Abstract
Pentosuria is one of four conditions hypothesized by Archibald Garrod in 1908 to be inborn errors of metabolism. Mutations responsible for the other three conditions (albinism, alkaptonuria, and cystinuria) have been identified, but the mutations responsible for pentosuria remained unknown. Pentosuria, which affects almost exclusively individuals of Ashkenazi Jewish ancestry, is characterized by high levels of the pentose sugar l-xylulose in blood and urine and deficiency of the enzyme l-xylulose reductase. The condition is autosomal-recessive and completely clinically benign, but in the early and mid-20th century attracted attention because it was often confused with diabetes mellitus and inappropriately treated with insulin. Persons with pentosuria were identified from records of Margaret Lasker, who studied the condition in the 1930s to 1960s. In the DCXR gene encoding l-xylulose reductase, we identified two mutations, DCXR c.583ΔC and DCXR c.52(+1)G > A, each predicted to lead to loss of enzyme activity. Of nine unrelated living pentosuric subjects, six were homozygous for DCXR c.583ΔC, one was homozygous for DCXR c.52(+1)G > A, and two were compound heterozygous for the two mutant alleles. l-Xylulose reductase was not detectable in protein lysates from subjects’ cells and high levels of xylulose were detected in their sera, confirming the relationship between the DCXR genotypes and the pentosuric phenotype. The combined frequency of the two mutant DCXR alleles in 1,067 Ashkenazi Jewish controls was 0.0173, suggesting a pentosuria frequency of approximately one in 3,300 in this population. Haplotype analysis indicated that the DCXR c.52(+1)G > A mutation arose more recently than the DCXR c.583ΔC mutation.
Keywords: dicarbonyl reductase, exome sequencing, glucuronic acid, history of genetics
In his 1908 Croonian Lectures to the Royal College of Physicians of London, Archibald Garrod introduced the concept of “inborn errors of metabolism” (1). He illustrated the concept with albinism, alkaptonuria, cystinuria, and pentosuria, each of which he postulated to be caused by recessive inheritance of a disease-specific chemical error (2). His concept was the first link between Mendelian factors, which had been rediscovered not long before (3), and enzyme chemistry, also then in its infancy (4). For three of the four traits described by Garrod, the underlying mutations have been identified (reviewed in ref. 5). In contrast, the molecular genetic basis of pentosuria remains unknown.
Pentosuria (MIM 260800), first described in 1892 (6), is characterized by high urinary excretion (1–4 gm/d) of the pentose sugar l-xylulose. In 1970, the critical enzyme deficiency was identified as l-xylulose reductase (7). The phenotype results from a defect in the glucuronic acid oxidation pathway (8). In this pathway, the carboxyl carbon atom of d-glucuronic acid is removed in a series of reactions, giving rise to the pentose l-xylulose, which is then converted to xylitol, and hence to d-xylulose, which may be phosphorylated to participate in reactions of the pentose phosphate pathway, leading to its conversion to hexose phosphate (9). In pentosuria, failure to convert l-xylulose to xylitol leads to accumulation of l-xylulose. Pentosuria is completely benign, but in the first half of the 20th century attracted attention because it was confused with diabetes (9). For as long as standard testing for urine sugars did not differentiate between glucose (the six-carbon sugar of diabetes mellitus) and pentose (the five-carbon sugar excreted in pentosuria), persons with pentosuria were often inappropriately treated with insulin, leading to hypoglycemic reactions. Once a specific test for glycosuria was developed, individuals with pentosuria no longer came to clinical attention. Pentosuria is found almost exclusively among persons of Ashkenazi Jewish ancestry. The confusion of pentosuria and glycosuria in this population in the early 20th century motivated the intensive study of pentosuria by Margaret Lasker (1884–1976), a clinical biochemist working at Montefiore Hospital in New York. Margaret Lasker developed an accurate method for testing for l-xylulose in urine (10), a major contribution to resolving diagnostic errors. Based on extensive pedigree and survival analyses of persons with pentosuria and their families, she concluded that the trait was inherited as an autosomal recessive (11) and had no impact on mortality (12). The gene DCXR, encoding l-xylulose reductase, was cloned in 2002 (13) and its tetrameric structure was elucidated in 2004 (14), but mutations responsible for pentosuria were not identified.
