Abstract
Recent evidence that some species can retranslocate boron as complexes with sugar alcohols in the phloem suggests a possible mechanism for enhancing boron efficiency. We investigated the relationship between sugar alcohol (sorbitol) content, boron uptake and distribution, and translocation of foliar-applied, isotopically enriched 10B in three lines of tobacco (Nicotiana tabacum) plants differing in sorbitol production. In tobacco line S11, transformed with sorbitol-6-phosphate dehydrogenase, the production of sorbitol was accompanied by an increase in the concentration of boron in plant tissues and an increased uptake of boron compared with either tobacco line A4, transformed with antisense orientation of sorbitol-6-phosphate dehydrogenase, or wild-type tobacco (line SR1, zero-sorbitol producer). Foliar application of 10B to mature leaves was translocated to the meristematic tissues only in line S11. These results demonstrate that the concentration of the boron-complexing sugar alcohol in the plant tissue has a significant effect on boron uptake and distribution in plants, whereas the translocation of the foliar-applied 10B from the mature leaves to the meristematic tissues verifies that boron is mobile in sorbitol-producing plants (S11) as we reported previously. This suggests that selection or transgenic generation of cultivars with an increased sugar alcohol content can result in increased boron uptake, with no apparent negative effects on short-term growth.
Current information implies that boron uptake is a passive process and that its rate is determined by the boron concentration in the medium and the formation of boron complexes in plants (Brown and Hu, 1994). Species and cultivars, however, differ significantly in boron uptake even when grown under identical environmental conditions that are characteristic of selective ion uptake. For example, Brown and Jones (1971) reported that the boron-efficient tomato cv Rutgers was 15 times more efficient in using boron from the medium than the boron-inefficient cv T3238. They concluded that cv T3238 lacks the ability to transport boron to the shoot. Nable et al. (1990) found that barley and wheat cultivars tolerant to boron-toxic soils accumulated less boron in the youngest expanded leaf than the less-tolerant cultivars. In both of these examples, species were grown under identical conditions and uptake kinetics were consistent with a passive process. The mechanisms underlying species differences in boron uptake are unknown, but may result from differences in membrane permeability (Nable and Paull, 1991), the ability of a species to translocate boron (Bellaloui and Brown, 1998) and mobilize it within the plant (Brown and Shelp, 1997), the formation of boron complexes in the cell (Hu and Brown, 1997), or other, as-yet-unidentified mechanisms.
Boron is generally considered an immobile element in most species, because it accumulates in leaves and does not retranslocate to other plant organs. The immobility of boron, however, is not a general phenomenon in all species. For example, Shelp et al. (1995) found that the concentration of boron in the floral parts of broccoli was higher than in the leaves, and that foliar-applied 10B was translocated to a small extent to florets mainly via the phloem. Further, it was reported that boron was highly mobile in apple (van Goor and van Lune, 1980), and that foliar-applied boron could be retranslocated from mature leaves of Prunus, Pyrus, and Malus species (Hanson, 1991; Picchioni et al., 1995). Recently, Brown and Hu (1996) observed that foliar-applied 10B was phloem mobile in sorbitol-rich species within Prunus, Pyrus, and Malus, but there was no significant translocation of the foliar-applied 10B in nonsorbitol species included in the study. The mobility of boron in sorbitol-rich species was subsequently verified by Hu et al. (1997), who isolated and characterized soluble boron complexes from the extrafloral nectar of peach and the phloem sap of celery. In peach and celery boron is translocated in the phloem as sorbitol-boron-sorbitol and mannitol-boron-mannitol, respectively (Hu et al., 1997).
The presence of high concentrations of sorbitol (Moing et al., 1992), the free phloem mobility of boron in sorbitol-rich species (Brown and Hu, 1996), and the finding that boron is present in the phloem as a sorbitol-boron-sorbitol complex (Hu et al., 1997) strongly suggest that polyols (sorbitol) play a significant role in boron transport in Prunus, Pyrus, and Malus species. Whether this phenomenon also influences boron uptake is unknown. However, the observation that boron uptake is partially determined by the underlying rate of boron-complex formation suggests that sugar alcohols may also affect boron uptake.
