Abstract
Serotonin (5-HT) plays a key role in early brain development, and manipulation of 5-HT levels during this period can have lasting neurobiological and behavioral consequences. It is unclear how perinatal exposure to drugs, such as selective serotonin reuptake inhibitors (SSRIs), impacts cortical neural network function and what mechanism(s) may elicit the disruption of normal neuronal connections/interactions. In this article, we report on cortical wiring organization after pre- and postnatal exposure to the SSRI citalopram. We show that manipulation of 5-HT during early development in both in vitro and in vivo models disturbs characteristic chemoarchitectural and electrophysiological brain features, including changes in raphe and callosal connections, sensory processing, and myelin sheath formation. Also, drug-exposed rat pups exhibit neophobia and disrupted juvenile play behavior. These findings indicate that 5-HT homeostasis is required for proper brain maturation and that fetal/infant exposure to SSRIs should be examined in humans, particularly those with developmental dysfunction, such as autism.
Keywords: oligodendrocyte, neurodevelopment, corpus callosum, sensory cortex, sexual dimorphism
Serotonin (5-HT) has long been postulated to play a trophic role in brain morphogenesis, including cell proliferation, migration, and differentiation. It is also known to be one of the first neurotransmitters to appear in the central nervous system (1, 2). An obvious question that can be raised relates to whether perinatal exposure to antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), can affect cortical circuit development and function. Perhaps one of the most intriguing previous findings related to 5-HT and early cortical organization was the observation from immunohistochemical and 5-HT transporter (SERT) binding studies that, at postnatal days 2–14 (P2–P14), rodent primary sensory cortex (notably layer IV of visual, auditory, and somatosensory areas) is transiently innervated by aggregates of fine grain-looking 5-HT–containing processes (3–5). Surprisingly, it became clear that, in early brain development, 5-HT is taken up into glutamatergic thalamocortical terminals (6, 7) and used in combination with the 5-HT 1B receptor on layer IV afferents (8). At present, the functional implication of such transient 5-HT expression and targeting, namely the primary sensory thalamocortical afferents during early development, remains unknown.
5-HT and Abnormal Brain Development.
Interestingly, manipulations of rodent brain 5-HT levels during early development, either through increases (produced in SERT or monoamine oxidase knockout mice) or decreases (produced by parachlorophenylalanine or other treatments), have been shown to produce the downstream effect of interfering with the formation of the whisker (barrel) representation in the primary somatosensory cortex and promoting aggressive and/or anxiety-related behaviors (9–14). Furthermore, early-life modification of 5-HT levels has been shown to cause overreaction to auditory or tactile sensory stimulation (15) and abnormal response properties of cortical neurons after sensory stimulus presentation (16–18). Clinically, 5-HT dysregulation has been shown to be involved in a variety of psychological and behavioral disorders, such as depression, anxiety, aggression, social interaction aversion, and obsessive compulsive disorder (19, 20). At present, SSRIs are a first-line pharmacological treatment for depressed pregnant mothers and/or children with these disorders. The current issue for consideration is whether such treatment is safe (21), especially to the developing brain, as modeled in rodents.
5-HT Dysfunction and Autism Spectrum Disorder (ASD).
There is some evidence that leads us to propose that dysfunction of the raphe 5-HT system during perinatal development may be one of the most important factors contributing to pervasive developmental disorders, especially to ASD. For instance, hyperserotonemia has been noted in autism subjects (22), and variants of the SERT gene (SLC6A4) significantly contribute to hyperserotonemia in autism (23). With PET scanning, altered 5-HT synthesis has been noted in autistic boys and girls vs. nonautistic siblings and epileptic children. Boys have also been found to have a slower rate of decline in 5-HT synthesis capacity than girls (24). A more recent study demonstrated that autistic children with decreases in left cortical α-methyl-l-tryptophan exhibit a higher prevalence of severe language impairment, whereas those with right cortical decreases more frequently display left and mixed handedness (25). Additionally, depletion of tryptophan has been found to increase various stereotyped behaviors in autistic children (26). These findings suggest that asymmetric development of the 5-HT system could lead to miswiring of neural circuits that specify hemisphere specialization. Interestingly, in addition to the more common deficits noted in ASD (abnormal socialization, impaired communication, repetitive interests, and motor behaviors), alterations in sensory perception have also been well documented (27). Although the precise etiology of such dysfunction is currently unknown, it is conceivable that abnormal development of the 5-HT system may be a key factor, especially because 5-HT is transiently expressed in glutamatergic terminals of primary sensory thalamocortical relays, further supporting the potential linkage between 5-HT and sensory dysfunction during early brain development.