Results
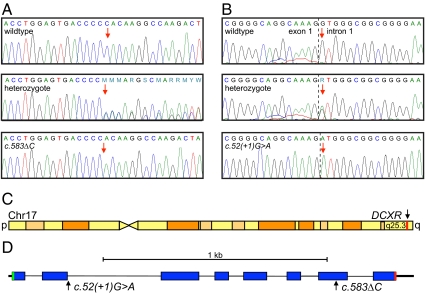
In the course of sequencing genomic DNA, we serendipitously discovered in an individual of Ashkenazi Jewish ancestry a heterozygous single base-pair deletion in DCXR (MIM 608347), which encodes l-xylulose reductase. The single base-pair deletion, chr17:79,994,115delG, corresponds to DCXR c.583ΔC (NM_016289) and leads to a stop six codons after the frameshift (DCXR p.His195fs6X) (Fig. 1A). We hypothesized that the predicted loss of more than 50 amino acids resulting from this mutation would abrogate l-xylulose reductase function and therefore could be the cause of pentosuria in homozygous individuals.
Fig. 1.
Pentosuria-associated mutations in the L-xylulose reductase gene DCXR. (A) Deletion of a single cytosine at chr17:79,994,115 (red arrow) corresponds to DCXR c.583ΔC. (B) Substitution at chr17:79,995,506 at the first base pair of intron 1 (red arrow) corresponds to DCXR c.52(+1)G > A. (C) Sketch of chromosome 17 indicating the position of DCXR in red. (D) Sketch of the DCXR gene, indicating the exon structure (blue bars), the translation start (green) and stop (red), and the positions of the two pentosuria-associated mutations.
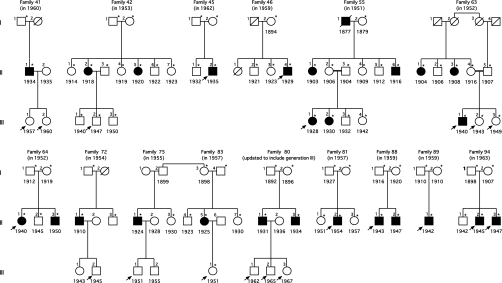
The challenge to linking the DCXR genotype to the pentosuria phenotype was that pentosurics are no longer identified because current routine urine tests do not reveal pentosuria and the condition is completely benign. However, in the hopes of future research on pentosuria, at the end of her career, Margaret Lasker bequeathed to one of the authors (A.G.M.) the data and records of more than 90 pentosuric families she had studied between 1930 and 1964. We were therefore able to search for surviving pentosuric probands and adult children of pentosurics from these families. We located and contacted informative members of 15 families, some of whom had been previously described in the literature (9, 12, 15–17). All families contacted agreed to participate in the present study (Fig. 2). Genomic DNA from all participants was genotyped for DCXR c.583ΔC by Sanger sequencing. Probands of six families (45.II-2, 46.II-4, 64.II-1, 88.II-1, 89.II-1, 94.II-2, and 94.II-3) were homozygous for this allele. In addition, children of deceased pentosuria probands from six other families (41.III-1, 41.III-2, 42.III-2, 72.III-2, 75-III.1, 80.III-1, 80.III-2, 80.III-3, and 83.III-1) were heterozygous for DCXR c.583ΔC. However, two pentosuric subjects (55.III-1 and 63.III-1) were heterozygous for DCXR c.583ΔC and one pentosuric subject (81.II-2) had only wild-type sequence at this site. To determine whether additional DCXR mutations were responsible for pentosuria in these individuals, we sequenced the complete DCXR gene from their genomic DNA and identified a second mutation at chr17:79,995,506C > T, equivalent to DCXR c.52(+1)G > A (Fig. 1 B–D). Among the three unresolved pentosuric subjects, one (81.II-2) was homozygous for DCXR c.52(+1)G > A and two (55.III-1 and 63.III-1) were compound heterozygotes for both DCXR c.583ΔC and DCXR c.52(+1)G > A.
Fig. 2.
Fifteen families from the series of Margaret Lasker, with pedigree structures as constructed by her at the dates indicated in parentheses. Asterisks (*) indicate persons tested for pentosuria in the 1950s; black symbols represent persons with pentosuria; white symbols represent persons without pentosuria; birth years are indicated under symbols. Arrows indicate participants in the present project. Recently available information indicates that families 75 and 83 are related, as shown. Family 80 has been updated to include participants born in the 1960s.