The aim of the present study was to determine if differences in sorbitol content affect boron uptake and translocation. To achieve this goal we determined boron uptake and translocation in tobacco (Nicotiana tabacum) genetically manipulated to produce sorbitol (S11) and contrasted this with the antisense strain, A4, and wild-type tobacco (strain SR1), in which sorbitol is absent.
MATERIALS AND METHODS
Plant Material
Three tobacco (Nicotiana tabacum) lines were selected for this study. The first line, S11, was genetically engineered to produce sorbitol using cDNA encoding NADP-dependent S6PDH (Tao et al., 1995). In the experiment of Tao et al. (1995), an apple cDNA encoding S6PDH was stably integrated and expressed in transgenic tobacco (line S11). They demonstrated that S6PDH was expressed in sufficient quantity for the synthesis of sorbitol in tobacco and suggested that S6PDH is a key enzyme for sorbitol synthesis in apple. The second line (positive control), A4, was genetically engineered to contain the antisense of the cDNA encoding S6PDH (Tao et al., 1995). The third line (negative control), SR1, is the wild type.
Seeds of tobacco lines S11, A4, and SR1 were germinated in Petri dishes. We treated seeds of strains S11 and A4 with kanamycin solution (100 mg L−) for 30 min, then placed them on moistened filter paper. Seeds of tobacco line SR1 were soaked in water for 30 min. We subsequently chilled all seeds at 5°C for 3 d, after which the seeds were transferred to a controlled-environment room for germination. After 7 d of germination, the seedlings were transplanted to a clean sand medium supplied with one-quarter-strength Hoagland solution (Hoagland and Arnon, 1950) under greenhouse conditions with natural lighting at a temperature of 30°C/15°C (day/night). The pH of the nutrient solution was adjusted to 5.5 to 6.5 and plants were irrigated twice a week. The nutrient irrigation was applied after the growth medium was rinsed with double-distilled water to avoid salt and boron accumulation. Isotopic boron (99.43% 10B:0.57% 11B) (Eagle Picher, Quapaw, OK) as boric acid was used as a tracer for uptake and translocation of boron.
We screened all of the transgenic plants for gene expression using the GUS assay according to the method of Jefferson (1987), and tested a number of nontransgenic tobacco (SR1, wild-type) plants as a negative control. For each experiment there were four replicates of each treatment and line combination. Replicates within treatments were assigned based on the activity of the GUS, such that the average GUS activity within a given treatment did not differ significantly from any other treatment.
Experiment 1: Uptake and Distribution of Boron
This experiment was designed to investigate whether sorbitol production and distribution is influenced by boron supply (0.1, 1.0, and 10 mg L−1), and whether soribitol affects the uptake and distribution of boron.
Two weeks after germination, plants were supplied with a concentration of 0.04 mg L−1 natural abundance boron as boric acid in a nutrient solution background (described above), for 2 weeks. After an additional 2 weeks, the boron source was changed to 99.43% 10B-enriched boric acid supplied at either 0.1, 1, or 10 mg L−1 with three-quarter-strength Hoagland solution (Hoagland and Arnon, 1950). Plants were harvested at 0 (before 10B treatment), 4, 12, 24, and 504 h after 10B-enriched boric acid application.
We determined the 10B uptake and distribution in plant tissues (mature leaves, meristematic tissues, stems, and roots) as described below. S11, was contrasted with the positive (A4) and negative (SR1) controls. Sorbitol concentrations were determined in all lines, as described below by Tao et al. (1995) and Greve and Labavitch (1991), and compared to boron uptake and distribution.