Experimentally, early exposure of rat pups to SSRIs has been shown to alter cortical barrel organization (28) as well as raphe circuits (29). More specifically, we reported recently (29) that chronic (P8–P21) neonatal racemic citalopram (CTM) treatment at doses that resulted in serum drug concentrations comparable to those obtained in clinical practice produces profound changes in 5-HT marker expression: a reduction of tryptophan hydroxylase (TPH) expression in midline subgroups of the raphe nuclear complex and a reduction in the density of SERT immunoreactive (-ir) fibers in cortex. This alteration seems to also persist into adulthood. Interestingly, these findings were obtained from male rats, in partial support of the notion that male subjects may be more vulnerable to disruptions of the 5-HT system than their female counterparts are, just as males are more likely to exhibit signs of ASD over females. In line with this view, recent reports have suggested that male pups have a much higher 5-HT level (showing a prominent peak at P3) compared with female pups (displaying a more gradual elevation at P5) (30), and depletion of 5-HT with the neurotoxin 5,7-dihydroxytryptamine in male mice on P1 preferentially affects spatial rearrangement and performance in object-novelty behavioral tests, i.e., evaluations of behavioral traits that are often implicated in ASD (31). At present, several obvious questions that have yet to be addressed are whether perinatal treatment with SSRIs produces ASD-like behavior in rodents, whether this type of drug exposure has the potential to alter corticocortical network function, and whether this treatment affects female pups to a similar degree. To this end, we have exposed experimental rodent subjects of both sexes either prenatally (E11–E19) or postnatally (P1–P7 or P8–P21) to CTM. We then examined behavioral consequences on treated subjects while they were still juveniles and cortical circuit organization (including ascending cortical inputs from the brainstem) after the animals reached adulthood.
Results
Perinatal CTM Exposure: Juvenile Play and Neophobia.
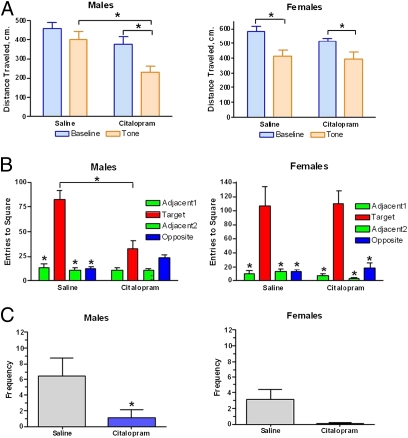
Our first priority was to examine rat pups that were perinatally exposed to CTM and determine whether they exhibit similar behavioral traits that are often seen in association with ASD. Specifically, we were interested in investigating juvenile play and neophobic behavior. It is well established that children with ASD display an inability to engage in play behavior with peers, avoid novelty, and have a compulsive need for an unchanging environment. Our data show that rat pups treated with CTM (10 mg/kg twice daily) as neonates (P8–P21) exhibited an exaggerated freezing response to a novel tone presented at P25 (Fig. 1A), suggesting a neophobic response to auditory stimuli. Moreover, CTM had a significant effect on the response of male rats but not female rats. Also, a clear reduction in the exploration of a novel object was evident at P39 (Fig. 1B). This behavioral change seems to exclusively affect the CTM-exposed male but not female rats. As such, these data indicate that perinatal CTM exposure may also predispose male rat pups to respond in a neophobic manner after the presentation of visual or tactile stimuli. The behavioral deficits in conspecific vs. object preference and familiar vs. unfamiliar scents noted in young animals persisted into adulthood (P60+) (Fig. S1). Furthermore, in comparisons of social play behavior between saline- and CTM-treated animals, we found that saline-treated animals were much more likely to engage in play behavior, whereas CTM-treated subjects were virtually uninterested in play. The disruption of juvenile play was prominent in males but not females (Fig. 1C). These results demonstrate that rat pups, when exposed perinatally to SSRIs, exhibit many behavioral traits often seen similarly in ASD.
Fig. 1.