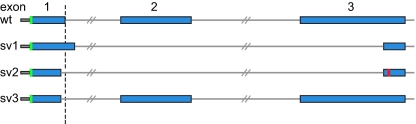
DCXR c.52(+1)G > A alters the splice donor site of DCXR intron 1 (Fig. 3). Analysis of DCXR cDNA from lymphoblast cell lines of proband 81.II-2 revealed aberrant splicing leading to multiple mutant DCXR transcripts. Mutant transcripts (n = 68) from this allele were cloned and sequenced. All clones had aberrant splicing at the 3′ end of exon 1. In contrast, in an individual without the DCXR c.52(+1)G > A mutation, 100% of transcripts (n = 76) spliced correctly at the 3′ end of exon 1.
Fig. 3.
Mutant transcripts from the DCXR c.52(+1)G > A allele. Transcripts (n = 68) from this allele were cloned and sequenced. Exons 1 to 3 are diagrammed for the wild-type sequence. The site of the splice mutation in the genomic sequence is illustrated with a dashed line. Splice variant 1 (sv1, 41% of clones) extends exon 1 by 17 bp, skips exon 2, and splices into exon 3 at a site 120 bp after the canonical splice site. This variant creates an in-frame deletion of 68 aa. Splice variant 2 (sv2, 29% of clones) deletes 6 bp at the end of exon 1 and splices into exon 3 at the site 120 bp from the canonical splice. This variant introduces a frame-shift and premature stop (shown in red). Splice variant 3 (sv3, 12% of clones) deletes 6 bp at the 3′ end of exon 1 and splices canonically into exon 2 and then exon 3, creating an in-frame deletion of Lys17 and Gly18. The remaining 18% of clones represented multiple transcripts, each present once or twice, and all with aberrant splicing at the 3′ end of exon 1.
Both pentosuria-associated DCXR mutations are likely to be deleterious to the structure and function of the DCXR protein. DCXR functions in vivo as a homotetramer (13), requiring the dinucleotide cofactor NADPH. Examination of the 3D structure of DCXR bound to NADP+ indicates that residues in the C-terminal region are important for interaction between individual monomers (14, 18). Given that the protein encoded by the DCXR c.583ΔC allele lacks more than 50 C-terminal amino acids of the 244-aa protein, formation of a functional tetramer by this allele is likely to be reduced or eliminated. The mutant transcripts generated by the DCXR c.52(+1)G > A allele (Fig. 3) are also likely to lead to loss of normal protein function. The deleted region of the first mutant transcript from DCXR c.52(+1)G > A encodes a large part of the NADP+ cofactor-binding domain (14, 18). The second mutant transcript from DCXR c.52(+1)G > A encodes only 15 wild-type amino acids. The third mutant transcript from DCXR c.52(+1)G > A leads to deletion of Lys17 and Gly18, altering the NADP+ contact residues at Lys17 and Ile19 (14).
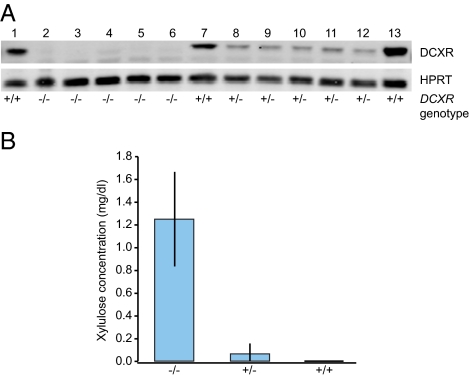
To investigate the effects of the mutations on DCXR protein expression, we analyzed lysates from lymphoblast cell lines of wild-type and mutant DCXR genotypes by immunoblotting. We used an antibody directed against an epitope predicted to be present in all mutant proteins except the 25-aa product of the DCXR c.52(+1)G > A allele. No DCXR protein was detectable in individuals with pentosuria of any genotype (Fig. 4A). This observation suggests that neither DCXR mutant allele produces stable protein. In addition, DCXR protein levels were substantially lower in heterozygous individuals than in individuals with normal DCXR genotypes, reflecting the heterozygote effect previously detected by enzyme assays (19).
Fig. 4.