Experiment 2: Translocation and Distribution of Boron
The aim of this experiment was to verify the role of sorbitol in boron translocation from the mature leaves to the meristematic tissues after foliar application of 10B.
Tobacco seeds were germinated and transplanted to sand culture, and were then supplied with a concentration of 0.1 mg L−1 boron as natural abundance boric acid. After 2 weeks of growth, the plants were supplied with a concentration of 1.0 mg L−1 boron in three-quarter-strength Hoagland solution. After 1 week of growth in 1 mg L1 boron, the mature leaves (three leaves from each plant) of S11, A4, and SR1 plants were treated (foliar application) with 10B-enriched (99.43 atom %) boric acid at a concentration of 700 mg L−1. The mature leaves were immersed in 700 mg L−1 10B with 0.05% (v/v) L-77 surfactant (Loveland Industries, Greeley, CO) for 15 s. Mature leaves and meristematic tissues of all lines were harvested at 0, 1, 12, 24, 48, and 240 h after foliar-applied 10B. Untreated plants of each line were used as controls.
Sampling and Plant Analysis
At each harvest, four individual plants (replicates) were selected from each line, and each plant was divided into mature leaves, meristematic tissues, stems, and roots. Roots were removed from the sand, cleaned of adhering substrate, and then collected without being washed. Preliminary experiments demonstrated that washing removes from 6% to 12% of root boron. This amount was highly variable between replicates. Replicate plants were more uniform in boron content when washing was omitted. We analyzed recently fully mature leaves. At each harvest mature leaves and meristematic tissues were divided into two groups: one for isotopic boron (10B) determination and the other for cell wall boron determination. We determined the isotopic boron content in mature leaves, meristematic tissues, stems, and roots of each plant, as described below.
Boron Analysis
Plant tissues were dry ashed at 500°C and analyzed for boron content using an inductively coupled plasma MS (model Elan 500, Perkin-Elmer, Norwalk, CT), as described by Brown and Hu (1994).
IM
IM of boron was calculated according to the equation of Williams (1948):
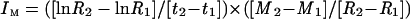 |
where R1 and R2 are the initial and final root dry weights at t1 and t2, respectively, and M1 and M2 are the initial (t1) and final (t2) boron contents.
Sorbitol Analysis
One gram of fresh tissue was homogenized with an ice-cold mortar and pestle in 10 mL of 80% ethanol. The extract was centrifuged and the supernatant dried by a stream of air. An internal standard, 250 μL of 50 μg of inositol, was added to the samples. The samples were dried again by a stream of air. Four-hundred microliters of acetic anhydride and 60 μL of 1-methyl imidazol were added to acetylate the sorbitol. After 10 min, the reaction was stopped by adding 2 mL of water. The acetylated sugars were partitioned in 2 mL of dichloromethane and dried. Acetylated samples were then dissolved in 100 μL of acetone and analyzed using a Perkin-Elmer gas chromatograph (model 8320). MS was carried out using a mass-selective detector (model 5970, Hewlett-Packard) to confirm the retention time (Greve and Labavitch, 1991; Tao et al., 1995).
Experimental Design
We used a randomized complete block design in this experiment. We repeated the experiment without the use of the antisense line. All values shown in tables and graphs represent the means of four replicates. Error bars indicate ses. We performed the statistical analysis with the General Linear Models procedure (SAS, 1982).
RESULTS
The plants were healthy throughout the experiment and did not show any symptoms of boron deficiency or toxicity.