Effect of neonatal (P8–P21) CTM exposure (20 mg/kg per d) on behavior. (A) Neonatal exposure preferentially decreases response (distance traveled) to a novel tone in male rats at P25. Data represent the mean ± SEM for 15–17 subjects. Baseline and tone conditions were presented for 120 s each. (B) In comparison with females, male CTM-exposed rats show a clear reduction in the exploration of a novel object at P39. Exploration of a novel object for 10 min was tested after a 10-min acclimation period. Data represent the mean ± SEM of 15–17 subjects. (C) Male rat pups that were exposed to CTM show virtually no participation in juvenile play in comparison with female CTM-exposed animals at P31. Data represent the mean ± SEM of 16–18 subjects. *P < 0.05, Bonferroni-corrected t test.
Altered Raphe Circuits.
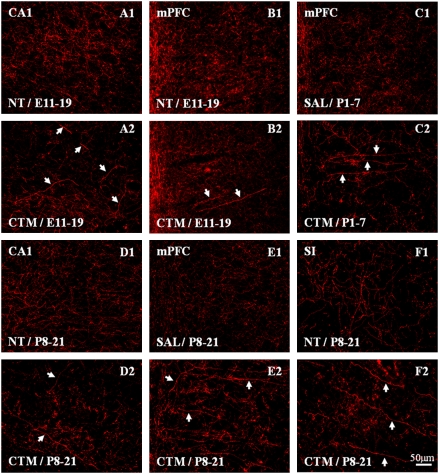
Our next goal was to investigate the serotonergic raphe system of males after perinatal SSRI treatment and determine the severity and selectivity of drug-induced changes in comparison with saline-exposed or no treatment rats as controls. Hence, we examined the morphology and density of SERT-ir fibers in the cerebral cortex as well as TPH expression in the raphe nuclear complex when the animals reached adulthood. Our data from three different exposure periods revealed dramatic reductions in SERT-ir cortical density in animals treated with CTM. Decreased density was noted in limbic areas, such as the hippocampus and medial prefrontal cortex, as well as in sensory territories of cortex, such as the primary somatosensory cortex and auditory cortex. The majority of SERT-ir axons took on a beaded or studded appearance (often lacking labeling in intervaricose segments), a morphology that contrasts with the typical arrangement of sequentially connected boutons in these fine-caliber fibers, supporting our previous findings noted in the medial prefrontal cortex and somatosensory cortex (29). In addition, a second axon profile was detected among the SERT-ir fiber network in neocortex after perinatal CTM exposure. These processes were rather thick, and many were devoid of well-defined synaptic swellings. Fibers in hippocampus were short and tortuous, whereas those in the neocortex tended to form long, radial, nonbranching arrays that traversed cortical layers perpendicular to the pial surface (Fig. 2). It is interesting to note that exposure to higher doses of CTM tends to promote a decrease in SERT-ir fiber density and an increase in the density of these abnormal-looking, thick, rod-like SERT-ir fibers in the hippocampus (32). Finally, such effects seem to selectively target the SERT-ir fibers but not the noradrenergic cortical fibers, such as those in the primary somatosensory cortex (Fig. S2).
Fig. 2.
Representative SERT expression in different cortical areas after CTM treatment at three different developmental periods (E11–E19, P1–P7, and P8–P21). Note the rather thick, rod-like SERT-ir fibers (indicated by arrows in A2, B2, C2, D2, E2, and F2) in drug-exposed males compared with the thinner, more evenly distributed fibers in saline-exposed (SAL) or no treatment (NT) rats (A1, B1, C1, D1, E1, and F1). (Scale bar: 50 μm.) CA1, hippocampus; mPFC, medial prefrontal cortex; SI, primary somatosensory cortex.
With regard to the serotonergic raphe nuclear complex, semiquantitative immunofluorescent analysis consistently revealed reductions in TPH labeling along the raphe midline, within both the dorsomedial and ventromedial subgroups of the dorsal raphe nucleus, as well as within the median raphe after perinatal CTM treatment (Fig. S3). Similar findings were obtained for the three treatment groups (regardless of time course of drug administration). It is also important to note that these affected subregions of the raphe are known to selectively target the sensory cerebral cortex and limbic structures such as the hippocampus (33, 34). At present, the biological mechanism for selective insult to the midline territory of the raphe complex is unknown.
Abnormal Callosal Connectivity.