DCXR protein levels and plasma xylulose levels associated with DCXR genotypes. (A) Western blot of DCXR protein from lymphoblasts. Subjects with +/+ genotypes (lanes 1, 7, and 13) have normal DCXR sequence. Subjects with −/− genotypes are homozygous for c.52(+1)G > A (lane 2), homozygous for c.583ΔC (lanes 3–5), or compound heterozygous for c.52(+1)G > A/c.583ΔC (lane 6). Subjects with +/− genotypes are heterozygous for a wild-type allele and c.583ΔC (lanes 8–12). DCXR protein is undetectable in all individuals homozygous or compound heterozygous for mutant DCXR genotypes and reduced in heterozygous individuals relative to those with normal genotypes. HPRT was used as a loading control. (B) Xylulose levels detected by HILIC-APCI-MS in plasma of subjects of the DCXR genotypes defined above. Plasma of individuals of mutant (−/−) DCXR genotypes contains elevated xylulose, whereas xylulose levels are extremely low in plasma of individuals with heterozygous (+/−) or wild-type (+/+) genotypes.
The definitive indication of pentosuria is elevated levels of l-xylulose in urine and blood (16, 20, 21). The individuals in our study had been last evaluated for pentosuria more than 50 y ago. To verify that they still expressed the condition and that the phenotype is indeed associated with mutations in the DCXR gene, we measured the level of xylulose in blood plasma of participants of various pentosuria genotypes using hydrophilic-interaction liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (HILIC-APCI-MS) (22). The amount of xylulose was at or below the limit of detection for the method (1.7 × 105 mg/dL) in plasma samples from control (+/+) or heterozygous (+/−) individuals (Fig. 4B, Figs. S1 and S2, and Table S1). The absence of xylulose accumulation in heterozygous individuals suggests that reduced levels of xylulose reductase are sufficient to convert xylulose to xylitol. In contrast, in all plasma samples from individuals homozygous for either DCXR mutation or compound heterozygous for the two mutations, the amount of xylulose ranged from 0.9 to 1.9 mg/dL. The HILIC-APCI-MS method does not distinguish between l- and d-xylulose, so it is necessary to assume that these levels of xylulose reflect elevated l-xylulose. These results confirm the original diagnoses of pentosuria in these individuals and support the conclusion that mutations in the DCXR gene are associated with the condition.
Given that we found two DCXR mutations for pentosuria in the Ashkenazi Jewish subjects from Margaret Lasker's studies, it was of interest to evaluate the historical origins of the two mutant DCXR alleles. To determine the frequencies of the two DCXR mutations in the Ashkenazi Jewish population, we genotyped these alleles in 1,067 individuals of Ashkenazi Jewish ancestry, identifying 29 individuals heterozygous for DCXR c.583ΔC and 8 individuals heterozygous for DCXR c.52(+1)G > A. No controls were homozygous or compound heterozygous for the DCXR alleles. The corresponding allele frequencies among controls are thus 0.0136 for DCXR c.583ΔC, 0.0037 for DCXR c.52(+1)G > A, and 0.0173 for the two alleles combined, leading to an expected frequency of pentosuria in this population of (0.0173)2 = 0.000300, or ∼1 in 3,300 individuals.
As a recessive trait, pentosuria appears more frequently in a historically endogamous population. Deviation of DCXR genotypes from Hardy-Weinberg equilibrium could be evaluated by comparing observed numbers of pentosuric probands of each genotype, with expected numbers based on allele frequencies among controls. No deviation from Hardy-Weinberg equilibrium was discernable in this small series (Table S2). It is interesting to note also that the two probands who were compound heterozygotes for the two mutant alleles (55.III-1 and 63.III-1) are children of the only consanguineous marriages in these 15 families, the opposite of the pattern expected if the prevalence of pentosuria depended on marriages of closely related individuals. The absence of assortative mating with respect to DCXR genotype was also supported by the geographic origins in Europe [as originally reported to Margaret Lasker (23)] of the families harboring each of the DCXR alleles. In the 19th century, ancestors of the study families and of controls carrying the DCXR alleles lived in all parts of central and eastern Europe, with no apparent regional differences by DCXR genotype (Table S3).