Experiment 1: Uptake and Distribution of Boron
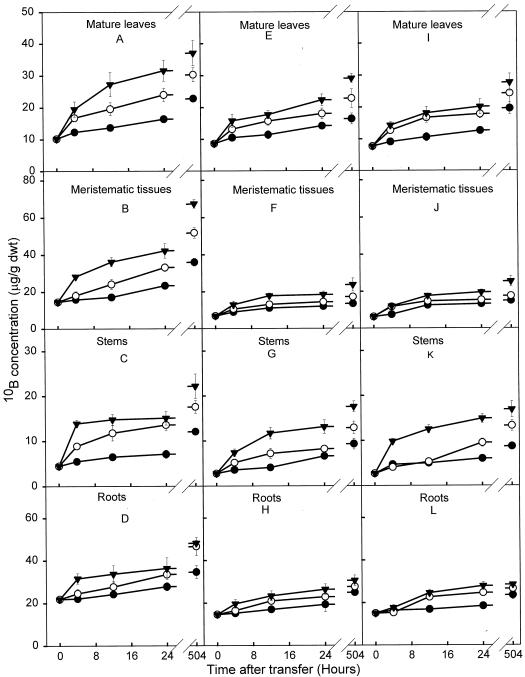
At 504 h the concentrations of 10B in all tissues of S11 plants were greater than in tissues of the A4 or SR1 plants at each solution concentration of boron (Fig. 1). This difference was particularly marked when plants were grown at 10 mg 10B L−1. In this treatment the highest concentrations of 10B were present in the meristematic tissue of S11, and were 300% higher than the 10B in the stems of S11 and approximately 300% higher than in any tissues of A4 or SR1. The distribution of boron in all plants changed with time and with the concentration of boron in the medium. With increasing boron concentrations and at later harvest dates, the proportion of boron found in aboveground plant parts (meristems and mature leaves) increased; this was particularly pronounced in S11 (Fig. 1).
Figure 1.
10B concentration in mature leaves, meristematic tissues, stems, and roots of tobacco plants. Transgenic S11, sense orientation (A–D); transgenic A4, antisense orientation (E–H); and wild-type SR1 (I–L) plants were grown in 0.1 (•), 1.0 (○), and 10 mg L−1 (▾) 10B for a period of 3 weeks. Bars represent means ± se of four replicates.
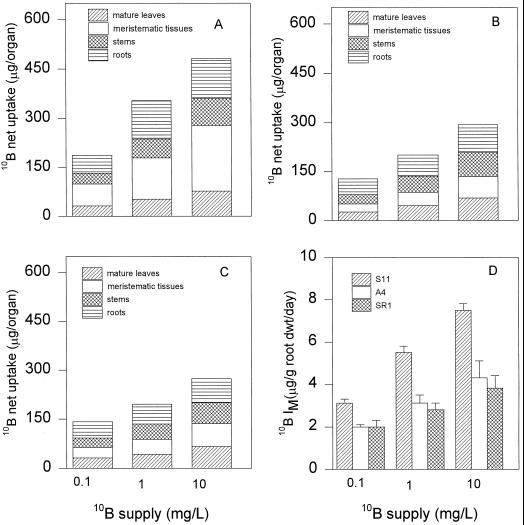
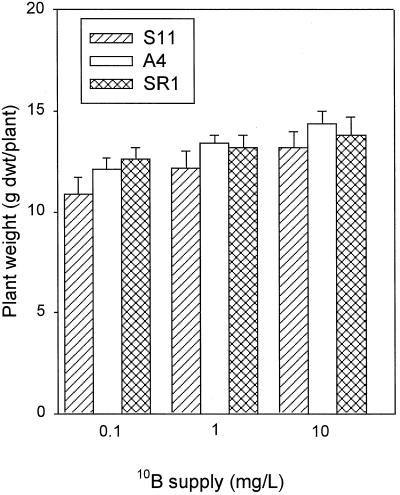
At all concentrations (0.1, 1.0, and 10 mg L−1) of 10B, the total net uptake of 10B (Fig. 2, A, B, and C) in S11 was higher than in A4 and SR1. In S11 the major site of 10B accumulation was in the meristematic tissues, whereas in A4 and SR1 the major site of 10B accumulation was in the mature leaves and roots. IM was also significantly higher in S11 than in A4 and SR1 (Fig. 2D). IM in S11 grown with 10 mg L−1 boron was 174% and 197% compared with IM in A4 and SR1. There was no difference in plant dry weight among the lines (Fig. 3). This indicates that the increase in 10B uptake in line S11 was due mainly to the ability of S11 to acquire more 10B from the medium, and was not a result of differences in plant growth.