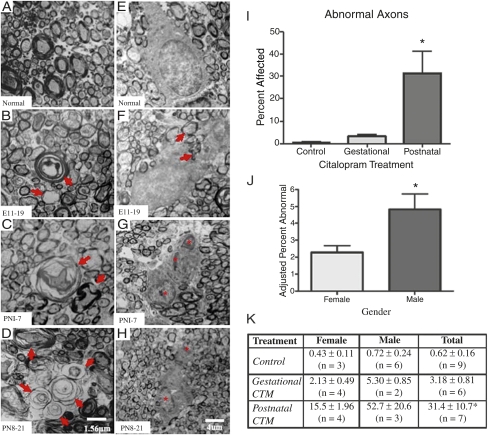
Given that manipulation of 5-HT during early development affects normal cortical barrel formation and sensory information processing, it is interesting that some of the most consistent pathologies observed in ASD are (i) the change in the size and shape of the corpus callosum and (ii) the apparent disconnection among long corticocortical pathways (35, 36). Hence, our next goal was to investigate whether perinatal CTM treatment affects the integrity of the corpus callosum. To address this issue, an ultrastructural study was undertaken to assess the architecture of callosal axons, namely the formation of myelin sheathes by oligodendrocytes (OLs). Our data revealed that perinatal exposure to CTM in rat pups alters the myelination of callosal axons and interferes with OL soma morphology (Fig. 3). Instances of hypo- and hypermyelination were detected, and lamellae separation was common in CTM-treated corpus callosum compared with normal controls (Fig. 3 A–D). On average, the number of abnormal callosal axons ranged from 21 to 62 per 1,000 in the CTM20 treatment group (20 mg/kg dose), whereas controls with the same sample size exhibited only 5–9 abnormal axons. These data suggest that SSRI administration induces a two- to sixfold increase in aberrant axon morphology. In addition, the postnatal treatment seems to exhibit more effects compared with the prenatal treatment (Fig. 3I).
Fig. 3.
Early-life CTM exposure on corpus callosum axons and OLs. (A–H) Electron photomicrographs of corpus callosum axons and OLs obtained from adult males treated at different developmental periods. Note the morphology of myelinated axons and OLs in controls (A and E) vs. subjects treated at E11–E19 (B and F), P1–P7 (C and G), and P8–P21 (D and H). B, C, and D demonstrate distortions in myelin sheathing, and F, G, and H illustrate abnormal OL morphologies. Multinucleated cells (indicated by asterisks) with inclusions (possibly enveloped myelinated axons, indicated by arrows) were observed in CTM-treated animals. (Scale bars: 1.56 μm (A–D); 4 μm (E–H). (I) Relative frequency of abnormal axons in three treatment groups (control, gestational, and postnatal). Data represent mean ± SEM of six to nine subjects per group (males and females combined). Gestational CTM treatment: maternal treatment from E11–E19 with 20 mg/kg per d CTM; postnatal CTM treatment: exposure of pups from P1 to P7 with 10 mg/kg per d CTM and from P8 to P21 with 20 mg/kg per d CTM. (J and K) Data are illustrated by sex. Where n = 2, values represent mean ± SD. For I, J, and K, analysis of covariance (ANCOVA) revealed a main effect of treatment period (F2, 18 = 13.332, P < 0.001) and a covariate effect of sex (F1, 18 = 5.262, P = 0.034). *P < 0.001 vs. control, Bonferroni-corrected t test.
Because boys are approximately three to four times more likely to exhibit ASD, we also wanted to examine whether CTM treatment produced a difference in the severity of effects between male and female rat pups. Our semiquantitative findings revealed that drug-treated male rats were more severely compromised (∼threefold difference) with a greater percentage of abnormal callosal axons: 5.8% of the axons that were sampled were malformed (see the description above), whereas only 1.8% of the axons sampled from drug-treated females were abnormal. A sex-based comparison of these results is shown in Fig. 3 J and K. With regard to OL somas, vacuole/lysosome inclusions were often observed in the OL cytoplasm of CTM-treated animals compared with controls (Fig. 3 E–H). Quite often, misplaced pieces of myelinated axon also appeared inside these inclusions. Another notable feature was that a greater preponderance of multinucleated OLs appeared in samples from drug-exposed than control subjects. In brief, our ultrastructural data provide an important clue that callosal axon myelination may be particularly vulnerable to perinatal CTM exposure. It is interesting to note that autophagic vacuoles have been observed in cortical neuron specimens obtained from brains of Alzheimer's patients (37). Because OLs from CTM-treated rats show an increased prevalence of vacuoles/lysosomal inclusions, it is very likely that these OLs are also undergoing a degenerating process.
