expression levels of osteopontin correlate with disease severity (R. Bataller et al, unpublished data, September 2011), indicating that osteopontin contributes to the pathogenesis of ALD. Blockade of osteopontin might be effective for amelioration of ALD.
Endocannabinoids
Endocannabinoids have been shown to be involved in the pathogenesis of ALD, signaling through cannabinoid receptor (CB) 1 and CB2.158 CB1-deficient mice are resistant whereas CB2-deficient mice are more susceptible to ethanol-induced fatty liver and hepatocellular damage.29,159 These findings indicate that CB1 antagonists and CB2 agonists could be therapeutic agents for the management of ALD. Because the neuropsychiatric side effects of CB1 antagonists limit their therapeutic potential for the treatment of liver disease, the peripherally restricted CB1 antagonists have been actively explored and might offer therapeutic benefits for patients with ALD.158
NOSTRIN
NOSTRIN regulates synthesis of nitric oxide, an effector of chronic liver diseases. Patients with ALD have increased hepatic levels of NOSTRIN protein and messenger RNA; this might contribute to the decreased enzymatic activity of endothelial nitric oxide synthase and was associated with more severe portal hyper-tension.160 Further studies are needed to determine whether NOSTRIN mediates the hemodynamic derangements observed in patients with AH. It could be a therapeutic target for reducing the increased hepatic resistance observed in patients with AH.
Conclusions
ALD is a major cause of advanced liver disease worldwide. Major advances in understanding its mechanisms of pathogenesis have been made at the experimental level using animal models. Translational studies, using human liver samples, have identified new therapeutic tar gets. However, translation of basic and translational research findings into new therapies has been modest. Future efforts should be directed toward identifying the main factors that promote disease in patients with moderate and severe ALD to develop new therapies.
Abbreviations used in this paper
- ACC
acetyl-CoA carboxylase
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- AMPK
AMP-activated protein kinase
- CB
cannabinoid receptor
- HSC
hepatic stellate cell
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- miRNA
microRNA
- PPAR
peroxisome proliferator-activated receptor
- SAMe
S-adenosylmethionine
- SREBP-1c
sterol regulatory element-binding protein 1c
- STAT3
signal transducer and activator of transcription 3
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 2.Yoon Y, Yi H. Liver cirrhosis mortality in the United States, 1970-2007 surveillance report #88. NIAAA homepage. 2010 Available at: http://pubs.niaaa.nih.gov/publications/surveillance88/Cirr07.htm. [Google Scholar]
- 3.Lieber CS, Jones DP, Decarli LM. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest. 1965;44:1009–1021. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieber CS. Susceptibility to alcohol-related liver injury. Alcohol Alcohol Suppl. 1994;2:315–326. [PubMed] [Google Scholar]
- 5.Thurman RG, Bradford BU, Iimuro Y, et al. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13(Suppl):S39–S50. [PubMed] [Google Scholar]
- 6.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 7.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng L, Crabb DW. Alcoholic liver disease. Curr Opin Gastroenterol. 2001;17:211–220. doi: 10.1097/00001574-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Arteel G, Marsano L, Mendez C, et al. Advances in alcoholic liver disease. Best Pract Res Clin Gastroenterol. 2003;17:625–647. doi: 10.1016/s1521-6918(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 10.Helman RA, Temko MH, Nye SW, et al. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311–321. doi: 10.7326/0003-4819-74-3-311. [DOI] [PubMed] [Google Scholar]
- 11.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto H, Machida K, Dynnyk A, et al. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–187. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27:44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- 14.Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 15.Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Lai KK, Verlinsky A, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatton J, Burton A, Nash H, et al. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–592. doi: 10.1111/j.1360-0443.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 18.Tian C, Stokowski RP, Kershenobich D, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 19.Stickel F, Buch S, Lau K, et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 20.Trépo E, Gustot T, Degré D, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–912. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Chang B, Li L, et al. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao B. Comprehensive handbook of alcohol related pathology. Vol. 2. Academic Press Inc; New York: 2005. Alcohol and hepatitis virus interactions in liver pathology; pp. 819–832. [Google Scholar]
- 23.Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Semin Liver Dis. 2009;29:188–199. doi: 10.1055/s-0029-1214374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res. 1979;20:289–315. [PubMed] [Google Scholar]