Figure 2.
Net uptake of 10B (after 10B treatment) per organ in S11 (A), A4 (B), SR1 (C), and IM (D) of tobacco plants grown in 0.1, 1.0, and 10 mg L−1 10B for a period of 3 weeks. Bars represent means of four replicates ± se.
Figure 3.
Total dry weight in S11, A4, and SR1. Plants were grown in 0.1, 1.0, and 10 mg L−1 10B for a period of 3 weeks. Bars represent means ± se of four replicates.
Sorbitol in Plant Tissues
No sorbitol was detected in either A4 or SR1. The concentration and content of sorbitol plants in S11 were higher in meristematic tissues compared with mature leaves, stems, or roots, and this pattern was observed when the plants were grown with 0.1, 1.0, or 10 mg L−1 10B in the medium (Table I). Total sorbitol (micromoles per plant) increased as 10B increased in the medium (Table I).
Table I.
Sorbitol in mature leaves, meristem tissues, stems, and roots of transgenic (sense) plants
| Organ | Sorbitol Concentration | Sorbitol Content | Total Sorbitol |
|---|---|---|---|
| μmol/g fresh wt | μmol/organ | μmol/plant | |
| 0.1 mg/mL 10B | |||
| Mature leaves | 0.37 ± 0.04 | 7.1 ± 0.5 | |
| Meristem tissues | 0.46 ± 0.03 | 12.0 ± 1.1 | 38.6 ± 1.4 |
| Stems | 0.30 ± 0.03 | 10.8 ± 1.1 | |
| Roots | 0.25 ± 0.04 | 8.7 ± 1.6 | |
| 1.0 mg/mL 10B | |||
| Mature leaves | 0.52 ± 0.03 | 11.0 ± 0.7 | |
| Meristem tissues | 0.63 ± 0.03 | 17.6 ± 2.4 | 55.3 ± 1.4 |
| Stems | 0.38 ± 0.03 | 14.7 ± 1.9 | |
| Roots | 0.33 ± 0.03 | 12.1 ± 1.3 | |
| 10 mg/mL 10B | |||
| Mature leaves | 0.87 ± 0.04 | 19.6 ± 1.6 | |
| Meristem tissues | 0.97 ± 0.04 | 29.1 ± 1.8 | 85.4 ± 6.3 |
| Stems | 0.57 ± 0.06 | 23.3 ± 3.3 | |
| Roots | 0.36 ± 0.02 | 13.7 ± 0.7 |
Plants were grown in 0.1, 1.0, and 10 mg/L-1 10B for 3 weeks. Values are means of four replicates ± se.
Correlation between Sorbitol and Boron Uptake
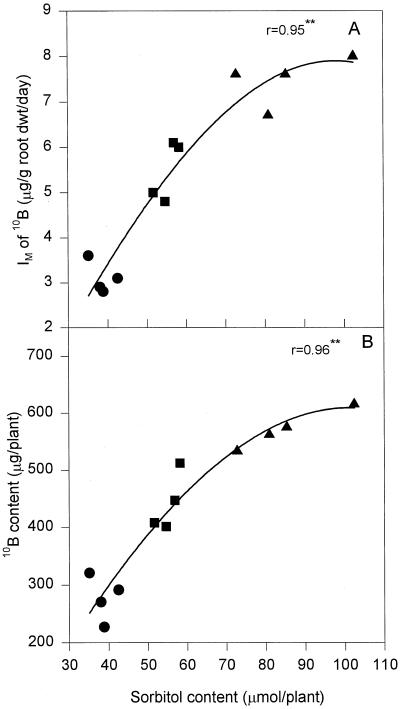
The 10B IM and the content of 10B in plant tissues were closely correlated with the content of sorbitol in the plant, and plants receiving the highest 10B treatment had both the highest IM of 10B and the highest tissue sorbitol content (Fig. 4).