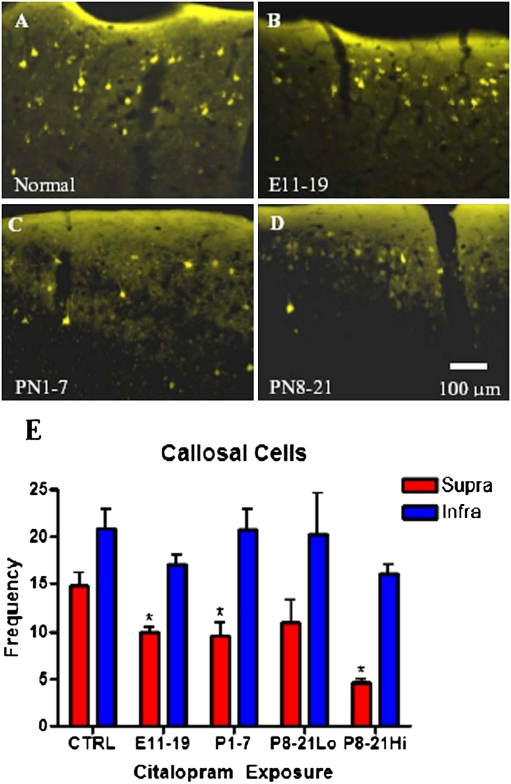
Providing that perinatal treatment with CTM interferes with the process of axon myelination, there is a strong possibility that such drug exposure may lead to abnormalities in neural network wiring. Of particular interest is whether transient blockade of 5-HT reuptake alters patterns of interhemispheric connectivity between the two sides of the cortex. To address this issue, retrograde tracers such as biotinylated dextran amine and/or Fluoro-Gold were applied to one cortical hemisphere in rat pups with three different time courses of CTM exposure compared with their controls. The somatosensory barrel cortex was well represented in the zone of tracer deposition. In the examination of cortical layers that provide projections to the corpus callosum, our quantitative analysis revealed that there was an overall reduction (∼50%) in the number of retrogradely labeled cells in layers II/III (supragranular layers) of primary somatosensory cortex but a less pronounced decrement in infragranular layers (V/VI) of CTM-exposed rats (Fig. 4E) compared with controls. Additionally, higher dose exposure from P8 to P21 seems to induce a more severe effect.
Fig. 4.
Representative photomicrographs of Fluoro-Gold retrogradely labeled callosal cells in the supragranular layer of the barrel cortex. Images were obtained from adult male rats that received CTM at different developmental periods. Note the reduction of callosal cells in the treatment groups [B, E11–E19; C, P1–P7; and D, P8–P21 (10 mg/kg)] compared with the normal subject, A. (Scale bar: 100 μm.) (E) Bar graph of data from both male and female subjects shows that the loss of retrogradely labeled callosal neurons is restricted to the supragranular layer. Values represent the mean ± SEM of 11 (CTRL), 4 (E11–E19; 20 mg/kg maternal dose), 5 (P1–P7; 10 mg/kg dose), 3 (P8–P21 Lo; 5 mg/kg dose), and 3 (P8–P21 Hi; 20 mg/kg dose) subjects per group. Repeated-measures ANOVA revealed a main effect of layer (F1, 21 = 38.791, P < 0.001) and CTM exposure (F4, 21 = 3.423, P = 0.026) but no interaction between layer and exposure. *P < 0.05 vs. control, a priori univariate F test. Supra- differed from infragranular under all exposure conditions (P < 0.05, a priori univariate F test).
OL Pathology.
We have also performed experiments with OL cell cultures. These cultures were derived from the optic nerve and/or forebrain areas to examine the effects of 5-HT on OLs. Our data indicate that treatment with concentrations of 5-HT in the range of 10–100 μM induces OL pathology. Such effects were not observed with a low concentration of 5-HT or in medium lacking 5-HT (Fig. S4). Namely, the processes of OL progenitor cells were shortened, distorted, and/or polarized compared with those grown in the absence of 5-HT. The same was observed in cultures of immature OLs (Fig. S5); however, ∼20–30% of OLs in this stage of development also exhibited evidence of apoptosis. Similar results were observed in OLs derived from cerebral cortex. Thus, we believe that certain aspects of this pathology are specific to the developmental stage of the OL, with immature OLs being more vulnerable to the effects of 5-HT. At present, the precise biological mechanism responsible for mediating the negative impact of 5-HT on OLs remains unknown.