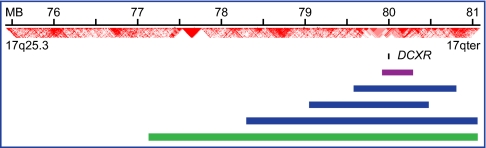
Relative ages of alternate alleles of a gene can be evaluated by homozygosity mapping of the critical genomic region. Lengths of homozygous intervals on chromosome 17q25.3 harboring DCXR were calculated for unrelated subjects homozygous for DCXR c.583ΔC, for the one subject homozygous for DCXR c.52(+1)G > A, and for unrelated Ashkenazi Jewish controls wild-type at DCXR (Fig. 5 and Table S4). The longer homozygous region surrounding DCXR c.52(+1)G > A indicates that this allele has a more recent origin than DCXR c.583ΔC. Longer homozygous regions at both mutant alleles compared with wildtype DCXR in Ashkenazi controls suggest that both DCXR mutations appeared well after the European origin of the Ashkenazi Jewish population.
Fig. 5.
Homozygosity mapping at DCXR on chromosome 17q25.3. The homozygous region surrounding DCXR for the subject homozygous for DCXR c.52(+1)G > A (green bar) is longer than those of subjects homozygous for DCXR c.583ΔC (blue bars), and much longer than those of Ashkenazi Jewish control subjects with wild-type DCXR sequences (mean length for 11 controls shown by the purple bar; details in Table S4). Position of the DCXR locus is indicated by the small black bar. Linkage disequilibrium values based on D′ from phased genotypes of individuals of European ancestry in the HapMap cohort (http://hapmap.ncbi.nlm.nih.gov) are shown in red. Relative lengths of the homozygous regions suggest that DCXR c.52(+1)G > A arose more recently than DCXR c.583ΔC and that both appeared after the European origin of the Ashkenazi Jewish population.
Discussion
The identification of DCXR c.583ΔC and DCXR c.52(+1)G > A as alleles responsible for pentosuria resolves the molecular genetics of Garrod's fourth inborn error of metabolism. That DCXR harbors the mutations responsible for pentosuria is not surprising, as deficiency of l-xylulose reductase has been known since 1970 to be the essential enzymatic defect in pentosuria (7) and the DCXR gene was cloned in 2002 (13). The missing piece of the puzzle—identification of the DCXR mutations responsible for pentosuria—could be supplied only by sequencing DCXR in pentosuric individuals. Because pentosuria no longer comes to medical attention, the association of phenotype and genotype was possible only because families with pentosuria studied more than 50 y ago remained accessible, thanks to the foresight of Margaret Lasker in bequeathing her records on pentosuric families to A.G.M. for potential future studies. The close association of DCXR genotype and loss of l-xylulose reductase enzyme activity is illustrated by the consistency of our allele frequency estimates based on genotype with previous estimates based on enzyme analysis. The frequency of pentosuria carrier status in the Ashkenazi Jewish population was calculated by Lane and Jenkins in 1985, based on the proportion of healthy medical students with reduced erythrocyte levels of l-xylulose reductase (19). Assuming that individuals with enzyme activity >2 SD below the sample mean were heterozygous for a pentosuria allele, these investigators estimated the allele frequency as 0.0127. Their result is close to our estimate of 0.0173 for the combined frequency for the two pentosuria alleles in the Ashkenazi Jewish population. Pentosuria has also been described in a large Lebanese kindred with relatives in Lebanon and South Africa (24, 25), in a Japanese family (26), and in a Canadian Indian of Athabascan ancestry in British Columbia (27). The alleles responsible for the phenotype in these individuals remain unknown.
The allele frequencies of the two DCXR mutations causing pentosuria in the Ashkenazi Jewish population follow a pattern characteristic of other rare recessive alleles in this population. As indicated in the Israeli National Genetic Database, for most of the “Jewish genetic diseases” two or more mutations in the same gene have been observed, one relatively common and the others much less common (http://www.goldenhelix.org/server/israeli/). This pattern is the the same for Tay Sachs disease, Canavan disease, Bloom syndrome, maple syrup urine disease, mucolipodosis IV, and Gaucher disease. For genetic diseases that are relatively common both in the Ashkenazi Jewish population and elsewhere (such as cystic fibrosis and connexin26-associated hearing loss), two or more mutations in the same gene have also been observed, the more common allele found throughout Europe and the other alleles specific to the Ashkenazi population. It has been suggested that for recessive diseases, an ancient mutation increases in frequency by selection, drift, or both, but rarer mutations become phenotypically evident through compound heterozygosity as the result of endogamy (28).