Figure 4.
Correlation between sorbitol content and IM of 10B (A) and 10B content (B) in S11. Plants were grown in 0.1 (•), 1.0 (▪), and 10 mg L−1 (▴) 10B for 3 weeks. **, P < 0.01.
Experiment 2: Translocation and Distribution of Boron
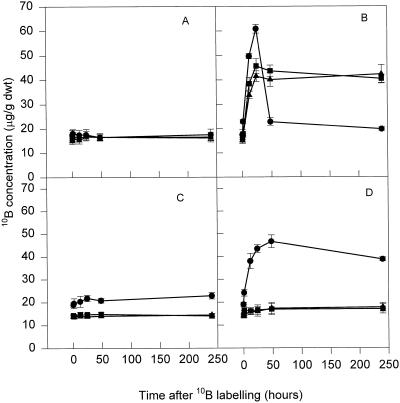
In lines A4 and SR1, foliar boron application increased boron concentration in treated leaves and had no effect on meristematic tissue leaves (Fig. 5, B and D). In line S11 foliar 10B application initially caused an increase in mature leaf 10B (24 h) followed by a decline in leaf 10B to control values by 48 h (Fig. 5B). Simultaneously, there was an increase in 10B in meristematic tissues of S11 (Fig. 5D). 10B in meristematic tissues of S11 increased from 19 μg g−1 dry weight at zero time to 46.6 μg g−1 dry weight (245%) by 48 h. This increase of 10B observed in the S11 meristematic tissues was not observed in the meristematic tissues of untreated S11, A4, or SR1 (Fig. 5, A and C) or treated A4 or SR1.
Figure 5.
Changes in 10B concentration in mature leaves in untreated (A) and treated (B) plants, and meristematic tissues in untreated (C) and treated (D) plants over a period of 250 h. 10B was applied to the mature leaves of S11 (•), A4 (▪), and SR1 (▴) at a concentration of 700 mg L−1.
DISCUSSION
Mature leaves, meristematic tissues, stems, and roots of transgenic (S11) tobacco plants had higher concentrations, greater net uptake, and IM of 10B than the antisense transformed or wild-type plants. There was a close, positive correlation between sorbitol production and total 10B content in the plant and IM of 10B. Foliar 10B labeling further demonstrated that the synthesis of sorbitol enhanced the transport of boron in S11.
These results suggest that the presence of sorbitol increased both boron uptake and transport, and support the results of Brown and Hu (1996), who demonstrated that the mobility of boron was mediated by the presence of sorbitol and the formation and transport of sorbitol-boron complexes in sorbitol-producing species.
The results presented here also suggest that the presence of boron-binding compounds in the cell may increase boron uptake. This conclusion is consistent with the current understanding of the mechanism of boron uptake, which is thought to be a nonmetabolic process determined in part by the formation of nonexchangeable boron complexes within the cytoplasm and cell wall (Brown and Hu, 1994). Although an increase in boron-binding compounds (e.g. sorbitol) in transgenic tobacco (S11) might be expected to increase boron uptake by maintaining a favorable gradient for boron diffusion into the plant, it should be noted that boron uptake occurs in tobacco plants containing no sorbitol. Clearly, sorbitol is not required for boron uptake, although its presence apparently enhances both uptake and translocation to the shoot. The influence of sorbitol production on boron uptake cannot be attributed to changes in cellular osmotic status, as the concentrations of sorbitol present are not osmotically significant.