Disturbed Tonotopic Organization.
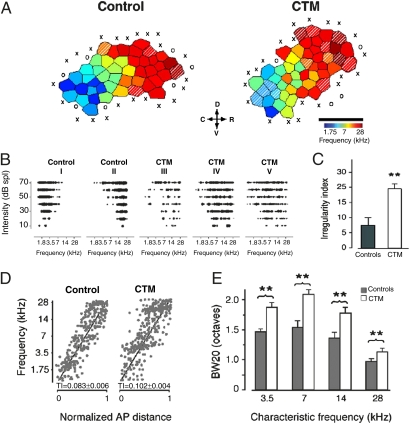
Because there is strong evidence of sensory dysfunction in individuals with ASD, especially related to language disturbances (25), we have conducted electrophysiological experiments to investigate the activity profile of cortical neurons in primary auditory cortex (A1). Rat pups of both sexes were treated postnatally (P8–P21) with CTM (5 mg/kg) or saline twice daily and then assessed at P22–P27. Our combined data (Fig. 5) reveal a distorted tonotopic organization in subjects exposed to CTM. Included in these findings are evidence of increased scatter in the characteristic-frequency map and the presence of neurons with abnormally broad and irregular receptive fields. In fact, we observed a 300% increase in irregularly shaped tuning curves. The average irregularity index was >4 per A1 map: controls (7.7 ± 3.4%) compared with CTM-treated rats (24.7 ± 2.8%) (P = 0.001; Fig. 5C). We also examined neural synchrony in A1 by simultaneously recording spontaneous neural firing during silent periods from pairs of neurons in CTM-exposed rats (64 recorded pairs) and controls (86 recorded pairs). Average cross-correlogram functions between −10- and +10-ms lags were significantly decreased in the CTM group (0.018 ± 0.0008) compared with controls (0.022 ± 0.0008) (Fig. S6A). This desynchronization in neural firing was observed for close and distant neuron pairs (Fig. S6B). These data suggest that CTM exposure led to an abnormal organization of intracortical networks, which play a key role in shaping individual receptive fields and global frequency representation during development. Not surprisingly, abnormal auditory processing, especially for complex sounds, is extremely common in ASD (38).
Fig. 5.
Degraded A1 frequency representation caused by CTM. (A) Representative A1 characteristic-frequency map from a control P22 rat (Left) and an age-matched 10 mg/kg CTM-treated rat (Right). Hatched areas represent cortical sites with an irregularity index (Materials and Methods) above 4. (Scale bar: 0.75 mm.) X, unresponsive cortical site; O, non-A1 cortical site (Materials and Methods). (B) Representative A1 receptive fields obtained in controls (I and II in characteristic-frequency map shown in A) and CTM-treated rats (III, IV, and V). (C) A threefold increase in irregularly shaped tuning curves (average irregularity index >4) was observed in CTM-treated vs. control subjects. (D) Distribution of all recorded characteristic frequencies in controls and CTM-treated rats plotted against a normalized tonotopic axis (Materials and Methods). The increased scatter of characteristic frequencies around the ideal tonotopic axis (black diagonal line) was quantified with the tonotopic index (TI), which was significantly higher in the CTM-treated group. (E) Distribution of tuning curve BW20 separated by characteristic frequency for controls and CTM-treated rats. (Controls: n = 14, 406 recorded sites; CTM-treated rats: n = 9, 407 recorded sites). Values shown are mean ± SEM. **P < 0.001, t test.
Discussion
We show here that rat pups perinatally exposed to the SSRI CTM exhibit impaired social behavior and response to novelty as well as abnormal raphe circuitry and cortical network function. In addition, perinatal drug exposure seems to more severely affect males than females, suggesting a sexually dimorphic response in our rodent model. Our results also indicate that early exposure to CTM may interfere with callosal OL maturation (as evidenced by abnormal myelin formation) and lead to a reduction in callosal connectivity. We believe that one of the ultimate consequences of this developmental defect is abnormal communication between the two cortical hemispheres. This view is reminiscent of MRI findings from ASD patients that show a reduction in the size of human corpus callosum and the loss of long-distance cortical connectivity (35, 36).