Risch et al. have suggested that founder alleles in the Ashkenazi Jewish population date to one of three time periods: the founding and expansion of the Jewish population in the Middle East >100 generations ago, the founding and expansion of the Ashkenazi Jewish population in Central Europe ∼50 generations ago, or the founding and expansion of the Jewish population in Lithuania and nearby regions of the Pale of Settlement ∼12 generations ago (29). As more heterozygous carriers of these DCXR alleles are identified, it will be interesting to determine the ages of these mutations relative to these periods of Jewish history in Europe.
The fact that pentosuria is a benign condition, with no detectable effect on health or lifespan, suggests that DCXR and the glucuronate oxidation pathway might be entirely expendable. However, it has been hypothesized that the enzyme encoded by DCXR has additional roles and may be important under certain conditions. In addition to l-xylulose, substrates of the enzyme include compounds with α-dicarbonyl groups (13). Reactive carbonyl compounds are produced by metabolic reactions and production is enhanced in response to oxidative stress. These carbonyl compounds combine with serum and tissue components to form advanced glycation end-products (30–32), accumulation of which are associated with diabetes and renal failure (30, 31, 33, 34). It is hypothesized that reduction of dicarbonyl compounds by DCXR could provide a detoxification function and result in reduced advanced glycation end-product accumulation (13). Consistent with this hypothesis, it was found that renal α-dicarbonyl compounds were decreased in DCXR-transgenic mice after renal unilateral ureteral obstruction (35), and that diabetic symptoms were reduced when DCXR-transgenic mice were crossed to the KK-Ay diabetic model (36). These results suggest that the level of xylulose reductase activity may be important under some disease conditions. With the identification of the mutations leading to pentosuria in the Ashkenazi Jewish population, it would be possible to test whether diabetic nephropathy is more frequent among Ashkenazi Jewish individuals with DCXR loss of function mutations.
The contribution of Garrod's inborn errors of metabolism has been more fully appreciated in recent years than during his lifetime (2, 4, 5). Garrod established the conceptual basis for the convergence of Mendelian genetics and chemistry. He recognized that metabolism was dynamic, and that a block in an otherwise normal pathway, causing a build-up of a metabolic intermediate, could lead to disease. He further understood that these errors could be inherited and were more frequent in consanguineous families and in communities with a history of endogamous marriages. He recognized the challenge of disentangling inherited and environmental causes of the same phenotype and of the importance of rare alleles, which he called “sports,” as the causes of these conditions (37). The identification of mutations responsible for the fourth of Garrod's original inborn errors of metabolism adds another chapter to this story and illustrates the power of modern genomics to elucidate the mechanism of mutational action.
Materials and Methods
Human Subjects and DNA.
Families were identified from the records of Margaret Lasker and contacted by letter. After explanation of the project by letter and by phone, subjects provided informed consent and blood samples. DNA was extracted from blood, and cell lines were established from lymphoblasts, both using standard methods. Control subjects were 1,067 unrelated individuals of Ashkenazi Jewish ancestry who had given prior permission for their anonymous DNA samples to be used for genomics projects in our laboratory. The project was approved by the Human Subjects Division of the University of Washington (protocol 27824).
DNA Sequencing.
For exome sequencing of the individual in whom heterozygosity at DCXR was first discovered, a library was prepared from genomic DNA extracted from blood and hybridized to cRNA oligonucleotide baits from the SureSelect Human All Exon kit (Agilent Technologies). Paired-end reads of 76 base pairs were generated on our Illumina GAIIx, as previously described (38). Average coverage for this sample was 125×. The variant chr17:79,994,115 delG was verified by Sanger sequencing. For Sanger sequencing of DCXR in all participants, DCXR exons were amplified by PCR and amplified products were sequenced on an ABI 3100 Genome Analyzer. Primers are listed in Table S5.
Transcript Analysis.
Transcripts produced by the DCXR c.52(+1)G > A allele were identified by amplifying and cloning exons 1 to 6 from lymphoblast cDNA, using PCR primers indicated in Table S5. Individual clones were sequenced.
Western Blotting.
Lymphoblast cell lysates (25 μg) were separated by SDS/PAGE, transferred to Immobilon-FL membrane (Millipore), and blotted with antibodies against DCXR (1:250, clone 6A6; Sigma-Aldrich) and HPRT (1:200, sc-20975; Santa Cruz Biotechnology). Proteins were detected with IRDye-conjugated secondary antibodies, followed by analysis with an Odyssey infrared imaging system (LI-COR Biosciences).