Both of the experiments that we conducted here and reported previously (Brown et al., 1999) demonstrate that sorbitol synthesis influences boron mobility. When foliar 10B was applied to mature leaves of S11, A4, and SR1 plants, meristematic tissues in S11 had the highest concentrations of 10B, whereas mature leaves of A4 and SR1 had the highest 10B concentrations. An increase in the proportion of boron allocated to meristematic tissues is typical of species in which sorbitol is abundant and boron is phloem mobile (Brown and Hu, 1996). Higher apical than basal concentrations of a nutrient are considered to be indicative of phloem mobility (Van Goor and Van Lune, 1980). Foliar 10B applications further verified the phloem mobility of 10B in strain S11. The rate at which 10B disappeared from leaves receiving 10B, and the appearance of 10B in nontreated meristematic tissues of S11, but not A4 or SR1, clearly confirm that the synthesis of sorbitol facilitated boron mobility.
The lack of mobility of foliar-applied 10B to the meristematic tissues in A4 and SR1 could be due to the high boron-fixing capacity of these lines, which would make the foliar-applied 10B and naturally root-absorbed boron unavailable for retranslocation (Brown and Hu, 1994). An alternative explanation is that the translocation of free boron as H3BO3 cannot take place because of the inherently high membrane permeability of H3BO3, which may lead to a leakage of free boron from the phloem vessels to the adjacent xylem vessel (Oertli and Richardson, 1970). The boron-sorbitol complex may facilitate boron translocation by preventing its complexation to insoluble compounds within the leaves, or the formation of sorbitol-boron complexes may alter the membrane permeability of boron, thereby overcoming the theoretical constraints to boron mobility proposed by Oertli and Richardson (1970).
The results of the current experiments suggest that the enhanced boron uptake in S11 tobacco plants resulted from increased uptake of boron by roots and by enhanced translocation of boron from roots to shoots. Together, these processes would reduce the concentration of free H3BO3 in the root-cell symplasm and hence favor increased boron uptake.
The production of sorbitol in line S11 was influenced by the presence of boron in the medium. The mechanism by which this occurred is unknown but may suggest that the formation of the sorbitol-boron complex favors further sorbitol synthesis by removing end-product inhibition. The concentration of sorbitol in S11 was approximately 0.3 to 1.0 μmol g−1 fresh weight, whereas boron was present at 0.1 to 0.5 μmol g−1 fresh weight. A preliminary experiment showed that with a high boron concentration in the medium (10 mg L-1), a majority of the cellular boron was soluble. If we presume that sorbitol and boron were localized together (the formation of the boron-sorbitol complex would favor this), then adequate boron was present to effectively complex most available sorbitol and favor more sorbitol production.
The genetic manipulation of tobacco to produce sorbitol clearly increased uptake and enhanced boron mobility. These two factors would be expected to result in overall improvement of the plants' ability to tolerate low-boron soils and to withstand brief periods of boron deficiency. Evidence suggests that boron plays a critical role in flowering and seed yield, and that short-term deficiencies of boron (as a result of drought, low transpiration, or rapid plant growth) can result in substantial yield reductions (Dell and Huang, 1997; Brown et al., 1999). Enhanced boron uptake and the ability to remobilize boron to supply reproductive boron requirements would clearly be a significant adaptive advantage with important agricultural implications. These advantages occurred with no apparent decrease in plant growth over the period used here (not shown). Long-term trials should be conducted to verify the utility of this approach in improving plant tolerance to a low-boron environment.
ACKNOWLEDGMENTS
We wish to thank Henry Fisk, Sandy Uratsu, David Zeng, and Carl Greve for technical assistance. We are grateful to Richard Bell, Murdoch University, and Hening Hu for their critical reading of the manuscript.
Abbreviations:
- IM
specific uptake rate
- S6PDH
sorbitol-6-P dehydrogenase
Footnotes
This work was supported by the U.S. Department of Agriculture (grant no. 9801010).