- 25.You M, Fischer M, Deeg MA, et al. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 26.Esfandiari F, Medici V, Wong DH, et al. Epigenetic regulation of hepatic endoplasmic reticulum stress pathways in the ethanolfed cystathionine beta synthase-deficient mouse. Hepatology. 2010;51:932–941. doi: 10.1002/hep.23382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- 28.Peng Z, Borea PA, Varani K, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhao XJ, Dong Q, Bindas J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrasek J, Dolganiuc A, Csak T, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649–660. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMullen MR, Pritchard MT, Wang Q, et al. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin M, Wheeler MD, Kono H, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 34.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You M, Matsumoto M, Pacold CM, et al. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 36.You M, Liang X, Ajmo JM, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 37.You MRC. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;2009:850–859. doi: 10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- 38.Horiguchi N, Wang L, Mukhopadhyay P, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Yu S, Rao S, Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3:561–572. doi: 10.2174/1566524033479537. [DOI] [PubMed] [Google Scholar]
- 41.Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 42.Galli A, Pinaire J, Fischer M, et al. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Zhuge J, Wang X, et al. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 44.You M, Considine RV, Leone TC, et al. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang X, Zhong W, Liu J, et al. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nath B, Levin I, Csak T, et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard MT, McMullen MR, Stavitsky AB, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–1126. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen JI, Roychowdhury S, McMullen MR, et al. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–674. doi: 10.1053/j.gastro.2010.04.041. 674 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaiser JP, Beier JI, Zhang J, et al. PKCepsilon plays a causal role in acute ethanol-induced steatosis. Arch Biochem Biophys. 2009;482:104–111. doi: 10.1016/j.abb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKim SE, Gabele E, Isayama F, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 53.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donohue TM., Jr Autophagy and ethanol-induced liver injury. World J Gastroenterol. 2009;15:1178–1185. doi: 10.3748/wjg.15.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu D, Wang X, Zhou R, et al. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 58.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farfan Labonne BE, Gutierrez M, Gomez-Quiroz LE, et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 60.Kendrick SF, O’Boyle G, Mann J, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988–1997. doi: 10.1002/hep.23572. [DOI] [PubMed] [Google Scholar]
- 61.Shen Z, Ajmo JM, Rogers CQ, et al. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 63.Tatsukawa H, Fukaya Y, Frampton G, et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology. 2009;136:1783–1795. doi: 10.1053/j.gastro.2009.01.007. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roychowdhury S, McMullen MR, Pritchard MT, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandal P, Park PH, McMullen MR, et al. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–1429. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller AM, Wang H, Bertola A, et al. Inflammation-associated IL-6/STAT3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in IL-10 deficient mice. Hepatology. 2011;54:846–856. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maltby J, Wright S, Bird G, et al. Chemokine levels in human liver homogenates: associations between GRO alpha and histopatho-logical evidence of alcoholic hepatitis. Hepatology. 1996;24:1156–1160. doi: 10.1053/jhep.1996.v24.pm0008903391. [DOI] [PubMed] [Google Scholar]
- 70.Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 71.Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Quiroz L, Bucio L, Souza V, et al. Interleukin 8 response and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde or lipopolysaccharide. Hepatol Res. 2003;26:134–141. doi: 10.1016/s1386-6346(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 73.Joshi-Barve S, Barve SS, Amancherla K, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 74.Joshi-Barve S, Barve SS, Butt W, et al. Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38:1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 75.Paik YH, Lee KS, Lee HJ, et al. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006;86:676–686. doi: 10.1038/labinvest.3700422. [DOI] [PubMed] [Google Scholar]
- 76.Maher JJ. Rat hepatocytes and Kupffer cells interact to produce interleukin-8 (CINC) in the setting of ethanol. Am J Physiol. 1995;269:G518–G523. doi: 10.1152/ajpgi.1995.269.4.G518. [DOI] [PubMed] [Google Scholar]