Quantification of Xylulose in Plasma.
The approach developed for this project for measurement of xylulose in plasma by the use of liquid chromatography-MS is described in SI Materials and Methods.
Haplotype Analysis.
Genomic DNA from subjects 45.II-2, 89.II-1, and 94.II-2, who are homozygous for DCXR c.583ΔC, and from 81.II-2, who is homozygous for DCXR c.52(+1)G > A, were genotyped with the use of the Affymetrix GeneChip 250K Nsp SNP array, as previously described (39). Boundaries of homozygous intervals were defined as the occurrence of two heterozygous markers in a ten-marker window (Table S4).
Supplementary Material
Acknowledgments
We thank Margaret Lasker for keeping detailed and careful records on pentosuria and for her generosity and foresight in passing them on to A.G.M many years ago for future research on this condition; the families studied by her for welcoming geneticists back into their lives after 50 years; and the participants in the Chaim Sheba 50th Anniversary Conference on the Genetics of Isolate and Migrant Populations, Herzliya, Israel, June 2011, for discussion and insight. This work was supported by unrestricted gifts to the M.C.K. laboratory and by National Institutes of Health Grants R01CA157744 and R01RR032708.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115888108/-/DCSupplemental.
References
- 1.Garrod AE. The Croonian lectures on inborn errors of metabolism, lecture IV. Lancet. 1908;2:214–220. [Google Scholar]
- 2.Bearn AG. Archibald Garrod and the Individuality of Man. Oxford: Clarendon Press; 1993. [Google Scholar]
- 3.De Vries H. The law of segregation of hybrids. Berichte der Deutschen Botanischem Gesellsshaft. 1900;18:83–90. German. [Google Scholar]
- 4.Harper PS. A Short History of Medical Genetics. Oxford: Oxford University Press; 2008. [Google Scholar]
- 5.Scriver CR. Garrod's Croonian Lectures (1908) and the charter ‘Inborn Errors of Metabolism’: Albinism, alkaptonuria, cystinuria, and pentosuria at age 100 in 2008. J Inherit Metab Dis. 2008;31:580–598. doi: 10.1007/s10545-008-0984-9. [DOI] [PubMed] [Google Scholar]
- 6.Salkowski E, Jastrowitz M. On a previously unobserved type of sugar in urine. Zbl med Wissensch. 1892;30:22–24. German. [Google Scholar]
- 7.Wang YM, Van Eys J. The enzymatic defect in essential pentosuria. N Engl J Med. 1970;282:892–896. doi: 10.1056/NEJM197004162821604. [DOI] [PubMed] [Google Scholar]
- 8.Lane AB. On the nature of L-xylulose reductase deficiency in essential pentosuria. Biochem Genet. 1985;23:61–72. doi: 10.1007/BF00499113. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt HH. Chapter 73. Pentosuria. 1978. Reprinted in Valle D, et al., eds. (2011) The Online Metabolic and Molecular Bases of Inherited Disease, www.ommbid.com. Accessed September 14, 2011.