LITERATURE CITED
- Bellaloui N, Brown PH. Cultivar differences in boron uptake and distribution in celery (Apium graveolens), tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) Plant Soil. 1998;198:153–158. [Google Scholar]
- Brown JC, Jones WE. Differential transport of boron in tomato (Lycopersiconesculentum Mill.) Physiol Plant. 1971;25:279–282. doi: 10.1104/pp.49.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Hu H, Dandekar A. Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron dificiency. Plant Physiol. 1999;119:17–20. doi: 10.1104/pp.119.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Hu H. Boron uptake by sunflower, squash and cultured tobacco cells. Physiol Plant. 1994;91:435–441. [Google Scholar]
- Brown PH, Hu H. Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol-rich species. Ann Bot. 1996;77:497–505. [Google Scholar]
- Brown PH, Shelp BJ. Boron mobility in plants. Plant Soil. 1997;193:85–101. [Google Scholar]
- Dell B, Huang L. Physiological response of plants to low boron. Plant Soil. 1997;193:103–120. [Google Scholar]
- Greve C, Labavitch JM. Cell wall metabolism in ripening fruit. V. Analysis of cell wall synthesis in ripening tomato pericarp tissue using a d-[U-13C]glucose tracer and gas chromatography- mass spectrometry. Plant Physiol. 1991;97:1456–1461. doi: 10.1104/pp.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EJ. Movement of boron out of tree fruit leaves. Hortic Sci. 1991;26:271–273. [Google Scholar]
- Hoagland DR, Arnon DI. The Water-Culture Method for Growing Plants without Soil. California Experiment Station Circular no. 347. The College of Agriculture, Berkeley: University of California; 1950. [Google Scholar]
- Hu H, Brown PH. Absorption of boron by plant roots. Plant Soil. 1997;193:49–58. [Google Scholar]
- Hu H, Penn SG, Lebrilla CB, Brown PH. Isolation and characterization of soluble B-complexes in higher plants. The mechanism of phloem mobility of boron. Plant Physiol. 1997;113:649–655. doi: 10.1104/pp.113.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–404. [Google Scholar]
- Moing A, Carbonne F, Rashad MH, Gaudilere JP. Carbon fluxes in mature peach leaves. Plant Physiol. 1992;100:1878–1884. doi: 10.1104/pp.100.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nable RO, Lance RCM, Cartwright B. Uptake of boron and silicon by barley genotypes with differing susceptibilities to boron toxicity. Ann Bot. 1990;66:83–90. [Google Scholar]
- Nable RO, Paull G (1991) Mechanism and genetics of tolerance to boron toxicity in plants. In Randall DD, Blevins DG, Miles CD, eds, Current Topics in Plant Biochemistry and Physiology, Vol 10. University of Missouri Press, Columbia, MS, pp 257–273
- Oertli JJ, Richardson WF. The mechanism of boron immobility in plants. Physiol Plant. 1970;23:108–116. [Google Scholar]
- Picchioni GA, Weinbaum SA, Brown PH. Retention and the kinetics of uptake and export of foliage-applied, labeled boron by apple, pear, prune, and sweet cherry leaves. J Am Soc Hortic Sci. 1995;120:28–35. [Google Scholar]
- SAS (1985) SAS User's Guide: Statistics. SAS Institute, Cary, NC, pp 433–506
- Shelp BJ, Marentes E, Kitheka AM, Vivekanandan P. Boron mobility in plants. Physiol Plant. 1995;94:356–361. [Google Scholar]
- Tao R, Uratsu LS, Dandekar AM. Sorbitol synthesis in transgenic tobacco with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Cell Physiol. 1995;36:525–532. doi: 10.1093/oxfordjournals.pcp.a078789. [DOI] [PubMed] [Google Scholar]
- van Goor BJ, van Lune P. Redistribution of potassium, boron, magnesium and calcium in apple trees determined by an indirect method. Physiol Plant. 1980;48:21–26. [Google Scholar]
- Williams RF. The effect of phosphorus supply on the rate of intake of phosphorus and nitrogen and upon certain aspects of phosphorus metabolism in gramineous plants. Austr J Sci Res B. 1948;1:333–361. [Google Scholar]