- 77.Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicol Mech Methods. 2007;17:431–440. doi: 10.1080/00952990701407702. [DOI] [PubMed] [Google Scholar]
- 78.Albano E, Vidali M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010;5:141–147. doi: 10.1007/s12263-009-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mottaran E, Stewart SF, Rolla R, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 80.Thiele GM, Duryee MJ, Willis MS, et al. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 2010;34:2126–2136. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 82.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saso K, Moehren G, Higashi K, et al. Differential inhibition of epidermal growth factor signaling pathways in rat hepatocytes by long-term ethanol treatment. Gastroenterology. 1997;112:2073–2088. doi: 10.1053/gast.1997.v112.pm9178701. [DOI] [PubMed] [Google Scholar]
- 84.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–878. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- 87.Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis. 2009;29:211–221. doi: 10.1055/s-0029-1214376. [DOI] [PubMed] [Google Scholar]
- 88.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 89.Jagavelu K, Routray C, Shergill U, et al. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inokuchi S, Tsukamoto H, Park E, et al. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mello T, Ceni E, Surrenti C, et al. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Radaeva S, Sun R, Jaruga B, et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 93.Muhanna N, Abu Tair L, Doron S, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–98. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 94.Jeong WI, Park O, Radaeva S, et al. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 95.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 97.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 98.McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 99.Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic onco-genesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shukla SD, Velazquez J, French SW, et al. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32:1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 101.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 102.Visone R, Petrocca F, Croce CM. Micro-RNAs in gastrointestinal and liver disease. Gastroenterology. 2008;135:1866–1869. doi: 10.1053/j.gastro.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 103.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;15:5633–5640. doi: 10.3748/wjg.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miranda RC, Pietrzykowski AZ, Tang Y, et al. MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res. 2010;34:575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bala S, Marcos M, Kodys K, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 107.Dolganiuc A, Petrasek J, Kodys K, et al. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung Y, Brown KD, Witek RP, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalakas E, Newsome PN, Boyle S, et al. Bone marrow stem cells contribute to alcohol liver fibrosis in humans. Stem Cells Dev. 2010;19:1417–1425. doi: 10.1089/scd.2009.0387. [DOI] [PubMed] [Google Scholar]
- 111.Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 112.Spahr L, Lambert JF, Rubbia-Brandt L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 113.Soyka M, Rosner S. Emerging drugs to treat alcoholism. Expert Opin Emerg Drugs. 2010;15:695–711. doi: 10.1517/14728214.2010.500811. [DOI] [PubMed] [Google Scholar]
- 114.Mohanty SR, LaBrecque DR, Mitros FA, et al. Liver transplantation for disulfiram-induced fulminant hepatic failure. J Clin Gastroenterol. 2004;38:292–295. doi: 10.1097/00004836-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 115.Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–2117. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- 116.Angulo P. Noninvasive assessment of fibrosis and steatosis in NASH and ASH. Gastroenterol Clin Biol. 2009;33:940–948. doi: 10.1016/j.gcb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Martinez SM, Crespo G, Navasa M, et al. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 118.Stickel F, Seitz HK. Alcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2010;24:683–693. doi: 10.1016/j.bpg.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Bergheim I, McClain CJ, Arteel GE. Treatment of alcoholic liver disease. Dig Dis. 2005;23:275–84. doi: 10.1159/000090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rongey C, Kaplowitz N. Current concepts and controversies in the treatment of alcoholic hepatitis. World J Gastroenterol. 2006;12:6909–6921. doi: 10.3748/wjg.v12.i43.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299–307. doi: 10.7326/0003-4819-113-4-299. [DOI] [PubMed] [Google Scholar]
- 122.Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 123.Rambaldi A, Saconato HH, Christensen E, et al. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167–1178. doi: 10.1111/j.1365-2036.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 124.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 125.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 126.Kendrick SF, Henderson E, Palmer J, et al. Theophylline improves steroid sensitivity in acute alcoholic hepatitis. Hepatology. 2010;52:126–131. doi: 10.1002/hep.23666. [DOI] [PubMed] [Google Scholar]