Perinatal exposure to SSRIs has recently been shown to alter social behavior in adult rodents (39, 40). Interestingly, the present investigation has demonstrated an increased incidence of neophobia and disrupted juvenile play when rat pups are exposed to CTM. Moreover, we have shown that reduced social play behavior is more obvious in drug-exposed male vs. female animals. Although we do not suggest that the juvenile play behavior that we assess corresponds to a specific trait dimension of ASD, our findings are consistent with the possibility that dysregulation/dysfunction of the 5-HT system during early brain development may be the critical contributing factor in the etiology of ASD. This notion is also supported by the fact that the altered behaviors were observed in CTM-exposed rats but not in non-SSRI perinatally exposed animals (29, 41), implicating a potential 5-HT linkage of social dysfunctioning seen in ASD (42).
Neuronal subgroups within the raphe complex have been shown to coexpress nitric oxide (NO) and 5-HT not only in their cell bodies but also in their axons (33). Taking this finding into consideration, one likely mechanism for such selective down-regulation of 5-HT marker proteins in midline subregions of the raphe (and their projections) may involve NO and/or neuronal NO synthase, the biosynthetic enzyme for NO. Neuronal NO synthase and SERT have recently been shown to reciprocally modulate each other's activity (43), and NO has been shown to inactivate TPH and generate nonfunctional chemical derivatives of 5-HT (44). At present, the role of NO in cortical circuit function after perinatal treatment with SSRIs remains to be elucidated.
Another issue that can be raised pertains to the mental health of females before and during pregnancy. It has been shown that gestational stress can place the unborn at a higher risk of developing a psychiatric disorder (45). Interestingly, a recent epidemiological study has stated that antidepressant use during pregnancy increased the incidence of ASDs by two- to threefold compared with a control population (46). Pregnant women are, therefore, being prescribed, or are continuing regimens of, SSRI medications to offset negative effects associated with stress and depression. Importantly, our present findings reveal that exposure to SSRIs can alter the course of development of the rodent fetal nervous system, including an impact on neural network function and behavior; whether such actions occur in humans is unknown. Further investigation is certainly warranted, considering that the prescription rate of SSRIs to pregnant females is on the rise, nearly doubling between 1995 and 2004 (47). To adequately address the recent and dramatic surge in pervasive developmental disorders (ASD in particular), we need to consider the physiological impact of therapeutic interventions during pregnancy as well as the biological environment imposed on the fetus by the mental state of the mother.
Materials and Methods
Experimental Animals and Drug Exposure.
Both male and female rat pups were used in the experimental procedures. The basic preparation of the SSRI, CTM, and animal care were performed as described previously (29, 32).
Behavioral Testing.
We conducted several specific social interaction tests while animals were in juvenile stages. Namely, locomotor response to a novel tone was tested on P25. Juvenile play was tested between P32 and P34. Response to a novel object was examined on P30 and P82.
Immunohistochemistry and Quantification.
Standard immunohistochemical methods were performed for SERT and TPH. Details of these procedures have been described previously (29, 32). Metamorph data acquisition software (Molecular Devices) was used for semiquantitative analysis.
Callosal Connectivity and Ultrastructural Analysis.
For changes in callosal connectivity, injections of retrograde tracers, such as Fluoro-Gold, were made into one hemisphere. The barrel field of the primary somatosensory cortex was a major target site for these injections. The numbers of labeled cells in both the supra- and infragranular layers were counted and analyzed.
Cell Culture and Immunohistochemistry.
OL progenitor cells were isolated from neonatal rat optic nerves as described previously (48). Both OL progenitor cells and immature OLs were treated with increasing concentrations of 5-HT (1, 10, and 100 μM) or the control medium. To identify cell developmental stages, NG2 and MBP antibodies were used. To detect cell death, the TUNEL technique was used.
Auditory Mapping.
These auditory experiments were conducted at P22–P27. Cortical responses were recorded with tungsten microelectrodes. Studies examined tonotopic organization, individual tuning curves, spontaneous neuronal spikes, and correlation patterns in neuronal firing. Data were collected and analyzed as described previously (49, 50).
Supplementary Material
Acknowledgments
We thank G. Hoskins, J. Zhang, T. Babcock, Donald Green, and S. Harrison for technical support. This research was supported by National Institutes of Health Grants RR017701 (to K.L.S. and I.A.P.), NS54278 and HD35496 (to Z.C.), and EUREKA MH084194 (to R.C.S.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109353108/-/DCSupplemental.