- 10.Lasker M, Enklewitz M. A simple method for the detection and estimation of l-xyloketose in urine. J Biol Chem. 1933;101:289–294. [Google Scholar]
- 11.Lasker M, Enklewitz M, Lasker GW. The inheritance of l-xyloketosuria (essential pentosuria) Hum Biol. 1936;8:243–255. [Google Scholar]
- 12.Lasker M. Mortality of persons with xyloketosuria; A follow-up study of a rare metabolic anomaly. Hum Biol. 1955;27:294–300. [PubMed] [Google Scholar]
- 13.Nakagawa J, et al. Molecular characterization of mammalian dicarbonyl/L-xylulose reductase and its localization in kidney. J Biol Chem. 2002;277:17883–17891. doi: 10.1074/jbc.M110703200. [DOI] [PubMed] [Google Scholar]
- 14.El-Kabbani O, et al. Crystal structure of human L-xylulose reductase holoenzyme: Probing the role of Asn107 with site-directed mutagenesis. Proteins. 2004;55:724–732. doi: 10.1002/prot.20047. [DOI] [PubMed] [Google Scholar]
- 15.Marble A. The diagnosis of the less common meliturias; Including pentosuria and fructosuria. Med Clin North Am. 1947;31:313–325. doi: 10.1016/s0025-7125(16)35836-9. [DOI] [PubMed] [Google Scholar]
- 16.Bozian RC, Touster O. Essential pentosuria: Renal or enzymic disorder. Nature. 1959;184(Suppl7):463–464. doi: 10.1038/184463a0. [DOI] [PubMed] [Google Scholar]
- 17.Dubach UC, Bickel H. Essential pentosuria in Switzerland. Schweiz Med Wochenschr. 1963;93:336–339.. German. [Google Scholar]
- 18.El-Kabbani O, Carbone V, Darmanin C, Ishikura S, Hara A. Structure of the tetrameric form of human L-Xylulose reductase: Probing the inhibitor-binding site with molecular modeling and site-directed mutagenesis. Proteins. 2005;60:424–432. doi: 10.1002/prot.20487. [DOI] [PubMed] [Google Scholar]
- 19.Lane AB, Jenkins T. Human L-xylulose reductase variation: Family and population studies. Ann Hum Genet. 1985;49:227–235. doi: 10.1111/j.1469-1809.1985.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 20.Levene PA, La Forge FB. Note on a case of pentosuria. J Biol Chem. 1914;18:319–327. [Google Scholar]
- 21.Flynn FV. Essential pentosuria. BMJ. 1955;1:391–395. doi: 10.1136/bmj.1.4910.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato Y, Numajiri Y. Chloride attachment negative-ion mass spectra of sugars by combined liquid chromatography and atmospheric pressure chemical ionization mass spectrometry. J Chromatogr. 1991;562:81–97. doi: 10.1016/0378-4347(91)80567-v. [DOI] [PubMed] [Google Scholar]
- 23.Lasker M. The geographic distribution of heterozygotes for essential pentosuria. Am J Phys Anthropol. 1952;10:258–259. [Google Scholar]
- 24.Khachadurian AK. Essential pentosuria. Am J Hum Genet. 1962;14:249–255. [PMC free article] [PubMed] [Google Scholar]
- 25.Politzer WM, Fleischmann H. L-xylulosuria in a Lebanese family. Am J Hum Genet. 1962;14:256–260. [PMC free article] [PubMed] [Google Scholar]
- 26.Soyama K, Furukawa N. A Japanese case of pentosuria. J Inherit Metab Dis. 1985;8:37. doi: 10.1007/BF01805483. [DOI] [PubMed] [Google Scholar]
- 27.Perry TL, Finch CA. Pentosuria in a North American Indian. Nature. 1967;216:1027–1028. doi: 10.1038/2161027a0. [DOI] [PubMed] [Google Scholar]
- 28.Zlotogora J. Multiple mutations responsible for frequent genetic diseases in isolated populations. Eur J Hum Genet. 2007;15:272–278. doi: 10.1038/sj.ejhg.5201760. [DOI] [PubMed] [Google Scholar]
- 29.Risch N, Tang H, Katzenstein H, Ekstein J. Geographic distribution of disease mutations in the Ashkenazi Jewish population supports genetic drift over selection. Am J Hum Genet. 2003;72:812–822. doi: 10.1086/373882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss MF, et al. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57:2571–2585. doi: 10.1046/j.1523-1755.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 31.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 33.Sell DR, Monnier VM. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990;85:380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makita Z, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 35.Odani H, et al. Suppression of renal alpha-dicarbonyl compounds generated following ureteral obstruction by kidney-specific alpha-dicarbonyl/L-xylulose reductase. Ann N Y Acad Sci. 2008;1126:320–324. doi: 10.1196/annals.1433.003. [DOI] [PubMed] [Google Scholar]
- 36.Sudo T, et al. Transgenic mice over-expressing dicarbonyl/L-xylulose reductase gene crossed with KK-Ay diabetic model mice: An animal model for the metabolism of renal carbonyl compounds. Exp Anim. 2005;54:385–394. doi: 10.1538/expanim.54.385. [DOI] [PubMed] [Google Scholar]
- 37.Garrod AE. Inborn Errors of Metabolism. , 2nd Ed. London: Oxford Univ Press; 1923. [Google Scholar]
- 38.Walsh T, et al. Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. Am J Hum Genet. 2010;87:90–94. doi: 10.1016/j.ajhg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renbaum P, et al. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am J Hum Genet. 2009;85:281–289. doi: 10.1016/j.ajhg.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.