- 127.di Mambro AJ, Parker R, McCune A, et al. In vitro steroid resistance correlates with outcome in severe alcoholic hepatitis. Hepatology. 2011;53:1316–1322. doi: 10.1002/hep.24159. [DOI] [PubMed] [Google Scholar]
- 128.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 129.De BK, Gangopadhyay S, Dutta D, et al. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613–1619. doi: 10.3748/wjg.15.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Whitfield K, Rambaldi A, Wetterslev J, et al. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev. 2009:CD007339. doi: 10.1002/14651858.CD007339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in nonresponders to corticosteroids. J Hepatol. 2008;48:465–470. doi: 10.1016/j.jhep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 132.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448–455. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 133.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 134.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cabre E, Rodriguez-Iglesias P, Caballeria J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 136.Foody W, Heuman DD, Mihas AA, et al. Nutritional therapy for alcoholic hepatitis: new life for an old idea. Gastroenterology. 2001;120:1053–1054. doi: 10.1016/s0016-5085(01)83918-4. [DOI] [PubMed] [Google Scholar]
- 137.Stickel F, Hoehn B, Schuppan D, et al. Review article: nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18:357–373. doi: 10.1046/j.1365-2036.2003.01660.x. [DOI] [PubMed] [Google Scholar]
- 138.Lu SC, Martinez-Chantar ML, Mato JM. Methionine adenosyltransferase and S-adenosylmethionine in alcoholic liver disease. J Gastroenterol Hepatol. 2006;21(Suppl 3):S61–S64. doi: 10.1111/j.1440-1746.2006.04575.x. [DOI] [PubMed] [Google Scholar]
- 139.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 140.Rambaldi A, Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst Rev. 2006:CD002235. doi: 10.1002/14651858.CD002235.pub2. [DOI] [PubMed] [Google Scholar]
- 141.Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry) Am J Transplant. 2010;10:138–148. doi: 10.1111/j.1600-6143.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 142.Dureja P, Lucey MR. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol. 2010;52:759–764. doi: 10.1016/j.jhep.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 143.Rambaldi A, Iaquinto G, Gluud C. Anabolic-androgenic steroids for alcoholic liver disease: a Cochrane review. Am J Gastroenterol. 2002;97:1674–1681. doi: 10.1111/j.1572-0241.2002.05826.x. [DOI] [PubMed] [Google Scholar]
- 144.Fede G, Germani G, Gluud C, et al. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst Rev. 2011;6:CD002800. doi: 10.1002/14651858.CD002800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mezey E, Potter JJ, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–46. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 146.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen-Khac E, Thevenot T, Piquet M, et al. Treatment of severe acute alcoholic hepatitis with corticoids plus N-acetyl cysteine versus corticoids alone: a multicentre, randomized, controlled trial. Hepatology. 2009;50:346A. [Google Scholar]
- 148.Bird GL, Sheron N, Goka AK, et al. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 149.Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–697. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 150.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Charbel Issa P, Chong NV, Scholl HP. The significance of the complement system for the pathogenesis of age-related macular degeneration — current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2011;249:163–174. doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 154.Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 155.Shiffman ML, Pockros P, McHutchison JG, et al. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor — a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:969–978. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 156.Ratziu V, Chojkiet M, Sheikh M, et al. Safety, tolerability and preliminary activity of GS-9450, a selective caspase inhibitor, in patients with non-alcoholic steatohepatitis. J Hepatol. 2010;52:S38. [Google Scholar]
- 157.Seth D, Gorrell MD, Cordoba S, et al. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol. 2006;45:306–320. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 158.Tam J, Liu J, Mukhopadhyay B, et al. Endocannabinoids in liver disease. Hepatology. 2011;53:346–355. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Louvet A, Teixeira-Clerc F, Chobert M, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating kupffer cell polarization in mice. Hepatology. 2011;54:1217–1226. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 160.Mookerjee RP, Wiesenthal A, Icking A, et al. Increased gene and protein expression of the novel eNOS regulatory protein NOSTRIN and a variant in alcoholic hepatitis. Gastroenterology. 2007;132:2533–2541. doi: 10.1053/j.gastro.2006.12.035. [DOI] [PubMed] [Google Scholar]