References
- 1.Azmitia EC. Modern view on an ancient chemical: Serotonin effects on proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:414–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 3.D'Amato RJ, et al. Ontogeny of the serotonergic projection to rat neocortex: Transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci USA. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- 5.Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat's somatosensory cortex. J Neurosci. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett-Clarke CA, Chiaia NL, Rhoades RW. Thalamocortical afferents in rat transiently express high-affinity serotonin uptake sites. Brain Res. 1996;733:301–306. doi: 10.1016/0006-8993(96)00791-3. [DOI] [PubMed] [Google Scholar]
- 7.Lebrand C, et al. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 8.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-HT transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cases O, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cases O, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 11.Persico AM, et al. Serotonin depletion and barrel cortex development: Impact of growth impairment vs. serotonin effects on thalamocortical endings. Cereb Cortex. 2000;10:181–191. doi: 10.1093/cercor/10.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Persico AM, et al. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT1A receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- 14.Jennings KA, et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahne D, et al. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75:403–410. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Seif I, Armstrong-James M. Differences in somatosensory processing in S1 barrel cortex between normal and monoamine oxidase A knockout (Tg8) adult mice. Cereb Cortex. 2001;11:26–36. doi: 10.1093/cercor/11.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Welker E, et al. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- 18.Esaki T, et al. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci USA. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GM. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: Assessing bioeffect and mechanisms of action. Int J Dev Neurosci. 2004;22:397–404. doi: 10.1016/j.ijdevneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Moses-Kolko EL, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: Literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. discussion 341–342. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho AM, et al. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9:264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- 24.Chugani DC, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Chandana SR, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 26.McDougle CJ, et al. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996;53:993–1000. doi: 10.1001/archpsyc.1996.01830110029004. [DOI] [PubMed] [Google Scholar]
- 27.Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150:151–161. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connell S, Karikari C, Hohmann CF. Sex-specific development of cortical monoamine levels in mouse. Brain Res Dev Brain Res. 2004;151:187–191. doi: 10.1016/j.devbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Hohmann CF, Walker EM, Boylan CB, Blue ME. Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Res. 2007;1139:163–177. doi: 10.1016/j.brainres.2006.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver KJ, Paul IA, Lin RCS, Simpson KL. Neonatal exposure to citalopram selectively alters the expression of the serotonin transporter in the hippocampus: Dose-dependent effects. Anat Rec (Hoboken) 2010;293:1920–1932. doi: 10.1002/ar.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson KL, Waterhouse BD, Lin RCS. Differential expression of nitric oxide in serotonergic projection neurons: Neurochemical identification of dorsal raphe inputs to rodent trigeminal somatosensory targets. J Comp Neurol. 2003;466:495–512. doi: 10.1002/cne.10912. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 35.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Welsh JP, Ahn ES, Placantonakis DG. Is autism due to brain desynchronization? Int J Dev Neurosci. 2005;23:253–263. doi: 10.1016/j.ijdevneu.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 38.Samson F, Mottron L, Jemel B, Belin P, Ciocca V. Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? J Autism Dev Disord. 2006;36:65–76. doi: 10.1007/s10803-005-0043-4. [DOI] [PubMed] [Google Scholar]
- 39.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 40.Homberg JR, Schiepers OJ, Schoeffelmeer AN, Cuppen E, Vanderschuren LJ. Acute and constitutive increases in central serotonin levels reduce social play behavior in peri-adolescent rats. Psychopharmacology (Berl) 2007;195:175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Porcel F, et al. Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat Rec (Hoboken) 2011;294:1726–1735. doi: 10.1002/ar.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossman JB, Carter A, Volkmar FR. Social behavior in autism. Ann N Y Acad Sci. 1997;807:440–454. doi: 10.1111/j.1749-6632.1997.tb51938.x. [DOI] [PubMed] [Google Scholar]
- 43.Chanrion B, et al. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci USA. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fossier P, et al. Nitric oxide transforms serotonin into an inactive form and this affects neuromodulation. Neuroscience. 1999;93:597–603. doi: 10.1016/s0306-4522(99)00165-7. [DOI] [PubMed] [Google Scholar]
- 45.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 46.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. July 4, 2011 doi: 10.1001/archgenpsychiatry.2011.73. 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 47.Bakker MK, Kölling P, van den Berg PB, de Walle HE, de Jong van den Berg LT. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang Y, Cai Z, Rhodes PG. Effect of tumor necrosis factor-α on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80:226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- 49.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 50.Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.